Abstract
Indoleamine 2,3 dioxygenase (IDO) is the rate-limiting enzyme in the kynurenine pathway, catabolizing tryptophan to kynurenine. Tryptophan depletion by IDO expressing tumors is a common mechanism of immune evasion inducing regulatory T cells and inhibiting effector T cells. As mammalian cells cannot synthesize tryptophan, it remains unclear how IDO positive tumor cells overcome the detrimental effects of local tryptophan depletion.
We demonstrate that IDO positive tumor cells express a novel amino acid transporter, which accounts for approximately 50% of the tryptophan uptake. The induced transporter is biochemically distinguished from the constitutively expressed tryptophan transporter System L by increased resistance to inhibitors of System L, resistance to inhibition by high concentrations of most amino acids tested, and high substrate specificity for tryptophan. Under conditions of low extracellular tryptophan, expression of this novel transporter significantly increases tryptophan entry into IDO positive tumors relative to tryptophan uptake through the low affinity System L alone, and further decreases tryptophan levels in the microenvironment. Targeting this additional tryptophan transporter could be a way of pharmacological inhibition of IDO mediated tumor escape.
These findings highlight the ability of IDO-expressing tumor cells to thrive in a tryptophan depleted microenvironment by expressing a novel, highly tryptophan-specific transporter, which is resistant to inhibition by most other amino acids. The additional transporter allows tumor cells to strike the ideal balance between supply of tryptophan essential for their own proliferation and survival, and depleting the extracellular milieu of tryptophan to inhibit T cell proliferation.
INTRODUCTION
Metabolism of tryptophan, like that of other essential amino acids, is tightly regulated. Its degradation through the kynurenine pathway is mediated by two functionally similar, but structurally unrelated, enzymes: Indoleamine 2,3-dioxygenase (IDO) and Tryptophan 2,3-dioxygenase (TDO)5 (1, 2). TDO is liver-specific and is expressed constitutively (3), whereas IDO is induced by IFN-γ in many cell types, including those of the epithelial and monocytic lineages (4, 5).
The IDO dependent kynurenine pathway is an important component of anti-bacterial innate immune responses, as depletion of intracellular tryptophan hinders replication of invading microorganisms (6). In recent years, the kynurenine pathway has been implicated in regulation of a number of immune processes, as a consequence of tryptophan depletion and/or metabolite toxicity (7-9). Placental expression of IDO prevents fetal rejection by suppressing the maternal immune response to fetal antigens (10). In keeping with this immunosuppressive function, loss of IDO expression or activity has been shown to enhance several autoimmune diseases, including experimental autoimmune encephalomyelitis (11, 12) and insulin-dependent diabetes mellitus (13), and to shorten graft survival (14, 15). The steroid dexamethasone was found to inhibit onset of allergic inflammation by inducing reverse signaling into plasmacytoid dendritic cells (pDC) and inducing expression of IDO (16). Induction of IDO in the context of experimental autoimmunity including graft versus host disease and diabetes can lead to amelioration of the disease (17, 18).
Van den Eynde and colleagues showed that many human tumors constitutively express IDO and that expression of IDO by immunogenic mouse tumor cells prevents their rejection by pre-immunized mice (19). IDO can be upregulated by human and murine dendritic cells (DC) on maturation (20-22) and IDO expressing regulatory DC were found to suppress T cell proliferation in mixed lymphocyte reactions in a manner that is reversed by the addition of tryptophan (23, 24). In mice, these cells were defined as a subset of pDC, found in abundance in tumor-draining lymph nodes (25). A functionally similar population of IDO+ pDC was found in human tumor-draining lymph nodes and was shown to negatively correlate with survival of melanoma patients (26). IDO expression by DC indicates that IDO activity, immunosuppression and tolerance could be linked (27). A population of DC constitutively expressing IDO has recently been described in the mesenteric lymph nodes of mice (28), and may play a role in maintenance of oral tolerance.
While it is well established that IDO expression by antigen presenting cells or tumors can inhibit immune responses, it is unclear whether IDO dependent immunosuppression is mediated by tryptophan depletion (9, 29) or by the toxicity of kynurenine and its downstream metabolites (8, 30). Although these two models are not mutually exclusive, they do have different implications for therapeutic approaches. Whilst not excluding the role of metabolite toxicity, this work particularly addresses mechanisms involving IDO dependent tryptophan depletion.
Tryptophan depletion is known to halt cell cycle progression by triggering the anti-proliferative GCN2 pathway in lymphocytes (31-33). However, there is currently little understanding of why some cells (such as T lymphocytes) are sensitive to tryptophan depletion, whereas others such as tumor cells are resistant to conditions of low tryptophan concentration. It is likely that regulation of tryptophan transport accounts for the differential susceptibility to tryptophan depletion between different cell types.
Amino acids are taken up by substrate-specific transmembrane transporters. In mammalian cells, transporter-mediated tryptophan uptake occurs mainly via the ubiquitously expressed, neutral amino acid transporter System L, which transports large hydrophobic amino acids in a sodium-independent manner (34, 35). System L is heterodimeric, composed of a heavy glycoprotein chain (CD98, encoded by SLC3A2), and one of two catalytic light chains LAT1 or LAT2, encoded by SLC7A5 and SLC7A8 genes (36, 37). System L is inhibited by 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) under sodium-free conditions (34, 38-40). Altered tryptophan uptake into IDO expressing cells could account for the ability of tumors to overcome the effects of tryptophan shortage. Consistent with this hypothesis, monocyte differentiation into macrophages, which is accompanied by induction of IDO, is associated with the up-regulation of a high affinity tryptophan transporter, in addition to System L (41).
In this paper, we demonstrate a link between IDO expression and transporter-mediated tryptophan uptake. Our results indicate that IDO expression, both in mouse and human tumor cells, results in modified tryptophan uptake through a novel transport system that has functional properties different from those of System L. These findings highlight a mechanism by which IDO positive tumors can survive under conditions of low tryptophan concentrations and may indeed contribute to IDO mediated depletion of tryptophan from the tumor microenvironment.
MATERIALS AND METHODS
Reagents
All chemicals including tryptophan, 1-methyl DL-tryptophan (1-MT) and BCH were purchased from Sigma Aldrich unless otherwise stated. Compounds sparingly soluble in aqueous solution (such as tryptophan) were dissolved in dimethyl sulfoxide prior to further dilution. 3H-L-tryptophan, 14C-L-histidine and 14C-L-lysine were obtained from Amersham Biosciences and American Radiolabeled Chemicals (Cardiff, UK). Human and mouse IFN-γ were from Peprotech. Anti-mouse IDO, anti-human CD3-APC and purified anti-human CD3 and CD28 antibodies were from Santa Cruz Biotechnology, BD Biosciences and Invitrogen. CFSE was from Molecular Probes, Invitrogen.
