Abstract
Migration of neurons and neural crest cells is of central importance to the development of nervous systems. In Caenorhabditis elegans, the QL neuroblast on the left migrates posteriorly, and QR on the right migrates anteriorly, despite similar lineages and birth positions with regard to the left–right axis. Initial migration is independent of a Wnt signal that controls later anterior–posterior Q descendant migration. Previous studies showed that the transmembrane proteins UNC-40/DCC and MIG-21, a novel thrombospondin type I repeat containing protein, act redundantly in left-side QL posterior migration. Here we show that the LAR receptor protein tyrosine phosphatase PTP-3 acts with MIG-21 in parallel to UNC-40 in QL posterior migration. We also show that in right-side QR, the UNC-40 and PTP-3/MIG-21 pathways mutually inhibit each other’s role in posterior migration, allowing anterior QR migration. Finally, we present evidence that these proteins act autonomously in the Q neuroblasts. These studies indicate an inherent left–right asymmetry in the Q neuroblasts with regard to UNC-40, PTP-3, and MIG-21 function that results in posterior vs. anterior migration.
CELL migration is a fundamental event in the development of nervous systems. In the vertebrate central nervous system, neurons and neuroblasts migrate radially to populate distinct layers in the cerebellar and cerebral cortices, and neural crest cells migrate along distinct paths in the vertebrate embryo to give rise to the peripheral nervous system. The Q neuroblasts in Caenorhabditis elegans are a useful model to study the migration of neuroblasts and neurons in the anterior–posterior axis. The Q neuroblasts are a bilaterally symmetric pair of cells in the posterior–lateral region of the animal, with QR on the right side and QL on the left side (Sulston and Horvitz 1977). The Q neuroblasts are born in embryogenesis and are the sisters of the V5 hypodermal seam cells. By 5 hr after hatching, QR has migrated anteriorly and divided over the V4 seam cell, and QL has migrated posteriorly and divided over the V5 seam cell (Honigberg and Kenyon 2000; Chapman et al. 2008; Dyer et al. 2010). The resulting Q cell descendants then undergo a pattern of migration, division, and programmed cell death resulting in three neurons each (AQR, SDQR, and AVM on the right from QR; and PQR, SDQL, and PVM on the left from QL) (Sulston and Horvitz 1977; Chalfie and Sulston 1981). The QR descendant AQR migrates the longest distance to a region near the anterior deirid ganglion in the head, and the QL descendant PQR migrates the longest distance posteriorly to the phasmid ganglion in the tail (Sulston and Horvitz 1977; White et al. 1986; Chapman et al. 2008). The posterior migration of QL descendants requires the activity of the MAB-5/Hox transcription factor, expression of which is induced in QL descendants by an EGL-20/Wnt signal emanating from the posterior (Chalfie et al. 1983; Kenyon 1986; Salser and Kenyon 1992; Harris et al. 1996; Whangbo and Kenyon 1999; Korswagen et al. 2000; Herman 2003; Eisenmann 2005). QR migrates anteriorly and does not normally receive this EGL-20/Wnt signal, and thus does not express MAB-5/Hox.
The initial anterior and posterior migrations of the QR and QL neuroblasts do not depend on MAB-5 or EGL-20/Wnt (Chapman et al. 2008), as QL and QR protrude and polarize normally in mab-5 and egl-20 mutants. While initial Q migration is independent of EGL-20/Wnt, the five Wnt genes are involved in subsequent Q descendant guidance along the anterior–posterior axis (Pan et al. 2006; Harterink et al. 2011; Zinovyeva et al. 2008).
The initial Q migrations can affect subsequent MAB-5 expression in the Q descendants (Chapman et al. 2008; Middelkoop et al. 2012). The extent of posterior protrusion correlates with mab-5 expression, with more mab-5 expression in cells that protrude posteriorly (Middelkoop et al. 2012), consistent with exposure to the posterior EGL-20/Wnt signal. QR is inherently less sensitive to the EGL-20/Wnt signal than QL (Whangbo and Kenyon 1999; Middelkoop et al. 2012), a difference that seems to be mediated by the MIG-21 molecule (i.e., in mig-21 mutants the differential sensitivity is abolished) (Middelkoop et al. 2012).
Previous studies have revealed mechanisms of initial Q neuroblast migration that is independent of EGL-20/Wnt and MAB-5/Hox. The transmembrane immunoglobulin superfamily receptor UNC-40/Deleted in Colorectal Cancer (DCC) controls the anterior–posterior protrusion and migration of both QR and QL (Honigberg and Kenyon 2000; Middelkoop et al. 2012). UNC-40/DCC is an UNC-6/Netrin receptor that regulates cell and growth cone migrations in the dorsal–ventral axis (Hedgecock et al. 1990; Keino-Masu 1996). UNC-6/Netrin is not involved with UNC-40/DCC in anterior–posterior Q migration (Honigberg and Kenyon 2000), nor does it act with UNC-40 in muscle arm extension (Alexander et al. 2009), suggesting that UNC-40/DCC might utilize other ligands in these processes.
To identify additional genes that might act with UNC-40 in initial Q protrusion and migration, we conducted a forward genetic screen for mutants with altered migrations of the QL and QR descendant neurons AQR and PQR, with the idea that they might also affect Q protrusion and migration. This screen identified three new mutations in the mig-21 gene (Du and Chalfie 2001), which encodes a small transmembrane molecule with two extracellular thrombospondin type I domains. MIG-21 was shown previously to affect Q protrusion and migration and Q descendant migration and to control differential sensitivity of QL and QR to the EGL-20/Wnt signal (Du and Chalfie 2001; Middelkoop et al. 2012).
A previous screen for Q descendant migration mutants identified qid-5(mu245) (Ch’ng et al. 2003), which we found caused misdirected AQR and PQR similar to unc-40 and mig-21. We sequenced the genome of a qid-5(mu245) strain and discovered that qid-5(mu245) is a new and potential null allele of the ptp-3 gene, which was previously implicated in Q protrusion and migration (Williams 2003) and which encodes a LAR-type receptor protein tyrosine phosphatase (Harrington et al. 2002; Ackley et al. 2005). The ptp-3 locus encodes a family of transmembrane molecules characterized by extracellular immunoglobulin and fibronectin type III repeats and two intracellular phosphatase domains. PTP-3/LAR-related molecules are involved in multiple aspects of nervous system development, including axon guidance, neurite development, and synaptic organization (Ackley et al. 2005; Johnson et al. 2006; Pawson et al. 2008; Hofmeyer and Treisman 2009; Wang et al. 2012), cell movements in gastrulation (Harrington et al. 2002), and germline stem cell maintenance (Srinivasan et al. 2012).
Analysis of double and triple mutants involving unc-40, ptp-3, and mig-21 revealed distinct interactions in QL vs. QR. In QL we found that UNC-40 and MIG-21 act redundantly in posterior migration, similar to a recently published study (Middelkoop et al. 2012). We also found that PTP-3 acts in parallel to UNC-40 in QL posterior migration and might act in the same pathway as MIG-21. Surprisingly, we found that the abnormal posterior migration of QR in ptp-3 and mig-21 mutants was suppressed by unc-40, and vice versa, leading us to speculate that in QR, UNC-40 and a PTP-3/MIG-21 pathway might mutually inhibit each other’s role in posterior migration, allowing for anterior migration of QR. Cell-specific rescue and RNAi experiments indicate that MIG-21, UNC-40, and PTP-3 can act cell autonomously in the Q cells to guide Q migrations.
In sum, we have identified three transmembrane molecules, UNC-40, MIG-21, and PTP-3, that control posterior Q cell migration. UNC-40 and MIG-21/PTP-3 act in parallel in QL and act as mutual inhibitors of one another in QR. These novel interactions between UNC-40, PTP-3, and MIG-21, indicate that complex transmembrane receptor interactions guide initial Q cell protrusion and migration, and that, despite apparent bilateral symmetry, QL and QR use inherently distinct mechanisms to guide initial anterior–posterior migration.
Materials and Methods
C. elegans genetics
All experiments were conducted at 20° using standard culture techniques (Sulston and Hodgkin 1988). The following mutations were used: LGI unc-40(e1430), unc-40(e271), unc-40(n324); LGII ptp-3(mu256), ptp-3(mu245), ptp-3(ok244), muIs32[mec-4::gfp]; LGIII mig-21(u787), mig-21(lq37), mig-21(lq78), mig-21(lq84); LGIV lqIs80[Pscm promoter::gfp::caax]; LGV sid-1(pk3321), lqIs58[Pgcy-32 promoter::cfp], ayIs9[Pegl-17::gfp] (Branda and Stern 2000); LG unassigned lqIs146[Pscm promoter::unc-40(RNAi)], lqEx661[Pscm promoter::mig-21(RNAi)], lqIs166[Pscm promoter::ptp-3(RNAi)]; lqIs151[Pscm promoter::unc-40(+)::gfp], lqEx637[Pscm promoter::ptp-3B(+)::gfp)], juIs197[ptp-3B(+)::gfp], lqEx593[mig-21(+)], lqEx712 [Pdpy-7::ptp-3B(+)], lqEx714 [Pegl-17::unc-40(+)], lqEx716 [Pegl-17::ptp3B(+)]. Trangenes were constructed by standard gonadal microinjection to produce extrachromosomal arrays and were stably integrated into the genome using standard trimethylpsoralen and ultraviolet light treatment (Mello and Fire 1995).
Isolation of mig-21 alleles and characterization of the mig-21 locus
The mig-21 alleles lq37, lq78, and lq84 were isolated in an EMS screen for new mutations that affect the positions of the Q cell descendants AQR and PQR (E. A. Lundquist, unpublished results). Single nucleotide polymorphism (SNP) mapping was used to assign these mutations to linkage groups as described in Davis et al. (2005). Briefly, males from the polymorphic Hawaiian strain CB4856 were mated to hermaphrodites harboring the new mutation in the N2 background, which also contained the lqIs58 V and lqIs80 IV marker transgenes. Single F1 heterozygous hermaphrodites were plated singly, and 10–20 F2 animals with AQR and PQR migration defects (mutant segregants) were subjected to PCR using the SNP primers described in Davis et al. (2005). The PCR products were digested with DraI restriction enzyme to detect the SNP. Relative agarose gel electrophoresis band intensities were used to determine which CB4856-associated SNP’s were underrepresented in the mutant segregants. lq37, lq78, and lq84 all showed linkage to SNP’s on LGIII, and each failed to complement each other and mig-21(u787) for AQR and PQR defects (data not shown). The mig-21 locus from each strain was amplified by polymerase chain reaction (PCR), and the PCR products were sequenced to identify the lesions associated with each allele (primer sequences available upon request). lq37 was a G-to-A transition (position LGIII 5,877,678 WS228) resulting in a C-to-Y missense change; lq78 was a G-to-A transition (position LGIII 5,877,466 WS228) resulting in a G-to-E missense; and lq84 was a G-to-A missense (position LGIII 5,877,289 WS228) resulting in an altered 3′ splice site.
A transgene containing the wild-type mig-21(+) locus was generated by amplifying and cloning the mig-21 gene from N2 genomic DNA. This fragment consisted of the entire region from the predicted upstream and downstream genes relative to F01F1.13 (LGIII 5,878,514 to 5,876,277, WormBase WS229). The region was sequenced to ensure that no mutations had been introduced by PCR. This construct (lqEx593) rescued the AQR and PQR migration defects of mig-21(u787).
