Abstract
The effect of recombinant Brugia malayi pepsin inhibitor (rBm33) on human monocytes/macrophages has been examined using THP-1 cells. THP-1 cells stimulated with rBm33 showed enhanced levels of expression of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) and diminished levels of IL-12, iNOS and anti-inflammatory cytokine (IL-10) expression suggesting the predominant features of Th1 response. Phorbol-12-myristate-13-acetate (PMA) treated THP-1 cells stimulated with rBm33 and subsequent incubation with GFP expressing Escherichia coli (E. coli) for 2 h enhanced the uptake of E. coli. Nitric oxide (NO) levels measured in the supernatants of these cultures did not show significant changes. Apoptotic studies with Peripheral Blood Mononuclear Cells (PBMCs) from normal individuals stimulated with rBm33 did not induce apoptosis of monocytes or lymphocytes. These observations suggest that rBm33 stimulates macrophages to induce Th1 response and does not promote apoptosis.
Keywords: Brugia malayi pepsin inhibitor, Monocyte, Macrophage, Cytokine, Phagocytosis, Apoptosis
1. Introduction
Human Lymphatic Filariasis is a tropical parasitic disease caused by Wuchereria bancrofti, Brugia malayi and Brugia timori. Clinically, the filarial patients are categorized as individuals with microfilariae (mf) and circulating antigen positive harboring active infection as microfilaremics (MF), symptomatic individuals (CP) with chronic disease are mf negative and circulating antigen negative and possess clinical manifestations such as lymphedema; Endemic normals (EN) are putatively immune individuals and are asymptomatic, amicrofilaremic and circulating antigen negative [1]. The long living filarial parasite adopts various strategies enabling them to escape the host defense system and favors survival within the host. Filarial parasites also induce profound dysfunction of antigen presenting cells (APC) such as dendritic cells, macrophages and langerhans cells in filarial patients [2]. Studies involving the use of filarial antigens have been shown selectively to induce naïve human T cell differentiation in vitro away from a Th1 phenotype and serve as a model to understand the immune responses to the early exposure of the filarial antigens and its subsequent activity in eliciting immune response [3]. Further, it has been shown that filarial antigen specific APC possessed diminished capacity to stimulate CD4+ T cells [4–6]. In order to study this complex immune regulatory network involved in the filarial pathology, characterization of the filarial antigens becomes obligatory.
Filarial Genome Project has provided the sequences of all proteins of the filarial parasites [7] and mostly their function needs to be evaluated. One of the shortcomings in filarial disease research is the availability of pure antigens for immunological investigations. In this regard, we have produced recombinant parasite and Wolbachia proteins like WbSXP-1, Bm33, WSP and WmHSP60 in our laboratory and have also investigated their serological and cellular responses using PBMCs of filarial patients. To be specific, Wolbachia proteins such as Wolbachia Surface Protein (WSP), Heat Shock Protein 60 (HSP 60) decreases CD4 + T cell activation and impairs the proliferative responses in filarial patients compared to the uninfected group of individuals thus contributing to the suppression of immune responses in patients [8,9]. Further, a hypodermal protein from L3 stage of the parasite, Bm33 was identified and was characterized [10]. Bm33 was produced using recombinant technology (rBm33) and was characterized to show high levels of antigen specific IgG4 antibodies in microfilaria positive individuals compared to the patients with chronic pathology [11]. Further, rBm33 induced early T cell activation in MF and CP patients followed by a decreased lymphoproliferation that contributes to the immune modulation in these individuals [12].
A lot of information is available on responses of lymphocyte population to parasite antigens in filarial patients, but data on monocyte and macrophage responses to the parasite antigens is meager. In addition, it becomes important to understand the mechanism by which the parasite evades the first line of defense to cause the hyporesponsiveness among the filarial patients which is the bottom line of filarial research. Filarial antigen WbSXP-1, induced modulation of the monocyte functions in filarial patients [13]. Filarial parasite B. malayi contains a homolog of human macrophage inhibitory factor that activates monocyte/macrophage and induces apoptosis indirectly through induction of cytokines and chemokines [14]. The cytokine environments present during the infectious state influence the macrophage activation either toward classical or alternative pathways.
Another class of molecules from filarial parasites that play a vital role in immune modulation includes protease inhibitors. Earlier reports have shown that pepsin inhibitor from a non-filarial nematode Ascaris suum (PI-3) act as an immunomodulator inhibiting Cathepsin E and antigen processing by T cells [15]. In this context, rBm33 showed striking similarities to PI-3, hence it was called pepsin inhibitor homolog. The biological activity of rBm33 as a human pepsin inhibitor was demonstrated [16] but its role in modulating APC function needs investigation. Thus, the present study investigates the effects of the rBm33 on THP-1 monocytes in vitro by the evaluating phagocytosis, cytokine, iNOS gene expression and examines the apoptosis of Peripheral Blood Mononuclear Cells (PBMCs) from normal individuals on rBm33 stimulation.
2. Materials & methods
2.1. Materials
RPMI 1640, Fetal Bovine Serum, Antibiotic and Antimycotic solution containing Penicillin E (10,000 U/ml), Streptomycin sulfate (10,000 μg/ml), Amphotericin (25 μg/ml) were obtained from GIBCO-BRL (Calif, U.S.A.), Lymphocyte separation medium (Pancoll, PAN BIOTECH, GmBH). HEPES was obtained from USB, Amersham Life sciences (Cleveland, OH, U.S.A.). NaHCO3 and Bovine Serum Albumin were obtained from Himedia Laboratories (Mumbai, India). Gentamicin was obtained from Ranbaxy Pharmaceuticals (New Delhi, India).
2.2. Study population
Patients were recruited through the Filariasis Control Unit under the Directorate of Public Health (Chennai, India) after obtaining informed oral consent with protocols approved by the Institutional Review Board of Anna University (Chennai). The individuals in the study were informed about the experiment by the DPH medical authorities, and only oral consent was possible as most of them were illiterate. The consent from all the volunteers was obtained during clinical examination, and it was documented as a spreadsheet. Standardized histories were obtained and physical examinations were done on all the participant residents during epidemiological surveys in and around Chennai, India, an area endemic for W. bancrofti infection. The NEN serum samples that were used in the study were a kind gift from Dr. Thomas B. Nutman (NIH, Bethesda, MD, U.S.A.). Four asymptomatic amicrofilaremic endemic normals (EN), four asymptomatic microfilaremics (MF) and four symptomatic amicrofilaremic individuals with chronic pathology (CP) were included and their sera were used to assess the immunoreactivity of rBm33. Further, five endemic normals were included for studies on apoptosis. All the individuals were screened for the presence of circulating filarial antigens by Og4C3 mAb ELISA, a marker of W. bancrofti infection and adult worm burden [17] (TropBio, Townsville, Queensland, Australia).
