Abstract
The mitochondrion is arguably the most complex organelle in the budding yeast cell cytoplasm. It is essential for viability as well as respiratory growth. Its innermost aqueous compartment, the matrix, is bounded by the highly structured inner membrane, which in turn is bounded by the intermembrane space and the outer membrane. Approximately 1000 proteins are present in these organelles, of which eight major constituents are coded and synthesized in the matrix. The import of mitochondrial proteins synthesized in the cytoplasm, and their direction to the correct soluble compartments, correct membranes, and correct membrane surfaces/topologies, involves multiple pathways and macromolecular machines. The targeting of some, but not all, cytoplasmically synthesized mitochondrial proteins begins with translation of messenger RNAs localized to the organelle. Most proteins then pass through the translocase of the outer membrane to the intermembrane space, where divergent pathways sort them to the outer membrane, inner membrane, and matrix or trap them in the intermembrane space. Roughly 25% of mitochondrial proteins participate in maintenance or expression of the organellar genome at the inner surface of the inner membrane, providing 7 membrane proteins whose synthesis nucleates the assembly of three respiratory complexes.
TO think about how mitochondrial proteins are synthesized, imported, and assembled, it is useful to have a clear picture of the organellar structures that they, along with membrane lipids, compose and the functions that they carry out. As almost every schoolchild learns, mitochondria carry out oxidative phosphorylation, the controlled burning of nutrients coupled to ATP synthesis. Since Saccharomyces cerevisiae prefers to ferment sugars, respiration is a dispensable function and nonrespiring mutants are viable [although they cannot undergo meiosis (Jambhekar and Amon 2008)]. However, mitochondria themselves are not dispensable. A substantial fraction of intermediary metabolism occurs in mitochondria (Strathern et al. 1982), and at least one of these pathways, iron–sulfur cluster assembly, is essential for growth (Kispal et al. 2005). Thus, any mutation that prevents the biogenesis of mitochondria by, for example, preventing the import of protein constituents from the cytoplasm, is lethal (Baker and Schatz 1991).
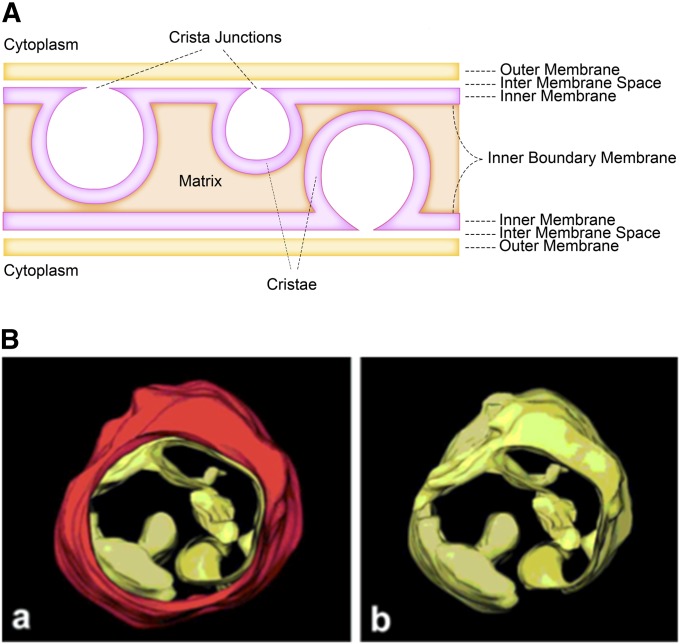
The mitochondria of S. cerevisiae are tubular structures at the cell cortex. While the number of distinct compartments can range from 1 to ∼50 depending upon conditions (Stevens 1981; Pon and Schatz 1991), continual fusion and fission events among them effectively form a single dynamic network (Nunnari et al. 1997). The outer membrane surrounds the tubules. The inner membrane has a boundary domain closely juxtaposed beneath the outer membrane and cristae domains that project internally from the boundary into the matrix (Figure 1A). The matrix is the aqueous compartment surrounded by the inner membrane. The aqueous intermembrane space lies between the membranes and is continuous with the space within cristae.
Figure 1 .
Overview of mitochondrial structure in yeast. (A) Schematic of compartments comprising mitochondrial tubules. The outer membrane surrounds the organelle. The inner membrane surrounds the matrix and consists of two domains, the inner boundary membrane and the cristae membranes, which are joined at cristae junctions. The intermembrane space lies between the outer membrane and inner membrane. (B) Electron tomograph image of a highly contracted yeast mitochondrion observed en face (a) with the outer membrane (red) and (b) without the outer membrane. Reprinted by permission from John Wiley & Sons from Mannella et al. (2001).
Inner membrane cristae are often depicted as baffles emanating from the boundary domain. However, electron tomography of mitochondria from several species, including yeast, shows that cristae actually emanate from the boundary membrane as narrow tubular structures at sites termed “crista junctions” and expand as they project into the matrix (Frey and Mannella 2000; Mannella et al. 2001) (Figure 1B). It seems clear that the boundary and cristae domains of the inner membrane have distinct compositions with respect to the respiratory complexes that are embedded preferentially in the cristae membrane domains, as well as other components (Vogel et al. 2006; Wurm and Jakobs 2006; Rabl et al. 2009; Suppanz et al. 2009; Zick et al. 2009; Davies et al. 2011).
The outer and inner boundary membranes are connected at multiple contact sites, at least some of which are involved in protein translocation and may be transient (Pon and Schatz 1991). In addition, there appear to be firm contact sites, not directly involved with protein translocation, preferentially colocalized with crista junctions (Harner et al. 2011a).
Overall, there appear to be ∼1000 distinct proteins in yeast mitochondria (Premsler et al. 2009). One series of proteomic studies on highly purified organelles identified 851 proteins thought to represent 85% of the total number of species (Sickmann et al. 2003; Reinders et al. 2006; Zahedi et al. 2006). Another study identified an additional 209 candidates (Prokisch et al. 2004). A computationally driven search for candidates involved in yeast mitochondrial function, coupled with experiments to assay respiratory function and maintenance of mitochondrial DNA (mtDNA), identified 109 novel candidates, although many of these may not be mitochondrial per se (Hess et al. 2009). Taking the boundary and cristae domains together, the inner membrane is the most protein-rich mitochondrial compartment, followed by the matrix (Daum et al. 1982).
Only eight of the yeast mitochondrial proteins detected in proteomic studies are encoded by mtDNA and synthesized within the organelle. They are hydrophobic subunits of respiratory complexes III (bc1 complex or ubiquinol-cytochrome c reductase), IV (cytochrome c oxidase), and V (ATP synthase), as well as a hydrophilic mitochondrial small subunit ribosomal protein. The remaining ∼99% of yeast mitochondrial proteins are encoded by nuclear genes, synthesized in cytoplasmic ribosomes, and imported into the organelle.
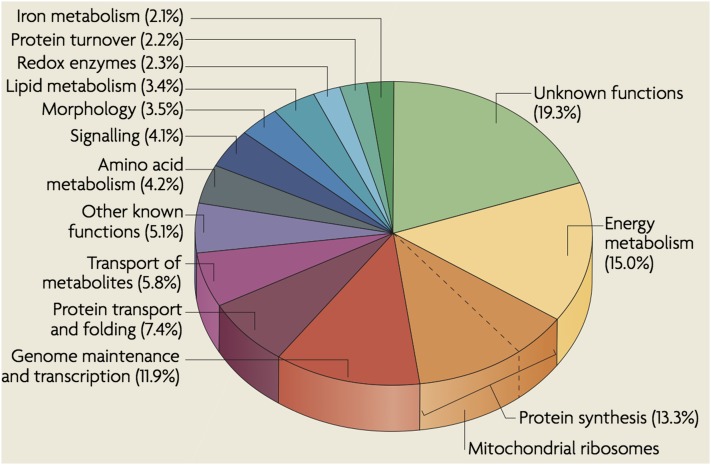
An overview of known nuclearly encoded mitochondrial protein functions (Figure 2) reveals that ∼25% of them are involved directly in genome maintenance and expression of the eight major mitochondrial genes (Schmidt et al. 2010). The functions of ∼20% of the proteins are not known. Fifteen percent are involved in the well-known processes of energy metabolism. Protein translocation, folding, and turnover functions occupy ∼10% of mitochondrial proteins.
Figure 2 .
Classification of identified mitochondrial proteins according to function. Reprinted by permission from Nature Publishing Group from Schmidt et al. (2010).
The following discussion reviews our understanding of the biogenesis of mitochondria starting on the outside, the cytoplasm, and working inward through the mitochondrial compartments.
Cytoplasmic Synthesis of Mitochondrial Proteins
Localization of some cytoplasmic messenger RNAs to mitochondria promotes import of the proteins that they encode
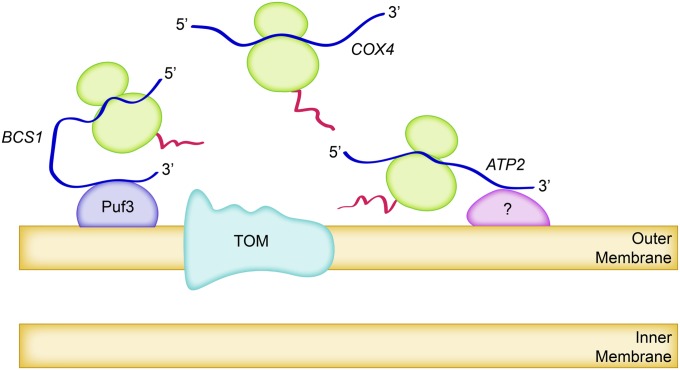
Expression of nuclear genes coding mitochondrial proteins begins with the transcription of messenger RNAs (mRNAs). (Mechanisms controlling the synthesis of these mRNAs are beyond the scope of this review.) It has been known for some time that the synthesis of proteins destined to reside in mitochondria can occur on polysomes bound to mitochondria or on other polysomes, usually referred to as “free polysomes” (Kellems et al. 1975; Ades and Butow 1980; Suissa and Schatz 1982). More recently, surveys of the intracellular locations of specific mRNAs encoding the bulk of the mitochondrial proteome have indicated a range, with approximately half of them selectively translated at the surface of the outer membrane, while translation of others occurs selectively on free polysomes or is not biased between mitochondrial and cytoplasmic locations (Corral-Debrinski et al. 2000; Marc et al. 2002; Garcia et al. 2007a; Saint-Georges et al. 2008; Gadir et al. 2011) (Figure 3). It is easy to imagine that the biological rationale for localized synthesis of organellar proteins is to promote their efficient import and assembly. The rationale for synthesizing roughly half of mitochondrial proteins on free cytoplasmic polysomes remains to be discerned.
Figure 3 .
Cytoplasmic synthesis of some mitochondrial proteins is localized to the organelles, while the synthesis of others is not. The figure depicts three examples: (1) The ATP2 mRNA is highly localized to mitochondria-bound polysomes (Garcia et al. 2007b), although factors required for this localization are unknown. (2) The BCS1 mRNA is also selectively found in mitochondria-bound polysomes, and its localization is partially dependent upon the mitochondrially localized RNA-binding protein Puf3 and the Puf3-binding sites in its 3′-UTR (Saint-Georges et al. 2008). (3) The COX4 mRNA is exclusively found on free polysomes, unassociated with mitochondria (Garcia et al. 2007b). The Atp2, Bcs1, and Cox4 proteins all traverse the outer membrane via the TOM complex pore.
What directs and tethers so many mitochondrially bound mRNAs to the outer surface of the organelles? Current evidence indicates the involvement of nucleotide signals in mRNA 3′-untranslated regions (3′-UTRs) that function prior to translation. In addition, the familiar (Pon and Schatz 1991) mitochondrial targeting signals in the amino acid sequences of the precursor proteins that the mRNAs encode also appear to contribute mRNA localization by mechanisms that are at least partially redundant—and poorly understood (Lithgow 2000).
The ATM1 mRNA, which encodes an essential inner membrane transporter protein, is among those that are highly enriched on mitochondrial-bound polysomes (Corral-Debrinski et al. 2000). By examining the localization of chimeric mRNAs, it was shown that the ATM1 3′-UTR was sufficient to direct mitochondrial localization of a reporter mRNA lacking any other mitochondria-related signals, although this did not lead to import of the GFP reporter protein itself into mitochondria. The 3′-UTR of the PGK1 mRNA, which encodes a soluble cytoplasmic protein, did not direct localization of the mRNA to mitochondria (Corral-Debrinski et al. 2000).
The physiological significance of mRNA localization signals in 3′-UTRs was demonstrated by a study in which the 3′-UTR of the ATP2 mRNA, which encodes the β-subunit of the F1 ATP synthase, was replaced by 3′-UTR of the ADH1 mRNA by alteration of the chromosomal ATP2 locus (Margeot et al. 2002). This alteration prevented normal growth on nonfermentable carbon sources, presumably due to decreased ATP synthase activity. The swap of 3′-UTRs did not affect the overall steady-state level of ATP2 mRNA. However, it did cause a large reduction in the fraction of ATP2 mRNA associated with mitochondria and a large increase of this mRNA in the free polysomal fraction.
The behavior of the Atp2 precursor protein translated from the altered mRNA was particularly interesting: while normal levels of protein were associated with mitochondria, it was overwhelmingly in the larger precursor form, retaining the 34-amino-acid N-terminal targeting signal (Margeot et al. 2002). This contrasted with Atp2 translated from the wild-type mRNA, which was overwhelmingly in the mature processed form. Furthermore, the pre-Atp2 protein translated from the altered mRNA appeared to be on the outside of the outer membrane. Taken together, these results suggest that localization of wild-type ATP2 mRNA to mitochondria via signals in its 3′-UTR promotes synthesis of an import-competent pre-Atp2 polypeptide. Synthesis of pre-Atp2 from an mRNA lacking this 3′-UTR on free polysomes yields a protein that binds the outer surface of mitochondria but fails to be imported efficiently, presumably due to altered structure or interactions with other proteins. Thus, mRNA localization directed by a signal in the ATP2 mRNA 3′-UTR promotes efficient translocation of the protein into mitochondria.
Based on this observation, one should use caution when conducting genetic experiments involving alteration of mitochondrial proteins by methods that also alter the 3′-UTRs of their mRNAs.
A large-scale survey of mRNA abundance in mitochondrially bound polysomes vs. free polysomes was carried out by hybridization to microarrays. After corrections for the amount of mRNA in each fraction, and for cross-contamination of fractions, the degree to which mRNAs encoding the yeast proteome are selectively localized to mitochondria was determined (Garcia et al. 2007b). The ATP2 mRNA, for example, ranks among the most highly localized to mitochondria, with 50% of the total bound to the organelles. At the other extreme of the distribution, none of the COX4 mRNA encoding cytochrome c oxidase subunit IV was found among mitochondria-bound polysomes (Figure 3). The degree of mitochondrial association for each mRNA was then scored by the rank of its ratio among all mRNAs (Garcia et al. 2007b). About half of the 423 mRNAs known to encode mitochondrial proteins at the time of this study were found to be preferentially associated with mitochondria, while relatively few mRNAs encoding known non-mitochondrial proteins were in this group (Marc et al. 2002). The subcellular mRNA distributions observed using genomic microarrays were confirmed by a focused study on 112 mRNAs encoding protein components of known mitochondrial complexes, using quantitative PCR to assay the fractions (Garcia et al. 2007a).
The notion that the strong association of specific mRNAs with mitochondria is correlated with the behavior of the proteins that they encode received striking support from a careful proteomic study of highly purified vesicles derived from the mitochondrial outer membrane (Zahedi et al. 2006). Forty-nine proteins, with a wide range of abundance, were found to be exposed on the outer surface of mitochondria. Surprisingly, 36 of these surface proteins were already well known to actually reside in internal mitochondrial compartments. Two such anomalous surface proteins, Atp2 and Cyb2, were experimentally shown to be unprocessed precursors of the mature internal proteins, and it is likely that at least several others are as well (Zahedi et al. 2006). Thus, a significant group of precursors, possibly awaiting import to internal destinations and processing, are bound to the surface of mitochondria. Significantly, 16 of these proteins are encoded by the 25 mRNAs most selectively localized to mitochondrial-bound polysomes as determined by Marc et al. (2002). Zahedi et al. (2006) performed comparisons of the entire yeast proteome, the mitochondrial proteome, and the internal mitochondrial proteins detected on the surface of the outer membrane, with the degree of mitochondrial localization of all yeast mRNAs as scored by rank (Marc et al. 2002). These distributions demonstrated a strong bias for localized mRNAs to encode internal proteins that were also detected at the surface.
Taken together, these findings appear to be paradoxical. If the purpose of localized translation at the surface of mitochondria is to promote efficient cotranslational import of proteins, then why should the locally synthesized proteins be preferentially found among the full-length unimported and unprocessed species detected on the organellar surface? Such molecules were clearly not cotranslationally imported. Perhaps these proteins, as a group, tend to rapidly adopt folded conformations that inhibit translocation. In this case, localized synthesis could be an adaptation that alleviates this problem, albeit incompletely, by facilitating cotranslational import of a significant fraction of molecules. It is currently unknown whether the fully synthesized precursor proteins bound to the mitochondrial outer surface are destined to be imported or degraded (Zahedi et al. 2006). The observation that unprocessed pre-Atp2 accumulates to an abnormally high level outside of mitochondria in cells translating a chimeric ATP2 mRNA lacking the localization signal in its 3′-UTR (Margeot et al. 2002) is consistent with the possibility that post-translational import of pre-ATP2 is inefficient in vivo, despite the fact that it occurs in vitro (Maccecchini et al. 1979). Perhaps the pre-ATP2 molecules detected on the surface of mitochondria were actually translated from those ATP2 mRNA molecules, ∼50% of the total, that were not localized to mitochondria-bound polysomes. In any event, it is clear that localized synthesis of pre-ATP2 somehow facilitates its import.
Another fascinating but imperfect correlation emerged from the ranking of mRNAs by their propensity to be mitochondrially localized. Those mRNAs found most selectively in mitochondria-bound polysomes tend to encode proteins whose evolutionary origins can be clearly traced to Bacteria and/or Archaea. Conversely, those mRNAs found most selectively in free polysomes tend to encode proteins lacking clear homologs in those phylogenetic domains and are therefore likely to be more recently evolved inventions of Eukarya (Marc et al. 2002; Garcia et al. 2007a). This correlation runs in parallel with the observation that the more locally synthesized proteins tend to be either ancient, conserved components of the mitochondrial genetic system or respiratory complexes or conserved proteins with roles in assembly of those core components (Margeot et al. 2005). In the case of cytochrome c oxidase, for example, proteins with bacterial orthologs that assemble mitochondrially coded core subunits in the inner membrane, insert metal cofactors, and synthesize the specific heme A cofactor are all selectively translated at the mitochondrial surface (although they do not all have clear bacterial or archaeal ancestors). In contrast, the eukaryotic-specific subunits of cytochrome c oxidase that surround the catalytic core of the enzyme are all selectively translated on free polysomes.
There are no obvious structural or chemical similarities among the set of proteins most selectively synthesized at the mitochondrial surface (Marc et al. 2002). So, what selective constraints maintain the localized translation of more anciently evolved proteins? It has been argued that synthesis localized to mitochondria may promote efficient assembly of core components of complexes by directing import of proteins to specific regions (Margeot et al. 2005; Garcia et al. 2007a). While this is an attractive hypothesis, there is no strong evidence that localized synthesis of any mitochondrial protein is spatially organized on the organellar surface. Nor is it obvious why nonlocalized translation of other essential but peripheral subunits of complexes would be advantageous. Interestingly, the mitochondria-bound mRNAs tend to be synthesized early during the yeast metabolic cycle (Tu et al. 2005; Lelandais et al. 2009).
Complex mechanisms for mRNA localization
Regardless of why some mRNAs are localized to mitochondria while others are not, the example of ATP2 demonstrates the importance of mRNA targeting for mitochondrial biogenesis. How are localized mRNAs brought and tethered to the organelles? mRNA 3′-UTRs contain information for localization in at least eight cases that have been experimentally examined (Corral-Debrinski et al. 2000; Marc et al. 2002; Margeot et al. 2002).
One factor with apparent roles in localization of many mRNAs encoding mitochondrial proteins is Puf3, a member of the Pumilio-homology domain family (PUF) of RNA-binding proteins. PUF family proteins are found in a wide variety of eukaryotes and carry out a wide variety of functions through their ability to mediate interactions between target RNAs and other proteins (Quenault et al. 2011). An initial survey of yeast PUF protein functions (Olivas and Parker 2000) revealed that a puf3Δ mutation strongly affected the COX17 mRNA, which encodes a (Eukarya-specific) mitochondrial copper-binding protein required for cytochrome c oxidase assembly (Glerum et al. 1996). The presence of Puf3 was shown to stimulate deadenylation and degradation of COX17 mRNA, but did not affect its translation as measured by the degree of polysome association or the level of accumulated Cox17 protein.
A genomic investigation of RNAs bound to Puf3 (as well as other members of the yeast Puf protein family) revealed a striking specificity: among 154 Puf3-binding mRNAs of known function, 135 encoded mitochondrial proteins (Gerber et al. 2004). Furthermore, mitochondrial proteins coded by 80 of the Puf3-binding mRNAs have roles in organellar translation (e.g., mitochondrial ribosomal proteins) while most of the rest participate in post-translational assembly functions. A Puf3-binding sequence was identified in the 3′-UTRs of these mRNAs (Gerber et al. 2004). This site occurs twice in the COX17 mRNA, and those sites are necessary for Puf3-dependent mRNA destabilization (Jackson et al. 2004).
The Puf3 protein itself was found to be located on the outer surface of mitochondria and visualized in puncta largely associated with mitochondrial tubules (Garcia-Rodriguez et al. 2007). Puf3 was also associated with Mdm12, a protein component of the tether that connects distinct sites on mitochondria with the endoplasmic reticulum (Garcia-Rodriguez et al. 2007; Kornmann et al. 2009). Consistent with a role in promoting degradation of mRNAs required for respiratory metabolism, and thereby affecting the production of respiratory complexes, overproduction of Puf3 caused a modest reduction in the growth of cells on the nonfermentable carbon source glycerol (Garcia-Rodriguez et al. 2007) and oxygen consumption (Chatenay-Lapointe and Shadel 2011). Log-phase cells lacking Puf3 contained elevated levels of respiratory complex subunits and exhibited increased rates of oxygen consumption (Chatenay-Lapointe and Shadel 2011). However, Puf3 appears to be more than simply a post-transcriptional repressor of mitochondrial functions since a puf3Δ also produces a very modest defect in growth on glycerol (Gerber et al. 2004).
Taken together, these findings suggest the possibility that Puf3 could have a direct role in localizing a number of mRNAs to the mitochondrial surface. Consistent with this possibility, a majority of those Puf3-binding mRNAs that encode known mitochondrial proteins (Gerber et al. 2004), and were examined for subcellular distribution (Marc et al. 2002), were among those selectively localized to mitochondria. Overall, it appears that about half of the mRNAs selectively localized to mitochondria contain Puf3-binding sites in their 3′-UTRs, and the localization of about half of those is significantly decreased in the absence of Puf3 (Saint-Georges et al. 2008). In addition, mutation of the Puf3-binding site in one such mRNA (BCS1) reduced its selective association with mitochondria by a factor of two as assayed both by quantitative PCR of the two polysome fractions and by quantitation of RNA granule location cytologically in FISH images (Saint-Georges et al. 2008) (Figure 3).
Similar results were obtained for a set of 24 mRNA-encoding mitochondrial proteins that were tagged with binding sites for an RNA-binding GFP fusion protein and visualized in granules (Gadir et al. 2011). These images suggest the possibility that mRNAs bound to the surface of mitochondria may not be evenly distributed on the organellar surface. Experiments to test whether these RNA granules colocalize with Puf3 puncta on mitochondria have not been reported. However, if they do, the fact that Puf3 associates with the mitochondrial-ER tether protein Mdm12 (Garcia-Rodriguez et al. 2007; Kornmann et al. 2009) would be consistent with reported partial colocalization of mitochondrial mRNA granules with ER (Gadir et al. 2011). Such localization of protein synthesis and presumably import could facilitate assembly of mitochondrial complexes, for example, mitochondrial ribosomes (Saint-Georges et al. 2008).
