Abstract
Mutations in the skeletal muscle voltage-gated calcium channel (CaV1.1) have been associated with hypokalemic periodic paralysis, but how the pathogenesis of this disorder relates to the functional consequences of mutations was unclear. In this issue of the JCI, Wu and colleagues recapitulate the disease by generating a novel knock-in CaV1.1 mutant mouse and use this model to investigate the cellular and molecular features of pathogenesis. They demonstrated an aberrant muscle cell current conducted through the CaV1.1 voltage-sensor domain (gating pore current) that explains an abnormally depolarized muscle membrane and the failure of muscle action potential firing during challenge with agents known to provoke periodic paralysis. Their work advances understanding of molecular and cellular mechanisms underlying an inherited channelopathy.
Ion channels are ubiquitous membrane proteins that confer selective ionic permeability to the plasmalemma or intracellular membranes and enable a wide variety of important physiological processes, including membrane excitability, synaptic transmission, signal transduction, cell volume regulation, and transcellular ion transport. The vital nature of ion channels is reflected by the existence of inherited disorders caused by mutations in genes that encode these proteins (1–5). These “channelopathies” represent more than 50 human genetic diseases, including several affecting skeletal muscle contraction, such as the periodic paralyses and nondystrophic myotonias (see “Muscle channelopathies”).

Hypokalemic periodic paralysis
Plasma membrane channels in skeletal muscle are essential for the generation and propagation of action potentials, leading to release of intracellular calcium through the process of excitation-contraction coupling. Ion channel dysfunction can hinder contraction by impairing action potential firing along the membrane. A characteristic symptom of this phenomenon is known as “periodic paralysis,” a form of paroxysmal weakness that occurs in the absence of neuromuscular junction or motor neuron disease. Periodic paralysis is most often thought of as an inherited disease, but certain acquired conditions can produce a similar phenotype. Disturbances in plasma potassium ion concentration often accompany bouts of weakness, and the direction of change has been used to classify the condition as hypokalemic, hyperkalemic, or normokalemic periodic paralysis (6). These conditions are not lethal, because respiratory muscles are spared.
Although the clinical features and inheritance pattern have been known since the first half of the twentieth century, the pathophysiology of periodic paralysis remained mysterious until approximately 30 years ago, when investigators in Germany published their electrophysiological observations on explanted intercostal muscle fibers from subjects with periodic paralysis (7). These studies revealed that resting membrane potential was less negative in fibers from hypokalemic periodic paralysis (HypoPP) subjects compared with normal muscle. Further, exposure of the fibers to low extracellular potassium concentration evoked a further depolarization, whereas this condition hyperpolarized normal muscle membranes. A similar paradoxical depolarization was observed after application of insulin. Both hypokalemia and insulin treatment rendered the fibers electrically inexcitable, owing to inactivation of voltage-gated sodium channels by the strongly depolarized membrane potential. By contrast, muscle fibers from subjects with hyperkalemic periodic paralysis (HyperPP) had normal resting membrane potentials but exhibited depolarization and inexcitability when exposed to elevated extracellular potassium (8). Tetrodotoxin, a highly specific blocker of voltage-gated sodium channels, reversed the depolarization occurring in HyperPP fibers but had no effect on HypoPP muscle. These observations established fundamental features of the pathogenesis of periodic paralysis but left unanswered key questions about the nature of the depolarizing stimulus in the hypokalemic variant.
Advances in the molecular genetics of periodic paralysis revealed that HyperPP is caused exclusively by mutations in SCN4A, the gene encoding the skeletal muscle voltage-gated sodium channel (NaV1.4), whereas HypoPP is caused most often by mutations in CACNA1S, encoding the pore-forming subunit of an L-type voltage-gated calcium channel (CaV1.1), and less commonly by SCN4A mutations (2). Although both syndromes are associated with notable allelic heterogeneity, CACNA1S and SCN4A mutations associated with HypoPP are clustered within voltage-sensor domains. A brief digression on the topic of voltage-gated channel structure and function will help clarify the pathophysiological significance of this mutation clustering.
