Abstract
The time course of and the influence of light intensity and light quality on the induction of a mitochondrial carbonic anhydrase (CA) in the unicellular green alga Chlamydomonas reinhardtii was characterized using western and northern blots. This CA was expressed only under low-CO2 conditions (ambient air). In asynchronously grown cells, the mRNA was detected 15 min after transfer from air containing 5% CO2 to ambient air, and the 21-kD polypeptide was detected on western blots after 1 h. When transferred back to air containing 5% CO2, the mRNA disappeared within 1 h and the polypeptide was degraded within 3 d. Photosynthesis was required for the induction in asynchronous cultures. The induction increased with light up to 500 μmol m−2 s−1, where saturation occurred. In cells grown synchronously, however, expression of the mitochondrial CA was also detected in darkness. Under such conditions the expression followed a circadian rhythm, with mRNA appearing in the dark 30 min before the light was turned on. Algae left in darkness continued this rhythm for several days.
Many unicellular green algae induce a CCM when cells grown under high-CO2 conditions (5% CO2) are transferred to low-CO2 conditions (ambient air) (Badger and Price, 1992). This mechanism allows the algal cell to concentrate the available carbon close to the active site of Rubisco, ensuring efficient photosynthesis even under CO2-limiting conditions. In Chlamydomonas reinhardtii at least seven polypeptides are synthesized during the induction of the CCM (Bailly and Coleman, 1988; Manuel and Moroney, 1988; Spalding and Jeffrey, 1989; Burow et al., 1996), but only one protein, a pCA, has been positively identified as a component of the CCM. Of the other low-CO2-induced polypeptides, one has been identified as an Ala aminotransferase (Chen et al., 1996), one has been proposed to be a chloroplast envelope carrier protein (Chen et al., 1997), and in an earlier report we identified one as an mtCA (Eriksson et al., 1996). Although these three proteins are all induced by low CO2, it is not known whether they play a direct role in the CCM. The identity, localization, and function of the other low-CO2-induced polypeptides have yet to be resolved.
pCA has been thoroughly studied, and many aspects of its induction have been characterized. It is encoded by two genes, Cah1 and Cah2, of which Cah1 is expressed only under low-CO2 conditions and Cah2 is expressed only under high-CO2 conditions (Fujiwara et al., 1990). The induction of Cah1 is inhibited by mixotrophic growth on acetate (Spalding and Ogren, 1982; Moroney et al., 1987; Fett and Coleman, 1994). In asynchronously grown C. reinhardtii, the expression of Cah1 is dependent on both light quality and light intensity. The protein is not expressed unless light of blue wavelengths is present (Dionisio et al., 1989b), and the expression increases with increasing light intensity (Dionisio et al., 1989a). In synchronously grown C. reinhardtii cultures, Cah1 is expressed mainly at the beginning of the light period. The first transcripts are seen 30 min before the light is turned on, indicating that the protein is under the control of a circadian clock (Rawat and Moroney, 1995; Fujiwara et al., 1996).
In the present study we characterized the regulation of expression of mtCA in response to CO2, red light, acetate, and light intensity under asynchronous growth, as well as the influence of light/dark cycles under synchronous growth.
MATERIALS AND METHODS
Algal Strain and Culture Conditions
Chlamydomonas reinhardtii, cell wall-deficient strain CW 92, was grown in batch cultures at 25°C under continuous light at an intensity of 150 μmol m−2 s−1. Bottles containing 800 mL of minimal medium were vigorously bubbled with air containing 5% CO2. For low-CO2 conditions, the cultures were bubbled with air. The major components of the medium were prepared according to the method of Sueoka (1960), and the trace element solution was prepared according to the method of Hutner et al. (1950). Light intensities were measured with an LI-185a quantum meter (Li-Cor, Lincoln, NE).
For time-course experiments of induction, cells were concentrated by centrifugation (5 min, 2000g), resuspended in fresh medium, and bubbled with air. Samples for northern- and western-blot analyses were withdrawn before the induction and 0.5, 1, 1.5, 3, 6, 12, and 24 h after the cells were transferred to air conditions. After 24 h of air bubbling, the culture was split into two fractions. One fraction was left under low-CO2 conditions for 1 week, and the other was transferred back to high-CO2 conditions. Samples for northern-blot analysis were withdrawn from the latter fraction 15, 30, 45, and 60 min after the transfer. Samples for western-blot analysis were collected 36, 48, 60, and 72 h after the low-to-high CO2 transfer.