It is important to bear in mind that Puf3 promotes degradation of at least some mitochondrially localized mRNAs (Olivas and Parker 2000; Jackson et al. 2004; Foat et al. 2005). Thus, even if Puf3 were not directly involved in localization, an mRNA stabilized in the absence of Puf3, or by mutation of its Puf3-binding site, could appear to be less selectively bound to mitochondria as measured by the ratio of its presence in bound vs. free polysomes. This would occur if an RNA’s abundance increased sufficiently to saturate other limiting localization factors on the organelle surface. The extent to which altered RNA stability may contribute to Puf3 dependence of localization has not been systematically explored. Nevertheless, it seems likely that Puf3 binding contributes directly to localization of those mRNAs bearing its binding site in addition to influencing their rates of degradation. How the interplay between these two activities influences protein import and assembly of mitochondrial complexes remains an open question (Quenault et al. 2011).
The existence of distinct mechanisms for RNA sequence-based mRNA recognition is indicated by the fact that the ATM1 and ATP2 mRNAs, whose 3′-UTRs clearly cause mitochondrial localization, lack Puf3-binding sites (Corral-Debrinski et al. 2000; Margeot et al. 2002; Saint-Georges et al. 2008). Selection of variant sequences derived from the ATP2 3′-UTR that functionally localize the mRNA suggest that both nucleotide sequence and secondary structural features play a role in its recognition (Liu and Liu 2007). However, no protein or other species that interact with this RNA element have been identified (Figure 3). Interestingly, the absence of Puf3 may reduce mitochondrial localization of the ATP2 mRNA, presumably by an indirect mechanism (Gadir et al. 2011), although this observation is inconsistent with an earlier report (Saint-Georges et al. 2008).
Tethering of mRNAs to mitochondria via nascent polypeptide chains
Actively translated mRNAs can be tethered to membranes via nascent polypeptide chains undergoing cotranslational membrane translocation. This appears to occur in the case of at least some mRNAs localized to mitochondria. A chimeric mRNA encoding the Atm1 N-terminal mitochondrial targeting signal fused to GFP, but with the ATM1 3′-UTR replaced by the PGK1 mRNA 3′-UTR, localized to mitochondria (Corral-Debrinski et al. 2000). Thus, the wild-type ATM1 mRNA on mitochondria appears to be localized both by an untranslated signal in its 3′-UTR and by the interaction of the polypeptide targeting signal with receptors on the outer mitochondrial surface and the protein import machinery.
In the case of an ATP2 mRNA lacking its normal 3′-UTR, residual localization to mitochondria required translation of both the N-terminal targeting sequence and sequences within the mature protein itself (Garcia et al. 2010). Normal association of the wild-type ATP2 mRNA also required one of the three Translocase of the Outer Membrane (TOM) complex outer membrane import receptor proteins, Tom70 (Table 1), and was reduced by mutation of the ATP2 translation initiation codon (Gadir et al. 2011).
Table 1 . Components of the TOM complex: transport of proteins through the outer membrane.
| Protein | ORF | Known function | Null phenotype |
|---|---|---|---|
| Mim1 | YOL026C | Insertion of transmembrane helix proteins into the outer membrane | Inviable |
| Mim2 | YLR099W-A | Insertion of transmembrane helix proteins into the outer membrane | Inviable |
| Tom5 | YPR133W-A | Viable, various defects | |
| Tom6 | YOR045W | Viable, various defects | |
| Tom7 | YNL070W | Viable, various defects | |
| Tom20 | YGR082W | Receptor for substrates with presequences | Viable, various defects |
| Tom22 | YNL131W | Central receptor facing cytoplasm and IMS; interaction with TIM23 complex | Viable, various defectsa |
| Tom40 | YMR203W | Translocation channel–β-barrel structure | Inviable |
| Tom70 | YNL121C | Receptor for substrates lacking presequences | Viable, various defects |
| Tom71 | YHR117W | Receptor | Viable, various defects |
Null was inviable in large-scale studies, but is viable if obtained by loss of TOM22 plasmid during mitotic growth (van Wilpe et al. 1999).
Deletion of another outer membrane import receptor protein, Tom20, was found to lower but not eliminate selective localization of most mRNAs associated with mitochondria (Eliyahu et al. 2010). While tom20Δ mutants are viable with a modest respiratory defect, and puf3Δ mutants are viable and almost wild type with respect to respiratory growth, a tom20Δ, puf3Δ double mutant was viable with a very tight respiratory defect. This synthetic respiratory phenotype is consistent with the picture of synergy in targeting of mRNAs to mitochondria by factors recognizing mRNA 3′-UTRs and the protein import machinery acting on nascent chains to promote efficient assembly of functional mitochondrial complexes. At the same time, the viability of the tom20Δ, puf3Δ double mutant demonstrates that protein import to mitochondria remains active by the action of partially redundant pathways for mRNA localization and precursor recognition.
Translocation and Membrane Insertion of Cytoplasmically Synthesized Mitochondrial Proteins
The distant ancestors of mitochondria were bacteria from the α-proteobacterial lineage (Gray et al. 2001). While the origins of all known extant eukaryotes trace back to organisms that contained both mitochondria and nucleo-cytoplasmic genetic systems related to Archaea, the events leading to endosymbiosis and the subsequent evolution of mitochondria as integrated cellular organelles have not been clearly discerned (Embley and Martin 2006). However, since bacteria are not known to import large polypeptides, their evolution into mitochondria apparently required the evolution of new mechanisms for the transport of cytoplasmically synthesized proteins across one or both of the mitochondrial (formerly bacterial) membranes. Some components of the present-day protein import machinery are clearly of bacterial origin. However, most appear to have distant bacterial homologs that do not participate in protein translocation or to have evolved de novo as endosymbionts became organelles (Dolezal et al. 2006; Kutik et al. 2009; Hewitt et al. 2011).
Transport of cytoplasmically synthesized mitochondrial proteins or their precursors across or into the outer membrane is carried out by the TOM complex, which includes both receptor proteins facing the cytoplasm and a pore in the membrane (Table 1). It is widely believed that the precursor proteins arrive at the organelle bound by chaperones and, in that state, are recognized by receptors of the TOM complex, although this has been demonstrated in only a few cases (Gautschi et al. 2001; Young et al. 2003).
Depending upon the nature of their targeting signals, proteins may be inserted into the outer membrane, translocated into the intermembrane space (IMS), or delivered to one of the two Translocase of the Inner Membrane (TIM) complexes for insertion into the inner membrane or translocation into the matrix (Pon and Schatz 1991; Neupert 1997; Voos et al. 1999). (While the nature of targeting signals for different compartments has been investigated intensively, it is important to note that they cannot be predicted solely from sequence information with a high degree of certainty.) A wide variety of different translocation and sorting events must be completed prior to, or concomitant with, the assembly of imported proteins into functional multimeric enzymes and higher-order complexes.
The literature on import of proteins into yeast mitochondria is extensive and has been extensively reviewed. Recent reviews present detailed descriptions of the components of import complexes and their functions (Young et al. 2003; Neupert and Herrmann 2007; Chacinska et al. 2009; Koehler and Tienson 2009; Mokranjac and Neupert 2009; Walther and Rapaport 2009; Endo and Yamano 2010; Schmidt et al. 2010; Dukanovic and Rapaport 2011; Gebert et al. 2011; Hewitt et al. 2011; Marom et al. 2011a; Riemer et al. 2011; Yogev and Pines 2011). Molecular structures of hydrophilic domains of proteins composing the import machinery are emerging, but as yet no full structures of mitochondrial translocation complexes are available (Endo et al. 2011), precluding, for the most part, precise biochemical descriptions of mechanisms. Outlined below are the routes taken by cytoplasmically synthesized proteins destined for the outer membrane, the intermembrane space, the inner membrane, and the matrix. The known pathways to these compartments overlap for most proteins as they traverse the outer membrane, but then become distinct.
Insertion of proteins into the outer membrane
All proteins entering mitochondria first encounter pre-existing outer membrane proteins and lipids. Thus, outer membrane proteins are crucial for all import, including the biogenesis of the outer membrane itself. All outer membrane proteins are synthesized in the cytoplasm, and none are known to be proteolytically cleaved during import or assembly (Schmidt et al. 2010). The signals that target these proteins are poorly understood but appear to reside in transmembrane domains (Mokranjac and Neupert 2009; Walther and Rapaport 2009).
Import and insertion of β-barrel proteins:
The overwhelming majority of cytoplasmically synthesized proteins that become incorporated into mitochondrial structures first associate with the organelles by interaction with the TOM complex in the outer membrane (Endo and Yamano 2010). Among them are the integral proteins of the mitochondrial outer membrane with β-barrel structures, including the most abundant, Por1 (porin) (Riezman et al. 1983), a voltage-gated anion channel. Another key β-barrel protein is Tom40 (Baker et al. 1990), which forms the TOM complex pores in the outer membrane through which most imported proteins pass (Hill et al. 1998; Künkele et al. 1998).
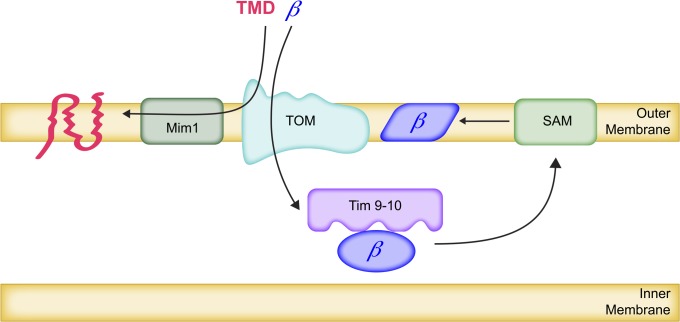
Both of these β-barrel proteins are translated on free cytoplasmic polysomes (Saint-Georges et al. 2008) and directed to mitochondria by unknown signals and mechanisms (Mokranjac and Neupert 2009). It is also unknown whether they arrive at the TOM complex associated with cytoplasmic chaperones. In any event, these apparently unfolded β-barrel proteins pass through the Tom40 pore of the TOM complex after interaction with the TOM receptor subunits Tom20 and Tom22 (Krimmer et al. 2001; Model et al. 2001) (Figure 4).
Figure 4 .
Insertion of proteins into the outer membrane. β-Barrel proteins are imported through the pores of the TOM complex in the outer membrane and then bound by IMS chaperone complexes comprising Tim9 and Tim10. The β-barrel-Tim9-Tim10 complexes bind to the inner surfaces of SAM complexes in the outer membrane, leading to insertion of β-barrel proteins into the outer membrane lipid bilayer. Some integral outer membrane proteins with multiple transmembrane domains (TMD) contact the Tom70 receptor and are then inserted into the bilayer from the outside through their interaction with multimeric complexes of Mim1.
At this point, the β-barrel proteins are present in the IMS, which is homologous to the periplasm of Gram-negative bacteria. In this soluble milieu, they are bound by hetero-hexameric chaperone complexes composed of the small proteins (∼100 amino acids each) Tim9-Tim10 and of Tim8-Tim13 (Hoppins and Nargang 2004; Wiedemann et al. 2004) (Table 2). The crystal structure of the Tim9-Tim10 hexamer reveals a propeller arrangement with 12 mobile α-helical tentacles descending from a core stabilized by intramolecular disulfide bonds in each subunit (Baker et al. 2009). The N-terminal tentacle of Tim9 is especially important for substrate binding in vivo. These chaperones accompany the β-barrel proteins back to the inner surface of the outer membrane where they are delivered to the Sorting and Assembly Machinery (SAM) complex, embedded in the outer membrane (also known as TOB for Topogenesis of β-Barrel proteins) (Paschen et al. 2003; Wiedemann et al. 2003a) (Table 3). The Sam35 component of the SAM complex recognizest β-barrel proteins by virtue of an amino acid sequence near their C termini (Wiedemann et al. 2003a; Kutik et al. 2008). The β-barrel proteins associate with the SAM complex from the IMS side and are then inserted laterally into the outer membrane lipid bilayer by an as-yet-unknown mechanism, where they assume β-barrel structure (Stroud et al. 2011) (Figure 4).
Table 2 . IMS import chaperones: delivery of hydrophobic proteins to the SAM complex (outer membrane) or TIM22 complex (inner membrane).
| Protein | ORF | Known function | Null phenotype |
|---|---|---|---|
| Tim8 | YJR135W-A | Complexed with Tim13 | Viable, various defects |
| Tim9 | YEL020W-A | Complexed with Tim10 | Inviable |
| Tim10 | YHR005C-A | Complexed with Tim9 | Inviable |
| Tim12 | YBR091C | Associated with Tim22 complex | Inviable |
| Tim13 | YGR181W | Complexed with Tim8 | Viable, various defects |
Table 3 . Components of the SAM complex: insertion of β-barrel proteins into the outer membrane.
| Protein | ORF | Known function | Null phenotype |
|---|---|---|---|
| Mdm10 | YAL010C | Viable, various defects | |
| Sam35 | YHR083W | Receptor | Inviable |
| Sam37 | YMR060C | Viable, various defects | |
| Sam50 | YNL026W | β-Barrel protein of SAM complex | Inviable |
The essential core component of the SAM complex, Sam50, is itself a β-barrel protein. Thus, the assembled and functional β-barrel proteins Tom40 and Sam50 are necessary for import and assembly of newly synthesized Tom40 and Sam50, as well as that of other outer membrane β-barrel proteins. Sam50 is homologous to the bacterial outer membrane protein Omp85, which has a similar function in the insertion of β-barrel proteins in the outer membrane of Gram-negative bacteria (Paschen et al. 2003; Gentle et al. 2004).
Insertion of other integral proteins into the outer membrane:
In addition to β-barrel proteins, the outer membrane contains integral proteins anchored in the lipid bilayer by one or more individual transmembrane domains. There appear to be multiple pathways for such proteins, and they are not well understood at present. At least some integral outer membrane proteins are exceptional in that they do not traverse the membrane via the Tom40 pore of the TOM complex. In the case of proteins with multiple membrane-spanning helices, the newly synthesized polypeptides first contact the outer membrane via the Tom70 receptor, but are then inserted into the bilayer from the outside, independently of Tom40, through their interaction with the multimeric complexes of Mim1 (Becker et al. 2011; Papic et al. 2011). Mim1 is a short (113 amino acids) single-spanning outer membrane protein (Ishikawa et al. 2004; Waizenegger et al. 2005) that forms dimers that organize into higher-order complexes (Popov-Čeleketić et al. 2008b) that were recently also shown to contain a second protein, Mim2 (YLR099W-A) (Dimmer et al. 2012). These Mim1-Mim2 complexes appear to have a membrane insertase function (Figure 4).
Mim1 is also required for insertion of at least some proteins with a single transmembrane domain near the their N termini, often termed signal anchored proteins. These include the outer membrane receptor proteins Tom20 and Tom70 (Becker et al. 2008; Hulett et al. 2008; Popov-Čeleketić et al. 2008b). Interestingly, insertion of these receptors does not depend upon their own receptor function (Ahting et al. 2005).
Mim1 itself has a conserved, centrally located transmembrane domain that is partially functional even in the absence of both flanking hydrophilic domains (Popov-Čeleketić et al. 2008b). The Mim1 C-terminal domain is exposed to the cytoplasm (Lueder and Lithgow 2009; Walther and Rapaport 2009). The pathway that Mim1 takes into the outer membrane has not yet been studied.
There is apparently at least one additional pathway into the outer membrane employed by proteins anchored in the membrane by a single transmembrane domain at their C termini, the so-called “tail-anchored proteins.” In the case of the tail-anchored protein Fis1, required for normal mitochondrial fission, outer membrane insertion is independent of all known components of the TOM and SAM complexes (Kemper et al. 2008). Furthermore, the insertion of Fis1 into lipid vesicle membranes with a low ergosterol content resembling the mitochondrial outer membrane indicates that lipid content may play a role in specificity in vivo. A possible role for Mim1 in Fis1 insertion was not tested. Another tail-anchored protein, the essential receptor Tom22, enters the membrane through the direct or indirect action of the SAM complex, after being recognized on the surface by TOM receptors (Stojanovski et al. 2007). Thus, the SAM complex may not be specific for the insertion of β-barrel proteins and may recognize substrates on either side of the membrane. The insertion of Tom22 is not dependent upon Mim1 (Becker et al. 2008).
Recently, evidence indicating the possibility of lateral diffusion of transmembrane domains out of the TOM complex has been reported (Harner et al. 2011b). Chimeric fusion proteins were trapped across the outer membrane by the folded structure of GFP on the outside and the multispanning inner membrane protein Tim23 inserted in the inner membrane. Fusion proteins with transmembrane domains accessible to the outer membrane were released from the TOM complex by an unknown mechanism. It remains to be determined whether any endogenous mitochondrial proteins employ this route into the outer membrane.
Import of proteins into the IMS
There are at least three mechanisms by which proteins are localized to the intermembrane space. Two involve covalent modifications of precursors after transit across the outer membrane by enzymes located in the intermembrane space there. The modifications stabilize folded structures that prevent retrograde transport out of the organelle. Trapping by noncovalent bonds may also occur in some cases. Finally, as discussed below in conjunction with transport to the inner membrane, some proteins are first targeted to the inner membrane and then released into the IMS by proteolytic cleavage.
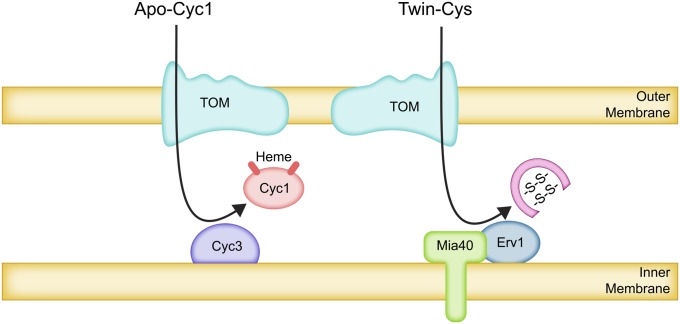
Covalent attachment of heme:
Cytochrome c (Cyc1 and Cyc7), which is located in the IMS, is perhaps the most intensively genetically analyzed S. cerevisiae protein (Sherman 2005). Surprisingly, the import of cytochrome c to the IMS is still relatively poorly understood. Cyc1 is largely synthesized on mitochondria-bound polysomes (Saint-Georges et al. 2008) and requires the TOM complex to traverse the outer membrane in a reaction that does not require ATP or an inner membrane potential (Diekert et al. 2001; Wiedemann et al. 2003b). However, the mechanism by which the TOM complex translocates apo-cytochrome c remains enigmatic. Blockage of the Tom40 pores used by other TOM substrates does not prevent import of apo-cytochrome c into membrane vesicles containing purified TOM complexes. Furthermore, removal of cytosolic domains of the TOM receptor subunits did not affect apo-cytochrome c import into mitochondria (Wiedemann et al. 2003b; Yamano et al. 2008). Nevertheless, complete removal of the Tom22 receptor did prevent import (Wiedemann et al. 2003b). Thus, Tom22 domains within the outer membrane or exposed on its inner surface may play a role in this as-yet-enigmatic translocation process. This behavior of apo-cytochrome c is unique among studied proteins.
Once in the IMS, apo-cytochrome c binds with the cytochrome c heme lyase, Cyc3, which is itself bound peripherally to the outer surface of the inner membrane (Dumont et al. 1991; Steiner et al. 1995; Bernard et al. 2005). Apo-cytochrome c is then irreversibly trapped by the covalent attachment of heme, which forms mature cytochrome c (Dumont et al. 1991) (Figure 5). The first 27 amino acids of cytochrome c contain the residues required for heme attachment, and they appear to be required for import (Wang et al. 1996). It is not clear whether this region is required for interaction with the TOM complex in addition to the heme lyase. In any event, apo-cytochrome c does not selectively partition to mitochondria in the absence of Cyc3, or if cyc1 mutations block the heme lyase reaction, although small amounts are associated with the organelles (Dumont et al. 1991). Interestingly, even when cyc1 mutations block heme attachment, overexpression of heme lyase increases partitioning of the mutant apo-cytochrome c to mitochondria, suggesting that protein–protein interactions alone initially sequester it (Dumont et al. 1991).
Figure 5 .
Trapping of proteins in the IMS by covalent modification. Apo-cytochrome c (Cyc1) traverses the outer membrane via the TOM complex by an unusual and poorly understood mechanism (see text). Covalent attachment of heme by the lyase (Cyc3), bound to the outer surface of the inner membrane, generates holo-cytochrome c. Holo-cytochrome c cannot translocate through the TOM complex and remains in the IMS. In an analogous mechanism, IMS proteins with twin-Cys residue pairs in reduced form are imported through the TOM complex and then oxidized by the Mia40-Erv1 disulfide relay system bound to the inner membrane. The internal disulfide bonds formed in the twin-Cys proteins prevent reverse translocation.
The cytochrome c heme lyase Cyc3 is itself imported into the intermembrane space via the TOM complex (Steiner et al. 1995). Based on studies with Neurospora crassa, a conserved targeting signal for heme lyases has been identified within its amino acid sequence, and this 60-residue region can target passenger proteins to the IMS (Diekert et al. 1999). This signal is believed to interact with cis and trans sites of the TOM complex and may also direct binding to unknown components of the inner membrane that could anchor it in the IMS.
Oxidation of paired cysteine residues to form disulfide bonds:
A second form of covalent modification that sequesters some IMS proteins is the generation of internal disulfide bonds between paired cysteine residues (CX3C or CX9C motifs) after import. There are at least 24 such proteins (Koehler and Tienson 2009), including the small chaperone proteins Tim8, Tim9, Tim10, Tim12, and Tim13 whose folded structures are known to be stabilized by intramolecular disulfide bonds (Baker et al. 2009). Like the Gram-negative bacterial periplasmic space (Messens and Collet 2006), the IMS is a more oxidizing environment than the cytoplasm (Hu et al. 2008) and contains enzymatic machinery for the controlled generation of intramolecular disulfide bonds (Koehler and Tienson 2009; Herrmann and Riemer 2012).
Some IMS proteins with paired Cys residues are synthesized on mitochondria-bound polysomes (e.g., Pet191, Cox23, Cox17), while others (e.g., Tim9, Tim13) are not (Saint-Georges et al. 2008). It is not clear what directs these proteins to mitochondria (Riemer et al. 2011). Import of Tim13 does not depend upon surface receptors of the TOM complex, but it apparently does enter mitochondria through the Tom40 pore of the TOM complex (Lutz et al. 2003). The Tom5 subunit of the TOM complex is also required (kurz et al. 1999). Chemical modification or mutation of the Cys residues prevented accumulation of Tim13 in the IMS (Lutz et al. 2003). Uptake of Tim10 was blocked if Cys residues were oxidized prior to import (Lu et al. 2004).
Mia40 is an essential protein bound to the outer surface of the inner membrane that is required for import of the essential Tim9-Tim10 chaperones and other twin-Cys proteins to the IMS (Chacinska et al. 2004; Naoe et al. 2004). These imported proteins associate with Mia40 via disulfide bonds. As one would expect, import of cytochrome c does not require Mia40 (Chacinska et al. 2004). A short peptide sequence containing a single Cys residue has been identified in several twin-Cys proteins that directs them to Mia40 in the IMS and binds covalently to it via a disulfide bond (Milenkovic et al. 2009; Sideris et al. 2009). This signal may also promote passage from the cytoplasm to the IMS, but it is not clear what outer membrane surface component could be involved in this recognition. The interaction of newly imported reduced twin-Cys substrates with oxidized Mia40 promotes folding of the substrate proteins and the formation of disulfide bonds, trapping the folded proteins in the intermembrane space (Banci et al. 2010) (Figure 5).
Reduced Mia40 is oxidized in turn by the essential intermembrane space protein Erv1, a conserved flavin-linked sulfhydryl oxidase (Mesecke et al. 2005). Electrons from the resulting reduced Erv1 can be accepted by cytochrome c and enter the respiratory chain or be accepted by molecular oxygen to form hydrogen peroxide that is metabolized by cytochrome c peroxidase (Bihlmaier et al. 2007; Dabir et al. 2007). This disulfide relay system has been reconstituted in vitro (Tienson et al. 2009).
