Abstract
After complement system (CS) activation, the sequential production of complement products increases cell injury and death through opsonophagocytosis, cytolysis, adaptive, and inflammatory cell responses. These responses potentiate cerebral ischemia-reperfusion (IR) injury after ischemic stroke and reperfusion. Activation of the CS via mannose binding lectin (MBL)-initiated lectin pathway is known to increase tissue damage in response to IR in muscle, myocardium and intestine tissue. In contrast, the contribution of this pathway to cerebral IR injury, a neutrophil-mediated event, is less clear. Therefore, we investigated the potential protective role of MBL deficiency in neutrophil-mediated cerebral injury after IR. Using an intraluminal filament method, neutrophil activation and cerebral injury were compared between MBL-deficient and wild type C57Bl/6 mice subjected to 60 minutes of MCA ischemia and reperfusion. Systemic neutrophil activation was not decreased in MBL-deficient animals after IR. In MBL-deficient animals, cerebral injury was significantly decreased only in the striatum (p < 0.05). Despite MBL deficiency, C3 depositions were evident in the injured hemisphere during reperfusion. These results indicate that while MBL deficiency results in a modest protection of a sub-cortical brain region during IR, redundant complement pathway activation may overwhelm further beneficial effects of MBL deficiency during reperfusion.
Keywords: Complement, ischemia, mannose binding lectin, neutrophil, reperfusion, stroke
INTRODUCTION
Stroke is currently the third leading cause of death in the US. Unfortunately, estimates indicate that as our population ages, the occurrence of stroke will dramatically increase 43% over the next sixteen years [1,2]. Stroke is also associated with significant disability and thus contributes to individual, family and societal health care burden. In ischemic stroke, by far the most common stroke etiology (89%) [2], a thrombus or embolus blocks a blood vessel responsible for delivering oxygen and nutrients to brain tissue. Occlusion of the middle cerebral artery, a common site of ischemic stroke, severely damages the sub-cortical brain region, where collateral circulation is limited. This area of irreversible ischemic injury is called the necrotic core. Surrounding the necrotic core, the penumbra contains viable tissue not yet irreversibly damaged because energy metabolism is maintained through collateral circulation [3]. Returning blood flow to ischemic tissue (reperfusion) is necessary to reduce expansion of the necrotic core to the cortical brain regions, thereby lessening ischemic injury. Unfortunately, the benefits of reperfusion are mitigated by a secondary injury to the penumbra which is, in part, related to acute and prolonged inflammatory events that damage viable brain cells [4–7]. This secondary injury is termed ischemia-reperfusion (IR) injury.
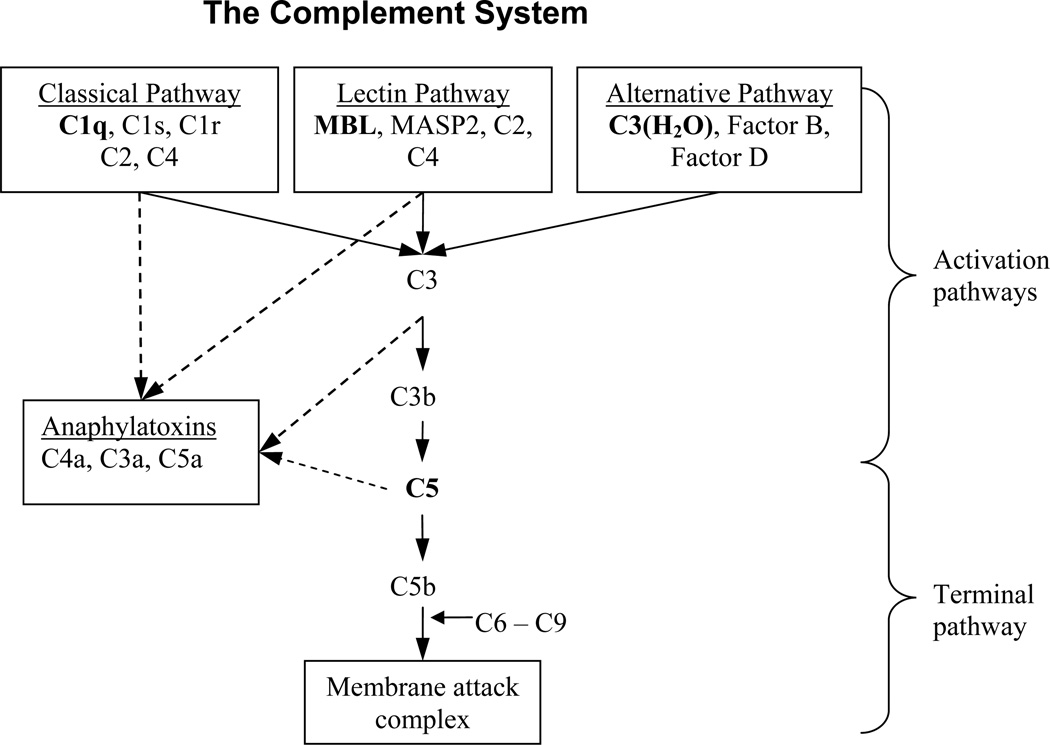
Activation of the complement system (CS) contributes to the inflammatory sequel of cerebral IR injury. The CS, a component of the innate immune system, is comprised of three activation pathways (alternative, classical and lectin) that lead to a common terminal pathway, summarized by Fig. (1). The alternative pathway is initiated by hydrolyzed C3 [C3(H2O)], factor B and factor D interactions. The classical pathway is initiated when systemically circulating C1q (a pathogen recognition molecule) interacts with antigen-antibody complexes on pathogenic cell surfaces. The lectin pathway, distinguished as independent from the classical pathway in the 1990s [8], is initiated when circulating mannose binding lectin (MBL) or ficolins interacts with sugar moieties present on invading bacteria or glycated molecules and damaged cells. Activation of alternative, classical, and lectin pathways may occur both simultaneously and independently of each other to result in C3 convertase formation and initiation of the terminal pathway [9,10]. Thus, as a result of CS activation, complement factors and fragments are sequentially produced, increasing cell death and cell clearing by opsonophagocytosis, cytolysis, adaptive, and inflammatory cell responses [9]. Important to cerebral IR injury, and thus, this study, is the central role of anaphylatoxins (complement fragment C3a and C5a) as potent inducers of neutrophil activation [11,12].
Fig. (1). Introduction to the complement system.
In the classical pathway, C1q recognizes and binds to immunoglobulins (IgG and IgM) presented on damaged or invading cells. The C1q-antibody complex activates serine proteases C1s and C1r which then combine C2 and C4 into the classical C3 convertase (C4b2a). In the lectin pathway, MBL recognizes and binds to glycans presented as antigens on damaged cells or microorganisms. The MBL-glycan complex activates serine protease MASP2 to create the lectin C3 convertase from C2 and C4 (C4b2a). The alternative pathway greatly differs from classical and lectin pathways. With increased concentrations of circulating C3(H2O), factors B and D spontaneously form the alternative C3 convertase (C3bBb). Anaphylatoxins C3a, C4a, and C5a are created through enzymatic cleavage of C3, C4, and C5; while C3b, C4b, and C5b are necessary for continued propagation of the CS toward the terminal pathway; C3a, C4a, and C5a carry out secondary CS actions to activate other immune systems. The sequential cascade of CS is highlighted by the solid arrows while the dotted arrows identify the origins of anaphylatoxin production (Fujita, 2004; Sunyer et al, 2005). The terminal pathway begins with C5 cleavage by C5 convertase and ends with MAC formation resulting in targeted cytolysis.