Voltage-gated channel structure and function
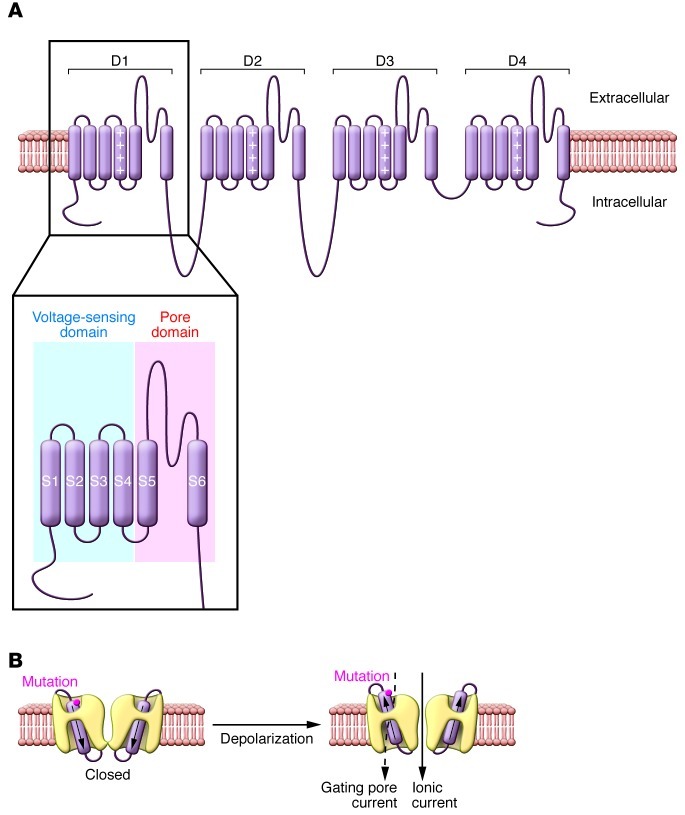
The sodium and calcium channels expressed in nerve, heart, and muscle belong to a superfamily of ion channels that are gated (opened and closed) by changes in membrane potential (9). The main pore-forming α-subunits have a four-fold symmetry consisting of structurally homologous domains (D1–D4), each containing four transmembrane segments that comprise the voltage-sensor domain (S1–S4) and a separate pore domain (S5–S6), important for determining ion selectivity (Figure 1A). The S4 segment, which functions as the main voltage-sensing element, is amphipathic, with basic amino acids (arginine or lysine) at every third position surrounded by hydrophobic residues. Activation of voltage-gated channels is evoked by a membrane depolarization that acts to propel the S4 segments in an outward direction away from the negative electrostatic cell interior. Subsequent conformational changes involving the S6 segment open the ion pore and permit rapid movement of ions through a passageway created by the pore domain.
Figure 1. Voltage-gated sodium and calcium channel structural domains and location of gating pore.
(A) Predicted transmembrane topology model of typical voltage-gated sodium or calcium channel. The S4 segments within voltage-sensing domains are indicated by a column of plus (+) signs. The inset illustrates locations of the separate voltage-sensing and pore domains that are repeated four times in the channel protein. (B) A cutaway view showing the pathway through which ionic current or gating pore currents are conducted. The cylinders within the channels represent the S4 segments, and the approximate location of the HypoPP mutation CaV1.1 R528H is indicated. Figure modified from the Journal of General Physiology (23).
The highly conserved S4 segment has received an enormous amount of attention for the past two decades, particularly with regard to the molecular motions that carry its positive charges through the membrane electric field (10, 11). One startling revelation regarding sodium and potassium channels was that the S4 segment becomes accessible to aqueous protein-modifying reagents during gating motions (12, 13). This observation led to the hypothesis that S4 segments travel through the membrane via a water-filled cavity. Even more intriguing was the observation that histidine substitutions for arginine residues within the S4 segment generate a proton pore that is separate from the main ion permeation pathway in the pore domain (14). The current flowing through the voltage-sensor pore (also known as the gating pore) was termed the “omega” or “gating pore” current (Figure 1B).