Although Erv1 does not contain the CX3C or CX9C motifs present in the other substrates of this system, its import does depend upon Mia40 action following TOM-dependent passage through the outer membrane (Terziyska et al. 2007). Interestingly, the import of another protein located partially in the IMS and lacking the closely paired Cys residue, Ccs1, depends upon Mia40 to form a disulfide bond, but that bond is not necessary for enzymatic activity (Gross et al. 2011; Klöppel et al. 2011). The import pathway followed by Mia40, and other proteins anchored in the inner membrane with hydrophilic domains in the IMS, will be described below.
Import of proteins into the inner membrane
The mitochondrial inner membrane contains a very wide variety of integral proteins. All studied cytoplasmically synthesized inner membrane proteins are recognized by receptors of the TOM complex and imported through its pores. They are then inserted into the inner membrane by one of three mechanisms, or combinations of them, depending upon the signals that they contain and their ultimate topology.
Insertion of metabolite carriers and other multispanning inner membrane proteins by the TIM22 insertase/translocase complex:
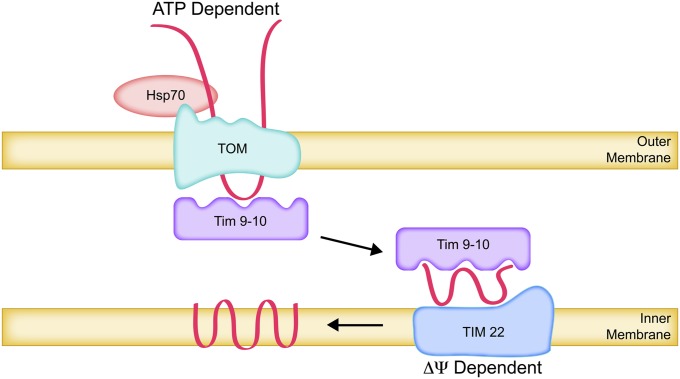
A major class of inner membrane proteins are imported and assembled into multispanning topologies without being proteolytically processed. At least 34 of these proteins are members of the metabolite carrier family (Palmieri et al. 2006), which includes the ATP/ADP carriers. Two other such proteins are Tim22 and Tim23, the essential pore-forming subunits of the TIM complexes described below.
These multispanning membrane proteins contain multiple internal mitochondrial targeting signals that generally flank transmembrane domains (Neupert and Herrmann 2007; Chacinska et al. 2009). Newly synthesized yeast carrier proteins have been shown to associate with the cytoplasmic chaperone Hsp70, which participates in their recognition by the Tom70 receptor subunit of the TOM complex (Young et al. 2003; Endo and Yamano 2010). ATP-dependent release from the Hsp70 chaperones allows the carrier proteins to enter the TOM complex pore (Figure 6). The N and C termini initially remain on the outside while internal regions containing recognition signals traverse the TOM complex as looped polypeptide chains through the Tom40 pore in the outer membrane (Wiedemann et al. 2001; Neupert and Herrmann 2007; Chacinska et al. 2009). The Tom40 pore appears to have specific interactions with different imported proteins and thus may also play an active role in substrate recognition (Gabriel et al. 2003; Sherman et al. 2006).
Figure 6 .
Insertion of multi-spanning carrier proteins into the inner membrane. Newly synthesized multi-spanning carrier proteins, complexed with cytoplasmic Hsp70, are recognized by the Tom70 receptor subunit of the TOM complex. ATP-dependent release from cytoplasmic Hsp70 leads to translocation through the TOM complex in a looped configuration and binding to the Tim9-Tim10 IMS chaperone complex. The multi-spanning proteins are delivered to the TIM22 insertase complex in the inner membrane, released from Tim9-Tim10, and inserted into the inner membrane by reactions that depend upon the Δψ potential across the inner membrane.
On the inside of the outer membrane, incoming carrier proteins are removed from the TOM complex by binding to the soluble essential Tim9-Tim10 chaperone complexes (Koehler et al. 1998; Sirrenberg et al. 1998; Curran et al. 2002; Vasiljev et al. 2004). Incoming Tim23 is preferentially bound by the homologous but dispensable Tim8-Tim13 chaperone complex (Davis et al. 2000; Paschen et al. 2000). The hydrophobic client proteins are thus transported through the aqueous intermembrane space (Figure 6). Their destination in the inner membrane is the TIM22 insertase/translocase complex (Sirrenberg et al. 1996; Kerscher et al. 1997) (Table 4). This complex has at its core a voltage-gated pore formed by the essential Tim22 protein (Kovermann et al. 2002), which is required for its own membrane insertion (Sirrenberg et al. 1998).
Table 4 . Components of the TIM22 complex: insertion of multispanning carrier proteins into the inner membrane.
| Protein | ORF | Known function | Null phenotype |
|---|---|---|---|
| Tim18 | YOR297C | Viable, various defects | |
| Tim22 | YDL217C | Core insertase of the complex | Inviable |
| Tim54 | YJL054W | Viable, various defects |
Carrier proteins, or other substrates, associated with the IMS chaperones Tim9-Tim10 or Tim8-Tim12 bind to the TIM22 complex on the outer surface of the inner membrane. The substrates are dissociated from the chaperones, a process that may involve redox reactions (Curran et al. 2004). Uptake of the substrates into the TIM22 complex, translocation of substrate domains through the membrane, and insertion of transmembrane domains into the membrane require the Δψ-membrane potential but not ATP (Rehling et al. 2003; Peixoto et al. 2007). The mechanistic details of these reactions are not well understood.
Functional carrier protein dimers appear to self-assemble in the membrane rapidly following insertion (Dyall et al. 2003). Tim22 is assembled into the TIM22 complex along with subunits that are inserted into the inner membrane by the pathway described below for proteins with cleavable presequences (Wagner et al. 2008).
Insertion of inner membrane spanning proteins with cleavable presequences by the TIM23 insertase/translocase complex:
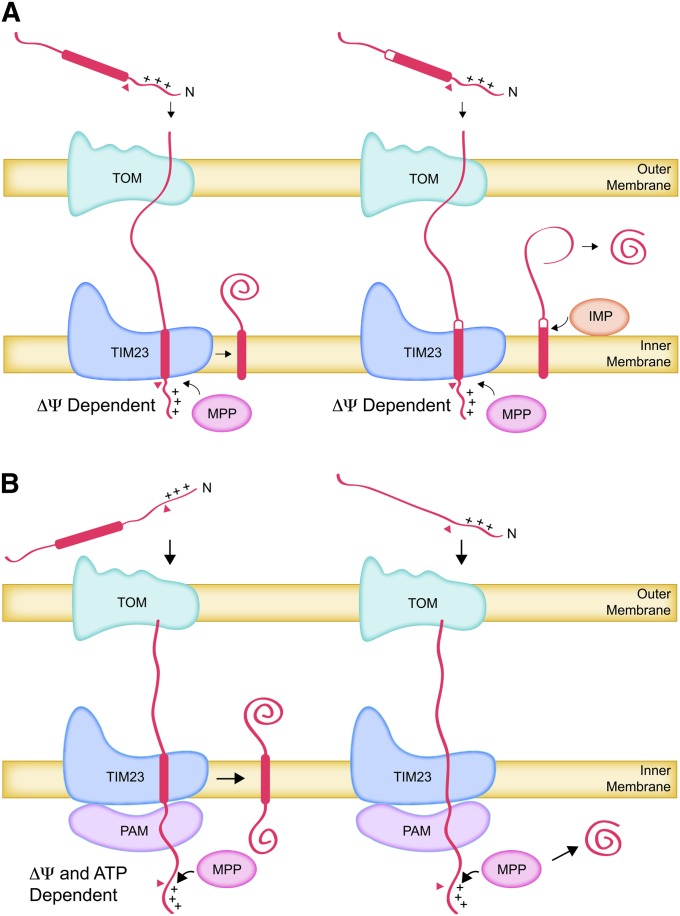
Sixty percent or more of all yeast mitochondrial proteins are synthesized as precursors whose N termini are cleaved during import (Vögtle et al. 2009). The N-terminal presequences typically contain targeting signals comprising amphipathic α-helices with positively charged and hydrophobic surfaces, although there is no consensus sequence. These “classical” targeting signals, which were reviewed in the previous edition of YeastBook (Pon and Schatz 1991) and elsewhere (Neupert 1997; Voos et al. 1999), are sufficient on their own to target proteins to the matrix, as discussed in the section below. However, these amphipathic α-helices can also be combined with nearby downstream hydrophobic sorting signals to form bipartite signals that direct proteins to the inner membrane (Figure 7A). Mia40 is such a protein, anchored in the inner membrane by the N-terminal hydrophobic sorting signal with its hydrophilic domains facing the IMS (Naoe et al. 2004; Neupert and Herrmann 2007). In addition, at least two well-studied IMS proteins, cytochrome b2 (Cyb2) and cytochrome c1 (Cyt1), adopt the same topology before being released from their N-terminal membrane anchors by the inner membrane protease (IMP: Imp1, Imp2, Som1) (Glick et al. 1992; Nunnari et al. 1993; Jan et al. 2000). In the case of Cyt1, a second internal sorting sequence near the C terminus inserts into the inner membrane by an unknown mechanism anchoring the hydrophilic N-terminal domain on the intermembrane space side (Arnold et al. 1998; Lange and Hunte 2002).
Figure 7 .
Import of proteins with amphipathic positively charged cleavable presequences. (A) ΔΨ-Dependent transport of proteins containing hydrophobic sorting signals (thick bars) to the inner membrane and IMS. The presequences are recognized by the Tom22 receptor of the TOM complex, pass through the TOM complex, and are recognized by the Tim50 subunit of the TIM23 complex. The presequence translocates through the TIM23 complex, driven electrophoretically by ΔΨ. This brings the hydrophobic sorting signal, located immediately downstream of the presequence, into the TIM23 complex. The presequence is removed by proteolysis and the sorting signal is inserted laterally into the inner membrane where it can function as a membrane anchor (left). Proteolytic cleavage at the outer surface of the inner membrane can release a soluble protein into the inner membrane space (right). (B) ΔΨ- and ATP-dependent transport to the inner membrane and the matrix. Presequences traverse the TOM and TIM23 complexes and are removed by proteolysis, as in A. If there is no hydrophobic sorting signal immediately downstream of the presequence, the incoming polypeptide is engaged by the PAM complexed with the inner surface of the TIM23 translocon. ATP hydrolysis by the Hsp70 (Ssc1) subunit of PAM translocates the polypeptide into the matrix. If a downstream hydrophobic sorting signal enters the TIM23 complex, it is released laterally into the inner membrane and translocation ceases (left). If there is no sorting signal, the entire polypeptide is translocated into the matrix (right).
Different presequence-containing proteins are synthesized on free or mitochondria-bound polysomes (the precursors of Mia40, Cyb2, and Cyt1 are all synthesized on mitochondria-bound polysomes) (Saint-Georges et al. 2008). There is relatively little information on the binding of cytoplasmic chaperones to presequence-containing precursors. However, in at least some cases, cytoplasmic Hsp70 (Ssa1–4) is required for import. This is thought to reflect the ability of Hsp70 to maintain precursors in partially unfolded states (Deshaies et al. 1988; Gautschi et al. 2001; Sass et al. 2003; Endo and Yamano 2010). While the pathways taken by these precursors to the outer surface of mitochondria are poorly understood, their pathways into the organelle have been the subject of intensive research.
A domain of the TOM receptor subunit Tom20 on the cytoplasmic side of the outer membrane recognizes the hydrophobic surfaces of presequence amphipathic α-helices (Abe et al. 2000; Yamamoto et al. 2011). The presequences are in turn bound by the Tom22 receptor subunit via electrostatic interactions and directed into the pore formed by Tom40 (Schmidt et al. 2010; Shiota et al. 2011). The Tom70 subunit is not thought to play a major role in recognition of presequence-containing precursors. However, while yeast cells survive without either Tom70 or Tom20, deletion of both is lethal, indicating that they can carry out redundant functions (Ramage et al. 1993).
The IMS side of the TOM complex interacts transiently with the major TIM complex, whose essential pore-forming subunit is Tim23 (Chacinska et al. 2009; Mokranjac and Neupert 2009; Marom et al. 2011a). This TIM23 complex (Table 5) has an essential receptor, Tim50, that seals the Tim23 pore in the absence of a substrate protein, preserving the inner membrane potential (Meinecke et al. 2006). Tim50 recognizes presequences emerging from the TOM complex and facilitates their transit to the pore (Yamamoto et al. 2002; Mokranjac et al. 2009; Tamura et al. 2009; Marom et al. 2011b; Schulz et al. 2011) in a reaction that must occur at translocation contact sites between outer and inner membranes (Pon et al. 1989).
Table 5 . Components of the TIM23 complex: transport of polypeptides through the inner membrane and lateral insertion of membrane anchors into the inner membrane.
| Protein | ORF | Known function | Null phenotype |
|---|---|---|---|
| Tim17 | YJL143W | Inviable | |
| Tim21 | YGR033C | Viable various defects | |
| Tim23 | YNR017W | Pore formation | Inviable |
| Tim44 | YIL022W | Tethers PAM to TIM23 complex | Inviable |
| Tim50 | YPL063W | Substrate receptor and pore gating | Inviable |
Passage of the presequence into and through the TIM23 complex pore is electrophoretically driven by the inner membrane electrical potential, Δψ, which is negative inside (Pon and Schatz 1991; Chacinska et al. 2009; Mokranjac and Neupert 2009; Marom et al. 2011a). It is independent of ATP hydrolysis (Glick et al. 1992). This transit of the positively charged presequence through the TIM23 complex can bring the downstream hydrophobic sorting signal into the TIM23 pore if the distance between them is short (Figure 7A). The presence of the hydrophobic sorting signal in the TIM23 channel prevents further translocation of the polypeptide chain. The presequence, now located in the matrix, is removed by sequence-specific activity of the soluble mitochondrial processing protease (MPP) (Pon and Schatz 1991; Taylor et al. 2001; Vögtle et al. 2009).
The “stop-transfer” activity of the sorting signal also triggers a lateral release of the polypeptide from the TIM23 complex, resulting in its insertion into the lipid bilayer of the inner membrane (Neupert and Herrmann 2007; Chacinska et al. 2009; Marom et al. 2011a) (Figure 7A). This lateral insertion reaction can be reconstituted in vitro with purified TIM23 complex components in lipid vesicles containing the mitochondria-specific lipid cardiolipin (van der Laan et al. 2007). Once embedded in the membrane, the sorting signal functions as a membrane anchor that eventually sequesters the rest of the polypeptide in the IMS after its passage through the TOM complex.
In the case of inner membrane proteins whose stop-transfer hydrophobic sorting signal is far downstream of the positively charged presequence, Δψ-dependent translocation of the presequence alone will not bring the sorting signal into the TIM23 translocase. For such proteins, the intervening residues must be pulled into the matrix by the ATP-driven presequence translocase-associated motor (PAM) until the stop-transfer sequence enters the TIM23 channel (Figure 7B).
The catalytic heart of the PAM complex (Table 6) is the essential mitochondrial Hsp70 protein Ssc1 (mtHsp70) (Kang et al. 1990; Manning-Krieg et al. 1991; Chacinska et al. 2009; Marom et al. 2011a). This ATP-hydrolyzing chaperone is a very abundant soluble constituent of the matrix with diverse roles in protein folding and assembly (Craig 1993; Hartl 1996; Voos and Röttgers 2002; Fontanesi et al. 2010b; Marom et al. 2011a). A fraction of the mtHsp70 molecules are bound to the TIM23 complex on the matrix side via an essential subunit, Tim44, which also contacts the incoming polypeptide (Slutsky-Leiderman et al. 2007; Marom et al. 2011b). Hydrolysis of ATP by mtHsp70 bound to the incoming chain provides the energy for translocation, although the molecular mechanism by which the released energy causes movement of the polypeptide chain remains a matter of debate (Chacinska et al. 2009; Marom et al. 2011a). In any event, repeated cycles of mtHsp70 molecules binding to the incoming chain, ATP hydrolysis, and ADP release effectively pull the chain through the TIM23 complex (Figure 7B). These cycles require the action of the essential nucleotide exchange factor Mge1 and the essential J-domain co-chaperone protein Pam18 that promotes ATP hydrolysis and thus stabilize interaction between mtHsp70 and the incoming polypeptide chain. An essential J-domain-like protein, Pam16, stabilizes association of Pam18 with the TIM23 complex (Frazier et al. 2004; Pais et al. 2011) and may regulate Pam18 activity (Marom et al. 2011a).
Table 6 . Components of the PAM complex: ATP-dependent pulling of proteins through the TIM23 complex into the matrix.
| Protein | ORF | Known function | Null phenotype |
|---|---|---|---|
| Mdj2 | YNL328C | Viable, various defects | |
| Mge1 | YOR232W | Nucleotide release factor for Ssc1 | Inviable |
| Pam16 | YJL104W | Inviable | |
| Pam17 | YKR065C | Interaction between TIM23 and PAM complexes | Viable |
| Pam18 | YLR008C | J protein co-chaperone for Ssc1 | Inviable |
| Ssc1 | YJR045C | Hsp70; ATP hydrolysis drives import of substrates into matrix | Inviable |
Entry of a trailing stop-transfer sequence into the TIM23 channel prevents further PAM-dependent uptake of the polypeptide chain. It is not known whether this is due to a signal transduced from the channel to the PAM motor to cease the ATP-driven cycle or to the generation of an energy barrier that prevents further import. Lateral movement of the stop-transfer sequence from the TIM23 channel into the membrane can then anchor the inner membrane to a protein with hydrophilic domains in the matrix (Gärtner et al. 1995) (Figure 7B).
Insertion of multispanning inner membrane proteins with presequences:
Relatively few studies have focused on this class of proteins. Presequences that are present on precursors of three multispanning inner membrane proteins—Mdl1, Oxa1, and Cox18—enter mitochondria through the TOM complex and pass through the TIM23 complex in a Δψ-dependent fashion (Herrmann et al. 1997; Frazier et al. 2003; Reif et al. 2005; Bohnert et al. 2010). Completed import and topogenesis also requires ATP and the PAM motor complex. However, different mechanisms appear to be responsible for insertion of different transmembrane domains, and they are not well characterized.
Mdl1 is an ABC-cassette transporter in the inner membrane that has six transmembrane domains and is oriented with its N and C termini on the matrix side (Young et al. 2001). Its import and completed topogenesis depends upon the TOM and TIM23 complexes as well as upon the pulling action of the PAM motor complex (Reif et al. 2005). The insertion of its first two transmembrane domains appears to depend upon the stop transfer mechanism since their topogenesis requires the inner membrane Δψ, but does not require the pulling action of the PAM motor complex. However, insertion of the third and fourth transmembrane domains is more complex. These regions are apparently pulled across the inner membrane by the PAM motor and then inserted into the membrane from the inside by the action of Oxa1 (Reif et al. 2005). Oxa1 is an inner membrane translocase/insertase known to export mitochondrially synthesized protein domains from the matrix (Bonnefoy et al. 2009). Since Oxa1 is homologous and functionally similar to bacterial YidC proteins, the insertion of imported domains back into the inner membrane from the inside is often referred to as “conservative sorting.”
Oxa1 has five transmembrane domains and is oriented in the inner membrane with its N terminus in the IMS and its C terminus in the matrix (Bonnefoy et al. 2009). During its import, the first Oxa1 transmembrane domain appears to cross the inner membrane, following the presequence. In a second step, the N-terminal domain is re-exported, concomitant with insertion of the first transmembrane domain by the translocase activity of pre-existing Oxa1 itself (Herrmann et al. 1997). It is not clearly established whether the other transmembrane domains are imported and then inserted from inside, transferred laterally into the membrane from the TIM23 complex, or perhaps inserted via some other pathway (Herrmann et al. 1997). The bacterial homolog of Oxa1, YidC, can promote lateral insertion of transmembrane domains from the Sec translocase into the bilayer (Dalbey and Kuhn 2004). This suggests the possibility that Oxa1 could carry out an analogous function with some substrates, in conjunction with the TIM23 complex (Reif et al. 2005). It is clear that Oxa1 cannot be absolutely required for its own topogenesis since nonrespiring oxa1Δ mutants can be restored to normal phenotype by reintroduction of a wild-type OXA1 gene (Bonnefoy et al. 1994).
Import of presequence-containing proteins to the matrix
A large fraction of presequence-containing precursors are targeted to the innermost mitochondrial compartment, the matrix. They contain amphipathic α-helices in their presequences but no stop-transfer sorting signals (Pon and Schatz 1991). Following synthesis on either bound or free polysomes, they traverse the TOM and TIM23 complexes as described above. After Δψ-dependent uptake of the presequence, ATP-dependent action of the PAM complex pulls the entire polypeptide into the matrix.
The pulling of entire proteins into the matrix by PAM depends upon at least partial unfolding of the C-terminal domains that are often still on the cytoplasmic side of the TOM complex when PAM engages the N-terminal end. This has been clearly demonstrated in in vitro reactions (Pon and Schatz 1991). The importance of this in vivo is demonstrated by the import of Fum1, the precursor of fumarase (Sass et al. 2003; Karniely et al. 2006). Wild-type Fum1 has the ability to fold rapidly into a stable conformation while the presequence is imported into the matrix and processed. Molecules that achieve this state fail to import and are released back into the cytoplasm in mature form by retrograde movement of their N termini. On the other hand, molecules whose C-terminal domains do not fold rapidly are pulled into the matrix. This is one of several mechanisms by which proteins can be localized both in mitochondria and in the cytoplasm (Yogev and Pines 2011).
Presequences of imported precursors are typically removed by the soluble MPP (Pon and Schatz 1991; Taylor et al. 2001). Many matrix proteins are further processed at their N termini by removal of a single residue or eight residues by the proteases Icp55 and Oct1, respectively (Vögtle et al. 2009, 2011). These alterations apparently serve to generate mature products with increased stability, following the bacterial N-end rules.
The folding of imported matrix proteins must be largely coupled to their interaction with, and release from, mtHsp70 associated with the PAM motor. Mitochondria also contain the essential chaperonin complex Hsp60-Hsp10 (Pon and Schatz 1991; Voos and Röttgers 2002), which is characteristic of bacteria and energy-transducing organelles. These chaperones, together with the dispensable Hsp78 and Pim1, homologs of bacterial ClpB and lon ATP-dependent proteases, assist in folding and maintenance of imported matrix proteins (Leonhardt et al. 1993; Suzuki et al. 1997; Bender et al. 2011).
Spatial distributions and regulation of import complexes
The import of presequence-containing proteins to the inner membrane and matrix requires at least transient interaction of some TOM complexes and TIM23 complexes at translocation contact sites between the membranes. By incubating isolated mitochondria with saturating amounts of an artificial presequence-containing protein that become trapped both outside the outer membrane and in the inner membrane, TOM complexes bound to TIM23 complexes can be detected. Using such a trap, it could be shown that all of the TIM23 complexes were associated with ∼25% of the TOM complexes (Dekker et al. 1997). Thus, at least under these conditions, the TIM23 complex must be located almost exclusively in the boundary domain of the inner membrane and form translocation contact sites with the outer membrane TOM complexes. However, in respiring wild-type cells, Tim23 appears to be only moderately enriched in the inner membrane boundary domain relative to cristae (Vogel et al. 2006).
The TIM23 complex must respond to signals in the substrate precursors that direct either their lateral insertion into the inner membrane or their complete translocation into the matrix. There is currently some dispute about whether the lateral insertion and matrix translocation functions are carried out by a single multi-functional TIM23-PAM complex with different conformations (Popov-Čeleketić et al. 2008a, 2011; Mokranjac and Neupert 2009) or by two forms of the TIM23 complex in dynamic equilibrium with each other (Chacinska et al. 2009, 2010; Schmidt et al. 2010). Two forms of static TIM23 complex were detected in solubilized extracts of mitochondria, depending upon whether they were trapped importing a precursor targeted for sorting to the outer surface of the inner membrane, which does not require the ATP-driven PAM motor, or for import into the matrix, which does require the motor (Chacinska et al. 2005, 2010). The isolated TIM23-sorting complexes contained the protein Tim21, but not PAM complex subunits. In contrast, the isolated TIM23 motor complexes contained PAM subunits but only low levels of Tim21. It has been suggested that the PAM motor could associate with the TIM23 channel if and when Tim21 is ejected preceding import into the matrix (Wiedemann et al. 2007).