In an unrelated experiment, the transcriptomes of L1 larvae ∼5 hr after hatching were sequenced using next-generation RNA seq on the Illumina Genome Analyzer IIx (GAIIx) platform (Cofactor Genomics, St. Louis). Sequencing reads were mapped to the C. elegans reference genome using TopHat (Langmead et al. 2009) and visualized using the Integrated Genomics Viewer 2.0.15 (Robinson et al. 2011; Thorvaldsdottir et al. 2012). The reads that mapped to the mig-21 locus are shown in Figure S1 and confirmed the gene structure diagrammed in Figure S2. The 3′ end of the gene structure is identical to that described in Middelkoop et al. (2012). The 5′ end differed from the Middelkoop et al. (2012) prediction, and was confirmed by a transcript sequencing read as shown on WormBase WS229 (read MM454_FPK17YK01AZZNG). The prediction described here contains a predicted N-terminal signal sequence not found in the Middelkoop et al. (2012) prediction.
ptp-3(mu245) identification
The previously identified qid-5(mu245) mutant affected AQR and PQR migration in a manner similar to mig-21. To identify the gene affected by qid-5(mu245), we subjected a strain harboring the mutation (LE2577; qid-5(mu245) muIs32 II; lqIs80 Iv; lqIs58 V) to genome resequencing using the Illumina GAIIx platform (special thanks to O. Hobert and A. Boyanov, Columbia University, New York). LE2577 reads were compared to the C. elegans reference genome using MAQgene (Bigelow et al. 2009), which also categorized the predicted effects the polymorphisms had on gene structure and function. LE2577 harbored a predicted C-to-A mutation (position LGII 10,995,118 in WS229) that resulted in a premature stop codon in the ptp-3 gene. qid-5(mu245) had been previously mapped to a region of LGII that contains ptp-3. qid-5(mu245) failed to complement ptp-3 for AQR and PQR migration defects (data not shown), indicating that mu245 is an allele of ptp-3.
Scoring AQR and PQR migration defects
Defects in AQR and PQR migration were quantified as described previously (Chapman et al. 2008). AQR and PQR were assayed using the gcy-32::cfp transgene lqIs58 in L4 or young adult animals. Five regions along the anterior–posterior axis of the animal were considered. Position 1 represents the wild-type position of AQR in the anterior deirid ganglion just posterior to the pharynx; position 2 represents a region anterior to the vulva but posterior to the anterior deirid; position 3 represents a region proximal to the vulva (∼10 AQR or PQR cell body widths to the anterior and posterior of the vulva); position 4 represents the birthplace of the Q neuroblasts near the posterior deirid ganglion; and position 5 represents the wild-type position of PQR posterior to the anus in the phasmid ganglion. At least 100 animals of each genotype were scored for AQR and PQR position relative to this scale. Only positions 1 and 5, the unambiguous positions of wild-type AQR and PQR, were used in statistical analysis. Significance was determined using Fisher’s exact test.
Scoring Q neuroblast migration defects
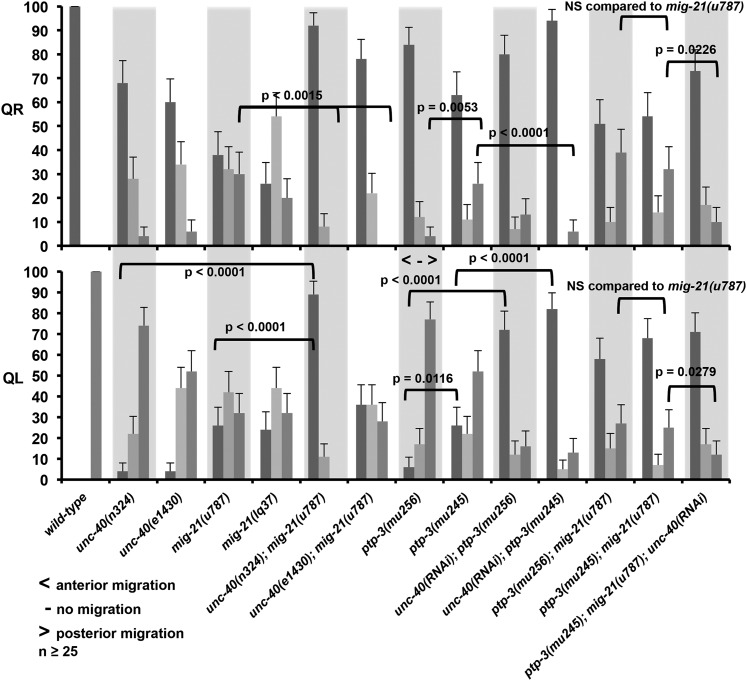
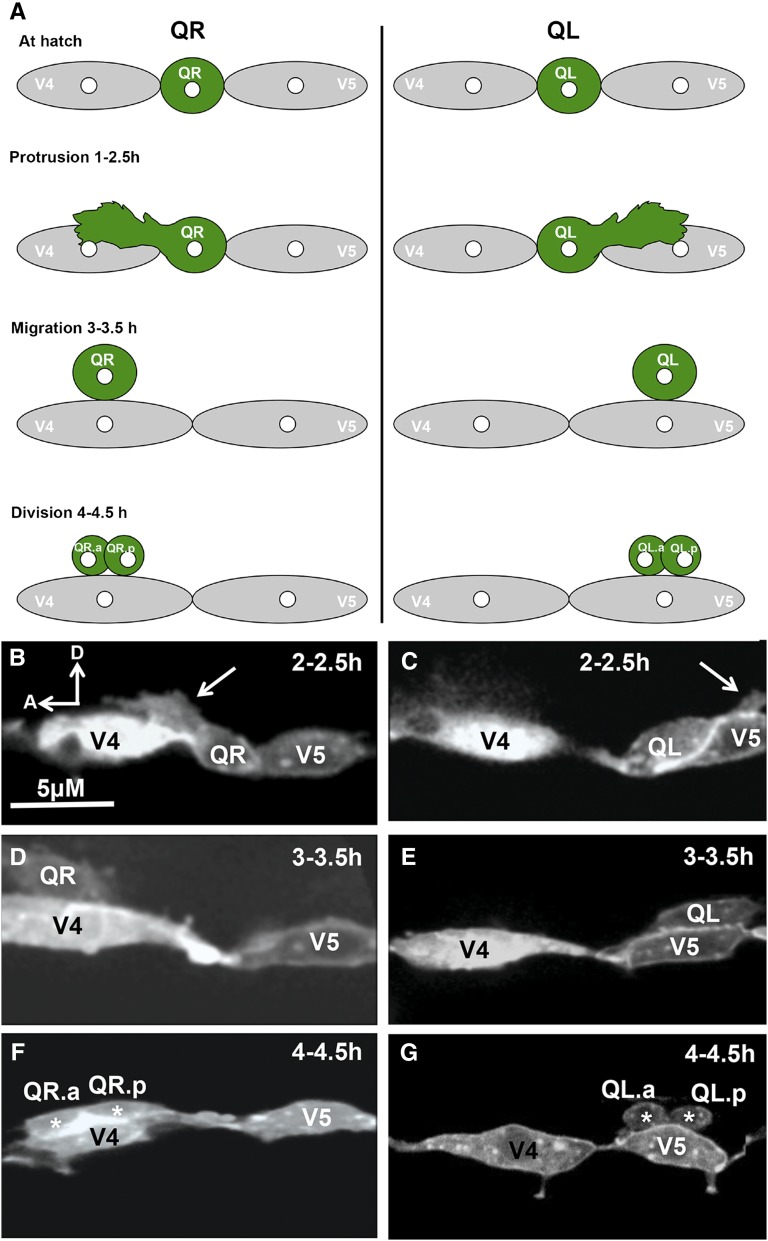
The protrusion and migration of the Q neuroblasts was quantified as described previously (Chapman et al. 2008). To synchronize animals, adults and larvae were washed from plates, leaving behind eggs. At one-half-hour time points, newly hatched larvae were washed from these plates and allowed to develop on freshly seeded NGM plates. Q cells were analyzed at 2, 3, and 4 hr after synchronization. At 2–2.5 hr posthatch, the Q neuroblasts extend anterior (QR) and posterior (QL) protrusions. By 3–3.5 hr, the Q neuroblasts have migrated above their respective seam cells (V4 for QR; V5 for QL). At 4–4.5 hr, the Q neuroblasts undergo their first division above the seam cells. Protrusion at 2–2.5 hr was scored as anterior if the cell protruded over the V4 seam, as no protrusion if it failed to protrude, and posterior if it protruded over the V5 seam cell. Migration at the 3- to 3.5-hr stage was scored as anterior if the Q cells migrated over the V4 seam cell, as migration failure if the Q cells failed to migrate, and as posterior if the Q cells migrated over the V5 seam cell. Position of division at 4–4.5 hr was scored as anterior if the Q cells divided over the V4 seam cell, as between if the Q cells divided between the V4 and V5 seam cells, and posterior if the Q cells divided over the V5 seam cell. At least 25 cells were scored for each genotype, and significance of difference in migration between genotypes was determined by Fisher’s exact analysis. Only position of division at 4–4.5 hr was included in Figure 6, but a table with all of the time-point data are included as Table S1.
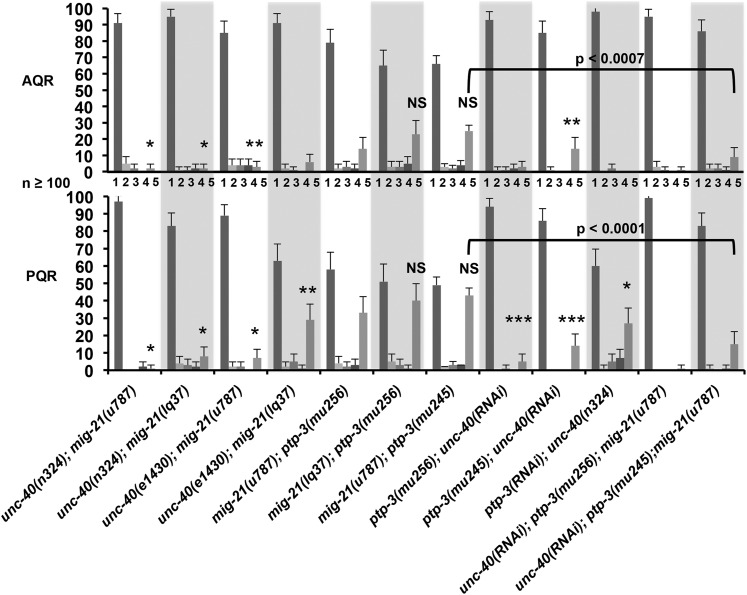
Figure 6 .
