Abstract
Purpose
From the randomized study SWOG 8794, we evaluated the effect of seminal vesicle involvement on outcomes and whether those patients benefited from post prostatectomy adjuvant radiation therapy.
Materials and Methods
SWOG 8794 randomized high risk (SV+ and/or capsular penetration and/or positive margins) post prostatectomy patients to radiation versus observation. 431 subjects with pathologically-advanced prostate cancer were randomized
Results
Median follow up is 12.2 years. A total of 139 patients had involved seminal vesicles with or without other risk factors. Compared to SV negative patients, survival was marginally worse for these patients (10 year overall survival 61% versus 74%). For all (radiated and observed) the SV negative patients, 5 and 10 year biochemical failure free survival (bffs) was 56% and 33%, respectively. For SV positive patients, it was 49% and 22%, respectively. The numbers for both groups were better if they received adjuvant radiation. (SV negative patients 68% and 39% and SV positive 62% and 36% 5 and 10 year bffs, respectively). Noteworthy is the administration of radiation negated the negative prognostic effect of positive seminal vesicles. Radiated patients were less likely to require subsequent androgen ablation (p=.01).
Conclusions
Although seminal vesicle involvement is a negative prognostic factor, long term control is possible, especially if patients are given adjuvant radiation therapy.
Keywords: prostatectomy, adjuvant radiation, positive seminal vesicles
Introduction
From the earliest studies of outcomes after radical prostatectomy for prostate cancer, seminal vesicle involvement has been recognized as a poor prognostic finding. (1) These patients often develop metastatic disease and are at high risk of dying from prostate cancer. (2,3) Some considered it a uniformly fatal finding (4). More contemporary studies with earlier diagnosed patients suggest the risk may not be so dire. (5,6) In addition, adjuvant radiation has been used in these patients, and on retrospective reviews, the results have been mixed, with some studies suggesting there might be a benefit (7,8), while others suggesting there is none (6). Therefore, there are two issues that need more clarification: the exact detriment to having pathologically positive seminal vesicles and whether adjuvant radiation benefits those patients. The gold standard for determining the potential benefit of any treatment is the randomized trial. There have been no randomized studies looking at this issue specifically, but Southwest Oncology Group Study 8794, which randomized patients to adjuvant radiation versus none after prostatectomy, included a significant number of these patients. (9,10) We reviewed the data to see how these patients fare compared to those high risk patients whose seminal vesicles were not involved (i.e. had only extracapsular extension and/or positive margins) and the level of benefit offered by adjuvant radiation in seminal vesicle positive patients.
Materials and Methods
SWOG 8794 was a prospective randomized study examining whether high risk post-prostatectomy patients would benefit from immediate (adjuvant) radiation therapy to the prostate fossa compared to no immediate treatment. Patients were required to have extracapsular tumor extension, positive surgical margins, or seminal vesicle involvement and histologically negative lymph nodes (although towards the end of the study, lymphadenectomy was not required for certain lower risk patients).
Although not an initial requirement, the protocol was amended shortly after opening to mandate prostate specific antigen (PSA) collection. Most patients had longitudinal PSA data. There was no restriction on PSA level at enrollment.
Radiation therapy was directed at the prostate fossa only. A four-field or arch technique was allowed. On the four field approach, this was defined as a 9×9 cm or 10×10 cm AP and PA portal. The only additional requirement on the lateral portals was an attempt to block at least part of the rectum. The prescribed dose was 60-64 Gray. The radiation data were reviewed for dosimetric and volumetric compliance.
Follow-up visits were scheduled every 3 months for one year, every 6 months for two years, and annually thereafter. At each visit, a PSA was obtained as were additional staging studies (e.g., bone scan) as clinically indicated. Biochemical failure was defined as a PSA > 0.4 ng/ml.