Surprisingly, the TIM23 complex has been found to associate with proton-pumping supercomplexes of the bc1 complex and cytochrome c oxidase. These interactions are facilitated by the Tim21 subunit (van der Laan et al. 2006), which also has a role in connecting the TOM complex to the TIM23 complex (Chacinska et al. 2005; Mokranjac et al. 2005), and by Pam16 and Pam18 (Wiedemann et al. 2007). The physiological relevance of this association is suggested by the observation that Tim21 accelerates Δψ-dependent precursor translocation only when the respiratory complexes are active (van der Laan et al. 2006). Thus, it appears that local increases in Δψ may affect the rate of presequence translocation through TIM23. Active TIM23 complexes must be located in the boundary domain of the inner membrane, while the respiratory complexes are selectively, but presumably not completely, located in the cristae domains (Vogel et al. 2006; Wurm and Jakobs 2006; Rabl et al. 2009; Zick et al. 2009; Davies et al. 2011).
A complex of inner membrane proteins present at the crista junctions, and required to form them, has been identified in several labs and named MINOS, MitOS, and MICOS (Harner et al. 2011a; Hoppins et al. 2011; von der Malsburg et al. 2011; Alkhaja et al. 2012). A core component of this complex, Fcj1, was previously shown to be required for crista junction formation (Rabl et al. 2009). The crista junctions are located at firm contact sites between the outer and inner membranes (Harner et al. 2011a). These firm contact sites apparently do not correspond to translocation contact sites between TOM and TIM23 complexes since TIM23-dependent precursor translocation is not directly affected by the absence of Fcj1 (von der Malsburg et al. 2011). However, Fcj1 interacts with Mia40 and with TOM complexes not associated with TIM23, facilitating Mia40-dependent import of soluble twin-Cys proteins to the IMS (von der Malsburg et al. 2011). This suggests that import of substrates dependent upon the Mia40-Erv1 disulfide relay system is selectively located near crista junctions. Cells lacking Fcj1 or other components of MINOS/MitOS/MICOS have respiratory defects, but are viable. Thus, this complex does not appear to be absolutely required for any essential import pathway.
Is the mitochondrial protein import machinery simply a conduit for any targeted protein that arrives at the outer membrane, or is its activity per se subject to modulation? Recent evidence indicates that TOM complex activity is regulated by phosphorylation of the Tom70 receptor by cAMP-dependent protein kinase A (PKA) (Schmidt et al. 2011). PKA activity is controlled such that its activity is high in the presence of glucose but low in nonfermentable carbon sources (Zaman et al. 2008). In the presence of glucose, PKA phosphorylates Tom40 residue Ser174 (among a wide variety of targets). This modification decreases the ability of Tom40 to interact with cytoplasmic Hsp70, the chaperone that delivers metabolite carrier proteins to the TOM complex (Young et al. 2003), decreasing the efficiency with which carrier proteins are imported to the inner membrane (Schmidt et al. 2011). Constitutive activation of PKA results in decreased levels of carrier proteins relative to mitochondrial proteins whose import is not affected by decreased Tom70 activity. Thus, this regulatory pathway has significant physiological effects on mitochondria.
Interestingly, another kinase, casein kinase 2, quantitatively phosphorylates two Ser residues of the central TOM receptor Tom22 and two residues of Mim1 (Schmidt et al. 2011). These modifications are required for normal assembly and activity of both proteins, and thus for the activity of the TOM complex. It is not clear whether these phosphorylations are modulated in response to environmental conditions.
Assembly of Complexes Containing Mitochondrially Synthesized Proteins
Mitochondrial gene expression in S. cerevisiae is required for active respiratory complexes located in the inner membrane, but is not required for any other physiological processes (Pon and Schatz 1991; Chacinska and Boguta 2000; Lipinski et al. 2010). The organellar genetic system produces only eight major proteins in S. cerevisiae. Seven are hydrophobic integral inner membrane proteins: Cob (Apo-cytochrome b), a subunit of the proton-pumping bc1 complex (respiratory complex III); Cox1, Cox2, and Cox3, subunits of proton-pumping cytochrome c oxidase (respiratory complex IV); and Atp6, Atp8, and Atp9, subunits of the F0 component of ATP synthase (respiratory complex V). These mitochondrially synthesized subunits are assembled with imported subunits to form the active enzymes. (S. cerevisiae does not have respiratory complex I, the proton-pumping NADH dehydrogenase found in most other eukaryotes. Succinate dehydrogenase, respiratory complex II, does not contain mitochondrial gene products.)
The eighth major mitochondrial gene product, Var1, is a small mitochondrial ribosomal subunit protein. Aside from Var1, all proteins known to be required for yeast mitochondrial gene expression are imported from the cytoplasm [with the exception of some minor proteins encoded by mitochondrial introns that facilitate intron splicing (Pon and Schatz 1991; Saldanha et al. 1993)]. Var1 is assembled inside the organelle with mitochondrially coded 15S rRNA and at least 33 imported ribosomal proteins coded by nuclear genes (Groot et al. 1979; Terpstra et al. 1979; Gan et al. 2002; Williams et al. 2005). The large mitochondrial ribosomal subunit is assembled from the mitochondrially coded 21S rRNA and at least 44 imported proteins (Gan et al. 2002). These organellar ribosomes do not share any known components with cytoplasmic ribosomes.
The assembly of yeast cytochrome c oxidase has been studied and reviewed extensively (Barrientos et al. 2002a, 2009; Carr and Winge 2003; Herrmann and Funes 2005; Khalimonchuk and Rodel 2005; Cobine et al. 2006; Fontanesi et al. 2006, 2008; Horn and Barrientos 2008; Mick et al. 2011; Soto et al. 2011). A striking fact to emerge from these studies is the specific involvement of >30 nuclear gene products that are not constituents of cytochrome c oxidase. They are required to produce the active enzyme by promoting specific reactions in the expression of genes; insertion of proteins into the inner membrane; insertion of heme, copper, zinc, and magnesium; and chaperoning assembly intermediates (Soto et al. 2011). The assembly of the cytochrome bc1 complex (Zara et al. 2009; Conte et al. 2011; Wagener et al. 2011; Smith et al. 2012) and the ATP synthase (Ackerman and Tzagoloff 2005; Rak et al. 2009) also requires similar assistance. This appears to contrast with the assembly of the TOM complex, for example. Its constituents are inserted into the outer membrane by general mechanisms and are believed to self-assemble once in the membrane (Becker et al. 2008, 2010). Furthermore, as discussed below, the mitochondrial synthesis of several respiratory complex subunits is coupled to assembly of those complexes.
At a higher level of assembly, the cytochrome bc1 complex and cytochrome c oxidase are organized together into supercomplexes that appear to facilitate efficient electron transport between them via cytochrome c (Heinemeyer et al. 2007; Stuart 2008), although this rationale has been questioned (Trouillard et al. 2011). Assembly of these supercomplexes has recently been shown to depend upon two supercomplex subunits, Rcf1 and Rcf2 (Chen et al. 2012; Strogolova et al. 2012; Vukotic et al. 2012).
Mitochondrial protein synthesis is membrane bound
Components of the mitochondrial genetic system have been identified chiefly through the isolation of mutants and the study of their phenotypes and of the proteins affected by their mutations (Tzagoloff and Dieckmann 1990). While mitochondrial protein synthesis can be studied in isolated intact organelles, no true in vitro translation system has been developed from yeast mitochondria. It appears that the mitochondrial genetic system is largely organized on the two-dimensional inner surface of the inner membrane, and an intact membrane [although not the membrane ΔΨ (Clarkson and Poyton 1989; He and Fox 1997)] may be required for protein synthesis. Indeed, the lipid composition of the inner membrane appears to affect mitochondrial protein synthesis (Marzuki and Hibbs 1986; Ostrander et al. 2001).
Mitochondrial ribosomes are bound to the inner membrane, preferentially to the cristae domains, apparently to facilitate cotranslational protein insertion (Vogel et al. 2006; Ott and Herrmann 2010). To date, three inner membrane proteins are known to interact with large mitochondrial ribosomal subunits and are thought to tether them to the membrane independently of nascent chains: they are Oxa1 (Jia et al. 2003, 2009; Szyrach et al. 2003), Mba1 (Ott et al. 2006; Gruschke et al. 2010), and Mdm38 (Frazier et al. 2006; Lupo et al. 2011). Oxa1 functions as a translocase/insertase-promoting export of mitochondrially coded hydrophilic domains, particularly those of Cox2, to the intermembrane space and insertion of transmembrane domains (He and Fox 1997; Hell et al. 1997, 2001). MbaI, a peripheral membrane protein, appears to be partially redundant in function with Oxa1 (Preuss et al. 2001; Ott et al. 2006). Similarly, Mdm38 promotes export of Cob and Atp6 domains (Frazier et al. 2006). Interestingly, Mdm38 is a bifunctional protein that also participates in K+/H+ exchange across the inner membrane (Nowikovsky et al. 2004; Froschauer et al. 2005; Zotova et al. 2010; Lupo et al. 2011).
The synthesis of mitochondrially coded proteins at the inner membrane surface appears to limit their contact with matrix chaperones. Membrane proteins emerging from mitochondrial ribosomes do not interact with mtHsp70 (Ssc1) (Ott et al. 2006). Furthermore, soluble reporter proteins synthesized inside mitochondria appear to fold more slowly than identical polypeptides synthesized in the cytoplasm and imported into the matrix via the mtHsp70-dependent TIM23/PAM machinery (Demlow and Fox 2003).
Channeling of mRNAs to the inner membrane
Mitochondrial DNA molecules are present in nucleoprotein aggregates, termed “nucleoids” (Meeusen et al. 1999; Kaufman et al. 2000; Kucej et al. 2008). A fraction of the ∼40 nucleoids per cell are tethered to the inner and outer membranes by a membrane-spanning protein complex (Aiken Hobbs et al. 2001; Boldogh et al. 2003; Meeusen and Nunnari 2003; Chen and Butow 2005). While the distribution of the mitochondrial RNA polymerase, Rpo41, among the nucleoids has not been reported, transcription likely occurs adjacent to the inner membrane. Furthermore, strong evidence suggests that mRNAs are channeled from RNA polymerase to the membrane-bound translation apparatus. Rpo41 interacts through its amino terminal domain with the soluble protein Mtf2 (Nam1), which in turn appears to chaperone processing of primary transcripts and delivery of mRNAs to the inner membrane surface, together with the membrane-associated proteins Sls1 and Rmd9 (Dieckmann and Staples 1994; Wallis et al. 1994; Rouillard et al. 1996; Rodeheffer et al. 2001; Bryan et al. 2002; Rodeheffer and Shadel 2003; Nouet et al. 2007; Williams et al. 2007) (Figure 8). Mtf2 (Nam1), Sls1, and Rmd9 are all required for normal mitochondrial translation.
Figure 8 .
Channeling of mitochondrially coded mRNAs from RNA polymerase (Rpo41) to membrane-bound ribosomes by Sls1, Mtf2, Rmd9, and mRNA-specific translational activators (TA). (The figure is not intended to suggest that mRNAs are translated while still emerging from RNA polymerase.)
The physiological importance of mitochondrial mRNA channeling from synthesis to translation is most clearly demonstrated by the fact that mutations in the mitochondrial RNA polymerase amino terminal domain that prevent interaction with Mtf2 (Nam1) severely reduce translation but do not prevent mRNA synthesis and accumulation (Rodeheffer and Shadel 2003). Thus, new primary transcripts appear to be directly transferred to Mtf2 (Nam1) from RNA polymerase for efficient gene expression, in conjunction with the interacting membrane protein Sls1 and perhaps Rmd9 among other factors. mRNAs do not, apparently, diffuse freely through the matrix.
Localization of protein synthesis by mRNA-specific translational activators
A peculiar feature of mitochondrial translation in S. cerevisiae and other ascomycetes is its dependence on translational activators for individual mRNAs (Fox 1996a; Coffin et al. 1997; Costanzo et al. 2000; Towpik 2005; Kühl et al. 2011) (Table 7). The roles of mRNA-specific translational activators have been most thoroughly explored for the expression of the S. cerevisiae mitochondrial genes encoding the three core cytochrome c oxidase subunits and apo-cytochrome b. The best-studied translational activators are membrane-bound proteins that recognize targets in the mitochondrial mRNA 5′-UTRs and interact with mitochondrial ribosomes, apparently promoting translation initiation (Fox 1996a; Rödel 1997; Towpik 2005). The translational activators for the COX1, COX2, and COX3 mRNAs are present at low levels that are rate limiting for gene expression (Fox 1996b; Steele et al. 1996; Green-Willms et al. 2001; Naithani et al. 2003; Perez-Martinez et al. 2009). Upsetting the balance of translational activators can interfere with cytochrome c oxidase biogenesis (Fiori et al. 2005).
Table 7 . Proteins that activate translation of specific mitochondrially coded mRNAs by various mechanisms.
| Mitochondrial mRNA | Activator proteins | Activator protein ORFs |
|---|---|---|
| ATP8-ATP6 | Aep3, Assembled F1 ATPase | YPL005W,YBL099W,YJR121W,YBR039W,YPL271W |
| ATP6 | Atp22 | YDR350C |
| ATP9 | Aep1, Aep2, Atp25 | YMR064W, YMR282C, YMR098C |
| COB | Cbp1, Cbp3, Cbp6, Cbs1, Cbs2 | YJL209W, YPL215W, YBR120C, YDL069C, YDR197W |
| COX1 | Pet54, Pet309, Mss51 | YGR222W, YLR067C, YLR203C |
| COX2 | Pet111 | YMR257C |
| COX3 | Pet54, Pet122, Pet494 | YGR222W, YER153C, YNR045W |
| VAR1 | Sov1 | YMR066W |
Mtf2 (Nam1) interacts physically with the activator Pet309, a pentatricopeptide repeat (PPR) protein that binds both the COX1 mRNA 5′-UTR and mitochondrial ribosomes (Manthey and Mcewen 1995; Naithani et al. 2003; Tavares-Carreon et al. 2008; F. Tavares-Carreon, A. Zamudio-Ochoa, Y. Camacho-Villasana, and X. Perez-Martinez; personal communication). PPR motifs have been identified in many organellar RNA-binding proteins with sequence-specific functions (Delannoy et al. 2007) and have been identified in eight mRNA-specific translational activators as well as Rmd9 and the Rpo41 amino terminal domain (Lipinski et al. 2011). Thus, it is likely that the COX1 mRNA is transferred from the RNA polymerase Rpo41 to Mtf2 (Nam1)/Sls1 and Rmd9 to Pet309 for translation by the ribosome. Indeed, Pet309 and at least two ribosomal proteins copurify with Rpo41 (Markov et al. 2009). Mtf2 (Nam1) also interacts with the PPR-containing COX2 mRNA-specific activator Pet111, and with Pet494, which activates COX3 mRNA translation, suggesting a similar pathway for those mRNAs (Naithani et al. 2003; Lipinski et al. 2011) (Figure 8).
The translational activators promoting synthesis of cytochrome c oxidase subunits have been shown to interact with each other (Brown et al. 1994; Naithani et al. 2003). Furthermore, a large protein complex can be isolated from mitochondria that contains Pet309 and the COB mRNA-specific activator Cbp1, as well as several unidentified proteins (Krause et al. 2004). These findings suggest that clusters of translational activators promote colocalized synthesis of mitochondrially coded respiratory complex subunits at distinct locations. Based on the levels of translational activator proteins, there appear to be at most a few hundred such sites per cell (Marykwas and Fox 1989; Fox 1996b; Ghaemmaghami et al. 2003; Naithani et al. 2003). It seems likely that these sites are adjacent to nucleoids.
The physiological significance of distinct sites or topologies for mitochondrial translation is supported by experiments in which Cox2 was translated in vivo from a chimeric mRNA with the 5′- and 3′-UTRs of the VAR1 mRNA, which normally flank the ORF encoding a ribosomal protein. While the rate of Cox2 synthesis was normal, Cox2 accumulation and cytochrome c oxidase activity were severely reduced (Sanchirico et al. 1998). The putative translational activator for the VAR1 mRNA is Sov1, a membrane-bound PPR protein (Sanchirico 1998; Lipinski et al. 2011; M. E. Sanchirico, A. R. Wiffen, T. D. Fox, and T. L. Mason; unpublished results), and translation of the chimeric mRNA appears to be membrane bound (Fiori et al. 2003). However, the VAR1 mRNA 5′-UTR apparently mislocalizes or misorients Cox2 synthesis on the inner membrane such that Cox2 cannot be efficiently assembled with other cytochrome c oxidase subunits and is largely degraded.
Interestingly, bacteria have also recently been found to employ signals embedded in mRNA sequences to localize translation of membrane proteins (Nevo-Dinur et al. 2011).
It has been suggested that localization of mitochondria-bound cytoplasmic protein synthesis and import could be coordinated with localization of mitochondrial protein synthesis to promote assembly of complexes containing both kinds of proteins (Garcia et al. 2007a). There is at present no evidence directly bearing on this interesting hypothesis.
Assembly of cytochrome c oxidase
The three mitochondrially coded subunits of yeast cytochrome c oxidase form its catalytic core (Tsukihara et al. 1996). They are surrounded by eight subunits imported from the cytoplasm (Taanman and Capaldi 1992; Soto et al. 2011). The order in which these subunits are assembled is not known, despite efforts to analyze subassemblies detected in mutants (Church et al. 2005; Horan et al. 2005). However, assembly is believed to be nucleated by mitochondrial synthesis of the core subunits, in particular Cox1 (Herrmann and Funes 2005; Fontanesi et al. 2008; Mick et al. 2011; Soto et al. 2011)
Cox1 is highly hydrophobic, with 12 transmembrane domains, and both termini are on the inside of the inner membrane. In the assembled enzyme Cox1 contains two heme A moieties and a copper atom that participate in electron transport (Tsukihara et al. 1995). Cox1 is believed to be cotranslationally inserted into the inner membrane by Oxa1 (Hell et al. 2001).
The synthesis of Cox1 is coupled to the formation of early assembly intermediates and is downregulated if assembly is artificially blocked by a variety of mutations (Barrientos et al. 2004; Shingu-Vazquez et al. 2010). This assembly-feedback regulation may prevent overproduction of Cox1 to protect yeast cells from damage due to pro-oxidant activity of unassembled Cox1 (Khalimonchuk et al. 2007). Mss51, a mitochondrial inner membrane protein with at least two functions, is a key factor in this regulatory circuit. Mss51 activates COX1 mRNA translation via its 5′-UTR, in conjunction with Pet309, and also binds to newly synthesized Cox1 (Decoster et al. 1990; Perez-Martinez et al. 2003, 2009; Zambrano et al. 2007) (Figure 9).
Figure 9 .
Assembly feedback control of Cox1 synthesis by Mss51 activities as a translational activator and assembly factor. Mitochondria translation of the COX1 mRNA is activated by the mRNA-specific activators Pet309 and Mss51 [Mss51 is associated with Hsp70 (Ssc1) throughout]. Synthesis of a new Cox1 polypeptide nucleates an early assembly intermediate containing Mss51 and the assembly factors Cox14 and Coa3. As additional assembly factors associate with newly synthesized Cox1, Mss51 is released from the assembly intermediates and is then available to initiate additional Cox1 synthesis.
Newly synthesized Cox1 nucleates the formation of an early pre-assembly complex with Mss51 and two short membrane proteins, Cox14 and Coa3 (Cox25) (Perez-Martinez et al. 2009; Fontanesi et al. 2010a; Mick et al. 2010). In addition, whether complexed with Cox1 or not, Mss51 is stoichiometrically associated with mtHsp70 (Ssc1) (Fontanesi et al. 2010b) (Figure 9). Both cox14 and coa3 deletions prevent association of Mss51 with Cox1 and allow uncontrolled Cox1 synthesis despite the fact that they prevent cytochrome c oxidase assembly (Perez-Martinez et al. 2009; Fontanesi et al. 2010a; Mick et al. 2010). Thus, sequestration of Mss51 in assembly intermediates containing Cox1 appears to prevent it from activating COX1 mRNA translation. Truncation of the C-terminal 11 residues of Cox1, normally exposed in the matrix, destabilizes this pre-assembly complex and disrupts assembly-feedback regulation, but does not prevent cytochrome c oxidase assembly (Shingu-Vazquez et al. 2010).
Assembly of Cox1 proceeds by the addition of the assembly factor Coa1 to the pre-assembly complex nucleated by Cox1, which in turn allows the addition of Shy1 (Mick et al. 2007, 2010; Pierrel et al. 2007; Khalimonchuk et al. 2010). Neither Coa1 nor Shy1 are completely essential for production of active cytochrome c oxidase [both are virtually dispensable in the D273-10B strain background (L. S. Burwell, Z. W. Via, and T. D. Fox, unpublished results)]. Mss51 is thought to be released from assembly intermediates in a step after Shy1 association, which may involve the addition of the imported cytochrome c oxidase subunits Cox5 (encoded by COX5A and COX5B) and Cox6 (Barrientos et al. 2002b; Mick et al. 2007; Shingu-Vazquez et al. 2010), although some evidence indicates that this occurs farther upstream (Khalimonchuk et al. 2010). The released Mss51 becomes free to activate another round of Cox1 synthesis, completing the regulatory circuit (Figure 9).
Cox1 assembly proceeds by the addition of redox cofactors. Shy1, together with Coa2, appears to promote the insertion of heme A into Cox1 (Pierrel et al. 2008; Bestwick et al. 2010). The copper-binding membrane protein Cox11 receives copper from the soluble shuttle protein Cox17 and inserts it into assembling Cox1 from the IMS side (Beers et al. 1997; Banting and Glerum 2006; Cobine et al. 2006; Khalimonchuk et al. 2010). The subunits Cox5 and Cox6, which directly contact Cox1, are believed to be associated at this stage with Cox1, Cox14, Shy1, Coa1, and Coa3 in a subassembly that awaits the addition of mitochondrially synthesized Cox2 and Cox3 (Horan et al. 2005; Fontanesi et al. 2010a; Khalimonchuk et al. 2010; Mick et al. 2010).
Cox2 has two transmembrane domains and acidic N- and C-terminal domains that are exported to the IMS side of the inner membrane prior to assembly (Tsukihara et al. 1996). The C-terminal domain binds two copper atoms. In S. cerevisiae, Cox2 is synthesized as a precursor with a 15-residue leader peptide (Pratje et al. 1983) whose length and sequence can be altered dramatically without destroying function (Bonnefoy et al. 2001). However, the mRNA sequence encoding the leader peptide contains a positive element that is required to antagonize negative elements downstream in the mRNA that otherwise prevent completed translation (Bonnefoy et al. 2001; Williams and Fox 2003). Taken together with the functional interactions of these elements with mitochondrial ribosomes (Williams et al. 2004, 2005; Prestele et al. 2009), these findings suggest the possibility that regulation of translation elongation could be coupled to steps in membrane topogenesis of pre-Cox2.
The pre-Cox2 N-terminal domain is cotranslationally exported by Oxa1 (He and Fox 1997; Hell et al. 1998; Bonnefoy et al. 2009). The leader peptide is rapidly processed in the IMS by the inner membrane protease in a reaction chaperoned by Cox20, an inner membrane protein whose topology resembles that of Cox2 (Nunnari et al. 1993; Hell et al. 2000; Jan et al. 2000).
Export of the acidic Cox2 C-terminal domain also requires Oxa1, but appears to be post-translational (He and Fox 1997; Fiumera et al. 2007). Its export also depends specifically upon another highly conserved inner membrane translocase, Cox18, which is paralogously related to Oxa1, and on two inner membrane proteins, Mss2 and Pnt1, which interact with Cox18 (He and Fox 1999; Broadley et al. 2001; Saracco and Fox 2002; Funes et al. 2004). The Cox20 chaperone also functions in C-tail export, probably by removing the exported protein from Cox18 (Fiumera et al. 2009; Elliott et al. 2012). Mature but unassembled Cox2 remains associated with Cox20 (Hell et al. 2000). Metallation of the Cox2 C-terminal domain is catalyzed by membrane-bound Sco1, which receives copper from the soluble Cox17 shuttle and then directly interacts with Cox2 (Lode et al. 2000; Cobine et al. 2006; Rigby et al. 2008). Metallation is believed to precede Cox2 addition to the Cox1 subassembly complex (Soto et al. 2011).