QR and QL migration defects. Graph plots genotype on the x-axis against percentage of QR and QL at 4–4.5 hr posthatching that had divided above V4 (anterior migration; bars with dark shading); between V4 and V5 (no migration; bars with light shading); and above V5 (posterior migration; bars with medium shading). Data for the 2- to 2.5-hr protrusion stage and the 3- to 3.5-hr migration stage are included in Supporting Information. The 2- to 2.5-hr and 3- to 3.5-hr data generally follow the trends seen with the 4- to 4.5-hr division stage data shown here. unc-40(RNAi) represents the cell-specific Pscm::unc-40(RNAi) transgene. The error bars represent two times the standard error of the proportion, and significances of difference were calculated by Fisher exact analysis. At least 25 animals were scored for each genotype.
ptp-3 and unc-40 transgenes
A transgene consisting of the wild-type ptp-3B gene and upstream region fused in frame to gfp (juIs197[ptp-3B::gfp]) (Ackley et al. 2005) rescued AQR and PQR defects of ptp-3(mu256). We amplified the coding region from this construct and placed it behind the scm promoter to drive ptp-3B::gfp expression specifically in the seam cells and early Q cells (lqEx637[scm promoter::ptp-3B::gfp]) (Chapman et al. 2008). Primer and plasmid sequences are available upon request. We created an scm promoter::unc-40::gfp transgene (lqIs151[scm promoter::unc-40(+)::gfp]) by amplifying the unc-40::gfp coding region from the previously described mec-4 promoter::unc-40::gfp plasmid (Levy-Strumpf and Culotti 2007) and placing it behind the scm promoter. The dpy-7 promoter (Gilleard et al. 1997) (LGX: 7,537,743–7,538,087; WS232) was amplified by PCR and placed upstream of ptp-3B(+) and the egl-17 promoter (Branda and Stern 2000; Cordes et al. 2006) (LGX: 485,131–489,794; WS232) was amplified by PCR and placed upstream of unc-40(+) and ptp-3B(+). The coding regions of these transgenes were sequenced to ensure that no errors had been introduced by PCR.
Transgenic RNA-mediated gene interference (RNAi)
We used a cell-specific transgenic RNAi approach as described previously (Esposito et al. 2007). Fragments of the mig-21, unc-40, and ptp-3 coding regions were amplified by PCR and inserted behind the scm promoter in a plasmid (primer and plasmid sequences available upon request). For each gene, a “sense” and “antisense” orientation relative to the scm promoter was isolated. An equimolar mixture of the sense and antisense plasmids was used to construct transgenic animals. These transgenic animals were predicted to express both sense and antisense RNAs driven by the scm promoter in the seam cells and Q cells, which was expected to trigger a double-stranded RNA response in these cells (RNAi). Using this approach, mig-21(RNAi) phenocopied mig-21 mutations in AQR and PQR migration. While ptp-3(RNAi) and unc-40(RNAi) had no effect on their own, they both enhanced the effects of mutations in the other. Transgenes were crossed into the sid-1(pk3321) background to test potential dsRNA spreading and systemic effects of the transgenes.
Results
mig-21 allele isolation and the mig-21 locus
In a screen for new mutations with AQR and PQR migration defects, we identified three new mutations, lq37, lq78, and lq84, that caused directional migration defects of both AQR and PQR. These three alleles mapped to linkage group III (see Materials and Methods) and failed to complement one another for AQR and PQR migration (data not shown), suggesting they affected the same gene. The mig-21 gene, which was previously shown to affect Q descendant migrations (Du and Chalfie 2001; Middelkoop et al. 2012), resides on linkage group (LG) III. mig-21(u787) failed to complement lq37, lq78, and lq84 for AQR and PQR migration defects (data not shown), indicating that these mutations were new alleles of mig-21.
mig-21 corresponds to the F01F1.13 gene in WormBase (Middelkoop et al. 2012). To confirm the mig-21 locus structure, we sequenced transcripts from early L1 animals using next generation sequencing (RNA seq) (see Materials and Methods). From these reads (Supporting Information, Figure S1) and from cDNAs reported on WormBase, a mig-21 gene structure was determined as depicted in Figures S1 and S2. The 3′ end (exons 2–5) was identical to that reported by Middelkoop et al. (2012). The first exon defined by RNA seq in our prediction differed from the first exon of the Middelkoop et al. (2012) model (Figure S1), which is upstream and nonoverlapping with our first exon. The initiator ATG in the Middelkoop et al. (2012) model is 167 bp upstream of our predicted ATG. No RNA seq reads aligned to this 167-bp upstream exon region (Figure S1), indicating that it is not actively transcribed and is not an exon of mig-21. A transcript with our predicted structure could be produced by transgenes described in Middelkoop et al. (2012), as these included our entire predicted region as well as the 167-bp upstream sequence.
The mig-21 locus described here can encode a 313-residue type I transmembrane molecule with an N-terminal signal sequence, two predicted extracellular thrombospondin type I domains, a transmembrane domain, and a short cytoplasmic tail with no obvious similarity to other molecules (Figure 1 and Figure S2). This MIG-21 molecule contained a predicted N-terminal signal sequence not found in the Middelkoop et al. (2012) prediction, supporting the accuracy of exon 1 in our gene model.
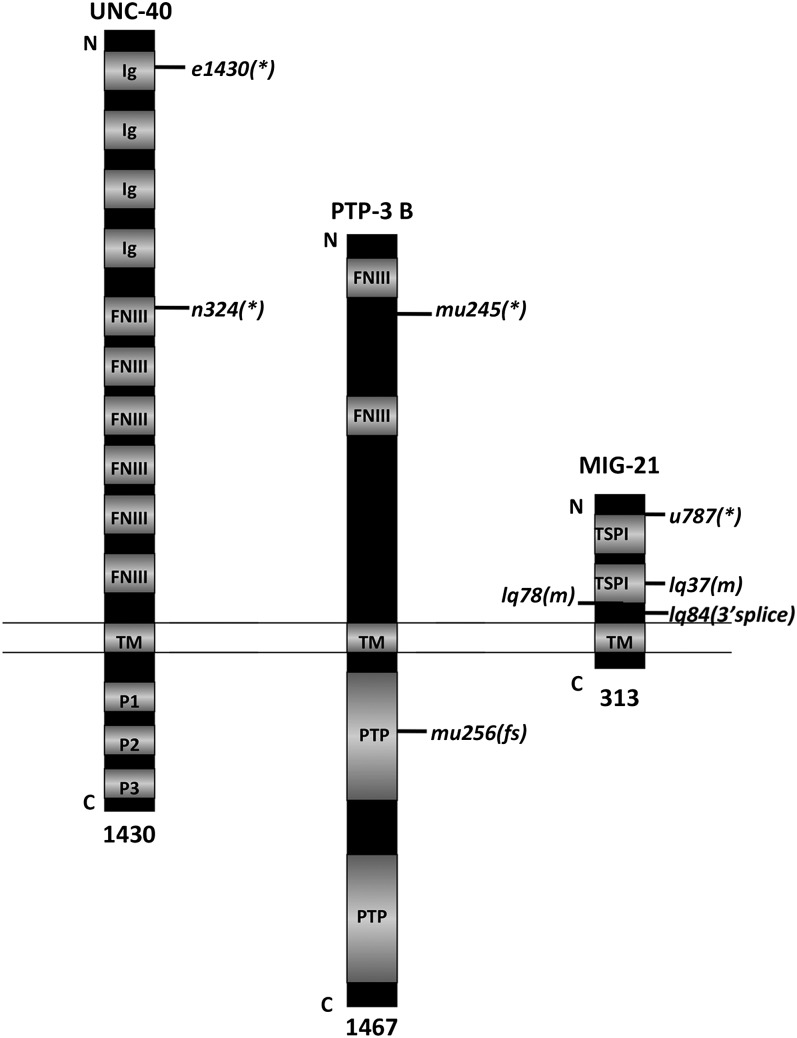
Figure 1 .
Three transmembrane molecules affect Q neuroblast protrusion and migration. Diagrams of UNC-40/DCC, PTP-3B/LAR, and MIG-21 are shown. The relative positions of nucleotide lesions associated with each mutation used here are indicated. *, nonsense premature stop codon; m, missense mutation; and 3′ splice, the mutation affects the 3′ splice site at that position in the transcript. Ig, immunoglobulin I domain; FNIII, fibronectin type III domain; TM, transmembrane domain; P1, P2, and P3, conserved, proline-rich domains; PTP, protein tyrosine phosphatase domain; TSPI, thrombospondin type I domain.
Sequencing of the mig-21 gene from lq37, lq78, and lq84 revealed nucleotide lesions associated with each allele (Figure S1 and Figure 1). lq37 contained a G-to-A transition resulting in a missense cysteine to phenylalanine change in the first thrombospondin type I domain. lq78 showed a G-to-A transition resulting in a glycine-to-glutamic acid missense change in an unconserved region of the predicted extracellular domain, and lq84 harbored a G-to-A transition in the 3′ splice site of the fourth intron. The previously characterized mig-21(u787) mutation is G-to-A transition, resulting in a premature stop codon (tryptophan 65).
MIG-21 controls AQR and PQR migration
Our mig-21 results reported here with the Q cell descendants AQR and PQR migration are similar to those reported in Middelkoop et al. (2012), who used the position of the QL.pax and QR.pax neurons (SDQL/R, AVM, and PVM) also derived from QL and QR. AQR and PQR were visualized with a gcy-32::cfp transgene described previously (Chapman et al. 2008). In wild type, the QR descendant AQR on the right migrates anteriorly to the anterior deirid ganglion, and the QL descendant PQR on the left migrates posteriorly to the phasmid ganglion in the tail (Figure 2, A–C). We found that mutations in mig-21 caused directional defects in both AQR and PQR migration, with PQR affected more strongly (Figures 2 and 3). PQR sometimes migrated anteriorly, and AQR sometimes migrated posteriorly. mig-21(u787), a predicted premature stop codon, was consistently the strongest allele, especially in AQR migration, and is likely a null (Figure 3). The other alleles lq37, lq78, and lq84 might be hypomorphic alleles. A transgene harboring a wild-type copy of the mig-21 locus (see Materials and Methods) rescued the AQR and PQR migration defects of mig-21(u787) (Figure 3). These results indicate that MIG-21 is involved in determining the direction of AQR and PQR migration.
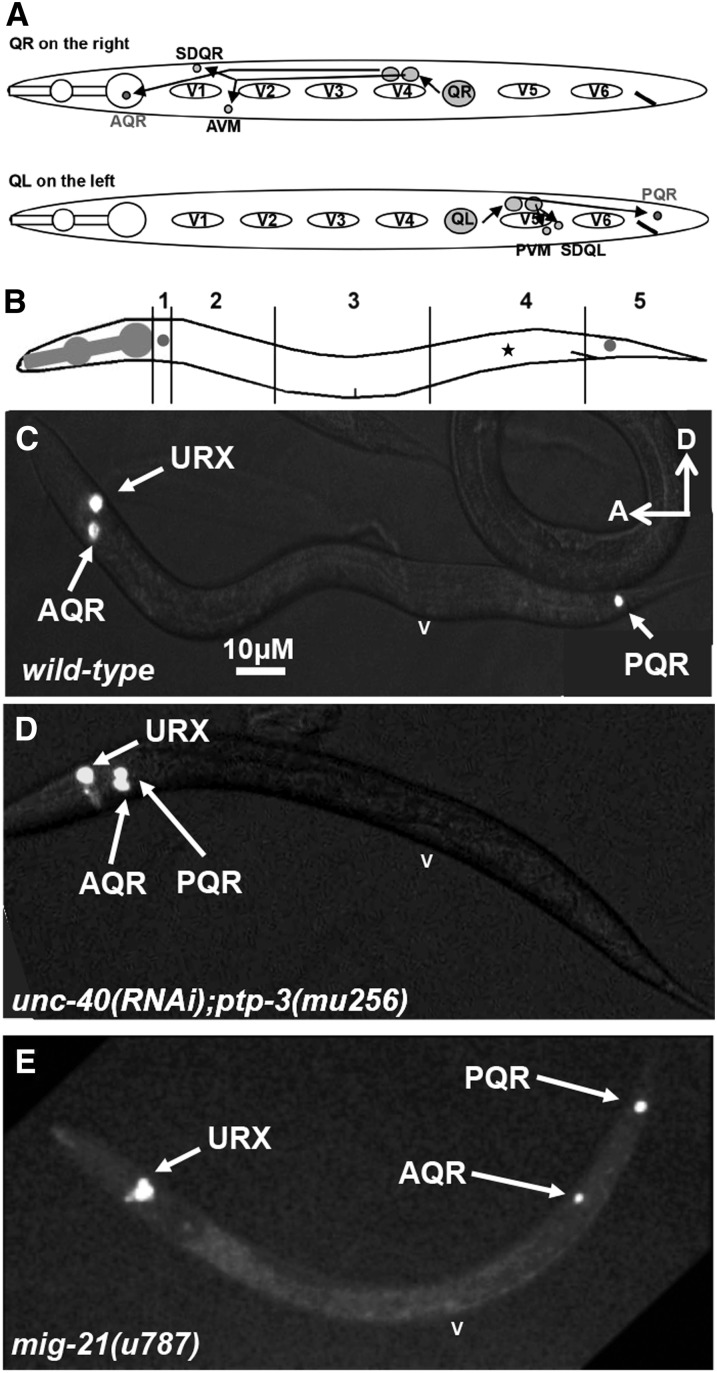
Figure 2 .
