Abstract
Despite growing evidence for adipose tissue regulation of bone mass, the role of the adipokine leptin in bone remodeling remains controversial. The majority of in vitro studies suggest leptin enhances osteoblastic proliferation and differentiation while inhibiting adipogenic differentiation from marrow stromal cells. Alternatively, some evidence demonstrates either no effect or a pro-apoptotic action of leptin on stromal cells. Similarly, in vivo work has demonstrated both positive and negative effects of leptin on bone mass. Most of the literature supports the idea that leptin suppresses bone mass by acting in the brainstem to reduce serotonin-dependent sympathetic signaling from the ventromedial hypothalamus to bone. However, other studies have found partly or entirely contrasting actions of leptin. Recently one study found a significant effect of surgery alone with intracerebroventricular administration of leptin, a technique crucial for understanding centrally-mediated leptin regulation of bone. Thus, two mainstream hypotheses for the role of leptin on bone emerge: 1) direct regulation through increased osteoblast proliferation and differentiation and 2) indirect suppression of bone formation through a hypothalamic relay. At the present time, it remains unclear whether these effects are relevant in only extreme circumstances (i.e. models with complete deficiency) or play an important homeostatic role in the regulation of peak bone acquisition and skeletal remodeling. Ultimately, determining the actions of leptin on the skeleton will be critical for understanding how the obesity epidemic may be impacting the prevalence of osteoporosis.
Keywords: Leptin, Bone remodeling, Adipocyte, Osteoblast, Sympathetic nervous system
1. Introduction
Bone remodeling is the process by which bone is resorbed by osteoclasts and then formed by osteoblasts. Remodeling is constantly occurring in order to respond to physical demand on bone and to maintain blood mineral homeostasis. Coupled remodeling is necessary for maintaining bone density, but with age, remodeling becomes uncoupled such that bone resorption outweighs formation and net bone loss occurs. Rapid bone loss in humans leads to a greater risk of osteoporosis that ultimately results in greater morbidity and poor quality of life. Thus, understanding the mechanisms that underlie osteoporosis is necessary in order to better treat patients and prevent fractures.
Bone marrow adiposity has been associated with low bone mass in age-related osteoporosis, as well as osteoporosis secondary to diseases like anorexia nervosa and drugs such as glucocorticoids [1–4]. Because adipocytes and osteoblasts arise from common precursors, much attention has been given to this association with the idea that perhaps adipose tissue plays a role (either positive or negative) in bone homeostasis. Additionally, it has recently been postulated that thermogenic brown adipose tissue has a positive effect on bone and that marrow adipocytes may have a more brown adipocyte-like phenotype in young healthy animals, which declines with age [5, 6]. Obese individuals have increased white adipose tissue and obesity increases fracture risk in women [7]. Thus, different types and depots of adipose tissue could have differential effects on bone mass.
Leptin, a peptide hormone secreted by adipocytes, has been found to have both positive and negative effects on bone mass. Despite the limited clinical evidence to support a significant role of leptin in modulating bone mass (discussed in Section 4.0), there is a wealth of evidence for modulation of bone mass by leptin from the rodent literature. Here, we describe how leptin impacts bone mass by examining two hypotheses for its actions: 1) leptin positively regulates bone formation through direct actions on bone and 2) leptin suppresses bone formation and increases resorption through a hypothalamic relay. Importantly, despite a growing body of literature, it remains difficult to compare studies conducted in different laboratories because methodologies differ vastly (from sex and age of mice, to housing conditions, to cell culture conditions and more). Notwithstanding the relative polarity of these two perspectives, we will also discuss the levels of evidence that are inconsistent and have yet to be reconciled.
2. Contradictory results from in vitro studies
Before discussing in vitro studies, it is important to note the normal ranges of serum leptin concentrations. In children and adolescents, leptin ranges from 0.7–6.2 ng/ml on average, depending on Tanner stage [8]. In adults, the average leptin ranges from 7.5 ± 9.3 ng/ml in normal weight individuals to 31.3 ± 24.1 ng/ml in obese individuals (mean ± SD) [9]. In rats and mice, serum leptin concentrations are similar to those of non-obese humans. Some, of the following reports use leptin concentrations that are supraphysiologic and therefore provide results from which it is difficult to draw meaningful conclusions. However, other studies use leptin concentrations closer to physiologic levels or use loss of function models, both of which contribute significantly to our understanding of the effects of leptin on bone cell biology.
2.1. In vitro evidence that leptin promotes osteoblast proliferation and differentiation
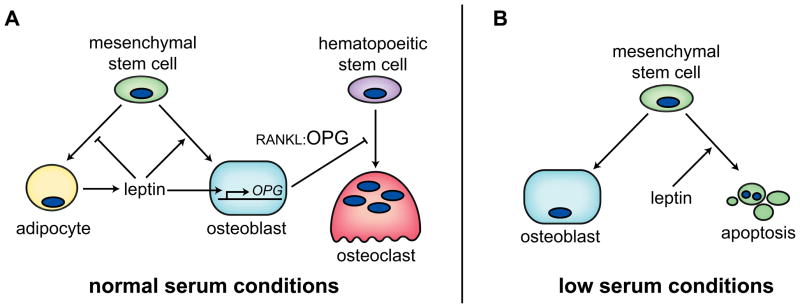
The first published indication that leptin could be an important regulator of bone mass surfaced in 1999 when Thomas et al. demonstrated that high levels of leptin (0.6–2.4 μg/ml) concentration-dependently increased osteoblast differentiation and blunted adipocyte differentiation from conditionally immortalized human marrow stromal cells (hMS2-12) in vitro [10]. Another supraphysiologic study demonstrated that 100 ng/ml leptin increased 3H-Thymidine incorporation into proliferating human osteoblasts derived from osteoarthritic patients [11]. This feature was accompanied by increased mineralized nodules in mature osteoblasts, increased mRNA expression of osteoblast differentiation markers, and a lower ratio of BAX/BCL-2 expression [11]. Cornish et al. demonstrated that physiologically relevantleptin concentrations as low as 1.6 ng/ml increased proliferation of rat calvarial osteoblasts, which were also shown to express the leptin receptor [55]. More recently, using a Cre-LoxP system, Scheller et al. deleted the long-form of the leptin receptor from bone marrow stromal cells in culture. Deletion of the leptin receptor delayed mineralization and increased adipogenesis [12]. Despite the high concentrations of leptin in some studies, others support a direct, positive effect of leptin on osteoblast proliferation and differentiation in congruence with a negative effect on adipogenic differentiation (Figure 1A).