Cell lines and culture medium
The EG7-OVA cell line was obtained from the American Type Culture Collection and is a mouse T lymphoma line transfected with a plasmid encoding chicken OVA. The Hela epithelial carcinoma cell line was from Cancer Research UK. EG7 cells were cultured in RPMI 1640 medium supplemented with 10% FCS, L-glutamine, 1% non-essential amino acids (v/v), 50U/ml penicillin, 50mg/ml streptomycin, 2-mercaptoethanol and 0.4mg/ml G418. Hela cells were cultured in MEM supplemented with 10% FCS, L-glutamine, 1% non-essential amino acids (v/v), 50U/ml penicillin and 50mg/ml streptomycin and 2-mercaptoethanol.
IDO expression
To induce expression of IDO tumor cells were transduced with recombinant lentiviral particles encoding IDO (exogenous expression) or treated with 1000U/ml recombinant IFN-γ for 48h (endogenous expression).
To generate lentiviral vectors encoding murine IDO, mouse intestinal mRNA was reverse-transcribed and the cDNA was used as a template for the amplification using the following forward and reverse primers: 5′CCTGATCACCACCATGGCACTCAGTAAAATATC 3′ 5′AACTCGAGCTAAGGCCAACTCAGAAGAGC 3′ The PCR product was ligated into the lentiviral vector pHR-SIN-BX-IRES-EM also containing the cDNA for GFP (42). 293T cells were used to produce viral particles. Control viral particles were also generated using a vector encoding GFP alone. Transduced EG7 cells were sorted on the basis of GFP expression and cloned by limiting dilution. IDO expression in EG7 clones was verified by RT-PCR and flow cytometry.
Hela cells expressing human IDO were made in a similar manner. Human IDO cDNA was amplified from mRNA of IFN-γ treated THP-1 cells using the following forward and reverse primers: 5′ AATGATCACCACCATGGCACACGCTATGG 3′ 5′GCCTCGAGTTAACCTTCCTTCAAAAGGGATTTC 3′ IDO expression in sorted, GFP positive, transduced Hela cells was verified by RT-PCR. Concentrations of tryptophan and kynurenine were measured in the culture supernatants by HPLC.
Detection of IDO protein by flow cytometry
For intracellular detection of IDO in transduced EG7 clones, cells were fixed with 2% paraformaldehyde, permeabilized with Saponin buffer (10mM Hepes, 5% FCS and 5mg/ml Saponin) and stained with a rabbit polyclonal anti-mouse IDO antibody (Santa Cruz) and Phycoerythrin (PE)-conjugated goat anti-rabbit IgG as a secondary antibody.
Measurement of IDO enzymatic activity
Cells were washed twice and resuspended in ice cold PBS with Pepstatin (1mg/ml) and Leupeptin (1mg/ml). Cells were lysed by repeated freeze/ thawing and centrifuged at 4°C for 5 min at 14000g. Lysate was stored at −20°C for use in enzymatic assays. The enzyme assays were initiated at 37°C by mixing equal volumes of lysate and incubation medium (100mM potassium phosphate buffer pH 6.5, 40mM ascorbic acid, 20mM methylene blue, 200 units/ml catalase and different concentrations of L-tryptophan). The reaction was terminated after 1h by addition of 30% (w/v) Trichloroacetic acid and the mixture incubated for a further 30 min at 50°C. After centrifugation, the clear supernatant was injected onto a Spherisorb S5-ODS1 column, 4.6mm×150mm (Waters, Milford, MA, USA) using an HPLC system consisting of a Knauer pump and variable wavelength detector and a BioTek 565 autosampler. The mobile phase consisted of 40mM sodium citrate buffer (pH 2.25), 50% methanol and 0.4mM SDS. Kynurenine and tryptophan were detected at 365 nm and 280 nm wavelengths respectively. Concentrations were normalized to the amount of protein in the cell lysate.
Measurement of amino acid uptake
Cells were cultured for 48h, then washed and incubated in PBS or choline buffer (pH 7.4, Choline Chloride, CaCl2, MgCl2, KCl, KH2PO4 (monobasic and dibasic)) for 20-30 minutes. 3H-L-tryptophan or 14C-L-Histidine was added at 50pmol/ml. After a fixed time at 37°C, uptake was terminated by layering the EG7 cells onto an oil layer before spinning in a microfuge to separate cells from the 3H-L-tryptophan-containing solution. Tryptophan uptake by EG7 cells was performed with duplicate samples.
For Hela cells, assays were performed in 6 or 12-well plates at 90-100% confluence. The uptake at 37°C was stopped by removing the supernatant and adding 20mM L-Lysine in ice-cold PBS. The cells were lysed with lysis buffer (1% NaOH, 0.1% SDS). 3H-L-tryptophan uptake was determined by liquid scintillation. To determine the period of time for which uptake was linear (initial rate), a time-course of 3H-L-tryptophan uptake was performed and showed that uptake increased in a linear fashion over the first 3 min. This time-point was used in subsequent experiments. Tryptophan uptake by Hela cells was performed with triplicate samples.
CFSE T cell proliferation
Hela cells were cultured for 72h and the supernatants harvested and filtered through a 0.2μm filter. 96 well flat bottom plates were coated with 5μg/ml anti-human CD3 and CD28 antibodies. PBL were isolated from the blood of healthy volunteers and labeled with 0.5μM CFSE, resuspended in pre-conditioned supernatant and 1-2×105 cells added to each well. In some wells the pre-conditioned supernatant had 25μM fresh L-tryptophan added. After 3-4d cells were harvested and stained with an anti human CD3-APC antibody (BD Biosciences). The number of T cell divisions was determined by flow cytometry, gating on propidium iodide negative (live) cells. The analyses were performed on duplicate wells.
RESULTS
IDO dependent modulation of tryptophan uptake by tumor cells
To study the relationship between IDO expression and tryptophan uptake, we first set up an in vitro cellular model for constitutive IDO expression. EG7 tumor cells were transduced with a lentiviral vector encoding the full-length mouse Ido with GFP. Wild type (WT) EG7 cells did not express detectable IDO; while IDO transduced EG7 cells expressed IDO at levels comparable to IFN-γ treated WT EG7 cells (Supplementary Fig. 1A). After cloning IDO-lentivirus transduced EG7 cells, five clones (clone 7, clone 12, clone 23, clone 25 and clone 26) were selected, based both on GFP expression (data not shown) and the level of intracellular IDO (Supplementary Fig. 1B). IDO protein expression was highest in clone 7 (C7), while clones 25 and 26 expressed the lowest amount of IDO. In these clones the level of IDO expression correlates with that of GFP expression.