Mixotrophic growth was obtained by the addition of sodium acetate to a final concentration of 20 mm. To prevent depletion of acetate and other nutrients, the cultures were diluted with fresh medium every 24 h. For studies of the induction at different light intensities, flasks with 80-mL cultures were positioned at different distances from the lamp (HPI-T 400 W, Philips, Eindhoven, The Netherlands) to obtain the desired light intensities.
To measure the induction under red light, high-CO2-grown cells were transferred to a DW3 oxygen electrode reaction chamber (Hansatech, King's Lynn, UK) and illuminated by light filtered through an interference filter (Schott, Mainz, Germany), transmitting light with a wavelength of 684 nm. The design of the DW3 oxygen electrode reaction chamber prevents ambient room light from reaching the sample. Light can only enter through a hole directly connected to the light source. The light intensity that was used for induction was 10 μmol m−2 s−1. Samples for western-blot analysis were withdrawn after 4 h of induction.
To obtain synchronized cultures, cells were grown under high-CO2 conditions for 3 d with 12-h light/12-h dark cycles. After the fourth light period, the culture was switched to low CO2 and divided into two fractions. One was left under the light/dark regime, and the other was transferred to continuous darkness. Samples for northern- and western-blot analyses were withdrawn during the light period 1, 6, and 11 h after the onset of light, and during the dark period 1, 6, and 11.5 h after the light was turned off. During the continuous dark treatments, the flasks were covered with three layers of aluminum foil, wrapped in black plastic, and placed in a darkroom. Cells were harvested under dim green light.
Western Analysis
The samples were separated by SDS-PAGE (12.5% polyacrylamide) as described by Laemmli (1970) using the Mini-Protean II slab-gel apparatus (Bio-Rad). The polypeptides were electroblotted to nitrocellulose filters for immunodetection with antisera raised against the mtCA (Villand et al., 1997). Horseradish peroxidase-linked secondary antibody and enhanced chemoluminescence western blotting detection reagents from Amersham were used for detection. The methods used were described by Harlow and Lane (1988). Protein concentrations of the samples were determined using the Bio-Rad protein assay according to the manufacturer's instructions.
Northern Analysis
RNA was isolated from 4 mL (5–10 μg chlorophyll mL−1) of culture using the TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Glyoxal-denatured northern-blot analysis was performed according to the method of Sambrook et al. (1989) with 32P-labeled Mca12 cDNA (Eriksson et al., 1996) as the probe. Hybridization and washing were done at 65°C. The hybridization signals were quantified with a PhosphorImager (Bio-Rad).
RESULTS AND DISCUSSION
Time Course of Induction and Degradation of mtCA
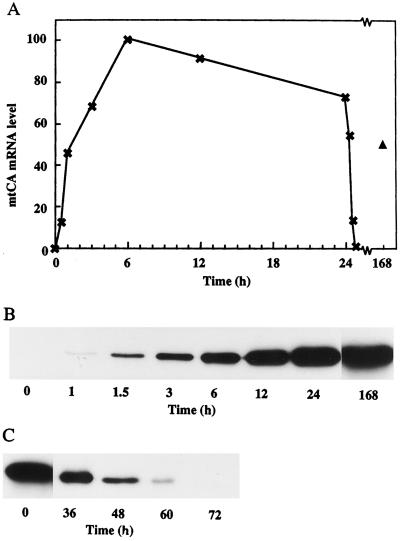
Experiments were performed to determine the time course of the induction of mtCA under low-CO2 conditions, as well as its repression when low-CO2 cells were transferred to conditions of high CO2 (Fig. 1). In the northern-blot hybridization experiments, transcripts were observed 30 min after the transfer to low-CO2 conditions (Fig. 1A). A rapid increase in the amount of transcript occurred during the first hours of induction, reaching a maximum after about 6 h. The amount of transcripts was slightly lower at 12 and 24 h after the transfer. Transcripts were also detected in cells grown for 1 week under low-CO2 conditions, but the amount had decreased to what is typically found after 1 h of induction. The 21-kD mtCA polypeptide was first observed 1 h after the induction, as revealed by western-blot analysis (Fig. 1B). The amount of the polypeptide increased during the first 24 h, after which the level remained relatively constant for at least 1 week. The difference in induction kinetics between mRNA and protein levels reflects the stability of the protein. When the protein is fully induced, less mRNA is needed to keep a constant pool of mtCA. When a low-CO2-adapted culture was switched back to high CO2, the mRNA disappeared after less then 1 h (Fig. 1A) and the amount of protein decreased gradually over 3 d (Fig. 1C).
