Abstract
Members of the Frizzled family of sevenpass transmembrane receptors signal via the canonical Wnt pathway and also via noncanonical pathways of which the best characterized is the planar polarity pathway. Activation of both canonical and planar polarity signaling requires interaction between Frizzled receptors and cytoplasmic proteins of the Dishevelled family; however, there has been some dispute regarding whether the Frizzled–Dishevelled interactions are the same in both cases. Studies looking at mutated forms of Dishevelled suggested that stable recruitment of Dishevelled to membranes by Frizzled was required only for planar polarity activity, implying that qualitatively different Frizzled–Dishevelled interactions underlie canonical signaling. Conversely, studies looking at the sequence requirements of Frizzled receptors in the fruit fly Drosophila melanogaster for canonical and planar polarity signaling have concluded that there is most likely a common mechanism of action. To understand better Frizzled receptor function, we have carried out a large-scale mutagenesis in Drosophila to isolate novel mutations in frizzled that affect planar polarity activity and have identified a group of missense mutations in cytosolic-facing regions of the Frizzled receptor that block Dishevelled recruitment. Interestingly, although some of these affect both planar polarity and canonical activity, as previously reported for similar lesions, we find a subset that affect only planar polarity activity. These results support the view that qualitatively different Frizzled–Dishevelled interactions underlie planar polarity and canonical Wnt signaling.
Keywords: planar polarity, planar cell polarity (PCP), Frizzled, Wnt signaling
FRIZZLED (Fz) proteins constitute a family of predicted sevenpass transmembrane receptors phylogenetically related to the G-protein coupled receptor (GPCR) superfamily and conserved throughout the animal kingdom (Barnes et al. 1998; Fredriksson et al. 2003; Wang et al. 2006). A characteristic feature is the amino-terminal extracellular domain containing a cysteine-rich domain (CRD) that mediates binding to ligands of the Wnt family (Bhanot et al. 1996; Wang et al. 1996). Whether Fz receptors act as bona fide GPCRs is controversial, although evidence that Fz proteins can act directly as ligand-dependent guanine nucleotide exchange factors for G-proteins, in the normal manner of GPCRs, has recently been presented (reviewed in Koval et al. 2011; Malbon 2011). Nevertheless, it remains unclear whether such an activity is obligatory for all or any of Fz receptor signaling functions.
Activation of Fz receptors leads to a variety of downstream responses, of which the best characterized is the so-called “canonical” signaling pathway that leads to stabilization and nuclear translocation of β-catenin and activation of transcription, in addition to a range of less-well-understood β-catenin-independent “noncanonical” outputs that include the so-called “Wnt/Ca2+” and planar cell polarity (PCP) pathways (reviewed in Angers and Moon 2009; Van Amerongen and Nusse 2009). Of particular interest is the role of the multidomain cytoplasmic proteins of the Dishevelled (Dsh in flies and frogs, Dvl in other vertebrates) family, which appear to mediate most—if not all—downstream responses to Fz receptors, by interacting with a variety of downstream effectors (reviewed in Wharton 2003; Wallingford and Habas 2005).
The fruit fly Drosophila melanogaster has been an important in vivo model for dissection of Fz function. The fruit fly genome encodes four Fz proteins, of which only two, Fz itself (also known as Fz1) and Fz2 are known to couple to downstream signaling pathways. Mutations in the fz gene result in viable adults that exhibit so-called “planar polarity” defects in the orientation in the plane of the tissue of cuticle structures such as hairs and bristles, and non-cell-autonomous effects are observed when loss of gene activity is studied in genetic mosaics (Gubb and García-Bellido 1982; Vinson and Adler 1987). Notably, a small class of fz alleles show only a cell-autonomous defect in cuticle polarity and do not show the nonautonomous effects, leading to the conclusion that fz has two activities that can be separated by mutation, functioning both in the transmission and in the reception/interpretation of planar polarity signals (Adler et al. 1987; Vinson and Adler 1987).
In addition to this role in planar polarity, Fz also acts redundantly with Fz2 to mediate canonical β-catenin-dependent signaling in response to binding of the Wnt ligand Wingless (Wg) (Bhat 1998; Kennerdell and Carthew 1998; Bhanot et al. 1999; Chen and Struhl 1999; Müller et al. 1999), and acting together with the co-receptor Arrow (Arr) a homolog of vertebrate LRP5/6 (Wehrli et al. 2000). Unlike Fz, Fz2 shows no activity in planar polarity specification; however, it has been implicated in other poorly understood signaling events (Cohen et al. 2002; Mathew et al. 2005; Mosca and Schwarz 2010).
The fruit fly genome encodes a single Dsh protein, which is absolutely required for both planar polarity and β-catenin-dependent signaling downstream of Fz and Fz2 (Klingensmith et al. 1994; Siegfried et al. 1994; Krasnow et al. 1995). Different domains of Dsh have been implicated in coupling to the two different downstream pathways: the Dishevelled, Egl-10, Pleckstrin (DEP) domain and to a lesser extent the PSD95, Dlg1, ZO-1 (PDZ) domain are required for planar polarity activity, whereas different regions of the DEP domain and the PDZ and DIX domains are needed for β-catenin-dependent signaling (Axelrod et al. 1998; Boutros et al. 1998; Penton et al. 2002).
Given the importance of Fz receptors, there is great interest in understanding their mechanism of function and, in particular, the structural requirements necessary to mediate Dsh recruitment and the different downstream responses. This has led to numerous studies in both Drosophila and other organisms, which are summarized below.
The amino-terminal extracellular domain of Fz receptors, including the 120 amino acid CRD that mediates binding to Wnt ligands, is generally assumed to be important for signaling activity, although the precise roles are controversial. For canonical function, the extracellular domains of Drosophila Fz and Fz2 are interchangeable, although that of Fz2 confers a greater ability to activate signaling, most likely due to a stronger affinity for Wnt ligands (Rulifson et al. 2000; Strapps and Tomlinson 2001). However, the CRD itself is at least partly dispensable for canonical activity (Chen et al. 2004; Povelones and Nusse 2005; Wu and Mlodzik 2008), leading to the suggestion that Wnt binding to the CRD does not itself stimulate Fz receptor activity but instead serves to locally increase the concentration of Wnt.
Wnt ligand binding does not seem to be necessary for planar polarity activity in Drosophila (Lawrence et al. 2002; Chen et al. 2008), and as no point mutations in the CRD have been isolated by random mutagenesis, it has been suggested that the CRD may not be required for planar polarity activity (Rulifson et al. 2000; Povelones et al. 2005). However, point mutations elsewhere in the extracellular domain, or deletion of the entire CRD, do affect planar polarity activity (Jones et al. 1996; Boutros et al. 2000; Chen et al. 2004; Wu et al. 2004; Povelones et al. 2005; Wu and Mlodzik 2008), and it has been proposed that it may directly mediate intercellular communication by interacting with the partner protein Strabismus in neighboring cells (Strutt and Strutt 2008; Wu and Mlodzik 2008), although others have disputed this (Chen et al. 2008).
Numerous lines of evidence indicate the importance of the seven transmembrane domains (hereafter TM1–7) and the associated three extracellular loops (ECL1–3) and intracellular loops (ICL1–3), collectively referred to as the 7TM region. Within the 7TM region, the extracellular loops appear to be least important, as in random mutagenesis experiments in Drosophila, only two point mutations have been isolated in these regions that only weakly affect receptor activity (Povelones et al. 2005), and in vitro mutagenesis of ECL1 and ECL3 in a vertebrate Fz receptor also does not significantly affect canonical activity (Cong et al. 2004). A small number of mutations in the TM domains that affect Drosophila Fz function have been isolated. Interestingly, none of these lie in TM1–3. Mutations in TM4–7 affect both canonical and planar polarity function, and almost all show reduced protein levels, possibly indicative of deficits in protein maturation or stability (Adler et al. 1994; Jones et al. 1996; Povelones et al. 2005).
The intracellular loops and intracellular C-terminal tail are the most intensively studied regions of Fz receptors, due to their possible roles in interacting with intracellular effectors of signaling. Systematic substitution of pairs of amino acids in ICL1–3 to alanines in a vertebrate Fz receptor has revealed that all three are required for canonical signaling (Cong et al. 2004). Similarly, studies of Drosophila Fz have also identified amino acid changes in ICL1–3 that affect both planar polarity and canonical signaling activity (Jones et al. 1996; Povelones et al. 2005; Wu et al. 2008).
The most critical region in the C-terminal tail appears to be the “KTxxxW” motif lying just downstream of the end of TM7 (Drosophila Fz residues 557–561). Mutation of the lysine, threonine, and tryptophan residues results in loss of canonical activity in vertebrate receptors (Umbhauer et al. 2000; Cong et al. 2004) and loss of canonical and planar polarity activity in Drosophila receptors (Wu et al. 2008). Also close to the end of TM7 and interleaved with the KTxxxW motif are two serine residues that have been identified as potential protein kinase C (PKC) phosphorylation sites, and such phosphorylations have been proposed to regulate Fz activity (Djiane et al. 2005). Conversely, there seems to be little essential function for residues downstream of the KTxxxW. Although this region has been implicated in the correct subcellular localization and canonical activity of Drosophila Fz2 (Wu et al. 2004; Wu et al. 2008), truncation of a Xenopus receptor does not alter canonical function, albeit in an overexpression assay (Umbhauer et al. 2000). The only conserved structural motif identified beyond the KTxxxW motif in Fz receptors is a predicted C-terminal PDZ-binding motif, and several studies in different organisms have found no role for this (Sawa et al. 1996; Itoh et al. 1998; Umbhauer et al. 2000; Strutt 2001; Wong et al. 2003; Wu et al. 2008), although another study has suggested a function in downregulating Drosophila Fz planar polarity activity (Djiane et al. 2005).
A major role of the intracellular loops and intracellular C-terminal tail is in mediating interactions with Dsh. In vertebrate receptors, mutations in ICL1, ICL3 and the KTxxxW motif have been shown to block Dsh binding (Umbhauer et al. 2000; Wong et al. 2003; Cong et al. 2004; Tauriello et al. 2012). Weak interactions occur between the PDZ domain of Dsh proteins and the KTxxxW region (Wong et al. 2003; Tauriello et al. 2012), but multiple lines of evidence suggest that the main interactions are mediated by the Dsh DEP domain (Axelrod et al. 1998; Rothbächer et al. 2000; Axelrod 2001; Shimada et al. 2001), most likely binding cooperatively to both the intracellular loops and the C-terminal tail (Tauriello et al. 2012).
As the same regions of Fz receptors mediate Dsh recruitment and signaling via both canonical and planar polarity pathways, the simplest model is that this recruitment is essential for activity in both cases. Nevertheless, it has also been proposed that membrane recruitment of Dsh may in fact be required only for planar polarity, as DEP domain lesions that block recruitment show a deficit in planar polarity but not canonical signaling, and the ability of vertebrate Fz homologs to recruit Dsh proteins does not necessarily equate with activation of canonical signaling (Axelrod et al. 1998; Boutros et al. 1998; Rothbächer et al. 2000; Axelrod 2001; Simons et al. 2009). Conversely, other studies suggest a correlation between the ability of Dsh proteins to bind Fz receptors, possibly via the PDZ rather than the DEP domain, and the ability to mediate canonical signaling (Umbhauer et al. 2000; Wong et al. 2003; Cong et al. 2004; Wu et al. 2008; Tauriello et al. 2012).