AQR and PQR migration in wild-type and mutants. (A) Simplified diagram of QR and QL migrations and divisions that result in three neurons from each. QR on the right migrates anteriorly above V4 and divides, and the daughters continue anterior migrations and divisions to produce three neurons PQR, SDQR, and AVM. QL on the left migrates posteriorly above V5, divides, and, in response to MAB-5 expression, the daughters continue posterior migration and division to produce PQR, SDQL, and PVM. (B) Diagram showing the scoring positions used in Figures 3 and 7–9 (see Materials and Methods). Position 1 is the normal final position of AQR near the anterior deirid ganglion, and position 5 is the normal final position of PQR behind the anus in the phasmid ganglion. The asterisk at position 4 represents the approximate birth place of the Q neuroblasts. Position 3 is proximate to the vulva, and position 2 is anterior to the vulva but still posterior to the anterior deirid ganglion. (C–E) Fluorescent micrographs of animals expressing cyan fluorescent protein (cfp) from the gcy-32 promoter in AQR, PQR, and the URXL/R neurons. v, position of the vulva. The scale bar in panel C represents 10 μM for panels C–E. In all micrographs, anterior is to the left, and dorsal is up.
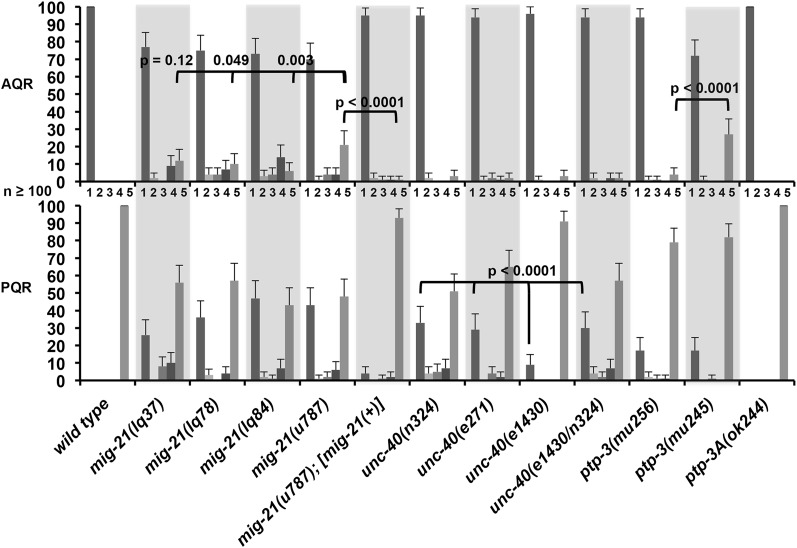
Figure 3 .
AQR and PQR migration defects in single mutants. The x-axis of the graph is the genotype, and the y-axis is the percentage of AQR or PQR neurons in each of the five positions (1–5) along the anterior–posterior as described in Figure 2 and Materials and Methods. Scale bars represent two times the standard error of the proportion, and significances of difference indicated were determined using the Fisher exact test. At least 100 animals of each genotype were scored.
MIG-21 controls early Q neuroblast migration
Previous results suggested that defects in AQR and PQR migration could be due to earlier defects in the protrusion and migration of the Q neuroblasts from which AQR and PQR are derived (Chapman et al. 2008; Dyer et al. 2010). We analyzed early Q cell protrusion and migration using the scm::GFP::CAAX reporter gene described previously (Chapman et al. 2008; Dyer et al. 2010). At 1–1.5 hr posthatching, protrusions start as small filopodial structures that later become large protrusions at 2–2.5 hr after hatching (Figure 4, A–C). QR protrudes anteriorly over the V4 seam cell and QL protrudes posteriorly over V5. At 3–3.5 hr after hatching, the Q cell bodies migrate to reside atop V4 (for QR) and V5 (for QL) (Figure 4, A, D, and E). At 4–4.5 hr after hatching, the Q cells undergo their first division atop the respective seam cells (Figure 4, A, F, and G).
Figure 4 .
Q neuroblast protrusion and migration in wild type. (A) Diagram of Q neuroblast protrusion and migration, with times after hatching indicated. (B–G) Micrographs of wild-type Q neuroblasts and seam cells V4 and V5 are shown at different stages scored (see Materials and Methods). The Pscm::gfp::caax transgene lqIs80 was used to visualize these cells. In all micrographs, anterior is to the left, and dorsal is up. (B and C) At 2–2.5 hr posthatching, QR sent a protrusion anteriorly over V4 and QL posteriorly over V5 (arrows). (D and E) At 3–3.5 hr, QR had migrated atop V4, and QL had migrated atop V5. (F and G) At 4–4.5 hr, QR had divided above V4 and QL atop V5 (daughter cells denoted by asterisks). Bar (in A), 5 μM for each panel.
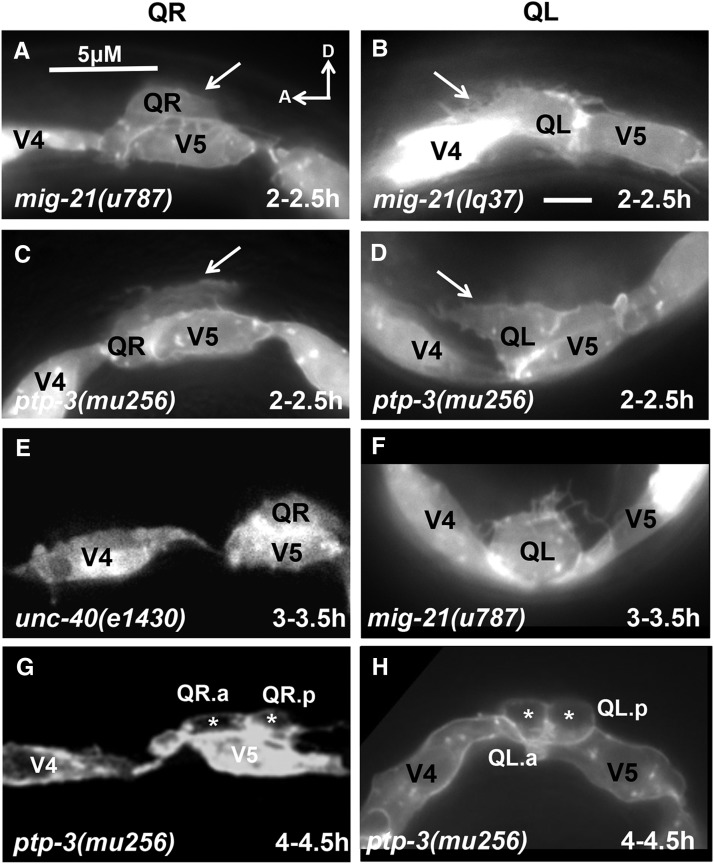
mig-21(u787) and mig-21(lq37) mutants displayed defects in the direction of Q neuroblast protrusion (Figure 5, A and B), similar to results in Middelkoop et al. (2012). QR sometimes protruded and migrated posteriorly, and QL sometimes protruded and migrated anteriorly. Directional protrusion defects were noted at the first sign of protrusion, suggesting that MIG-21 affects the initial decision about direction of protrusion. We also noted that some protrusions were shorter compared with wild type. mig-21 Q cell bodies also migrated in the wrong direction and divided atop the incorrect seam cell; QR sometimes migrated posteriorly and divided over V5, and QL sometimes migrated posteriorly and divided over V4. In some cases MIG-21 Q cells failed to migrate (Figure 5F) and divided between the V4 and V5 cells or on their posterior or anterior edges, respectively.
Figure 5 .
Q neuroblast polarization and migration in mutants. Images are as described in Figure 4, but in different mutant backgrounds. In all micrographs, anterior is to the left, and dorsal is up. (A–C) Images at 2–2.5 hr posthatching. (A) QR neuroblast protruded to the posterior over V5 in mig-21(u787) (arrow points to the protrusion). (B) QL neuroblast protruded to the anterior over V4 in a mig-21(lq37) mutant (arrow). (C) QR protruded posteriorly over V5 in a ptp-3(mu256) mutant. (D) QL protruded anteriorly (arrow). (E and F) Images at 3–3.5 hr posthatching. (E) QR migrated posteriorly atop the V5 seam cell in an unc-40(e1430) mutant. (F) QL failed to migrate and resided between V4 and V5 in mig-21(u787). (G and H) Images at 4–4.5 hr posthatching. (G) QR had divided atop V5 in ptp-3(mu256) (daughter cells denoted by asterisks). (H) QL divided between V4 and V5 seam cells in ptp-3(mu256).
Defects in mig-21 at the stage of division (4–4.5 hr after hatching) are quantified in Figure 6. QL migration was essentially randomized in both mig-21(u787) and mig-21(lq37), and QR was less severely affected. These data are consistent with Middelkoop et al. (2012). We also quantified initial protrusion at 2–2.5 hr and migration at 3–3.5 hr, and similar trends were observed (see Table S1).
UNC-40/DCC controls early Q neuroblast migration
Previous studies have shown that the immunoglobulin superfamily receptor molecule UNC-40/Deleted in Colorectal Cancer controls early Q neuroblast protrusion (Honigberg and Kenyon 2000; Middelkoop et al. 2012). We found similar results using the scm::GFP::CAAX reporter to visualize the Q cells (Chapman et al. 2008). In unc-40 mutants, Q cells often initially protruded and migrated in the wrong direction and failed to migrate fully atop the seam cells before dividing (Figures 5E and 6). While similar to mig-21, the penetrance of the unc-40(n324) and unc-40(e1430) phenotypes was consistently lower than those of mig-21(u787) and mig-21(lq37).
unc-40 also displayed defects in AQR and PQR direction and extent of migration (Figure 3). PQR was more strongly affected than AQR, and the defects were less penetrant than those in mig-21. PQR migration defects were significantly less severe in unc-40(e1430) compared to unc-40(n324), suggesting that unc-40(e1430) might retain some function, although AQR migration and QL/QR migration were not significantly different between unc-40(e1430) and unc-40(n324). A third unc-40 allele, e271, caused defects similar to unc-40(n324) that were significantly more severe than unc-40(e1430) (Figure 3). Furthermore, the n324/e1430 trans-heterozygote resembled n324 alone (Figure 3). Together, these data suggest that unc-40(e1430) is a hypomorph, although it is also possible that unc-40(e1430) carries a linked recessive suppressor mutation not found in the other strains.