Consistent with the level of IDO expression, clone 7 displayed the highest rate of kynurenine production as determined by HPLC, while clones 25 and 26 showed the lowest (Supplementary Fig. 1C) at different tryptophan concentrations. As a control, EG7 cells were transduced with a lentivirus encoding GFP alone. These cells neither produce IDO transcript nor have IDO activity (data not shown).
Uptake of 3H-L-tryptophan in sodium-containing and sodium-free buffer showed that tryptophan uptake in EG7 cells was sodium-independent (data not shown). Therefore subsequent experiments were conducted under sodium-free conditions. Initial time course experiments indicated that the absolute rate of tryptophan uptake was similar in all of the cell-types tested and was not influenced by expression of IDO (data not shown).
IDO dependent tryptophan uptake is not mediated solely by System L
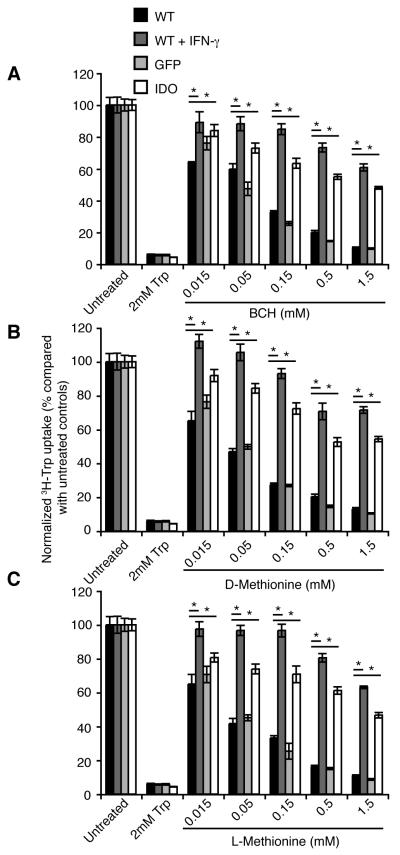
We next compared tryptophan uptake in WT, GFP controls and IDO positive C7 cells. The effect of unlabeled tryptophan as an inhibitor was compared between the different cell types and there was no apparent difference between IDO positive and negative cells (Fig. 1A). To determine whether tryptophan transport in IDO positive, EG7-C7 tumor cells was mediated solely through the ubiquitous System L transporter or whether other transporters were induced, the potent and selective inhibitor of System L, BCH was tested under sodium-free conditions (34, 38-40) (Fig. 1B). High concentrations of BCH completely abolished transporter-mediated 3H-L-tryptophan uptake in EG7-WT, GFP and IDO positive cells. In contrast, inhibition of tryptophan uptake at lower BCH concentrations was significantly greater in WT and GFP cells than in the IDO positive C7 cells. Using these data we calculated that the inhibition constants (Ki) for the EG7-GFP and WT cells for BCH was 12μM, while for the IDO expressing EG7 cells the Ki was 80μM, indicating the presence of a BCH-resistant component to tryptophan uptake in IDO positive cells.
Figure 1. IDO positive EG7 cells express an alternative BCH resistant tryptophan transporter in addition to System L.
3H-Tryptophan uptake was measured over 3 minutes in EG7 WT, GFP-transduced or IDO-transduced (C7) cells after incubating for 30 minutes in sodium and amino acid free buffer. Uptake measurements were performed in duplicate and were measured in the presence of different concentrations of different inhibitors as indicated. A. Unlabelled tryptophan competes with the 3H-tryptophan for uptake through all tryptophan transporters and has a similar effect on tryptophan uptake by each cell type. B. BCH inhibits tryptophan uptake through System L, and is more potent at inhibiting uptake in IDO negative tumor cells. C. D-Met and D. the isomer L-Met inhibit tryptophan uptake through LAT1 mediated transporters and are more effective in IDO negative cells. Data are normalized to uptake in untreated cells and are shown as the means of duplicate samples from at least two combined independent experiments ± SE. Statistical significance <0.05 as assessed using Student’s T-test is indicated by *. Data are representative of at least three independent experiments.
We then examined 3H-tryptophan uptake in the presence of a panel of inhibitors, shown to discriminate between various members of the LAT transporter family (43). Experiments were performed using a combination of BCH together with an excess of the following inhibitors: methyl-aminoisobutyric acid (MeAIB) that inhibits System-A transporters such as ATA2, N-ethyl maleimide (NEM) that inhibits LAT2, 3 and 4 and D-Methionine (D-Met) that inhibits LAT1 (43). Use of either NEM or MeAIB with BCH at high concentrations completely inhibited tryptophan uptake in WT and GFP-expressing cells, while in IDO positive cells a component of the uptake was resistant to inhibition (data not shown). In the presence of both BCH and D-Met, inhibition of 3H-tryptophan uptake was complete in both the WT and GFP-transduced cells, while a significant proportion of 3H-tryptophan uptake was still present in IDO expressing cells (data not shown).
We titrated the effect of D-Met or L-Met on tryptophan uptake in the absence of BCH. Both isomers inhibited over 80% of the tryptophan uptake both in WT and GFP-expressing cells at 0.05mM, while uptake in EG7-C7 cells was maintained at values greater than 40% (Fig. 1C&D). The Ki for D-Met, calculated based on a single tryptophan uptake system, was approximately 10-fold higher in the IDO positive cells than the IDO negative cells (100-300μM and 10-30μM, respectively). When the Ki were calculated using an equation based on two independent systems with different affinities, the data for the WT and GFP-positive cells indicated the presence of a single transporter while IDO positive C7 cells appeared to have more than one tryptophan transporter, the first with the same affinity for D- and L-Met as in the WT and GFP cells, and an additional transporter with a 10-fold lower affinity for D- and L-Met (36).
In conclusion, these data indicate that tryptophan transport in IDO positive cells occurs through two separate mechanisms: the BCH and D/L-Met-sensitive System L (CD98 and LAT1/2) and an additional transporter that is relatively resistant to BCH and D/L-Met inhibition. NEM or MeAIB alone, known to inhibit LAT2, 3 and 4 or System A respectively, had minimal effect on tryptophan uptake in IDO positive cells (data not shown), suggesting the additional tryptophan uptake observed is unlikely to be mediated via one of the other LATs.