With regard to the requirement of Fz receptors to interact with Dsh in planar polarity signaling, an intriguing observation is that mutation of proline-278 in ICL1 of Drosophila Fz gives rise to a protein expressed at normal levels but cannot recruit Dsh to cell membranes (Jones et al. 1996; Axelrod 2001; Amonlirdviman et al. 2005) and that this leads specifically to a disruption of the cell-autonomous planar polarity activity of fz but does not disrupt cell–cell transmission of polarity information. Similarly, a mutation in the KTxxxW motif of Drosophila Fz that blocks Dsh binding also results in a protein that is deficient only in cell-autonomous planar polarity activity (Wu et al. 2008; Wu and Mlodzik 2008). Taken together these results suggest that it may be a general property of Fz receptors that they need to bind to Dsh to mediate cell-autonomous planar polarity activity, but not to pass polarity information to neighboring cells (Amonlirdviman et al. 2005).
Based on the described studies, two groups have proposed similar models for Fz receptor function, which suggest that there is a common mechanism underlying activation of canonical signaling and planar polarity specification. This is based on the twin observations of a correlation between the strength of deficit in canonical signaling or planar polarity activity for individual fz mutant alleles (Povelones et al. 2005) and that a failure to recruit Dsh to membranes equates with failure to signal in both pathways, suggesting that common Fz–Dsh interactions underlie both activities (Wu et al. 2008).
Although these models are plausible, they are based on the analysis of relatively small numbers of mutations (Table 1). It remains a major question whether the primary role of the intracellular loops and the C-terminal intracellular domain is to recruit Dsh and whether a failure to do so always similarly compromises both canonical and planar polarity activities. Similarly, there is still only sparse evidence for the suggestion that receptors that localize to the membrane but fail to recruit Dsh always show a deficit in only cell-autonomous planar polarity function. Other outstanding issues that might be answered by analysis of a larger number of mutations include the roles of the extracellular domain and CRD, the extracellular loops, and the C-terminal tail after the KTxxxW motif.
Table 1 . Properties of amino acid changes in Drosophila Fz.
| Allele | Amino acid change | Region of protein | Origin | Planar polarity phenotype | Planar polarity nonautonomy | Canonical phenotype | Protein levels | Extra tarsal jointsa | Canonical phenotype (wing margin)a | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| fzP21 | I70del | CRD | EMS | Strongb | Yesb | Noneb | 2.9 ± 0.6 | Strong | fz21 (FlyBase) | |
| fzHE11 | C229Y | Extra | EMS | Strongb | Yesb | Weakc | Normalb | 2.1 ± 0.6 | ||
| Weakc | ||||||||||
| fzI12 | P278L | ICL1 | EMS | Strongb | Nob | Moderatec | Normalb, c | 2.0 ± 0.2 | Intermediate | fz16, fz19 (FlyBase) |
| fzJ22 | Moderateb | |||||||||
| fzF31 | P278S | ICL1 | EMS | Moderated | Noe | Weakc | Normalc | 1.6 ± 0.5 | No | fz13, fz20 (FlyBase) |
| fzN21 | Weakc | |||||||||
| fzR53 | A374G | ICL2 | EMS | Weakb,c | Partialb | Weakc | Normalb,c | fz24 (FlyBase) | ||
| fzTT | H383Y | TM4 | ENU | Weakc | Weakc | Normalc | ||||
| fzSY | G405D | ECL2 | ENU | Weakc | Weakc | |||||
| fzED | C412R | ECL2 | ENU | Weakc | Weakc | Normalc | ||||
| fzGL31 | P429L | TM5 | EMS | Strongb | Yesb | Strongc | Lowerb,c | |||
| V454F | ICL3 | EMS | Moderateb | |||||||
| fzHB41 | V454F | ICL3 | EMS | Strongb | Yesb | Lowerb | ||||
| fzHB52 | ||||||||||
| fzR54 | M469R | ICL3 | EMS | Strongd | Yese | Moderatec | Lowerc | 2.8 ± 0.4 | Strong | fz25 (FlyBase) |
| W500R | ECL3 | Moderateb | ||||||||
| fzM469R | M469R | ICL3 | Transgene | Strongf | Strongf | αTub84B promoter | ||||
| fzKW | I472K | TM6 | ENU | Moderatec | Moderatec | Lowerc | 2.3 ± 0.5 | |||
| fzHD21 | G485R | TM6 | EMS | Moderateb | Yesb | Moderatec (25°) | Lowerb,c (25°) | Temperature sensitive | ||
| Weakc (25°) | ||||||||||
| fzH51 | W500X | ECL3 | EMS | Strongb | Yesb | Normal (smaller)b | 3.9 ± 0.3 | Strong | fz15 (FlyBase) | |
| fzHC52 | G545R | TM7 | EMS | Strongb | Yesb | Lowerb | ||||
| fzMP | G545E | TM7 | ENU | Moderatec | Moderatec | Lowerc | ||||
| fzΔSKT | Δ S555 –T557 | C-term | Transgene | Strongf | Strongf | αTub84B promoter | ||||
| fzΔSWRNF | Δ S560 –F564 | C-term | Transgene | Strongf | Nog | Strongf | αTub84B promoter | |||
| fz565Stop | V565X | C-term | Transgene | Nonef | Weakf | αTub84B promoter |
Materials and Methods
Fly strains and transgenes
Fly strains are described in FlyBase, except for transgenes generated for this study. P{w+, Act5C-fz} flies were generated by cloning the wild-type fz coding sequence into the vector pP{w+, Act5C-FRT-PolyA-FRT} (Strutt 2001), generating transgenic flies, and excising the FRT-PolyA-FRT cassette in vivo. Other fz transgenes were generated by in vitro mutagenesis of the fz coding sequence fused to EGFP or EYFP (Clontech) and cloning into pP{w+, Act5C-FRT-PolyA-FRT}. fz2 transgenes were generated by adding the coding sequence for three Myc epitope tags just upstream of the mature peptide, preceded by the signal peptide sequence from the rat CD2 protein. In vitro mutagenesis was used to introduce any desired changes, before cloning into a transformation vector under control of the armadillo promoter as previously described (Strutt 2001).
For use in the mutagenesis screen, a P{w+, Act5C-fz} transgene insertion on the second chromosome was selected that, when crossed into a fzP21 background, showed no phenotype in the adult leg, notum, or eye down a dissecting microscope. Inspection of adult wings on the compound microscope showed that this transgene rescues through much of the wing, but does show weak swirls toward the distal tip, possibly due to overexpression of fz. This transgene also fully rescues the wing margin phenotype of fzP21 fz2C1 clones. Levels of expression in the pupal wing were verified by Western blotting using rabbit anti-Fz (Bastock and Strutt 2007) and mouse monoclonal anti-actin AC-40 (Sigma) as loading control.
Mutagenesis to identify loss-of-function mutations in a fz transgene
Males of genotype w1118; P{w+, Act5C-fz}; fzP21 were mutagenized with either 35 mM ethyl methanesulphonate (EMS) or 4 mM N-ethyl-N-nitrosourea (ENU) as described (Grigliatti 1998) and mated to females of genotype w1118; fzP21/TM3, P{w+; hs-hid}, Sb. Males were remated to fresh females every day, before being discarded on the fourth day. Progeny were heat-shocked during larval stages for 2 hr at 38° to kill TM3, P{w+; hs-hid}, Sb bearing progeny. Remaining progeny of genotype w1118; P{w+, Act5C-fz}/+; fzP21 were scored for visible planar polarity phenotypes under a dissecting microscope once they emerged as adults. Flies showing a planar polarity phenotype were recrossed to flies of genotype w1118; fzP21 to select for mutations that could be recovered in the germline.
Analysis of mutations isolated
To identify changes in the coding region of mutagenized transgenes, genomic DNA was isolated from 20 adult flies and the fz coding region isolated by PCR using primers specific for flanking regions in the transgene, before sequencing using internal primers.
Adult tarsal phenotypes were assessed in males of genotype w1118; P{w+, Act5C-fz}/+; fzP21 raised at 25°. Rescue of canonical signaling activity in the adult wing margin was assessed in flies of genotype y; P{w+, Act5C-fz*}/vgQ1206-GAL4, UAS-flp; fzP21 fz2C1 ri FRT2A/hsCD2, y+ ri FRT2A, which harbor clones of fzP21 fz2C1 homozygous mutant cells that can be identified in the wing margin by lack of expression of yellow, if the clones survive to adulthood by virtue of being rescued by the presence of the P{w+, Act5C-fz*} transgene. Pupal wing planar polarity phenotypes were assessed in flies of genotype y w Ubx-FLP/+; P{w+, Act5C-fz*}/+; fzP21 FRT80B; arm-lacZ FRT80B. Activities of in vitro mutagenized transgenes were analyzed in the same way, using transgene insertions on the second chromosome. Similarly, activities of existing mutations in the fz locus were analyzed using the same genotypes, except the fz allele was substituted for fzP21 and no fz transgene was present. Mosaic patches of expression from transgenes expressing GFP-tagged proteins were generated by crossing the transgene-bearing flies to Ubx-FLP (to excise any FRT-PolyA-FRT cassette that might be present) and the Δ2–3 transposase source (to mobilize the transgene, thus causing mosaic expression).
To assess numbers of extra tarsal joints, adult male first legs from at least 10 flies were examined under the compound microscope and the number of joints between tarsal segments counted (with partial joints that extended at least halfway into the segment being counted as 1). In Tables 1 and 3, the number of extra tarsal joints is expressed as the average plus or minus the standard deviation. To assess the strength of canonical signaling the defect in the anterior wing margin was examined under the compound microscope. At least six wings were examined for each genotype and the proportions of the wing margin containing yellow mutant bristles and “notches” with no bristles present were measured. The proportions of the wing margin showing either notching or rescued patches of yellow bristles varied widely both within and between genotypes, probably due to stochastic variation in rate and time of clone induction. For rescue using P{w+, Act5C-fz} transgenes, genotypes that showed on average >10% notching of the anterior wing margin were scored as “strong,” and those with no notches and ∼20% or more occupied by yellow mutant bristles (representing rescued fz fz2 tissue) were scored as “rescued,” with those with smaller notched regions and/or less rescued tissue scored as “intermediate” (see Supporting Information, Table S1). For rescue using P{w+, ArmP-Myc-fz2} transgenes (which express relatively weakly, such that a wild-type coding region rescues notching, but gives only ∼10% of the anterior wing margin containing yellow mutant bristles), >10% notching was scored as strong and 0–10% notching as “moderate.”
