Abstract
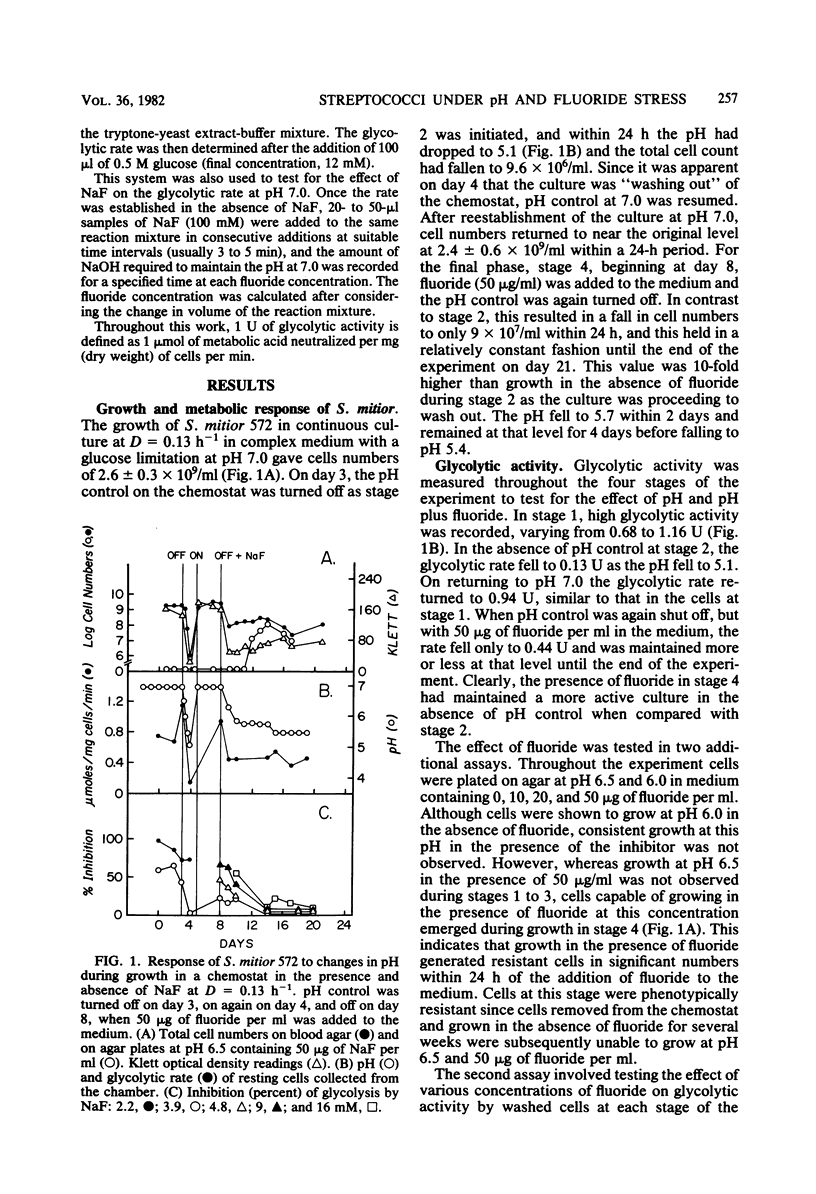
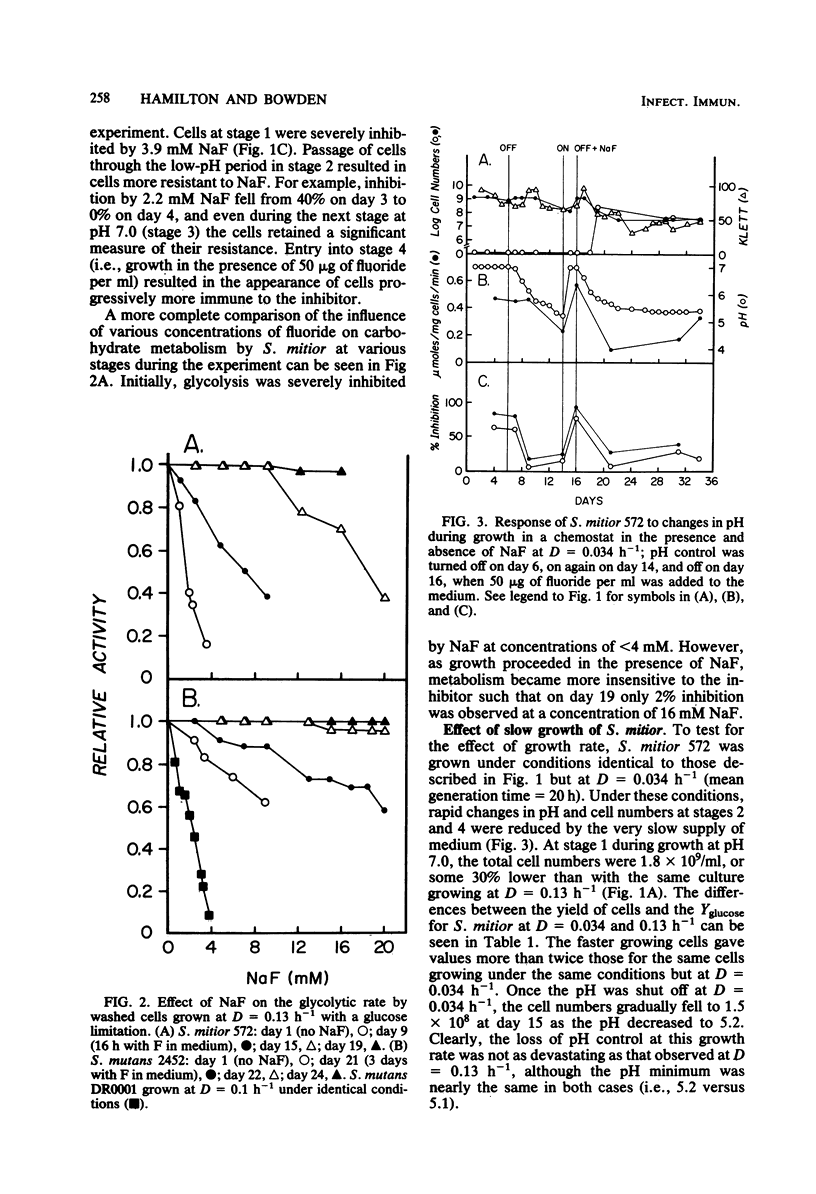
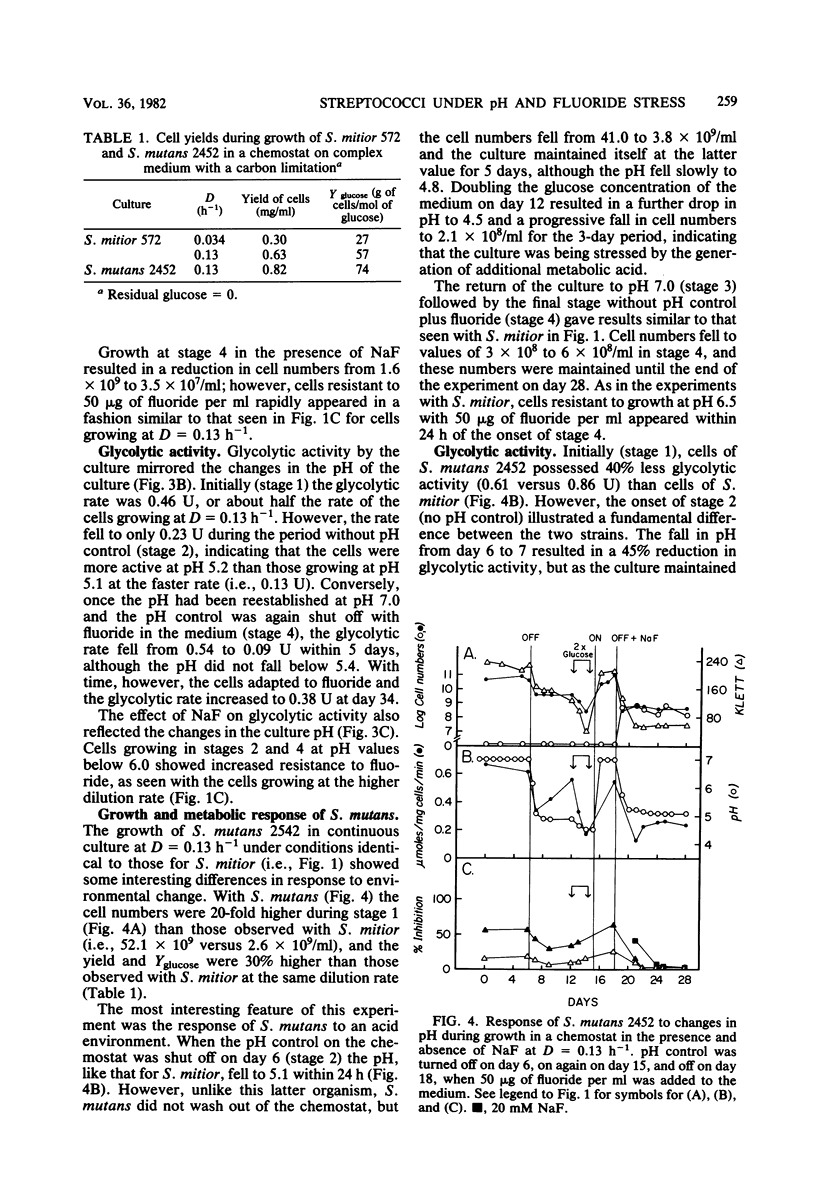
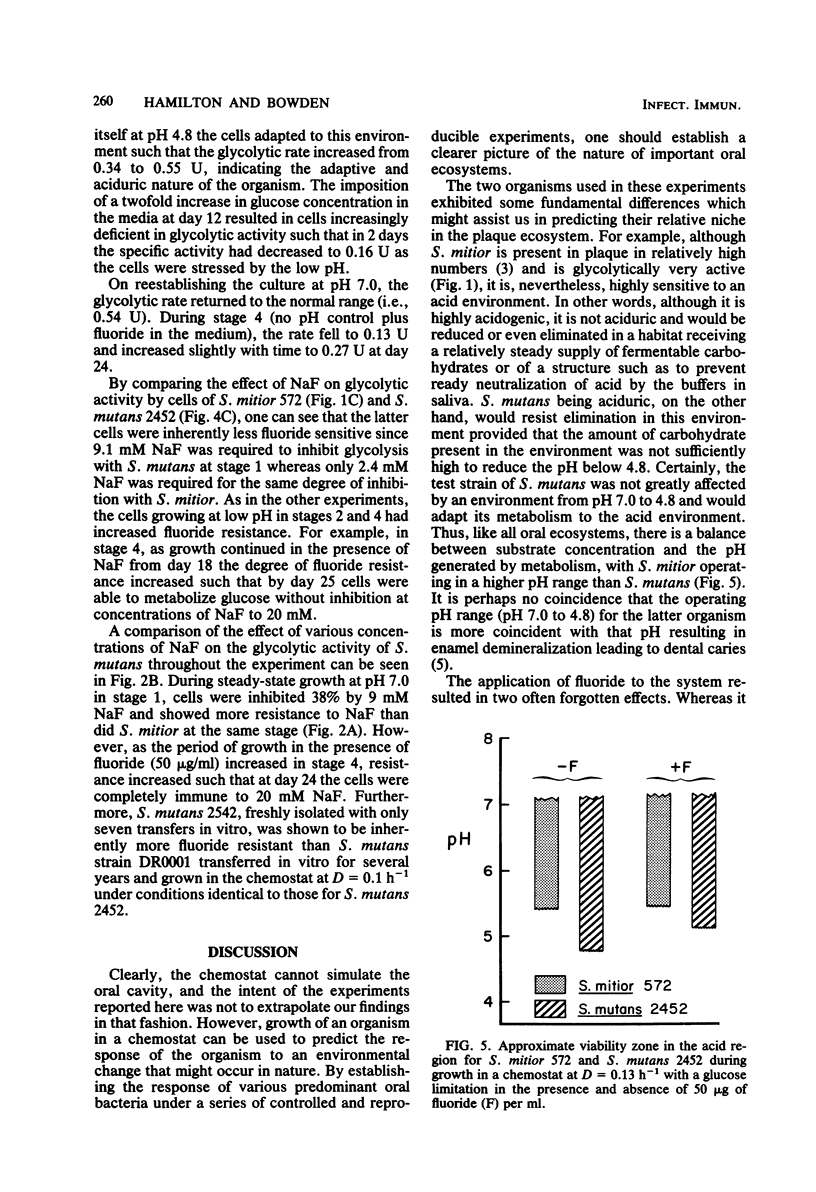
A study was undertaken to compare the effects of pH and fluoride on the growth and metabolic properties of Streptococcus mutans 2452 and Streptococcus mitior 572, strains recently isolated from 8-year-old school children and grown in continuous culture with a glucose limitation. Each experiment had four consecutive stages of growth: (i) pH 7.0, (ii) no pH control, (iii) pH 7.0, and (iv) no pH control plus 50 μg of fluoride per ml in the medium. At a dilution rate (D) of 0.13 h −1, cells of S. mitior possessed high glycolytic activity at pH 7.0 in the initial stage, but were washing out of the chemostat within 24 h after the pH control was shut off and the pH fell to 5.1. Once the culture was reestablished at pH 7.0, fluoride (50 μg/ml) was added to the medium and the pH control was again turned off. Whereas cell numbers fell from 24.0 × 108 to 0.9 × 108/ml within 24 h, the culture remained relatively constant during