It is well known that activated neutrophils, a component of cell mediated inflammatory responses, increase cerebral IR injury [5–7]. Activated neutrophils firmly adhere to injured blood vessel endothelium thereby localizing the responses of these inflammatory cells (vessel plugging, release of reactive oxygen species and proteases) to distinct sites of injury. Up-regulation of surface expression of CD11b on systemic neutrophils facilitates their firm adhesion to activated vessel endothelium and diapedesis into brain parenchyma. Thus CD11b is a measure both of neutrophil activation and their potential for increasing cerebral IR injury [13].
Investigations examining complement-mediated cerebral IR injury after ischemic stroke and reperfusion burgeoned approximately ten years ago. Initial lines of inquiry examined the effects of total CS protein depletion, inhibition of all three activating pathways or inhibition of the terminal pathway on cerebral IR injury; the majority of these studies found significant neuroprotection [14–20]. Inhibition of all three activation pathways also decreased neutrophil accumulation and the production of free radicals and lipid peroxidase in brain tissue after cerebral IR [14,16,19,21]. Studies that followed focused on determining the contribution of individual pathway activation to cerebral IR injury [14,18,22]. Limited evidence now suggests that complement mediated injury in cerebral IR is independent of C1q (classical pathway activation) thus bringing to attention the possible role of either the alternative and lectin activation pathways [18,22]. In tissues other than the brain, most notably the heart, skeletal muscle, kidney and intestine, investigators have examined the contribution of MBL-initiated lectin pathway activation to IR injury and reported significant organ protection in the MBL-deficient mouse [23–28].
To date, the role of the lectin activation pathway in cerebral IR injury is limited [29]. Furthermore, while both the CS and neutrophil activation clearly contribute to cerebral IR injury, a relationship between the two has not yet been well defined in ischemic stroke and reperfusion. Therefore the purpose of this study was to determine if MBL-initiated lectin pathway activation of the CS significantly contributes to neutrophil activation and resultant cerebral injury after ischemic stroke and reperfusion. We hypothesized that in the absence of MBL, CS activation would be significantly decreased during cerebral ischemia and reperfusion and that the resulting decrease in anaphylatoxin production would limit neutrophil activation, and thus significantly attenuate cerebral injury.
MATERIALS AND METHODS
Animals
C57Bl/6 (MBL+/+) mice or C57Bl/6 mice with targeted mutations to both the MBL A and MBL C genes (MBL−/−) were purchased from Jackson Laboratories, (Bar Harbor, ME). This MBL-deficient mouse model, first described by Shi et al, has been used extensively to investigate MBL activation during IR injury in organ systems other than the brain [25,27,30]. All animal experiments were performed in compliance with the University of Arizona Institutional Animal Care and Use Committee.
Experiments
MBL, C1q and factor D gene expression (activators of the lectin, classical, and alternative pathway, respectively) has not been adequately described after experimental cerebral IR. In an initial set of experiments, gene expression of MBL-A, MBL-C, C1q, and factor D was measured after cerebral IR (in MBL+/+) and compared to animals undergoing a sham procedure. In addition, as both CS and neutrophil activation are each implicated as mechanisms of reperfusion cerebral IR injury, it was also important to first establish whether products produced via CS activation could impact neutrophil activation in the mouse. In vitro studies were undertaken to investigate the effects of C3a and C5a on neutrophil activation in mouse whole blood.
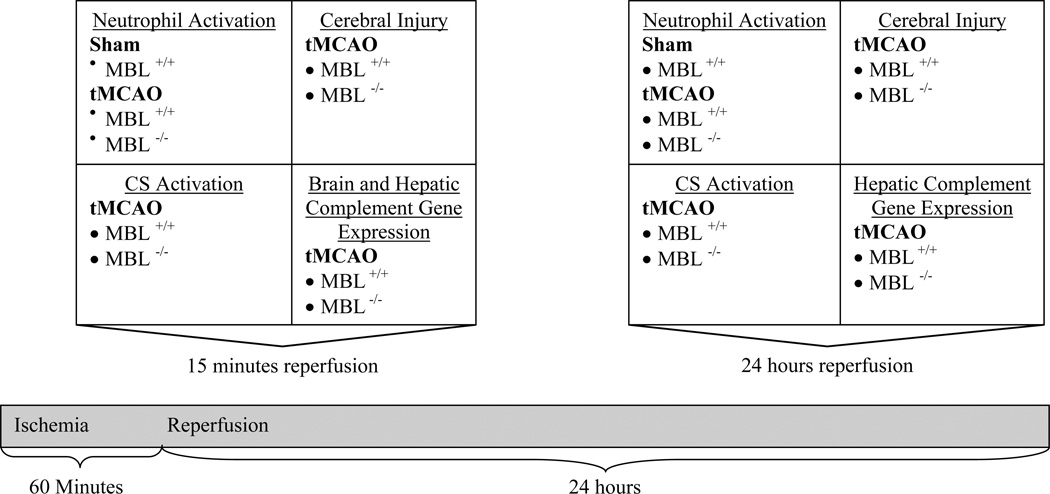
Fig. (2) summarizes the key experiments conducted in both MBL+/+ and MBL−/− animals. To investigate the role of MBL-initiated CS activation on neutrophil activation during cerebral IR injury systemic neutrophil CD11b expression was measured in MBL+/+ and MBL−/− animals after ischemic stroke and reperfusion. The effect of MBL deficiency on cerebral injury, measured by infarct volume and brain edema, and on CS activation, measured by cerebral deposition of C3, was also evaluated after ischemic stroke and reperfusion. Finally, to investigate effects of MBL deficiency on classical and alternative complement activation after ischemic stroke and reperfusion, we compared mRNA expression of C1q and factor D between MBL−/− and MBL+/+ mice.
Fig. (2).
Experimental design.
Temporary Middle Cerebral Artery Occlusion
The temporary middle cerebral artery occlusion (tMCAO) and sham procedures were performed as previously described [13]. In brief, after anesthesia induction, cranial Doppler probe (PeriFlux System 5000, North Royalton, OH) placement and ventral neck incision, the right common and external common artery (ECA) were isolated and the ECA was cauterized and cut. For the tMCAO procedure, a prepared filament (blunted, silicone coated 6-0 nylon, 0.21–0.25 mm) was introduced via the ECA stub, the common carotid artery was tied, and the filament was advanced to the ostea of the middle cerebral artery. No filament was placed for the sham procedure. Cerebral ischemia was confirmed by a sudden reduction (70% of baseline) in relative cerebral blood flow (rCBF) measured by doppler probe. To model severe ischemic stroke, the filament remained in place for 60 minutes while animals remained under anesthesia. Sham animals remained under anesthesia for comparable time periods without intraluminal filament placement. After the ischemic period, the filament was withdrawn and the common carotid artery was untied to initiate reperfusion. A return of rCBF to at least 70% of baseline was required for study inclusion. Once affixed to the cranium, the laser doppler probe remained in place allowing for continuous rCBF monitoring throughout the ischemic period and for 15 minutes post reperfusion. Average rCBF was recorded at fifteen minute intervals in all animals.
Real Time RT-PCR
Gene expression of MBL-A, MBL-B, C1q, and factor D was examined to elucidate modalities of CS activation during cerebral IR injury. Real time RT- PCR was used to assess gene expression in MBL+/+ and MBL−/− animals that underwent either sham or tMCAO surgery. C1q and factor D mRNA expression was assessed after ischemia and 15 minutes of reperfusion in brain and liver tissue collected from MBL+/+ and MBL−/− animals. Hepatic MBL-A and -C mRNA expression was assessed in MBL+/+ animals after ischemia and either 15 minutes or 24 hours of reperfusion. Total RNA was isolated from tissues using TriZol (Invitrogen, Carlsbad, CA) extraction followed by a lithium chloride extraction protocol [31]. Isolated RNA was quantified and assessed for purity on a spectrophotometer (Biophotometer 6131, Eppendorf, Hauppauge, NY) and reverse transcribed to cDNA (100ng/µl) using an iScript kit (BioRad, Hercules, CA). TaqMan (Applied Biosystems, Carlsbad, CA) primer probes for the genes of interest are summarized in Table 1. The PCR reaction contained 10µl of 2× iQ Supermix (BioRad), 9µl RNase-free water and 1µl of the TaqMan probe of interest for a total volume of 19µl. All reactions were run in triplicate with the following program: 95°C for 2 min followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. Cycle threshold (CT) for each experiment sample was determined after standardization of cDNA content and normalization using β-actin. Fold change between specific comparison groups were calculated as 2−ΔΔCt [31].