Table 3. Summary of properties of fz mutations examined in this study.
| Allele | Amino acid change | Region of protein | Planar polarity phenotype (extra tarsal joints) | Canonical phenotype (wing margin) | Frizzled localization | Dishevelled localization | Flamingo localization | Planar polarity nonautonomy |
|---|---|---|---|---|---|---|---|---|
| Alleles isolated by mutagenesis of Act5C-fz transgene on second chromosome | ||||||||
| 68G2 | C53F | CRD | 2.7 ± 0.5 | Intermediate | Lost/Cytoplasmic | Lost | Reduced | Strong |
| 70A2.1 | C53Y | CRD | 3.5 ± 0.5 | Strong | Lost/Cytoplasmic | Lost | Reduced | Strong |
| 26C1 | C107Y | CRD | 2.9 ± 0.3 | Strong | Lost/Cytoplasmic | Lost | Reduced | Strong |
| 25A3 | P112L | CRD | 2.3 ± 0.8 | Intermediate | Lost/Cytoplasmic | Lost | Reduced | Strong |
| 73A2 | R274C | ICL1 | 1.6 ± 0.5 | Rescued | At junctions | Lost | At junctions | None |
| 27C2 | R274H | ICL1 | 0.8 ± 0.4 | Rescued | At junctions | Lost | At junctions | None |
| 39B2 | 1.1 ± 0.3 | Rescued | At junctions | Lost | At junctions | None | ||
| 17A1 | P278L | ICL1 | 1.8 ± 0.4 | Intermediate | At junctions | Lost | At junctions | None |
| 30E2 | 2.3 ± 0.8 | Intermediate | At junctions | Lost | At junctions | None | ||
| 18B3 | P278S | ICL1 | 1.0 ± 0.0 | Rescued | At junctions | Reduced | At junctions | None |
| 79F3 | 1.0 ± 0.0 | Rescued | At junctions | Reduced | At junctions | None | ||
| 8A4 | E279K | ICL1 | 3.3 ± 0.8 | Rescued | At junctions | Lost | At junctions | None |
| 4C3 | E373K | ICL2 | 1.0 ± 0.0 | Rescued | At junctions | Reduced | At junctions | None |
| 11A2 | 0.9 ± 0.3 | Rescued | At junctions | Reduced | At junctions | None | ||
| 72A4 | H383L | TM4 | 1.3 ± 0.5 | Rescued | At junctions | Reduced | At junctions | None |
| 37E2 | H383Y | TM4 | 1.7 ± 0.5 | Rescued | At junctions | Lost | At junctions | None |
| 26C2 | P390L | TM4 | 2.4 ± 0.5 | Intermediate | Reduced/Cytopl. | Lost | Reduced | Moderate |
| 30A3 | 2.3 ± 0.5 | Intermediate | Reduced/Cytopl. | Lost | Reduced | Moderate | ||
| 8F2 | G444R | TM5 | 1.9 ± 0.6 | Strong | At junctions | Lost | At junctions | None |
| 70B4 | I451F | ICL3 | 2.1 ± 0.6 | Rescued | At junctions | Lost | At junctions | None |
| 50E1 | M469K | ICL3 | 2.3 ± 0.5 | Strong | At junctions | Lost | At junctions | None |
| 7A3 | M469T | ICL3 | 1.0 ± 0.0 | Rescued | At junctions | Lost | At junctions | Mild |
| G477A | TM6 | |||||||
| 39D1 | I472K | TM6 | 1.8 ± 0.8 | Rescued | At junctions | Lost | At junctions | None |
| 76B1 | G545R | TM7 | 2.6 ± 0.9 | Strong | Lost/Cytoplasmic | Lost | Reduced | Strong |
| 19D3 | Y553X | C-term | 2.3 ± 0.8 | Intermediate | Reduced/Cytopl. | Lost | Reduced | Moderate |
| 25B2 | S554F | C-term | 2.3 ± 0.7 | Strong | At junctions | Lost | At junctions | None |
| 6E3 | W561X | C-term | 2.4 ± 0.5 | Rescued | At junctions | Lost | At junctions | None |
| 14B2 | 2.2 ± 0.4 | Rescued | At junctions | Lost | At junctions | None | ||
| 47B3 | 2.9 ± 0.4 | Intermediate | Lost | Lost | Reduced | Strong | ||
| 70A2.3 | W561R | C-term | 2.1 ± 0.3 | Rescued | At junctions | Lost | At junctions | None |
| Existing fz alleles | ||||||||
| fzP21 | I70del | CRD | 2.9 ± 0.6 | Strong | Lost | Lost | Reduced | Strong |
| fzJ22 | P278L | ICL1 | 2.0 ± 0.2 | Intermediate | At junctions | Lost | At junctions | None |
| fzN21 | P278S | ICL1 | 1.6 ± 0.5 | Rescued | ND | ND | ND | ND |
| fzR54 | M469R | ICL3 | 2.8 ± 0.4 | Strong | Reduced | Lost | Reduced | Moderate |
| W500R | ECL3 | |||||||
| fzH51 | W500X | ECL3 | 3.9 ± 0.3 | Strong | Lost | Lost | ND | ND |
| In vitro generated mutations in Act5C-fz-EGFP transgene insertions | ||||||||
| fzIE > AA #4 | I375A | ICL2 | 2.8 ± 0.4 | Strong | ND | ND | ND | ND |
| E376A | ||||||||
| fzIE > AA #5 | I375A | ICL2 | 2.7 ± 0.5 | Strong | ND | ND | ND | ND |
| E376A | ||||||||
| fzKL > AA #2 | K464A | ICL3 | 2.5 ± 0.5 | Strong | ND | ND | ND | ND |
| L465A | ||||||||
| fzKL > AA #3 | K464A | ICL3 | 2.6 ± 0.7 | Strong | ND | ND | ND | ND |
| L465A | ||||||||
| fzAAXXXA #1 | K556A | C term | 2.8 ± 0.4 | Strong | Reduced | Lost | Reduced | Strong |
| T557A | ||||||||
| W561A | ||||||||
| fzAAXXXA #2 | K556A | C term | 3.0 ± 0.02 | Strong | ND | ND | ND | ND |
| T557A | ||||||||
| W561A | ||||||||
| fzW561Y #1 | W561Y | C term | 0.1 ± 0.3 | Rescued | ND | ND | ND | ND |
| fzW561Y #7 | W561Y | C term | 0.1 ± 0.3 | Rescued | ND | ND | ND | ND |
To verify that the tarsal joint phenotype of fz mutations correlates with the strength of the ommatidial polarity defect, eyes from three mutations isolated in the screen that gave different strength tarsal phenotypes were sectioned. 7A3 gives a weak tarsal phenotype (1.0 extra joints) and showed a weak ommatidial polarity defect (9% misoriented), 14B2 gives an intermediate tarsal phenotype (2.2 extra joints) and an intermediate ommatidial polarity defect (28% misoriented) and 24C1 gives a strong tarsal phenotype (3.4 extra joints) and a strong ommatidial polarity defect (35% misoriented).
Pupal wings were dissected, immunolabeled, and imaged as previously described (Strutt 2001). Phalloidin conjugated to Alexa-568 (Molecular Probes) was used to label F-actin. Primary antibodies used were mouse monoclonal anti-ßgal (Promega), rabbit anti-ßgal (Cappel), rabbit anti-GFP (Abcam), mouse monoclonal anti-Arm [Developmental Studies Hybridoma Bank (DSHB)], mouse monoclonal anti-Fmi#74 (DSHB) (Usui et al. 1999), rabbit anti-Fz (Bastock and Strutt 2007), and rat anti-Dsh (Strutt et al. 2006). Adult wings and legs were mounted in Gary’s Magic Mountant (GMM).
Modeling of Fz receptor structure
No experimental structure of Fz is yet known, although those of other distantly related GPCRs including rhodopsin (Palczewski et al. 2000) and β2-adrenergic receptor (Cherezov et al. 2007) are available. Therefore, to investigate the possible structural consequences of the missense mutations isolated, the sequence of Fz was submitted to the iterative threading assembly refinement (I-TASSER) WebServer (Zhang 2007; Roy et al. 2010), which used multiple threading alignments and structure assembly simulations to produce a homology-based model of the protein. The figures based on the model were made using Pymol (http://www.pymol.org). The resulting model conforms to the expected 7TM pattern, with seven transmembrane helices, three intracellular loops, and a cytoplasmic C-terminal region. Note that in Tables 1, 2, and 3, the positions of residues to transmembrane regions or loops are based on predictions of protein topology generated using TMHMM (Krogh et al. 2001) and that these predictions do not precisely agree with the predictions from the homology model (e.g., TMHMM places M469 in ICL3 rather than within a transmembrane helix). The degree of conservation at each position as shown in Figure 6D was calculated from the SIFT data (Ng and Henikoff 2001). The SIFT algorithm assigns each possible substitution at a particular residue a “normalized” value such that if the substitution is completely tolerated the value is 1.0 and if not tolerated at all the value is 0. The sum of the 20 probabilities for each possible residue substitution was calculated, and the reciprocal of this sum was used as a measure of conservation.
Table 2. Summary of nucleotide and amino acid changes induced in the Act5C-fz transgene.
| Allelea | Nucleotide change | Mutagen | Amino acid change | Region | SIFT scoreb |
|---|---|---|---|---|---|
| 22E1 | G5A | EMS | W2X | Extra | |
| 6H4 | C40T | EMS | Q14X | Extra | |
| 68G2 | G158T | ENU | C53F | Extra (CRD) | 0.00 |
| 70A2.1 | G158A | ENU | C53Y | Extra (CRD) | 0.00 |
| 26C1 | G320A | EMS | C107Y | Extra (CRD) | 0.00 |
| 25A3 | C335T | EMS | P112L | Extra (CRD) | 0.00 |
| 3A2 | C355T | EMS | R119X | Extra (CRD) | |
| 19B2 | ΔT413–G708 | EMS | M138–172Xc | Extra (CRD) | |
| 49D2 | C739T | ENU | R247X | TM1 | |
| 19A3 | G746A | EMS | W249X | TM1 | |
| 37F3 | G746A | EMS | W249X | TM1 | |
| 27C2 | G821A | EMS | R274H | ICL1 | 0.00 |
| 39B2 | G821A | ENU | R274H | ICL1 | 0.00 |
| 73A2 | C820T | ENU | R274C | ICL1 | 0.00 |
| 17A1 | C833T | EMS | P278L | ICL1 | 0.00 |
| 30E2 | C833T | EMS | P278L | ICL1 | 0.00 |
| 18B3 | C832T | EMS | P278S | ICL1 | 0.03 |
| 79F3 | C832T | ENU | P278S | ICL1 | 0.03 |
| 8A4 | G835A | EMS | E279K | ICL1 | 0.01 |
| 68B1 | T869A | ENU | L290X | TM2 | |
| 20F4 | C985A | EMS | Q329X | ECL1 | |
| 9C2 | C994T | EMS | R332X | ECL1 | |
| 12F1 | G1061A | EMS | W354X | TM3 | |
| 16A3 | T1091A | EMS | L364X | TM3 | |
| 15D4 | G1110A | EMS | W370X | ICL2 | |
| 53D3 | G1110A | ENU | W370X | ICL2 | |
| 4C3 | G1117A | EMS | E373K | ICL2 | 0.00 |
| 11A2 | G1117A | EMS | E373K | ICL2 | 0.00 |
| 37E2 | C1147T | EMS | H383Y | TM4 | 0.00 |
| 72A4 | A1148T | ENU | H383L | TM4 | 0.00 |
| 24C1 | G1161A | EMS | W387X | TM4 | |
| 34A3 | G1160A | EMS | W387X | TM4 | |
| 63E1 | G1159A | ENU | W387X | TM4 | |
| 26C2 | C1169T | EMS | P390L | TM4 | 0.00 |
| 30A3 | C1169T | ENU | P390L | TM4 | 0.00 |
| 8F2 | G1330A | EMS | G444R | TM5 | 0.00 |
| 70B2 | A1351T | ENU | I451F | ICL3 | 0.00 |
| 5E2 | ΔG1382–T1394 | EMS | T462S–464Xc | ICL3 | |
| 50E1 | T1406A | ENU | M469K | ICL3 | 0.00 |
| 7A3 | T1406C, G1430C | EMS | M469T, G477A | ICL3, TM6 | 0.00, 0.01 |
| 29D2 | C1411T | EMS | R471X | TM6 | |
| 39D1 | T1415A | EMS | I472K | TM6 | 0.05 |
| 6D2 | ΔT1421–C1425 | EMS | F475W–496Xc | TM6 | |
| 63D2 | T1457A | ENU | L486X | TM6 | |
| 8D4 | G1500A | EMS | W500X | ECL3 | |
| 53B4 | G1500A | ENU | W500X | ECL3 | |
| 78A1 | G1500A | ENU | W500X | ECL3 | |
| 76B1 | G1633C | ENU | G545R | TM7 | 0.00 |
| 19D3 | T1659A | EMS | Y553X | Intra | |
| 14D3 | C1661T | EMS | S554F | Intra | 0.00 |
| 25B2 | C1661T | EMS | S554F | Intra | 0.00 |
| 6E3 | G1682A | EMS | W561X | Intra | |
| 14B2 | G1683A | EMS | W561X | Intra | |
| 47B3 | G1683A | ENU | W561X | Intra | |
| 70A2.3 | T1681C | ENU | W561R | Intra | 0.00 |
Missense mutations indicated in boldface type.
