Abstract
Systemic juvenile idiopathic arthritis (SJIA), a subtype of juvenile idiopathic arthritis, is characterized by systemic features, such as spiking fever, salmon-colored macular rash, serositis, lymphadenopathy, hepatosplenomegaly, and joint inflammation. It is also often complicated with growth retardation, osteoporosis, and sometimes macrophage activation syndrome (MAS) develops, a potentially fatal disease. Pathogenesis of SJIA and MAS is not yet fully understood, but activation of the innate immune system, which causes phagocytosis by dendritic cells, monocytes, and macrophages to produce proinflammatory cytokines such as interleukin-6 (IL-6), IL-1β and IL-18, is thought to be a primary abnormality associated with SJIA. Dysregulated production of IL-6 plays a major role in the development of systemic clinical features. The blockade of IL-6 might thus represent a novel strategy for the treatment of SJIA. Several phase II and III clinical trials of a humanized anti-IL-6 receptor antibody, tocilizumab, proved its outstanding efficacy and tolerable safety profile for SJIA refractory to conventional treatment regimens. This resulted in the approval of tocilizumab for the treatment of SJIA in Japan, India, the EU and the USA.
Keywords: Interleukin-6, a humanized anti-interleukin-6 receptor antibody, systemic juvenile idiopathic arthritis, tocilizumab
Introduction
Juvenile idiopathic arthritis (JIA) is not a single disease, but a term that encompasses all forms of arthritis that begin before a patient is 16 years old, persist for more than 6 weeks and are of unknown origin [Prakken et al. 2011; Mellins et al. 2011]. Systemic juvenile idiopathic arthritis (SJIA), currently classified as a subtype of JIA by the International League of Associations for Rheumatology (ILAR) [Petty et al. 2004], is characterized by systemic features, such as daily spiking fever, salmon-colored macular rash, serositis, lymphadenopathy, hepatosplenomegaly and arthritis. Although its precise pathogenesis remains to be determined, marked activation of the innate immune system and the absence of autoantibodies have led to the hypothesis that SJIA can be categorized as an autoinflammatory syndrome [Ramanan and Grom, 2005, Mellins et al. 2011] and that interleukin-6 (IL-6) plays a major role in its development [De Benedetti and Martini, 2005; Heriln, 2009; Yokota and Kishimoto, 2010]. In this review, we present recent findings regarding the significant pathogenic role of IL-6 in the development of SJIA and the outstanding clinical efficacy of the IL-6 blocking strategy for SJIA. Additionally, one of the most severe diseases complicated with SJIA, macrophage activation syndrome (MAS), will be discussed from the viewpoint of dysregulation of proinflammatory cytokines.
Pathologic role of interleukin-6 on systemic juvenile idiopathic arthritis
Clinical manifestations of systemic juvenile idiopathic arthritis and proinflammatory cytokines
Systemic manifestations with a quotidian, spiking fever, fatigue, anorexia, nonfixed erythematosus skin rash, and polyarthritis are characteristic features of SJIA [Prakken et al. 2011; Mellins et al. 2011]. SJIA is often accompanied by serositis, generalized lymphadenopathy and hepatosplenomegaly. It is also associated with the complications of growth retardation [Still, 1941], osteoporosis [Lang et al. 1995], and compression fracture of the spine. In addition, some patients develop amyloid A amyloidosis due to long-term uncontrolled status [Obici et al. 2005] or MAS [Sawhney et al. 2001; Grom and Mellins, 2010]. Laboratory findings show marked increases in acute phase proteins such as C-reactive protein (CRP) and serum amyloid A (SAA), leukocytosis, predominantly neutrophilia, thrombocytosis, anemia, hypoalbuminemia, and elevated liver transaminases. These clinical symptoms and abnormal laboratory findings are mainly mediated by the dysregulated and continuous production of IL-6. In cases complicated with MAS, pancytopenia, especially severe thrombocytopenia, increased levels of fibrin/fibrinogen degradation products, marked elevations of aspartate aminotransferase and lactate dehydrogenase, high levels of urinary β2 microglobulin and serum ferritin, and then low levels of total cholesterol and high levels of triglyceride are sequentially found. These laboratory abnormalities in MAS can be attributable to the dysregulated production of proinflammatory cytokines such as IL-1β, IL-18, interferon γ, tumor necrosis factor α (TNFα) as well as IL-6 [Yokota et al. 2004].