PTP-3/LAR controls early Q neuroblast migration
To identify other molecules with roles in Q migration similar to MIG-21 and UNC-40, we assayed mutants that had been shown previously to affect Q descendant migrations. qid-5(mu245) was shown to affect the placement of the AVM and PVM Q descendants (Ch’ng et al. 2003). We found that qid-5(mu245) also affected AQR and PQR direction and extent of migration similar to unc-40 and mig-21. We used next generation sequencing to determine the genome sequence of a qid-5(mu245) strain (see Materials and Methods). qid-5(mu245) had been previously mapped to the region of linkage group II (Ch’ng et al. 2003), and in this region of the genome we discovered a premature stop codon in the ptp-3 gene. ptp-3 had been shown previously to affect Q and descendant migrations (Williams 2003), and we found that qid-5(mu245) failed to complement ptp-3(mu256) for AQR and PQR migration (data not shown).
The previously described ptp-3(mu256) allele is a single nucleotide insertion in the coding region for the first phosphatase domain (Ackley et al. 2005) (Figure 1 and Figure S3). ptp-3(mu245) introduces a TCA/serine to TAA premature stop at codon 905 in the ptp-3A open reading frame and is predicted to affect all known ptp-3 transcripts except the shortest, ptp-3C (Figure 1 and Figure S3). As described below, the ptp-3B transcript is the relevant transcript for Q cell migration. ptp-3B encodes an isoform of a LAR receptor tyrosine phosphatase-like molecule and consists of an extracellular domain with two fibronectin III repeats, a transmembrane domain, and an intracellular domain with two tyrosine phosphatase domains (Figure 1) (Harrington et al. 2002).
Both ptp-3(mu245) and ptp-3(mu256) caused defects in AQR and PQR migration that resembled mig-21 and unc-40 (Figure 3). ptp-3(mu256) was shown previously to affect early Q direction and extent of protrusion (Williams 2003), and we found that ptp-3(mu245) also affected initial Q direction of protrusion and migration similar to unc-40 and mig-21 (Figure 6). The ptp-3(ok244) deletion allele affects only a subset of ptp-3 predicted transcripts with extended 5′ exons (ptp-3A, D, and E; WormBase). ptp-3(ok244) caused no defects in AQR or PQR migration (Figure 3) or in early Q neuroblast protrusion and migration (data not shown), suggesting that the ptp-3A, D, and E products are not involved in Q migrations. The ptp-3B transcript is the only known transcript affected by mu245 and not affected by ok244 (Figure S3), suggesting that ptp-3B is the relevant isoform in Q and descendant migrations. As shown below, a ptp-3B transgene rescued ptp-3(mu256), consistent with this notion.
ptp-3(mu245) displayed significantly more posteriorly directed QR cells and AQR neurons and anterior QL migration than ptp-3(mu256) (Figures 3 and 6), suggesting that ptp-3(mu245) might be a stronger loss-of-function allele than ptp-3(mu256) and might be a null for the affected isoforms, including ptp-3B. While unc-40, ptp-3, and mig-21 mutations all affected Q and descendant migrations in a similar manner, mig-21 and ptp-3 mutants generally had stronger effects than unc-40 mutants.
MIG-21 and UNC-40 act redundantly in posterior QL migration
mig-21 and unc-40 mutations both displayed defects in QL posterior protrusion and migration, with some QL cells protruding and migrating to the anterior. mig-21(u787); unc-40(n324) double mutants had QL migration defects that were significantly and synergistically more severe than either mutant alone (Figure 6). This phenotypic synergy suggests that UNC-40 and MIG-21 normally act redundantly to control QL posterior migration. These results are consistent with Middelkoop et al. (2012), who also found that MIG-21 and UNC-40 redundantly control posterior QL protrusion. The unc-40(e1430); mig-21(u787) defects were not significantly different from mig-21(u787) alone (Figure 6), supporting the idea that unc-40(e1430) is a hypomorph.
PQR migration was also significantly more severely affected in double mutants of two distinct unc-40 and mig-21 alleles than the single mutants alone (Figure 7). In unc-40(n324); mig-21(u787) doubles, the putative double null, only 2% of PQR neurons migrated posteriorly. These results indicate that UNC-40 and MIG-21 redundantly control posterior protrusion and migration of QL and posterior migration of PQR.
Figure 7 .
AQR and PQR migration in double and triple mutants. Genotypes are listed on the x-axis, and the percentage of AQR and PQR in the five positions along the body axis are shown on the y-axis. The five positions (1–5) are described in Materials and Methods and Figure 2. Asterisks denote statistical significance compared to single mutant genotypes displayed in Figure 3 as determined by Fisher exact analysis. The table below describes the significance of each asterisk. Error bars represent two times the standard error of the proportion. At least 100 animals were scored for each genotype.
Anterior QR migration is regulated through mutual antagonism of MIG-21 and UNC-40
mig-21 and unc-40 mutants both displayed abnormal posterior protrusion and migration of QR, which normally migrates anteriorly (Figure 6). Surprisingly, unc-40; mig-21 double mutants displayed significantly reduced posterior QR protrusion and migration compared to either single mutant alone (Figure 6). In fact, in no unc-40; mig-21 double mutant scored did we observe a QR that had migrated posteriorly. These data suggest that wild-type UNC-40 activity was required for posterior QR migration in mig-21 mutants, and that wild-type MIG-21 activity was required for posterior QR migration in unc-40 mutants. These results imply that in QR, MIG-21 and UNC-40 might normally inhibit the other’s role in posterior migration, resulting in the normal anterior migration of QR. When either MIG-21 or UNC-40 is missing, the other is free to drive posterior protrusion and migration.
A similar trend was observed in AQR migration, as significantly fewer AQRs migrated posteriorly in the unc-40; mig-21 double mutants compared to mig-21 alone (Figure 7). The exception was the unc-40(e1430); mig-21(lq37) combination, which was not significantly different from mig-21(lq37) alone, again suggesting that unc-40(e1430) is a hypomorph.
In sum, these experiments indicate that MIG-21 and UNC-40 are required for posterior Q cell and descendant migration. In QL, MIG-21 and UNC-40 act redundantly to drive posterior protrusion and migration. In QR, MIG-21 and UNC-40 might reciprocally inhibit each other, allowing anterior protrusion and migration. In other words, mutation of one revealed a latent ability of QR to protrude and migrate posteriorly, which was dependent on the other.
PTP-3 acts redundantly with UNC-40 in QL, similar to MIG-21
We found that the unc-40(n324); ptp-3(mu256) double mutant resulted in embryonic lethality (data not shown), and we were unable to score Q cells and descendants in these mutants. To circumvent this lethality, we used a transgenic RNAi approach to knock down unc-40 and ptp-3 in the seam cells and Q cells based on the approach described in Esposito et al. (2007) (see Materials and Methods). Plasmids were generated to drive expression of sense and antisense RNA complementary to the unc-40 and ptp-3 genes under the control of the seam cell promoter (scm promoter), which is active in the seam cells and early Q cells (Terns et al. 1997; Chapman et al. 2008). Animals were made transgenic with a mix of the sense and antisense plasmids, and the resulting transgenes were used in analysis. On their own, scm::ptp-3(RNAi) and scm::unc-40(RNAi) lines, from hereon called ptp-3(RNAi) and unc-40(RNAi), showed no defects in QR or QL migration (data not shown). However, unc-40(RNAi); ptp-3(mu256) and unc-40(RNAi); ptp-3(mu245) showed significantly more anterior QL migration compared to ptp-3(mu256) and ptp-3(mu245) alone (Figure 6). PQR migration was similarly affected (Figure 7). These results indicate that UNC-40 and PTP-3 act redundantly in QL protrusion and migration. unc-40(RNAi) had no effect on its own, indicating that unc-40(RNAi) eliminated some, but not all, unc-40 activity. ptp-3(RNAi) also had no effect alone but enhanced the PQR migration defects of unc-40(n324) (Figure 7). These results indicate that PTP-3 and UNC-40 redundantly control the posterior protrusion and migration of QL and the posterior migration of PQR, similar to MIG-21 and UNC-40.
PTP-3 and UNC-40 display mutual antagonism in QR, similar to MIG-21 and UNC-40
The unc-40(RNAi); ptp-3(mu245) double mutant displayed significantly fewer posterior QR and AQR migrations compared to ptp-3(mu245) alone (Figures 6 and 7), suggesting mutual antagonism in posterior QR migration as described for UNC-40 and MIG-21. unc-40(RNAi) in the ptp-3(mu256) hypomorphic background resulted in fewer posterior QR, but this was not statistically significant. This could be due to the fact that neither unc-40(RNAi) nor ptp-3(mu256) completely eliminates function of either gene, and the hypomorphic ptp-3(mu256) had fewer posterior QR/AQR than the null ptp-3(mu245).
In reciprocal experiments targeting ptp-3 with RNAi, we found that ptp-3(RNAi); unc-40(n324) animals showed no posterior AQR migration compared to 3% for unc-40(n324) alone (Figure 7). While not statistically significant, this result is consistent with PTP-3 function being required for posterior AQR migration in unc-40 mutants.
In sum, interactions between ptp-3 and unc-40 were similar to those observed between mig-21 and unc-40: redundancy in QL/PQR posterior migration and mutual suppression of posterior QR/AQR migration.
PTP-3 and MIG-21 act in the same genetic pathway
ptp-3; mig-21 double mutants showed no significant change in percentages of QL and QR that migrated to the posterior compared to single mig-21(u787) mutants, suggesting that they act in the same pathway (Figure 6). This is in contrast to unc-40(n324); mig-21(u787) double mutants, which strongly synergized in QL, suggesting action in parallel pathways (Figure 6). AQR and PQR defects in mig-21; ptp-3 doubles were also not different from mig-21 alone (Figure 7). The lack of strong genetic enhancement in mig-21; ptp-3 double mutants indicates that PTP-3 and MIG-21 act in the same pathway in Q migrations.
The triple unc-40(RNAi); ptp-3(mu245); mig-21(u787) displayed significantly more QL and PQR defects than ptp-3(mu245); mig-21(u787), indicating redundancy between unc-40 and ptp-3/mig-21 (Figures 6 and 7). Triple mutant ptp-3(mu245); mig-21(u787); unc-40(RNAi) QL and PQR defects were not significantly more severe than unc-40(n324); mig-21(u787) or unc-40(RNAi); ptp-3 doubles (Figures 6 and 7), consistent with MIG-21 and PTP-3 acting in the same pathway in parallel to UNC-40. The unc-40(RNAi); ptp-3(mu245); mig-21(u787) triple also displayed significantly fewer posterior QR and AQR migrations than ptp-3(mu245); mig-21(u787) (Figures 6 and 7), indicating mutual antagonism of UNC-40 and PTP-3/MIG-21 in QR and AQR posterior migration.
This genetic analysis indicates UNC-40, MIG-21, and PTP-3 all function to promote posterior Q cell and Q descendant migration. IN QL, MIG-21 and PTP-3 act in the same pathway in parallel to UNC-40. In QR, UNC-40 and MIG-21/PTP-3 might define two pathways that mutually antagonize the other’s activity in posterior migration, such that anterior migration of these cells can result.
UNC-40, MIG-21, and PTP-3 can act autonomously in the Q cells
We drove the expression of the unc-40 coding region fused to green fluorescent protein (gfp) in the seam cells and Q cells using the scm promoter (see Materials and Methods). This construct was expressed in these cells, and UNC-40::GFP accumulated at the cell margins of the seam cells and Q cells (Figure 8A). Furthermore, Pscm::unc-40::gfp rescued the AQR and PQR migration defects of unc-40(n324) (Figure 8D), suggesting that UNC-40 activity in the seam cells and/or Q cells is sufficient for AQR and PQR migration.