SIFT score is shown for missense mutations and represents the normalized probability of the amino acid substitution occurring in a homologous protein (Ng and Henikoff 2001). Substitutions with probabilities of ≤0.05 are predicted to be deleterious.
Deletions that cause frameshifts. In 6D2, amino acids from codon 475 onward are replaced with WTVHSARRGITGLLVLRVLQLX. In 5E2, amino acids from codon 462 onward are replaced with SDX. In 19B2, amino acids from codon 138 are replaced with ILPGERLDCSSILGWILGSGLCCQLLVYGAHLLDX.
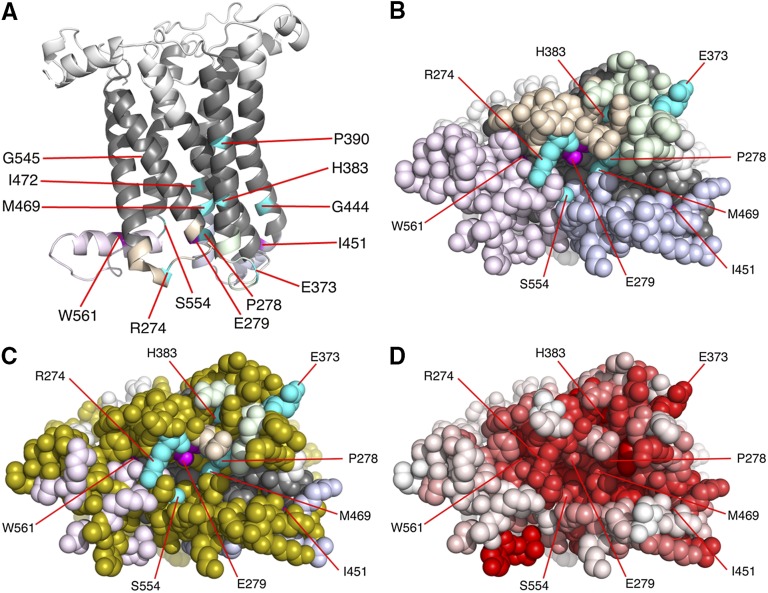
Figure 6 .
Views of the Fz homology model. (A) Cartoon representation of the model with extracellular loops colored in white, transmembrane helices in dark gray, ICL1 in light orange, ICL2 in light green, ICL3 in light blue, and the C-terminal tail in light purple. Residues where mutation gave a strong effect on planar polarity but not on canonical activity are colored in magenta and the remaining residues in turquoise. (B) Space filling representation, orthogonal to A, of the cytoplasmic face of the model, colored as in A. (C) View as in B but with residues colored olive that cannot be mutated by GC:AT substitutions to give a deleterious change according to the SIFT algorithm (Ng and Henikoff 2001). (D) View as in B and C but with amino acids colored on a scale from red (highly conserved) to white (unconserved). Degree of conservation is based on using the SIFT algorithm to determine how tolerant a position is to a change in identity of the amino acid present in the wild-type Fz coding sequence.
Results
Generation of novel fz mutations in vivo by chemical mutagenesis
To further explore Fz receptor function, we generated a large collection of randomly induced mutations that compromise Drosophila fz planar polarity activity. As fz is not essentially required for viability, such mutations can be easily scored in an “F1” screen on the basis of polarity defects on the adult cuticle (Adler et al. 1987; Jones et al. 1996; Povelones et al. 2005). However, unlike in previous studies, we did not screen for mutations in the endogenous fz locus, but instead assayed for loss of activity of a transgene located on the second chromosome expressing a fz cDNA under the heterologous Actin5C (Act5C) promoter. Immunoblot analysis shows that a single copy of this transgene expresses Fz at severalfold the level found in wild-type flies (Figure S1); nevertheless, the transgene fully rescues the fz null Planar polarity phenotype in adult tissues (Figure 1A) with the exception of a region toward the distal tip of the wing (see Materials and Methods) and also rescues lack of fz fz2 activity in canonical signaling in the wing (Figure 1G). This transgene-based strategy has the advantage that due to the lack of introns in the transgene, it is easier to identify the induced DNA sequence changes, and the presence of the transgene on the second chromosome greatly simplifies testing for a deficit in canonical signaling activity in subsequent assays.
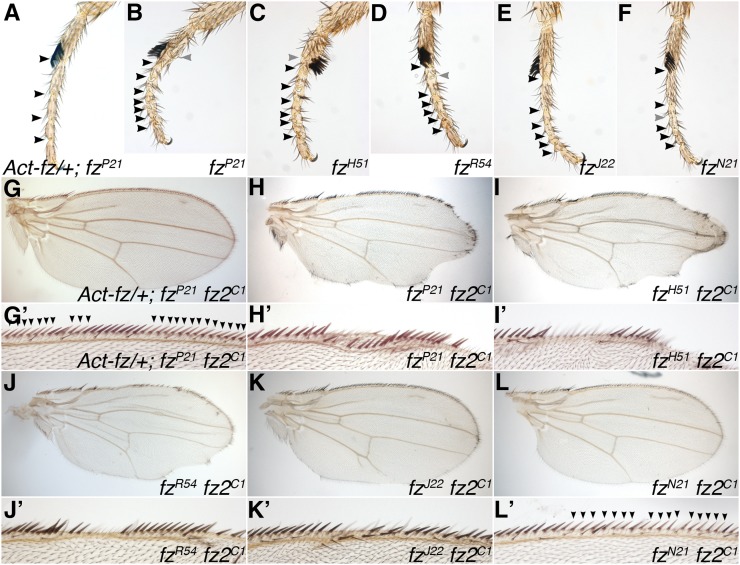
Figure 1 .
Adult planar polarity and canonical signaling phenotypes of existing fz alleles. (A–F) Distal regions of adult male first legs of the indicated genotypes. Solid arrowheads indicate complete joints between tarsal segments, and shaded arrowheads indicate partially formed joints. Note complete rescue of ectopic joint phenotype seen in fzP21 by the Act5C-fz transgene. (G–L) Whole adult male wings, or enlargements of distal margin (G’–L’), containing loss-of-function clones lacking activity of an allelic series of fz alleles in combination with fz2C1. Distal is to the right and anterior is up. Clones marked by presence of yellow bristles (lighter in color and indicated by solid arrowheads in G’–L’). Most alleles cause notching or distortion of the wing margin with loss of yellow bristles in the mutant tissue; however, rescue by the Act5C-fz transgene (G) or the presence of fzN21 (L) leads to rescue of yellow bristles in clone tissue indicating that canonical signaling activity is present.
Initially mutations were induced by exposure of flies to the alkylating agent EMS, which induces largely GC:AT DNA base changes (Ashburner et al. 2005). However, such changes are predicted only to result in deleterious missense mutations in ∼30% of amino acids in the Fz coding sequence (Figure S2). We therefore switched to the mutagen ENU, which is reported to induce both GC:AT and AT:GC changes (Ashburner et al. 2005) and potentially raises the number of codons that are predicted to suffer a deleterious change to ∼55% (Figure S2).
A total of ∼100,000 F1 progeny of the appropriate genotype from fathers treated with EMS were screened, as well as ∼110,000 from fathers treated with ENU. A total of 148 single flies showing a putative fz lesion were identified, as assayed by the appearance of a planar polarity phenotype visible on the notum, legs, or eyes down a dissecting microscope. After recrossing to confirm the presence of a phenotype and transmission through the germline, a total of 55 stable lines were recovered bearing mutations that lead to a loss of fz Planar polarity activity (Table 2).
The coding sequence of the fz transgene in these strains was isolated by PCR and sequenced, and in all cases at least one DNA change was found. Nonsense mutations leading to a premature stop codon and truncation of the predicted protein product were found in 25 strains and missense mutations leading to an amino acid change were found in 27 strains. Three strains harbored deletions leading to a change of reading frame and shortly afterward a premature stop codon. ENU did not yield AT:GC changes or missense mutations at a higher rate than EMS (Table 2). Notably, all of the amino acid mutations produced by the missense mutations are predicted to be mutagenic, using the SIFT tool, which sorts intolerant from tolerant substitutions on the basis of conservation of the particular amino acid in related proteins (Ng and Henikoff 2001). This is consistent with the induced mutations being the cause of the mutant phenotype.
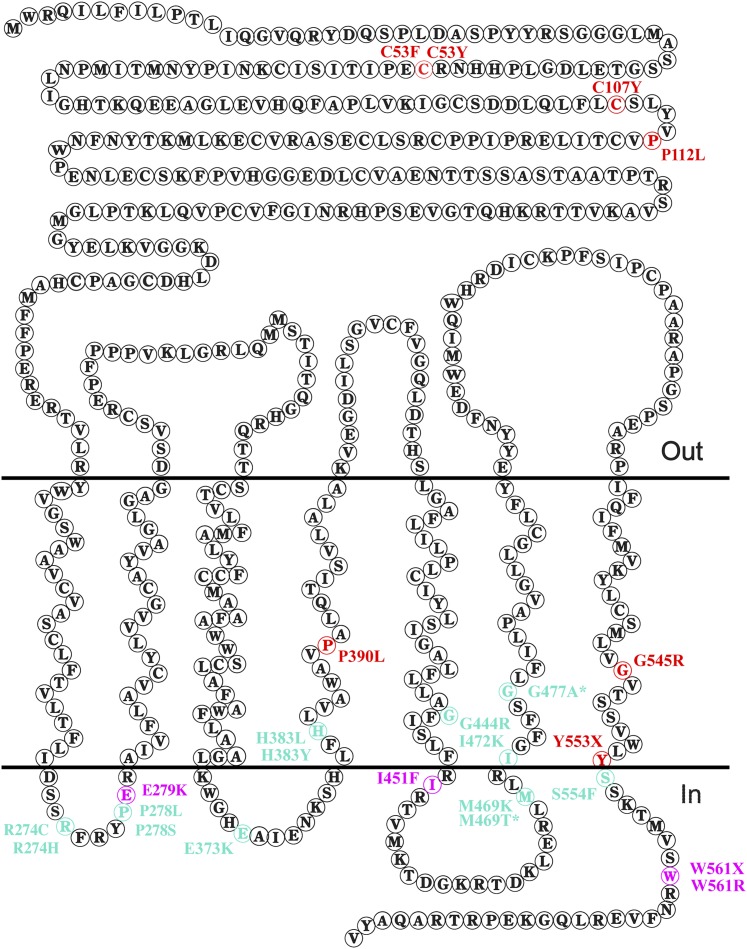
Focusing on the missense mutations, and nonsense mutations lying in or beyond the end of TM7, it is notable that as for the previous two screens (Jones et al. 1996; Povelones et al. 2005) (Table 1), most of our mutations map to the intracellular loops and C-terminal tail (Figure 2). However, we also obtained four lying in the extracellular CRD (C53F, C53Y, C107Y, and P112L). In common with the previous studies, we found no missense mutations in TM1–3, but also in common with these studies did find mutations in TM4–7. Overall we have approximately doubled the number of missense mutations in fz identified by chemical mutagenesis (compare Tables 1–3).