Statistical methods
The primary study endpoint was metastasis-free survival, defined as the time from randomization to first evidence of metastatic disease or death due to any cause. The study design details have been previously published (9). Additional secondary endpoints included PSA failure-free interval which was defined for men with a post-surgical PSA of 0.4 ng/ml or lower as the time to first occurrence of a PSA greater than 0.4 ng/ml or death due to any cause. Recurrent disease was defined as any evidence of measurable or evaluable (e.g., bone lesions) disease, but excluding isolated PSA failure, and recurrence-free survival was defined as the first evidence of any objective recurrence (not including PSA) or death due to any cause. Patients without the event of interest were censored at their last contact date (last PSA assessment date for PSA failure). The methods of Kaplan and Meier were used to generate the time-to-event curves (Figures 1-4).
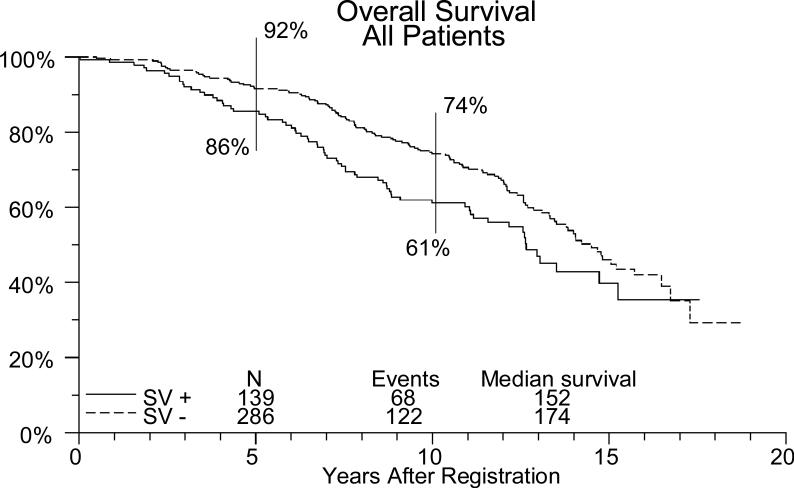
Figure 1.
Overall survival in high risk patients, impact of SV involvement.
Results
Patient characteristics and overall outcomes for the entire study have been previously reported (9,10). 425 patients were eligible and had PSA levels obtained on follow-up, with 374 having immediate post prostatectomy PSA data. Of these, 348 with post-surgical PSA ≤ 0.4 ng/mL were included in the biochemical failure analysis. Median follow-up was 12.2 years. A total of 139 patients had positive seminal vesicles, with or without capsular penetration or positive margins. Patient characteristics are shown in table 1. Seventy one patients were randomized to receive radiation and 68 were randomized to initial observation. Of the patients in the observation group, 20 (29%) subsequently received radiation and 31 (46%) received androgen ablation. In the radiation group, 14 (20%) subsequently received androgen ablation. Patients on the observation arm were more likely to report the use of subsequent androgen ablation, and initiated this therapy significantly earlier than patients on adjuvant RT (p=.01 by stratified log-rank test).
Table 1.
Characteristics of eligible subjects.
| Characteristic | Observation (n=211) | Adjuvant Radiotherapy (n=214) |
|---|---|---|
| Age (median; range) | 65.8 (47.4, 79.2) | 64.1 (43.8, 78.0) |
| Race | ||
| White | 140 (67 percent) | 154 (72 percent) |
| African American | 42 (20 percent) | 41 (19 percent) |
| Preoperative hormonal therapy use | ||
| Yes | 17 (8 percent) | 19 (9 percent) |
| No | 193 (92 percent) | 195 (91 percent) |
| Extent of disease | ||
| Beyond capsule or positive margin | 142 (68 percent) | 143 (67 percent) |
| Seminal vesicle invasion | 23 (11 percent) | 22 (10 percent) |
| Beyond capsule, positive margin and seminal vesicle invasion | 45 (21 percent) | 49 (23 percent) |
| Gleason Grade ** | ||
| Gleason ≤ 6 | (46 percent) | (57 percent) |
| Gleason 7 | (38 percent) | (34 percent) |
| Gleason 8-10 | (16 percent) | (9 percent) |
| PSA at diagnosis | ||
| < 10 ng/ml | (53 percent) | (51 percent) |
| ≥ 10 ng/ml | (47 percent) | (59 percent) |
| PSA after radical prostatectomy* | ||
| < 0.2 ng/ml | (68 percent) | (65 percent) |
| ≥ 0.2 ng/ml | (32 percent) | (36 percent) |
Available in 376 subjects
Available in 325 subjects
In these high risk patients, there was a trend to improved survival if the seminal vesicles were negative (ie. They just had capsular extension/positive margins) (figure 1) For patients without seminal vesicle involvement, the overall 5 and 10 year biochemical failure free survival was 56% and 33%, respectively. For those that received radiation, it was 68% and 39%, respectively and for those observed it was 45% and 27% respectively. (table 2, figure 2). Overall, for patients with seminal vesicle involvement, the 5 and 10 year biochemical failure free survival were 49% and 22%, respectively. For the radiation patients it was 62% and 36%, respectively, and for observation it was 36% and 12% respectively. In multivariate Cox regression analyses adjusting for treatment arm, patients with positive seminal vesicles had significantly worse outcomes (overall survival, biochemical failure-free survival, metastasis-free survival, local recurrence-free survival) than those that did not have involvement (p<.05 for each endpoint).