Figure 1.
In vitro data supports a direct role of leptin on bone cells. Two opposing bodies of literature: (A) Under normal serum conditions, leptin induces proliferation of osteoblasts and suppression of adipocyte differentiation. Additional evidence demonstrates that leptin suppresses osteoclast recruitment by enhancing osteoblast OPG expression. (B) Under low serum conditions, leptin has either no effect on osteoblast differentiation, or induces apoptosis of osteoblast precursors.
2.2. In vitro evidence for a negative or no effect of leptin on bone marrow stromal cells
In contrast to the above findings leptin concentrations as low as 16 ng/ml (10−8 M) induced apoptosis of human bone marrow stromal cells (hBMSCs), which in turn reduced alkaline phosphatase activity and osteocalcin secretion [13]. These cells which were obtained from ribs during open thoracotomy in patients without any preexisting bone disease expressed leptin receptor by RT-PCR. Kim et al. found that leptin reduced cell viability by formazan dye formation, flow cytometry and nuclear morphology, and this cell death was dependent upon increased activity of caspase-9 and caspase-3 [13]. Based on findings from various inhibitor studies, they proposed that leptin signaling activates JAK/STAT1 and ERK/cPLA2/Cytochrome c signaling to induce apoptosis of hBMSCs [13] (Figure 1B). Ducy et al. failed to find leptin receptor expression after 27 cycles of RT-PCR in mouse calvaria, long bones or primary osteoblasts [14]. Additionally, the group found neither an effect of 5-day supraphysiologic 1.2 μg/ml leptin on mouse primary osteoblast differentiation nor a difference between primary osteoblasts isolated from wild type and db/db (leptin receptor deficient) mice[ 14].
Interestingly, both of the above experiments were performed in cultures with low fetal calf serum: Kim et al. used 0.5% serum for 24 hours before and during leptin treatment and Ducy et al. used 0.5% during 5-day leptin treatment. In contrast, the experiments outlined in Section 2.1 were performed in standard serum conditions (i.e. 10% FCS) and leptin treatment was of longer duration [10]. Because leptin is present in serum, using low serum conditions would better control leptin concentrations, which is important for understanding acute effects of leptin treatment (i.e. apoptosis, changes in signaling, gene expression). On the other hand, the outcomes of longer experiments examining the effects of leptin on collagen synthesis and mineralization may be altered by the choice of low serum conditions. Low serum (2%) conditions have been shown to be beneficial for adipogenic differentiation [15] and are detrimental for osteoblast differentiation in general. Ideally, an experiment should examine osteoblast proliferation, differentiation and apoptosis with varying concentrations of leptin, and low and high serum conditions. A suitable serum concentration should be found before treating with leptin, to ensure the reduction in serum does not deleteriously affect the culture system. It may be that the level of leptin in culture medium (which would be subphysiologic) could be used as a starting point for concentration-dependent effects. A soluble leptin antibody could also be used to quench leptin signaling in culture and determine if its absence affects cellular properties. The possibility that serum concentrations play a major role in the effects of leptin in vitro are intriguing, but have never been reported directly.
2.3. Leptin regulation of osteoclastogenesis – in vitro
Some of the above studies have also examined how leptin modulates osteoclast recruitment and function. Ducy et al. found no difference in osteoclast differentiation and function in bone marrow cultures from leptin-deficient ob/ob or db/db mice compared to wild type [14]. Cornish et al. found significant suppression of tartrate-resistant acid phosphatase (TRAP) positive osteoclasts in bone marrow cultures treated with physiologic concentrations of leptin, indicating an inhibitory role for leptin in osteoclast recruitment [55]. In a different experimental design, Holloway, et al demonstrated that concentrations ≥ 16 ng/ml leptin dose-dependently decreased osteoclast number and percent resorption in human peripheral blood mononuclear cells (PBMCs) treated with sRANKL and hM-CSF [16]. However, when PBMCs were washed to remove non-adherent or weakly adherent cells, or when PBMCs were first purified to include only CD14+ cells, leptin did not decrease osteoclastogenesis. In fact, leptin treatment of unwashed PBMC cultures increased osteoprotegerin (OPG) expression, suggesting contaminating cells in the culture system were responsible for the response to leptin (Figure 1A). However, there was also a leptin-induced reduction in receptor activator of nuclear factor kappa-b (RANK) expression in CD14+ cells, suggesting direct leptin effects could in part account for the reduced osteoclast differentiation from PBMCs [16].
Thus far, in vitro studies have provided us with contradictory insights about leptin’s actions on bone (Figure 1). Taken together the in vitro data, using physiologically relevant leptin concentrations support the notion that leptin directly promotes osteoblast proliferation and differentiation while inhibiting osteoclastogenesis, likely through osteoblast-dependent mechanisms. That being said, powerful in vivo studies examining leptin administration and mice deficient in leptin and/or leptin receptor point to a different mechanism of leptin action on bone i.e. leptin acting via the central nervous system to regulate bone metabolism.