Recent pathological analyses have disclosed that SJIA differs from other forms of JIA [Prakken et al. 2011; Mellins et al. 2011; Lin et al. 2011]. SJIA is an autoinflammatory disease with abnormalities in the innate immune system through which the aberrant activation of dendritic cells and phagocytes, including monocytes and macrophages, becomes involved in the release of proinflammatory cytokines such as IL-1β, IL-6, IL-18 and proinflammatory S100-proteins. This contrasts the imbalance between autoreactive T helper 1 (Th1)/Th17 and regulatory T cells (Tregs) which appears to play a major part in the development of oligoarticular and polyarticular JIA [Nistala and Wedderburn, 2009].
Biological activities of interleukin-6 in patients with systemic juvenile idiopathic arthritis
Human IL-6 is a typical cytokine featuring redundancy and pleiotropy [Kishimoto, 1989]. It performs a wide variety of functions by acting on lymphocytes, hepatocytes, hematopoietic progenitor cells, fibroblasts, and other cells [Kishimoto, 2005]. IL-6 induces the differentiation of activated B cells into immunoglobulin-producing cells, and is also a regulatory cytokine for the differentiation of naïve CD4-positive T cells. While IL-6, combined with transforming growth factor β (TGFβ), is essential for Th17 differentiation from naïve CD4-positive T cells, it also inhibits the generation of Tregs induced by TGFβ [Korn et al. 2009], leading to an imbalance between Th17 and Tregs. It has recently been suggested that this imbalance may cause several autoimmune and chronic inflammatory diseases, including oligoarticular and polyarticular JIA [Kimura and Kishimoto, 2010; Tanaka et al. 2012]. In hepatocytes, IL-6 induces a broad spectrum of acute phase proteins such as CRP, SAA, haptoglobin, a1-antitrypsin, antichymotrypsin, fibrinogen, and hepcidin along with IL-1β [Hagihara et al. 2005; Nishikawa et al. 2008], while it reduces the production of albumin, fibronectin, and transferrin [Heinrich et al. 1990]. High levels of hepcidin, an iron regulatory peptide hormone, block iron transporter ferroportin 1 on macrophages, hepatocytes and gut epithelial cells. This leads to hypoferremia and anemia of chronic inflammation [Nemeth et al. 2004; Ganz and Nemeth, 2011]. Long-lasting high concentrations of SAA result in amyloid A (AA) amyloidosis [Obici et al. 2005]. When acting on synovial fibroblasts, IL-6 induces osteoclast-like cell formation [Kotake et al. 1996] and also generates the receptor activator of nuclear factor κB ligand [Palmqvist et al. 2002], which is an essential factor for the differentiation and activation of osteoclasts and bone resorption. This eventually leads to joint destruction and osteoporosis. IL-6 also inhibits early differentiation of chondrogenic progenitor cells [Nakajima et al. 2009a] and causes growth impairment in IL-6 transgenic mice as a result of a reduction in insulin-like growth factor-1 (IGF-1) and circulating IL-6 levels which correlate negatively with IGF-1 levels [De Benedetti et al. 1997a]. In addition, the inhibitory effect of IL-6 on IGF-1 activity is mediated by the activation of suppressor of cytokine signaling 3 [Al-Shanti and Stewart, 2011]. Lastly, IL-6 promotes the final maturation of megakaryocytes to platelets [Ishibashi et al. 1989]. These biological activities of IL-6 are responsible for development of the systemic features, complications, and abnormal laboratory findings of SJIA.
Serum IL-6 levels are elevated in patients with SJIA [De Benedetti et al. 1991, 1994], increase before each fever spike and correlate with systemic disease activity [De Benedetti et al. 1991]. IL-6 levels are reportedly higher in patients with SJIA than in those with polyarticular JIA and correlate with disease activity [Rooney et al. 1995]. Synovial fluid levels of IL-6 have also been found to be elevated in patients with SJIA [De Benedetti et al. 1997b]. Peripheral blood mononuclear cells from patients with SJIA were observed to produce increased levels of IL-6 [Muller et al. 1998; Pignatti et al. 2001], and to cause the —174G allele of the IL-6 gene to confer susceptibility to SJIA [Fishman et al. 1998; Ogilvie et al. 2003]. Taken together, systemic features of SJIA can be attributable to the dysregulation of IL-6 production.