Table 2 (a).
Effect of positive seminal vesicles on outcome in high risk patients. For SV negative patients, they were capsule and/or margin positive. In the SV positive patients, 45 (32%) were SV positive only (with negative capsule and/or margins).
| Number of patients | 5 yr bffs | 10 yr bffs | 5 yr OS | 10 yr OS | 10 yr mets free | 10 yr LF free | |
|---|---|---|---|---|---|---|---|
| All SV + | 139 (108 for bffs) | 49% | 22% | 86% | 61% | 56% | 51% |
| SV+ C/M+ | 94 (76 for bffs) | 53% | 11% | 88% | 59% | 54% | 50% |
| SV+ C/M - | 45 (32 for bffs) | 40% | 35% | 80% | 65% | 61% | 54% |
| All SV – C/M + | 286 (240 for bffs) | 56% | 33% | 92% | 74% | 70% | 66% |
| Radiation SV + | 71 (54 for bffs) | 62% | 36% | 84% | 71% | 66% | 64% |
| Radiation SV – | 143 (119 for bffs) | 68% | 39% | 93% | 76% | 73% | 71% |
| Observation SV + | 68 (54 for bffs) | 36% | 12% | 87% | 51% | 47% | 38% |
| Observation SV – | 143 (121 for bffs) | 45% | 27% | 90% | 73% | 67% | 61% |
Bffs= biochemical free survival. This is the number of patients that had complete PSA data.
SV+ = positive seminal vesicles. SV- = negative seminal vesicles (by study parameters these patients had capsular penetration and/or positive margins)
C/M+ = capsular penetration and/or positive margins
C/M- = no capsular penetration and/or positive margins (SV + only)
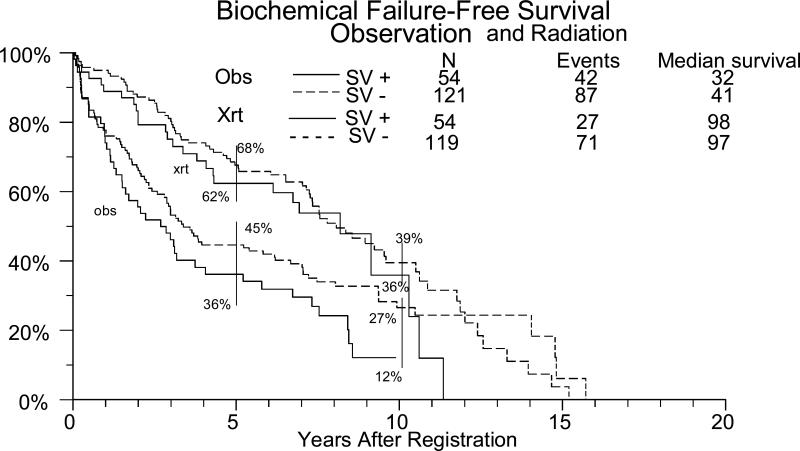
Figure 2.
Biochemical failure free survival for radiation and observation high risk patients as influenced by seminal vesicle involvement.