Insertion of the seven transmembrane domains of Cox3, whose amino terminal residues remain on the matrix side, is assisted by Oxa1 (Tsukihara et al. 1996; Hell et al. 2001). There are no known specific chaperones involved in further assembly of Cox3 into the catalytic core of cytochrome c oxidase. Nor are there data on the addition of imported subunits to form the active enzyme.
Assembly of the bc1 complex
The catalytic core of the bc1 complex comprises three subunits. Cob (cytochrome b) is mitochondrially synthesized and embedded in the membrane with its two noncovalently bound heme B moieties. The imported subunits Cyt1 (cytochrome c1) and Rip1 (Rieske 2Fe-2S protein) are anchored to the membrane with their hydrophilic domains bearing heme C and 2Fe-2S cluster redox cofactors, respectively, in the IMS (Solmaz and Hunte 2008; Zara et al. 2009; Smith et al. 2012). Seven additional imported proteins that do not directly participate in electron transfer complete the bc1 complex structure, which forms a dimer. Analysis of subassemblies detected in mutants indicates that Cob may initially associate with two imported subunits, Qcr7 and Qcr8, while Cyt1 associates with Cor1 and Cor2 (Crivellone et al. 1988; Zara et al. 2004, 2007). Association of these subcomplexes, facilitated by the assembly factor Bca1 (Mathieu et al. 2011), forms a nearly complete species that subsequently accepts Rip1.
Cob has eight transmembrane domains, and both termini are on the inside of the inner membrane. Its two heme B moieties are inserted by an unknown mechanism. Oxa1 participates in its membrane insertion (Hell et al. 2001). Translation of the COB mRNA is specifically activated through its 5′-UTR by Cbs1, Cbs2, and Cbp1 (Rödel 1997; Islas-Osuna et al. 2002). In addition, synthesis of Cob is promoted by two proteins, Cbp3 and Cbp6, that bind to mitochondrial ribosomes, associate with newly synthesized Cob after release from the ribosome, and together with Cob form a preassembly complex with the assembly factor Cbp4 (Gruschke et al. 2011). While this mechanism couples translation to assembly, Cob synthesis is not reduced by a feedback mechanism when assembly of the bc1 complex is disrupted.
The complex import pathway of Cyt1, resulting in a hydrophilic N-terminal domain in the IMS with a C-tail anchor, was described above. Covalent attachment of heme to this hydrophilic domain is catalyzed by the lyase Cyt2 either during or after maturation of Cyt1 (Bernard et al. 2003).
The maturation of Rip1 is particularly interesting. This protein is initially fully imported into the matrix (Hartl et al. 1986). Since Fe-S cluster biogenesis occurs in the matrix, it is believed that assembly of the Rip1 polypeptide with its 2Fe-2S cluster occurs after this initial import reaction (Kispal et al. 1999; Lill 2009; Wagener et al. 2011). In any event, the C-terminal domain of Rip1 is subsequently exported through the inner membrane to the IMS. This export reaction specifically requires Bcs1, a member of the AAA family of ATPases that is tethered to the inner surface of the inner membrane (Nobrega et al. 1992; Wagener et al. 2011). Most mitochondrial AAA proteins are hexameric complexes with a central compartment capable of ATP-dependent unfolding, proteolysis, and chaperone-like activity (Fiumera et al. 2009; Tatsuta and Langer 2009; Truscott et al. 2010). In the case of Bcs1, it appears that an enlarged central compartment translocates the folded C-terminal domain of Rip1 bearing its 2Fe-2S cluster across the membrane and releases the Rip1 N-terminal transmembrane domain laterally into the membrane (Wagener et al. 2011). The matrix protein Mzm1 facilitates this export of Rip1 from the matrix (Atkinson et al. 2010, 2011), which must occur prior to its assembly into the bc1 complex.
Assembly of the ATP synthase
The raison d’être of the respiratory chain is the synthesis of ATP. This reaction is carried out by the well-known F1F0 ATP synthase. The F0 sector in the inner membrane captures energy of the proton gradient as rotary motion and turns the central stalk of the F1 sector, which projects into the matrix. Rotation of the central stalk relative to the catalytic sites in the Atp13-Atp23 (α3β3) hexamer of F1, which is fastened to nonrotating proteins in F0 by the peripheral stalk or stator, drives the synthesis of ATP from ADP and inorganic phosphate (Stock et al. 1999; von Ballmoos et al. 2009). In S. cerevisiae, all subunits of F1 and of the stator are imported (Velours and Arselin 2000; Ackerman and Tzagoloff 2005). Three hydrophobic core proteins of F0,—Atp6, Atp8, and Atp9—are mitochondrial gene products, while two others are imported. Current evidence indicates that the complete ATP synthase is assembled from modular subassemblies, such that the proton-conducting pore of F0, formed by the interface between Atp6 and Atp9 (Fillingame and Dmitriev 2002), is formed at the last step (Rak et al. 2009, 2011) (Figure 10). This hypothesis predicts that the proton pore is immediately coupled to the catalytic machinery at the moment that it is formed, avoiding the deleterious effects of unregulated proton leakage across the inner membrane.
Figure 10 .
Assembly of ATP synthase from modular subassemblies. The imported subunits of the F1 complex (green) are assembled in the matrix with the help of specific assembly chaperones (see text for details). Assembled F1 activates mitochondrial translation of the dicistronic mRNA encoding Atp8 and Atp6, which nucleate the assembly of imported subunits into the stator module (red and tan). The Atp9 ring (blue) is assembled from monomers and then joined with F1. Finally, association of the stator with the F1-Atp9 ring subassembly generates the ATP synthase proton pore at the same time that ATPase activity is coupled to the inner membrane proton gradient. Reprinted by permission from Macmillan Publishers Ltd. from Rak et al. (2011).
One pre-assembly module is F1 itself, which assembles in the absence of F0 or the stator (Schatz 1968; Tzagoloff 1969; Velours and Arselin 2000). The newly imported subunit Atp1 is bound stoichiometrically in a dimeric assembly intermediate with the specific chaperone Atp12, while the newly imported Atp2 subunit is similarly bound by its chaperone Atp11 (Ackerman and Tzagoloff 1990; Ludlam et al. 2009). Release of these F1 subunits from their chaperones to allow the formation of Atp1-Atp2 dimers and hexamers requires the presence of the subunit Atp3 (γ), a component of the central stalk. In the case of Atp1, structural evidence strongly suggests that Atp3 binding to Atp1 directly displaces the chaperone Atp12 (Ludlam et al. 2009). An additional protein, Fmc1, is required to generate Atp1-Atp2 dimers at high temperature (Lefebvre-Legendre et al. 2001), and Hsp90 chaperones have also been implicated in this process (Francis and Thorsness 2011). Addition of the central stalk proteins Atp16 (δ) and Atp15 (ε) has not been examined.
A second pre-assembly module is the rotating component of F0, comprising a decameric ring of the highly hydrophobic mitochondrially coded Atp9 (subunit c) embedded in the membrane (Stock et al. 1999; Rak et al. 2011). [The assembled ring structure is not dissociated by treatment with SDS (Herrmann et al. 1994; Rak et al. 2011).] Translation and/or stability of the ATP9 mRNA requires the specific activators Aep1, Aep2, and Atp25 (Ackerman et al. 1991; Finnegan et al. 1991; payne et al. 1993; Ziaja et al. 1993; Ellis et al. 1999; Zeng et al. 2008; Rak et al. 2009). Atp25 is particularly interesting as the 60-kDa protein is cleaved roughly in half to yield a C-terminal fragment that is required for ATP9 mRNA stability and/or translation and an N-terminal fragment that is not required for Atp9 synthesis but appears to promote assembly of the Atp9 ring (Zeng et al. 2008). Insertion of Atp9 into the inner membrane and assembly of the ring structure does not require Oxa1 (Lemaire et al. 2000; Jia et al. 2007; Mathieu et al. 2010) or the other mitochondrially coded F0 subunits Atp6 and Atp8 (Rak et al. 2011). However, Oxa1 does have a role in promoting association of the Atp9 ring with a single Atp6 molecule late in the assembly of ATP synthase (Jia et al. 2007)
The assembled F1 and Atp9 ring modules associate to form a larger pre-assembly complex independently of other F0 subunits through interaction of the central stalk subunits Atp3 and Atp15 with the ring (von Ballmoos et al. 2009; Rak et al. 2011).
A third pre-assembly module has at its core the mitochondrially synthesized F0 components Atp6 and Atp8. Both of these two proteins are translated from the only dicistronic yeast mitochondrial mRNA, ensuring that their synthesis is colocalized (Dieckmann and Staples 1994). Translation of the downstream coding sequence, ATP6, requires the specific activator Atp22 acting on a region upstream of the coding sequence (Zeng et al. 2007c). However, translation of the upstream ATP8-coding sequence does not require Atp22. Interestingly, translation of both coding sequences requires the presence of F1 or an F1 pre-assembly complex, but does not depend upon F1 ATPase catalytic activity (Rak and Tzagoloff 2009). The target upon which F1 acts is in an untranslated regions(s) of the dicistronic mRNA, but has not been further localized. Whatever the mechanism of this translational control, it couples the production of the Atp6-Atp8 preassembly module to the presence of the imported subunits of one of its assembly partners.
Translation of the ATP6 sequence yields pre-Atp6, with an N-terminal leader peptide (Michon et al. 1988). While partially dispensable, this leader peptide does promote efficient assembly of ATP synthase (Zeng et al. 2007a). After the N terminus is translocated through the inner membrane by an unknown mechanism, the Pre-Atp6 leader peptide is specifically cleaved by Atp23, a metalloprotease (Osman et al. 2007; Zeng et al. 2007b).
Immediately after their synthesis, Atp6 and Atp8 associate in the inner membrane with each other, forming a pre-assembly module that lacks Atp9 and F1 subunits (Rak et al. 2011). This complex also contains at least two proteins that comprise the peripheral stator stalk (Atp4 and Atp7) and Atp10, a specific chaperone protein known to bind Atp6 and promote its final assembly with the Atp9 ring (Tzagoloff et al. 2004; Rak et al. 2011). Additional stator subunits were not identified in this pre-assembly complex (or group of similarly sized complexes) nor was the Atp23 protease, which, like Atp10, binds to Atp6 and promotes association of Atp6 and Atp9 by a mechanism independent of its catalytic activity in cleaving the pre-Atp6 leader peptide (Osman et al. 2007; Zeng et al. 2007b). Further work will be required to understand the assembly of the stator and the nature of the pre-assembly module containing Atp6, Atp8, and, presumably, the imported subunits of F0.
Despite current uncertainties, final assembly of the complete F1F0 ATP synthase appears to involve the joining of two pre-assembly complexes produced by independent pathways: the F1-Atp9 ring complex and an Atp6-Atp8-stator complex (Figure 10). Such a mechanism would form, in a single step, both the complete F0 rotary motor and the stator bridge between the F1 and F0 sectors, coupling the proton-motive force to ATP synthesis (Rak et al. 2011).
Assembled ATP synthase monomers associate with each other via contacts between their single Atp6 subunits and other components of their F0 sectors and stators within the membrane (Wagner et al. 2010; Velours et al. 2011). The ATP synthase dimers in turn form larger chain-like oligomeric structures that apparently help to maintain membrane curvature of cristae (Stuart 2008; Rabl et al. 2009; Velours et al. 2009; Zick et al. 2009).
Perspective
There is no gainsaying the utility of Saccharomyces as a system for exploring biological mechanisms. The importance of this is compounded by the evolutionary conservation of so many cellular mechanisms among eukaryotes, including humans. The synthesis, import, and assembly of mitochondrial proteins can be largely included with other such conserved processes.
The TOM, SAM, TIM22, and TIM23 complexes appear to be nearly universally present in eukaryotes (Paschen et al. 2003; Dolezal et al. 2006; Kutik et al. 2009; Hewitt et al. 2011; Rada et al. 2011) [the TIM22 complex appears to be absent in some, such as Trypanosomes (Schneider et al. 2008)]. The core components of these complexes have maintained significant sequence conservation. Furthermore, expression of a mitochondrially targeted protein from one species in cells of another typically results in correct protein localization by the host import machinery.
Similarly, many of the accessory proteins required to assemble respiratory complexes have apparent or confirmed orthologs in humans (Ackerman and Tzagoloff 2005; Soto et al. 2011; Wagener et al. 2011). Particularly striking is the fact that the human orthologs of Oxa1 (Bonnefoy et al. 1994) and Cox18 (Gaisne and Bonnefoy 2006) can support assembly of cytochrome c oxidase and respiratory growth in the corresponding yeast mutants. Furthermore, the ability of the human OXA1 mRNA to direct synthesis of the functional mitochondrial protein depends upon interaction of the human mRNA 3′-UTR with yeast mitochondria, suggesting conservation in the mechanisms of mRNA targeting (Sylvestre et al. 2003).
In contrast to the clear conservation of protein import and assembly mechanisms, mitochondrial genome structures and their expression systems are highly divergent among eukaryotic phyla (Burger et al. 2003). Nevertheless, the strategy of linking transcription and translation together at the inner membrane, identified in yeast, appears to be employed by mammals for the synthesis and assembly of highly hydrophobic mitochondrial gene products (Weraarpachai et al. 2009; Sasarman et al. 2010; Brown et al. 2011; He et al. 2012).
Given the increasingly evident role of a broad array of mitochondrial functions in the maintenance of healthy human cells, tissues, and bodies (Nunnari and Suomalainen 2012), detailed studies of mechanisms underlying mitochondrial activities in yeast should remain a high priority.
Acknowledgments
Research in the author’s laboratory is supported by National Institutes of Health grant R01-GM29362.
Footnotes
Communicating editor: A. Hinnebusch
Literature Cited
- Abe Y., Shodai T., Muto T., Mihara K., Torii H., et al. , 2000. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell 100: 551–560 [DOI] [PubMed] [Google Scholar]
- Ackerman S. H., Tzagoloff A., 1990. Identification of two nuclear genes (ATP11, ATP12) required for assembly of the yeast F1-ATPase. Proc. Natl. Acad. Sci. USA 87: 4986–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman S. H., Tzagoloff A., 2005. Function, structure, and biogenesis of mitochondrial ATP synthase. Prog. Nucleic Acid Res. Mol. Biol. 80: 95–133 [DOI] [PubMed] [Google Scholar]
- Ackerman S. H., Gatti D. L., Gellefors P., Douglas M. G., Tzagoloff A., 1991. ATP13, a nuclear gene of Saccharomyces cerevisiae essential for the expression of subunit 9 of the mitochondrial ATPase. FEBS Lett. 278: 234–238 [DOI] [PubMed] [Google Scholar]
- Ades I. Z., Butow R. A., 1980. The products of mitochondria-bound cytoplasmic polysomes in yeast. J. Biol. Chem. 255: 9918–9924 [PubMed] [Google Scholar]
- Ahting U., Waizenegger T., Neupert W., Rapaport D., 2005. Signal-anchored proteins follow a unique insertion pathway into the outer membrane of mitochondria. J. Biol. Chem. 280: 48–53 [DOI] [PubMed] [Google Scholar]
- Aiken Hobbs A. E., Srinivasan M., McCaffery J. M., Jensen R. E., 2001. Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability. J. Cell Biol. 152: 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhaja A. K., Jans D. C., Nikolov M., Vukotic M., Lytovchenko O., et al. , 2012. MINOS1 is a conserved component of Mitofilin complexes and required for mitochondrial function and cristae organization. Mol. Biol. Cell 23: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold I., Folsch H., Neupert W., Stuart R. A., 1998. Two distinct and independent mitochondrial targeting signals function in the sorting of an inner membrane protein, cytochrome c1. J. Biol. Chem. 273: 1469–1476 [DOI] [PubMed] [Google Scholar]
- Atkinson A., Khalimonchuk O., Smith P., Sabic H., Eide D., et al. , 2010. Mzm1 influences a labile pool of mitochondrial zinc important for respiratory function. J. Biol. Chem. 285: 19450–19459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson A., Smith P., Fox J. L., Cui T. Z., Khalimonchuk O., et al. , 2011. The LYR protein Mzm1 functions in the insertion of the Rieske Fe/S protein in yeast mitochondria. Mol. Cell. Biol. 31: 3988–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K. P., Schatz G., 1991. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature 349: 205–208 [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schaniel A., Vestweber D., Schatz G., 1990. A yeast mitochondrial outer membrane protein essential for protein import and cell viability. Nature 348: 605–609 [DOI] [PubMed] [Google Scholar]
- Baker M. J., Webb C. T., Stroud D. A., Palmer C. S., Frazier A. E., et al. , 2009. Structural and functional requirements for activity of the Tim9-Tim10 complex in mitochondrial protein import. Mol. Biol. Cell 20: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banci L., Bertini I., Cefaro C., Cenacchi L., Ciofi-Baffoni S., et al. , 2010. Molecular chaperone function of Mia40 triggers consecutive induced folding steps of the substrate in mitochondrial protein import. Proc. Natl. Acad. Sci. USA 107: 20190–20195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banting G. S., Glerum D. M., 2006. Mutational analysis of the Saccharomyces cerevisiae cytochrome c oxidase assembly protein Cox11p. Eukaryot. Cell 5: 568–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A., Barros M. H., Valnot I., Rotig A., Rustin P., et al. , 2002a. Cytochrome oxidase in health and disease. Gene 286: 53–63 [DOI] [PubMed] [Google Scholar]
- Barrientos A., Korr D., Tzagoloff A., 2002b. Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh’s syndrome. EMBO J. 21: 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A., Zambrano A., Tzagoloff A., 2004. Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 23: 3472–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A., Gouget K., Horn D., Soto I. C., Fontanesi F., 2009. Suppression mechanisms of COX assembly defects in yeast and human: insights into the COX assembly process. Biochim. Biophys. Acta 1793: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T., Pfannschmidt S., Guiard B., Stojanovski D., Milenkovic D., et al. , 2008. Biogenesis of the mitochondrial TOM complex: Mim1 promotes insertion and assembly of signal-anchored receptors. J. Biol. Chem. 283: 120–127 [DOI] [PubMed] [Google Scholar]
- Becker T., Guiard B., Thornton N., Zufall N., Stroud D. A., et al. , 2010. Assembly of the mitochondrial protein import channel: role of Tom5 in two-stage interaction of Tom40 with the SAM complex. Mol. Biol. Cell 21: 3106–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T., Wenz L. S., Kruger V., Lehmann W., Muller J. M., et al. , 2011. The mitochondrial import protein Mim1 promotes biogenesis of multispanning outer membrane proteins. J. Cell Biol. 194: 387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers J., Glerum D. M., Tzagoloff A., 1997. Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J. Biol. Chem. 272: 33191–33196 [DOI] [PubMed] [Google Scholar]
- Bender T., Lewrenz I., Franken S., Baitzel C., Voos W., 2011. Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease. Mol. Biol. Cell 22: 541–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard D. G., Gabilly S. T., Dujardin G., Merchant S., Hamel P. P., 2003. Overlapping specificities of the mitochondrial cytochrome c and c1 heme lyases. J. Biol. Chem. 278: 49732–49742 [DOI] [PubMed] [Google Scholar]
- Bernard D. G., Quevillon-Cheruel S., Merchant S., Guiard B., Hamel P. P., 2005. Cyc2p, a membrane-bound flavoprotein involved in the maturation of mitochondrial c-type cytochromes. J. Biol. Chem. 280: 39852–39859 [DOI] [PubMed] [Google Scholar]
- Bestwick M., Khalimonchuk O., Pierrel F., Winge D. R., 2010. The role of Coa2 in hemylation of yeast Cox1 revealed by its genetic interaction with Cox10. Mol. Cell. Biol. 30: 172–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihlmaier K., Mesecke N., Terziyska N., Bien M., Hell K., et al. , 2007. The disulfide relay system of mitochondria is connected to the respiratory chain. J. Cell Biol. 179: 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert M., Rehling P., Guiard B., Herrmann J. M., Pfanner N., et al. , 2010. Cooperation of stop-transfer and conservative sorting mechanisms in mitochondrial protein transport. Curr. Biol. 20: 1227–1232 [DOI] [PubMed] [Google Scholar]
- Boldogh I. R., Nowakowski D. W., Yang H. C., Chung H., Karmon S., et al. , 2003. A protein complex containing Mdm10p, Mdm12p and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery. Mol. Biol. Cell 14: 4618–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy N., Kermorgant M., Groudinsky O., Minet M., Slonimski P. P., et al. , 1994. Cloning of a human gene involved in cytochrome oxidase assembly by functional complementation of an oxa1− mutation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 91: 11978–11982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy N., Bsat N., Fox T. D., 2001. Mitochondrial translation of Saccharomyces cerevisiae COX2 mRNA is controlled by the nucleotide sequence specifying the pre-Cox2p leader peptide. Mol. Cell. Biol. 21: 2359–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy N., Fiumera H. L., Dujardin G., Fox T. D., 2009. Roles of Oxa1-related inner-membrane translocases in assembly of respiratory chain complexes. Biochim. Biophys. Acta 1793: 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley S. A., Demlow C. M., Fox T. D., 2001. Peripheral mitochondrial inner membrane protein, Mss2p, required for export of the mitochondrially coded Cox2p C-tail in Saccharomyces cerevisiae. Mol. Cell. Biol. 21: 7663–7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. G., Costanzo M. C., Fox T. D., 1994. Interactions among three proteins that specifically activate translation of the mitochondrial COX3 mRNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 14: 1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Tkachuk A. N., Shtengel G., Kopek B. G., Bogenhagen D. F., et al. , 2011. Superresolution fluorescence imaging of mitochondrial nucleoids reveals their spatial range, limits, and membrane interaction. Mol. Cell. Biol. 31: 4994–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan A. C., Rodeheffer M. S., Wearn C. M., Shadel G. S., 2002. Sls1p is a membrane-bound regulator of transcription-coupled processes involved in Saccharomyces cerevisiae mitochondrial gene expression. Genetics 160: 75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G., Gray M. W., Lang B. F., 2003. Mitochondrial genomes: anything goes. Trends Genet. 19: 709–716 [DOI] [PubMed] [Google Scholar]
- Carr H. S., Winge D. R., 2003. Assembly of cytochrome c oxidase within the mitochondrion. Acc. Chem. Res. 36: 309–316 [DOI] [PubMed] [Google Scholar]
- Chacinska A., Boguta M., 2000. Coupling of mitochondrial translation with the formation of respiratory complexes in yeast mitochondria. Acta Biochim. Pol. 47: 973–991 [PubMed] [Google Scholar]
- Chacinska A., Pfannschmidt S., Wiedemann N., Kozjak V., Sanjuan Szklarz L. K., et al. , 2004. Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J. 23: 3735–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacinska A., Lind M., Frazier A. E., Dudek J., Meisinger C., et al. , 2005. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell 120: 817–829 [DOI] [PubMed] [Google Scholar]
- Chacinska A., Koehler C. M., Milenkovic D., Lithgow T., Pfanner N., 2009. Importing mitochondrial proteins: machineries and mechanisms. Cell 138: 628–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacinska A., van der Laan M., Mehnert C. S., Guiard B., Mick D. U., et al. , 2010. Distinct forms of mitochondrial TOM-TIM supercomplexes define signal-dependent states of preprotein sorting. Mol. Cell. Biol. 30: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenay-Lapointe M., Shadel G. S., 2011. Repression of mitochondrial translation, respiration and a metabolic cycle-regulated gene, SLF1, by the yeast Pumilio-family protein Puf3p. PLoS ONE 6: e20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. J., Butow R. A., 2005. The organization and inheritance of the mitochondrial genome. Nat. Rev. Genet. 6: 815–825 [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Taylor E. B., Dephoure N., Heo J. M., Tonhato A., et al. , 2012. Identification of a protein mediating respiratory supercomplex stability. Cell Metab. 15: 348–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church C., Goehring B., Forsha D., Wazny P., Poyton R. O., 2005. A role for Pet100p in the assembly of yeast cytochrome c oxidase: interaction with a sub-assembly that accumulates in a pet100 mutant. J. Biol. Chem. 280: 1854–1863 [DOI] [PubMed] [Google Scholar]