Pharmacodynamics of interleukin-6 and development of tocilizumab
IL-6 transduces its signal through the IL-6 receptor (IL-6R) system which consists of two chains; namely, transmembrane IL-6R, an 80 kDa IL-6-binding subunit with a short cytoplasmic domain [Yamasaki et al. 1988], and gp130, a 130 kDa transmembrane glycoprotein [Hibi et al. 1990]. After the binding of IL-6 to transmembrane IL-6R, the resultant IL-6/IL-6R complex associates with gp130, and the activated IL-6 receptor complex is then formed as a hexameric structure comprising two molecules each of IL-6, IL-6R and gp130 [Murakami et al. 1993; Boulanger et al. 2003]. IL-6 can bind to soluble IL-6R (sIL-6R) and the resultant IL-6/sIL-6R complex also can associate with gp130. Thus, even in cells lacking transmembrane IL-6R, the IL-6/sIL-6R complex can transduce the IL-6 signal onto gp130-expressing cells [Kishimoto et al. 1994]. Because of the biological activities of IL-6 and its pathological role, it was expected that an IL-6 blockade might constitute a novel treatment strategy for various autoimmune and chronic inflammatory disorders, including SJIA [De Benedetti and Martini, 2005; Tanaka et al. 2012]. Consequently, tocilizumab, which is a humanized anti-IL-6R monoclonal antibody of the immunoglobulin (Ig) G1 class, was developed. It was generated by grafting the complementarity determining regions of a mouse anti-human IL-6R antibody onto human IgG1. Tocilizumab completely blocks IL-6-mediated signal transduction by inhibiting IL-6 binding to transmembrane and soluble IL-6R [Sato et al. 1993].
Macrophage activation syndrome and proinflammatory cytokines
MAS is a clinical syndrome caused by the excessive activation and proliferation of well differentiated macrophages. It is the most severe complication during the course of SJIA. Typically, patients with SJIA become acutely ill with persistent fever, sickness-type behaviors such as sleep, lethargy and anorexia, lymphadenopathy, and hepatosplenomegaly. Laboratory investigation reveals profound depression of one or more blood cell lines, paradoxical improvement of CRP and erythrocyte sedimentation rate, and activation of the coagulation/fibrinolysis system (increased fibrinogen/fibrin-derived products, FDP and D-dimer). Serum levels of FDP and D-dimer were increased and accompanied by the increased excretion of urinary β2-microglobulin, which is a light chain of human leukocyte antigen class I molecule and a marker protein of massive interferon γ (IFNγ) production. Along with the increase in serum ferritin levels (ferritin is a marker protein of TNFα in serum) [Tran et al. 1997], high levels of lactate dehydrogenase and aspartate aminotransferase rather than alanine aminotransferase (ALT) could be detected, reflecting overall cell apoptosis due to mitochondrial permeability transition by TNFα [Bradham et al. 1998]. Proinflammatory cytokines such as IFNγ and TNFα are attributable to the activation of endothelial cells, and they then play a central role in the differential effects on the coagulation and fibrinolysis [Levi et al. 2003]. Thus, increased levels of cytokine-induced proteins (ferritin and urinary β2 microglobulin), and abnormalities of the clotting profile progress to disseminated intravascular coagulopathy. As a result, multiple organ failure commonly occurs.
Clinical efficacy, safety and tolerability of tocilizumab for systemic juvenile idiopathic arthritis
Phase II trials of tocilizumab for systemic juvenile idiopathic arthritis in Japan and the UK
A phase II for the clinical trial of tocilizumab was performed for 11 Japanese children with severe and active SJIA refractory to high-dose, long-term corticosteroids [Yokota et al. 2005]. The study (MRA011JP), designated as a dose-escalating trial, started with three 2 mg/kg infusions of tocilizumab at 2-week intervals. CRP levels were positive at least 5 days after the initial and second injections of tocilizumab so the dose was increased to 4 mg/kg and was administered three times every 2 weeks. If CRP concentrations did not improve, then three infusions of 8 mg/kg were administered every 2 weeks. Efficacy was evaluated every 2 weeks according to responses on the JIA core set of improvement criteria and the results of laboratory tests [Giannini et al. 1997]. It was found that tocilizumab abruptly reduced disease activity in 10 of the 11 children, but levels of inflammatory reactants fluctuated until the proper tocilizumab dose for each child was reached. Two weeks after the third fixed dose of tocilizumab, 90.9% of all patients had a 30% improvement response, 90.9% had a 50% improvement response, and 63.6% had a 70% improvement response (Figure 1). None of the patients were withdrawn during the study period. The adverse events (AEs) included upper respiratory tract infection, pustules on extremities, and eczema. All laboratory abnormalities were mild with increases in total cholesterol, mild decreases in γ globulin, transient, very mild increases in hepatic enzymes, and glycosuria. Tocilizumab was thus considered safe and well tolerated by the patients.