Table 1.
Inducible Gene Expression of MBL A and C after Ischemic Stroke and Reperfusion
| MBL +/+ | Gene Description | Brain | Liver | |
|---|---|---|---|---|
| Gene type, accession no. | 15 minutes | 15 minutes | 24 hours | |
| NM_010775 | Mannose binding lectin A (MBL A) | 24.8 ± 13.1 | 12.1 ± 0.8*** | 36.6 ± 8.5* |
| NM_010776 | Mannose binding lectin B (MBL C) | 122.6 ± 116.9 | 114.4 ± 5.9** | 147.5 ± 38.7 |
Values are expressed as fold change (mean ± SEM) in MBL A and MBL C gene transcripts in MBL+/+ animals that underwent MCAOR procedure (n = 3) as compared to MBL+/+ animals that underwent the sham procedure (n = 3). Gene expression was measured after 15 minutes of reperfusion in the brain and both 15 minutes and 24 hours of reperfusion in the liver to assess for both local and systemic CS responses to ischemic stroke. Statistical significance between MCAOR vs. sham was determined with Student-t test (* p = 0.05, ** p < 0.01, *** p < 0.001).
In vitro Complement Mediated Neutrophil Activation
In order to determine the extent to which C3a and C5a induced neutrophil CD11b expression, blood was collected and pooled from six naïve (not subjected to tMCAO) MBL+/+ animals and incubated in-vitro with different concentrations of each complement factor. Incubations were conducted in triplicate. Complement factors were prepared and stored according to manufactures’ recommendations. Neutrophil CD11b expression in whole blood after in vitro stimulation with murine C5a and C3a has not yet been described in the mouse. The range of C5a and C3a concentrations used in this in vitro study were based on those known to activate human neutrophils [12].
A midline dorsal abdominal incision was made in anesthetized animals and blood was drawn from the ascending vena cava using a 23-ga needle into a 1 ml syringe prepared for a 1:10 citrate/blood dilution (Sigma, MO). Whole blood (50ul) was immediately aliquotted into Eppendorf tubes and incubated with an equal volume of either murine C5a (0.005ng/ml to 15ng/ml; BD Pharmingen, San Jose, CA) or murine C3a (3ng/ml to 300ng/ml; BD Pharmingen). Blood incubated with an equal volume of phosphate-buffered saline or lipopolysaccharide (LPS, 0.1ng/ml, final concentration) served as negative and positive controls, respectively. All whole blood aliquots were incubated with complement proteins for thirty minutes followed by flow cytometry processing for analysis of neutrophil CD11b expression (see below). Total incubation time prior to sample fixation with 500 µl 1% paraformaldehyde solution was 55 minutes. Data acquisition was performed immediately after sample fixation.
Post-Stroke In vivo Complement Mediated Neutrophil Activation
To assess the role of lectin complement activation on systemic neutrophil activation responses after transient ischemic stroke, data were collected in two sets of experiments. In a first set of animals, data were collected after ischemia and 15 minutes of reperfusion in three groups of animals: sham procedure in MBL+/+ animals (n = 6), tMCAO procedure in MBL+/+ animals (n = 8), and tMCAO procedure in MBL−/− animals (n = 7). In a second set of animals, data were collected after ischemia and 24 hours post-reperfusion in the same three groups: sham procedure in MBL+/+ animals (n = 7), tMCAO procedure in MBL+/+ animals (n = 7), and tMCAO procedure in MBL−/− animals (n = 7). In all animals, blood was drawn from the abdominal aorta as described above. After blood collection, whole blood (50 µl) was aliquot into Eppendorf tubes for flow cytometry processing and analysis (detailed below).
Flow Cytometry
Whole blood collected for in vitro and in vivo experiments described above was processed for quantification of neutrophil CD11b expression using flow cytometry according to the method previously described [13]. In brief, a PE-Cy5-labeled neutrophil population was identified by its characteristic forward and side scatter. FITC conjugated antibody was used to measure neutrophil surface expression of CD11b within the identified (gated) neutrophil population. To measure neutrophil surface expression of CD11b, FITC-conjugated antibody bound to neutrophil CD11b was excited at 488nm; a 520nm emission was detected by FL1 detectors (FACSCalibur, BD Biosciences, San Jose, CA). After data were acquired from 10,000 events, the geometric mean of CD11b-FITC fluorescence was multiplied by the percentage of cells that were positively fluoresced (percent positive) to yield a total fluorescence intensity (TFI) value for each neutrophil population. Neutrophil populations were independently assessed for each in vitro and in vivo experimental condition and data are reported as mean TFI ± SEM.
Cerebral Injury
Cerebral injury (brain infarct volume and edema) was assessed in MBL+/+ (n = 10) and MBL−/− (n = 8) animals subjected to 60 minutes of ischemia. After 24 hours of reperfusion, brains were rapidly removed from euthanized animals, sliced in 4 coronal sections (2mm) and incubated with 1% 2, 3, 5-triphenyltetrazolium chloride (TTC) as described previously [13]. After overnight incubation in 10% formalin, color digital images of the anterior and posterior aspect of each slice were acquired (HP Scanjet 4850) and saved as TIFF image files at 600 dots per inch. Images were assessed in a blinded fashion using Image J software (NIH) for infarct volume and edema. Anatomical areas (cortex and striatum) in the ipsilateral (affected) and contralateral (non-affected) hemispheres were identified using a mouse brain stereotaxic atlas reference. Infarct volume is reported as a percent of the contralateral hemisphere. Edema is reported as the percent increase in volume between the ipsilateral and contralateral hemispheres ([ipsilateral hemispheric volume - contralateral hemispheric volume)/contralateral hemispheric volume] * 100) [13].
Immunofluorescence
C3 deposition in brain tissue was assessed histologically after 15 minutes and 24 hours of reperfusion in MBL+/+ (n = 2/time group) and MBL−/− (n = 3/time group) animals subjected to 60 minutes of ischemia. Brains were sliced in 2 mm coronal sections and rapidly frozen in an isopentane slurry and stored at −80 °C prior to cryostat slicing. Using a cryostat, 2µm sections were cut beginning at 4mm from the frontal pole. C3 deposits in brain sections were identified by direct IF staining using FITC-conjugated C3 (MP Biomedicals, Solon, OH. C3-FITC was prepared according to manufacturer’s recommendations. A 1:10 dilution (100µl rehydrated FITC-labeled antibody: 900µl PBS) was applied to tissue sections, incubated in a humid chamber for 30 minutes and then rinsed in PBS. Stained tissue was fixed in 100% ethanol and coverslip placed with Mowial. Phase and immunofluorescence images were captured in five consistent locations (3 areas in the cortex and 2 areas in the striatum) using a Leica phase microscope and RT color spot camera (Diagnostic Instruments, Sterling Heights, MI). C3 positive cells were counted in the five images (40X) per hemisphere (ipsilateral and contralateral). Mean number of C3 fluoresced cells/field were calculated for each hemisphere in each experimental group.
Statistical Analysis
All data are presented as the mean ± standard error of mean (SEM). Statistical significance was determined by Student’s t-test or ANOVA with Welch correction, when unequal variances were present, and post-hoc testing, as appropriate, using SPSS [16] software.