Figure 2 .
Topology of Fz and the location of selected mutations. The diagram depicts the amino acid sequence of Fz, showing the positions of amino acids in the extracellular region (top, “Out”), the transmembrane regions (middle), and the intracellular loops and C-terminal tail (bottom, “In”). Colored amino acids indicate missense mutations and nonsense mutations in TM7 and further C-terminal, isolated in our screen. Red indicates amino acid changes that give rise to Fz proteins that do not stably localize to junctions. Turquoise and magenta indicate changes where Fz protein still localizes to junctions at apparently normal levels and that show either phenotypes consistent with similar defects in canonical and planar polarity activity (turquoise) or a strong defect in planar polarity activity but normal canonical activity (magenta).
Assays for phenotypic analysis of in vivo and in vitro-generated fz mutations
To characterize the mutations isolated, we carried out a number of assays, looking both at adult phenotypes and effects on planar polarity establishment in the developing pupal wing. Truncations in the Fz protein prior to TM7 are known to cause complete loss of activity, with rare exceptions that may be caused by translational read-through of stop codons (Jones et al. 1996; Povelones et al. 2005). We therefore focused our analysis of alleles from our screen on missense mutations, and nonsense mutations that introduce stop codons only after TM7 (Figure 2 and Table 3). We also carried out some assays on existing fz alleles and a number of transgenes expressing in vitro mutagenized forms of fz, which we generated.
Assaying planar polarity activity using an adult phenotype:
To quantify the strength of the adult planar polarity phenotype, we initially sought to establish a simple and robust assay. Although planar polarity is most often studied in the wing, in this tissue the main phenotype is a swirling of the trichomes, which is difficult to quantify, and so in past studies the alternative measure of the number of cells showing multiple trichomes (“multiple hair cells”) has been used (Adler et al. 1987; Povelones et al. 2005). However, the production of multiple trichomes is believed to be a only secondary effect of altering hair polarity (Adler et al. 1987; Wong and Adler 1993) and is also very sensitive to genetic modifiers in the background of the stock (Adler et al. 1987; Jones et al. 1996). An alternative tissue where planar polarity defects can be readily quantified is the adult eye, where numbers of ommatidia showing polarity defects can be counted (e.g., Zheng et al. 1995), but this requires the production of semithin histological sections. For these reasons, we turned to a third adult tissue, the leg. fz mutant flies show polarity defects in their legs both in terms of the orientation of bristles, but also in the arrangement of the five tarsal segments at the distal end of the leg, exhibiting extra and mirror-image duplicated joints between segments (Held et al. 1986). We note that the number of additional joints (above the normal number of four) correlates well with the allele strengths determined in other assays (Figure 1, B–F, and Table 1; see Materials and Methods).
Assaying canonical signaling activity using an adult phenotype:
We also quantified the ability of the mutated transgenes to transduce canonical Wg signaling. In a previous study this was done by measuring the ability of maternally contributed fz activity to rescue the cuticle phenotype of fz fz2 double mutant embryos (Povelones et al. 2005). In our experiments, as the mutations were induced in a transgene inserted in a different chromosome from the fz and fz2 loci, we were able to directly assay for the rescuing ability of zygotic gene activity. As it is a well-characterized, easy to score phenotype (Figure 1, G–L), we measured the ability of Fz Fz2 transduction of Wg signaling to specify bristle fate in the adult wing margin (Chen and Struhl 1999; Chen et al. 2004).
Investigating pupal wing phenotypes:
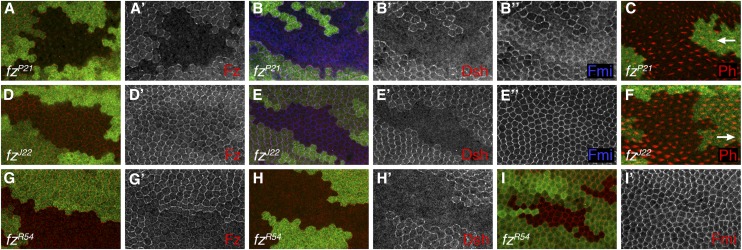
To gain insights into the mechanistic basis underlying the defects in canonical and planar polarity signaling observed, we studied effects on protein distribution and trichome polarity in the pupal wing. As points of reference we use existing endogenous alleles (Figure 3, A–I), in particular the strong fzP21 allele that harbors a nonsense mutation producing a early truncation of the protein and the cell-autonomous fzJ22 allele that has the P278L substitution in ICL1 that is known to block Dsh recruitment to junctions (Jones et al. 1996; Axelrod 2001).
Figure 3 .
Pupal wing phenotypes of existing fz alleles. (A–I) Confocal microscope images of 28 hr (A, B, D, E, G, H, I) or 32 hr (C and F) pupal wings containing loss-of-function clones for the indicated genotypes marked by lack of lacZ expression (green labeling), showing distribution of Fz (red or white in A, D, G), Dsh (red or white in B, E, H), Fmi (blue or white in B, E, I), or labeled with phalloidin to reveal F-actin (red in C and F). Distal is to the right; arrows in C and F indicate trichome polarity in nonmutant cells on distal side of clones, showing nonautonomous effect on polarity of fzP21 but not fzJ22.
Marked mosaic patches (“clones”) of cells lacking endogenous fz activity were generated in wings uniformly expressing the mutagenized transgene, and four characteristics were assessed:
-
1.
The levels and distribution of Fz protein produced by the transgene in otherwise fz mutant tissue. This gives a measure of both the amounts of Fz produced that can correctly localize to the adherens junctions regions and the ability of this protein to localize asymmetrically in the plane of the tissue and correctly specify planar polarity (Strutt 2001). Previous studies have measured total levels of mutant Fz proteins by Western blotting (Jones et al. 1996; Povelones et al. 2005). However, we believe that measuring junctional levels by immunolabeling is a more relevant assay, as this appears to be the functional population, at least for planar polarity activity.
-
2.
The distribution of Dsh in the junctional region. In the absence of fz activity, Dsh is not recruited to junctions, but additionally there are Fz mutant proteins that localize to junctions but fail to recruit Dsh (Axelrod 2001; Shimada et al. 2001; Amonlirdviman et al. 2005; Wu et al. 2008).
-
3.
The distribution of Fmi in the junctional region. Reducing fz activity is known to result in Fmi remaining localized to the adherens junction region of cells, but losing its characteristic asymmetric distribution to proximal and distal cell boundaries (Usui et al. 1999). Strong loss of fz activity also results in reduction of the levels of Fmi at junctions and some redistribution to apical cell membranes (Strutt and Strutt 2008).
-
4.
The orientation of trichomes around the fz clones expressing the mutated transgene: alleles that disrupt nonautonomous function result in trichomes outside the fz clone pointing inwards toward the clone (Vinson and Adler 1987; Adler et al. 1997).
Comparison of the effects of different mutations on fz activity
As expected, the missense alleles from our screen had a range of strengths of planar polarity defect, showing from about one to almost four additional tarsal joints (Table 3 and Figure S3). Comparison with existing strong fz alleles such as fzP21, fzH51, and fzR54 (Adler et al. 1987; Jones et al. 1996), which show around three or more additional joints (Figure 1, B–D, Tables 1 and 3), suggests that our strongest missense alleles can be considered to lack planar polarity activity. Three missense changes isolated in our screen also exist in extant endogenous alleles, these being P278L (fzJ22, fzI12 and 17A1, 30E2), P278S (fzF31, fzN21 and 18B3, 79F3), and I472K (fzKW and 39D1). These provide points of comparison for the effects of amino acid changes in the context of endogenous alleles or our fz transgenes. The endogenous alleles generally show slightly higher numbers of additional joints (∼0.5; Tables 1 and 3), suggesting a weaker effect of changes in the context of the transgene, most likely due to higher expression levels than from the endogenous locus. Thus our screen will probably tend to underestimate the effects of particular amino acid changes.
Strong loss-of-function mutations:
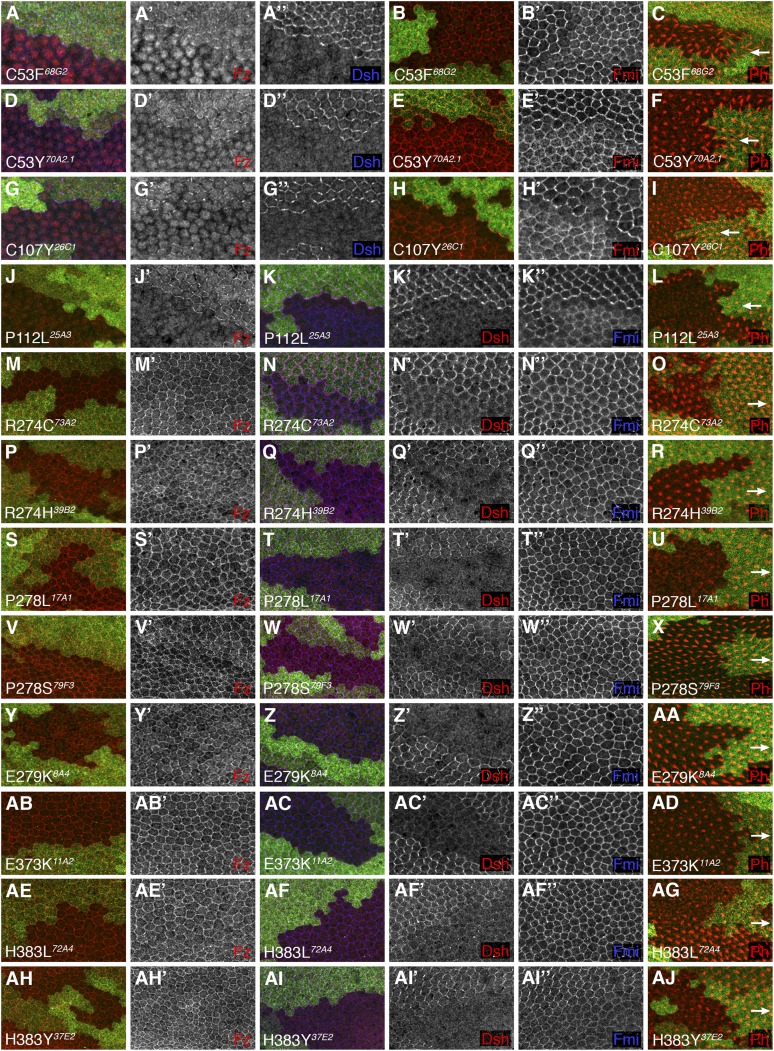
Nine alleles from our screen, affecting seven residues, behaved as strong loss-of-function mutations, showing defects in canonical signaling and autonomous and nonautonomous planar polarity activities (marked in Figure 2 and Figure S3). Considering the pupal wing phenotypes, all behave qualitatively the same as strong loss-of-function alleles such as fzP21, showing little or no Fz or Dsh at junctions, reduced symmetric Fmi junctional labeling and significant Fmi localized apically, and repolarization of trichomes outside the clone (Table 3, Figure 3, A–C, Figure 4, and Figure 5). Four such substitutions lay in the CRD (C53F, C53Y, C107Y, P112L) and from the Fz immunolabeling appeared to still produce a protein that could be seen in the cytoplasm but failed to localize to junctions. Three mutations are substitutions in TM4 and TM7 (P390L, G545R) and a truncation at the start of the C-terminal tail (Y553X), which again appear to compromise junctional localization or stability of the Fz protein. In addition, a similar result was seen for one of our mutant lines bearing a more C-terminal truncation of the tail (mutation 47B3 containing a W561X change); however, as two other lines that bear the same mutation (6E3, 14B2) show Fz immunolabeling at junctions, we conclude that 47B3 most likely bears a second-site regulatory mutation that fails to produce Fz protein.