Expression of IDO in Hela cells inhibits T cell proliferation in vitro
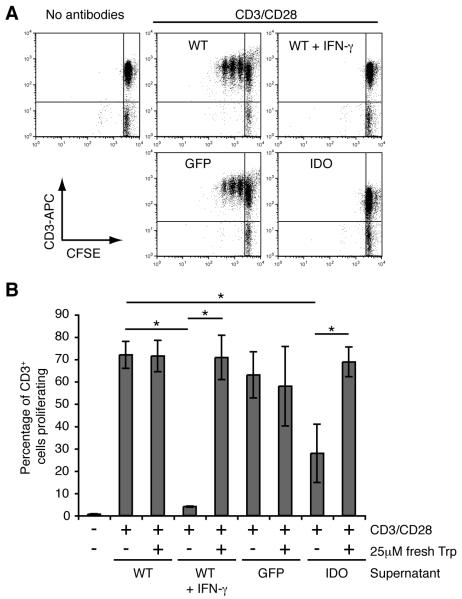
To establish whether results obtained using mouse tumors could be extended to human tumor cells, we generated a lentiviral vector encoding human IDO and used this to transduce Hela cells. Untransduced Hela cells were also treated with IFN-γ, to induce endogenous IDO expression. RT-PCR and q-RT-PCR confirmed the expression of IDO mRNA (data not shown), while analysis of the culture supernatants confirmed conversion of tryptophan to kynurenine in cultures of IDO transduced and IFN-γ-treated Hela cells, but not with untreated or GFP-transduced cells (Supplementary Fig. 1D). To confirm functional differences between IDO positive and negative Hela cells in their ability to reduce tryptophan levels in tissue culture medium, T cell proliferation in the presence of medium from IDO positive and IDO negative Hela cells was compared (Fig. 2). IDO positive or negative Hela cells were cultured for 72h in complete MEM in the presence or absence of IFN-γ. Peripheral blood lymphocytes purified from healthy donors and labeled with CFSE, were cultured in the pre-conditioned media in 96 well plates pre-coated with anti-CD3 and anti-CD28 antibodies (Fig. 2A&B). T cell proliferation as indicated by CFSE dilution was completely inhibited when cells were cultured in supernatant from IDO positive Hela cells, while they proliferated when cultured in supernatant from WT or GFP cells (Fig. 2A). T cell proliferation was restored by diluting the IDO conditioned supernatant 1:2 with fresh complete MEM or by conditioning the MEM with IDO positive cells in the presence of the IDO inhibitor 1-MT (data not shown). Finally we used pre-conditioned MEM that was reconstituted with 25μM L-tryptophan (Fig. 2B). T cell proliferation in conditioned MEM from IDO positive cells reconstituted with tryptophan was restored to the same levels as seen in WT cells (Fig. 2B), despite the presence of kynurenine metabolites, suggesting that depletion of tryptophan is likely to be the mechanism of T cell inhibition in this model.
Figure 2. Inhibition of T cell proliferation by IDO positive Hela cells by depletion of tryptophan.
Peripheral blood lymphocytes were labeled with CFSE and cultured in preconditioned supernatants from Hela cells in 96 well flat-bottomed plates, pre-coated with PBS or with antibodies against human CD3 and CD28. After 4d the cells were harvested, stained with anti human CD3-APC and acquired on the flow cytometer (FACScalibur), gating on propidium iodide negative (live) cells and analyzed using FlowJo software. A. Representative dot plots are shown from cells cultured in conditioned supernatant from WT, WT + IFN-γ, GFP or IDO Hela cells, with CFSE against CD3-APC and T cell proliferation is shown by dilution of CFSE. B. T cell proliferation in pre-conditioned supernatant can be restored to normal levels by adding 25μM fresh L-tryptophan to the T cell cultures. Data are shown as percentage of CD3+ T cells that have undergone proliferation and are the means of duplicate wells from two independent experiments combined ± SE. The data shown are representative from at least three experiments. Statistical significance <0.05 as assessed using Student’s T-test is indicated by *.
Expression of human IDO by Hela cells induces expression of a BCH resistant tryptophan transporter
Having established that IDO positive Hela cells can deplete tryptophan from tissue culture medium, we sought to determine whether (in line with our previous results with murine cells) in addition to IDO dependent tryptophan degradation, IDO positive Hela cells could further reduce tryptophan in the medium by up-regulating a second tryptophan transporter. To address this possibility, we compared the effects of BCH and D- and L-Met on 3H-tryptophan uptake by Hela cells (Fig. 3A-C). 3H-tryptophan uptake by WT and GFP-transduced cells was strongly inhibited by BCH (Fig. 3A), D-Met (Fig. 3B) and L-Met (Fig. 3C), but less so by MeAIB or NEM (data not shown). In contrast, each of the three inhibitors was significantly less effective at inhibiting 3H-tryptophan uptake by IDO transduced cells. Similar effects were seen with cells expressing endogenous IDO in response to IFN-γ treatment. The Ki for BCH was calculated to be approximately 300 and 600μM for IDO and IFN-γ treated cells respectively, while for control cells it was 12μM. For D- and L-Met, similar differences in the calculated Ki were observed. Similar to murine tumor cells, tryptophan uptake by IDO positive Hela cells and the effects of D-Met were independent of both sodium concentration (Supplementary Fig. 2A-D) and external pH (data not shown).
Figure 3. Human tumor cells expressing either IDO through transfection or in response to IFN-γ express a BCH resistant tryptophan transporter.
Measurement of transporter-mediated 3H-L-tryptophan uptake was measured under initial rate conditions over 3 min. Hela cells were cultured for 48h before the assay and tryptophan uptake by IDO-lentivirus transduced or IFN-γ treated Hela cells was measured in the presence of a panel of System L and LAT inhibitors including: A. BCH, B. D-Met and C. L-Met. Plotted data are normalized and are the means of triplicate measurements ± SE. Statistical significance <0.05 as assessed using Student’s T-test is indicated by *. Data are representative of at least three independent experiments.
These data indicate that similarly to the results obtained with murine tumor cells, tryptophan uptake by human tumor cells transduced with IDO contains two components, one that is probably System L, as defined by its high sensitivity to BCH and D-Met, and a second that is relatively insensitive to either BCH or D-Met. Similar effects were seen when the tumor cells were treated with IFN-γ to induce expression of IDO.
Characterization of the amino acid specificity of the IDO induced tryptophan transporter
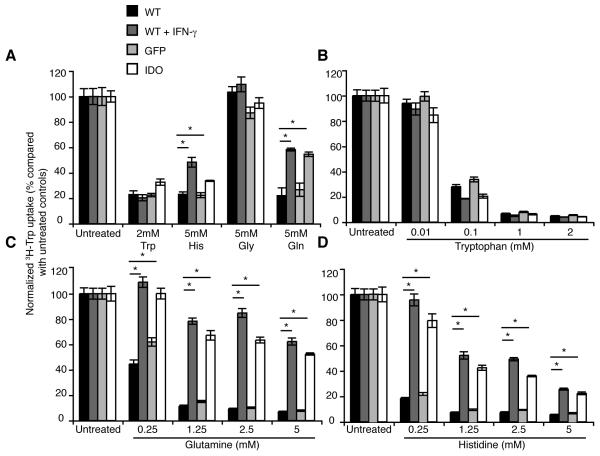
We performed a number of 3H-tryptophan uptake experiments in the presence of competing unlabelled amino acids. Initially we used an excess concentration of a panel of amino acids including tryptophan, histidine, glycine and glutamine (Fig. 4A). Unlabeled tryptophan effectively competed for uptake with the 3H-tryptophan to a similar extent in all cell-types, irrespective of IDO expression. Mediated tryptophan uptake ranged from 80% to greater than 95% in different experiments.