- Clarkson G. H. D., Poyton R. O., 1989. A role for membrane potential in the biogenesis of cytochrome c oxidase subunit II, a mitochondrial gene product. J. Biol. Chem. 264: 10114–10118 [PubMed] [Google Scholar]
- Cobine P. A., Pierrel F., Winge D. R., 2006. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim. Biophys. Acta 1763: 759–772 [DOI] [PubMed] [Google Scholar]
- Coffin J. W., Dhillon R., Ritzel R. G., Nargang F. E., 1997. The Neurospora crassa cya-5 nuclear gene encodes a protein with a region of homology to the Saccharomyces cerevisiae PET309 protein and is required in a post-transcriptional step for the expression of the mitochondrially encoded COXI protein. Curr. Genet. 32: 273–280 [DOI] [PubMed] [Google Scholar]
- Conte L., Trumpower B. L., Zara V., 2011. Bcs1p can rescue a large and productive cytochrome bc(1) complex assembly intermediate in the inner membrane of yeast mitochondria. Biochim. Biophys. Acta 1813: 91–101 [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Blugeon C., Jacq C., 2000. In yeast, the 3′ untranslated region or the presequence of ATM1 is required for the exclusive localization of its mRNA to the vicinity of mitochondria. Mol. Cell. Biol. 20: 7881–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. C., Bonnefoy N., Williams E. H., Clark-Walker G. D., Fox T. D., 2000. Highly diverged homologs of Saccharomyces cerevisiae mitochondrial mRNA-specific translational activators have orthologous functions in other budding yeasts. Genetics 154: 999–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., 1993. Chaperones: helpers along the pathways to protein folding. Science 260: 1902–1903 [DOI] [PubMed] [Google Scholar]
- Crivellone M. D., Wu M., Tzagoloff A., 1988. Assembly of the mitochondrial membrane system: analysis of structural mutants of the yeast coenzyme QH2-cytochrome c reductase complex. J. Biol. Chem. 263: 14323–14333 [PubMed] [Google Scholar]
- Curran S. P., Leuenberger D., Oppliger W., Koehler C. M., 2002. The Tim9p-Tim10p complex binds to the transmembrane domains of the ADP/ATP carrier. EMBO J. 21: 942–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S. P., Leuenberger D., Leverich E. P., Hwang D. K., Beverly K. N., et al. , 2004. The role of Hot13p and redox chemistry in the mitochondrial TIM22 import pathway. J. Biol. Chem. 279: 43744–43751 [DOI] [PubMed] [Google Scholar]
- Dabir D. V., Leverich E. P., Kim S. K., Tsai F. D., Hirasawa M., et al. , 2007. A role for cytochrome c and cytochrome c peroxidase in electron shuttling from Erv1. EMBO J. 26: 4801–4811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E., Kuhn A., 2004. YidC family members are involved in the membrane insertion, lateral integration, folding, and assembly of membrane proteins. J. Cell Biol. 166: 769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G., Böhni P., Schatz G., 1982. Import of proteins into mitochondria: cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257: 13028–13033 [PubMed] [Google Scholar]
- Davies K. M., Strauss M., Daum B., Kief J. H., Osiewacz H. D., et al. , 2011. Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. USA 108: 14121–14126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. J., Sepuri N. B., Holder J., Johnson A. E., Jensen R. E., 2000. Two intermembrane space TIM complexes interact with different domains of Tim23p during its import into mitochondria. J. Cell Biol. 150: 1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoster E., Simon M., Hatat D., Faye G., 1990. The MSS51 gene product is required for the translation of the COX1 mRNA in yeast mitochondria. Mol. Gen. Genet. 224: 111–118 [DOI] [PubMed] [Google Scholar]
- Dekker P. J. T., Martin F., Maarse A. C., Bomer U., Muller H., et al. , 1997. The tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16: 5408–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy E., Stanley W. A., Bond C. S., Small I. D., 2007. Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem. Soc. Trans. 35: 1643–1647 [DOI] [PubMed] [Google Scholar]
- Demlow C. M., Fox T. D., 2003. Activity of mitochondrially synthesized reporter proteins is lower than imported proteins, and is increased by lowering cAMP in glucose-grown Saccharomyces cerevisiae cells. Genetics 165: 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R., 1988. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332: 800–805 [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Staples R. R., 1994. Regulation of mitochondrial gene expression in Saccharomyces cerevisiae. Int. Rev. Cytol. 152: 145–181 [DOI] [PubMed] [Google Scholar]
- Diekert K., Kispal G., Guiard B., Lill R., 1999. An internal targeting signal directing proteins into the mitochondrial intermembrane space. Proc. Natl. Acad. Sci. USA 96: 11752–11757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert K., de Kroon A. I., Ahting U., Niggemeyer B., Neupert W., et al. , 2001. Apocytochrome c requires the TOM complex for translocation across the mitochondrial outer membrane. EMBO J. 20: 5626–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer K. S., Papic D., Schumann B., Sperl D., Krumpe K., et al. , 2012. A crucial role of Mim2 in the biogenesis of mitochondrial outer membrane proteins. J. Cell Sci. 125: 3464–3473 [DOI] [PubMed] [Google Scholar]
- Dolezal P., Likic V., Tachezy J., Lithgow T., 2006. Evolution of the molecular machines for protein import into mitochondria. Science 313: 314–318 [DOI] [PubMed] [Google Scholar]
- Dukanovic J., Rapaport D., 2011. Multiple pathways in the integration of proteins into the mitochondrial outer membrane. Biochim. Biophys. Acta 1808: 971–980 [DOI] [PubMed] [Google Scholar]
- Dumont M. E., Cardillo T. S., Hayes M. K., Sherman F., 1991. Role of cytochrome c heme lyase in mitochondrial import and accumulation of cytochrome c in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 5487–5496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall S. D., Agius S. C., De Marcos Lousa C., Trezeguet V., Tokatlidis K., 2003. The dynamic dimerization of the yeast ADP/ATP carrier in the inner mitochondrial membrane is affected by conserved cysteine residues. J. Biol. Chem. 278: 26757–26764 [DOI] [PubMed] [Google Scholar]
- Eliyahu E., Pnueli L., Melamed D., Scherrer T., Gerber A. P., et al. , 2010. Tom20 mediates localization of mRNAs to mitochondria in a translation-dependent manner. Mol. Cell. Biol. 30: 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott L. E., Sarraco S. A., Fox T. D., 2012. Multiple roles of the Cox20 chaperone in assembly of Saccharomyces cereviae cytochrome c oxidase. Genetics 190: 559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T. P., Lukins H. B., Nagley P., Corner B. E., 1999. Suppression of a nuclear aep2 mutation in Saccharomyces cerevisiae by a base substitution in the 5′-untranslated region of the mitochondrial oli1 gene encoding subunit 9 of ATP synthase. Genetics 151: 1353–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embley T. M., Martin W., 2006. Eukaryotic evolution, changes and challenges. Nature 440: 623–630 [DOI] [PubMed] [Google Scholar]
- Endo T., Yamano K., 2010. Transport of proteins across or into the mitochondrial outer membrane. Biochim. Biophys. Acta 1803: 706–714 [DOI] [PubMed] [Google Scholar]
- Endo T., Yamano K., Kawano S., 2011. Structural insight into the mitochondrial protein import system. Biochim. Biophys. Acta 1808: 955–970 [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Dmitriev O. Y., 2002. Structural model of the transmembrane Fo rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ. Biochim. Biophys. Acta 1565: 232–245 [DOI] [PubMed] [Google Scholar]
- Finnegan P. M., Payne M. J., Kermidaris E., Lukins H. B., 1991. Characterization of a yeast nuclear gene, AEP2, required for accumulation of mitochondrial mRNA encoding subunit-9 of the ATP synthase. Curr. Genet. 20: 53–61 [DOI] [PubMed] [Google Scholar]
- Fiori A., Mason T. L., Fox T. D., 2003. Evidence that synthesis of the Saccharomyces cerevisiae mitochondrially-encoded ribosomal protein Var1p may be membrane localized. Eukaryot. Cell 2: 651–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori A., Perez-Martinez X., Fox T. D., 2005. Overexpression of the COX2 translational activator, Pet111p, prevents translation of COX1 mRNA and cytochrome c oxidase assembly in mitochondria of Saccharomyces cerevisiae. Mol. Microbiol. 56: 1689–1704 [DOI] [PubMed] [Google Scholar]
- Fiumera H. L., Broadley S. A., Fox T. D., 2007. Translocation of mitochondrially synthesized Cox2p domains from the matrix to the intermembrane space. Mol. Cell. Biol. 27: 4664–4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera H. L., Dunham M. J., Saracco S. A., Butler C. A., Kelly J. A., et al. , 2009. Translocation and assembly of mitochondrially coded Saccharomyces cerevisiae cytochrome c oxidase subunit Cox2 by Oxa1 and Yme1 in the absence of Cox18. Genetics 182: 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foat B. C., Houshmandi S. S., Olivas W. M., Bussemaker H. J., 2005. Profiling condition-specific, genome-wide regulation of mRNA stability in yeast. Proc. Natl. Acad. Sci. USA 102: 17675–17680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi F., Soto I. C., Horn D., Barrientos A., 2006. Assembly of mitochondrial cytochrome c oxidase, a complicated and highly regulated cellular process. Am. J. Physiol. Cell Physiol. 291: c1129–c1147 [DOI] [PubMed] [Google Scholar]
- Fontanesi F., Soto I. C., Barrientos A., 2008. Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life 60: 557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi F., Clemente P., Barrientos A., 2010a. Cox25 teams up with Mss51, Ssc1 and Cox14 to regulate mitochondrial cytochrome C oxidase subunit 1 expression and assembly in Saccharomyces cerevisiae. J. Biol. Chem. 286: 555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi F., Soto I. C., Horn D., Barrientos A., 2010b. Mss51 and Ssc1 facilitate translational regulation of cytochrome c oxidase biogenesis. Mol. Cell. Biol. 30: 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., 1996a. Genetics of mitochondrial translation, pp. 733–758 in Translational Control, edited by Hershey J. W. B., Matthews M. B., Sonenberg N. Cold Spring Harbor Press, Cold Spring Harbor, NY [Google Scholar]
- Fox T. D., 1996b. Translational control of endogenous and recoded nuclear genes in yeast mitochondria: regulation and membrane targeting. Experientia 52: 1130–1135 [DOI] [PubMed] [Google Scholar]
- Francis B. R., Thorsness P. E., 2011. Hsp90 and mitochondrial proteases Yme1 and Yta10/12 participate in ATP synthase assembly in Saccharomyces cerevisiae. Mitochondrion 11: 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier A. E., Chacinska A., Truscott K. N., Guiard B., Pfanner N., et al. , 2003. Mitochondria use different mechanisms for transport of multispanning membrane proteins through the intermembrane space. Mol. Cell. Biol. 23: 7818–7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier A. E., Dudek J., Guiard B., Voos W., Li Y., et al. , 2004. Pam16 has an essential role in the mitochondrial protein import motor. Nat. Struct. Mol. Biol. 11: 226–233 [DOI] [PubMed] [Google Scholar]
- Frazier A. E., Taylor R. D., Mick D. U., Warscheid B., Stoepel N., et al. , 2006. Mdm38 interacts with ribosomes and is a component of the mitochondrial protein export machinery. J. Cell Biol. 172: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey T. G., Mannella C. A., 2000. The internal structure of mitochondria. Trends Biochem. Sci. 25: 319–324 [DOI] [PubMed] [Google Scholar]
- Froschauer E., Nowikovsky K., Schweyen R. J., 2005. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves Yol027/Letm1 proteins. Biochim. Biophys. Acta 1711: 41–48 [DOI] [PubMed] [Google Scholar]
- Funes S., Nargang F. E., Neupert W., Herrmann J. M., 2004. The Oxa2 protein of Neurospora crassa plays a critical role in the biogenesis of cytochrome oxidase and defines a ubiquitous subbranch of the Oxa1/YidC/Alb3 protein family. Mol. Biol. Cell 15: 1853–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel K., Egan B., Lithgow T., 2003. Tom40, the import channel of the mitochondrial outer membrane, plays an active role in sorting imported proteins. EMBO J. 22: 2380–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadir N., Haim-Vilmovsky L., Kraut-Cohen J., Gerst J. E., 2011. Localization of mRNAs coding for mitochondrial proteins in the yeast Saccharomyces cerevisiae. RNA 17: 1551–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisne M., Bonnefoy N., 2006. The COX18 gene, involved in mitochondrial biogenesis, is functionally conserved and tightly regulated in humans and fission yeast. FEMS Yeast Res. 6: 869–882 [DOI] [PubMed] [Google Scholar]
- Gan X., Kitakawa M., Yoshino K., Oshiro N., Yonezawa K., et al. , 2002. Tag-mediated isolation of yeast mitochondrial ribosome and mass spectrometric identification of its new components. Eur. J. Biochem. 269: 5203–5214 [DOI] [PubMed] [Google Scholar]
- Garcia M., Darzacq X., Delaveau T., Jourdren L., Singer R. H., et al. , 2007a. Mitochondria-associated yeast mRNAs and the biogenesis of molecular complexes. Mol. Biol. Cell 18: 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Darzacq X., Devaux F., Singer R. H., Jacq C., 2007b. Yeast mitochondrial transcriptomics. Methods Mol. Biol. 372: 505–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Delaveau T., Goussard S., Jacq C., 2010. Mitochondrial presequence and open reading frame mediate asymmetric localization of messenger RNA. EMBO Rep. 11: 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodriguez L. J., Gay A. C., Pon L. A., 2007. Puf3p, a Pumilio family RNA binding protein, localizes to mitochondria and regulates mitochondrial biogenesis and motility in budding yeast. J. Cell Biol. 176: 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner F., Voos W., Querol A., Miller B. R., Craig E. A., et al. , 1995. Mitochondrial import of subunit Va of cytochrome c oxidase characterized with yeast mutants: independence from receptors but requirement for matrix hsp70 translocase function. J. Biol. Chem. 270: 3788–3795 [DOI] [PubMed] [Google Scholar]
- Gautschi M., Lilie H., Funfschilling U., Mun A., Ross S., et al. , 2001. RAC, a stable ribosome-associated complex in yeast formed by the DnaK-DnaJ homologs Ssz1p and zuotin. Proc. Natl. Acad. Sci. USA 98: 3762–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert N., Ryan M. T., Pfanner N., Wiedemann N., Stojanovski D., 2011. Mitochondrial protein import machineries and lipids: a functional connection. Biochim. Biophys. Acta 1808: 1002–1011 [DOI] [PubMed] [Google Scholar]
- Gentle I., Gabriel K., Beech P., Waller R., Lithgow T., 2004. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164: 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. P., Herschlag D., Brown P. O., 2004. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2: E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., et al. , 2003. Global analysis of protein expression in yeast. Nature 425: 737–741 [DOI] [PubMed] [Google Scholar]
- Glerum D. M., Shtanko A., Tzagoloff A., 1996. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem. 271: 14504–14509 [DOI] [PubMed] [Google Scholar]
- Glick G. S., Brandt A., Cunningham K., Müller S., Hallberg R. L., et al. , 1992. Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell 69: 809–822 [DOI] [PubMed] [Google Scholar]
- Gray M. W., Burger G., Lang B. F., 2001. The origin and early evolution of mitochondria. Genome Biol. 2: REVIEWS1018
- Green-Willms N. S., Butler C. A., Dunstan H. M., Fox T. D., 2001. Pet111p, an inner membrane-bound translational activator that limits expression of the Saccharomyces cerevisiae mitochondrial gene COX2. J. Biol. Chem. 276: 6392–6397 [DOI] [PubMed] [Google Scholar]
- Groot G. S. P., Mason T. L., Van Harten-Loosbrock N., 1979. Var1 is associated with the small ribosomal subunit of mitochondrial ribosomes in yeast. Mol. Gen. Genet. 174: 339–342 [DOI] [PubMed] [Google Scholar]
- Gross D. P., Burgard C. A., Reddehase S., Leitch J. M., Culotta V. C., et al. , 2011. Mitochondrial Ccs1 contains a structural disulfide bond crucial for the import of this unconventional substrate by the disulfide relay system. Mol. Biol. Cell 22: 3758–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruschke S., Groene K., Heublein M., Holz S., Israel L., et al. , 2010. Proteins at the polypeptide tunnel exit of the yeast mitochondrial ribosome. J. Biol. Chem. 285: 19022–19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruschke S., Kehrein K., Rompler K., Grone K., Israel L., et al. , 2011. Cbp3-Cbp6 interacts with the yeast mitochondrial ribosomal tunnel exit and promotes cytochrome b synthesis and assembly. J. Cell Biol. 193: 1101–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner M., Korner C., Walther D., Mokranjac D., Kaesmacher J., et al. , 2011a. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 30: 4356–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner M., Neupert W., Deponte M., 2011b. Lateral release of proteins from the TOM complex into the outer membrane of mitochondria. EMBO J. 30: 3232–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., 1996. Molecular chaperones in cellular protein folding. Nature 381: 571–579 [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Schmidt B., Wachter E., Weiss H., Neupert W., 1986. Transport into mitochondria and intramitochondrial sorting of the Fe/S protein of ubiquinol-cytochrome c reductase. Cell 47: 939–951 [DOI] [PubMed] [Google Scholar]
- He J., Cooper H. M., Reyes A., Di Re M., Sembongi H., et al. , 2012. Mitochondrial nucleoid interacting proteins support mitochondrial protein synthesis. Nucleic Acids Res. 40: 6109–6121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Fox T. D., 1997. Membrane translocation of mitochondrially coded Cox2p: distinct requirements for export of amino- and carboxy-termini, and dependence on the conserved protein Oxa1p. Mol. Biol. Cell 8: 1449–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Fox T. D., 1999. Mutations affecting a yeast mitochondrial inner membrane protein, Pnt1p, block export of a mitochondrially synthesized fusion protein from the matrix. Mol. Cell. Biol. 19: 6598–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer J., Braun H. P., Boekema E. J., Kouril R., 2007. A structural model of the cytochrome C reductase/oxidase supercomplex from yeast mitochondria. J. Biol. Chem. 282: 12240–12248 [DOI] [PubMed] [Google Scholar]
- Hell K., Herrmann J., Pratje E., Neupert W., Stuart R. A., 1997. Oxa1p mediates the export of the N- and C-termini of pCoxII from the mitochondrial matrix to the intermembrane space. FEBS Lett. 418: 367–370 [DOI] [PubMed] [Google Scholar]
- Hell K., Herrmann J. M., Pratje E., Neupert W., Stuart R. A., 1998. Oxa1p, an essential component of the N-tail protein export machinery in mitochondria. Proc. Natl. Acad. Sci. USA 95: 2250–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell K., Tzagoloff A., Neupert W., Stuart R. A., 2000. Identification of Cox20p, a novel protein involved in the maturation and assembly of cytochrome oxidase subunit 2. J. Biol. Chem. 275:4571–4578 [DOI] [PubMed] [Google Scholar]
- Hell K., Neupert W., Stuart R. A., 2001. Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 20: 1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. M., Funes S., 2005. Biogenesis of cytochrome oxidase-sophisticated assembly lines in the mitochondrial inner membrane. Gene 354: 43–52 [DOI] [PubMed] [Google Scholar]
- Herrmann J. M., Riemer J., 2012. Mitochondrial disulfide relay: redox-regulated protein import into the intermembrane space. J. Biol. Chem. 287: 4426–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. M., Stuart R. A., Craig E. A., Neupert W., 1994. Mitochondrial heat shock protein 70, a molecular chaperone for proteins encoded by mitochondrial DNA. J. Cell Biol. 127: 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. M., Neupert W., Stuart R. A., 1997. Insertion into the mitochondrial inner membrane of a polytopic protein, the nuclear-encoded Oxa1p. EMBO J. 16: 2217–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D. C., Myers C. L., Huttenhower C., Hibbs M. A., Hayes A. P., et al. , 2009. Computationally driven, quantitative experiments discover genes required for mitochondrial biogenesis. PLoS Genet. 5: e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt V., Alcock F., Lithgow T., 2011. Minor modifications and major adaptations: the evolution of molecular machines driving mitochondrial protein import. Biochim. Biophys. Acta 1808: 947–954 [DOI] [PubMed] [Google Scholar]
- Hill K., Model K., Ryan M. T., Dietmeier K., Martin F., et al. , 1998. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395: 516–521 [DOI] [PubMed] [Google Scholar]
- Hoppins S. C., Nargang F. E., 2004. The Tim8-Tim13 complex of Neurospora crassa functions in the assembly of proteins into both mitochondrial membranes. J. Biol. Chem. 279: 12396–12405 [DOI] [PubMed] [Google Scholar]
- Hoppins S., Collins S. R., Cassidy-Stone A., Hummel E., Devay R. M., et al. , 2011. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell Biol. 195: 323–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan S., Bourges I., Taanman J. W., Meunier B., 2005. Analysis of COX2 mutants reveals cytochrome oxidase subassemblies in yeast. Biochem. J. 390: 703–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D., Barrientos A., 2008. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life 60: 421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Dong L., Outten C. E., 2008. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J. Biol. Chem. 283: 29126–29134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett J. M., Lueder F., Chan N. C., Perry A. J., Wolynec P., et al. , 2008. The transmembrane segment of Tom20 is recognized by Mim1 for docking to the mitochondrial TOM complex. J. Mol. Biol. 376: 694–704 [DOI] [PubMed] [Google Scholar]
- Ishikawa D., Yamamoto H., Tamura Y., Moritoh K., Endo T., 2004. Two novel proteins in the mitochondrial outer membrane mediate beta-barrel protein assembly. J. Cell Biol. 166: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas-Osuna M. A., Ellis T. P., Marnell L. L., Mittelmeier T. M., Dieckmann C. L., 2002. Cbp1 is required for translation of the mitochondrial cytochrome b mRNA of Saccharomyces cerevisiae. J. Biol. Chem. 277: 37987–37990 [DOI] [PubMed] [Google Scholar]
- Jackson J. S., Jr, Houshmandi S. S., Lopez Leban F., Olivas W. M., 2004. Recruitment of the Puf3 protein to its mRNA target for regulation of mRNA decay in yeast. RNA 10: 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambhekar A., Amon A., 2008. Control of meiosis by respiration. Curr. Biol. 18: 969–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan P.-S., Esser K., Pratje E., Michaelis G., 2000. Som1, a third component of the yeast mitochondrial inner membrane peptidase complex that contains Imp1 and Imp2. Mol. Gen. Genet. 263: 483–491 [DOI] [PubMed] [Google Scholar]
- Jia L., Dienhart M., Schramp M., McCauley M., Hell K., et al. , 2003. Yeast Oxa1 interacts with mitochondrial ribosomes: the importance of the C-terminal region of Oxa1. EMBO J. 22: 6438–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Dienhart M. K., Stuart R. A., 2007. Oxa1 directly interacts with Atp9 and mediates its assembly into the mitochondrial F1Fo-ATP synthase complex. Mol. Biol. Cell 18: 1897–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Kaur J., Stuart R. A., 2009. Mapping the yeast Oxa1-mitochondrial ribosome interface and identification of MrpL40, a ribosomal protein in close proximity to Oxa1 and critical for oxidative phosphorylation complex assembly. Eukaryot. Cell 8: 1792–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P.-J., Ostermann J., Shilling J., Neupert W., Craig E. A., et al. , 1990. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature 348: 137–142 [DOI] [PubMed] [Google Scholar]