3. Evidence from in vivo models of leptin/leptin receptor deficiency support a centrally-mediated effect of leptin on bone
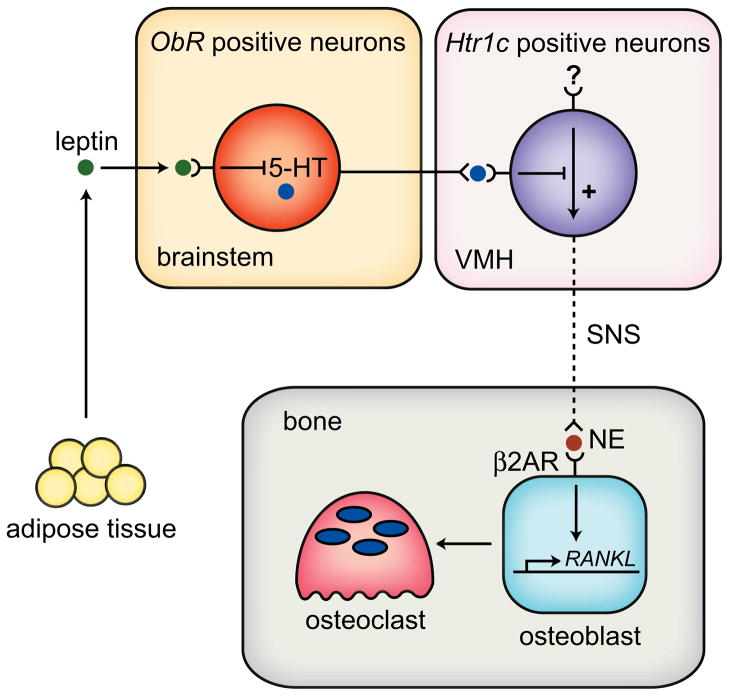
It is important to note that the majority of the findings outlined in the following subsection (3.1) are reported from the laboratory of Ducy and Karsenty. The overarching theme of their work is that leptin’s effects on bone are mediated solely through the sympathetic nervous system. More recently they have demonstrated suppression of serotonin signaling in the brain, which, in turn, removes the serotonin-induced suppression of sympathetic nervous system activity to bone. Osteoblasts respond to the sympathetic nervous system via the β2-adrenergic receptor (Adrb2) by suppressing bone formation and elevating resorption (through the RANK ligand (RANKL)/OPG pathway; see Figure 2). Other laboratories have identified contrasting phenotypes and these have been given equal consideration (Section 3.2). Finally, a gene therapy approach has demonstrated that intracerebroventricular (icv) administration of vehicle for leptin alone has an negative effect on bone, which poses a question as to whether central regulation of bone mass can be studied with the icv administration technique (Section 3.2.3).
Figure 2.
Leptin regulation of the sympathetic nervous system control of bone mass. Leptin, secreted from adipose tissue, crosses the blood brain barrier and acts through the leptin receptor (ObR) to inhibit serotonin (5-HT) production in serotonin-containing neurons in the brainstem. Normally, serotonin would be secreted from these nerve terminals in the ventromedial hypothalamus (VMH) to suppress sympathetic activity to bone. However, under leptin-induced inhibition of serotonin synthesis, the sympathetic nervous system (SNS) signals to osteoblasts by releasing norepinephrine (NE) onto β2-adrenergic receptors (Adrb2). This in turn suppresses bone formation and increases resorption through increased RANKL expression.
3.1. Leptin acts centrally to suppress bone formation and increase resorption
In vivo, Ducy et al. first observed that ob/ob (leptin deficient) and db/db (leptin receptor deficient) mice had high trabecular bone mass in the proximal tibia and vertebrae due to high osteoblast activity despite their hypogonadal state [14]. This paradox could not be solely attributed to their high body weight in part because young ob/ob mice were not yet obese yet had high bone mass and that bone mass could be suppressed in the ob/ob, db/db and wild type mice by icv administration of leptin. This group proposed that leptin acted centrally to indirectly affect bone in a manner analogous to its effects on appetite and energy expenditure. Takeda et al, demonstrated with several mouse models that leptin’s antiosteogenic functions in the vertebrae were not mediated through the same pathways as its anorexic fuctions [17]. Importantly, gold thioglucose (GTG) –mediated disruption of glucose-sensitive neurons in the hypothalamus resulted in high vertebral bone volume fraction (BV/TV) phenotype similar to that of ob/ob mice. Additionally, GTG-treated ob/ob mice were resistant to the bone effects (but not the anorexigenic effects) of icv leptin administration. Mice with a deletion of dopamine β-hydroxylase (DBH), the enzyme responsible for catecholamine synthesis, had high vertebral BV/TV similar to that of ob/ob mice and were resistant to icv leptin-induced bone loss. Furthermore, Takeda et al, demonstrated the expression of Adrb2 in primary mouse osteoblasts and on osteoblasts in histological sections. Next, the authors demonstrated that administration of isoproterenol, a β-adrenergic receptor agonist reduced vertebral BV/TV and bone formation rate in both wild type and ob/ob mice. Conversely, propranolol (β-adrenergic receptor blocker) treatment of wild type and ovariectomized mice elevated bone volume and bone formation rate (BFR), suggesting a role for sympathetically-mediated suppression of bone formation in both resting and ovariectomozed states. Despite the vast number and scope of experiments performed, phenotyping of these bones was not particularly robust in that only vertebrae were examined and no micro-architectural studies were performed [17]. The group maintained that leptin’s major effects on bone were centrally mediated, as icv leptin did not alter serum leptin levels and overexpression of leptin from the Col2.3 promoter did not alter bone mass, although it is unclear whether the amount of leptin overexpression and the localization (ie. intracellular vs extracellular) was biologically significant [17]. In sum, these experiments described a robust role for the sympathetic nervous system and β-adrenergic signaling in bone, one that could be mediated in part by leptin.