Figure 4 .
Pupal wing phenotypes of fz clones in presence of indicated mutagenized transgenes. (A–AJ) Confocal microscope images of 28-hr (A, B, D, E, G, H, J, K, M, N, P, Q, S, T, V, W, Y, Z, AB, AC, AE, AF, AH, AI) or 32-hr (C, F, I, L, O, R, U, X, AA, AD, AG, AJ) pupal wings containing loss-of-function clones of fzP21 marked by lack of lacZ expression (green labeling), in the presence of the mutagenized transgenes bearing the indicated amino acid change. Immunolabeling reveals distribution of Fz (red or white in A, D, G, J, M, P, S, V, Y, AB, AE, AH), Dsh (blue or white in A, D, G; red or white in K, N, Q, T, W, Z, AC, AF, AI), Fmi (red or white in B, E, H; blue or white in K, N, Q, T, W, Z, AC, AF, AI). Phalloidin labeling reveals distribution of F-actin (red in C, F, I, L, O, R, U, X, AA, AD, AG, AJ). Distal is to the right; arrows indicate trichome polarity in nonmutant cells on distal side of clones, showing nonautonomous effect on polarity of C53F, C53Y, C107Y, and P112L amino acid changes.
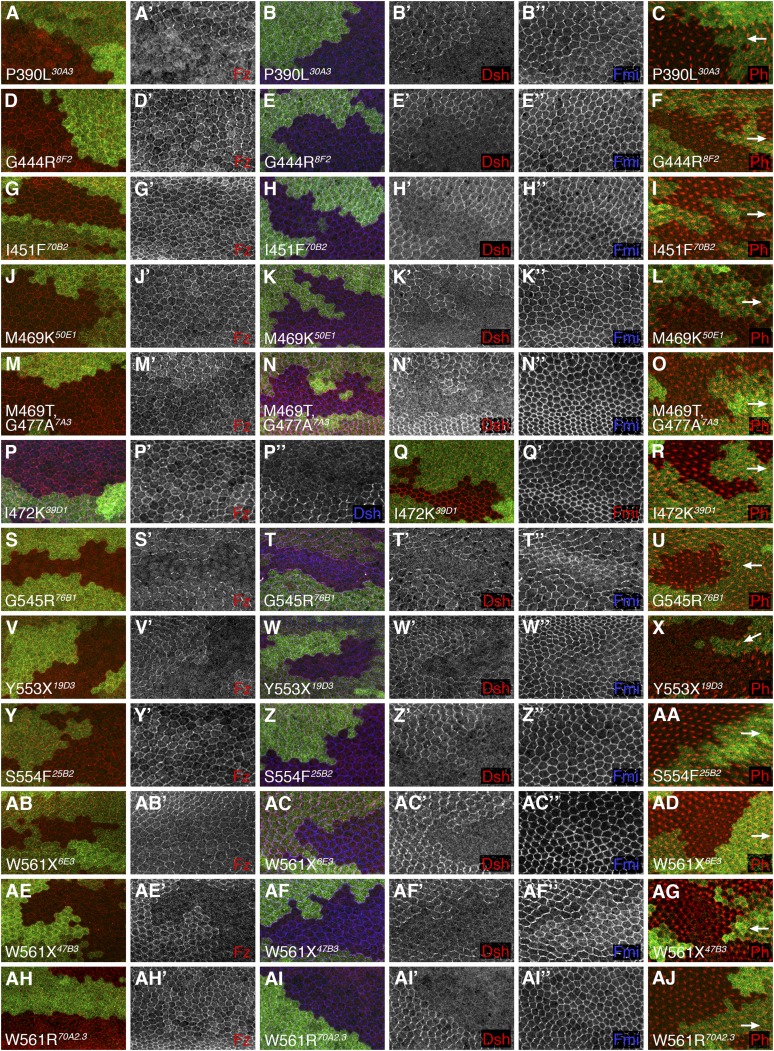
Figure 5 .
Pupal wing phenotypes of fz clones in presence of indicated mutagenized transgenes. (A–AJ) Confocal microscope images of 28 hr (A, B, D, E, G, H, J, K, M, N, P, Q, S, T, V, W, Y, Z, AB, AC, AE, AF, AH, AI) or 32 hr (C, F, I, L, O, R, U, X, AA, AD, AG, AJ) pupal wings containing loss-of-function clones of fzP21 marked by lack of lacZ expression (green labeling), in the presence of the mutagenized transgenes bearing the indicated amino acid change. Immunolabeling reveals distribution of Fz (red or white in A, D, G, J, M, P, S, V, Y, AB, AE, AH), Dsh (blue or white in P; red or white in B, E, H, K, N, T, W, Z, AC, AF, AI), Fmi (red or white in Q; blue or white in B, E, H, K, N, T, W, Z, AC, AF, AI). Phalloidin labeling reveals distribution of F-actin (red in C, F, I, L, O, R, U, X, AA, AD, AG, AJ). Distal is to the right; arrows indicate trichome polarity in nonmutant cells on distal side of clones, showing nonautonomous effect on polarity of P390L, G545R, and Y553X. One of three W561 mutations also showed loss of Fz at junctions and resulted in nonautonomous defects, but as the other two W561 mutations produced visible protein it is likely that this allele contains other regulatory mutations.
Mutations that produce Fz protein but fail to recruit Dsh:
The remaining amino acid changes induced in our screen that we analyzed in the pupal wing were 16 unique missense mutations affecting 12 different amino acids, all lying close to or within ICL1–3 or the C-terminal tail of Fz (turquoise and magenta in Figures 2 and 6A–C). Interestingly, all of these resemble the cell-autonomously acting fzJ22 allele (Figure 3, D–F), producing Fz protein that localizes symmetrically at apicolaterally junctions together with Fmi, failing to recruit Dsh to junctions and not causing nonautonomous trichome polarity defects in surrounding tissue (Table 3, Figures 4 and 5). In addition, the same phenotype was seen for two of our mutant lines that bear a W561X mutation that truncates the C-terminal tail within the KTxxxW motif.
Notably, alleles in this class showed a significant variation in strength of the polarity phenotype, ranging between around one and three additional tarsal segments. This may indicate that there are subtle differences in the ability of the proteins to localize to junctions or recruit Dsh, which are not easily detected by immunolabeling, but which influence the level of activity.
Surprisingly, although this class of alleles shows negligible ability to recruit Dsh to junctions, many fully rescue fz fz2 activity in wing margin patterning, indicating that they retain canonical signaling ability. In particular, five alleles with strong planar polarity defects (i.e., more than two extra tarsal segments) completely rescue fz fz2 clones (magenta in Figure 2 and Figure S3).
An important issue that might affect these results is that expression levels from our fz transgene may not be appropriate for rescuing canonical activity. We note that when assaying for planar polarity activity, our transgene insertions provide better rescue than endogenous alleles, and this may also account for the observed rescue of canonical activity. To investigate further, we tested the canonical signaling strength of existing fz alleles, by recombining them with a strong fz2 mutation and generating loss-of-function clones in the wing margin. Under these conditions, fzJ22 behaved similarly to alleles isolated in our screen bearing the same P278L mutation, showing only weak notching. Similarly, fzN21, bearing a P278S change, showed full rescue, as seen for P278S-bearing alleles from our screen. We conclude that the ability of this class of alleles to rescue canonical signaling is not simply an artifact of our assay system.
Mapping the position of missense mutations on a Fz homology model
To seek to understand the different behaviors of the isolated missense mutations in and around the 7TM region, we generated a homology-based model of the structure of Drosophila Fz (see Materials and Methods) and mapped the positions of the affected residues onto this (Figure 6, A and B). All of the mutated residues are situated in the lower half of the molecule, that is, toward the cytoplasmic face of the receptor (Figure 6A). Six of these residues (H383, P390, G444, M469, I472, and G545) are positioned in transmembrane helices and their mutation most likely affects signaling indirectly by altering the precise positioning of exposed amino acids in the intracellular loops. The other seven mutated residues are situated within the intracellular loop regions or in the C-terminal tail. A space-filling representation of the cytoplasmic face (Figure 6B) shows that two of the non-TM residues (I451, W561) are not exposed on the cytoplasmic face but appear to face upward toward the membrane (Figure 6A), and so their effect on binding is also likely to be indirect. Another three residues (E279, M469, and S554) line the inside of a deep surface pocket formed at the interface of the three intracellular loops and the C-terminal region (Figure 6B). The remaining amino acids (R274, E373, H383, and P278) form a distinct, broader cluster of residues situated close to one side of the deep pocket (Figure 6B, turquoise).
Comparison with in vitro generated mutations in fz
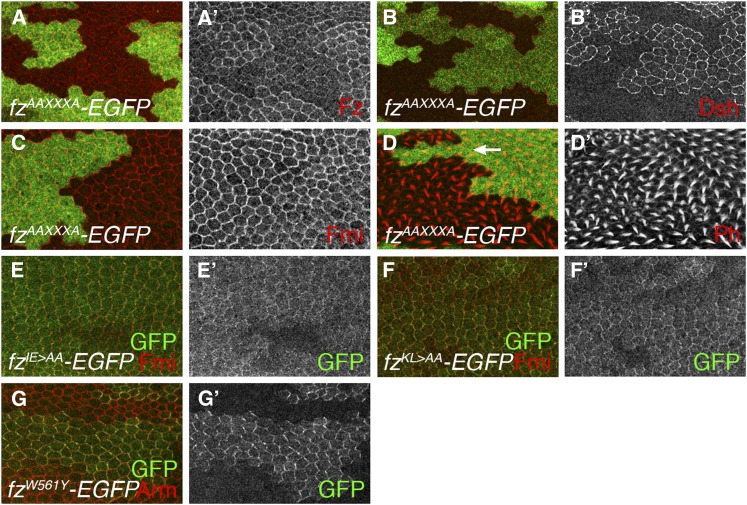
As previous reports have suggested a correlation between the ability of Fz receptors to recruit Dsh and function in canonical signaling, we sought to investigate the matter further by making more extreme changes to residues implicated in interacting with Dsh. On the basis of mutations made in vertebrate receptors that blocked canonical signaling, we substituted two alanines into ICL2 or ICL3 or replaced the KTxxxW motif with AAxxxA (Umbhauer et al. 2000; Cong et al. 2004). These mutations resulted in moderate to strong reduction in planar polarity activity (Table 3, Figure 7, A and D) and no longer retained canonical signaling activity (Table 3). However, when we examined the subcellular localization of the mutated proteins, we found that they localized poorly to junctions (Figure 7, A, E, and F), suggesting that their conformation or stability may be compromised.
Figure 7 .
Activity of in vitro mutagenized fz transgenes in the pupal wing. (A–D) Confocal microscope images of 28 hr (A, B, C) or 32 hr (D) pupal wings containing loss-of-function clones of fzP21 marked by lack of lacZ expression (green labeling), in the presence of an Act5C-fzAAXXXA-EGFP transgene, immunolabeled for distribution of Fz (red or white in A), Dsh (red or white in B), Fmi (red or white in C), or with phalloidin labeling to show distribution of F-actin (red or white in D). Note nonautonomous trichome phenotype in tissue on distal side of clone (arrow in D). (E–G) Mosaic patches of expression (green or white labeling) of indicated mutant Fz protein from an Act5C-fz-EGFP transgene in a wild-type background, colabeled for Fmi (red in E and F) or Armadillo (red in G). Note clear asymmetric localization of FzW561Y to junctions, compared to poorer localization of FzIE > AA and FzKL > AA.