Figure 4. Tryptophan uptake by IDO negative, but not IDO positive Hela cells is inhibited in the presence of glutamine or histidine.
3H-Tryptophan uptake was measured in the presence of unlabeled amino acids to determine the amino acid specificity of the induced transporter in IDO positive cells. A. Tryptophan uptake was measured in the presence of high concentrations of a panel of unlabeled amino acids individually including L-tryptophan, histidine, glycine and glutamine. Tryptophan uptake was inhibited in all cell types tested equally by tryptophan, while glycine had no effect. Histidine or glutamine inhibited tryptophan uptake less effectively by IDO positive compared with IDO negative cells. Titration experiments were performed measuring tryptophan uptake in the presence of a range of concentrations of: B. tryptophan, C. glutamine or D. histidine, indicating equal inhibition by tryptophan across cell types while incomplete inhibition of tryptophan uptake in IDO positive Hela cells with either glutamine or histidine. Plotted data are normalized and the means of triplicate measurements ± SE and are representative of at least two independent experiments. Statistical significance <0.05 as assessed using Student’s T-test is indicated by *. Experiments were performed in 12 well plates.
In contrast, 5mM glycine had no effect on tryptophan uptake in any of the cell-types tested. However with 5mM histidine or 5mM glutamine, we noticed a reproducible difference between IDO positive and negative Hela cells. Tryptophan uptake was completely inhibited in IDO negative cells with a high concentration of histidine or glutamine, while a proportion of the tryptophan uptake was retained in IDO positive Hela cells (Fig. 4A).
To determine the affinity of the different amino acids for the tryptophan transporters we used a range of concentrations of tryptophan, glutamine or histidine. There was little difference between cell types in the inhibition by unlabeled tryptophan at a range of concentrations (Fig. 4B). In contrast, the lower concentrations of glutamine almost completely abrogated tryptophan uptake in WT or GFP transduced Hela cells, while even at 5mM over 50% of tryptophan uptake was maintained in IDO positive cells (Fig. 4C). A qualitatively similar effect was seen for 5mM histidine (Fig. 4D). The calculated Ki for tryptophan was almost identical in each cell type, while for both glutamine and histidine there was a 10-fold increase in the Ki for IDO positive cells compared with IDO negative controls. These data indicate that tryptophan transport occurs through two distinct systems, one of higher and one of lower affinity for histidine, and that there are both glutamine sensitive and glutamine insensitive components of tryptophan transport. Tryptophan uptake by murine EG7–IDO positive cells was also resistant to inhibition by glutamine, while uptake in WT and GFP-transduced cells was inhibited (data not shown).
As a control for non-specific effects, we measured uptake of other amino acids. We found that tryptophan, histidine or glutamine inhibited uptake of 14C-histidine, showing that IDO expression did not affect histidine uptake (data not shown).
We also performed experiments using the human prostate adenocarcinoma cell line PC-3 and found that, similar to that seen in Hela cells, expression of IDO by PC-3 cells either by transfection or indirectly through treatment of the cells with IFN-γ led to the upregulation of a glutamine-resistant tryptophan transport phenotype (Supplementary Figure 3). These data, together with the mouse EG7 cell data, suggest that induction of such a transporter may be a common response to expression of IDO across different tumour cells, although whether expression is restricted to tumours of certain cellular origins remains to be established.
Using the property of glutamine resistance to functionally characterize the IDO induced tryptophan transporter in tumor cells demonstrates a high degree of selectivity for aromatic amino acids
Once we had found that tryptophan uptake by WT and GFP-transduced Hela cells was almost completely abrogated with 2.5-5mM unlabeled glutamine, while a significant component was resistant to inhibition in IDO positive cells, we screened a panel of representative amino acids, as well as BCH in the presence of 2.5mM glutamine for effects on 3H-tryptophan (and 14C-histidine) uptake (Supplementary Table I).
The first observation of note is that the induced transporter is relatively tryptophan and isomer specific. Tryptophan uptake was reduced to baseline in IDO positive cells (in the presence of 2.5mM glutamine) by 2mM unlabelled L-tryptophan, while there was minimal inhibition by D-tryptophan. In IFN-γ treated cells we observed additional effects on tryptophan uptake in the presence of D-tryptophan (Supplementary Table I), possibly resulting from trans-stimulation—the mechanism by which transport of one amino acid stimulates transport of another amino acid, sometimes through secondary or tertiary transporters, as previously described for the cationic CAT family of heterodimeric amino acid transporters (36). It is highly likely that the profile of amino acid transporters present in IFN-γ treated cells is significantly more complex than those in IDO-transfected cells and it is perhaps unsurprising that the degree and pattern of trans-stimulation by different amino acids is different between the two cell types.
There was no additional inhibition of tryptophan uptake by BCH in IDO positive Hela cells in the presence of glutamine. Together with the almost complete inhibition in IDO negative cells, this indicates that glutamine is likely to inhibit tryptophan uptake via System L. It also confirms that the additional transporter in IDO positive cells has properties distinct from those of System L. Other than L-tryptophan, the only amino acid that had any detectable effect on tryptophan uptake in both IFN-γ-treated and IDO transduced Hela cells was phenylalanine, which inhibited tryptophan uptake by over 50%.
We then measured the affinity for tryptophan of the IDO induced transporter in tumor cells. Tryptophan uptake by WT and GFP-transduced cells was completely abolished in the presence of glutamine alone, and was not reduced further by the addition of unlabeled tryptophan. In contrast, in IDO positive tumor cells in the presence of glutamine a clear reduction in tryptophan uptake was observed as the concentration of unlabelled tryptophan increased (Fig. 5A). The Ki of the induced glutamine-resistant 3H-tryptophan transport in IDO positive tumors was 2.3-4.2μM.
Figure 5. 3H-Tryptophan uptake by IDO positive cells in the presence of unlabeled glutamine is inhibited by a range of aromatic amino acids.