This same group went further to demonstrate the relationship between serum leptin levels and bone mass using transgenic mice with 3-fold (serum amyloid P component (SAP)-driven (liver)) and 300-fold (ApoE-driven) overexpression of serum leptin [18]. In addition to significantly reducing fat pad weight and food intake, both of these models reduced BV/TV of the vertebrae (−25% and −21%, respectively). Similarly, reduction of free serum leptin levels by overexpression of the soluble leptin receptor increased BV/TV in ob/+ mice by 13% [18]. In 2005, Elefteriou et al. published that mice with a deletion of the β2-adrenergic receptor (Adrb2−/−) had high bone mass in the vertebrae and femur and that the induction of cAMP by isoproterenol (β-adrenergic receptor agonist) in wild type osteoblast cultures was blocked by the absence of Adrb2 [19]. Additionally, deletion of Adrb2 had no effect on osteoclast cultures, but did significantly reduce osteoclast surface and activity in vivo. Their group went further to demonstrate that isoproterenol administered to osteoblasts in vitro induced Rankl expression, and that this effect was also dependent on Adrb2. Vertebral BV/TV was not suppressed in response to leptin in Adrb2−/−- mice, suggesting that leptin-dependent effects on bone were via the sympathetic nervous system and β2-adrenergic signaling in osteoblasts (Figure 2). Additionally, leptin effects were found to be in part mediated by circulating cocaine and amphetamine regulated transcript (CART) [19, 20]. However, once again there were not complete microarchitectural and histomorphometric analyses of leptin-treated Adrb2−/− mice. In a later study, Kajimura et al. found high vertebrae and tibia trabecular bone mass in 24-week old mice with Adrb2 deleted from osteoblasts using Col1a1(2.3 kb)-Cre [21]. Additionally, 12-week old osteoblast-Adrb2−/− mice were protected from changes in BV/TV, BFR and other histomorphometric parameters after icv leptin infusion.
In order to understand the mechanism of reduced bone formation by sympathetic signaling, Fu et al. demonstrated that clock genes were expressed and active in osteoblasts, and that absence of clock genes (Period (Per) 1−/−;Per2−/− mice and Cryptochrome (Cry) 1−/−;Cry2−/− mice) resulted in a high bone mass phenotype (with increased bone formation) that was resistant to central effects of leptin [22]. In fact, in response to icv leptin, Per1−/−;Per2m/m (deletion of PAS domain of Per2) mice had elevated vertebral BV/TV and osteoblast number from administration of leptin, which is in stark contrast to the result in wild type mice. One important conclusion of this paper is that in the presence of clock genes, β-adrenergic signaling suppresses osteoblast proliferation. Alternately, if clock genes are suppressed or absent, β-adrenergic signaling can induce AP-1 transcription factors and promote osteoblast proliferation.
The fundamental question remained as to whether leptin exerted its effects on bone solely through centrally-mediated mechanisms, or if leptin affected bone directly. As discussed in Section 2, evidence from in vitro models was contradictory and many in the field were convinced that leptin had direct effect on bone. Shi et al. answered by conditionally deleting the leptin receptor in osteoblasts using α1(I)Collagen-Cre and in neurons using SynapsinI-Cre [23]. They found no difference in vertebrae trabecular histomorphometry when leptin-receptor was deleted in osteoblasts, although long bone morphology was not shown. In contrast, deletion of leptin receptor in neurons increased bone mass in the vertebrae and the femur, supporting a role for a primarily centrally-mediated action of leptin.
More recently, Karsenty et al. observed that despite the absence of the leptin receptor on the neurons of the ventromedial hypothalamus (a site necessary for brain-bone interaction), leptin delivery to the brain still altered bone remodeling [24]. Therefore, Yadav et al set out to determine where in the brain leptin was acting [25]. Through deletions of the enzymes responsible for serotonin synthesis in the gut (tryptophan hydroxylase (Tph) 1) and brain (Tph2), the group demonstrated that deletion of brain derived serotonin (BDS), through knockout of Tph2, resulted in low vertebral and tibia trabecular bone mass. This phenotype was opposite that of the ob/ob mice: Tph2−/− mice had low bone formation and increased resorption. Interestingly, deletion of BDS increased sympathetic activity (marked by epinephrine levels and Ucp1 expression in brown adipose tissue). Additionally, crossing Tph2−/− mice with mice heterozygous for the β2-adrenergic receptor (Adrb2+/−) rescued the low bone mass phenotype, suggesting that the absence of serotonin signaling in the brain elevates sympathetic activity to bone. The group went on to demonstrate that the serotonin receptor Htr2c was expressed in the ventromedial hypothalamus (VMH) and that its deletion resulted in low bone mass similar to that of Tph2−/− mice.
But a fundamental question remained: Where did the serotonin come from that activated the VMH? Tph2 expressing neurons were found to originate in the brainstem and continue to the ventromedial hypothalamus and icv leptin administration decreased brainstem Tph2 expression. Additionally, deletion of the leptin receptor in serotonin expressing neurons (via the Tph2 promoter) prevented leptin-induced STAT3 signaling in Tph2 positive neurons in the brainstem. Thus, the group concluded that leptin signaling in the brainstem prevents serotonin synthesis, which in turn elevates sympathetic output to bone [25]. Importantly, Yadav, et al also determined that leptin inhibited food intake and increased energy expenditure through inhibition of serotonin signaling to Htr1a and Htr2b receptors in the arcuate neurons supporting a central role for serotonin in leptin signaling. [25]. In contrast, Lam, et al. demonstrated that serotonin was not required for the full leptin effects on appetite [26]. Further studies will be important to better understand these concepts, which are beyond the scope of this review.