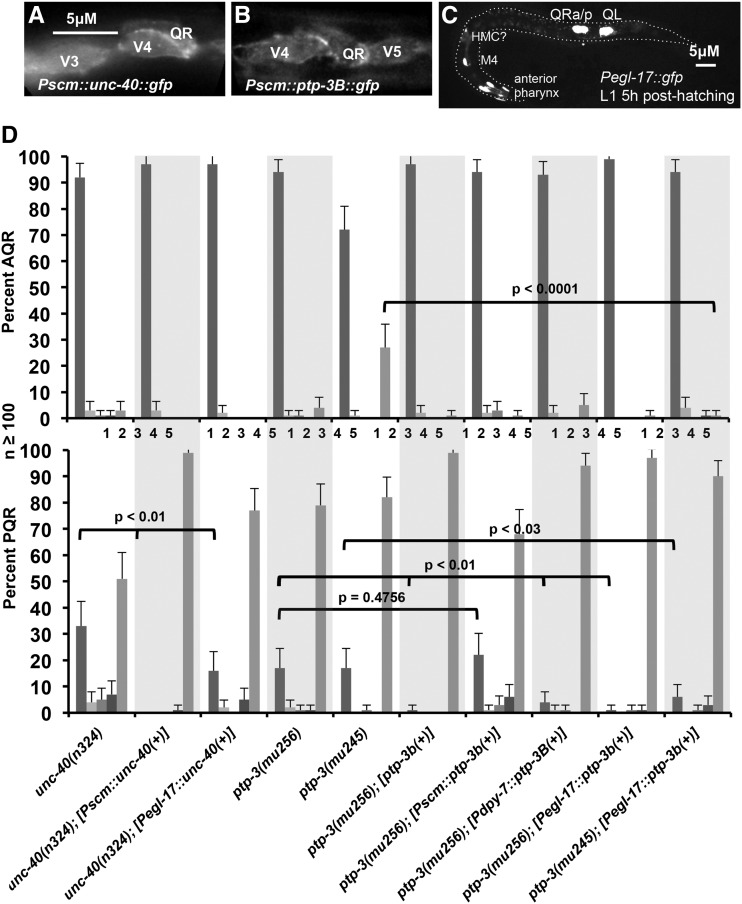
Figure 8 .
Rescue of AQR and PQR defects using cell-specific transgenes. In all micrographs, anterior is to the left, and dorsal is up. (A) Micrograph showing UNC-40::GFP expression from the scm::unc-40::gfp transgene (lqIs151) in seam cells (V3–V5) and QR at 2–2.5 hr after hatching. UNC-40::GFP accumulated at the cell margins. Bar, 5 μM for A and B. (B) Expression of PTP-3B::gfp from the scm::ptp-3B::gfp transgene (lqEx637) in the seam cells V4 and V5 and QR at 2–2.5 hr after hatching. PTP-B::gfp accumulated at cell margins similar to UNC-40::GFP. (C) Pegl-17::gfp expression from the ayIs9 transgene in an early L1 larva 5 hr after hatching. Q cells have migrated, and QR has divided. In the anterior, expression is observed in some anterior pharyngeal cells, the M4 pharyngeal neuron, and a cell whose position and morphology resemble the head mesodermal cell (HMC). Weak fluorescence along the length of the animal is autofluorescence from the gut. Dashed line indicates the approximate outline of the body of the animal. (D) Graph is shown relating genotypes on the x-axis with the percentage of AQR and PQR in the five positions as described in Materials and Methods and Figure 2. [Pscm::unc-40(+)] is the Pscm promoter::unc-40::gfp transgene lqIs151, shown in A; [ptp-3B(+)] is the full length ptp-3B::gfp under its own promoter (juIs179), and [Pscm::ptp-3B(+)] is the Pscm::ptp-3B::gfp transgene (lqEx637) shown in B (see Materials and Methods). Statistical significances of difference were determined by Fisher exact analysis, and error bars represent two times the standard error of the proportion.
We also generated a transgene driving unc-40 expression in the Q cells and not the seam cells using the egl-17 promoter (Branda and Stern 2000; Cordes et al. 2006). At the time of Q cell migration and division in early L1 (0–6 hr posthatching), the egl-17 promoter drove gfp expression in the Q cells in the posterior, with no detectable expression in other posterior cells (Figure 8C and Middelkoop et al. 2012). In the anterior, some pharyngeal cells, the M4 pharyngeal neuron, and a cell that might be the head mesodermal cell expressed egl-17::gfp (Figure 8C). After Q migration and division (6–10 hr posthatching), egl-17::gfp expression was detected in the P cells (the vulval precursor cells) in the posterior (data not shown). However, at the time of Q migration, egl-17::gfp expression was limited to the Q cells in the posterior.
The Pegl-17::unc-40 transgene rescued AQR and PQR migration defects of unc-40(n324) (Figure 8D). As the egl-17 promoter was active only in Q cells in the posterior at the time of Q migration and division, Q cell expression is the most likely source for unc-40 rescue. Although non-Q sources cannot be definitively excluded, this result suggests that UNC-40 acts autonomously in the Q cells.
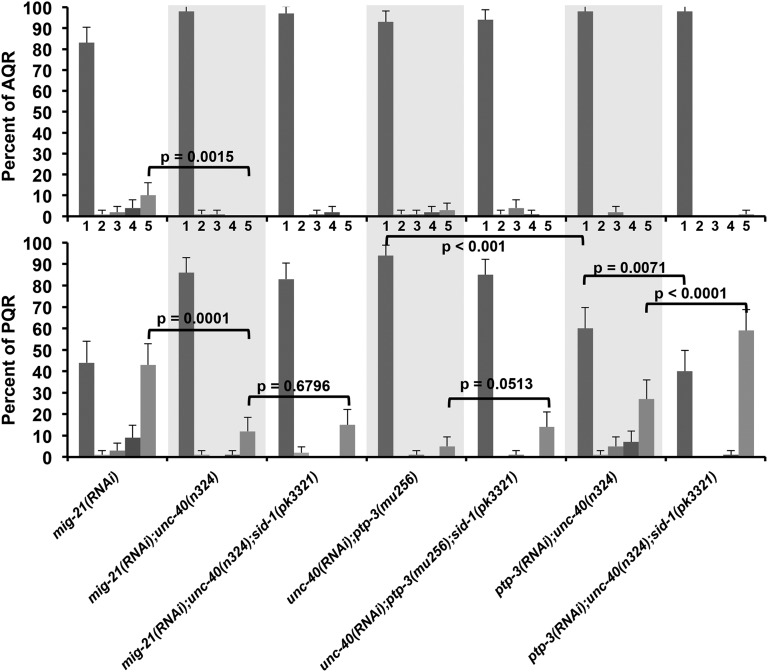
unc-40 RNAi driven by the scm promoter enhanced QL/QR and AQR/PQR migration defects of ptp-3 and mig-21 (Figure 7), suggesting that UNC-40 acts in the seam cells and/or Q cells. However, RNAi can spread from one tissue to another (systemic RNAi) (Tabara et al. 1998; Timmons et al. 2001), so it is possible that RNAi expressed from the scm promoter might spread to other tissues, and that knockdown in other tissues is responsible for AQR and PQR migration defects. The SID-1 protein is required for spreading of RNAi, as SID-1 affects the ability of cells to accumulate extracellular dsRNA molecules (Winston et al. 2002). Previous studies have used transgenic expression of sid-1 to sensitize neurons to systemic RNAi (Calixto et al. 2010). Here, we test whether SID-1 function is required for the effects of cell-specific RNAi expressed by the scm promoter. We tested the efficacy of unc-40(RNAi) enhancement of ptp-3(mu256) in a sid-1(pk3321) background and found that AQR and PQR defects were slightly but not significantly reduced compared to the sid-1(+) background (Figure 9). Together with rescue of unc-40 mutants by the Pscm::unc-40 and Pegl-17::unc-40 construct, these data indicate that UNC-40 acts in the Q cells in AQR and PQR migration.
Figure 9 .
Cell autonomous RNAi of mig-21, unc-40, and ptp-3 and the effects of sid-1(pk3321). The x-axis shows genotypes analyzed, and the y-axis shows the percentage of AQR and PQR cells in each of the five positions as described in Materials and Methods and Figure 2. mig-21(RNAi) represents the scm::mig-21(RNAi) transgene lqEx661; unc-40(RNAi) represents the scm::unc-40(RNAi) transgene lqIs146; and ptp-3(RNAi) represents the scm::ptp-3(RNAi) transgene lqIs166 (see Materials and Methods). Statistical significances of difference were determined by Fisher exact analysis, and error bars represent two times the standard error of the proportion.
MIG-21 expression in the Q cells using the egl-17 promoter rescued defects, indicating that MIG-21 acts autonomously in the Q cells (Middelkoop et al. 2012). We were unable to obtain cell-specific rescue of mig-21 with a transgene containing the scm promoter driving the mig-21(+) coding region fused to gfp, nor could we detect GFP expression. The endogenous expression of mig-21 is transient in the Q neuroblasts at the time that they are extending protrusions (1–2 hr after hatching) and diminishes rapidly as the cell bodies migrate (Middelkoop et al. 2012). Possibly, the Pscm::mig-21::gfp construct is not expressed at the correct time or level to rescue mig-21 mutants.
We found that mig-21 RNAi expressed from the scm promoter caused AQR and PQR migration defects (Figure 9). Furthermore, mig-21(RNAi) significantly enhanced PQR migration defects of unc-40(n324), and unc-40(n324) significantly suppressed AQR posterior migration of mig-21(RNAi), similar to mig-21(u787) (Figure 9). unc-40(n324); mig-21(RNAi); sid-1(pk3321) mutant AQR and PQR defects were not significantly different from the sid-1(+) background (Figure 9), suggesting that mig-21 knockdown in the seam cells and/or Q cells was causing the defects. This is consistent with previous studies showing cell autonomy of mig-21 function in the Q cells (Middelkoop et al. 2012).
Similar studies were conducted on ptp-3. A transgene containing the endogenous ptp-3B gene under its own promoter fused to gfp (see Materials and Methods) rescued AQR and PQR migration defects of ptp-3(mu256), as did expression from the dpy-7 promoter active in all hypodermis including the seam cells and the early Q cells (Gilleard et al. 1997) (Figure 8C). Q-cell–specific Pegl-17::ptp-3B expression also rescued ptp-3(mu256) and ptp-3(mu245) AQR and PQR migration defects, suggesting autonomy of ptp-3 function in the Q cells. However, expression of ptp-3B::gfp from the scm seam cell promoter did not significantly rescue ptp-3(mu256) (Figure 8C), despite robust expression of PTP-3B::GFP in the seam and Q cells and cell margin accumulation (Figure 8B). Furthermore, ptp-3 RNAi driven by the scm promoter enhanced PQR migration defects of unc-40(n324) but the enhancement was abolished in a sid-1(pk3321) mutant background (Figure 9). That Pegl-17::ptp-3B rescued ptp-3 mutants argues that ptp-3 can act cell autonomously, as the egl-17 promoter was active only in the Q cells in the posterior at the time of initial Q migration (Figure 8C). However, expression in other cells driven by the egl-17 promoter (pharyngeal cells, the M4 neuron, and later P cell expression) cannot be excluded. Lack of rescue by Pscm::ptp-3B and sid-1 sensitivity of scm promoter-driven RNAi suggest that ptp-3 might also have a nonautonomous role in Q cell migration.