A. Unlabelled tryptophan in the presence of 2.5mM glutamine inhibits tryptophan uptake by IDO positive cells with a Ki of 2.3-3.2μM. Curve fitting in the presence of glutamine and tryptophan was performed using Sigmaplot. Individual data points are indicated by open (Hela WT) or filled (GFP) triangles or by open (WT + IFN-γ) or filled circles (IDO). The IDO induced tryptophan transporter is inhibited by some of the aromatic amino acids and their derivatives. B. tyrosine, C. phenylalanine and D. L-Dopa were tested in the presence of 2.5mM glutamine at a range of concentrations. Plotted data are normalized and the means of triplicate measurements ± SE and are representative of at least two independent experiments. Statistical significance <0.05 as assessed using Student’s T-test is indicated by *. Experiments were performed in 12 well plates.
We measured 3H-tryptophan uptake in the presence of glutamine with a titration of tyrosine (Fig. 5B), phenylalanine (Fig. 5C), or L-3,4-dihydroxyphenylalanine (L-Dopa) (Fig. 5D). Both phenylalanine and L-Dopa inhibited the glutamine-insensitive tryptophan flux in IDO positive cells, while tyrosine had little additional effect, suggesting that the induced tryptophan transporter has specificity for more hydrophobic, aromatic amino acids, although qualitatively the effect of tryptophan was of significantly higher affinity.
Together these data indicate that tumor cells can adapt to the relative scarcity of tryptophan in their microenvironment by modifying their capacity for tryptophan uptake by up-regulating a second tryptophan transporter. This mechanism ensures sufficient tryptophan is taken up by tumor cells for optimal protein synthesis, while further depleting the tumor microenvironment of tryptophan and thus inhibiting T cell proliferation.
DISCUSSION
In this paper, we have examined the link between IDO activity and transporter-mediated tryptophan uptake. Our experiments have established that transporter-mediated tryptophan uptake is regulated by the expression of IDO in tumor cells. Our central finding is that IDO activity alters tryptophan uptake in human and mouse tumor cells by inducing a second sodium-independent, glutamine-resistant tryptophan transporter, that is distinct from the constitutively expressed System L.
We have shown that the IDO induced tryptophan transporter in IDO positive EG7 and Hela cells is tryptophan-specific, as the uptake of other amino acids, including lysine and histidine, was similar between IDO positive and IDO negative cells. The observation that excess glutamine effectively inhibited tryptophan uptake by IDO negative tumor cells, while only partially inhibiting uptake in their IDO positive counterparts, provided us with a useful tool to dissect the specificity of the induced transporter. These data indicated that the IDO induced transporter has a high degree of selectivity and a higher affinity for tryptophan than System L, as defined by the observation that 3H-tryptophan uptake in the presence of glutamine is efficiently inhibited by unlabeled tryptophan, and with lower affinity by phenylalanine or L-Dopa, but by none of the other amino acids tested.
Upregulation of a highly tryptophan-specific IDO induced transporter, as compared with the broader amino acid uptake mediated by System L (44), has two main advantages for IDO positive tumor cells. Firstly, it ensures that tryptophan uptake in an environment depleted of tryptophan is not impaired by the competitive transport of other, more abundant, amino acids. Secondly, it further contributes to the IDO dependent tryptophan depletion from the tumor microenvironment, hence further impairing T cell proliferation.
Under ‘normal’ plasma concentrations of tryptophan (approximately 60μM) (45), expression of an additional high affinity transporter would have little effect on the total tryptophan uptake by cells. However, its effect becomes increasingly more important for tryptophan uptake as the local extracellular tryptophan concentration decreases. Previous work has shown that tryptophan depletion to concentrations below 10μM reduces T cell proliferation, while below 1μM T cell proliferation is completely inhibited (46). We confirmed using an in vitro model that depletion of tryptophan by IDO positive tumor cells inhibited T cell proliferation and that replacement of fresh tryptophan or inhibition of IDO was sufficient to restore proliferation to normal levels. In the presence of an additional transporter, the permeability for tryptophan entry at low tryptophan concentrations would be substantially greater. It follows that the additional transporter would increase both depletion of local extracellular tryptophan by IDO positive cells and availability of tryptophan for metabolism by IDO positive tumors. It is notable that the affinity of tryptophan for the novel transporter (3μM) falls precisely into the critical concentration range for the inhibition of T cell proliferation (1-10μM). Interestingly, preliminary data suggests that a glutamine-resistant tryptophan transporter is unlikely to be expressed by activated human T cells as tryptophan uptake in such cells is highly sensitive to inhibition by glutamine (data not shown). It is also known that activation of T cells leads to rapid upregulation of System L (CD98 and LAT1) (47). Indeed CD98 is widely recognized as a marker of activated T cells.
Our findings and the previously published results indicating the induction of a high affinity, tryptophan transporter in IDO positive macrophages (41) highlight the presence of different IDO inducible tryptophan transporters in non-malignant and rapidly proliferating malignant cells, which may be necessary for the different physiological requirements for tryptophan by different cell types. Importantly we also showed that physiological stimuli capable of inducing IDO expression, such as IFN-γ, also led to a similar modification in the tryptophan transport phenotype. It is interesting to note that in microarray experiments, IFN-γ treated Hela cells appear to be highly metabolically active and upregulate, at least at the mRNA level, expression of a large number of transporters for a range of different nutrients (Datasets: GSE11299. Location available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE11299 (48)).
System L comprises a heavy chain (CD98/4F2hc) paired with one of two catalytic light chains, either LAT1 or LAT2, that define the transport specificity. We demonstrated that none of the components of System L are likely to contribute to formation of the novel IDO induced transporter as we found no evidence for significantly increased expression of CD98, LAT1 or LAT2 either at RNA or protein levels (data not shown), in addition to the decreased sensitivity to inhibition by BCH or D-Met. We also consider that it is unlikely that the CD98-independent transporters LAT3 (49, 50) and LAT4 (51) play a role in tryptophan uptake in IDO positive tumor cells because LAT3 is inhibited by BCH and has been shown to transport leucine, isoleucine, valine, phenylalanine, and methionine (49), while LAT4 can transport leucine, isoleucine, phenylalanine, and methionine and to a lesser degree tryptophan, proline and arginine. Transport via LAT2, 3 and 4 is significantly inhibited by NEM (43), while we found little effect of NEM on tryptophan uptake by IDO positive tumor cells (data not shown). Finally we also found no evidence for an increase in LAT3 or LAT4 expression in IDO positive EG7 cells either at RNA or protein levels (data not shown).
Other possible candidates for the IDO induced tryptophan transporter include a member of the monocarboxylate family, TAT1, also known as MCT10 or SLC16A10 (52), suggested to play a role in tryptophan uptake by HepG2 cells (53). MCT10 has been shown to be specific for aromatic amino acids including tryptophan, phenylalanine and tyrosine (52). However, MCT10 can also transport the iodothyronine thyroid hormones (54), which can inhibit tryptophan uptake (53), and we have no evidence for differences in the levels of mRNA of MCT10 between IDO positive and negative Hela cells (data not shown). We also examined the effect of the different thyroid hormones on tryptophan uptake in Hela cells in the presence of glutamine and found little inhibition (Supplementary Fig. 2E) suggesting that MCT10 is unlikely to be the candidate molecule in IDO positive tumors.