2.3. Cell culture
Human Monocytic cell line (THP-1) was obtained from National Centre for Cell Science (NCCS, Pune, India). The cells were maintained in RPMI 1640 containing 10% heat inactivated Fetal Bovine Serum (FBS) at 37 °C in 5% CO2 incubator for propagation and used for experiments.
2.4. Antigens and mitogens
rBm33 was expressed and purified as described previously [11]. Briefly, pRSET-A-Bm33 plasmid was transformed into the host such as BL21 (DE3) for large-scale expression of the recombinant protein induced with 1 mM IPTG (CalBiochem, San Diego, CA, U.S.A.). The expressed rBm33 was purified by Immobilized Metal Affinity Chromatography. The endotoxin contamination in rBm33 was assessed by Limulus Amoebocyte Lysate (LAL) assay, which showed < 1 pg of LPS/10 μg of protein. 100 ng/ml of Escherichia coli Lipopolysaccharide (LPS, Sigma chem, St. Louis, MO, U.S.A.) served as the positive control to stimulate THP-1 monocytes. Purified Protein Derivative from Mycobacterium. tuberculosis (PPD, Statens Serum Institute) a non-parasite antigen control was used at concentration of 10 μg/ml and rBm33 was used at a concentration of 10 μg/ml respectively. 100 μM Cycloheximide (Sigma chemicals, St. Louis, MO, U.S.A.) was used to induce apoptosis of PBMCs.
2.5. Immunoreactivity of purified rBm33 with pooled filarial patient sera
Reactivity of purified rBm33 was confirmed using filarial patients’ sera by Western Blot analysis. Briefly, purified rBm33 (25 μg) loaded in each lane was resolved on 12% SDS-PAGE and transferred on to a nitrocellulose membrane (Hybond; Amersham Pharmacia, Sunnyvale, CA, U.S.A.) using a semi-dry blotting apparatus (Amersham Pharmacia) at a constant voltage of 20 V and 150 mA for 1.5 h. The membrane was blocked for 2 h at 37 °C using 5% skimmed milk powder in 1 × PBS and washed thrice in 0.05% Tween-20 in PBS/PBS for 10 min. The membrane was cut into strips and probed with NEN, EN, MF and CP sera (1:50). A strip probed with mouse anti-histidine monoclonal antibody (1:5000) was used as a positive control. Subsequently, the strips probed with patient serum and anti-histidine were incubated with ALP conjugates of goat anti-human IgG and goat anti-mouse IgG (1:10,000) (Sigma–Aldrich)respectively and were finally developed with 66 μl of Nitro Blue Tetrazolium and 33 μl of 5-bromo-4-chloro-3-indolyl phosphate salt (USB, Cleveland, Ohio, U.S.A.) as substrate.
2.6. THP-1 cells stimulation with LPS, PPD and rBm33
THP-1 monocytes (0.1 × 106 cells/ml/well) were cultured as duplicates in 24 well tissue culture plate (Nunc, Rochester, NY) with LPS (100 ng/ml), PPD and rBm33 (10 μg/ml) at 37 °C, 5% CO2. After 24 h of incubation, the cells were harvested by centrifugation. Culture supernatants and pellets were collected for assessment of cytokines.
2.6.1. RNA preparation
Stimulated and unstimulated THP-1 monocytes were lysed using reagents of a commercial kit (RNeasy mini kit, Qiagen). Total RNA was extracted according to the manufacturer's protocol (RNeasy mini kit, Qiagen) and RNA was dissolved in 20 μl of RNase-free water.
2.6.2. cDNA synthesis
Reverse transcription of RNA was performed in a final volume of 20 μl containing 0.25 mM mix of the four deoxynucleotide triphosphates (dATP, dGTP, dTTP and dCTP) (New England Biolabs, MA, USA), 1X reverse transcriptase buffer (50 mM Tris–HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2), 8 mM DTT, 20 U RNase inhibitor (New England Biolabs) and 200 U of MMLV-reverse transcriptase (New England Biolabs) and followed by incubation of the tubes at 37 °C for 60 min. Moloney murine leukemia virus reverse transcriptase is an RNA-dependent DNA polymerase which has RNase H activity. The reverse transcription reaction was stopped by heating the tubes at 90 °C for 5 min. The cDNAs were snap-chilled in ice for 5–10 min and stored at –20 °C until use.
2.6.3. Real-time PCR for cytokine and iNOS gene expression
Real-time quantitative RT-PCR was performed in an ABI7700 sequence detection system (Applied Biosystems, Fullerton, CA, U.S.A.) using TaqMan Assays-on-Demand reagents for IL-1β, IL-12, IL-6, TNF-α, IL-10, iNOS and endogenous 18S ribosomal RNA control. Quantification of gene expression was performed using the comparative CT method (Sequence Detector User Bulletin 2, Applied Biosystems) and reported as the fold change relative to the house keeping gene. To calculate the fold change, CT of the house keeping gene (18s rRNA) was subtracted from the CT of the target gene to yield the ΔCT. Change in the expression of the normalized target gene as a result of antigenic exposure was expressed as 2–ΔΔCT, where ΔΔCT = ΔCT of stimulated –ΔCT of unstimulated cells. Results are expressed as mean ± SD of three independent experiments.
2.6.4. ELISA
The levels of cytokines (TNF-α and IL-10) in the pooled culture supernatants from 3 independent experiments were measured using Bioplex multiplex cytokine ELISA assay system (Biorad, Hercules, CA).
2.7. Phagocytosis assay and NO levels in culture supernatants
THP-1 cells (0.1 × 106 cells/ml/well) were seeded on 24 well plate (Nunc, Rochester, NY) and 10 μg/ml Phorbol 12-myristate 13-acetate (PMA, Sigma chemicals, St. Louis, MO, U.S.A.) was used to differentiate the monocytes to macrophages. Differentiation of PMA treated cells was enhanced after the 72 h stimulus by removal of PMA containing media then incubating the cells in fresh Complete RPMI 1640 (PMAr THP-1 cells).
PMAr THP-1 cells were used for Phagocytosis assay. The rested cells were stimulated with mitogens such as LPS (100 ng/ml), PPD and rBm33 (10 μg/ml) for 24 h of incubation. Unstimulated cells were kept as control. The treated and untreated cells were challenged with green fluorescence protein expressing E. coli (E. coli-GFP) following the incubation. Macrophages were incubated with E. coli-GFP at in the ratio of 1:10. The time point of measurement of phagocytosis was 2 h after incubation. The cells were washed with 1 X PBS to remove any extracellular bacteria. The cells were then subjected to Fluorescence Microscopic observation (NIKON India Pvt Ltd). Percentage phagocytosis was determined by counting the number of bacteria in 200 THP-1 macrophages, and NO production after phagocytosis was determined by Griess method described previously [18]. Sodium nitro prusside (SNP, 300 μM) was used as positive control for the Griess assay. Results are expressed as mean ± SD of five independent experiments.