RESULTS
Hepatic MBL-A and -C mRNA Expression Increases after Ischemic Stroke and Reperfusion
Hepatic expression of MBL-A mRNA expression significantly increased in MBL+/+ mice after both 15 minutes (p < 0.05) and 24 hours (p = 0.05) of reperfusion as compared to sham animals. Hepatic MBL-C mRNA was significantly increased after 15 minutes (p < 0.05) but not 24 hours (p = 0.06) of reperfusion when compared to sham animals (Table 1). MBL-A and -C gene expression also appeared to be induced locally in the injury brain although these increases did not achieve significance when compared to sham animals (Table 1).
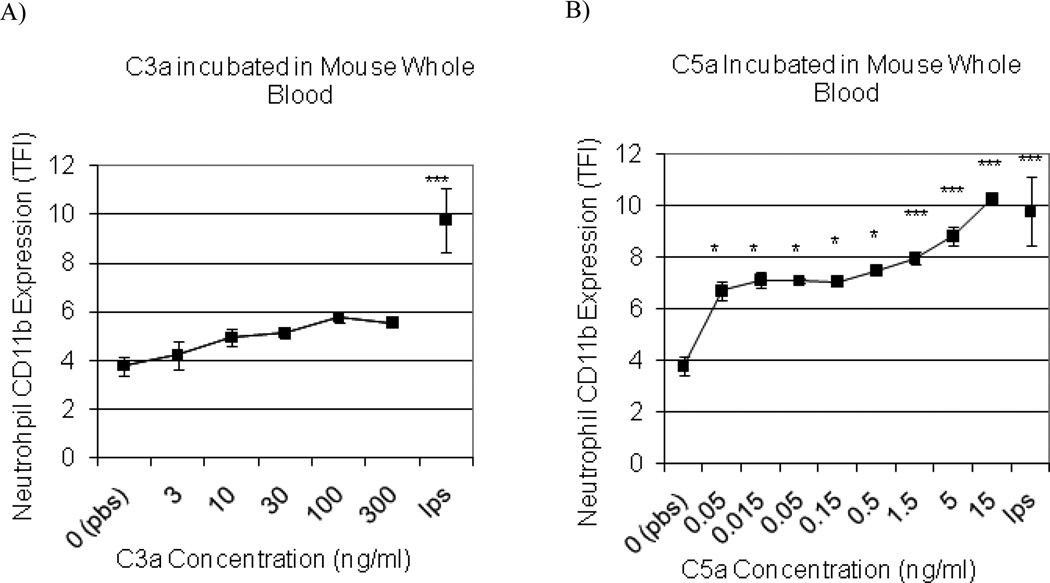
Complement Peptide C5a Increases Neutrophil Expression of CD11b
C3a also did not alter neutrophil CD11b when whole blood was incubated in vitro over a range of C3a concentrations (Fig. 3B). In contrast, a significant concentration-dependent increase in CD11b expression occurred in response to C5a incubation (Fig. 3C). C5a was a potent inducer of CD11b expression.
Fig. (3). Anaphylatoxin C5a increases neutrophil CD11b expression.
Mouse whole blood was incubated with anaphylatoxins C3a (A) and C5a (B) to determine the dose response of neutrophil CD11b expression. Negative (PBS) and positive controls (LPS [10 µg/0.1ml]) were included. Statistical significance versus PBS incubation was denoted at p < 0.05 (*) and p < 0.001 (***) (n = 3 for each incubation; data are mean TFI ± SEM).
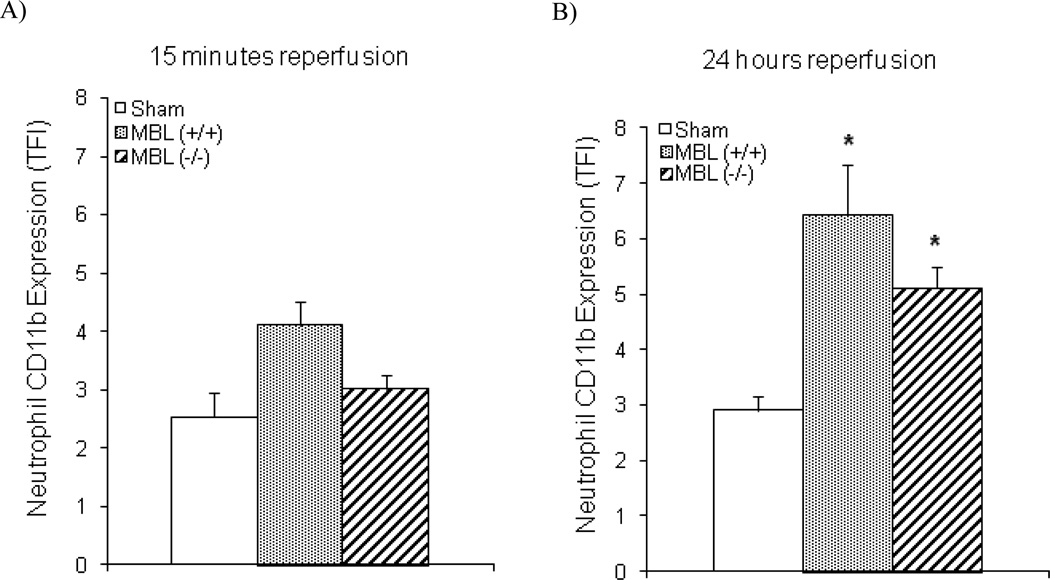
Cerebral Ischemia and Reperfusion Induces Neutrophil CD11b Expression in the Absence of MBL
To investigate a potential role for lectin pathway activation of the CS in the systemic activation of neutrophils during ischemic stroke and reperfusion, CD11b expression on circulating neutrophils was measured in three groups of animals (MBL+/+ sham, MBL+/+ animals with tMCAO, and MBL−/− animals with tMCAO) after fifteen minutes (Fig. 4A) or twenty-four hours (Fig. 4B) of reperfusion. After ischemia and only 15 minutes of reperfusion, neutrophil surface expression of CD11b was not statistically different than shams in either MBL+/+ (p = 0.21) or MBL−/− mice (p = 0.31) subjected to tMCAO. After 24 hours of reperfusion, CD11b expression did increase significantly on circulating neutrophils in the MBL+/+ mice when compared to sham (p = 0.001). CD11b expression was also significantly increased in the MBL−/− mice at twenty-four hours of reperfusion (p < 0.05) and, contrary to our hypothesis, was no different than in the MBL+/+ mice.
Fig. (4). Neutrophil activation post-reperfusion of ischemic stroke.
A) Neutrophil expression of CD11b was assessed in three groups after ischemia and 15 minutes of reperfusion to assess neutrophil activation: MBL+/+ animals with sham procedure (n = 6), MBL+/+ animals with tMCAO procedure (n = 8), and MBL−/− animals with tMCAO procedure (n = 8). B) Data were also collected in the same groups after 24 hours of reperfusion (n = 7 for all groups). Neutrophil CD11b expression was significantly increased in both MBL+/+ and MBL−/− animals after 24 hours of reperfusion. Statistical significance versus sham was denoted at p < 0.05 (*) (data are mean TFI ± SEM).