Figure 1.
Time-course experiments of the induction and degradation of mtCA. A, Northern analysis of the relative amounts of the mtCA mRNA. High-CO2-grown cells were transferred to low-CO2 conditions at time 0. After 24 h, the culture was transferred back to high-CO2 conditions. The triangle represents cells left on low CO2 for 1 week. All lanes on the northern blot contained 5 μg of total RNA. B, Western blot of the induction of mtCA. Numbers represent hours after transfer to low CO2. All lanes contained 0.5 μg of protein. C, Western blot of the degradation of mtCA. Numbers represent hours after transfer from low CO2 to high CO2. All lanes contained 0.5 μg of protein.
There are two genes that encode pCA in C. reinhardtii: one is expressed under low CO2 and one is expressed under high CO2 (Fujiwara et al., 1990). Transcripts for the low-CO2-induced form, Cah1, are present 1 h after the switch to low CO2, reaching a maximum after 2 h. When switched back to high CO2, the mRNA disappears after 1 h. The time scale for the regulation of the Cah2 gene is similar, but this gene is induced by high CO2 and repressed by low CO2, and its mRNA is present in lower amounts. The Cah1 gene product is detectable after 2 h, and the amount of protein increased for 6 h, the longest time of induction examined (Dionisio-Sese et al., 1990). The similarities of the time courses for the induction of mtCA and pCA may indicate that mtCA also has an important function in the CCM.
Expression of mtCA under Mixotrophic Growth
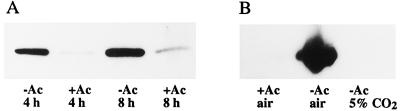
When the growth medium was supplemented with acetate, the induction of mtCA was almost totally inhibited (Fig. 2A). Only a small amount of mtCA was observed on western blots, as compared with cells transferred to low-CO2 conditions in the absence of acetate. In another set of experiments, a culture of low-CO2-adapted phototrophic cells was split into three fractions. One was left under low-CO2 phototrophic conditions, a second was left at low CO2 but supplemented with acetate, and a third was transferred to high-CO2 conditions (Fig. 2B). After 3 d, no mtCA could be detected in the acetate-supplemented culture or in the culture transferred back to high CO2. However, the amount of mtCA in the control culture was unchanged.
Figure 2.
Western-blot analysis of the induction and degradation of mtCA under mixotrophic and phototrophic growth. A, Amount of mtCA in cells adapted to low-CO2 conditions for 4 or 8 h in the absence (−Ac) or presence (+Ac) of acetate in the growth medium. All lanes contained 0.5 μg of protein. B, Amount of mtCA in low-CO2-adapted phototrophic cells after transfer to three different growth conditions for 3 d.
Mixotrophic growth on acetate has also been reported to repress the induction of pCA and the CCM (Spalding and Ogren, 1982; Moroney et al., 1987; Fett and Coleman, 1994). A possible explanation for the lack of induction under low-CO2 conditions in acetate-supplemented medium is that when acetate is metabolized CO2 is produced. This CO2 release might be sufficient to mimic high-CO2 conditions.
Light-Intensity Dependence
Using a reporter gene system, we found that the expression of the mtCA genes depends on light intensity (Villand et al., 1997). In the present study we examined the dependence of light intensity in more detail, both at the mRNA and protein levels, to determine whether the light-regulated induction is controlled at the level of transcription or at the level of translation.