2.8. Effect of rBm33 on the apoptosis of PBMCs from healthy subjects
PBMCs were isolated from heparinized venous blood from five endemic normals by density centrifugation as described previously [12]. Cells obtained by Ficoll-Hypaque density centrifugation were suspended in complete RPMI 1640 medium and the cells were counted in hemocytometer by trypan blue exclusion and seeded in culture plates. Apoptotic studies were carried out using the isolated PBMCs by performing AO/EtBr staining, DNA fragmentation assay and FACS using Annexin-V/PI labeling of cells.
2.8.1. Acridine orange/ethidium bromide staining
PBMCs (1 × 106 cells/ml/well) were seeded in 24 well plate (Nunc, Rochester, NY) and stimulated in the presence and absence of 100 μM Cycloheximide (CHX, Sigma, St. Louis, U.S.A.), 10 μg/ml of PPD and rBm33 for 24 h. Later, AO/EtBr dye mix (Dye mix: 100 μg/ml of AO and 100 μg/ml of EtBr, Himedia laboratories, India) of about 1 μl was prepared in 1 × PBS. Cells were incubated with the dye mix for 15–30 min and were taken for Fluorescence microscopic (Nikon India Pvt Ltd) observation at 400× magnification for any morphological changes. Viable cells appear with normal nuclei (Bright green), Viable cells with apoptotic nuclei (Bright green with condensed or fragmented nuclei), and necrotic cells appear bright orange.
2.8.2. DNA fragmentation analysis
PBMCs were seeded at a concentration of 2 × 106 cells/ml/well in 24 well tissue culture plate (Nunc, Rochester, NY) in the presence and absence of CHX (100 μM), PPD, rBm33 (10 μg/ml) for 24 h were harvested and centrifuged. Cells were collected, and genomic DNA was extracted as described [19]. The extracted DNA was analyzed on 1.2% Agarose gel electrophoresis.
2.8.3. Quantitative apoptotic cell death assay
Apoptosis was measured in PBMCs seeded as duplicates in 24 well tissue culture plate (NUNC, Rochester, NY) at a concentration of 0.5 × 106 cells/ml/well incubated with and without CHX (100 μM), PPD (10 μg/ml) and rBm33 (10 μg/ml) at appropriate concentration for 24 h of incubation. Staining with Annexin-V-FITC (BD Pharmingen, Sanjose, CA, U.S.A.) and Propidium iodide (PI, MERCK, Germany) identified early apoptotic and late apoptotic cells respectively. PBMCs were identified on the basis of light scatter properties. PBMCs were harvested following the incubation and washed with ice cold 2 × PBS prior to binding buffer(10 mM HEPES pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) wash. The washed cells were incubated with 2 μl of Annexin-V-FITC for 10–20 min and later with PI for 15–20 min. The stained cells were washed with 2X PBS to remove any background staining and were analyzed by using a FACS Calibur instrument (BD Biosciences, Sanjose, CA, U.S.A.). About 50,000 PBMCs were gated and analyzed using Flow Jo Software and the data for stimulated condition were expressed as percent apoptosis. Dual color dot blots were analyzed for Annexin-V/PI labeled cells and % early apoptotic (Annexin V+ PI-) and late apoptotic (Annexin V+ PI+) cells determine stages of apoptosis.
2.9. Statistical analysis
Statistical analysis was carried out using Graphpad Prism version 5.0 for Windows (Graphpad Software, San Diego CA, U.S.A., www.graphpad.com). Comparison among the stimulated cultures for Cytokine analysis, Phagocytosis assay, Griess assay and FACS analysis comparisons were performed using the non-parametric one-way ANOVA test, Friedman's test and Dunn's tests for comparisons. A P value of ≤0.05 was considered statistically significant.
3. Results
3.1. Reactivity of rBm33 with pooled sera from filarial patients
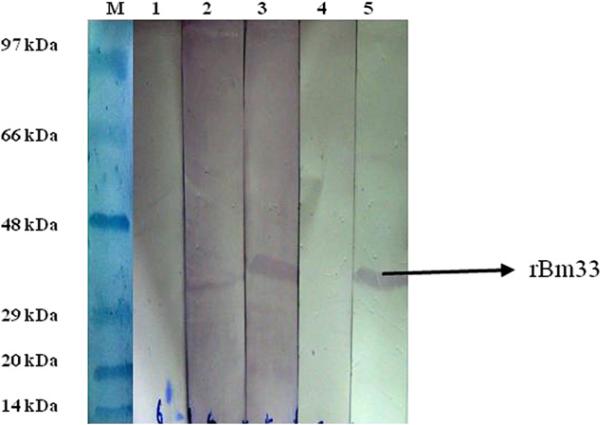
Bm33 gene was identified from the L3 cDNA libraryof B. malayi and cloned into pRSET-A and rBm33 was purified by immobilized metal affinity chromatography as reported previously [11]. Sera from microfilaria positive patients, chronic pathology reacted with rBm33 in Western Blot while asymptomatic amicrofilaremic individuals (EN) and non-endemic normal (NEN) did not exhibit any reactivity (Fig.1).
Fig. 1.
Western Blot showing the reactivity of recombinant pure Bm33 with patients’ sera. Lane M: molecular weight marker; Lane 1: purified Bm33 probed with pooled sera from endemic normals (1:50 dilution); Lane 2: purified Bm33 probed with pooled sera of patients with chronic pathology (1:50 dilution); Lane 3: purified Bm33 probed with pooled sera of microfilaria positive individuals (1:50 dilution); Lane 4: purified Bm33 probed with pooled sera from non-endemic normals (1:50 dilution); Lane 5: purified Bm33 probed with mouse anti-histidine monoclonal antibody (1:5000 dilution).