MBL Deficiency Decreases Infarct Size in the Striatum
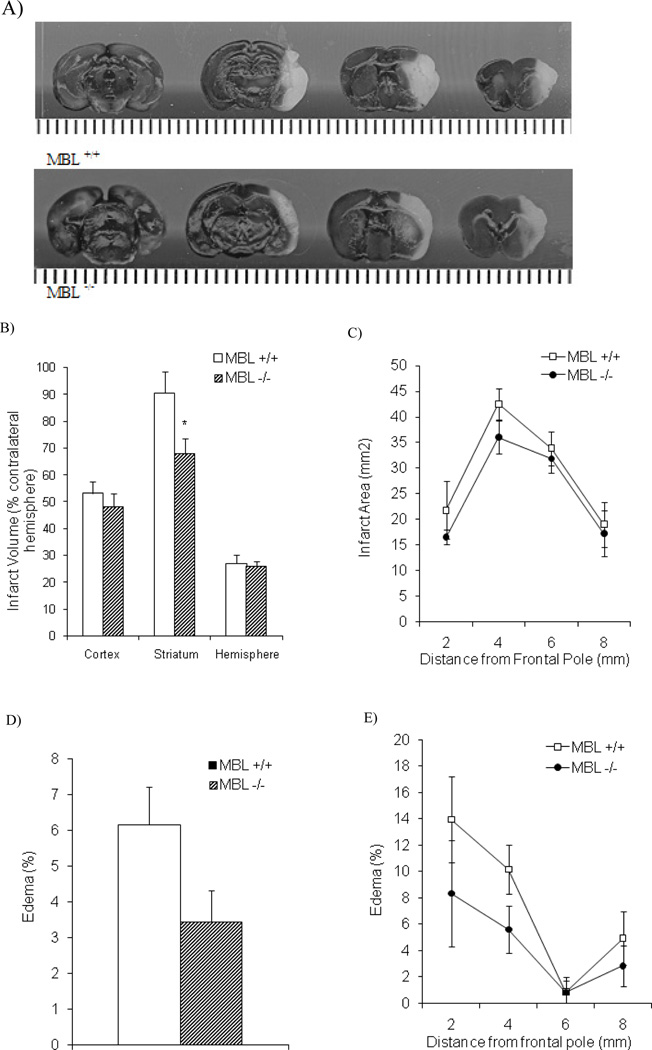
To determine whether MBL deficiency could be cerebroprotective, independent of effects on neutrophil activation, we next evaluated the effect of MBL deficiency on actual brain injury, as measured by infarct volume after twenty-four hours of reperfusion following ischemia. As is evident from representative images of brain injury in MBL+/+ (upper panel) and MBL−/− (lower panel) animals after tMCAO (Fig. 5A), infarct volume in the striatum was significantly decreased in the MBL−/− group (26.1% reduction), compared to the MBL+/+ group (p < 0.05) (Fig. 5B). In contrast, there was a small but non-significant reduction in infarct volume in the cortex (12.7%) and no difference in total hemisphere infarct volume in the MBL−/− group when compared to the MBL+/+ group (Fig. 5B). Further analysis of the distribution of infarction volume following tMCAO, which was greatest in middle coronal sections 4–6mm from the frontal lobe of MBL +/+ mice (Fig. 5C), revealed small but non-significant decreases in total infarct area in the frontal portion of the brain of MBL −/− mice where the cortex and striatum are proportionately greatest (0–4 mm from frontal lobe).
Fig. (5). Cerebral injury in MBL-deficient animals after ischemic stroke and reperfusion.
A) Representative images and of cerebral injury after tMCAO procedure and 24 hours of reperfusion in MBL+/+ (upper panel) and MBL−/− (lower panel). Quantitative analysis of cerebral injury in MBL+/+ and MBL+/+ animals is presented as follows: B) Infarct volume (% contralateral hemisphere) in the cortex, striatum, and total hemisphere (n = 10/MBL+/+, n = 8/c). Analysis of infarct volume illustrate that striatal injury was significantly decreased in MBL−/− versus MBL+/+ animals. C) Total infarct area (mm2) in serial coronal sections in MBL+/+ (n = 10) and MBL−/− (n = 8) groups. Edema present in brain tissue is presented as follows: D) Edema (%) in MBL+/+ (n = 10) and MBL−/− (n = 8) groups. Analysis of hemispheric edema data illustrate that there was no significant reduction in edema between MBL+/+ and MBL−/− (p = 0.07) animals after ischemic stroke and reperfusion. (E) Edema (%) in serial coronal sections in MBL+/+ (n = 10) and MBL−/− (n = 8) groups. Statistical significance versus MBL+/+ was denoted at p < 0.05 (*) (data are mean ± SEM).
Brain edema in MBL deficient mice was decreased by 44% when compared to the MBL+/+ group. This change did not quite reach statistical significance with two tailed testing (p = .07) (Fig. 5D). As with infarction volume, this reduction in edema observed in MBL −/− mice also appeared most marked in the frontal portion of the brain (Fig. 5E).
The Complement System is Activated during Cerebral IR Despite MBL Deficiency
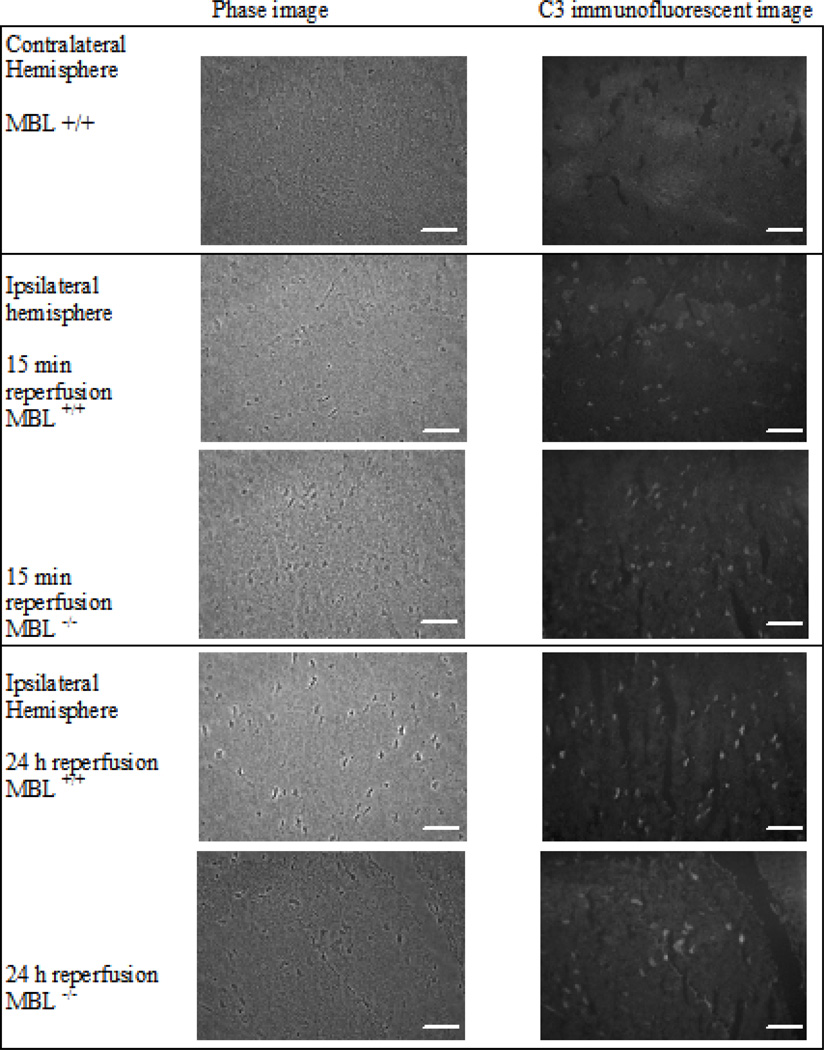
Because MBL deficiency had only a modest effect in limiting cerebral injury, we next assessed CS activation in response to IR injury. Immunofluorescent C3 deposits were not observed in the uninjured contralateral hemispheres of MBL+/+ or MBL−/− mice after ischemia and reperfusion (15 minutes or 24 hours) (Fig. 6). In contrast, distinct C3 deposits, which primarily localized to neurons, were evident in the injured ipsilateral hemispheres of both MBL+/+ and MBL−/− mice. The number of C3 positive cells in the ipsilateral cortex and striatum of MBL+/+ and MBL−/− mice following tMCAO were no different at 15 minutes (31.1 ± 19.5, 17.4 ± 11.9 respectively) or 24 hours (25.0 ± 5.2, 21.0 ± 9.4 respectively) of reperfusion.