For patients with positive seminal vesicles the effect of radiation on outcomes is shown in table 3 and figure 2. The difference in local recurrence-free and biochemical failure-free survival between those that underwent radiation and those that were observed was statistically significant at p<.05. The differences in overall and metastasis-free survival, however, were not statistically significant in this subgroup, although there was a trend (figure 3). Eleven patients (16%) in the radiation group had developed metastasis and 18 (25%) had developed local recurrence. In the observation group 19 (28%) developed metastasis and 33 (49%) developed local recurrence. Ten year metastasis free survival was 66 % for the radiation patients and 47% for the observation patients. (table 3).
Table 3.
Seminal vesicle positive patients. Hazard ratios and p-values for pattern of failure.
| N | Median Event-free (yrs) | 5-year Event-free | 10-year Event-free | HR (95% CI)* | p* | |
|---|---|---|---|---|---|---|
| Overall Survival | ||||||
| RT | 71 | 13.5 | 84% | 71% | 1.0 (ref) | -- |
| Obs | 68 | 10.9 | 87% | 51% | 1.54 (0.95, 2.49) | 0.08 |
|
Metastasis-Free Survival | ||||||
| RT | 71 | 13.5 | 76% | 66% | 1.0 (ref) | -- |
| Obs | 68 | 8.6 | 72% | 47% | 1.51 (0.94, 2.41) | 0.09 |
|
Local Recurrence-Free Survival | ||||||
| RT | 71 | 12.7 | 74% | 61% | 1.0 (ref) | -- |
| Obs | 68 | 6.8 | 56% | 34% | 1.78 (1.15, 2.74) | 0.009 |
|
Biochemical Failure-Free Survival | ||||||
| RT | 54 | 8.2 | 62% | 36% | 1.0 (ref) | -- |
| Obs | 54 | 2.7 | 36% | 12% | 2.33 (1.41, 3.85) | 0.001 |
Hazard ratios and p values from proportional hazards regression models comparing outcomes among SV+ patients on observation vs. adjuvant RT. Ref= reference.
Figure 3.
High risk patients with positive seminal vesicles. Impact of radiation on survival.
In testing for interaction between seminal vesicle positivity and treatment arm, in no case was the interaction test significant, indicating that seminal vesicle positive patients responded to the same degree as the other patients to adjuvant radiation therapy (although as noted above, the overall outcome was worse).
We also analyzed the effect of preoperative PSA (<10 vs. >10 ng/ml), post operative PSA (≤0.2 vs. >0.2 ng/ml), patient age, and Gleason score (<7 vs. >6) . Undetectable (≤0.2 ng/ml) post operative PSA and patient age < 65 were found to be significantly associated with better outcomes (OS, metastasis-free survival, local recurrence-free survival, and biochemical failure-free survival). These associations were not modified by either seminal vesicle positivity or treatment assignment.
Discussion
The advantage of this data from SWOG 8794 is that it is one of the largest single data sets for seminal vesicle positive patients and the only one reported based on randomized data. The disadvantage is that the questions we are pursuing specifically regarding seminal vesicle positive disease was not a specified study endpoint, so the study was not powered to fully address these issues. Therefore we must recognize that we can only make general observations, but the information should be less biased than that found in retrospective studies.
From the earliest data analyses of prostatectomy patients, it was clear that seminal vesicle involvement was a poor prognostic sign. The consideration was that most patients with seminal vesicle involvement were destined to fail and had a high risk for cancer death (1-4). This impression was carried into contemporary times with the conclusion that seminal vesicle positive disease was tantamount to metastatic disease (12). As a result, there already has been a trend to offer some of these patients adjuvant treatment to try to change the outcome. This usually consists of androgen ablation or radiation. With the selection bias inherent as to who got adjuvant treatment versus those that didn't, observations from the retrospective series need to be made cautiously. In general, patients from contemporary series seem to do a little better than those historically. In the older series, clinical failure rates of 66% (1) and metastatic rates of 50% (2) were reported and survival at 7 years (32%) was less than half that of seminal vesicle negative patients (67%) (3) More contemporary studies of prostatectomy patients that didn't receive adjuvant treatment show biochemical control rates at 5 years usually in the 30-50% range (5,6,11,12) This is consistent with our findings where 5 year freedom from biochemical failure is 49% and 10 year was 22%. All of our patients had at least some high risk features, but the presence of seminal vesicle involvement led to statistically worse (p <0.05) outcomes. Still, the biochemical control rate at 5 years was 56% and 33% at 10 years (table 2).