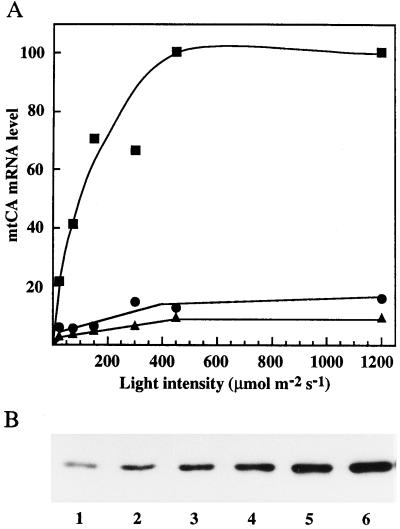
Using asynchronous cultures, no induction of mtCA mRNA was detected in darkness, but within 15 min after transfer to low-CO2 conditions the mtCA mRNA was observed (Fig. 3A). When mRNA levels were monitored 1 h after the transfer, the light response of mtCA expression resembled the light-response curve for photosynthesis, with saturation occurring at approximately 500 μmol m−2 s−1. Western-blot analysis of cells grown for 2 h under low-CO2 conditions revealed a similar pattern at the protein level (Fig. 3B).
Figure 3.
Induction of mtCA under different light intensities. A, The relative amount of mtCA mRNA after transfer to low-CO2 conditions for 15 (▴), 30 (•), and 60 (▪) min under different light intensities. All lanes on the northern blot contained 5 μg of total RNA. B, Western blot of mtCA after 2 h of induction to low-CO2 conditions under different light intensities. The light intensities were: lane 1, 25; lane 2, 75; lane 3, 150; lane 4, 300; lane 5, 450; and lane 6, 1200 μmol m−2 s−1. All lanes contained 0.5 μg of protein.
In asynchronous cultures the induction of Cah1 not only requires low CO2 but also light and a functioning photosynthetic apparatus (Spalding and Ogren, 1982; Spencer et al., 1983; Dionisio-Sese et al., 1990). Dionisio et al. (1989a) observed that the induction of Cah1 was stimulated by higher light intensities. A low induction was observed at 2 μmol m−2 s−1, but the induction increased dramatically up to 70 μmol m−2 s−1, which was the highest light intensity used. They also observed that when 10 μm DCMU (an inhibitor of the photosynthetic electron transport chain) was added before the carbon switch, no induction of the CA occurred.
These results show that the expression of both mtCA and pCA are dependent on photosynthetic electron transport. However, whether this is because the induction is regulated by the redox state of the electron transport chain, the production of energy, or some other aspect of photosynthesis cannot be determined from these experiments. The fact that light dependency is observed at the mRNA level shows that the regulation is not at the level of translation.
Light-Quality Dependence
pCA has been shown to be regulated by light quality (Dionisio et al., 1989b). No induction was observed at 25 μmol m−2 s−1 red light. However, if the red light was supplemented with 6 μmol m−2 s−1 blue light, induction occurred. In the present study we examined the induction of mtCA under 10 μmol m−2 s−1 red light (684 nm; Fig. 4). A clear induction was seen at this low light intensity, demonstrating that the expression of mtCA is not regulated by differences in the light quality in the same way as pCA expression.
Figure 4.
The induction of mtCA under red light. Western-blot analysis of samples withdrawn from the culture before (5% CO2) and after (air) 4 h of induction under red light (684 nm) of 10 μmol m−2 s−1. Both lanes contained 0.5 μg of protein.
Circadian Rhythm of mtCA mRNA Expression
If a plant is grown under a light/dark regime, many genes are expressed in a diurnal manner. For some of these genes, the oscillating expression follows a circadian rhythm. A circadian rhythm has a period of about 24 h and is governed by an endogenous clock that is reset every day by an exogenous stimulus such as light. If this stimulus is withdrawn, the circadian rhythm continues to oscillate but with a decreasing amplitude. A circadian rhythm is also temperature compensated and will proceed at almost the same rate even if the ambient temperature is changed. A diurnal rhythm, however, will not continue in the absence of the entraining stimulus and is not temperature compensated (Pittendrigh, 1960).