Fig. (6). Histopathological and immunofluorescent evaluation of infarcted brain tissue.
Contralateral (Row 1) and ipsilateral hemispheres were assessed for C3 deposits in four different animal groups: Row 2) ischemia and 15 minutes of reperfusion in MBL+/+ animals (n = 2), Row 3) ischemia and 15 minutes of reperfusion in MBL−/− animals (n = 3), Row 4) ischemia and 24 hours of reperfusion in MBL+/+ animals (n = 2), and Row 5) ischemia and 24 hours of reperfusion in MBL−/− animals (n = 3). Phase images (column 1) and C3-FITC images (column 2) were captured using a 40X magnification. The contralateral hemisphere image is representative for all groups. C3 deposits, marked by the fluorescent C3-conjugated antibody and white spots present in infarcted tissue, were observed in all groups. Scale marker is 50 microns.
Hepatic C1q mRNA Expression Decreases after Ischemic Stroke and Reperfusion
We next investigated gene expression of C1q and factor D, important components of classical and alternative CS pathways, in response to stroke and reperfusion injury in wild type MBL+/+ and MBL−/− mice (Table 2). Factor D gene expression in MBL+/+ animals was not statistically different after ischemic stroke and reperfusion in either brain or liver tissue when compared to the sham group. In addition, hepatic factor D expression after ischemic stroke and reperfusion was unaffected by MBL deficiency. Similarly, brain C1q gene expression was also unaffected by ischemic stroke and reperfusion and unaffected by MBL deficiency. However, hepatic C1q gene expression was significantly decreased after 15 minutes of reperfusion in both MBL+/+ and MBL−/− mice, (p < 0.01 and p < 0.001 respectively). After 24 hours of reperfusion, C1q gene expression was not different in either MBL group when compared to sham.
Table 2.
Inducible Factor D and C1q Gene Expression after Ischemic Stroke and Reperfusion in MBL+/+ and MBL−/− Animals
| Gene type, Accession no. | Gene Description | Length of Reperfusion | MBL+/+ | MBL −/− | |
|---|---|---|---|---|---|
| Liver | |||||
| NM_013459 | factor D (fD) | 15 min | 0.5 ± 0.5 | 3.9 ± 2.8 | |
| 24 hour | 2.8 ± 2.16 | 0.7 ± 0.3 | |||
| NM_007572 | C1q | 15 min | 0.7 ± 0.0** | 0.5 ± 0.03*** | |
| 24 hour | 0.7 ± 0.2 | 0.5 ± 0.3 | |||
| Brain | |||||
| NM_013459 | factor D (fD) | 15 min | ND | ND | |
| 24 hour | ND | ND | |||
| NM_007572 | C1q | 15 min | 0.4 ± 0.2 | ± 0.2 | |
Values are expressed as fold change (mean ± SEM) of factor D and C1q gene transcripts in MBL+/+ and MBL−/− animals that underwent the tMCAO procedure (n = 3/group) as compared to MBL+/+ animals that underwent the sham procedure (n = 3) (ND = transcripts not detected). Gene expression was measured after 15 minutes of reperfusion in the brain and both 15 minutes and 24 hours of reperfusion in the liver. Normalized data are presented for all complement factors. Statistical significance was determined with analysis of variance with Welch correction (a correction for unequal variances) and Bonferroni post-hoc where appropriate. Statistical significance (** p < 0.01, *** p < 0.001) between groups are reported vs. sham. There was no statistically significant alteration in Factor D or C1q gene transcripts between MBL+/+ and MBL−/− groups in liver or brain tissue.
DISCUSSION
To our knowledge, this is the first study to investigate the postulate that MBL-initiated lectin pathway activation of the CS enhances neutrophil-mediated IR injury after ischemic stroke. It is well known that activated neutrophils significantly increase cerebral injury after IR via multiple mechanisms including: the “no-reflow” phenomena, caused by neutrophil microvascular occlusion; release of toxic substances such as ROS and proteases; and systemic and local release of pro-inflammatory cytokines [7]. A sustained reduction of blood flow combined with release of neutrophil products (ROS, proteases, cytokines), during reperfusion, extends damage to the blood brain barrier, vascular endothelium, and, eventually, brain parenchyma [32].
CS activation is known to enhance neutrophil activation, mediated primarily by anaphylatoxin C5a [11,12] and, critically, MBL-initiation of the CS has proven to play a role in IR injury in every vascular bed studied to date [23,28]. Mechanistically, in response to IR injury, MBL is thought to have two roles: first, to mark cells for phagocytosis and second, to initiate the complement system via the lectin pathway resulting in cytolysis, increased anaphylatoxin production and increased inflammatory responses. Evidence suggests that, during reperfusion, interactions between MBL and IgM initiate complement activation and tissue damage [33,34]. Specifically, IgM antibodies bind to antigens expressed on injured tissue and vascular endothelium [28] and contain mannose terminating glycans which affect antibody solubility [35]. Systemically circulating and locally produced MBL recognizes these glycan domains and binds to IgM. Subsequently, these MBL-IgM interactions initiate the lectin pathway and sequential production of anaphylatoxins (C3a and C5a) during IR.
The liver is the primary site of MBL-A and -C production although these gene products are also known to be produced in other tissues, including the brain [36]. To our knowledge, these studies provide the first evidence that hepatic expression of MBL-A and MBL -C is significantly increased after ischemic stroke and reperfusion. Thus, while serum MBL was not measured in this study, because gene expression of MBL-A and MBL- C correlate with serum MBL concentrations [24], these changes in hepatic gene expression suggest that circulating levels of MBL may be significantly increased very early in response to cerebral IR injury and remain elevated for up to 24 hours.
As the effects of anaphylatoxins C3a and C5a on neutrophil activation in mouse whole blood had not previously been reported, it was important to verify their effects in this study. Previously, in human whole blood, neutrophil activation has been reported in response to C3a or C5a with C5a being the most potent inducer of neutrophil activation as compared to C3a, including concentration-dependent increases in neutrophil CD11b expression [11,12]. In the current study, similar to the findings in human studies, we found that C5a, but not C3a, potently increases neutrophil surface expression of CD11b in mice. These novel data therefore suggest that neutrophil activation in mice models that of humans and can similarly be induced via CS activation and subsequent production of anaphylatoxin C5a.
Previously, we demonstrated that neutrophil CD11b expression was significantly increased in a mouse model of ischemic stroke and reperfusion [13]. However, contrary to our hypothesis that the MBL pathway would contribute significantly to CS activation and thus neutrophil activation, MBL deficiency was not associated with a reduction in CD11b expression following cerebral IR injury. Although neutrophil accumulation is also an important measure, the cross-reactivity between neutrophils that have migrated into brain tissue and resident microglia impedes any ability, using conventional methods, to distinguish between the two similar cell populations [37–39]. While flow cytometry assesses the first step in the migration of neutrophils to tissue, cell activation, it is a more accurate method that reflects subsequent tissue accumulation.
MBL deficiency significantly decreased infarct volume in the striatum, the small sub-cortical region of the brain where the necrotic core is located following tMCAO, a brain region that suffers the majority of the ischemic insult [3]. This novel finding is indicative that MBL deficiency has a cerebroprotective role during brain ischemia. In contrast, in brain regions more subject to reperfusion and therefore increased inflammatory events augmented by reperfusion injury, cortical region or affected total hemisphere, we did not observe significant protection of MBL-deficient mice. This notion of ischemic versus IR specific effects of MBL is supported by the findings of Yager et al., who examined cortex damage after a traumatic brain injury in the same MBL−/− mouse model [40]. In findings that are similar to our own, Yager et al. found that MBL deficiency did not protect the cortex after traumatic brain injury. In combination, these findings suggest that CS activation may occur in a differential manner during ischemic injury as compared to IR injury, postulates that warrant further investigation.