3.2. Differential cytokines, iNOS gene expression in rBm33 stimulated monocytes
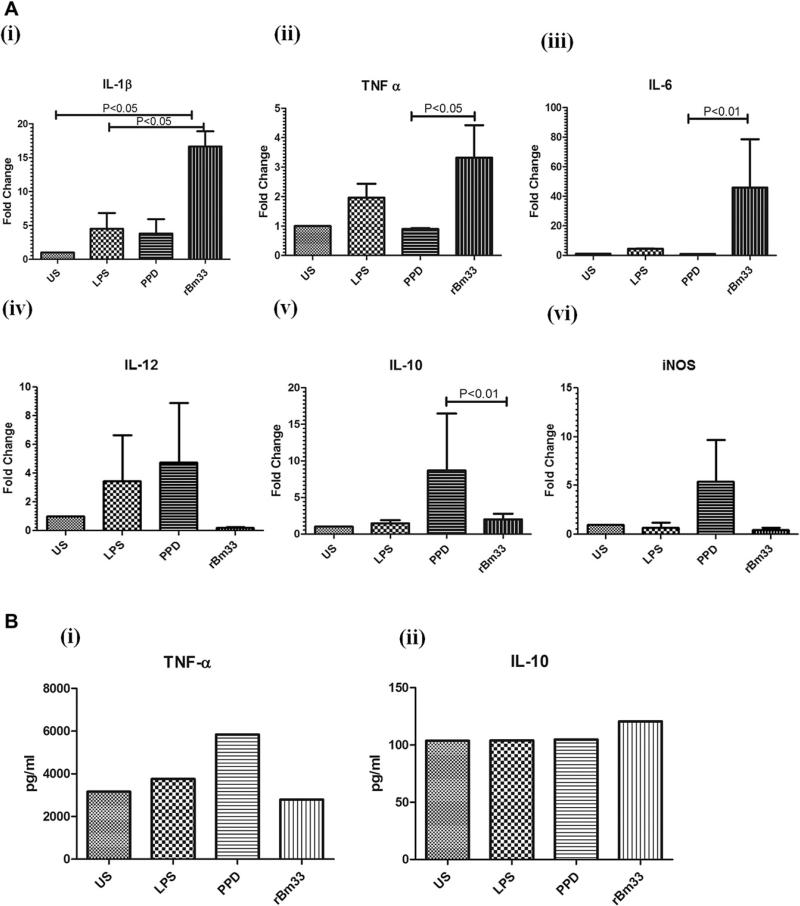
To assess the expression pattern of pro-inflammatory (IL-1β, TNF-α, IL-6, IL-12), anti-inflammatory (IL-10) cytokines and iNOS expression at mRNA level, THP-1 monocytes was examined following 24 h stimulation with LPS, PPD and rBm33by Real Time PCR (Fig. 2A)(i)–(vi)).
Fig. 2.
Cytokine and iNOS gene expression in THP-1 cells (A) Expression of cytokines (i) IL-1β, (ii)TNF-α, (iii) IL-6, (iv) IL-12, (v) IL-10, (vi) iNOS upon stimulation with 100 ng/ml Lipopolysaccharide (LPS), 10 μg/ml Purified Protein derivative (PPD) from M. tuberculosis, 10 μg/ml recombinant Bm33 (rBm33) in THP-1 cells (0.1 × 106 cells/ml), 24 h by Real-Time PCR. Antigen-induced fold change was plotted for the values on the Y-axis upon unstimulated cells (US), LPS, PPD, rBm33. Each bar represents expression levels as fold change. Fold change (ΔCT) = CT of the house keeping gene (18s rRNA) – CT of the target gene. Change in the expression of the normalized target gene as a result of antigenic exposure (2–ΔΔCT), where ΔΔCT = ΔCT of stimulated – ΔCT of unstimulated. Values are mean ± S.D of three independent experiments. (B) rBm33 induced release of cytokine levels (i) TNF-α and (ii) IL-10 in the pooled culture supernatants of three independent gene expression experiments by Bioplex multiplex cytokine ELISA assay system.
rBm33 induced significantly increased production of IL-1β gene expression (Geometric mean (GM) fold change of 16.6 in rBm33 vs 1.0 in Unstimulated THP-1 monocytes (US); P < 0.05) (Fig. 2A i) and LPS showed increased IL-1β expression compared to US (GM fold change of 4.50 vs 1 in US).TNF-α expression increased upon rBm33 stimulation (GM fold change of 3.32 in rBm33 vs 0.90 in PPD; P < 0.05) as shown in (Fig. 2A ii).Similarly, IL-6 gene expression upon rBm33 stimulation was elevated (GM fold change of 45.87 in rBm33 vs 0.93 in PPD; P < 0.01) compared to that of controls LPS and PPD (Fig. 2A iii). There was no significant alteration in the IL-12 gene expression levels with respect to rBm33 (GM fold change of 0.18 in rBm33 vs 1.0 in US) stimulation of THP-1 cells whereas LPS and PPD showed apparent increase (GM fold change 3.47 in LPS vs 4.77 in PPD) in IL-12 gene expression (Fig. 2A iv). IL-10 gene expression was significantly low in rBm33 when compared to the control antigen PPD (GM fold change 2 in rBm33 vs 8.69 in PPD; P < 0.01) (Fig. 2A v). Thus, rBm33 stimulated predominant Th1 response in THP-1 monocytes was found to be antigen specific.
iNOS gene expression was unaltered in rBm33, LPS induced and Unstimulated THP-1 monocytes while PPD stimulation resulted in five fold increase in the iNOS gene expression (Fig. 2A vi).
In addtition to this, the levels of cytokines (TNF-α and IL-10) in the pooled culture supernatants of rBm33 stimulated THP-1 monocytes from 3 independent experiments showed an apparent increase in IL-10 levels(Fig. 2B ii) when compared to controls (Unstimulated, LPS, PPD) while TNF-α did not show any change (Fig. 2B i).
3.3. rBm33 enhanced phagocytic activity in THP-1 cells
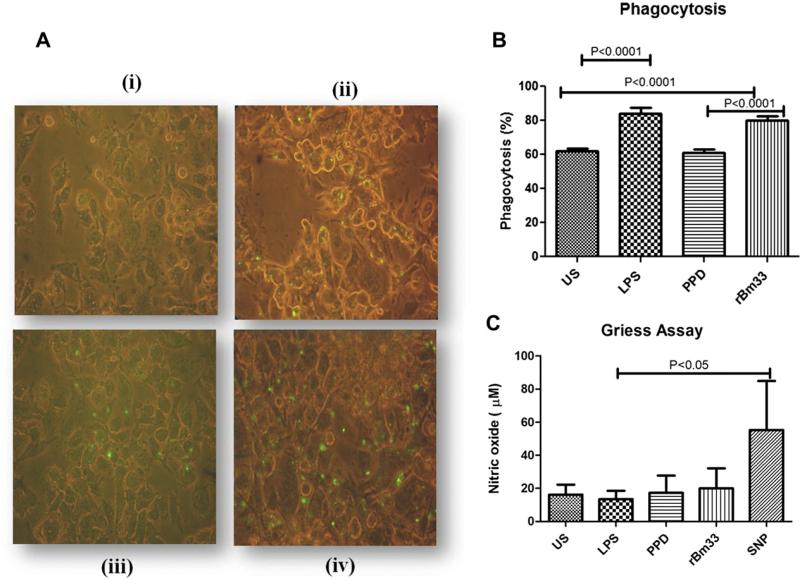
THP-1 cells were maintained in the conditioned medium and were CD 14 positive. For Phagocytosis experiments, THP-1 cells stimulated with Phorbol-12-myristate-13-acetate (PMA) for 72 h differentiated to macrophages were used and these cells were also CD 14 positive. LPS stimulated THP-1 macrophages (Fig. 3A) compared to unstimulated cultures (P < 0.0001, US Vs LPS) showed increased phagocytosis as evident from the GFP expressing E. coli uptake. Similar trend was observed with rBm33 (P < 0.0001, US Vs rBm33) and PPD showed phagocytosis similar to that of unstimulated macrophages with the order as LPS > rBm33 > PPD = US (Fig. 3B).