There are various prognostic factors on univariate and multivariate analysis that have been identified in the retrospective studies. Given the small numbers in most of the studies and their retrospective nature, these factors are not always consistent, but more commonly include Gleason score (usually >6), pre surgery PSA (10 ng/ml usually the cutoff) and margin status (positive worse). More variable is age and extracapsular extension. With these factors, there can be identified patients that will do well. For example, for Gleason <7 and margin negative, a five year biochemical failure free survival of 69 % has been reported (compared to 8% if margin was positive) (5). Unfortunately, patients with such favorable prognostic factors are in the distinct minority (<15%) and the remainder still face a high failure rate. In our series, we did not have enough patients to perform a meaningful analysis of this small subgroup. In our series, the pretreatment PSA and pathology Gleason score did not influence outcomes.
Regarding the effect of adjuvant radiation therapy, there have been some retrospective studies evaluating its role SV positive patients. Unfortunately, the numbers are very small and in some cases, seminal vesicle positive patients are a small part of a larger retrospective study. In two representative studies, adjuvant radiation reduced the five year biochemical failure rate from 92% to 20% (7) and 82% to 40% (8). On the contrary, another study (5) showed that those that received adjuvant radiation did worse (58% 4 year biochemical failure rate) than those that didn't (48% 4 year biochemical failure rate), although they acknowledged that there may have been some selection biases. Fortunately, with our series, we now have randomized data that not only shows a clear benefit for high risk patients in general, but also for those that specifically have seminal vesicle involvement. Five year biochemical failure free survival was improved over observation alone from 36% to 62% at 5 years and from 12% to 36% at 10 years with the addition of adjuvant radiation. The radiation clearly reduced the rate of detectable local and biochemical failure, but there was only a trend for improvement in metastasis free survival and overall survival (table 3, figure 3). The latter was likely effected by the infrequency of metastasis and the high use of subsequent salvage radiation and/or androgen ablation in the observation group.
Interestingly, overall it appears that the seminal vesicle positive patients have a greater benefit to radiation than those that have only extracapsular extension/positive margins. For the capsule/margin positive only patients, radiation therapy improved the biochemical control by 50% at 5 years and 40% at 10 years. In those that also had positive seminal vesicles, the control at 5 years was increased by 70% and by 10 years 200%. In fact, with radiation, the ultimate control between the SV + and the SV- groups is very similar, with a 36% and 39% 10 year biochemical control, respectively. It appears that the radiation has negated some of the worst prognostic effects of seminal vesicle positivity. This causes a marked departure from conventional wisdom that the primary risk in SV positive patients is systemic, when this data would strongly suggest that it is local. It is worth remembering that this was prostate bed only radiation with a modest dose. It is intriguing to consider that pelvic radiation and higher doses could further improve these results.
In summary, the presence of positive seminal vesicles does result in somewhat worse outcomes, but ten year survival is still likely (61%). Previously we have shown that high risk post prostatectomy patients clearly benefit from adjuvant radiation (9,10) with a decrease in biochemical failure, local failure and metastatic disease. With the exception of metastatic disease, we have confirmed those findings in seminal vesicle positive patients. Radiation therapy is the only modality that has been shown in randomized studies to accomplish this. Therefore, as with all high risk prostatectomy patients, seminal vesicle positive patients should be given the option of adjuvant radiation.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA14028, CA58416, CA58658, CA42777, CA27057, CA46136, CA35431, CA58882, CA12644, CA58861, CA35090, CA37981, CA76429, CA04919, CA76132, CA35119, CA35178, CA35176, CA46282, CA67575, CA45377, CA46113, CA74647, CA35261, CA04920, CA20319, CA76447, CA58723, CA12213, CA22433, CA46441, CA21661, CA23318, CA66636, CA11083.