To explore the possibility that CS activation in the brain persists despite MBL deficiency, C3 deposits were assessed in brain tissue after ischemic stroke and reperfusion. C3, common to all three activating pathways, deposits on injured cells after CS activation marking them for phagocytosis. Others have used immunohistochemistry techniques to measure C3 deposits in situ after injury or infection as a measure of non-pathway specific complement activation after IR [18,25,27,41]. Similar to the findings of others [18], in the current study, after either 15 minutes or 24 hours of ischemic stroke and reperfusion, C3 deposits were, in fact, present within damaged areas of brain tissue of all animals. In combination with neutrophil activation data, these findings suggest that CS activation, and therefore anaphylatoxin production and C3 deposition in brain tissue after ischemic stroke and reperfusion, still occurs in the absence of MBL and must therefore occur via initiation of the alternative and classical pathway.
Gene expression of factor D and C1q, activators of the alternative and classical activation pathways respectively, has not been examined in the setting of cerebral IR. Although systemic factor D secretion originates primarily from adipocytes [42], the factor D gene is also expressed in hepatocytes, spleen and intestine [43]. In a mouse model, hepatic sources of factor D transcripts can be modulated by a high fat fed diet. To investigate localized and hepatic alterations of factor D in response to ischemic stroke and reperfusion, gene transcripts were measured in brain and liver tissue. Our findings indicate that brain and liver were not significant sources of increased factor D after ischemic stroke and reperfusion as factor D transcripts were not measurable in brain tissue and, in liver tissue, were unaltered by tMCAO. Alternatively, any changes to systemic concentrations of factor D during cerebral IR may originate from adipocytes.
In contrast to factor D, C1q gene transcripts were robustly detected from liver tissue (data not shown). Although hepatocytes are capable of secreting C1q, the primary source of C1q is from differentiated macrophages. In the liver, Kuffer cells, the resident liver macrophage, modulate mRNA production and associated C1q secretion in response to acute injury [44]. Interestingly, in contrast to enhanced MBL gene expression, hepatic C1q gene expression was decreased in both MBL+/+ animals and MBL−/− animals immediately after (15 minutes) reperfusion when compared to sham. Similar to this finding, Arbrust et al. have demonstrated in vitro that C1q gene expression and secretion from Kuffer cells are decreased after LPS challenge of injured hepatic tissue suggesting that classical pathway activation is suppressed to prevent overwhelming CS activa-tion during acute injury [44]. However, this initial suppres-sion of activation and subsequent early protection may not be present with extended reperfusion. Further exploration of the cooperative responses of the CS activation pathways, over time, after ischemic stroke and reperfusion is clearly warranted.
To date, the contributions of each of the three individual CS activation pathways to cerebral injury after ischemic stroke and reperfusion have not been thoroughly explored. Ideally, direct inhibition of MBL following cerebral IR is needed, but specific inhibitors of murine MBL-A and -C do not exist at the present time. Instead, the majority of cerebral IR injury research has centered on non-specific inhibition of all three CS activation pathways. The simultaneous inhibition of the three activation pathways (via C1-INH) significantly reduces total infarct volume and neutrophil infiltration into the affected hemisphere after ischemic stroke and reperfusion [14–22]. In contrast, in the single study evaluating genetic deletion of a single pathway, C1q-initiated classical pathway, there was no reduction in hemispheric infarct volume after cerebral ischemia and reperfusion [18]. Similarly, in our studies, while deletion of only the MBL-initiated lectin pathway did reduce striatum infarct volume, it did not decrease total hemispheric infarct volume. Taken together, these results support the postulate that, unlike other organs, deletion of a single activating pathway, e.g. either the lectin or classical pathway, does not yield significant attenuation of reperfusion injury in the cortex or total ischemic hemisphere [18,19,22]. Alternatively, it is possible that the alternative pathway, not yet studied in isolation in stroke, plays a critical role in IR injury that is unique to the brain.
In addition to redundant complement pathway activation, brain tissue heterogeneity may explain the lack of MBL-mediated protection in the cortex observed in this study [45,46]. Several lines of evidence support this notion. While few have compared the convolution of inflammatory responses and regional tissue damage after ischemic stroke and reperfusion, Maxon-Emre & Schlicher propose that microglial responses to transient ischemic injury are region-specific [38]. Additional evidence suggests that, compared to the cortex, white matter (i.e. striatum) may be uniquely vulnerable to inflammatory mediators due to increased axonal and myelin damage [47,48]. Local production of CS proteins and CS activation occurs within brain tissue [49–51] after injury and anaphylotoxin receptors C5aR and C3aR are present on neurons and glial cells that initiate inflammatory responses [52]. Although not yet investigated, local CS protein expression and CS activation may also vary by region after ischemic stroke and reperfusion. The results of the present study provide evidence that the brain striatum is at increased risk for MBL-mediated tissue damage after IR. These findings support the possibility of region and/or cell specific responses to complement mediated brain injury.
In summary, we demonstrated in a mouse model of ischemic stroke and reperfusion that MBL deficiency may protect brain striatum during ischemia, however, with reperfusion, MBL deficiency does not protect cortical and hemispheric injury. We suggest that redundant complement pathway activation during reperfusion may overwhelm any protective responses that occur during ischemia. Further research is needed to characterize the relative contributions of single and combined complement pathway activation on regional, temporal, and reperfusion responses to cerebral injury after ischemic stroke.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants NIH F31-NR010658-01 (Morrison), Sigma Theta Tau Beta Mu Chapter Student Research Grant, University of Arizona College of Nursing Emmons Student Dissertation Award. Sarver Heart Center Hudson/Lovaas Endowment. Immunofluorescent staining by the TACMASS Core (Tissue Acquisition and Cellular/Molecular Analysis Shared Service) was supported by the Arizona Cancer Center Support Grant, NIH CA023074.