Fig. 3.
Phagocytosis assay (A) Photomicrograph of one of the representative experiment of five independent experiments of THP-1 monocytic cells differentiated into macrophages by PMA and incubated with stimulants for 24 h. Subsequently green fluorescent protein expressing E. coli was added and incubated for 2 h to examine phagocytosis by fluorescent microscopy represented by green dots. (i)Unstimulated cells (US) X400, (ii) Lipopolysaccharide (LPS,100 ng/ml) X400, (iii) Purified Protein Derivative (PPD, 10 μg/ml) from M. tuberculosis X400, (iv) Recombinant Bm33 (rBm33,10 μg/ml) X400. (B) Percent phagocytosis = (number of bacteria/200 macrophages) × 100 determined from (A), values are mean ± S.D of five independent experiments. (C) Nitric oxide (NO) levels in the culture supernatants of phagocytosis assay. Nitric oxide release was measured in PMA rested THP-1 cells unstimulated cells (US), PMA rested THP-1 cells stimulated with Lipopolysaccharide (LPS, 100 ng/ml), purified protein derivative (PPD, 10 μg/ml) from M. tuberculosis, recombinant Bm33 (rBm33, 10 μg/ml) and sodium nitro prusside (SNP, 300 μM) as positive control and incubated for 24 h and culture supernatants were collected and NO measured by Griess Assay. Values are mean ± S.D of five independent experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
As expected, SNP served as a positive control and resulted in high NO levels compared to that noticed with LPS (P < 0.05). NO levels in the culture supernatants of the PPD or LPS or rBm33 stimulated cultures post phagocytosis did not show any change (Fig. 3C).
3.4. Assessment of rBm33 induced apoptosis in human PBMCs
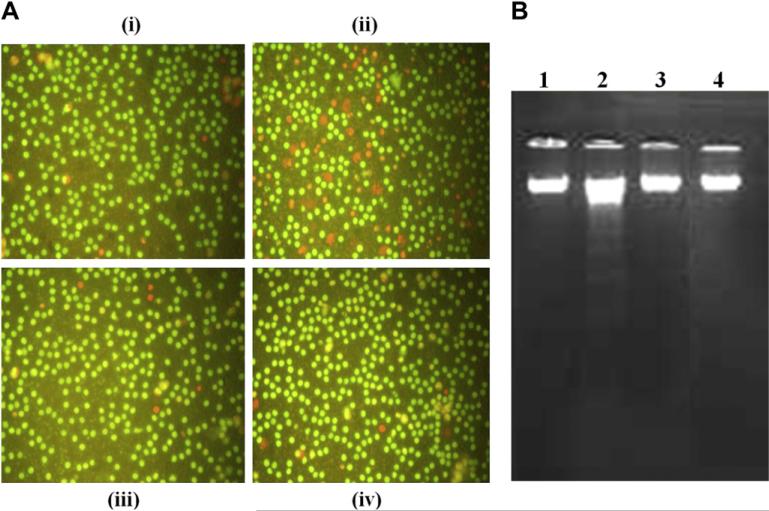
To examine the effect of rBm33 on the survival of PBMCs, morphological change due to cell death was observed under fluorescence microscope. An intact morphology with cells stained green was observed in the unstimulated and antigens (PPD and rBm33) stimulated cells in contrast to cells treated with CHX which showed a typical characteristic of cell death and stained yellow or orange (Fig. 4A(i)–(iv)). DNA fragmentation analysis substantiated AO/EtBr staining results that, only CHX induced apoptosis and showed typical laddering pattern (Fig. 4B).
Fig. 4.
Apoptosis assessment in stimulated PBMCs (A) Photomicrograph of one of the representative experiment of five independent experiments of acridine orange/ethidium bromide staining: PBMCs were cultured in the medium for 24 h without any stimulation (i) Unstimulated cells (US), (ii) in the presence of Cycloheximide (CHX, 100 μM), (iii) Purified Protein Derivative (PPD, 10 μg/ml) from M. tuberculosis, (iv) Recombinant Bm33, (rBm33,10 μg/ml) and morphological changes observed by fluorescence microscopy with acridine orange and ethidium bromide staining. (B) DNA fragmentation assay: PBMCs were cultured in the medium for 24 h. Lane 1: Unstimulated PBMCs (US), Lane 2: PBMC with Cycloheximide (CHX, 100 μM), Lane 3: PBMC with Purified Protein Derivative from M. tuberculosis (PPD, 10 μg/ml), Lane 4: PBMC with recombinant Bm33 (rBm33, 10 μg/ml). The classical DNA ladder assay shown by 1.2% agarose gel electrophoresis.