Independent laboratories have also performed work that supports the centrally-mediated effect of leptin on trabecular bone. Pogoda et al. demonstrated suppressed BFR and enhanced bone loss from OVX when sheep (n=3) were treated with icv leptin [27]. The same group also demonstrated high BFR and BV/TV, as well as accelerated fracture healing in ob/ob and db/db mice compared to C57BL/6J [28]. Unfortunately, the mice in this study were obtained from separate breeding colonies, therefore introducing the possibility that the observed high BV/TV phenotype was not as significant as described. Baldock et al. also demonstrated that ob/ob mice had femoral trabecular bone mass that was higher than their wildtype littermates [29]. Sato et al. also demonstrated trabecular bone loss from icv leptin in wildtype and ob/ob mice and that this was dependent on presence of the anorexigenic neuropeptide neuromedin U [30]. Additional reports involving hypothalamic leptin gene therapy also support the notion of a centrally-mediated effect on bone, and these will be discussed in Section 3.2.3. Taken together, the majority of published literature examining the in vivo phenotype of ob/ob mice demonstrates high trabecular bone mass in the vertebrae and long bones, with a reduction in trabecular bone after icv leptin.
3.2. Alternative results/interpretations
Although the above work (Section 3.1) describes a novel and intriguing mechanism for leptin-dependent regulation of bone mass, some laboratories have failed to fully recapitulate results from these experiments. Confounding factors that become apparent are differing measurements (i.e. histomorphometry, microCT and DEXA) and different modes of treatment (i.e. icv injection of leptin, icv leptin gene therapy, peripheral administration of leptin). The following work either agrees only in part, or disagrees with the work outlined above.
3.2.1. Leptin stimulates bone growth and increases strength
In 2000, Steppan et al. demonstrated that ob/ob mice have reduced femoral length compared to lean littermates and this can be partially rescued by daily ip injections of 50 μg leptin [32]. Similarly, subcutaneous leptin injections increased growth plate thickness in wild type Swiss mice [55]. Collectively, these results suggested that absence of leptin stunts growth while replacement or addition of exogenous leptin stimulates growth. In 2002, Cornish et al. showed that despite reduced body weight due to its effects on appetite, there were no significant changes on osteoblast and osteoclast parameters with four weeks of leptin injections (43 μg/day). Intriguingly there was reduced bone fragility in adult male Swiss mice, which could have been attributed to a non-significant increase in the thickness of the cortices [55].
Recently, Bartell et al. demonstrated that icv injection of leptin (0.38 or 1.5 μg/d for 12 days) resulted in increased aBMD, aBMC and tibia MAR in 15-week old female ob/ob mice compared to ob/ob mice treated with artificial cerebrospinal fluid (aCSF) [31]. However, a separate experiment in the same article demonstrated a much smaller effect of 1.5 μg/d leptin on aBMC and no significant effect on aBMD. Unfortunately, the authors did not publish microarchitectural data or static and dynamic histomorphometry, although they did demonstrate that leptin increased tibia and vertebrae MAR. It is difficult to compare this study to the complimentary study of Ducy et al because it is of shorter duration (12 d vs 28 d) and had higher leptin dose (0.38–1.5 μg/d vs 192 μg/d) [14]. To confound matters further, neither of the studies had a single bone measurement in common: Bartell et al. published only aBMD, aBMC and MAR (vertebrae and tibia), while Ducy et al published only vertebrae BV/TV from histomorphometry. Although the MAR data between the two groups are not consistent, it is possible that vertebrae BV/TV could be reduced while whole body BMD and BMC are increased by leptin administration, which collectively suggests leptin has negative effect on trabecular bone and a positive effect on cortical bone.
3.2.2. Cancellous bone density and remodeling
Steppan et al. also demonstrated that 4 week old male ob/ob mice had low trabecular and cortical mineral content in the femur, and that this could be partially rescued after three weeks by daily intraperitoneal (ip) injections of 50 μg leptin [32]. Although lacking in any detailed histomorphometric or microarchitectural analyses, this result is completely opposite of that of Ducy, et al and represents the beginning of years of controversy over leptin’s role in bone remodeling [14, 32]. Also in contrast to the work in Section 3.1, Williams et al. recently demonstrated that mice lacking the functional leptin receptor (db/db) have low tibia trabecular BV/TV and cortical thickness at 11 weeks of age [33]. Trabecular BV/TV was not significantly different in the L5 vertebra of these mice, but trabecular thickness was substantially reduced (p=0.002). The low trabecular bone mass was associated with low osteocalcin, but these results were not paired with static or dynamic histomorphometry, making it difficult to determine the mechanism of bone changes. Furthermore, the mice were purchased from separate colonies of db/db and C57BL/6J mice, and not intercrossed to breed littermates. This alone could have an enormous impact on the skeletal phenotype and could explain why the opposite phenotype was found compared to Ducy, et al. [14, 33].
In 2004 Hamrick et al. showed that male ob/ob mice have high bone mass in the vertebra, which is consistent with the original findings of Ducy et al., but low bone mass in the proximal femur and femur midshaft [14, 34]. Consistent with previous icv treatment results, subcutaneous infusion of leptin in wild type mice resulted in reduced proximal tibia trabecular bone forming surface [35]. However, in this study leptin replacement resulted in much higher bone forming surface in leptin-treated ob/ob compared to untreated ob/ob mice. Another important difference from Ducy, et al was that untreated ob/ob mice had reduced bone-forming surfaces compared to untreated wild type. Thus, in an attempt to reconcile these differences, it was postulated that leptin had alternate effects on bone formation depending on location (axial vs appendicular skeleton) and route of administration (central vs peripheral) [34]. However, this does not completely explain differences found between the two research groups.