Because these S4 segment histidine substitutions created unnatural channels, astute researchers investigating the functional consequences of channelopathy-associated mutations recognized that this mechanism might explain the pathophysiology of HypoPP. Specifically, sodium channel mutations associated with HypoPP that replace S4 segment arginine residues create channels that conduct an anomalous inward current at resting membrane potentials (15–18). The in vivo relevance of this mechanism was demonstrated subsequently using a mouse model of the disease (NaV1.4 R669H knock-in), in which an anomalous inward current was detected in muscle cells at hyperpolarized potentials (19). These investigations offered a molecular explanation for HypoPP caused by sodium channel mutations, but did not address what happens with the more common calcium channel mutations. Furthermore, prior studies of mutations engineered in human CaV1.1 did not reveal a consistent and compelling pattern of channel dysfunction that would explain the phenotype, in part because of the difficulty of expressing this channel in heterologous cell systems.
Calcium channel mutant mice
To address the pathogenesis of HypoPP caused by CACNA1S mutations, Wu and colleagues in the laboratory of Stephen Cannon report in this issue of the JCI the investigation of a novel knock-in mouse model of the disease (20). Mice were generated that express the most common human HypoPP mutation (CaV1.1 R528H), a histidine substitution for the outermost arginine residue of the D2/S4 segment in CaV1.1. Although animals did not exhibit spontaneous attacks of weakness, muscle strength was reduced more severely in male mice, consistent with the reduced penetrance in females observed for human HypoPP (6). Muscles from knock-in mice exhibited features previously observed in human HypoPP fibers, including reduced contractile force and paradoxical membrane depolarization evoked by low extracellular potassium or by glucose and insulin challenge. Muscle fibers from homozygous knock-in R528H mice exhibited a –15-mV depolarization of the resting membrane potential similar to human HypoPP fibers. In addition, these mice exhibited a chronic vacuolar myopathy similar to that observed in patients with this disorder.
A critical observation made by these investigators was the presence of an anomalous inward current in mutant mouse muscle fibers consistent with gating pore current rather than ionic current conducted through a known ion channel (20). These findings offered a mechanistic link between calcium channel mutation and attacks of muscle weakness. Anomalous gating pore current creates a precarious balance between the inward and outward currents that maintain the membrane potential. Factors such as hypokalemia that transiently depress potassium currents, which are required to maintain a normal resting membrane potential, can render the muscle severely depolarized and, consequently, inexcitable owing to inactivation of sodium channels.
Now that a common mechanism of evoking gating pore current has been established in HypoPP, several new and intriguing questions emerge. Can aberrant gating pore current be selectively blocked, and would this prevent attacks of paralysis? Do carbonic anhydrase inhibitors such as acetazolamide and dichlorphenamide, which are effective treatments for HypoPP regardless of genotype, affect gating pore current directly or indirectly? Are there triggering factors that act specifically by potentiating gating pore current? Finally, what is the basis for vacuolar myopathy, and why is there a sex-biased penetrance of mutations? These and other important questions can now be addressed using the mouse model developed by Wu and colleagues.
Although HypoPP was the first channelopathy for which aberrant gating pore current was implicated in disease pathogenesis, many other S4 mutations in voltage-gated sodium, calcium, and potassium channels have been found in other inherited disorders of membrane excitability, including epilepsy and cardiac arrhythmia syndromes (21). Are some of these conditions also “gating pore-opathies”? A recent study demonstrating that an S4 segment mutation in the cardiac sodium channel (NaV1.5 R219H) associated with dilated cardiomyopathy evokes an aberrant proton current (22) suggests that this may be a more widespread phenomenon.
Acknowledgments
The author is supported by grants from the NIH (HL083374, NS032387).
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(12):4333–4336. doi:10.1172/JCI66535.
See the related article beginning on page 4580.