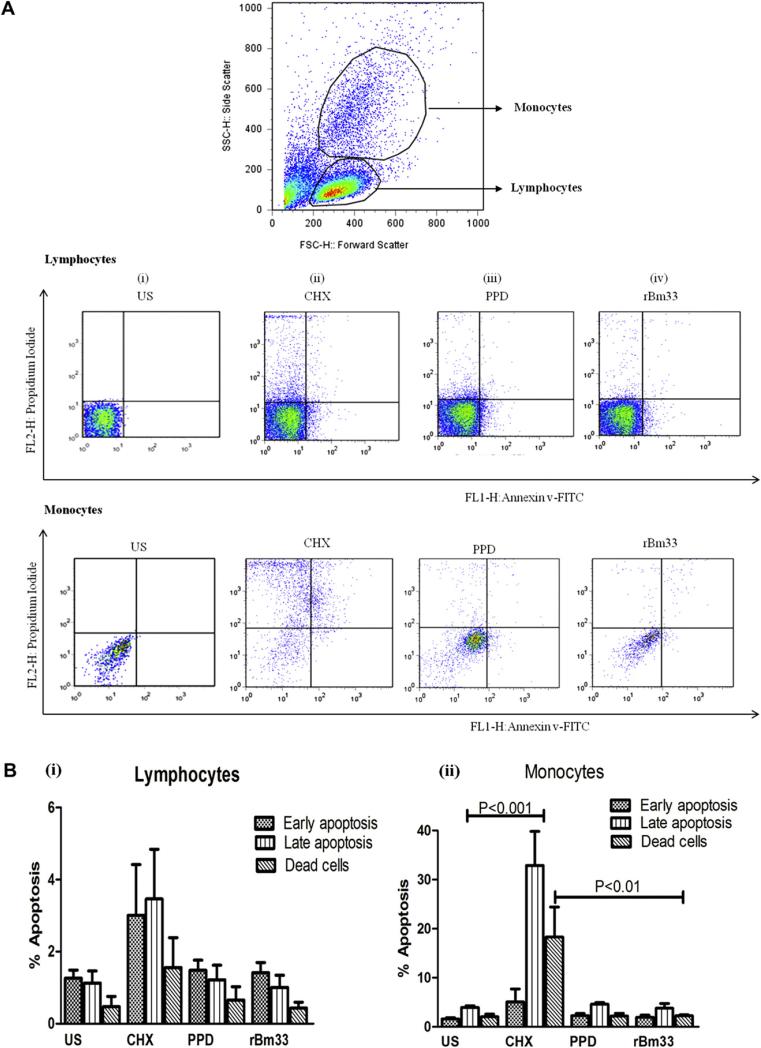
To quantify the apoptotic cell death in the stimulated PBMCs, Annexin V-FITC and Propidium Iodide were used for Fluorescence Activated Cell Sorting analysis where PBMCs were outlined and gated using FACS dot blot (Fig. 5A). Lymphocytes and monocytes were gated separately in PBMCs to quantify apoptosis. Early apoptotic cells were Annexin V+/PI – and late apoptotic cells were Annexin V-/PI + and dead cells were Annexin V /PI +. In the lymphocyte populations, neither early nor late apoptotic cells were observed in the unstimulated or antigen (CHX, PPD, rBm33) stimulated cultures. A similar trend was noticed with monocyte populations also. However, CHX induced significantly high early and late apoptotic cells in monocytes (GM fold change of 3.46 in CHX vs 1.1 in US; P < 0.001) compared to that seen with the lymphocytes (Fig. 5B). The percentage of dead cells (monocytes) were significantly low in rBm33 stimulated cells when compared to the control CHX (GM fold change of 0.44 in rBm33 vs 1.55 in CHX; P < 0.01) as shown in Fig. 5Bii. These findings corroborate with morphological and DNA fragmentation observations. Thus, rBm33 did not induce apoptosis of PBMCs.
Fig. 5.
Flow cytometric analysis of apoptosis (A) Representative distributions of the fluorescence intensity of Annexin V-FITC and propidium iodide binding of human lymphocytes and monocytes that were flow cytometrically gated using the forward- and side-light scatters in human Peripheral Blood Mononuclear Cells (PBMCs). PBMCs cultured in the presence of medium only, (i) Unstimulated cells (US),(ii) Cycloheximide (CHX, 100 μM), (iii) Purified Protein Derivative from M. tuberculosis (PPD,10 μg/ml), (iv) Recombinant Bm33 (rBm33,10 μg/ml). (B) Quantitative analysis of apoptosis by FACS: (i) Percentage of apoptosis in lymphocyte population of PBMCs, (ii) Percentage of apoptosis in monocyte population of PBMCs. Values are mean ± S.D obtained from five independent experiments.
4. Discussion
The antigen specific hyporesponsiveness in filarial infection has been attributed to a multitude of factors, one of which being altered monocyte function. Monocytes are antigen presenting cells involved in T cell activation through the production of cytokines that evokes an immune response. It would have been appropriate to use purified monocytes from filarial patients to examine the effect of recombinant parasite antigen on them in the present study. However, it has become difficult to enroll microfilaria positive individuals as their numbers have diminished following mass DEC treatment in endemic areas as a mandate of WHO to eliminate human lymphatic filariasis by 2020. Further heterogeneous population of monocytes, their yield from the blood, their differential responses to antigens and the variation in patients’ samples make it difficult to adopt it as an in vitro system for evaluating the effects of parasite derived recombinant proteins. Hence, the THP-1 monocyte cell line have been used, as they possess the characteristics of human monocytes and offers a better model system to study native monocytes derived macrophages [20]. The third larval stage (L3) of filarial parasites is infectious and presumably, the target of protective immunity [21]. Recombinant protein has the advantage that it can be produced with sufficient purity and concentrations for immunological investigations and is unlikely to show batch to batch variations. The immunoblot analysis with MF patients’ sera showed better reactivity with rBm33 compared to other groups this is further evident from the sero-logical studies where rBm33 specific IgG4 was high in MF compared to other groups as assessed by ELISA [11]. rBm33 contained negligible amounts of LPS and the assessment of IL-4 gene expression in PBMCs of EN following the stimulation with rBm33 in the presence and absence of polymyxin B suggest that the effect of rBm33 is unlikely due to LPS (data not shown).
THP-1 monocytes stimulated with rBm33 showed enhanced gene expression of pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α and apparently low IL-10 gene expression. IL-6 is the principal cytokine produced by monocytes in 2 h upon in vitro stimulation [22] and hence its gene expression level is high in rBm33 stimulated monocytes. Regulatory cytokine, i.e. IL-12 gene expression decreased upon rBm33 stimulation of THP-1 cells, and this may be due to the lack of signals for IL-12 production, i.e. IFN-γ, bacterial products, and is produced by activated T cells [23]. Similar observations have been reported with THP-1 cell system using three stages of live parasites which showed microfilariae (mf) stimulated pro-inflammatory cytokine production more efficiently than third larval stage (L3) and adult stage of the parasites [24]. Besides this, the cytokine levels in rBm33 stimulated pooled culture supernatants showed difference in the gene and protein expression that could be attributed to post transcriptional, translational regulation and sensitivity of the assay. This might, however, necessitate a closer look by monitoring the time kinetics of expression. Thus, it appears that cytokine responses are specific to filarial antigen (rBm33) and PPD stimulated THP-1 monocytes showed a different pattern of cytokine expression. Similarly, it has been reported earlier that non-parasite protein (PPD) showed an unimpaired immune responses in infected group of individuals [25] while cytokine impairment was specific to filarial parasites in the same group of individuals. As expected, THP-1 cells upon LPS stimulation induced Th1 response in monocytes by inducing proinflammatory cytokines (IL-1β, TNF-α, IL-6 and IL-12) [26].
The NO producing enzyme, iNOS gene expression was noticeable in rBm33 stimulated THP-1 monocytes. However, the existence of the NO synthase (NOS) pathway in human monocytes/ macrophages remains a subject of controversy, despite an increasing number of reports suggesting that human monocytes produce NO in vitro in response to various stimuli [27]. Further, low NO production in the rBm33 stimulated cultures could also contribute to the increased Th1 polarization similar to the study carried out in the macrophages of XID mice, gerbils and humans during the prepatent period of filarial infection [28–30]. Thus, it appears that rBm33 induces classical pathway of macrophage activation.