the following 6 days despite the fall in pH to 5.4. The cells from this culture also maintained an intermediate glycolytic rate of 0.44 μmol mg−1 min−1. The cells in this latter stage developed phenotypic resistant to fluoride at concentrations up to 16 mM. Growth of S. mitior at D = 0.034 h−1 resulted in a slower response to environmental change such that cells were able to grow to pH values as low as 5.2 in the absence of fluoride. In contrast to S. mitior, S. mutans 2452 under the same conditions at D = 0.13 h−1 grew to higher cell numbers and higher yields and was able to maintain significant cell numbers to pH 4.8 once the pH control was shut off in the presence and absence of fluoride. S. mutans had 40% less glycolytic activity but was fourfold more resistant to fluoride at the start of the experiment, and cells were shown to adapt to growth at low pH and to fluoride at levels as high as 20 mM. This fluoride resistance by freshly isolated S. mutans 2452 was significantly higher than that of S. mutans DR0001 grown under identical conditions in the chemostat. S. mutans DR0001 is a strain which has been subcultured in vitro for several years. This study demonstrated that S. mutans 2452 was more aciduric than S. mitior 572 and, unlike the latter organism, could grow at pH values below 5.1. The addition of fluoride to the medium stabilized the S. mitior culture in the absence of pH control, indicating that whereas fluoride does suppress growth and glycolytic activity it also results in higher environmental pH values, which permit the survival of the less aciduric bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden G. H., Odlum O., Nolette N., Hamilton I. R. Microbial populations growing in the presence of fluoride at low pH isolated from dental plaque of children living in an area with fluoridated water. Infect Immun. 1982 Apr;36(1):247–254. doi: 10.1128/iai.36.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Effects of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res. 1977;11 (Suppl 1):262–291. doi: 10.1159/000260304. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Growth characteristics of adapted and ultraviolet-induced mutants of Streptococcus salivarius resistant to sodium fluoride. Can J Microbiol. 1969 Mar;15(3):287–295. doi: 10.1139/m69-052. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINGSLEY G. R., GETCHELL G. Direct ultramicro glucose oxidase method for determination of glucose in biologic fluids. Clin Chem. 1960 Oct;6:466–475. [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. The predominant cultivable flora of carious plaque and carious dentine. Caries Res. 1973;7(3):201–216. doi: 10.1159/000259844. [DOI] [PubMed] [Google Scholar]
- Thott E. K., Folke L. E., Sveen O. B. A microbiologic study of human fissure plaque. Scand J Dent Res. 1974;82(6):428–436. doi: 10.1111/j.1600-0722.1974.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Aasenden R., Peebles T. C. Lactobacilli in human dental plaque and saliva. J Dent Res. 1981 Jan;60(1):2–5. doi: 10.1177/00220345810600010401. [DOI] [PubMed] [Google Scholar]
- Williams R. A. The growth of Lancefield group D streptococci in the presence of sodium fluoride. Arch Oral Biol. 1967 Jan;12(1):109–117. doi: 10.1016/0003-9969(67)90147-1. [DOI] [PubMed] [Google Scholar]