References
- 1.George AL., Jr Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115(8):1990–1999. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jurkat-Rott K, Lehmann-Horn F. Muscle channelopathies and critical points in functional and genetic studies. J Clin Invest. 2005;115(8):2000–2009. doi: 10.1172/JCI25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 2005;115(8):2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115(8):2018–2024. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jentsch TJ, Maritzen T, Zdebik AA. Chloride channel diseases resulting from impaired transepithelial transport or vesicular function. J Clin Invest. 2005;115(8):2039–2046. doi: 10.1172/JCI25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon SC, George AL. Diseases of the Nervous System, Clinical Neuroscience and Therapeutic Principles. 2002. Pathophysiology of myotonia and periodic paralysis. In: Asbury AK, Mckhann GM, McDonald WI, Goadsby PJ, McArthur JC, eds. pp. 1183–1206. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; [Google Scholar]
- 7.Rüdel R, Lehmann-Horn F, Ricker K, Kuther G. Hypokalemic periodic paralysis: in vitro investigation of muscle fiber membrane parameters. Muscle Nerve. 1984;7(2):110–120. doi: 10.1002/mus.880070205. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann-Horn F, et al. Two cases of adynamia episodica hereditaria: In vitro investigation of muscle cell membrane and contraction parameters. Muscle Nerve. 1983;6(2):113–121. doi: 10.1002/mus.880060206. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong CM, Hille B. Voltage-gated ion channels and electrical excitability. Neuron. 1998;20(3):371–380. doi: 10.1016/S0896-6273(00)80981-2. [DOI] [PubMed] [Google Scholar]
- 10.Swartz KJ. Sensing voltage across lipid membranes. Nature. 2008;456(7224):891–897. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bezanilla F. How membrane proteins sense voltage. Nature Rev Mol Cell Biol. 2008;9(4):323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 12.Yang NB, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16(1):113–122. doi: 10.1016/S0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- 13.Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the shaker K+ channel S4. . Neuron. 1996;16(2):387–397. doi: 10.1016/S0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 14.Starace DM, Stefani E, Bezanilla F. Voltage-dependent proton transport by the voltage sensor of the shaker K+ channel. . Neuron. 1997;19(6):1319–1327. doi: 10.1016/S0896-6273(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 15.Sokolov S, Scheuer T, Catterall WA. Gating pore current in an inherited ion channelopathy. Nature. 2007;446(7131):76–78. doi: 10.1038/nature05598. [DOI] [PubMed] [Google Scholar]
- 16.Sokolov S, Scheuer T, Catterall WA. Depolarization-activated gating pore current conducted by mutant sodium channels in potassium-sensitive normokalemic periodic paralysis. Proc Natl Acad Sci U S A. 2008;105(50):19980–19985. doi: 10.1073/pnas.0810562105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Struyk AF, Cannon SC. A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore. . J Gen Physiol. 2007;130(1):11–20. doi: 10.1085/jgp.200709755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Struyk AF, Markin VS, Francis D, Cannon SC. Gating pore currents in DIIS4 mutations of NaV1.4 associated with periodic paralysis: saturation of ion flux and implications for disease pathogenesis. . J Gen Physiol. 2008;132(4):447–464. doi: 10.1085/jgp.200809967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, et al. A sodium channel knockin mutant (NaV1.4-R669H) mouse model of hypokalemic periodic paralysis. . J Clin Invest. 2011;121(10):4082–4094. doi: 10.1172/JCI57398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu F, et al. A calcium channel mutant mouse model of hypokalemic periodic paralysis. J Clin Invest. 2012;122(12):4580–4591. doi: 10.1172/JCI66091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurkat-Rott K, Groome J, Lehmann-Horn F. Pathophysiological role of omega pore current in channelopathies. Front Pharmacol. 2012;3:112. doi: 10.3389/fphar.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosselin-Badaroudine P, et al. A Proton Leak current through the cardiac sodium channel is linked to mixed arrhythmia and the dilated cardiomyopathy phenotype. PLoS One. 2012;7(5):e38331. doi: 10.1371/journal.pone.0038331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahern CA, Horn R. Specificity of charge-carrying residues in the voltage sensor of potassium channels. J Gen Physiol. 2004;123(3):205–216. doi: 10.1085/jgp.200308993. [DOI] [PMC free article] [PubMed] [Google Scholar]