The presence of a tryptophan transporter upregulated in conditions of tryptophan depletion is consistent with previously published reports describing the induction of arginase expression and degradation of arginine upon macrophage activation, and the resulting up-regulation of a second arginine transporter CAT2 in addition to the constitutively expressed cationic transporter CAT1 (55, 56). Together this suggests that modulation of the expression of amino acid transporters by cells may be a generalizable mechanism linking the function of intracellular immunoregulatory catabolic enzymes with nutrient availability and transport.
It is as yet unclear whether additional amino acid transporters are expressed in other IDO positive cells such as matured DC, DC resident in the mesenteric lymph nodes known to constitutively express IDO (28), or TDO positive cells such as those in the liver or in tumor cells expressing IDO2 (57, 58). It is distinctly possible that the activity of other catabolic enzymes such as TDO or IDO2, possibly through localized tryptophan depletion or accumulation of metabolites, may indeed drive modifications in the repertoire of amino acid transporters expressed by certain cell types. The mechanism of induction of the novel tryptophan transporter and candidate molecules are currently under investigation. However, tryptophan uptake in human placenta (a site of high IDO activity) is mediated by a number of different transporters at different poles of the cell (34). It is also likely that other cell types, such as myeloid derived suppressor cells, that are expanded during infectious diseases and cancer growth and that suppress T cell proliferation by a combination of arginase and inducible nitric oxide synthase expression (59), may require an altered amino acid uptake capacity. Indeed it has been demonstrated that myeloid derived suppressor cells can regulate T cell function by depletion of cystine through differential expression of transporters, thereby acting as a sink for cystine in the microenvironment (60).
Finally our data have potential clinical implications, as identification of small molecule inhibitors of the IDO dependent tryptophan transporter expressed by tumor cells would be of therapeutic importance, not only to relieve IDO dependent immunosuppression, but also to enhance the sensitivity of IDO positive tumor cells to tryptophan shortage, slowing down tumor growth. Further experiments are warranted to identify this novel IDO induced tryptophan transporter, to characterize its expression and assess its potential as a therapeutic target.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Mary Collins (University College, London) for providing the pHR-SIN-BX-IRES-GFP vector, Mariolina Salio (University of Oxford) for providing the mouse intestinal mRNA used to amplify mouse Ido and the GFP expressing EG7 cells, and Kunimasa Yan (Department of Pediatrics, Kyorin University School of Medicine) for the anti-LAT3 antibody.
Abbreviations used in this paper
5.
- TDO
Tryptophan 2,3-dioxygenase
- DC
dendritic cells
- pDC
plasmacytoid dendritic cells
- 1-MT
1-methyl DL-tryptophan
- BCH
2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid
- WT
wild type
- MeAIB
methyl-aminoisobutyric acid
- NEM
N-ethyl maleimide
- D/L-Met
D- or L-methionine
- L-Dopa
L-3,4-dihydroxyphenylalanine
Footnotes
This work was funded by The Ludwig Institute for Cancer Research and the Medical Research Council. SL was funded by a studentship awarded by the Algerian Ministry of Higher Education and Scientific Research.
REFERENCES
- 1.Brady FO. Inhibition of rabbit intestinal indoleamine 2,3-dioxygenase by copper chelators. FEBS Lett. 1975;57:237–240. doi: 10.1016/0014-5793(75)80307-3. [DOI] [PubMed] [Google Scholar]
- 2.Brady FO. Tryptophan 2,3-dioxygenase: a review of the roles of the heme and copper cofactors in catalysis. Bioinorg Chem. 1975;5:167–182. doi: 10.1016/s0006-3061(00)80058-7. [DOI] [PubMed] [Google Scholar]
- 3.Hayaishi O, Hirata F, Fujiwara M, Senoh S, Tokuyama T. Indoleamine 2,3-dioxygenase. Note II. Biological function. Acta Vitaminol Enzymol. 1975;29:291–293. [PubMed] [Google Scholar]
- 4.Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents of induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987;144:1147–1153. doi: 10.1016/0006-291x(87)91431-8. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida R, Imanishi J, Oku T, Kishida T, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci USA. 1981;78:129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlin JM, Ozaki Y, Byrne GI, Brown RR, Borden EC. Interferons and indoleamine 2,3-dioxygenase: role in antimicrobial and antitumor effects. Experientia. 1989;45:535–541. doi: 10.1007/BF01990503. [DOI] [PubMed] [Google Scholar]
- 7.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 8.Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183–190. doi: 10.1007/978-1-4615-0135-0_21. [DOI] [PubMed] [Google Scholar]
- 9.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. The Journal of Experimental Medicine. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science (New York, N.Y) 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 11.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–196. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 13.Grohmann U, Fallarino F, Bianchi R, Vacca C, Orabona C, Belladonna ML, Fioretti MC, Puccetti P. Tryptophan catabolism in nonobese diabetic mice. Adv Exp Med Biol. 2003;527:47–54. doi: 10.1007/978-1-4615-0135-0_5. [DOI] [PubMed] [Google Scholar]
- 14.Alexander AM, Crawford M, Bertera S, Rudert WA, Takikawa O, Robbins PD, Trucco M. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes. 2002;51:356–365. doi: 10.2337/diabetes.51.2.356. [DOI] [PubMed] [Google Scholar]
- 15.Gorczynski RM, Hadidi S, Yu G, Clark DA. The same immunoregulatory molecules contribute to successful pregnancy and transplantation. Am J Reprod Immunol. 2002;48:18–26. doi: 10.1034/j.1600-0897.2002.01094.x. [DOI] [PubMed] [Google Scholar]
- 16.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nature Medicine. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 17.Fallarino F, Volpi C, Zelante T, Vacca C, Calvitti M, Fioretti MC, Puccetti P, Romani L, Grohmann U. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol. 2009;183:6303–6312. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- 18.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–5070. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Medicine. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 20.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIlroy D, Tanguy-Royer S, Le Meur N, Guisle I, Royer PJ, Leger J, Meflah K, Gregoire M. Profiling dendritic cell maturation with dedicated microarrays. J Leukoc Biol. 2005;78:794–803. doi: 10.1189/jlb.0105029. [DOI] [PubMed] [Google Scholar]
- 22.Wirleitner B, Reider D, Ebner S, Bock G, Widner B, Jaeger M, Schennach H, Romani N, Fuchs D. Monocyte-derived dendritic cells release neopterin. J Leukoc Biol. 2002;72:1148–1153. [PubMed] [Google Scholar]