Figure 1.
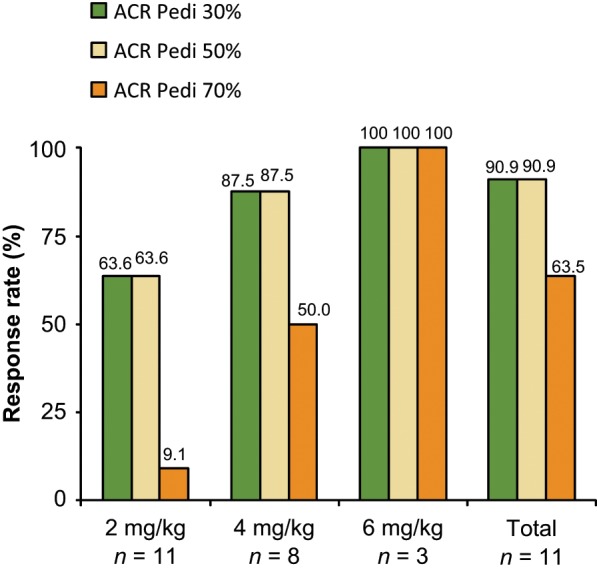
Achievement of American College of Rheumatology Pediatric (ACR Pedi) 30%, 50%, and 70% improvement response to phase II dose-escalating study of tocilizumab in 11 children with systemic juvenile idiopathic arthritis (SJIA).
The tocilizumab treatments (three doses each at 2, 4, and 8 mg/kg) yielded significantly better outcomes for all endpoints according to the ACR Pedi core set criteria. Of the 11 children requiring 2 mg/kg tocilizumab, seven (63.6%) achieved both 30% and 50% improved responses, while one child (9.1%) achieved a 70% improved response at the last observation. Seven of the eight children (87.5%) infused three times with 4 mg/kg tocilizumab had both 30% and 50% improved responses, and four of these eight children (50.0%) had a 70% improvement response at the last observation. All three children who received three doses of 8 mg/kg tocilizumab had 30%, 50%, and 70% improvement responses 2 weeks after the last infusion of tocilizumab [Yokota et al. 2005].
In the UK and France, 18 Caucasian patients with SJIA were enrolled in an open-label, single-dose trial (LRO320) [Woo et al. 2005]. Three patients were excluded due to protocol violations. The patients were divided into three groups receiving 2 (n = 4), 4 (n = 6) or 8 mg/kg (n = 5) of tocilizumab. Tocilizumab appeared to be dramatically effective, with clinical and laboratory responses observed by means of a 48 h post- infusion. These improvements continued for up to 8 weeks. Eleven patients (73.3%) showed an ACR Pedi 30% response and eight (53.3%) an ACR Pedi 50% response. As for safety, 59 AEs were recorded for 15 patients, including gastrointestinal disorders, laboratory abnormalities such as increases in hepatic enzymes, respiratory disorders and infection/infestations, but the majority of these AEs were mild. No serious bacterial infections were documented.