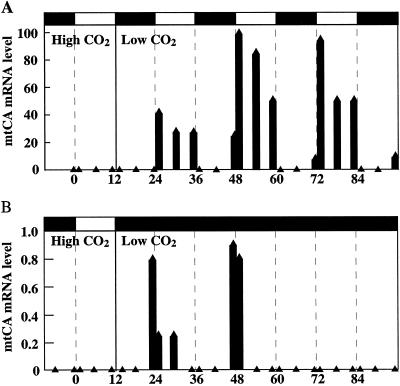
Figure 5 shows that the expression of mtCA is at least to some extent controlled by a circadian clock when the cells are grown synchronously in a 12-h light/12-h dark growth cycle. Cells were grown in high CO2 and switched to low CO2 at the beginning of the dark period. The culture was divided into two fractions: one was left in a light/dark cycle and the other was transferred to continuous darkness. In both fractions, expression of the CA could be detected 30 min before the start of the light period. The expression of the gene in the cells left under the light/dark regime reached a maximum during the early light period and decreased during the day (Fig. 5A). During the dark period the expression was completely arrested. No mRNA was seen 1 h after the light was turned off; however, 30 min before the next light period, the mRNA could be detected again. In the cells kept in continuous darkness (Fig. 5B), mRNA was detected just before and just after the light should have been turned on (−0.5 and 1 h) during the following 2 d. Although the induction was about 2 orders of magnitude less than the corresponding expression in light, induction for several days under continuous darkness shows that expression of mtCA is to some extent controlled by a circadian clock and is not dependent only on the presence of photosynthesis.
Figure 5.
Northern-blot analyses of mtCA mRNA levels in synchronously grown cells. After three light/dark cycles, the culture was transferred to low CO2 at the beginning of the fourth dark period. A, Cells left under a light/dark regime. B, Cells transferred to continuous darkness. Samples for mRNA analyses were withdrawn 1, 6, and 11 h into the light period and 1, 6, and 11.5 h into the dark period. All lanes on the northern blot contained 5 μg of total RNA.
It has been demonstrated that Cah1 is under the control of a circadian clock (Rawat and Moroney, 1995; Fujiwara et al., 1996). When Rawat and Moroney (1995) cultured low-CO2-adapted C. reinhardtii cells synchronously under a 12-h light/12-h dark growth regime, they observed that the Cah1 gene showed a diurnal expression, with transcripts detected 1 h before the light was turned on. They also observed that the pCA was expressed at the end of the first dark period when high-CO2-grown synchronous cells were switched to low CO2 30 min into the dark period. Fujiwara et al. (1996) also grew low-CO2 cells synchronously under a 12-h light/12-h dark growth regime and monitored the diurnal expression of Cah1. They then split the culture into two fractions: one left in continuous light and the other in continuous darkness. In both cultures the oscillating expression of the pCA gene was seen for 3 d, confirming that the gene was under circadian control. In both studies the oscillating expression of the Cah1 gene reached a maximum during the early light period, decreased during the day, and stayed at a very low level during the dark period.
CONCLUSIONS
The mtCA is expressed only under low-CO2 conditions, and the expression is inhibited by acetate. Light and photosynthesis are absolute requirements for the induction of mtCA under asynchronous growth. One hour after the transfer, the light response of mtCA induction resembles the light-response curve for photosynthesis, with saturation occurring at approximately 500 μmol m−2 s−1. Photosynthesis is not an absolute requirement for induction under synchronous growth. Under such conditions a low expression is seen when cells are transferred to continuous darkness, indicating that at least some of the expression is under the control of a circadian clock. The only difference in regulation between mtCA and pCA is that the induction of mtCA seems not to be dependent on light of blue wavelengths. This similarity in regulation is surprising, considering that the promoter regions of the mtCA and the pCA show no sequence similarity (Villand et al., 1997). It should also be stressed that mtCA and pCA probably have two very different roles in the cell: pCA supplies CO2 at the plasma membrane, and mtCA has been suggested to be important for buffering the mitochondrial matrix (Eriksson et al., 1996). The similarity in induction of mtCA and pCA and the close correlation to the induction of the CCM may indicate that mtCA has an important function in the CCM.
ACKNOWLEDGMENT
The authors thank Dr. Youn-Il Park for assistance with experiments on light quality.
Abbreviations:
- CA
carbonic anhydrase
- CCM
CO2-concentrating mechanism
- mtCA
mitochondrial CA
- pCA
periplasmic CA
Footnotes
In accordance with the Commission on Plant Gene Nomenclature's guidelines, we have decided to change the names of the genes encoding the mtCA from β-CA1 and β-CA2 to Mca1 and Mca2.