Discussion
Previous results showed that MIG-21 and UNC-40 act redundantly in posterior QL migration (Middelkoop et al. 2012). Our results presented here confirm this finding in QL and suggest that MIG-21 and UNC-40 might repress each other in posterior QR migration, allowing QR to migrate anteriorly (Figure 9). In the mig-21; unc-40 double null mutant, there was no posterior migration of either QL or QR, consistent with a central role of UNC-40 and MIG-21 in promoting posterior migration. The LAR receptor tyrosine phosphatase PTP-3 has been implicated in Q cell and descendant migration (Williams 2003). We present evidence here that UNC-40 and PTP-3 act redundantly in posterior QL migration, and that MIG-21 and PTP-3 might act in the same pathway in parallel to UNC-40. unc-40 RNAi knockdown consistently reduced posterior QR migration in ptp-3, similar to unc-40 suppression of this defect in mig-21, and mig-21; ptp-3 double mutants showed posterior QR migration similar to either single alone. Thus, MIG-21 and PTP-3 might act in the same pathway in parallel to UNC-40 in Q migration (Figure 10).
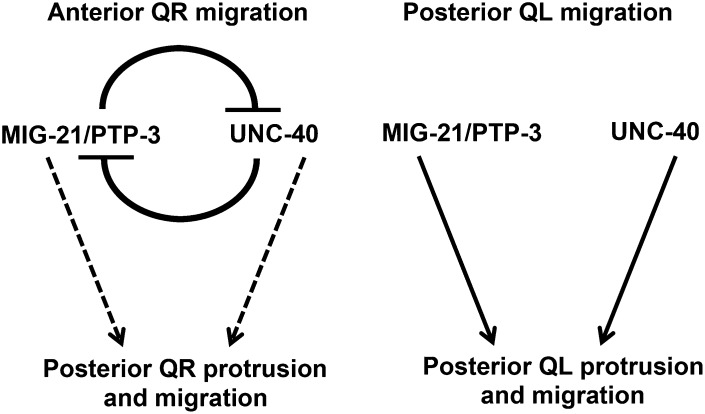
Figure 10 .
Model of MIG-21, UNC-40, and PTP-3 in Q neuroblast protrusion and migration. (A) Genetic interactions indicate that MIG-21, UNC-40, and PTP-3 are all required for posterior migration. In QL, which normally protrudes and migrates posteriorly, UNC-40 acts in parallel to a pathway involving MIG-21 and PTP-3, as unc-40 enhanced both mig-21 and ptp-3, but mig-21 and ptp-3 did not significantly enhance each other. In QR, which normally protrudes and migrates anteriorly, UNC-40 and a pathway involving MIG-21 and PTP-3 mutually repress each other in QR posterior protrusion and migration, resulting in anterior protrusion and migration. Mutations in one pathway resulted in posterior QR protrusion and migration that was dependent on the function of the other.
The initial protrusion and migration of QL and QR affect the subsequent expression of MAB-5 in the Q descendants and thus their anterior posterior migration (Chapman et al. 2008; Middelkoop et al. 2012). QR is inherently less sensitive than QL to the EGL-20/Wnt signal that activates MAB-5 expression (Whangbo and Kenyon 1999), and MIG-21 appears to mediate this differential sensitivity (Middelkoop et al. 2012). That AQR and PQR defects generally follow the trend of QL and QR migration defects in these mutants is consistent with this idea that initial Q protrusion and migration affects mab-5 expression and subsequent Q descendant migration.
MIG-21 and UNC-40 mutual inhibition in QR
We found that unc-40; mig-21 double mutants displayed no posterior QR migration, in contrast to each single mutant alone, which showed significant QR posterior migration. This result suggests that UNC-40 and MIG-21 can promote posterior migration in QR as in QL, but in QR they mutually repress each other’s activity, allowing anterior migration (Figure 10). In a mig-21 mutant, UNC-40 was free to promote posterior QR migration; and in an unc-40 mutant, MIG-21 was free to promote posterior QR migration. Thus, QL and QR have an inherently distinct mechanism involving UNC-40, PTP-3, and MIG-21 to control posterior vs. anterior migration. In both QL and QR, these three molecules promote posterior migrations. However, in QR, UNC-40 and MIG-21 repress each other’s activity, allowing QR to migrate anteriorly. The nature of this inherent difference in UNC-40 and MIG-21 function in QL vs. QR is not understood, but it might be that QL and QR express distinct cytoplasmic molecules that govern this differential action, or that there are differences in the extracellular environments on the left vs. the right side, leading to this effect. There is precedence for differences in QL vs. QR, as QR is inherently less sensitive than QL to the EGL-20/Wnt signal that induces MAB-5/Hox expression (Whangbo and Kenyon 1999; Middelkoop et al. 2012). Indeed, MIG-21 itself is involved in this differential sensitivity to EGL-20/Wnt, positioning MIG-21 as a key regulator of differential responses in initial Q migrations as well as subsequent MAB-5–dependent Q descendant migrations.
While Middelkoop et al. (2012) did not address posterior QR migration directly, their data (figure 6A of Middelkoop et al. 2012) includes posteriorly directed QR cells in mig-21(u787) mutants (∼15–30%), with fewer in unc-40(e1430); mig-21(u787) (∼0–3%), consistent with our results. They also reported posterior AQR migration in mig-21(u787), which was abolished in unc-40(e1430); mig-21(u787), consistent with our results and with their finding that unc-40(e1430); mig-21(u787) mutants showed little or no mab-5 expression due to nearly complete anterior QR and QL migration.
unc-40; ptp-3 embryonic lethality
In our genetic analyses, we found that unc-40; ptp-3 double mutants resulted in embryonic lethality. We did not characterize this lethality here, but ptp-3 has been shown previously to cause defects in embryonic cell movements in gastrulation resulting in low-penetrance embryonic lethality and to act redundantly with other signaling molecules (e.g., VAB-1/Ephrin) in this process (Harrington et al. 2002). Our results indicate that UNC-40 might have a role in gastrulation cell movements in parallel to PTP-3, resulting in embryonic lethality in the double mutant.
Our genetic analysis suggests that unc-40(e1430) retains some function and is a hypomorph. PQR migration defects were significantly weaker in unc-40(e1430) compared to unc-40(n324) and unc-40(e271), and an n324/e1430 trans-heterozygote was more severe than unc-40(e1430) and resembled unc-40(n324) and unc-40(e271) homozygotes. While there is no evidence of alternative splicing or alternative exon use in unc-40, it is possible that this happens at a low frequency that has not been detected molecularly, leading to functional transcripts in the unc-40(e1430) background.
PTP-3/LAR controls Q migration
The LAR receptor protein tyrosine phosphatase PTP-3 was previously implicated in Q migration (Harrington et al. 2002; Williams 2003; Ackley et al. 2005). We found that qid-5(mu245) (Ch’ng et al. 2003) was a new allele of ptp-3. mu245 was a premature stop codon early in the ptp-3B coding region (exon 4), which encodes an extracellular portion of the molecule (Figure 1). mu245 also affects the ptp-3A, D, and E isoforms. However, these isoforms are not relevant to Q migration, as ptp-3(ok244), a deletion that affects these isoforms but not ptp-3B, caused no defects in QL, QR, AQR, or PQR migration. Thus, ptp-3B is the relevant isoform in Q migration. One caveat is that the shortest ptp-3C isoform is not affected by mu245 or ok244, so it is possible that ptp-3C also contributes to Q migration.
ptp-3(mu245) mutants were significantly more severe than ptp-3(mu256) mutants in QL and QR migration defects, suggesting that ptp-3(mu256) is a hypomorph. ptp-3(mu256) is a single nucleotide insertion in the coding region for the first intracellular phosphatase domain (Ackley et al. 2005), and is predicted to cause frameshifts and premature stops in all known ptp-3 isoforms, including the shortest ptp-3C. It is surprising that a frameshift mutation in a region shared by all isoforms might be a hypomorph. One explanation is that the ptp-3(mu256) transcript is subject to nonsense-mediated mRNA decay, affecting all isoforms and functions of the locus. It is also conceivable that the mu256 insertion is spliced out of some transcripts using cryptic splice sites, or that the intracellular phosphatase domains of PTP-3 are not required for its function in Q migration, and that despite a premature stop codon, some PTP-3 is made in ptp-3(mu256), which lacks the intracellular domains. Consistent with these latter possibilities, ptp-3(mu256) animals displayed some anti–PTP-3 immunoreactivity using an antiserum against the entire intracellular region, including the region before the mu256 insertion (Ackley et al. 2005). In photoreceptor axon pathfinding in Drosophila, the catalytic activity of the dLAR phosphatase is not required, but the intracellular domains are still required to serve scaffolding functions (Hofmeyer and Treisman 2009).
PTP-3 and MIG-21 might act in the same pathway in parallel to UNC-40
Genetic data presented here suggest that MIG-21 and PTP-3 might act in the same pathway in parallel to UNC-40. The ptp-3(mu245) phenotype resembled mig-21(u787) in severity QR migration defects compared to unc-40(n324) (ptp-3 and mig-21 had more severe QR and AQR defects than did unc-40), and mig-21 and ptp-3 behaved in a similar manner in genetic interactions with unc-40. In QL, unc-40 enhanced both mig-21 and ptp-3, but ptp-3 did not enhance mig-21. Furthermore, unc-40 reduced posterior QR migration in both mig-21 and ptp-3, and QR posterior migration resembled ptp-3 and mig-21 alone in the ptp-3; mig-21 double mutants. Finally, the triple mutant ptp-3(mu245); mig-21(u787); unc-40(RNAi) was not significantly different than the ptp-3(mu245); unc-40(RNAi) double mutant. Taken together, these data suggest that PTP-3 and MIG-21 might act together in a pathway in parallel to UNC-40.
UNC-40, PTP-3, and MIG-21 can act autonomously in the Q cells
We found that UNC-40::GFP expressed in the Q cells using the egl-17 promoter rescued AQR and PQR migration defects of unc-40(n324) animals. Furthermore, unc-40(RNAi) and mig-21(RNAi) expressed by the scm promoter enhanced AQR and PQR migration defects similar to unc-40 and mig-21 alleles and was not sensitive to sid-1(pk3321). These results suggest that UNC-40 and MIG-21 act autonomously in the Q cells.
Rescue of ptp-3 mutants by expression of ptp-3B from the egl-17 promoter active in the Q cells argues that ptp-3 can act autonomously in the Q cells. However, expression in the Q cells and the seam cells by the scm promoter did not rescue. Possibly, the timing or levels of ptp-3B expression from the scm promoter were not conducive to ptp-3 rescue. It is also possible transgenic PTP-3B expression on the neighboring seam cells might inhibit PTP-3B function in the Q cells, possibly through a homophilic interaction, suggesting a possible nonautonomous role of PTP-3B. The sensitivity of scm promoter driven ptp-3 RNAi to sid-1(pk3321) also hints at a possible nonautonomous role of PTP-3. Thus, the Pegl-17::ptp-3B rescue experiments argue that ptp-3 can act autonomously in the Q cells, but ptp-3 might also have nonautonomous roles as well.