REFERENCES
- 1.Broderick JP, William M. Feinberg Lecture: stroke therapy in the year 2025: burden, breakthroughs, and barriers to progress. Stroke. 2004;35(1):205. doi: 10.1161/01.STR.0000106160.34316.19. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Zaharchuk G, Yamada M, Sasamata M, Jenkins BG, Moskowitz MA, Rosen BR. Is all perfusion-weighted magnetic resonance imaging for stroke equal? The temporal evolution of multiple hemodynamic parameters after focal ischemia in rats correlated with evidence of infarction. J Cerebr Blood F Met. 2000;20(9):1341. doi: 10.1097/00004647-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21(5):401. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ritter LS, Orozco JA, Coull BM, McDonagh PF, Rosenblum WI. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke. 2000;31(5):1153. doi: 10.1161/01.str.31.5.1153. [DOI] [PubMed] [Google Scholar]
- 6.Ruehl ML, Orozco JA, Stoker MB, McDonagh PF, Coull BM, Ritter LS. Protective effects of inhibiting both blood and vascular selectins after stroke and reperfusion. Neurol Res. 2002;(3):226. doi: 10.1179/016164102101199738. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu JH, Thiel S, Wiedemann H, Timpl R, Reid KB. Binding of the pentamer/hexamer forms of mannan-binding protein to zymosan activates the proenzyme C1r2C1s2 complex, of the classical pathway of complement, without involvement of C1q. J Immunol. 1990;144(6):2287. [PubMed] [Google Scholar]
- 9.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev. 2002;2(5):346. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 10.Sunyer JO, Boshra H, Li J. Evolution of anaphylatoxins, their diversity and novel roles in innate immunity: insights from the study of fish complement. Vet Immunol Immunop. 2005;108(1–2):77. doi: 10.1016/j.vetimm.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Foreman KE, Glovsky MM, Warner RL, Horvath SJ, Ward PA. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20(1):1. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- 12.Furebring M, Hakansson L, Venge P, Sjolin J. C5a, interleukin-8 and tumour necrosis factor-alpha-induced changes in granulocyte and monocyte expression of complement receptors in whole blood and on isolated leukocytes. Scand J Immunol. 2006;63(3):208. doi: 10.1111/j.1365-3083.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 13.Morrison H, McKee D, Ritter L. Systemic neutrophil activation in a mouse model of ischemic stroke and reperfusion. Biol Res Nurs. doi: 10.1177/1099800410384500. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akita N, Nakase H, Kaido T, Kanemoto Y, Sakaki T. Protective effect of C1 esterase inhibitor on reperfusion injury in the rat middle cerebral artery occlusion model. Neurosurgery. 2003;52(2):395. doi: 10.1227/01.neu.0000043710.61233.b4. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson C, Zhu H, Qiao F, et al. Complement-dependent P-selectin expression and injury following ischemic stroke. J Immunol. 2006;177(10):7266. doi: 10.4049/jimmunol.177.10.7266. [DOI] [PubMed] [Google Scholar]
- 16.Costa C, Zhao L, Shen Y, et al. Role of complement component C5 in cerebral ischemia/reperfusion injury. Brain Res. 2006;1100(1):142. doi: 10.1016/j.brainres.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Figueroa E, Gordon LE, Feldhoff PW, Lassiter HA. The administration of cobra venom factor reduces post-ischemic cerebral injury in adult and neonatal rats. Neuro Sci Lett. 2005;380(1–2):48. doi: 10.1016/j.neulet.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Mocco J, Mack WJ, Ducruet AF, et al. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99(2):209. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- 19.Storini C, Rossi E, Marrella V, et al. C1-inhibitor protects against brain ischemia-reperfusion injury via inhibition of cell recruitment and inflammation. Neurobiol Dis. 2005;19:10. doi: 10.1016/j.nbd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Harhausen D, Khojasteh U, Stahel PF, et al. Membrane attack complex inhibitor CD59a protects against focal cerebral ischemia in mice. J Neuroinflamm. 2010;7:15. doi: 10.1186/1742-2094-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Kim LJ, Mealey R, et al. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285(5427):595. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 22.De Simoni MG, Rossi E, Storini C, Pizzimenti S, Echart C, Bergamaschini L. The powerful neuroprotective action of C1-inhibitor on brain ischemia-reperfusion injury does not require C1q. Am J Pathol. 2004;164(5):1857. doi: 10.1016/S0002-9440(10)63744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan RK, Ibrahim SI, Takahashi K, et al. The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J Immunol. 2006;177(11):8080. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 24.de Vries B, Walter SJ, Peutz-Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA. The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury. Am J Pathol. 2004;165(5):1677. doi: 10.1016/S0002-9440(10)63424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart ML, Ceonzo KA, Shaffer LA, et al. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol. 2005;174(10):6373. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 26.Moller-Kristensen M, Wang W, Ruseva M, et al. Mannan-binding lectin recognizes structures on ischaemic reperfused mouse kidneys and is implicated in tissue injury. Scand J Immunol. 2005;61(5):426. doi: 10.1111/j.1365-3083.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 27.Walsh MC, Bourcier T, Takahashi K, et al. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175(1):541. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Takahashi K, Alicot EM, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177(7):4727. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 29.Cervera A, Planas AM, Justicia C, et al. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS One. 2010;5(2):e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L, Takahashi K, Dundee J, et al. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J Exp Med. 2004;199(10):1379. doi: 10.1084/jem.20032207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54(11):3452. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 32.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. Am J Physiol. 2009;297(5):H1853. doi: 10.1152/ajpheart.00049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211(10):759–766. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Arnold JN, Dwek RA, Rudd PM, Sim RB. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett. 2006;106(2):103. doi: 10.1016/j.imlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Wagner S, Lynch NJ, Walter W, Schwaeble WJ, Loos M. Differential expression of the murine mannose-binding lectins A and C in lymphoid and nonlymphoid organs and tissues. J Immunol. 2003;170(3):1462. doi: 10.4049/jimmunol.170.3.1462. [DOI] [PubMed] [Google Scholar]
- 37.Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7(4):378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moxon-Emre I, Schlichter LC. Evolution of inflammation and white matter injury in a model of transient focal ischemia. J Neuropathol Exp Neurol. 2010;69(1):1–15. doi: 10.1097/NEN.0b013e3181c3ce6c. [DOI] [PubMed] [Google Scholar]
- 39.Schilling M, Strecker JK, Ringelstein EB, Schabitz WR, Kiefer R. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009;1289:79–84. doi: 10.1016/j.brainres.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 40.Yager PH, You Z, Qin T, et al. Mannose binding lectin gene deficiency increases susceptibility to traumatic brain injury in mice. J Cerebr Blood F Met. 2008;28(5):1030. doi: 10.1038/sj.jcbfm.9600605. [DOI] [PubMed] [Google Scholar]
- 41.La Bonte LR, Davis-Gorman G, Stahl GL, McDonagh PF. Complement inhibition reduces injury in the type 2 diabetic heart following ischemia and reperfusion. Am J Physiol. 2008;294(3):H1282. doi: 10.1152/ajpheart.00843.2007. [DOI] [PubMed] [Google Scholar]
- 42.Spiegelman BM, Frank M, Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983;258(16):10083–10089. [PubMed] [Google Scholar]
- 43.Recinos A, Carr BK, Bartos DB, et al. Liver gene expression associated with diet and lesion development in atherosclerosis-prone mice: induction of components of alternative complement pathway. Physiol Genomics. 2004;19(1):131–142. doi: 10.1152/physiolgenomics.00146.2003. [DOI] [PubMed] [Google Scholar]
- 44.Armbrust T, Nordmann B, Kreissig M, Ramadori G. C1Q synthesis by tissue mononuclear phagocytes from normal and from damaged rat liver: up-regulation by dexamethasone, down-regulation by interferon gamma, and lipopolysaccharide. Hepatology. 1997;26(1):98–106. doi: 10.1053/jhep.1997.v26.pm0009214457. [DOI] [PubMed] [Google Scholar]
- 45.Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH., Jr Differential regional vulnerability in transient focal cerebral ischemia. Stroke. 1982;13(3):339–346. doi: 10.1161/01.str.13.3.339. [DOI] [PubMed] [Google Scholar]
- 46.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;689:701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 47.Hughes PM, Anthony DC, Ruddin M, et al. Focal lesions in the rat central nervous system induced by endothelin-1. J Neuropathol Exp Neurol. 2003;62(12):1276–1286. doi: 10.1093/jnen/62.12.1276. [DOI] [PubMed] [Google Scholar]
- 48.Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27(9):1641–1646. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- 49.Thomas A, Gasque P, Vaudry D, Gonzalez B, Fontaine M. Expression of a complete and functional complement system by human neuronal cells in vitro. Int Immunol. 2000;12(7):1015. doi: 10.1093/intimm/12.7.1015. [DOI] [PubMed] [Google Scholar]
- 50.Seyfarth J, Garred P, Madsen HO. Extra-hepatic transcription of the human mannose-binding lectin gene (mbl2) and the MBL-associated serine protease 1–3 genes. Mol Immunol. 2006;43(7):962. doi: 10.1016/j.molimm.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen ED, Loberg EM, Vege E, Daha MR, Maehlen J, Mollnes TE. In situ deposition of complement in human acute brain ischaemia. Scand J Immunol. 2009;69(6):555–562. doi: 10.1111/j.1365-3083.2009.02253.x. [DOI] [PubMed] [Google Scholar]
- 52.Van Beek J, Bernaudin M, Petit E, et al. Expression of receptors for complement anaphylatoxins C3a and C5a following permanent focal cerebral ischemia in the mouse. Exp Neurol. 2000;161(1):373. doi: 10.1006/exnr.1999.7273. [DOI] [PubMed] [Google Scholar]