References
- 1.Schellhammer PF. Radical prostatectomy. Patterns of local failure and survival in 67 patients. Urology. 1988 Mar;31(3):191–7. doi: 10.1016/0090-4295(88)90137-9. [DOI] [PubMed] [Google Scholar]
- 2.Fowler JE, Mills SE. Operable prostatic carcinoma: correlations among clinical stage, pathological stage, gleason histological score and early disease-free survival. J Urol. 1985 Jan;133(1):49–52. doi: 10.1016/s0022-5347(17)48778-7. [DOI] [PubMed] [Google Scholar]
- 3.Byar DP, Mostofi FK. Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies. Examined by the step-section technique. Cancer. 1972 Jul;30(1):5–13. doi: 10.1002/1097-0142(197207)30:1<5::aid-cncr2820300103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Jewett HJ, Eggleston JC, Yawn DH. Radical prostatectomy in the management of carcinoma of the prostate: probable causes of some therapeutic failures. J Urol. 1972 Jun;107(6):1034–40. doi: 10.1016/s0022-5347(17)61201-1. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Aronson WJ, Presti JC, Jr, Amling CL, Terris MK, Trock B, Kane CJ. Predictors of prostate-specific antigen progression among men with seminal vesicle invasion at the time of radical prostatectomy. Cancer. 2004 Apr 15;100(8):1633–8. doi: 10.1002/cncr.20122. [DOI] [PubMed] [Google Scholar]
- 6.Eggener SE, Roehl KA, Smith ND, Antenor JA, Han M, Catalona WJ. Contemporary survival results and the role of radiation therapy in patients with node negative seminal vesicle invasion following radical prostatectomy. J Urol. 2005 Apr;173(4):1150–5. doi: 10.1097/01.ju.0000155158.79489.48. [DOI] [PubMed] [Google Scholar]
- 7.Vargas C, Kestin LL, Weed DW, Krauss D, Vicini FA, Martinez AA. Improved biochemical outcome with adjuvant radiotherapy after radical prostatectomy for prostate cancer with poor pathologic features. Int J Radiat Oncol Biol Phys. 2005 Mar 1;61(3):714–24. doi: 10.1016/j.ijrobp.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Lee HM, Solan MJ, Lupinacci P, Gomella LG, Valicenti RK. Long-term outcome of patients with prostate cancer and pathologic seminal vesicle invasion (pT3b): effect of adjuvant radiotherapy. Urology. 2004 Jul;64(1):84–9. doi: 10.1016/j.urology.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chen G, Swanson G, Canby-Hagino E, Crawford ED. Adjuvant radiotherapy for locally-advanced prostate cancer: results of a randomized, prospective clinical trial. JAMA. 2006;296:2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 10.Swanson GP, Hussey MA, Tangen CM, Chin J, Messing E, Canby-Hagino E, Forman JD, Thompson IM, Crawford ED. Predominant failure in post prostatectomy patients is local: analysis of patterns of failure in SWOG 8794. J Clin Oncol. 2007;25(16):2225–9. doi: 10.1200/JCO.2006.09.6495. [DOI] [PubMed] [Google Scholar]
- 11.Tefilli MV, Gheiler EL, Tiguert R, Banerjee M, Sakr W, Grignon DJ, Pontes JE, Wood DP., Jr Prognostic indicators in patients with seminal vesicle involvement following radical prostatectomy for clinically localized prostate cancer. J Urol. 1998 Sep;160(3 Pt 1):802–6. doi: 10.1016/S0022-5347(01)62791-5. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CW, Anastasiadis AG, McKiernan JM, Salomon L, Eaton S, Goluboff ET, Olsson CA, Benson MC. Prognostic indicators for long term outcome following radical retropubic prostatectomy for prostate cancer involving the seminal vesicles. Urol Oncol. 2004 Mar-Apr;22(2):107–11. doi: 10.1016/S1078-1439(03)00138-8. Erratum in: Urol Oncol. 2004 May-Jun;22(3):275.