Scheller et al. found that conditional deletion of the leptin receptor gene from the Col3.6 promoter (osteoblasts, adipocytes and some chondrocytes) increased trabecular bone volume fraction similar to the findings of Ducy, et al [12, 14]. However, these findings suggest that leptin signaling is important for regulating skeletal function directly and independent from the CNS effects. However, deletion the leptin receptor in osteoblasts using the Col2.3 promoter caused no significant effect on femoral trabecular bone parameters, although the animal numbers in the Scheller experiment were small (n=3–6) [12]. Another confounding factor in these studies is that this group recently reported that Cre expression, from both the Col3.6 and Col2.3 promoters, was capable of causing recombination in the brain, making it impossible to determine whether their observed effects were due to central or peripheral leptin signaling, or both [36].
Several studies have also examined the effects of peripheral leptin administration on bone in different scenarios. While some studies have found a beneficial effect of leptin on bone, others have found little or no effect. In 2001, Burguera et al demonstrated that subcutaneous administration of 100 μg/day leptin to ovariectomized rats significantly reduced body weight gain and cancellous bone loss from ovariectomy [37]. Similarly, 50 μg/kg leptin infusion slowed loss from hind limb suspension by preventing complete suppression of bone formation [38]. In normal rats, however, continuous leptin infusion at the same dose did not affect proximal tibia bone mineral density (BMD), whereas 500 μg/kg per day suppressed BMD and BFR [38], suggesting a profound dose-dependent effect of leptin. Additionally, infusion of leptin was not capable of preventing type 1 diabetes-induced cancellous bone loss, but did prevent marrow adiposity [39]. Subcutaneous infusion of 10 μg/d leptin to ob/ob mice increased aBMD, aBMC, and tibia MAR [31]. Although intriguing, none of these studies rule out that the effect of leptin is centrally-mediated.
3.2.3. New lessons learned from central leptin gene therapy – does icv administration cause an effect independent of the active agent?
Iwaniec et al, examined how leptin affects bone via the hypothalamus in a completely different way: through recombinant adeno-associted virus-leptin (rAAV-lep) gene therapy [42]. This method delivers either green fluorescent protein (GFP) or leptin expression to the hypothalamus via icv injection. Delivery of leptin expression in the hypothalamus of ob/ob mice rescued the shorter femur length in ob/ob compared to wild type [42]. Here, the ob/ob mice had high BV/TV in the femur and vertebrae compare to wild type, consistent with Ducy, et al, and leptin gene therapy significantly reduced BV/TV in both sites by 15 weeks [14]. At 30 weeks, this effect was less prominent in the distal femur but remained strong in the vertebrae, although continuation of hypothalamic leptin expression was only verified at 15 weeks and not at 30 weeks [41]. Overall, the findings are consistent with those of Ducy et al. [14], but Iwaniec et al. did not measure markers of bone remodeling which could support/refute the proposed mechanism of leptin-induced sympathetic output to bone causing reduced bone formation [42]. Additionally, rAAV-lep caused a significant decrease in blood glucose and insulin levels (in wt, ob/ob, and Akita mice (a model of insulin-dependent diabetes)) [43]. Circulating osteocalcin was low in ob/ob (compared to wild type) and hypothalamic leptin increased osteocalcin in wt, ob/ob, and Akita mice compared to untreated, genotype-matched controls [43].
Newer findings from Iwaniec et al, have shed even more light on the mechanism of bone changes from hypothalamic leptin gene therapy in wild type animals. Three-month old rats were treated with either the GFP or leptin containing rAAV vectors for either 5 or 10 weeks and compared to untreated rats [44]. Similar to their previous findings, 5-week leptin gene therapy reduced cancellous bone volume fraction in the vertebrae and distal femur by micro-computed tomography (μCT), and in the proximal tibia by histomorphometry. Additionally, leptin reduced proximal tibia growth plate and hypertrophic zone thickness. Static measurements of bone remodeling demonstrated that there was no effect of leptin on osteoclast perimeter. Importantly, both rAAV-GFP and rAAV-lep increased osteoblast perimeter and reduced adipocyte number in the proximal tibia, as well as reduced cortical thickness in the midshaft of the femur. Next, the group examined the same parameters after 10 weeks of icv vector administration. Strikingly, none of the above stated differences between the vector-GFP and the leptin over-expression remained after 10 weeks. Additionally, after 18 weeks of the same treatment in 9 month old rats, no differences were observed between non-treated and leptin treated histomorphometric parameters [44]. Although the authors did not verify leptin expression in the hypothalamus at these time points, they did observe consistently reduced body mass, white adipose tissue mass, and serum leptin levels, which suggests continued expression of the recombinant leptin. In another study, the group showed that leptin expression in the hypothalamus had no effect on bone changes from ovariectomy in rats, while it did reduce food intake and fat mass accumulation [45]. In all, the findings from retroviral leptin expression suggest that hypothalamic leptin effects on bone turnover could be acute in nature and are possibly enhanced by the surgery itself.
4.0. The problem of leptin resistance
Although analyzing clinical data is not the main focus of this review, it is important to point out some of the limitations that arise when comparing leptin effects on bone in rodents vs. humans. Leptin resistance is a term used to describe the failure of endogenous hyperleptinemia and/or exogenous leptin administration to reduce food intake and body mass as would be expected [46]. For example, obese subjects have high circulating leptin, but no apparent responsiveness to the hormone. However, as pointed out by Myers et al., because of the evolutionary significance of protection against undernutrition over protection against overnutrition, it may be that the physiologic maximum range for leptin action is at the level found in obese patients. In other words, low leptin may have a more biologically significant effect than high leptin [46]. This point aside, the idea that the overwhelming majority of obese individuals and animals that gain weight on high fat diet are leptin resistant makes interpretation of studies examining the effects of leptin on bone in these models particularly challenging. For example at first glance, one would expect that based on the majority of mouse data, hyperleptinemia in obese individuals would cause bone loss. This is not the case, however, and has been attributed to the leptin resistance of the individuals.