Increase uptake of GFP expressing E. coli in rBm33 stimulated PMAr THP-1 macrophages compared to PPD demonstrates that this parasite antigen enhances the phagocytic capacity of macrophages. In vivo studies also showed a significant correlation between increased phagocytic and microbicidal activity with Brugia pahangi L3 induced activation of macrophages in the peritoneal cavity of jirds [31]. Further, percentage phagocytosis of rBm33 induced THP-1 macrophages was comparable to LPS stimulated ones. Studies have shown that LPS can induce macrophage activation by triggering inflammatory cytokines, augmenting phagocytosis, increases chemotactic activity and its oxidative ability [32]. Macrophages can be activated to kill parasites suggests that these cells may be responsible for some of the pathology associated with filariasis [6]. This correlates well with the fact that rBm33 promotes pro-inflammatory cytokine production by THP-1 cells and thus involves in inducing protective immunity. In this study, NO production in the culture supernatants post phagocytosis did not exhibit any trend among the stimulated cultures and thus correlated with results of apoptosis as low levels of NO contribute to the killing of intracellular bacteria and the increased levels may damage or induce apoptosis of the cells itself [33]. Further, NO production from human macrophages in response to stimulus requires synergistic stimulation with cytokines [34].
Apoptotic pathways have not been examined at in detail in human filarial infections. Studies with several experimental models suggested the role of CD 4+ T cells apoptosis in proliferative defect associated with the filarial patients [35,36]. We have previously shown using PBMCs that rBm33 induced unsustained early T cell activation in the MF individuals and one possibility for this could be the apoptosis of T cells in such individuals [12]. Similar studies have been carried out previously with live L3 microfilariae of B. malayi, which showed early Natural killer cell activation followed by NK cell apoptosis [37]. Further, it has also been shown that filaria-infected patients have impaired monocyte function such as their inability to produce cytokines/chemokine in response to activating stimuli and expressing more pro-apoptotic genes and adhesion molecules such as ICAM-1 [38].
In order to examine this, PBMCs from normal individuals free of filarial infection was chosen as a system for the study on apoptosis with rBm33 and the PBMCs offer a good source of lymphocytes and monocytes. The percentage of live cells in culture gradually decreased with increase of time and so 24 h of incubation was chosen to study apoptosis in PBMCs. rBm33 and PPD stimulated PBMCs did not exhibit any morphological and physiological changes due to apoptosis. However, CHX an inducer of apoptosis showed the above mentioned changes. Further, in flow cytometric analysis it was found that rBm33 did not induce apoptosis of both monocyte and lymphocyte population of PBMCs in contrast to CHX inducing apoptosis of monocytes. In-depth studies on rBm33 induced apoptotic pathway, the receptors involved in the process and time kinetic responses in the context of apoptosis could answer this in detail.
Thus, the present study shows that rBm33 stimulated monocytes/macrophages by elevated expression of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6), diminished levels of anti-inflammatory cytokine (IL-10), and enhanced phagocytic activity. This demonstrates that rBm33 induced predominant features of Th1 type of immune response in THP-1 monocytes and contributes to T cell activation thereby enhancing cell mediated immune response. Further, rBm33 did not promote apoptosis of monocytes or lymphocytes. However, these findings when evinced with rBm33 induced isolated monocytes from filarial patients would offer more evidence that APCs are one component in the cascade of events of T-cell responses to filarial antigens.
Acknowledgments
The authors wish to thank Dr. Thomas B. Nutman (NIH, Bethesda, MD, USA) for providing reagents in the present work. This work was supported by grants from the Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi. This work received partial support from the National Institutes of Health through NIAID/TRC ICER programme.
Abbreviations
- APC
Antigen presenting cells
- cDNA
Complementary DNA
- CHX
Cycloheximide
- CP
Chronic pathology
- EN
Endemic normal
- FCS
Fetal calf serum
- IFN-γ
Interferon gamma
- IL
Interleukin
- LPS
Lipopolysaccharide
- MF
Microfilaremics
- mf
Microfilariae
- PBMC
Peripheral blood mononuclear cell
- PMA
Phorbol-12-myristate-13-acetate
- PPD
Purified protein derivative
- rBm33
Recombinant Brugia malayi pepsin inhibitor
References
- 1.Freedman DO. Immune dynamics in the pathogenesis of human lymphatic filariasis. Parasitol Today. 1998;14:229–34. doi: 10.1016/s0169-4758(98)01244-7. [DOI] [PubMed] [Google Scholar]
- 2.Semnani RT, Nutman TB. Towards an understanding of the interaction between filarial parasites and host antigen —presenting cells. Immunol Rev. 2004;201:127–38. doi: 10.1111/j.0105-2896.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 3.Steel C, Nutman TB. Helminth antigens selectively differentiate unsensitized CD45RA+ CD4+ human T cells in vitro. J Immunol. 1998;160:351–60. [PubMed] [Google Scholar]
- 4.Semnani RT, Liu AY, Sabzevari H, Kubofcik J, Zhou J, Gilden JK, et al. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J Immunol. 2003;4:1950–60. doi: 10.4049/jimmunol.171.4.1950. [DOI] [PubMed] [Google Scholar]
- 5.Semnani RT, Sabzevari H, Iyer R, Nutman TB. Filarial antigens impair the function of human dendritic cells during differentiation. Infect Immun. 2001;69:5813–22. doi: 10.1128/IAI.69.9.5813-5822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JE, Lawrence RA, Maizels RM. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–51. doi: 10.1093/intimm/8.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Williams SA, Lizotte-Waniewski MR, Foster J, Guiliano D, Daub J, Scott AL, et al. The filarial genome project: analysis of the nuclear, mitochondrial and endosymbiont genomes of Brugia malayi. Int J Parasitol. 2000;30:411–9. doi: 10.1016/s0020-7519(00)00014-x. [DOI] [PubMed] [Google Scholar]