- 23.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. International Immunology. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 24.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr., Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science (New York, N.Y) 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 25.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of Clinical Investigation. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JR, Dalton RR, Messina JL, Sharma MD, Smith DM, Burgess RE, Mazzella F, Antonia SJ, Mellor AL, Munn DH. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Laboratory investigation; a journal of technical methods and pathology. 2003;83:1457–1466. doi: 10.1097/01.lab.0000090158.68852.d1. [DOI] [PubMed] [Google Scholar]
- 27.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 28.Onodera T, Jang MH, Guo Z, Yamasaki M, Hirata T, Bai Z, Tsuji NM, Nagakubo D, Yoshie O, Sakaguchi S, Takikawa O, Miyasaka M. Constitutive expression of IDO by dendritic cells of mesenteric lymph nodes: functional involvement of the CTLA-4/B7 and CCL22/CCR4 interactions. J Immunol. 2009;183:5608–5614. doi: 10.4049/jimmunol.0804116. [DOI] [PubMed] [Google Scholar]
- 29.Attwood JT, Munn DH. Macrophage suppression of T cell activation: a potential mechanism of peripheral tolerance. Int Rev Immunol. 1999;18:515–525. doi: 10.3109/08830189909088496. [DOI] [PubMed] [Google Scholar]
- 30.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. The Journal of Experimental Medicine. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Fioretti MC, Puccetti P. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. 2006;17:58–60. doi: 10.1016/j.trim.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Manlapat AK, Kahler DJ, Chandler PR, Munn DH, Mellor AL. Cell-autonomous control of interferon type I expression by indoleamine 2,3-dioxygenase in regulatory CD19+ dendritic cells. Eur J Immunol. 2007;37:1064–1071. doi: 10.1002/eji.200636690. [DOI] [PubMed] [Google Scholar]
- 33.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Kudo Y, Boyd CA. Characterisation of L-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J Physiol. 2001;531:405–416. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 36.Deves R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- 37.Hediger MA, Kanai Y, Lee WS, Wells RG. Identification of a new family of proteins involved in amino acid transport. Soc Gen Physiol Ser. 1993;48:301–314. [PubMed] [Google Scholar]
- 38.Christensen HN, Handlogten ME, Thomas EL. Na plus-facilitated reactions of neutral amino acids with a cationic amino acid transport system. Proc Natl Acad Sci USA. 1969;63:948–955. doi: 10.1073/pnas.63.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen HN, Thomas EL, Handlogten ME. Features of amino acid structure enhancing or obstructing cosubstrate reactivity of Na+ in transport. Biochim Biophys Acta. 1969;193:228–230. doi: 10.1016/0005-2736(69)90079-0. [DOI] [PubMed] [Google Scholar]
- 40.Kudo Y, Boyd CA. The role of L-tryptophan transport in L-tryptophan degradation by indoleamine 2,3-dioxygenase in human placental explants. J Physiol. 2001;531:417–423. doi: 10.1111/j.1469-7793.2001.0417i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seymour RL, Ganapathy V, Mellor AL, Munn DH. A high-affinity, tryptophan-selective amino acid transport system in human macrophages. J Leukoc Biol. 2006;80:1320–1327. doi: 10.1189/jlb.1205727. [DOI] [PubMed] [Google Scholar]
- 42.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M, Thrasher AJ. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Human Gene Therapy. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 43.Vumma R, Wiesel FA, Flyckt L, Bjerkenstedt L, Venizelos N. Functional characterization of tyrosine transport in fibroblast cells from healthy controls. Neuroscience Letters. 2008;434:56–60. doi: 10.1016/j.neulet.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 44.Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- 45.Kudo Y, Boyd CA, Sargent IL, Redman CW. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. American Journal of Obstetrics and Gynecology. 2003;188:719–726. doi: 10.1067/mob.2003.156. [DOI] [PubMed] [Google Scholar]
- 46.Kudo Y, Boyd CA, Sargent IL, Redman CW. Tryptophan degradation by human placental indoleamine 2,3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001;535:207–215. doi: 10.1111/j.1469-7793.2001.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nii T, Segawa H, Taketani Y, Tani Y, Ohkido M, Kishida S, Ito M, Endou H, Kanai Y, Takeda E, Miyamoto K. Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation. Biochem J. 2001;358:693–704. doi: 10.1042/0264-6021:3580693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni Z, Abou El Hassan M, Xu Z, Yu T, Bremner R. The chromatin-remodeling enzyme BRG1 coordinates CIITA induction through many interdependent distal enhancers. Nature Immunology. 2008;9:785–793. doi: 10.1038/ni.1619. [DOI] [PubMed] [Google Scholar]
- 49.Babu E, Kanai Y, Chairoungdua A, Kim DK, Iribe Y, Tangtrongsup S, Jutabha P, Li Y, Ahmed N, Sakamoto S, Anzai N, Nagamori S, Endou H. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278:43838–43845. doi: 10.1074/jbc.M305221200. [DOI] [PubMed] [Google Scholar]
- 50.Fukuhara D, Kanai Y, Chairoungdua A, Babu E, Bessho F, Kawano T, Akimoto Y, Endou H, Yan K. Protein characterization of NA+-independent system L amino acid transporter 3 in mice: a potential role in supply of branched-chain amino acids under nutrient starvation. The American Journal of Pathology. 2007;170:888–898. doi: 10.2353/ajpath.2007.060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- 52.Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH, Endou H. Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J Biol Chem. 2001;276:17221–17228. doi: 10.1074/jbc.M009462200. [DOI] [PubMed] [Google Scholar]
- 53.Ritchie JW, Taylor PM. Tryptophan and iodothyronine transport interactions in HepG2 human hepatoma cells. Amino Acids. 2009 doi: 10.1007/s00726-009-0344-6. [DOI] [PubMed] [Google Scholar]
- 54.Friesema EC, Jansen J, Jachtenberg JW, Visser WE, Kester MH, Visser TJ. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Molecular Endocrinology (Baltimore, Md) 2008;22:1357–1369. doi: 10.1210/me.2007-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeramian A, Martin L, Arpa L, Bertran J, Soler C, McLeod C, Modolell M, Palacin M, Lloberas J, Celada A. Macrophages require distinct arginine catabolism and transport systems for proliferation and for activation. Eur J Immunol. 2006;36:1516–1526. doi: 10.1002/eji.200535694. [DOI] [PubMed] [Google Scholar]
- 56.Yeramian A, Martin L, Serrat N, Arpa L, Soler C, Bertran J, McLeod C, Palacin M, Modolell M, Lloberas J, Celada A. Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J Immunol. 2006;176:5918–5924. doi: 10.4049/jimmunol.176.10.5918. [DOI] [PubMed] [Google Scholar]
- 57.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 58.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 59.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Grone HJ, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. The Journal of Clinical Investigation. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.