- Karniely S., Regev-Rudzki N., Pines O., 2006. The presequence of fumarase is exposed to the cytosol during import into mitochondria. J. Mol. Biol. 358: 396–405 [DOI] [PubMed] [Google Scholar]
- Kaufman B. A., Newman S. M., Hallberg R. L., Slaughter C. A., Perlman P. S., et al. , 2000. In organello formaldehyde crosslinking of proteins to mtDNA: identification of bifunctional proteins. Proc. Natl. Acad. Sci. USA 97: 7772–7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems R. E., Allison V. F., Butow R. A., 1975. Cytoplasmic type 80S ribosomes associated with yeast mitochondria IV: attachment of ribosomes to the outer membrane of isolated mitochondria. J. Cell Biol. 65: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper C., Habib S. J., Engl G., Heckmeyer P., Dimmer K. S., et al. , 2008. Integration of tail-anchored proteins into the mitochondrial outer membrane does not require any known import components. J. Cell Sci. 121: 1990–1998 [DOI] [PubMed] [Google Scholar]
- Kerscher O., Holder J., Srinivasan M., Leung R. S., Jensen R. E., 1997. The Tim54p-Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane. J. Cell Biol. 139: 1663–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalimonchuk O., Rodel G., 2005. Biogenesis of cytochrome c oxidase. Mitochondrion 5: 363–388 [DOI] [PubMed] [Google Scholar]
- Khalimonchuk O., Bird A., Winge D. R., 2007. Evidence for a Pro-oxidant Intermediate in the Assembly of Cytochrome Oxidase. J. Biol. Chem. 282: 17442–17449 [DOI] [PubMed] [Google Scholar]
- Khalimonchuk O., Bestwick M., Meunier B., Watts T. C., Winge D. R., 2010. Formation of the redox cofactor centers during Cox1 maturation in yeast cytochrome oxidase. Mol. Cell. Biol. 30: 1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G., Csere P., Prohl C., Lill R., 1999. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 18: 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G., Sipos K., Lange H., Fekete Z., Bedekovics T., et al. , 2005. Biogenesis of cytosolic ribosomes requires the essential iron-sulphur protein Rli1p and mitochondria. EMBO J. 24: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel C., Suzuki Y., Kojer K., Petrungaro C., Longen S., et al. , 2011. Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol. Mol. Biol. Cell 22: 3749–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler C. M., Tienson H. L., 2009. Redox regulation of protein folding in the mitochondrial intermembrane space. Biochim. Biophys. Acta 1793: 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler C. M., Jarosch E., Tokatlidis K., Schmid K., Schweyen R. J., et al. , 1998. Import of mitochondrial carriers mediated by essential proteins of the intermembrane space. Science 279: 369–373 [DOI] [PubMed] [Google Scholar]
- Kornmann B., Currie E., Collins S. R., Schuldiner M., Nunnari J., et al. , 2009. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovermann P., Truscott K. N., Guiard B., Rehling P., Sepuri N. B., et al. , 2002. Tim22, the essential core of the mitochondrial protein insertion complex, forms a voltage-activated and signal-gated channel. Mol. Cell 9: 363–373 [DOI] [PubMed] [Google Scholar]
- Krause K., Lopes de Souza R., Roberts D. G., Dieckmann C. L., 2004. The mitochondrial message-specific mRNA protectors Cbp1 and Pet309 are associated in a high-molecular weight complex. Mol. Biol. Cell 15: 2674–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimmer T., Rapaport D., Ryan M. T., Meisinger C., Kassenbrock C. K., et al. , 2001. Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the TOM complex. J. Cell Biol. 152: 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucej M., Kucejova B., Subramanian R., Chen X. J., Butow R. A., 2008. Mitochondrial nucleoids undergo remodeling in response to metabolic cues. J. Cell Sci. 121: 1861–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl I., Dujeancourt L., Gaisne M., Herbert C. J., Bonnefoy N., 2011. A genome wide study in fission yeast reveals nine PPR proteins that regulate mitochondrial gene expression. Nucleic Acids Res. 39: 8029–8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künkele K. P., Juin P., Pompa C., Nargang F. E., Henry J. P., et al. , 1998. The isolated complex of the translocase of the outer membrane of mitochondria. Characterization of the cation-selective and voltage-gated preprotein-conducting pore. J. Biol. Chem. 273: 31032–31039 [DOI] [PubMed] [Google Scholar]
- Kurz M., Martin H., Rassow J., Pfanner N., Ryan M. T., 1999. Biogenesis of Tim proteins of the mitochondrial carrier import pathway: differential targeting mechanisms and crossing over with the main import pathway. Mol. Biol. Cell 10: 2461–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutik S., Stojanovski D., Becker L., Becker T., Meinecke M., et al. , 2008. Dissecting membrane insertion of mitochondrial beta-barrel proteins. Cell 132: 1011–1024 [DOI] [PubMed] [Google Scholar]
- Kutik S., Stroud D. A., Wiedemann N., Pfanner N., 2009. Evolution of mitochondrial protein biogenesis. Biochim. Biophys. Acta 1790: 409–415 [DOI] [PubMed] [Google Scholar]
- Lange C., Hunte C., 2002. Crystal structure of the yeast cytochrome bc1 complex with its bound substrate cytochrome c. Proc. Natl. Acad. Sci. USA 99: 2800–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre-Legendre L., Vaillier J., Benabdelhak H., Velours J., Slonimski P. P., et al. , 2001. Identification of a nuclear gene (FMC1) required for the assembly/stability of yeast mitochondrial F(1)-ATPase in heat stress conditions. J. Biol. Chem. 276: 6789–6796 [DOI] [PubMed] [Google Scholar]
- Lelandais G., Saint-Georges Y., Geneix C., Al-Shikhley L., Dujardin G., et al. , 2009. Spatio-temporal dynamics of yeast mitochondrial biogenesis: transcriptional and post-transcriptional mRNA oscillatory modules. PLOS Comput. Biol. 5: e1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire C., Hamel P., Velours J., Dujardin G., 2000. Absence of the mitochondrial AAA protease Yme1p restores ATPase F0 subunit accumulation in an oxa1 deletion mutant of Saccharomyces cerevisiae. J. Biol. Chem. 275: 23471–23475 [DOI] [PubMed] [Google Scholar]
- Leonhardt S. A., Fearon K., Danese P. N., Mason T. L., 1993. HSP78 encodes a yeast mitochondrial heat shock protein in the clp family of ATP-dependent proteases. Mol. Cell. Biol. 13: 6304–6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., 2009. Function and biogenesis of iron-sulphur proteins. Nature 460: 831–838 [DOI] [PubMed] [Google Scholar]
- Lipinski K. A., Kaniak-Golik A., Golik P., 2010. Maintenance and expression of the S. cerevisiae mitochondrial genome: from genetics to evolution and systems biology. Biochim. Biophys. Acta 1797: 1086–1098 [DOI] [PubMed] [Google Scholar]
- Lipinski K. A., Puchta O., Surandranath V., Kudla M., Golik P., 2011. Revisiting the yeast PPR proteins: application of an iterative hidden Markov model algorithm reveals new members of the rapidly evolving family. Mol. Biol. Evol. 28: 2935–2948 [DOI] [PubMed] [Google Scholar]
- Lithgow T., 2000. Targeting of proteins to mitochondria. FEBS Lett. 476: 22–26 [DOI] [PubMed] [Google Scholar]
- Liu J. M., Liu D. R., 2007. Discovery of a mRNA mitochondrial localization element in Saccharomyces cerevisiae by nonhomologous random recombination and in vivo selection. Nucleic Acids Res. 35: 6750–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode A., Kuschel M., Paret C., Rödel G., 2000. Mitochondrial copper metabolism in yeast: interaction between Sco1p and Cox2p. FEBS Lett. 485: 19–24 [DOI] [PubMed] [Google Scholar]
- Lu H., Allen S., Wardleworth L., Savory P., Tokatlidis K., 2004. Functional TIM10 chaperone assembly is redox-regulated in vivo. J. Biol. Chem. 279: 18952–18958 [DOI] [PubMed] [Google Scholar]
- Ludlam A., Brunzelle J., Pribyl T., Xu X., Gatti D. L., et al. , 2009. Chaperones of F1-ATPase. J. Biol. Chem. 284: 17138–17146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueder F., Lithgow T., 2009. The three domains of the mitochondrial outer membrane protein Mim1 have discrete functions in assembly of the TOM complex. FEBS Lett. 583: 1475–1480 [DOI] [PubMed] [Google Scholar]
- Lupo D., Vollmer C., Deckers M., Mick D. U., Tews I., et al. , 2011. Mdm38 is a 14–3-3-like receptor and associates with the protein synthesis machinery at the inner mitochondrial membrane. Traffic 12: 1457–1456 [DOI] [PubMed] [Google Scholar]
- Lutz T., Neupert W., Herrmann J. M., 2003. Import of small Tim proteins into the mitochondrial intermembrane space. EMBO J. 22: 4400–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G., 1979. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc. Natl. Acad. Sci. USA 76: 343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A., Pfeiffer D. R., Bradshaw P. C., Moraru I. I., Slepchenko B., et al. , 2001. Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life 52: 93–100 [DOI] [PubMed] [Google Scholar]
- Manning-Krieg U. C., Scherer P. E., Schatz G., 1991. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 10: 3273–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey G. M., McEwen J. E., 1995. The product of the nuclear gene PET309 is required for translation of mature mRNA and stability or production of intron-containing RNAs derived from the mitochondrial COX1 locus of Saccharomyces cerevisiae. EMBO J. 14: 4031–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc P., Margeot A., Devaux F., Blugeon C., Corral-Debrinski M., et al. , 2002. Genome-wide analysis of mRNAs targeted to yeast mitochondria. EMBO Rep. 3: 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeot A., Blugeon C., Sylvestre J., Vialette S., Jacq C., et al. , 2002. In Saccharomyces cerevisiae, ATP2 mRNA sorting to the vicinity of mitochondria is essential for respiratory function. EMBO J. 21: 6893–6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeot A., Garcia M., Wang W., Tetaud E., di Rago J. P., et al. , 2005. Why are many mRNAs translated to the vicinity of mitochondria: a role in protein complex assembly? Gene 354: 64–71 [DOI] [PubMed] [Google Scholar]
- Markov D. A., Savkina M., Anikin M., Del Campo M., Ecker K., et al. , 2009. Identification of proteins associated with the yeast mitochondrial RNA polymerase by tandem affinity purification. Yeast 26: 423–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom M., Azem A., Mokranjac D., 2011a. Understanding the molecular mechanism of protein translocation across the mitochondrial inner membrane: still a long way to go. Biochim. Biophys. Acta 1808: 990–1001 [DOI] [PubMed] [Google Scholar]
- Marom M., Dayan D., Demishtein-Zohary K., Mokranjac D., Neupert W., et al. , 2011b. Direct interaction of mitochondrial targeting presequences with purified components of the TIM23 complex. J. Biol. Chem. 286: 43809–43815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marykwas D. L., Fox T. D., 1989. Control of the Saccharomyces cerevisiae regulatory gene PET494: transcriptional repression by glucose and translational induction by oxygen. Mol. Cell. Biol. 9: 484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuki S., Hibbs A. R., 1986. Are all mitochondrial translation products synthesized on membrane-bound ribosomes? Biochim. Biophys. Acta 866: 120–124 [DOI] [PubMed] [Google Scholar]
- Mathieu L., Bourens M., Marsy S., Hlavacek O., Panozzo C., et al. , 2010. A mutational analysis reveals new functional interactions between domains of the Oxa1 protein in Saccharomyces cerevisiae. Mol. Microbiol. 75: 474–488 [DOI] [PubMed] [Google Scholar]
- Mathieu L., Marsy S., Saint-Georges Y., Jacq C., Dujardin G., 2011. A transcriptome screen in yeast identifies a novel assembly factor for the mitochondrial complex III. Mitochondrion 11: 391–396 [DOI] [PubMed] [Google Scholar]
- Meeusen S., Nunnari J., 2003. Evidence for a two membrane-spanning autonomous mitochondrial DNA replisome. J. Cell Biol. 163: 503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen S., Tieu Q., Wong E., Weiss E., Schieltz D., et al. , 1999. Mgm101p is a novel component of the mitochondrial nucleoid that binds DNA and is required for the repair of oxidatively damaged mitochondrial DNA. J. Cell Biol. 145: 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke M., Wagner R., Kovermann P., Guiard B., Mick D. U., et al. , 2006. Tim50 maintains the permeability barrier of the mitochondrial inner membrane. Science 312: 1523–1526 [DOI] [PubMed] [Google Scholar]
- Mesecke N., Terziyska N., Kozany C., Baumann F., Neupert W., et al. , 2005. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121: 1059–1069 [DOI] [PubMed] [Google Scholar]
- Messens J., Collet J. F., 2006. Pathways of disulfide bond formation in Escherichia coli. Int. J. Biochem. Cell Biol. 38: 1050–1062 [DOI] [PubMed] [Google Scholar]
- Michon T., Galante M., Velours J., 1988. NH2-terminal sequence of the isolated yeast ATP synthase subunit 6 reveals post-translational cleavage. Eur. J. Biochem. 172: 621–625 [DOI] [PubMed] [Google Scholar]
- Mick D. U., Wagner K., van der Laan M., Frazier A. E., Perschil I., et al. , 2007. Shy1 couples Cox1 translational regulation to cytochrome c oxidase assembly. EMBO J. 26: 4347–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick D. U., Vukotic M., Piechura H., Meyer H. E., Warscheid B., et al. , 2010. Coa3 and Cox14 are essential for negative feedback regulation of COX1 translation in mitochondria. J. Cell Biol. 191: 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick D. U., Fox T. D., Rehling P., 2011. Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat. Rev. Mol. Cell Biol. 12: 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic D., Ramming T., Muller J. M., Wenz L. S., Gebert N., et al. , 2009. Identification of the signal directing Tim9 and Tim10 into the intermembrane space of mitochondria. Mol. Biol. Cell 20: 2530–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model K., Meisinger C., Prinz T., Wiedemann N., Truscott K. N., et al. , 2001. Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nat. Struct. Biol. 8: 361–370 [DOI] [PubMed] [Google Scholar]
- Mokranjac D., Neupert W., 2009. Thirty years of protein translocation into mitochondria: unexpectedly complex and still puzzling. Biochim. Biophys. Acta 1793: 33–41 [DOI] [PubMed] [Google Scholar]
- Mokranjac D., Popov-Celeketić D., Hell K., Neupert W., 2005. Role of Tim21 in mitochondrial translocation contact sites. J. Biol. Chem. 280: 23437–23440 [DOI] [PubMed] [Google Scholar]
- Mokranjac D., Sichting M., Popov-Celeketić D., Mapa K., Gevorkyan-Airapetov L., et al. , 2009. Role of Tim50 in the transfer of precursor proteins from the outer to the inner membrane of mitochondria. Mol. Biol. Cell 20: 1400–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naithani S., Saracco S. A., Butler C. A., Fox T. D., 2003. Interactions among COX1, COX2 and COX3 mRNA-specific translational activator proteins on the inner surface of the mitochondrial inner membrane of Saccharomyces cerevisiae. Mol. Biol. Cell 14: 324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoe M., Ohwa Y., Ishikawa D., Ohshima C., Nishikawa S., et al. , 2004. Identification of Tim40 that mediates protein sorting to the mitochondrial intermembrane space. J. Biol. Chem. 279: 47815–47821 [DOI] [PubMed] [Google Scholar]
- Neupert W., 1997. Protein import into mitochondria. Annu. Rev. Biochem. 66: 863–917 [DOI] [PubMed] [Google Scholar]
- Neupert W., Herrmann J. M., 2007. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76: 723–749 [DOI] [PubMed] [Google Scholar]
- Nevo-Dinur K., Nussbaum-Shochat A., Ben-Yehuda S., Amster-Choder O., 2011. Translation-independent localization of mRNA in E. coli. Science 331: 1081–1084 [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Nobrega M. P., Tzagoloff A., 1992. BCS1, a novel gene required for the expression of functional Rieske iron-sulfur protein in Saccharomyces cerevisiae. EMBO J. 11: 3821–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouet C., Bourens M., Hlavacek O., Marsy S., Lemaire C., et al. , 2007. Rmd9p controls the processing/stability of mitochondrial mRNAs and its overexpression compensates for a partial deficiency of Oxa1p in Saccharomyces cerevisiae. Genetics 175: 1105–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowikovsky K., Froschauer E. M., Zsurka G., Samaj J., Reipert S., et al. , 2004. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J. Biol. Chem. 279: 30307–30315 [DOI] [PubMed] [Google Scholar]
- Nunnari J., Suomalainen A., 2012. Mitochondria: in sickness and in health. Cell 148: 1145–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J., Fox T. D., Walter P., 1993. A mitochondrial protease with two catalytic subunits of nonoverlapping specificities. Science 262: 1997–2004 [DOI] [PubMed] [Google Scholar]
- Nunnari J., Marshall W. F., Straight A., Murray A., Sedat J. W., et al. , 1997. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol. Biol. Cell 8: 1233–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivas W., Parker R., 2000. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J. 19: 6602–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C., Wilmes C., Tatsuta T., Langer T., 2007. Prohibitins interact genetically with Atp23, a novel processing peptidase and chaperone for the F1Fo-ATP synthase. Mol. Biol. Cell 18: 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander D. B., Zhang M., Mileykovskaya E., Rho M., Dowhan W., 2001. Lack of mitochondrial anionic phospholipids causes an inhibition of translation of protein components of the electron transport chain. A yeast genetic model system for the study of anionic phospholipid function in mitochondria. J. Biol. Chem. 276: 25262–25272 [DOI] [PubMed] [Google Scholar]
- Ott M., Herrmann J. M., 2010. Co-translational membrane insertion of mitochondrially encoded proteins. Biochim. Biophys. Acta 1803: 767–775 [DOI] [PubMed] [Google Scholar]
- Ott M., Prestele M., Bauerschmitt H., Funes S., Bonnefoy N., et al. , 2006. Mba1, a membrane-associated ribosome receptor in mitochondria. EMBO J. 25: 1603–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais J. E., Schilke B., Craig E. A., 2011. Reevaluation of the role of the Pam18:Pam16 interaction in translocation of proteins by the mitochondrial Hsp70-based import motor. Mol. Biol. Cell 22: 4740–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F., Agrimi G., Blanco E., Castegna A., Di Noia M. A., et al. , 2006. Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim. Biophys. Acta 1757: 1249–1262 [DOI] [PubMed] [Google Scholar]
- Papic D., Krumpe K., Dukanovic J., Dimmer K. S., Rapaport D., 2011. Multispan mitochondrial outer membrane protein Ugo1 follows a unique Mim1-dependent import pathway. J. Cell Biol. 194: 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen S. A., Rothbauer U., Kaldi K., Bauer M. F., Neupert W., et al. , 2000. The role of the TIM8–13 complex in the import of Tim23 into mitochondria. EMBO J. 19: 6392–6400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen S. A., Waizenegger T., Stan T., Preuss M., Cyrklaff M., et al. , 2003. Evolutionary conservation of biogenesis of beta-barrel membrane proteins. Nature 426: 862–866 [DOI] [PubMed] [Google Scholar]
- Payne M. J., Finnegan P. M., Smooker P. M., Lukins H. B., 1993. Characterization of a second nuclear gene, AEP1, required for expression of the mitochondrial OLI1 gene in Saccharomyces cerevisiae. Curr. Genet. 24: 126–135 [DOI] [PubMed] [Google Scholar]
- Peixoto P. M., Grana F., Roy T. J., Dunn C. D., Flores M., et al. , 2007. Awaking TIM22, a dynamic ligand-gated channel for protein insertion in the mitochondrial inner membrane. J. Biol. Chem. 282: 18694–18701 [DOI] [PubMed] [Google Scholar]
- Perez-Martinez X., Broadley S. A., Fox T. D., 2003. Mss51p promotes mitochondrial Cox1p synthesis and interacts with newly synthesized Cox1p. EMBO J. 22: 5951–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martinez X., Butler C. A., Shingu-Vazquez M., Fox T. D., 2009. Dual functions of Mss51 couple synthesis of Cox1 to assembly of cytochrome c oxidase in Saccharomyces cerevisiae mitochondria. Mol. Biol. Cell 20: 4371–4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrel F., Bestwick M. L., Cobine P. A., Khalimonchuk O., Cricco J. A., et al. , 2007. Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 26: 4335–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrel F., Khalimonchuk O., Cobine P. A., Bestwick M., Winge D. R., 2008. Coa2 is an assembly factor for yeast cytochrome c oxidase biogenesis facilitating the maturation of Cox1. Mol. Cell. Biol. 28: 4927–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon L., Schatz G., 1991. Biogenesis of yeast mitochondria, pp. 333–406 in The Molecular and Cellular Biology of the Yeast Saccharomyces: Genome Dynamics, Protein Synthesis and Energetics, edited by Broach J. R., Pringle J. R., Jones E. W. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Pon L., Moll T., Vestweber D., Marshallsay B., Schatz G., 1989. Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J. Cell Biol. 109: 2603–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov-Čeleketić D., Mapa K., Neupert W., Mokranjac D., 2008a. Active remodelling of the TIM23 complex during translocation of preproteins into mitochondria. EMBO J. 27: 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov-Čeleketić J., Waizenegger T., Rapaport D., 2008b. Mim1 functions in an oligomeric form to facilitate the integration of Tom20 into the mitochondrial outer membrane. J. Mol. Biol. 376: 671–680 [DOI] [PubMed] [Google Scholar]
- Popov-Čeleketić D., Waegemann K., Mapa K., Neupert W., Mokranjac D., 2011. Role of the import motor in insertion of transmembrane segments by the mitochondrial TIM23 complex. EMBO Rep. 12: 542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratje E., Mannhaupt G., Michaelis G., Beyreuther K., 1983. A nuclear mutation prevents processing of a mitochondrially encoded membrane protein in Saccharomyces cerevisiae. EMBO J. 2: 1049–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premsler T., Zahedi R. P., Lewandrowski U., Sickmann A., 2009. Recent advances in yeast organelle and membrane proteomics. Proteomics 9: 4731–4743 [DOI] [PubMed] [Google Scholar]
- Prestele M., Vogel F., Reichert A. S., Herrmann J. M., Ott M., 2009. Mrpl36 is important for generation of assembly competent proteins during mitochondrial translation. Mol. Biol. Cell 20: 2615–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss M., Leonhard K., Hell K., Stuart R. A., Neupert W., et al. , 2001. Mba1, a novel component of the mitochondrial protein export machinery of the yeast Saccharomyces cerevisiae. J. Cell Biol. 153: 1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokisch H., Scharfe C., Camp D. G., II, Xiao W., David L., et al. , 2004. Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol. 2: 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenault T., Lithgow T., Traven A., 2011. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol. 21: 104–112 [DOI] [PubMed] [Google Scholar]
- Rabl R., Soubannier V., Scholz R., Vogel F., Mendl N., et al. , 2009. Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. J. Cell Biol. 185: 1047–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P., Dolezal P., Jedelsky P. L., Bursac D., Perry A. J., et al. , 2011. The core components of organelle biogenesis and membrane transport in the hydrogenosomes of Trichomonas vaginalis. PLoS ONE 6: e24428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M., Tzagoloff A., 2009. F1-dependent translation of mitochondrially encoded Atp6p and Atp8p subunits of yeast ATP synthase. Proc. Natl. Acad. Sci. USA 106: 18509–18514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M., Zeng X., Briere J. J., Tzagoloff A., 2009. Assembly of F0 in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1793: 108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak M., Gokova S., Tzagoloff A., 2011. Modular assembly of yeast mitochondrial ATP synthase. EMBO J. 30: 920–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G., 1993. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 12: 4115–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P., Model K., Brandner K., Kovermann P., Sickmann A., et al. , 2003. Protein insertion into the mitochondrial inner membrane by a twin-pore translocase. Science 299: 1747–1751 [DOI] [PubMed] [Google Scholar]
- Reif S., Randelj O., Domanska G., Dian E. A., Krimmer T., et al. , 2005. Conserved mechanism of Oxa1 insertion into the mitochondrial inner membrane. J. Mol. Biol. 354: 520–528 [DOI] [PubMed] [Google Scholar]
- Reinders J., Zahedi R. P., Pfanner N., Meisinger C., Sickmann A., 2006. Toward the complete yeast mitochondrial proteome: multidimensional separation techniques for mitochondrial proteomics. J. Proteome Res. 5: 1543–1554 [DOI] [PubMed] [Google Scholar]