In comparison, a single point mutation in an equivalent transgenic construct (W561Y) produced a protein that localizes asymmetrically to junctions and can fully rescue canonical signaling and also shows only negligible planar polarity defects (Table 3 and Figure 7G). This supports the previous finding in vertebrates that changing this tryptophan residue in the KTxxxW motif to a tyrosine residue does not disrupt canonical signaling of Fz receptors (Cong et al. 2004).
Comparison with in vitro-generated mutations in fz2
As Fz is not the primary receptor acting in canonical Wg signaling in flies, we also analyzed the effect of amino acid substitutions in Fz2 on Wg signal transduction. In the context of a vertebrate receptor, mutations in all three intracellular loops and the KTxxxW motif in the C-terminal tail have been shown to be important (Umbhauer et al. 2000; Cong et al. 2004). We generated a series of changes in the intracellular loops and KTxxxW motif of Fz2, and as a control also mutated the fourth conserved cysteine of the CRD (C118Y, equivalent to C107Y in Fz). These proteins were expressed in flies under a heterologous promoter. To increase the sensitivity of the assay, the relatively weak armadillo promoter was used, which provides a level of activity such that a wild-type Fz2 coding sequence barely rescues the wing notch phenotype of fz fz2 clones (see Materials and Methods). To control for effects of insertion site, at least two transgene insertions were assayed for each mutation.
As expected, our results show a requirement for C118 in canonical signaling (Table 4) and also confirm roles for residues in ICL1–3, including the equivalent of the P278L mutation in Fz (P349L). We also found a critical role for the initial lysine of the KTxxxW motif (K608A), but only a partial role for the threonine (T609A) and tryptophan residues (W613A), in disagreement with the previous report that the tryptophan was essential for canonical signaling (Umbhauer et al. 2000).
Table 4. Summary of properties of fz2 mutations examined in this study.
| Allele | Amino acid change | Region of protein | Equivalent change in Fz | Canonical phenotype (wing margin) |
|---|---|---|---|---|
| fz2C118Y #6 | C118Y | CRD | C107Y | Strong |
| fz2C118Y #8 | C118Y | CRD | C107Y | Strong |
| fz2R345A #1 | R345A | ICL1 | R274A | Moderate |
| fz2R345A #2 | R345A | ICL1 | R274A | Strong |
| fz2P349L #3 | P349L | ICL1 | P278L | Strong |
| fz2P349L #7 | P349L | ICL1 | P278L | Strong |
| fz2IT > AA #1 | I433A, T434A | ICL2 | I375A, E376A | Strong |
| fz2IT > AA #3 | I433A, T434A | ICL2 | I375A, E376A | Strong |
| fz2L528A #2 | L528A | ICL3 | L465A | Strong |
| fz2L528A #3 | L528A | ICL3 | L465A | Strong |
| fz2K608A #5 | K608A | C-term | K556A | Strong |
| fz2K608A #8 | K608A | C-term | K556A | Strong |
| fz2T609A #5 | T609A | C-term | T557A | Moderate |
| fz2T609A #7 | T609A | C-term | T557A | Moderate |
| fz2W613A #2 | W613A | C-term | W561A | Moderate |
| fz2W613A #8 | W613A | C-term | W561A | Moderate |
As the assay method and set of mutations analyzed was not the same for Fz and Fz2, the results cannot be directly compared. Nevertheless, the equivalent C107Y/C118Y, P278L/P349L. and IE > AA/IT > AA ICL2 changes were tested in both contexts and in each case cause a moderate to strong loss of canonical signaling activity. Overall these results suggest that there are similar sequence requirements in the Fz and Fz2 receptors for canonical signaling.
Discussion
Previous data have led to two contrasting models for how Fz interacts with Dsh, and how this affects canonical and planar polarity activities. On one hand it was suggested that as mutant forms of Dsh that are not recruited to membranes by Fz receptors can still mediate canonical signaling, Dsh recruitment was absolutely required only for planar polarity activity (Axelrod et al. 1998; Boutros et al. 1998; Rothbächer et al. 2000; Simons et al. 2009). These observations suggest that Fz receptors exhibit different mechanisms of action when activating different downstream signaling pathways via Dsh, which fits with the differential requirement for Wnt ligands and coreceptors in canonical and noncanonical signaling. Conversely, others have suggested that there is a requirement for Dsh binding to Fz for canonical signaling (Umbhauer et al. 2000; Wong et al. 2003; Cong et al. 2004; Wu et al. 2008), and together with other data this has led to the suggestion of a common mode of Fz receptor activation (Povelones et al. 2005).
It should be noted that it may be important to make the distinction between the ability of Fz receptors to recruit Dsh to membranes, possibly in stoichiometric quantities, as observed in planar polarity complex formation, and the possibility of more transient Fz-Dsh interactions that might conceivably underlie canonical signaling. Indeed, much of the evidence for recruitment of Dsh to membranes during canonical signaling comes from overexpression experiments. For instance, in Drosophila this model was proposed on the basis of experiments in which GFP-tagged Dsh was recruited by mutant Fz receptors overexpressed in a stripe of cells in the wing (Wu et al. 2008). Such methodologies may mask quantitative or qualitative differences in Fz–Dsh interactions.
By analyzing a much larger collection of missense alleles than was previously available, we present evidence that supports the first model of different modes of receptor activation. Specifically, we have identified amino acid changes in the intracellular loops and the proximal C-terminal tail that strongly affect planar polarity activity but are functional for canonical activity in a wing margin assay. Nevertheless, we did also find missense alleles that affected both canonical and planar polarity activity.
Povelones et al. (2005) based their hypothesis of a common mechanism of Fz receptor action on the observation that a range of fz alleles showed quantitatively similar defects in canonical and planar polarity signaling. Many of the alleles analyzed either were truncations early in the coding sequence or showed reduced protein levels, and so a deficit in both pathways is not surprising. However, six were missense alleles showing normal protein levels. One of these is fzJ22, which both in our hands and the previous study showed a moderate defect in both planar polarity and canonical signaling. Notably, the remaining five (fzHE11, fzF31, fzR53, fzTT, fzED) showed only weak defects in planar polarity and also only weak defects in canonical signaling as assayed by the maternal contribution assay used. Using our wing margin assay for canonical signaling, we find that fzN21, which harbors the same change as fzF31 (P278S), gives no defect in canonical signaling. Similarly, we obtained alleles with H383Y and H383L changes, comparable to the fzTT allele with a H383Y change, and these again showed no defect in canonical signaling. From this we conclude that the maternal effect assay used by Povelones et al. (2005) to assay canonical signaling is more sensitive to weak deficits in activity, possibly due to the more stringent requirement for maternally provided gene function to rescue a zygotic phenotype.
Taking our data and that of Povelones et al. together, there is clear evidence for amino acid changes that affect both canonical and noncanonical signaling to similar degrees, but we have also found examples of changes that have strong effects on planar polarity without a similar strong effect on canonical signaling (E279K, I451F, W561R, W561X). This class of amino acid changes that shows large differences between planar polarity and canonical activity is seen only relatively rarely following chemical mutagenesis, most likely explaining why it was not previously detected.
Our favored model is that there are different modes of interaction of Fz with Dsh that confer different signaling specificities. Notably, the ability to recruit Dsh to junctions, as seen during planar polarity specification, appears to require some specific residues in the Fz intracellular loops and C-terminal tail that have no major requirement in canonical signaling (magenta in Figures 2 and 6). We believe that two of these residues (I451 and W561) may be required for conformational changes in Fz that promote planar polarity activity, as in our model of the Fz structure they do not lie on a cytosolic-facing interaction surface (Figure 6B). A further residue that when mutated gave a strong effect on planar polarity but not on canonical activity (E279) protrudes into the deep central pocket in our model (Figure 6B) and thus might be part of a specific binding surface that interacts directly with Dsh only when the Fz receptor adopts a certain conformation. Alternatively, these residues could be involved in recruiting other factors that promote planar polarity activity. For instance, stable recruitment of Dsh into planar polarity complexes correlates with its phosphorylation (Axelrod 2001; Shimada et al. 2001) and thus these residues could be involved in recruiting or activating a kinase.
If we consider the positions within our model of the Fz structure of missense alleles that do not show large differences of activity between planar polarity and canonical activity (turquoise in Figure 6), then again some lie in transmembrane regions or deep pockets and are unlikely to directly affect Dsh binding (Figure 6A). However, others lie close together on the cytosolic face of Fz (Figure 6B). This region could plausibly form part of an interaction surface for Dsh that is required for both canonical and planar polarity activity. It is important to note that the interaction surfaces on the cytosolic face of Fz are likely to be much more extensive than revealed by the small number of missense alleles that we obtained. Many residues on the predicted cytosolic surface cannot be mutated to give deleterious changes by the GC:AT substitutions most commonly induced by EMS or ENU mutagenesis (olive in Figure 6C). Furthermore, many residues in the region are highly conserved in homologous proteins, suggesting functional conservation (deep pink and red in Figure 6D).
It is notable that we failed to obtain any missense alleles that caused a defect in planar polarity but gave rise to Fz proteins still able to recruit Dsh to junctions. This is consistent with the primary role of the intracellular regions of Fz in planar polarity signaling being to recruit Dsh, although we also note that in some cases a failure to recruit Dsh leads to a relatively mild planar polarity phenotype, leaving open the possibility that loss of Dsh recruitment is not in itself the cause of the signaling defect. Our data also provide strong evidence for the hypothesis that Fz receptors that cannot stably recruit Dsh, but still localize to cell junctions, can participate in only non-cell-autonomous planar polarity signaling (Amonlirdviman et al. 2005). We identified 13 more alleles that show this property. This further cements the view that the primary role of Dsh is in intracellular amplification and interpretation of polarity cues (Krasnow et al. 1995; Strutt and Strutt 2007), most likely by promoting clustering of planar polarity protein complexes in cell membranes (Strutt et al. 2011).
Several other points emerge from our analysis of novel fz alleles. First, we isolated four different changes in the CRD, all of which affected both canonical and planar polarity activity, and three altering cysteine residues suggested to be involved in disulphide bond formation based on the crystal structure of vertebrate CRDs (Dann et al. 2001). However, these all also prevented Fz localization to junctions, suggesting that a properly folded CRD is required for normal trafficking to the cell surface, similar to what was previously observed for a mutation in the CRD of Fz2 (Chen et al. 2004). In theory we might have expected to isolate point mutations in the CRD that block the reported intercellular interaction with Stbm in neighboring cells (Strutt and Strutt 2008; Wu and Mlodzik 2008), but in practice it may not be possible to separate this activity from the requirement for the CRD in membrane targeting. Second, we failed to isolate any mutations in the extracellular loops, and although this may reflect our screen being slightly less sensitive to planar polarity defects than that previously performed by Povelones et al. (2005), it nevertheless suggests that these regions are less sensitive to changes in amino acid identity. Finally, we found no evidence for an essential requirement of the Fz C-terminal tail downstream of the KTxxxW motif: this is consistent with the general lack of sequence conservation for this region among Fz family receptors.
Supplementary Material
Acknowledgments
We thank Paul Adler, Roel Nusse, Gary Struhl, Bloomington, and the Developmental Studies Hybridoma Bank for reagents, Paul Adler for the diagram of Fz used in Figure 2, and Helen Strutt for assistance with immunoblotting and comments on the manuscript. This work was supported by a Wellcome Trust Senior Fellowship to D.S., a project grant from Cancer Research UK, and a studentship from the Biotechnology and Biological Sciences Research Council. Confocal facilities were provided by the Wellcome Trust and Yorkshire Cancer Research.