- 8.Shiny C, Krushna NSA, Haripriya K, Babu S, Elango S, Manokaran G, et al. Recombinant Wolbachia Surface Protein (WSP) induced T cell responses in Wuchereria bancrofti infections. Parasitol Res. 2011a doi: 10.1007/s00436-011-2553-7. Epub Ahead print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiny C, Krushna NSA, Babu S, Elango S, Manokaran G, Narayanan RB. Recombinant Wolbachia Heat Shock Protein 60 (HSP60) mediated immune responses in patients with lymphatic filariasis. Microb Infect. 2011b;13:1221–31. doi: 10.1016/j.micinf.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dissanayake S, Xu M, Nkenfou C, Piessens WF. Molecular cloning and sero-logical characterization of Brugia malayi pepsin inhibitor homolog. Mol Biochem Parasitol. 1993;62:143–6. doi: 10.1016/0166-6851(93)90191-y. [DOI] [PubMed] [Google Scholar]
- 11.Krushna NSA, Shiny C, Dharanya S, Sindhu A, Aishwarya S, Nararyanan RB. Immunolocalization and serum antibody responses to Brugia malayi pepsin inhibitor homolog (Bm-33). Microbiol Immunol. 2009;53:173–83. doi: 10.1111/j.1348-0421.2009.00114.x. [DOI] [PubMed] [Google Scholar]
- 12.Krushna NSA, Shiny C, Manokaran G, Elango S, Babu S, Narayanan RB. Immune responses to recombinant Brugia malayi pepsin inhibitor homolog (Bm-33) in patients with human lymphatic filariaisis. Parasitol Res. 2011;108:407–15. doi: 10.1007/s00436-010-2081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasisekhar B, Aparna M, Augustin DJ, Kaliraj P, Kar SK, Nutman TB, et al. Diminished monocyte function in microfilaremic patients with lymphatic filariasis and its relationship to altered lymphoproliferative responses. Infect Immun. 2005b;73:3385–93. doi: 10.1128/IAI.73.6.3385-3393.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zang XX, Taylor P, Wang JM, Meyer DJ, Scott AL, Walkinshaw MD, et al. Homologs of human macrophage migration inhibitory factors from a parasitic nematode. J Biol Chem. 2002;277:44261–7. doi: 10.1074/jbc.M204655200. [DOI] [PubMed] [Google Scholar]
- 15.Kageyama T. Molecular cloning, expression and characterization of an Ascaris inhibitor for pepsin and cathepsin E. Eur J Biochem. 1998;253:804–9. doi: 10.1046/j.1432-1327.1998.2530804.x. [DOI] [PubMed] [Google Scholar]
- 16.Krishna NR, Krushna NS, Narayanan RB, Rajan SS, Gunasekaran K. Expression, purification and characterization of refolded rBm-33 (pepsin inhibitor homologue) from Brugia malayi: a human lymphatic filarial parasite. Protein Express Purif. 2011;79:245–50. doi: 10.1016/j.pep.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Chanteau S, Moulia-Pelat JP, Glaziou P, Nguyen NL, Luquiaud P, Plichart C, et al. Og4C3 circulating antigen: a marker of infection and adult worm burden in Wuchereria bancrofti filariasis. J Infect Dis. 1994;170:247–50. doi: 10.1093/infdis/170.1.247. [DOI] [PubMed] [Google Scholar]
- 18.Krushna NSA, Shiny C, Verma P, Nithya D, Basker P, Elango S, et al. Wuchereria bancrofti: diminished platelet activation in filarial patients. Exp Parasitol. 2010;125:114–23. doi: 10.1016/j.exppara.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Yoganandhan K, Sathish S, Narayanan RB, Sahul Hameed AS. A rapid nonenzymatic method of DNA extraction for PCR detection of white spot syndrome virus in shrimp. Aquacult Res. 2003;34:1093–7. [Google Scholar]
- 20.Auwerx J. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 21.Devaney E, Osborne J. The third-stage larva (L3) of Brugia: its role in immune modulation and protective immunity. Microbes Infect. 2000;2:1363–71. doi: 10.1016/s1286-4579(00)01290-9. [DOI] [PubMed] [Google Scholar]
- 22.Aarden LA, DeGroot ER, Schaap DL, Landsorp PJ. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987;17:1411–6. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 24.Dixit S, Gaur1 RL, Khan MA, Saxena JK, Murthy PSR, Murthy PK. Inflammatory antigens of Brugia malayi and their effect on rodent host Mastomys coucha. Parasite Immunol. 2004;26:397–407. doi: 10.1111/j.0141-9838.2004.00725.x. [DOI] [PubMed] [Google Scholar]
- 25.Babu S, Bhat SQ, Pavan Kumar N, Lipira AB, Kumar S, Karthik C, et al. Filarial lymphedema is characterized by antigen-specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis. 2009;3:e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007;8:1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dugas B, Mossalayi MD, Damais C, Kolb JP. Nitric oxide production by human monocytes: evidence for a role of CD23. Immunol Today. 1995;16:574–80. doi: 10.1016/0167-5699(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 28.Mukhopadhyay S, George A, Bal V, Ravindran B, Rath S. Bruton's tyrosine kinase deficiency in macrophages inhibits nitric oxide generation leading to enhancement of IL-12 induction. J Immunol. 1999;163:1786–92. [PubMed] [Google Scholar]
- 29.Nasarre C, Krahenbuhl JL, Klei TR. Down regulation of macrophage activation in Brugia pahangi-infected jirds (Meriones unguiculatus). Infect Immun. 1998;66:1063–9. doi: 10.1128/iai.66.3.1063-1069.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denis M. Human monocytes/macrophages: no or no no? J Leukoc Biol. 1994;55:682–4. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 31.Jeffers GW, Klei TR, Enright FM. Activation of jird (Meriones unguiculatus) macrophages by the filarial parasite Brugia pahangi. Infect Immun. 1984;43:43–48. doi: 10.1128/iai.43.1.43-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu TT, Chen TL, Chen RM. Lipopolysaccharide triggers macrophage activation of inflammatory cytokine expression, chemotaxis, phagocytosis, and oxidative ability via a toll-like receptor 4-dependent pathway: validated by RNA interference. Toxicol Lett. 2009;191:195–202. doi: 10.1016/j.toxlet.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Marriott HM, Bingle CD, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 2004;18:1126–8. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- 34.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 35.Jenson JS, O'Connor R, Osborne J, Devaney E. Infection with Brugia microfilaria induces apoptosis of CD4+ T lymphocytes: a mechanism of immune unresponsiveness in filariasis. Eur J Immunol. 2002;32:858–67. doi: 10.1002/1521-4141(200203)32:3<858::AID-IMMU858>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Connor RAO, Jenson JS, Osborne J, Devaney E. An enduring association? Microfilaria and immunosuppression in lymphatic filariasis. Trends Parasitol. 2003;19:565–70. doi: 10.1016/j.pt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Babu S, Blauvelt CP, Nutman TB. Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J Immunol. 2007;179:2445–56. doi: 10.4049/jimmunol.179.4.2445. [DOI] [PubMed] [Google Scholar]
- 38.Semnani RT, Keiser PB, Coulibaly YI, Keita F, Diallo AA, Traore D, et al. Filaria-induced monocyte dysfunction and its reversal following treatment. Infect Immun. 2006;74:4409–17. doi: 10.1128/IAI.01106-05. [DOI] [PMC free article] [PubMed] [Google Scholar]