Most known roles of LAR have been shown to be or are presumed to be cell autonomous (for examples, see Srinivasan et al. 2012; Wang et al. 2012). Our studies indicate that PTP-3B can act autonomously in the Q cells, possibly as a receptor. However, our data hint that ptp-3 might also have a nonautonomous role, possibly as a ligand. The transmembrane PTP-3 molecule could play a juxtacrine role, requiring cell–cell contact. It is also possible that the extracellular domain of PTP-3 is cleaved and serves as a diffusible signaling molecule. Indeed, the extracellular domain of mammalian LAR is shed in response to calcium ionophores, phorbol esters, and EGF receptor activity mediated by the alpha-secretase matrix metalloprotease ADAM-17/TACE (Aicher et al. 1997; Ruhe et al. 2006; Haapasalo et al. 2007). These studies show that ectodomain shedding is important for the regulation of the intracellular domain of LAR (an autonomous function), but it is possible that the cleaved ectodomain plays a nonautonomous signaling role.
We envision a mechanism in which UNC-40/DCC, PTP-3/LAR, and MIG-21 act as receptors for an extracellular signal that controls Q migration, and that these molecules interact with each other distinctly in QR vs. QL (Figure 10). Our genetic analysis suggests that PTP-3 and MIG-21 are in the same genetic pathway in parallel to UNC-40. It is possible that these molecules interact physically as co-receptors in complexes that regulate responses to anterior–posterior guidance cues.
Many genes have been identified that control early Q neuroblast migrations, yet none encode a molecule that acts nonautonomously as a guidance signal. The transmembrane CUB-domain containing molecule MIG-13 nonautonomously guides Q descendants in the anterior–posterior axis and is present on the commissures of motor axons in an anterior-to-posterior gradient along the animal (Sym et al. 1999), but MIG-13 does not affect early Q neuroblast migration. Further studies of genes affecting this process might reveal a signaling molecule that controls Q neuroblast migration via the parallel PTP-3/LAR and UNC-40/DCC pathways.
Supplementary Material
Acknowledgments
The authors thank B. Ackley, J. Culotti, G. Garriga, and J. Teuliere for plasmids and reagents; O. Hobert, A. Boyanov, and M. Doitsidou for next generation sequencing assistance; S. Macdonald for assistance with TopHat and IGV2.0.15; J. Dyer for assistance with Q neuroblast scoring; E. Struckhoff for technical assistance; and members of the Lundquist and Ackley labs for discussion and critical feedback on this project. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources (NCRR). This work was supported by NIH grants R01NS040945 and R21NS070417 to E.A.L., and NIH grant P20 RR016475 from the Kansas Infrastructure Network for Biomedical Research Excellence program of the NCRR.
Footnotes
Communicating editor: K. Kemphues
Literature Cited
- Ackley B. D., Harrington R. J., Hudson M. L., Williams L., Kenyon C. J., et al. , 2005. The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J. Neurosci. 25: 7517–7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher B., Lerch M. M., Muller T., Schilling J., Ullrich A., 1997. Cellular redistribution of protein tyrosine phosphatases LAR and PTPsigma by inducible proteolytic processing. J. Cell Biol. 138: 681–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M., Chan K. K., Byrne A. B., Selman G., Lee T., et al. , 2009. An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development 136: 911–922 [DOI] [PubMed] [Google Scholar]
- Bigelow H., Doitsidou M., Sarin S., Hobert O., 2009. MAQGene: software to facilitate C. elegans mutant genome sequence analysis. Nat. Methods 6: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda C. S., Stern M. J., 2000. Mechanisms controlling sex myoblast migration in Caenorhabditis elegans hermaphrodites. Dev. Biol. 226: 137–151 [DOI] [PubMed] [Google Scholar]
- Calixto A., Chelur D., Topalidou I., Chen X., Chalfie M., 2010. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods 7: 554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng Q., Williams L., Lie Y. S., Sym M., Whangbo J., et al. , 2003. Identification of genes that regulate a left-right asymmetric neuronal migration in Caenorhabditis elegans. Genetics 164: 1355–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Sulston J., 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82: 358–370 [DOI] [PubMed] [Google Scholar]
- Chalfie M., Thomson J. N., Sulston J. E., 1983. Induction of neuronal branching in Caenorhabditis elegans. Science 221: 61–63 [DOI] [PubMed] [Google Scholar]
- Chapman J. O., Li H., Lundquist E. A., 2008. The MIG-15 NIK kinase acts cell-autonomously in neuroblast polarization and migration in C. elegans. Dev. Biol. 324: 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes S., Frank C. A., Garriga G., 2006. The C. elegans MELK ortholog PIG-1 regulates cell size asymmetry and daughter cell fate in asymmetric neuroblast divisions. Development 133: 2747–2756 [DOI] [PubMed] [Google Scholar]
- Davis M. W., Hammarlund M., Harrach T., Hullett P., Olsen S., et al. , 2005. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Chalfie M., 2001. Genes regulating touch cell development in Caenorhabditis elegans. Genetics 158: 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer J. O., Demarco R., Lundquist E. A., 2010. Distinct roles of Rac GTPases and the UNC-73/Trio and PIX-1 Rac GTP exchange factors in neuroblast protrusion and migration in C. elegans. Small GTPases 1: 44–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann D. M., 2005. Wnt signaling (June 25, 2005), WormBook, ed. The C. elegans Research Community WormBook, doi/10.1895/wormbook.1.7.1, http://www.wormbook.org
- Esposito G., Di Schiavi E., Bergamasco C., Bazzicalupo P., 2007. Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395: 170–176 [DOI] [PubMed] [Google Scholar]
- Gilleard J. S., Barry J. D., Johnstone I. L., 1997. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol. Cell. Biol. 17: 2301–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo A., Kim D. Y., Carey B. W., Turunen M. K., Pettingell W. H., et al. , 2007. Presenilin/gamma-secretase-mediated cleavage regulates association of leukocyte-common antigen-related (LAR) receptor tyrosine phosphatase with beta-catenin. J. Biol. Chem. 282: 9063–9072 [DOI] [PubMed] [Google Scholar]
- Harrington R. J., Gutch M. J., Hengartner M. O., Tonks N. K., Chisholm A. D., 2002. The C. elegans LAR-like receptor tyrosine phosphatase PTP-3 and the VAB-1 Eph receptor tyrosine kinase have partly redundant functions in morphogenesis. Development 129: 2141–2153 [DOI] [PubMed] [Google Scholar]
- Harris J., Honigberg L., Robinson N., Kenyon C., 1996. Neuronal cell migration in C. elegans: regulation of Hox gene expression and cell position. Development 122: 3117–3131 [DOI] [PubMed] [Google Scholar]
- Harterink M., Kim D. H., Middelkoop T. C., Doan T. D., van Oudenaarden A., et al. , 2011. Neuroblast migration along the anteroposterior axis of C. elegans is controlled by opposing gradients of Wnts and a secreted Frizzled-related protein. Development 138: 2915–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4: 61–85 [DOI] [PubMed] [Google Scholar]
- Herman M. A., 2003. Wnt signaling in C. elegans, pp. 187–212 in Wnt Signaling in Development, edited by M. Kühl. Landes Biosciences, Georgetown, TX
- Hofmeyer K., Treisman J. E., 2009. The receptor protein tyrosine phosphatase LAR promotes R7 photoreceptor axon targeting by a phosphatase-independent signaling mechanism. Proc. Natl. Acad. Sci. USA 106: 19399–19404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg L., Kenyon C., 2000. Establishment of left/right asymmetry in neuroblast migration by UNC-40/DCC, UNC-73/Trio and DPY-19 proteins in C. elegans. Development 127: 4655–4668 [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Tenney A. P., Ghose A., Duckworth A. M., Higashi M. E., et al. , 2006. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron 49: 517–531 [DOI] [PubMed] [Google Scholar]
- Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S.-Y., et al. , 1996. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87: 175–185 [DOI] [PubMed] [Google Scholar]
- Kenyon C., 1986. A gene involved in the development of the posterior body region of C. elegans. Cell 46: 477–487 [DOI] [PubMed] [Google Scholar]
- Korswagen H. C., Herman M. A., Clevers H. C., 2000. Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature 406: 527–532 [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S. L., 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Strumpf N., Culotti J. G., 2007. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat. Neurosci. 10: 161–168 [DOI] [PubMed] [Google Scholar]
- Mello C., Fire A., 1995. DNA transformation. Methods Cell Biol. 48: 451–482 [PubMed] [Google Scholar]
- Middelkoop T. C., Williams L., Yang P. T., Luchtenberg J., Betist M. C., et al. , 2012. The thrombospondin repeat containing protein MIG-21 controls a left-right asymmetric Wnt signaling response in migrating C. elegans neuroblasts. Dev. Biol. 361: 338–348 [DOI] [PubMed] [Google Scholar]
- Pan C. L., Howell J. E., Clark S. G., Hilliard M., Cordes S., et al. , 2006. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev. Cell 10: 367–377 [DOI] [PubMed] [Google Scholar]
- Pawson C., Eaton B. A., Davis G. W., 2008. Formin-dependent synaptic growth: evidence that Dlar signals via Diaphanous to modulate synaptic actin and dynamic pioneer microtubules. J. Neurosci. 28: 11111–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. T., Thorvaldsdottir H., Winckler W., Guttman M., Lander E. S., et al. , 2011. Integrative genomics viewer. Nat. Biotechnol. 29: 24–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe J. E., Streit S., Hart S., Ullrich A., 2006. EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell. Signal. 18: 1515–1527 [DOI] [PubMed] [Google Scholar]
- Salser S. J., Kenyon C., 1992. Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature 355: 255–258 [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Mahowald A. P., Fuller M. T., 2012. The receptor tyrosine phosphatase Lar regulates adhesion between Drosophila male germline stem cells and the niche. Development 139: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J., Hodgkin J., 1988. Methods, pp. 587–606 in The Nematode Caenorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sulston J. E., Horvitz H. R., 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156 [DOI] [PubMed] [Google Scholar]
- Sym M., Robinson N., Kenyon C., 1999. MIG-13 positions migrating cells along the anteroposterior body axis of C. elegans. Cell 98: 25–36 [DOI] [PubMed] [Google Scholar]
- Tabara H., Grishok A., Mello C. C., 1998. RNAi in C. elegans: soaking in the genome sequence. Science 282: 430–431 [DOI] [PubMed] [Google Scholar]
- Terns R. M., Kroll-Conner P., Zhu J., Chung S., Rothman J. H., 1997. A deficiency screen for zygotic loci required for establishment and patterning of the epidermis in Caenorhabditis elegans. Genetics 146: 185–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdottir H., Robinson J. T., Mesirov J. P., 2012. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., Court D. L., Fire A., 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112 [DOI] [PubMed] [Google Scholar]
- Wang F., Wolfson S. N., Gharib A., Sagasti A., 2012. LAR receptor tyrosine phosphatases and HSPGs guide peripheral sensory axons to the skin. Curr. Biol. 22: 373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whangbo J., Kenyon C., 1999. A Wnt signaling system that specifies two patterns of cell migration in C. elegans. Mol. Cell 4: 851–858 [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. 314: 1–340 [DOI] [PubMed] [Google Scholar]
- Williams L., 2003. A genetic analysis of the left-right asymmetric polarizations and migrations of the Q neuroblasts in C. elegans. Ph.D. Thesis, University of California, San Francisco
- Winston W. M., Molodowitch C., Hunter C. P., 2002. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295: 2456–2459 [DOI] [PubMed] [Google Scholar]
- Zinovyeva A. Y., Yamamoto Y., Sawa H., Forrester W. C., 2008. Complex network of Wnt signaling regulates neuronal migrations During Caenorhabditis elegans. Dev. Genet. 179: 1357–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.