- Riemer J., Fischer M., Herrmann J. M., 2011. Oxidation-driven protein import into mitochondria: insights and blind spots. Biochim. Biophys. Acta 1808: 981–989 [DOI] [PubMed] [Google Scholar]
- Riezman H., Hay R., Gasser S., Daum G., Schneider G., et al. , 1983. The outer membrane of yeast mitochondria: isolation of outside-out sealed vesicles. EMBO J. 2: 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby K., Cobine P. A., Khalimonchuk O., Winge D. R., 2008. Mapping the functional interaction of Sco1 and Cox2 in cytochrome oxidase biogenesis. J. Biol. Chem. 283: 15015–15022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer M. S., Shadel G. S., 2003. Multiple interactions involving the amino-terminal domain of yeast mtRNA polymerase determine the efficiency of mitochondrial protein synthesis. J. Biol. Chem. 278: 18695–18701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer M. S., Boone B. E., Bryan A. C., Shadel G. S., 2001. Nam1p, a protein involved in RNA processing and translation, is coupled to transcription through an interaction with yeast mitochondrial RNA polymerase. J. Biol. Chem. 276: 8616–8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel G., 1997. Translational activator proteins required for cytochrome b synthesis in Saccharomyces cerevisiae. Curr. Genet. 31: 375–379 [DOI] [PubMed] [Google Scholar]
- Rouillard J. M., Dufour M. E., Theunissen B., Mandart E., Dujardin G., et al. , 1996. SLS1, a new Saccharomyces cerevisiae gene involved in mitochondrial metabolism, isolated as a synthetic lethal in association with an SSM4 deletion. Mol. Gen. Genet. 252: 700–708 [DOI] [PubMed] [Google Scholar]
- Saint-Georges Y., Garcia M., Delaveau T., Jourdren L., Le Crom S., et al. , 2008. Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS ONE 3: e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha R., Mohr G., Belfort M., Lambowitz A. M., 1993. Group I and group II introns. FASEB J. 7: 15–24 [DOI] [PubMed] [Google Scholar]
- Sanchirico M. E., 1998. Understanding Mitochondrial Biogenesis Through Gene Relocation. Ph.D. Thesis, University of Massachusetts, Amherst, MA [Google Scholar]
- Sanchirico M. E., Fox T. D., Mason T. L., 1998. Accumulation of mitochondrially synthesized Saccharomyces cerevisiae Cox2p and Cox3p depends on targeting information in untranslated portions of their mRNAs. EMBO J. 17: 5796–5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracco S. A., Fox T. D., 2002. Cox18p is required for export of the mitochondrially encoded Saccharomyces cerevisiae Cox2p C-tail, and interacts with Pnt1p and Mss2p in the inner membrane. Mol. Biol. Cell 13: 1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman F., Brunel-Guitton C., Antonicka H., Wai T., Shoubridge E. A., et al. , 2010. LRPPRC and SLIRP interact in a ribonucleoprotein complex that regulates posttranscriptional gene expression in mitochondria. Mol. Biol. Cell 21: 1315–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass E., Karniely S., Pines O., 2003. Folding of fumarase during mitochondrial import determines its dual targeting in yeast. J. Biol. Chem. 278: 45109–45116 [DOI] [PubMed] [Google Scholar]
- Schatz G., 1968. Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic “petite” mutant of Saccharomyces cerevisiae. J. Biol. Chem. 243: 2192–2199 [PubMed] [Google Scholar]
- Schmidt O., Pfanner N., Meisinger C., 2010. Mitochondrial protein import: from proteomics to functional mechanisms. Nat. Rev. Mol. Cell Biol. 11: 655–667 [DOI] [PubMed] [Google Scholar]
- Schmidt O., Harbauer A. B., Rao S., Eyrich B., Zahedi R. P., et al. , 2011. Regulation of mitochondrial protein import by cytosolic kinases. Cell 144: 227–239 [DOI] [PubMed] [Google Scholar]
- Schneider A., Bursac D., Lithgow T., 2008. The direct route: a simplified pathway for protein import into the mitochondrion of trypanosomes. Trends Cell Biol. 18: 12–18 [DOI] [PubMed] [Google Scholar]
- Schulz C., Lytovchenko O., Melin J., Chacinska A., Guiard B., et al. , 2011. Tim50’s presequence receptor domain is essential for signal driven transport across the TIM23 complex. J. Cell Biol. 195: 643–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman E. L., Taylor R. D., Go N. E., Nargang F. E., 2006. Effect of mutations in Tom40 on stability of the translocase of the outer mitochondrial membrane (TOM) complex, assembly of Tom40, and import of mitochondrial preproteins. J. Biol. Chem. 281: 22554–22565 [DOI] [PubMed] [Google Scholar]
- Sherman F., 2005. The importance of mutation, then and now: studies with yeast cytochrome c. Mutat. Res. 589: 1–16 [DOI] [PubMed] [Google Scholar]
- Shingu-Vazquez M., Camacho-Villasana Y., Sandoval-Romero L., Butler C. A., Fox T. D., et al. , 2010. The carboxyl-terminal end of Cox1 is required for feedback-assembly regulation of Cox1 synthesis in Saccharomyces cerevisiae mitochondria. J. Biol. Chem. 285: 34382–34389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota T., Mabuchi H., Tanaka-Yamano S., Yamano K., Endo T., 2011. In vivo protein-interaction mapping of a mitochondrial translocator protein Tom22 at work. Proc. Natl. Acad. Sci. USA 108: 15179–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann A., Reinders J., Wagner Y., Joppich C., Zahedi R., et al. , 2003. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA 100: 13207–13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideris D. P., Petrakis N., Katrakili N., Mikropoulou D., Gallo A., et al. , 2009. A novel intermembrane space-targeting signal docks cysteines onto Mia40 during mitochondrial oxidative folding. J. Cell Biol. 187: 1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirrenberg C., Bauer M. F., Guiard B., Neupert W., Brunner M., 1996. Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature 384: 582–585 [DOI] [PubMed] [Google Scholar]
- Sirrenberg C., Endres M., Folsch H., Stuart R. A., Neupert W., et al. , 1998. Carrier protein import into mitochondria mediated by the intermembrane proteins Tim10/Mrs11 and Tim12/Mrs5. Nature 391: 912–915 [DOI] [PubMed] [Google Scholar]
- Slutsky-Leiderman O., Marom M., Iosefson O., Levy R., Maoz S., et al. , 2007. The interplay between components of the mitochondrial protein translocation motor studied using purified components. J. Biol. Chem. 282: 33935–33942 [DOI] [PubMed] [Google Scholar]
- Smith P. M., Fox J. L., Winge D. R., 2012. Biogenesis of the cytochrome bc1 complex and role of assembly factors. Biochim. Biophys. Acta 1817: 276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmaz S. R., Hunte C., 2008. Structure of complex III with bound cytochrome c in reduced state and definition of a minimal core interface for electron transfer. J. Biol. Chem. 283: 17542–17549 [DOI] [PubMed] [Google Scholar]
- Soto I. C., Fontanesi F., Liu J., Barrientos A., 2011. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta 1817: 883–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele D. F., Butler C. A., Fox T. D., 1996. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc. Natl. Acad. Sci. USA 93: 5253–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Zollner A., Haid A., Neupert W., Lill R., 1995. Biogenesis of mitochondrial heme lyases in yeast. Import and folding in the intermembrane space. J. Biol. Chem. 270: 22842–22849 [DOI] [PubMed] [Google Scholar]
- Stevens B., 1981. Mitochondrial structure, pp. 471–504 in The Molecular Biology of the Yeast Saccharomyces, Life Cycle and Inheritance, edited by Strathern J. N., Jones E. W., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Stock D., Leslie A. G., Walker J. E., 1999. Molecular architecture of the rotary motor in ATP synthase. Science 286: 1700–1705 [DOI] [PubMed] [Google Scholar]
- Stojanovski D., Guiard B., Kozjak-Pavlovic V., Pfanner N., Meisinger C., 2007. Alternative function for the mitochondrial SAM complex in biogenesis of alpha-helical TOM proteins. J. Cell Biol. 179: 881–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Jones E. W., Broach J. R. (), 1982. The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Strogolova V., Furness A., Robb-McGrath M., Garlich J., Stuart R. A., 2012. Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Mol. Cell. Biol. 32: 1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud D. A., Becker T., Qiu J., Stojanovski D., Pfannschmidt S., et al. , 2011. Biogenesis of mitochondrial beta-barrel proteins: the POTRA domain is involved in precursor release from the SAM complex. Mol. Biol. Cell 22: 2823–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R. A., 2008. Supercomplex organization of the oxidative phosphorylation enzymes in yeast mitochondria. J. Bioenerg. Biomembr. 40: 411–417 [DOI] [PubMed] [Google Scholar]
- Suissa M., Schatz G., 1982. Import of proteins into mitochondria. Translatable mRNAs for imported mitochondrial proteins are present in free as well as mitochondria-bound cytoplasmic polysomes. J. Biol. Chem. 257: 13048–13055 [PubMed] [Google Scholar]
- Suppanz I. E., Wurm C. A., Wenzel D., Jakobs S., 2009. The m-AAA protease processes cytochrome c peroxidase preferentially at the inner boundary membrane of mitochondria. Mol. Biol. Cell 20: 572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki C. K., Rep M., van Dijl J. M., Suda K., Grivell L. A., et al. , 1997. ATP-dependent proteases that also chaperone protein biogenesis. Trends Biochem. Sci. 22: 118–123 [DOI] [PubMed] [Google Scholar]
- Sylvestre J., Margeot A., Jacq C., Dujardin G., Corral-Debrinski M., 2003. The role of the 3′ untranslated region in mRNA sorting to the vicinity of mitochondria is conserved from yeast to human cells. Mol. Biol. Cell 14: 3848–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyrach G., Ott M., Bonnefoy N., Neupert W., Herrmann J. M., 2003. Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 22: 6448–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taanman J. W., Capaldi R. A., 1992. Purification of yeast cytochrome c oxidase with a subunit composition resembling the mammalian enzyme. J. Biol. Chem. 267: 22481–22485 [PubMed] [Google Scholar]
- Tamura Y., Harada Y., Shiota T., Yamano K., Watanabe K., et al. , 2009. Tim23-Tim50 pair coordinates functions of translocators and motor proteins in mitochondrial protein import. J. Cell Biol. 184: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T., Langer T., 2009. AAA proteases in mitochondria: diverse functions of membrane-bound proteolytic machines. Res. Microbiol. 160: 711–717 [DOI] [PubMed] [Google Scholar]
- Tavares-Carreon F., Camacho-Villasana Y., Zamudio-Ochoa A., Shingu-Vazquez M., Torres-Larios A., et al. , 2008. The pentatricopeptide repeats present in Pet309 are necessary for translation but not for stability of the mitochondrial COX1 mRNA in yeast. J. Biol. Chem. 283: 1472–1479 [DOI] [PubMed] [Google Scholar]
- Taylor A. B., Smith B. S., Kitada S., Kojima K., Miyaura H., et al. , 2001. Crystal structures of mitochondrial processing peptidase reveal the mode for specific cleavage of import signal sequences. Structure 9: 615–625 [DOI] [PubMed] [Google Scholar]
- Terpstra P., Zanders E., Butow R. A., 1979. The association of var1 with the 38S mitochondrial ribosomal subunit in yeast. J. Biol. Chem. 254: 12653–12661 [PubMed] [Google Scholar]
- Terziyska N., Grumbt B., Bien M., Neupert W., Herrmann J. M., et al. , 2007. The sulfhydryl oxidase Erv1 is a substrate of the Mia40-dependent protein translocation pathway. FEBS Lett. 581: 1098–1102 [DOI] [PubMed] [Google Scholar]
- Tienson H. L., Dabir D. V., Neal S. E., Loo R., Hasson S. A., et al. , 2009. Reconstitution of the mia40-erv1 oxidative folding pathway for the small tim proteins. Mol. Biol. Cell 20: 3481–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towpik J., 2005. Regulation of mitochondrial translation in yeast. Cell. Mol. Biol. Lett. 10: 571–594 [PubMed] [Google Scholar]
- Trouillard M., Meunier B., Rappaport F., 2011. Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of the respiratory chain. Proc. Natl. Acad. Sci. USA 108: E1027–E1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott K. N., Lowth B. R., Strack P. R., Dougan D. A., 2010. Diverse functions of mitochondrial AAA+ proteins: protein activation, disaggregation, and degradation. Biochem. Cell Biol. 88: 97–108 [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., et al. , 1995. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 Å. Science 269: 1069–1074 [DOI] [PubMed] [Google Scholar]
- Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., et al. , 1996. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272: 1136–1144 [DOI] [PubMed] [Google Scholar]
- Tu B. P., Kudlicki A., Rowicka M., McKnight S. L., 2005. Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science 310: 1152–1158 [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., 1969. Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J. Biol. Chem. 244: 5027–5033 [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L., 1990. PET genes of Saccharomyces cerevisiae. Microbiol. Rev. 54: 211–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Barrientos A., Neupert W., Herrmann J. M., 2004. Atp10p assists assembly of Atp6p into the F0 unit of the yeast mitochondrial ATPase. J. Biol. Chem. 279: 19775–19780 [DOI] [PubMed] [Google Scholar]
- van der Laan M., Wiedemann N., Mick D. U., Guiard B., Rehling P., et al. , 2006. A role for Tim21 in membrane-potential-dependent preprotein sorting in mitochondria. Curr. Biol. 16: 2271–2276 [DOI] [PubMed] [Google Scholar]
- van der Laan M., Meinecke M., Dudek J., Hutu D. P., Lind M., et al. , 2007. Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat. Cell Biol. 9: 1152–1159 [DOI] [PubMed] [Google Scholar]
- van Wilpe S., Ryan M. T., Hill K., Maarse A. C., Meisinger C., et al. , 1999. Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase. Nature 401: 485–489 [DOI] [PubMed] [Google Scholar]
- Vasiljev A., Ahting U., Nargang F. E., Go N. E., Habib S. J., et al. , 2004. Reconstituted TOM core complex and Tim9/Tim10 complex of mitochondria are sufficient for translocation of the ADP/ATP carrier across membranes. Mol. Biol. Cell 15: 1445–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velours J., Arselin G., 2000. The Saccharomyces cerevisiae ATP synthase. J. Bioenerg. Biomembr. 32: 383–390 [DOI] [PubMed] [Google Scholar]
- Velours J., Dautant A., Salin B., Sagot I., Brethes D., 2009. Mitochondrial F1F0-ATP synthase and organellar internal architecture. Int. J. Biochem. Cell Biol. 41: 1783–1789 [DOI] [PubMed] [Google Scholar]
- Velours J., Stines-Chaumeil C., Habersetzer J., Chaignepain S., Dautant A., et al. , 2011. Evidence of the proximity of ATP synthase subunits 6 (a) in the inner mitochondrial membrane and in the supramolecular forms of Saccharomyces cerevisiae ATP synthase. J. Biol. Chem. 286: 35477–35484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel F., Bornhovd C., Neupert W., Reichert A. S., 2006. Dynamic subcompartmentalization of the mitochondrial inner membrane. J. Cell Biol. 175: 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vögtle F. N., Wortelkamp S., Zahedi R. P., Becker D., Leidhold C., et al. , 2009. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 139: 428–439 [DOI] [PubMed] [Google Scholar]
- Vögtle F. N., Prinz C., Kellermann J., Lottspeich F., Pfanner N., et al. , 2011. Mitochondrial protein turnover: role of the precursor intermediate peptidase Oct1 in protein stabilization. Mol. Biol. Cell 22: 2135–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ballmoos C., Wiedenmann A., Dimroth P., 2009. Essentials for ATP synthesis by F1F0 ATP synthases. Annu. Rev. Biochem. 78: 649–672 [DOI] [PubMed] [Google Scholar]
- von der Malsburg K., Muller J. M., Bohnert M., Oeljeklaus S., Kwiatkowska P., et al. , 2011. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev. Cell 21: 694–707 [DOI] [PubMed] [Google Scholar]
- Voos W., Röttgers K., 2002. Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim. Biophys. Acta 1592: 51–62 [DOI] [PubMed] [Google Scholar]
- Voos W., Martin H., Krimmer T., Pfanner N., 1999. Mechanisms of protein translocation into mitochondria. Biochim. Biophys. Acta 1422: 235–254 [DOI] [PubMed] [Google Scholar]
- Vukotic M., Oeljeklaus S., Wiese S., Vogtle F. N., Meisinger C., et al. , 2012. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab. 15: 336–347 [DOI] [PubMed] [Google Scholar]
- Wagener N., Ackermann M., Funes S., Neupert W., 2011. A pathway of protein translocation in mitochondria mediated by the AAA-ATPase Bcs1. Mol. Cell 44: 191–202 [DOI] [PubMed] [Google Scholar]
- Wagner K., Gebert N., Guiard B., Brandner K., Truscott K. N., et al. , 2008. The assembly pathway of the mitochondrial carrier translocase involves four preprotein translocases. Mol. Cell. Biol. 28: 4251–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K., Perschil I., Fichter C. D., van der Laan M., 2010. Stepwise assembly of dimeric F(1)F(o)-ATP synthase in mitochondria involves the small F(o)-subunits k and i. Mol. Biol. Cell 21: 1494–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizenegger T., Schmitt S., Zivkovic J., Neupert W., Rapaport D., 2005. Mim1, a protein required for the assembly of the TOM complex of mitochondria. EMBO Rep. 6: 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis M. G., Groudinsky O., Slonimski P. P., Dujardin G., 1994. The NAM1 protein (NAM1p), which is selectively required for cox1, cytb and atp6 transcript processing/stabilisation, is located in the yeast mitochondrial matrix. Eur. J. Biochem. 222: 27–32 [DOI] [PubMed] [Google Scholar]
- Walther D. M., Rapaport D., 2009. Biogenesis of mitochondrial outer membrane proteins. Biochim. Biophys. Acta 1793: 42–51 [DOI] [PubMed] [Google Scholar]
- Wang X., Dumont M. E., Sherman F., 1996. Sequence requirements for mitochondrial import of yeast cytochrome c. J. Biol. Chem. 271: 6594–6604 [PubMed] [Google Scholar]
- Weraarpachai W., Antonicka H., Sasarman F., Seeger J., Schrank B., et al. , 2009. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 41: 833–837 [DOI] [PubMed] [Google Scholar]
- Wiedemann N., Pfanner N., Ryan M. T., 2001. The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria. EMBO J. 20: 951–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N., Kozjak V., Chacinska A., Schonfisch B., Rospert S., et al. , 2003a. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424: 565–571 [DOI] [PubMed] [Google Scholar]
- Wiedemann N., Kozjak V., Prinz T., Ryan M. T., Meisinger C., et al. , 2003b. Biogenesis of yeast mitochondrial cytochrome c: a unique relationship to the TOM machinery. J. Mol. Biol. 327: 465–474 [DOI] [PubMed] [Google Scholar]
- Wiedemann N., Truscott K. N., Pfannschmidt S., Guiard B., Meisinger C., et al. , 2004. Biogenesis of the protein import channel Tom40 of the mitochondrial outer membrane: intermembrane space components are involved in an early stage of the assembly pathway. J. Biol. Chem. 279: 18188–18194 [DOI] [PubMed] [Google Scholar]
- Wiedemann N., van der Laan M., Hutu D. P., Rehling P., Pfanner N., 2007. Sorting switch of mitochondrial presequence translocase involves coupling of motor module to respiratory chain. J. Cell Biol. 179: 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. H., Fox T. D., 2003. Antagonistic signals within the COX2 mRNA coding sequence control its translation in Saccharomyces cerevisiae mitochondria. RNA 9: 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. H., Perez-Martinez X., Fox T. D., 2004. MrpL36p, a highly diverged L31 ribosomal protein homolog with additional functional domains in Saccharomyces cerevisiae mitochondria. Genetics 167: 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. H., Bsat N., Bonnefoy N., Butler C. A., Fox T. D., 2005. Alteration of a novel dispensable mitochondrial ribosomal small subunit protein, Rsm28p, allows translation of defective COX2 mRNAs. Eukaryot. Cell 4: 337–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. H., Butler C. A., Bonnefoy N., Fox T. D., 2007. Translation initiation in Saccharomyces cerevisiae mitochondria: functional interactions among mitochondrial ribosomal protein Rsm28p, initiation factor 2, methionyl-tRNA-formyltransferase, and novel protein Rmd9p. Genetics 175: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm C. A., Jakobs S., 2006. Differential protein distributions define two sub-compartments of the mitochondrial inner membrane in yeast. FEBS Lett. 580: 5628–5634 [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Esaki M., Kanamori T., Tamura Y., Nishikawa S., et al. , 2002. Tim50 is a subunit of the TIM23 complex that links protein translocation across the outer and inner mitochondrial membranes. Cell 111: 519–528 [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Itoh N., Kawano S., Yatsukawa Y., Momose T., et al. , 2011. Dual role of the receptor Tom20 in specificity and efficiency of protein import into mitochondria. Proc. Natl. Acad. Sci. USA 108: 91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano K., Yatsukawa Y., Esaki M., Hobbs A. E., Jensen R. E., et al. , 2008. Tom20 and Tom22 share the common signal recognition pathway in mitochondrial protein import. J. Biol. Chem. 283: 3799–3807 [DOI] [PubMed] [Google Scholar]
- Yogev O., Pines O., 2011. Dual targeting of mitochondrial proteins: mechanism, regulation and function. Biochim. Biophys. Acta 1808: 1012–1020 [DOI] [PubMed] [Google Scholar]
- Young J. C., Hoogenraad N. J., Hartl F. U., 2003. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112: 41–50 [DOI] [PubMed] [Google Scholar]
- Young L., Leonhard K., Tatsuta T., Trowsdale J., Langer T., 2001. Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science 291: 2135–2138 [DOI] [PubMed] [Google Scholar]
- Zahedi R. P., Sickmann A., Boehm A. M., Winkler C., Zufall N., et al. , 2006. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol. Biol. Cell 17: 1436–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S., Lippman S. I., Zhao X., Broach J. R., 2008. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42: 27–81 [DOI] [PubMed] [Google Scholar]
- Zambrano A., Fontanesi F., Solans A., de Oliveira R. L., Fox T. D., et al. , 2007. Aberrant translation of cytochrome c oxidase subunit 1 mRNA species in the absence of Mss51p in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 18: 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zara V., Palmisano I., Conte L., Trumpower B. L., 2004. Further insights into the assembly of the yeast cytochrome bc1 complex based on analysis of single and double deletion mutants lacking supernumerary subunits and cytochrome b. Eur. J. Biochem. 271: 1209–1218 [DOI] [PubMed] [Google Scholar]
- Zara V., Conte L., Trumpower B. L., 2007. Identification and characterization of cytochrome bc(1) subcomplexes in mitochondria from yeast with single and double deletions of genes encoding cytochrome bc(1) subunits. FEBS J. 274: 4526–4539 [DOI] [PubMed] [Google Scholar]
- Zara V., Conte L., Trumpower B. L., 2009. Biogenesis of the yeast cytochrome bc1 complex. Biochim. Biophys. Acta 1793: 89–96 [DOI] [PubMed] [Google Scholar]
- Zeng X., Kucharczyk R., di Rago J. P., Tzagoloff A., 2007a. The leader peptide of yeast Atp6p is required for efficient interaction with the Atp9p ring of the mitochondrial ATPase. J. Biol. Chem. 282: 36167–36176 [DOI] [PubMed] [Google Scholar]
- Zeng X., Neupert W., Tzagoloff A., 2007b. The metalloprotease encoded by ATP23 has a dual function in processing and assembly of subunit 6 of mitochondrial ATPase. Mol. Biol. Cell 18: 617–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Hourset A., Tzagoloff A., 2007c. The Saccharomyces cerevisiae ATP22 gene codes for the mitochondrial ATPase subunit 6-specific translation factor. Genetics 175: 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Barros M. H., Shulman T., Tzagoloff A., 2008. ATP25, a new nuclear gene of Saccharomyces cerevisiae required for expression and assembly of the Atp9p subunit of mitochondrial ATPase. Mol. Biol. Cell 19: 1366–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaja K., Michaelis G., Lisowksy T., 1993. Nuclear control of the messenger RNA expression for mitochondrial ATPase subunit 9 in a new yeast mutant. J. Mol. Biol. 229: 909–916 [DOI] [PubMed] [Google Scholar]
- Zick M., Rabl R., Reichert A. S., 2009. Cristae formation-linking ultrastructure and function of mitochondria. Biochim. Biophys. Acta 1793: 5–19 [DOI] [PubMed] [Google Scholar]
- Zotova L., Aleschko M., Sponder G., Baumgartner R., Reipert S., et al. , 2010. Novel components of an active mitochondrial K(+)/H(+) exchange. J. Biol. Chem. 285: 14399–14414 [DOI] [PMC free article] [PubMed] [Google Scholar]