LITERATURE CITED
- Badger MR, Price GD. The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol Plant. 1992;84:606–615. [Google Scholar]
- Bailly J, Coleman JR. Effect of CO2 concentration on protein biosynthesis and carbonic anhydrase expression in Chlamydomonas reinhardtii. Plant Physiol. 1988;87:833–840. doi: 10.1104/pp.87.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow MD, Chen ZY, Mouton TM, Moroney JV. Isolation of cDNA clones of genes induced upon transfer of Chlamydomonas reinhardtii cells to low CO2. Plant Mol Biol. 1996;31:443–448. doi: 10.1007/BF00021807. [DOI] [PubMed] [Google Scholar]
- Chen Z-Y, Burow MD, Mason CB, Moroney JV. A low-CO2 inducible gene encoding an alanine:α-ketoglutarate aminotransferase in Chlamydomonas reinhardtii. Plant Physiol. 1996;112:677–684. doi: 10.1104/pp.112.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-Y, Lavigne LL, Mason CB, Moroney JV. Cloning and overexpression of two cDNAs encoding the low-CO2-inducible chloroplast envelope protein LIP-36 from Chlamydomonas reinhardtii. Plant Physiol. 1997;114:265–273. doi: 10.1104/pp.114.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio ML, Tsuzuki M, Miyachi S. Light requirement for carbonic anhydrase induction in Chlamydomonas reinhardtii. Plant Cell Physiol. 1989a;30:207–213. [Google Scholar]
- Dionisio ML, Tsuzuki M, Miyachi S. Blue light induction of carbonic anhydrase activity in Chlamydomonas reinhardtii. Plant Cell Physiol. 1989b;30:215–219. [Google Scholar]
- Dionisio-Sese ML, Fukuzawa H, Miyachi S. Light-induced carbonic anhydrase expression in Chlamydomonas reinhardtii. Plant Physiol. 1990;94:1103–1110. doi: 10.1104/pp.94.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Karlsson J, Ramazanov Z, Gardeström P, Samuelsson G. Discovery of an algal mitochondrial carbonic anhydrase: molecular cloning and characterization of a low-CO2-induced polypeptide in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1996;93:12031–12034. doi: 10.1073/pnas.93.21.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett JP, Coleman JR. Regulation of periplasmic carbonic anhydrase expression in Chlamydomonas reinhardtii by acetate and pH. Plant Physiol. 1994;106:103–108. doi: 10.1104/pp.106.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Fukuzawa H, Tachiki A, Miyachi S. Structure and differential expression of two genes encoding carbonic anhydrase in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1990;87:9779–9783. doi: 10.1073/pnas.87.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Ishida N, Tsuzuki M. Circadian expression of the carbonic anhydrase gene, Cah1, in Chlamydomonas reinhardtii. Plant Mol Biol. 1996;32:745–749. doi: 10.1007/BF00020215. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hutner SH, Provasoli L, Schatz A, Haskins CP. Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc Am Philos Soc. 1950;94:152–170. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manuel LJ, Moroney JV. Inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1988;88:491–496. doi: 10.1104/pp.88.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Kitayama M, Togasaki RK, Tolbert NE. Evidence for inorganic carbon transport by intact chloroplasts of Chlamydomonas reinhardtii. Plant Physiol. 1987;83:460–463. doi: 10.1104/pp.83.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Rawat M, Moroney JV. The regulation of carbonic anhydrase and ribulose-1,5-bisphosphate carboxylase/oxygenase activase by light and CO2 in Chlamydomonas reinhardtii. Plant Physiol. 1995;109:937–944. doi: 10.1104/pp.109.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Spalding MH, Jeffrey M. Membrane-associated polypeptides induced in Chlamydomonas by limiting CO2 concentrations. Plant Physiol. 1989;89:133–137. doi: 10.1104/pp.89.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding MH, Ogren WL. Photosynthesis is required for induction of the CO2-concentrating system in Chlamydomonas reinhardtii. FEBS Lett. 1982;145:41–44. [Google Scholar]
- Spencer KG, Kimpel DL, Fisher ML, Togasaki RK, Miyachi S. Carbonic anhydrase in Chlamydomonas reinhardtii. II. Requirements for carbonic anhydrase induction. Plant Cell Physiol. 1983;24:301–304. [Google Scholar]
- Sueoka N. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1960;46:83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villand P, Eriksson M, Samuelsson G. Carbon dioxide and light regulation of promoters controlling the expression of mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Biochem J. 1997;327:51–57. doi: 10.1042/bj3270051. [DOI] [PMC free article] [PubMed] [Google Scholar]