Footnotes
Communicating editor: B. Sullivan
Literature Cited
- Adler P. N., Charlton J., Vinson C., 1987. Allelic variation at the Frizzled locus of Drosophila. Dev. Genet. 8: 99–119 [Google Scholar]
- Adler P. N., Charlton J., Jones K. H., Liu J., 1994. The cold-sensitive period for frizzled in the development of wing hair polarity ends prior to the start of hair morphogenesis. Mech. Dev. 46: 101–107 [DOI] [PubMed] [Google Scholar]
- Adler P. N., Krasnow R. E., Liu J., 1997. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr. Biol. 7: 940–949 [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K., Khare N. A., Tree D. R. P., Chen W.-S., Axelrod J. D., et al. , 2005. Mathematical modeling of planar cell polarity to understand domineering non-autonomy. Science 307: 423–426 [DOI] [PubMed] [Google Scholar]
- Angers S., Moon R. T., 2009. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10: 468–477 [DOI] [PubMed] [Google Scholar]
- Ashburner M., Golic K. G., Hawley R. S., 2005. Drosophila: A Laboratory Handbook, Ed. 2 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Axelrod J. D., 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signalling. Genes Dev. 15: 1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. D., Miller J. R., Shulman J. M., Moon R. T., Perrimon N., 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12: 2610–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. R., Duckworth D. M., Beeley L. J., 1998. Frizzled proteins constitute a novel family of G protein-coupled receptors, most closely related to the secretin family. Trends Pharmacol. Sci. 19: 399–400 [DOI] [PubMed] [Google Scholar]
- Bastock R., Strutt D., 2007. The planar polarity pathway promotes coordinated cell migration during Drosophila oogenesis. Development 134: 3055–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P., Brink M., Samos C. H., Hsieh J.-C., Wang Y., et al. , 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382: 225–230 [DOI] [PubMed] [Google Scholar]
- Bhanot P., Fish M., Jemison J. A., Nusse R., Nathans J., et al. , 1999. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development 126: 4175–4186 [DOI] [PubMed] [Google Scholar]
- Bhat K. M., 1998. frizzled and frizzled 2 play a partially redundant role in Wingless signaling and have similar requirements to Wingless in neurogenesis. Cell 95: 1027–1036 [DOI] [PubMed] [Google Scholar]
- Boutros M., Paricio N., Strutt D. I., Mlodzik M., 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94: 109–118 [DOI] [PubMed] [Google Scholar]
- Boutros M., Mihaly J., Bouwmeester T., Mlodzik M., 2000. Signaling specificity by frizzled receptors in Drosophila. Science 288: 1825–1828 [DOI] [PubMed] [Google Scholar]
- Chen C.-M., Struhl G., 1999. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126: 5441–5452 [DOI] [PubMed] [Google Scholar]
- Chen C. M., Strapps W., Tomlinson A., Struhl G., 2004. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc. Natl. Acad. Sci. USA 101: 15961–15966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-S., Antic D., Matis M., Logan C. Y., Povelones M., et al. , 2008. Asymmetric homotypic interactions of the atypical cadherin Flamingo mediate intercellular polarity signaling. Cell 133: 1093–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., et al. , 2007. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318: 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. D., Mariol M.-C., Wallace R. M. H., Weyers J., Kamberov Y. G., et al. , 2002. Dwnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev. Cell 2: 437–448 [DOI] [PubMed] [Google Scholar]
- Cong F., Schweizer L., Varmus H., 2004. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115 [DOI] [PubMed] [Google Scholar]
- Dann C. E., Hsieh J. C., Rattner A., Sharma D., Nathans J., et al. , 2001. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412: 86–90 [DOI] [PubMed] [Google Scholar]
- Djiane A., Yogev S., Mlodzik M., 2005. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121: 621–631 [DOI] [PubMed] [Google Scholar]
- Fredriksson R., Lagerstrom M. C., Lundin L. G., Schioth H. B., 2003. The G-protein-coupled receptors in the human genome form five main families: phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63: 1256–1272 [DOI] [PubMed] [Google Scholar]
- Grigliatti T. A., 1998. Mutagenesis, pp. 55–83 in Drosophila: A Practical Approach, Ed. 2, edited by D. B. Roberts. Oxford University Press, Oxford [Google Scholar]
- Gubb D., García-Bellido A., 1982. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68: 37–57 [PubMed] [Google Scholar]
- Held L. I., Duarte C. M., Derakhshanian K., 1986. Extra tarsal joints and abnormal cuticular polarities in various mutants of Drosophila melanogaster. Roux’s Arch. Dev. Biol. 195: 145–157 [DOI] [PubMed] [Google Scholar]
- Itoh K., Jacob J., Sokol S. Y., 1998. A role for Xenopus Frizzled 8 in dorsal development. Mech. Dev. 74: 145–157 [DOI] [PubMed] [Google Scholar]
- Jones K. H., Liu J., Adler P. N., 1996. Molecular analysis of EMS-induced frizzled mutations in Drosophila melanogaster. Genetics 142: 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell J. R., Carthew R. W., 1998. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the Wingless pathway. Cell 95: 1017–1026 [DOI] [PubMed] [Google Scholar]
- Klingensmith J., Nusse R., Perrimon N., 1994. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 8: 118–130 [DOI] [PubMed] [Google Scholar]
- Koval A., Purvanov V., Egger-Adam D., Katanaev V. L., 2011. Yellow submarine of the Wnt/Frizzled signaling: submerging from the G protein harbor to the targets. Biochem. Pharmacol. 82: 1311–1319 [DOI] [PubMed] [Google Scholar]
- Krasnow R. E., Wong L. L., Adler P. N., 1995. dishevelled is a component of the frizzled signaling pathway in Drosophila. Development 121: 4095–4102 [DOI] [PubMed] [Google Scholar]
- Krogh A., Larsson B., Von Heijne G., Sonnhammer E. L., 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305: 567–580 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Casal J., Struhl G., 2002. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 129: 2749–2760 [DOI] [PubMed] [Google Scholar]
- Malbon C. C., 2011. Wnt signalling: the case of the ‘missing’ G-protein. Biochem. J. 433: e3–e5 [DOI] [PubMed] [Google Scholar]
- Mathew D., Ataman B., Chen J., Zhang Y., Cumberledge S., et al. , 2005. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 310: 1344–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca T. J., Schwarz T. L., 2010. The nuclear import of Frizzled2-C by Importins-beta11 and alpha2 promotes postsynaptic development. Nat. Neurosci. 13: 935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H., Samanta R., Wieschaus E., 1999. Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development 126: 577–586 [DOI] [PubMed] [Google Scholar]
- Ng P. C., Henikoff S., 2001. Predicting deleterious amino acid substitutions. Genome Res. 11: 863–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., et al. , 2000. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289: 739–745 [DOI] [PubMed] [Google Scholar]
- Penton A., Wodarz A., Nusse R., 2002. A mutational analysis of dishevelled in Drosophila defines novel domains in the Dishevelled protein as well as novel suppressing alleles of axin. Genetics 161: 747–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M., Nusse R., 2005. The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling. EMBO J. 24: 3493–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M., Howes R., Fish M., Nusse R., 2005. Genetic evidence that Drosophila frizzled controls planar cell polarity and Armadillo signaling by a common mechanism. Genetics 171: 1643–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbächer U., Laurent M. N., Deardorff M. A., Klein P. S., Cho K. W. Y., et al. , 2000. Dishevelled phosphorylation, subcellular localisation and multimerisation regulate its role in early embryogenesis. EMBO J. 19: 1010–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Kucukural A., Zhang Y., 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5: 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson E. J., Wu C.-H., Nusse R., 2000. Pathway specificity by the bifunctional receptor Frizzled is determined by affinity for Wingless. Mol. Cell 6: 117–126 [PubMed] [Google Scholar]
- Sawa H., Lobel L., Horvitz H. R., 1996. The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev. 10: 2189–2197 [DOI] [PubMed] [Google Scholar]
- Shimada Y., Usui T., Yanagawa S., Takeichi M., Uemura T., 2001. Asymmetric co-localisation of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarisation. Curr. Biol. 11: 859–863 [DOI] [PubMed] [Google Scholar]
- Siegfried E., Wilder E. L., Perrimon N., 1994. Components of wingless signalling in Drosophila. Nature 367: 76–80 [DOI] [PubMed] [Google Scholar]
- Simons M., Gault W. J., Gotthardt D., Rohatgi R., Klein T. J., et al. , 2009. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat. Cell Biol. 11: 286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strapps W. R., Tomlinson A., 2001. Transducing properties of Drosophila Frizzled proteins. Development 128: 4829–4835 [DOI] [PubMed] [Google Scholar]
- Strutt D. I., 2001. Asymmetric localisation of Frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7: 367–375 [DOI] [PubMed] [Google Scholar]
- Strutt D., Strutt H., 2007. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev. Biol. 302: 181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H., Strutt D., 2008. Differential stability of Flamingo protein complexes underlies the establishment of planar polarity. Curr. Biol. 18: 1555–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H., Price M. A., Strutt D., 2006. Planar polarity is positively regulated by casein kinase Iε in Drosophila. Curr. Biol. 16: 1329–1336 [DOI] [PubMed] [Google Scholar]
- Strutt H., Warrington S. J., Strutt D., 2011. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev. Cell 20: 511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriello D. V., Jordens I., Kirchner K., Slootstra J. W., Kruitwagen T., et al. , 2012. Wnt/beta-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl. Acad. Sci. USA 109: E812–E820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M., Djiane A., Goisset C., Penzo-Mendez A., Riou J. F., et al. , 2000. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in Frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 19: 4944–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T., Shima Y., Shimada Y., Hirano S., Burgess R. W., et al. , 1999. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98: 585–595 [DOI] [PubMed] [Google Scholar]
- Van Amerongen R., Nusse R., 2009. Towards an integrated view of Wnt signaling in development. Development 136: 3205–3214 [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Adler P. N., 1987. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329: 549–551 [DOI] [PubMed] [Google Scholar]
- Wallingford J. B., Habas R., 2005. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 132: 4421–4436 [DOI] [PubMed] [Google Scholar]
- Wang Y., Macke J. P., Abella B. S., Andreasson K., Worley P., et al. , 1996. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 271: 4468–4476 [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Liu T., Malbon C. C., 2006. Structure-function analysis of Frizzleds. Cell. Signal. 18: 934–941 [DOI] [PubMed] [Google Scholar]
- Wehrli M., Dougan S. T., Caldwell K., O’keefe L., Schwartz S., et al. , 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407: 527–530 [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Jr, 2003. Runnin’ with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253: 1–17 [DOI] [PubMed] [Google Scholar]
- Wong H. C., Bourdelas A., Krauss A., Lee H. J., Shao Y., et al. , 2003. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell 12: 1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. L., Adler P. N., 1993. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Mlodzik M., 2008. The Frizzled Extracellular Domain Is a Ligand for Van Gogh/Stbm during Nonautonomous Planar Cell Polarity Signaling. Dev. Cell 15: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Klein T. J., Mlodzik M., 2004. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2: E158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Jenny A., Mirkovic I., Mlodzik M., 2008. Frizzled-Dishevelled signaling specificity outcome can be modulated by Diego in Drosophila. Mech. Dev. 125: 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., 2007. Template-based modeling and free modeling by I-TASSER in CASP7. Proteins 69(Suppl 8): 108–117 [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhang J., Carthew R. W., 1995. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development 121: 3045–3055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.