In a recent study of elderly adults, leptin levels were not associated with fracture risk [47]. These patients had an average BMI near 27 kg/m2, which is overweight and potentially leptin resistant, but the absence of association existed even at the tertile with the lowest leptin and BMI (23 and 24 kg/m2 in women and men, respectively). In a small study of 54 postmenopausal women, bone mineral content and bone mineral density correlated with weight, fat mass and to a lesser degree leptin levels [48]. After correcting for fat mass, however, the correlation between leptin and bone mineral content was lost, suggesting that leptin itself did not independently determine bone mass. Additionally, no correlation was found between leptin and markers of bone resorption (urinary deoxypyridinoline and hydroxyproline) or formation (serum osteocalcin) [48]. Again, BMI in this study was variable, ranging from 15.8–42.9 kg/m2, therefore it is difficult to draw the conclusion that leptin has no effect on bone if some of these individuals are indeed leptin resistant. A similar story can be told in animal models in which leptin levels are high due to dietary obesity. For example, Turner et al. found that bone metabolism is not altered in rats when leptin levels are increased from high fat feeding [49].
5.0. Conclusions
In this review we examined the published literature surrounding the role of leptin in modulating bone mass. In vitro, the majority of studies suggest leptin positively regulates osteoblast proliferation and suppresses osteoblast-dependent osteoclast recruitment. In vivo, the majority of the work demonstrates a negative role for leptin by enhancing sympathetic output to bone from the hypothalamus (by suppressing serotonin synthesis in the brainstem). Others have described site-dependent effects of leptin: i.e. leptin-deficiency causes high bone mass in the axial skeleton and low bone mass in the appendicular skeleton. Finally, leptin gene therapy in the brain leads to long standing suppression of food intake and white adipose tissue mass, but only a transient decrease in bone density. This last point suggests that the effects of leptin delivery to the brain could be more due to the surgery and administration of leptin rather than the actions of leptin itself.
Notwithstanding, one could conceptualize that since extremely high doses of leptin results in cancellous bone loss and absence of leptin results in increased cancellous bone, these effects are complimentary to each other and consistent with a significant effect of leptin on bone mediated through the sympathetic nervous system. However, when leptin levels hover near normal (as in low-dose studies and/or wild type mice), the effects of leptin on bone appear to be more complex and/or less significant. It could be that the temporal changes in leptin affect bone remodeling as well, and that these might differ in individuals, strains or colonies of mice. Importantly, it is still not clear what happens to adrenergic output in the central nervous system when there are high circulating leptin levels, as is often seen in human obesity [50]. Notwithstanding, it is likely that leptin does enhance sympathetic signaling. Therefore one could postulate that leptin administration would have a more potent effect in mice that are already exposed to some stress (i.e. frequent handling, single housing, male fighting). In fact, it was recently demonstrated that common stressors in laboratory rodents elevate sympathetic activity differently depending on strain and that enriched environments can reduce peripheral adiposity and lower leptin levels [51, 52]. These findings could also be confounded by stress-induced increases in glucocorticoids, which can cause osteoporosis in humans and rodents, and are in part responsible for age-related bone loss [53, 54]. Thus, simply understanding the effect of one gene on bone remodeling is becoming particularly complex and dependent on variables that are difficult to control between laboratories. It is clear that leptin does have an effect on bone. Whether this effect presents itself only in extreme circumstances or is physiologically relevant during homeostatic functions has yet to be fully elucidated.
Highlights.
The role of leptin in bone remains controversial
Some studies suggest leptin promotes osteoblast proliferation and differentiation
Other studies suggest leptin inhibits bone formation through hypothalamic relay
Acknowledgments
This work was supported by Grant Number AR061932 to KJM from the National Institute Of Arthritis And Musculoskeletal And Skin Diseases, by Grant Number AG040219 to CJR from the National Institute On Aging, and by Grant Number DK092759 to CJR from the National Institute Of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The authors thank Casey Doucette and Anyonya Guntur for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, Rosen CJ, Gordon CM. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95:407–413. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Jin L, Cui Q, Wang GJ, Balian G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int. 2005;16:101–108. doi: 10.1007/s00198-004-1649-7. [DOI] [PubMed] [Google Scholar]
- 5.Motyl KJ, Rosen CJ. Temperatures rising: brown fat and bone. Discov Med. 2011;11:179–185. [PMC free article] [PubMed] [Google Scholar]
- 6.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2011 doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, Pfeilschifter J, Silverman S, Diez-Perez A, Lindsay R, Saag KG, Netelenbos JC, Gehlbach S, Hooven FH, Flahive J, Adachi JD, Rossini M, Lacroix AZ, Roux C, Sambrook PN, Siris ES. Obesity Is Not Protective against Fracture in Postmenopausal Women: GLOW. Am J Med. 2011;124:1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82:2904–2910. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- 9.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 10.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 11.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 12.Scheller EL, Song J, Dishowitz MI, Soki FN, Hankenson KD, Krebsbach PH. Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells. 2010;28:1071–1080. doi: 10.1002/stem.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim GS, Hong JS, Kim SW, Koh JM, An CS, Choi JY, Cheng SL. Leptin induces apoptosis via ERK/cPLA2/cytochrome c pathway in human bone marrow stromal cells. J Biol Chem. 2003;278:21920–21929. doi: 10.1074/jbc.M204598200. [DOI] [PubMed] [Google Scholar]
- 14.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 15.Nagasaki H, Shang Q, Suzuki T, Hashimoto H, Yoshimura T, Kondo TA, Ozaki T, Maruyama S, Jomori T, Oiso Y, Hamada Y. Low-serum culture system improves the adipogenic ability of visceral adipose tissue-derived stromal cells. Cell Biol Int. 2011;35:559–568. doi: 10.1042/CBI20100406. [DOI] [PubMed] [Google Scholar]
- 16.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 17.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 18.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson C, Nakao K, Capeau J, Karsenty G. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 20.Singh MK, Elefteriou F, Karsenty G. Cocaine and amphetamine-regulated transcript may regulate bone remodeling as a circulating molecule. Endocrinology. 2008;149:3933–3941. doi: 10.1210/en.2008-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, Guo XE, Karsenty G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208:841–851. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Yadav VK, Suda N, Liu XS, Guo XE, Myers MG, Jr, Karsenty G. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105:20529–20533. doi: 10.1073/pnas.0808701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam DD, Leinninger GM, Louis GW, Garfield AS, Marston OJ, Leshan RL, Scheller EL, Christensen L, Donato J, Jr, Xia J, Evans ML, Elias C, Dalley JW, Burdakov DI, Myers MG, Jr, Heisler LK. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 2011;13:584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pogoda P, Egermann M, Schnell JC, Priemel M, Schilling AF, Alini M, Schinke T, Rueger JM, Schneider E, Clarke I, Amling M. Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res. 2006;21:1591–1599. doi: 10.1359/jbmr.060709. [DOI] [PubMed] [Google Scholar]
- 28.Beil FT, Barvencik F, Gebauer M, Beil B, Pogoda P, Rueger JM, Ignatius A, Schinke T, Amling M. Effects of increased bone formation on fracture healing in mice. J Trauma. 2011;70:857–862. doi: 10.1097/TA.0b013e3181de3dd9. [DOI] [PubMed] [Google Scholar]
- 29.Baldock PA, Sainsbury A, Allison S, Lin EJ, Couzens M, Boey D, Enriquez R, During M, Herzog H, Gardiner EM. Hypothalamic control of bone formation: distinct actions of leptin and y2 receptor pathways. J Bone Miner Res. 2005;20:1851–1857. doi: 10.1359/JBMR.050523. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya K, Takeda S. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13:1234–1240. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- 31.Bartell SM, Rayalam S, Ambati S, Gaddam DR, Hartzell DL, Hamrick M, She JX, Della-Fera MA, Baile CA. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26:1710–1720. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- 32.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 33.Williams GA, Callon KE, Watson M, Costa JL, Ding Y, Dickinson M, Wang Y, Naot D, Reid IR, Cornish J. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011;26:1698–1709. doi: 10.1002/jbmr.367. [DOI] [PubMed] [Google Scholar]
- 34.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Rosen CJ, Motyl KJ. No bones about it: insulin modulates skeletal remodeling. Cell. 2010;142:198–200. doi: 10.1016/j.cell.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Scheller EL, Leinninger GM, Hankenson KD, Myers MG, Jr, Krebsbach PH. Ectopic expression of col2.3 and col3.6 promoters in the brain and association with leptin signaling. Cells Tissues Organs. 2011;194:268–273. doi: 10.1159/000324745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/endo.142.8.8346. [DOI] [PubMed] [Google Scholar]
- 38.Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-1 pathway. Endocrinology. 2007;148:3419–3425. doi: 10.1210/en.2006-1541. [DOI] [PubMed] [Google Scholar]
- 39.Motyl KJ, McCabe LR. Leptin treatment prevents type I diabetic marrow adiposity but not bone loss in mice. J Cell Physiol. 2009;218:376–384. doi: 10.1002/jcp.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, Clemmons DR, Rosen CJ. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc Natl Acad Sci U S A. 2010;107:10508–10513. doi: 10.1073/pnas.1000788107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueno N, Dube MG, Inui A, Kalra PS, Kalra SP. Leptin modulates orexigenic effects of ghrelin and attenuates adiponectin and insulin levels and selectively the dark-phase feeding as revealed by central leptin gene therapy. Endocrinology. 2004;145:4176–4184. doi: 10.1210/en.2004-0262. [DOI] [PubMed] [Google Scholar]
- 42.Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28:1012–1019. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalra SP, Dube MG, Iwaniec UT. Leptin increases osteoblast-specific osteocalcin release through a hypothalamic relay. Peptides. 2009;30:967–973. doi: 10.1016/j.peptides.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwaniec UT, Boghossian S, Trevisiol CH, Wronski TJ, Turner RT, Kalra SP. Hypothalamic leptin gene therapy prevents weight gain without long-term detrimental effects on bone in growing and skeletally mature female rats. J Bone Miner Res. 2011;26:1506–1516. doi: 10.1002/jbmr.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson MA, Iwaniec UT, Turner RT, Wronski TJ, Kalra SP. Effects of increased hypothalamic leptin gene expression on ovariectomy-induced bone loss in rats. Peptides. 2011;32:1575–1580. doi: 10.1016/j.peptides.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers MG, Jr, Heymsfield SB, Haft C, Kahn BB, Laughlin M, Leibel RL, Tschop MH, Yanovski JA. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 2012;15:150–156. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, Kanaya AM, Harris TB, Bauer DC, Cauley JA. Adipokines and the risk of fracture in older adults. J Bone Miner Res. 2011;26:1568–1576. doi: 10.1002/jbmr.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998;63:456–458. doi: 10.1007/s002239900557. [DOI] [PubMed] [Google Scholar]
- 49.Turner RT, Iwaniec UT. Moderate weight gain does not influence bone metabolism in skeletally mature female rats. Bone. 2010;47:631–635. doi: 10.1016/j.bone.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity (Silver Spring) 2006;14(Suppl 5):254S–258S. doi: 10.1038/oby.2006.319. [DOI] [PubMed] [Google Scholar]
- 51.van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5:139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 52.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.den Uyl D, Bultink IE, Lems WF. Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep. 2011;13:233–240. doi: 10.1007/s11926-011-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson DE, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]