Phase III trials of tocilizumab for systemic juvenile idiopathic arthritis in Japan and the EU
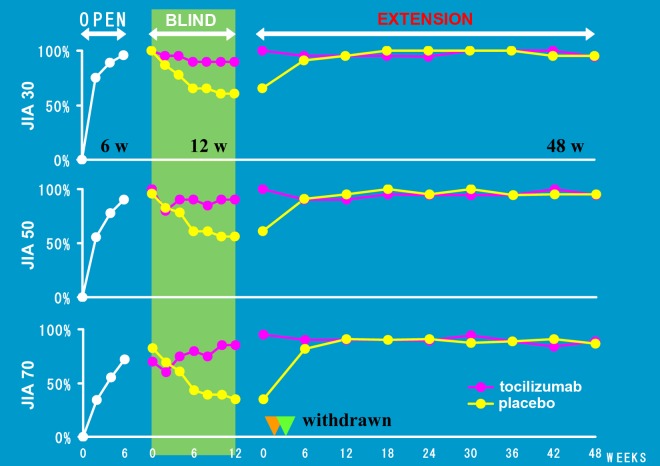
A phase III trial (MRA316JP) was conducted to investigate the efficacy and safety of tocilizumab for 56 patients with SJIA who had been refractory to conventional treatment [Yokota et al. 2008a]. The study consisted of three phases: an open-label lead-in phase of 6 weeks; a double- blind, randomized, placebo-controlled phase of 12 weeks; and an open-label extension phase of at least 48 weeks. Since phase II of the study for tocilizumab abruptly reduced disease activity in most children with SJIA, the double-blind part of the phase III trial was designed as a withdrawal study. To overcome the ethical issues for treating children, the double-blind part occurred after the first 6 weeks of being an open-label lead-in phase. A dose of 8 mg/kg of tocilizumab was administered intravenously every 2 weeks. At the end of the first open-label phase, ACR Pedi 30%, 50%, and 70% responses were achieved in 91%, 86%, and 68% of the enrolled patients respectively. In the double-blind, placebo-controlled phase, 17% of children in the placebo group maintained an ACR Pedi 30% response compared with 80% of children in the tocilizumab group. By week 48 of the open-label extension phase, ACR Pedi 30%, 50%, and 70% responses were achieved by 98%, 94%, and 90% of the 48 patients respectively (Figure 2). Improvements in osteoporosis and catch-up growth in children with retarded growth were also observed. The efficacy of tocilizumab increased continuously over time. During the open-label lead-in phase, two serious AE were recorded. One was an anaphylactoid reaction and the other was a gastrointestinal hemorrhage from chronic ulceration. Additionally, two serious AEs (infectious mononucleosis for a patient treated with tocilizumab and herpes zoster for a patient administered with placebo) which occurred during the double-blind phase resulted in the two patients having to be withdrawn from the study. However, most of the other AEs were mild or moderate in severity and no deaths or cases of MAS occurred.
Figure 2.
Phase III trial of tocilizumab for systemic juvenile idiopathic arthritis in Japan.
A phase III trial consisted of three phases: an open-label lead-in phase of 6 weeks (OPEN); a double-blind, randomized, placebo-controlled phase of 12 weeks (BLIND); and an open-label extension phase of at least 48 weeks (EXTENSION).The double-blind part was designed as a withdrawal study after the first 6 weeks of being in a lead-in phase [Yokota et al. 2008].
In addition, pretreatment radiographical findings of joint destruction (such as joint space narrowing, subchondral bone cysts and erosion), all diminished after tocilizumab treatment [Inaba et al. 2011]. Also, osteoporosis showed marked amelioration in seven patients with SJIA in association with improvement of reduced serum cartilage oligomeric matrix protein levels [Nakajima et al. 2009b]. Based on its outstanding efficacy and tolerable safety for SJIA, tocilizumab was approved as the first biological drug for the treatment of SJIA in Japan in 2008. The recommended dosing of tocilizumab is 8 mg/kg every 2 weeks but when the response is not adequate, tocilizumab can be administered at this rate every week. According to the Japanese guidelines for using tocilizumab for SJIA [Yokota et al. 2011], a warning is indicated for patients with refractory SJIA who have persistent inflammation and clinical symptoms requiring prolonged use of corticosteroids (0.2 mg/kg/day or more of prednisolone), who experience severe adverse reactions induced by corticosteroids, or who require high-dose corticosteroids to suppress inflammation and clinical symptoms to render the disease inactive, but which could sooner or later lead to corticosteroid-induced adverse reactions.
The double-blind, placebo–controlled, randomized phase III TENDER trial (WA18221), the first part of this 5-year ongoing multinational study, examined the safety and efficiency of intravenously administered placebo or tocilizumab in 112 children with active SJIA for 12 weeks [De Benedetti et al. 2010]. The primary endpoint of this part of the study was to find the proportion of subjects achieving at least ACR Pedi 30% response plus an absence of fever at week 12. Children were randomized to receive either a placebo or tocilizumab at two different doses based on body weight. The doses used in the study were based on pharmacokinetic modeling for children in earlier studies. Tocilizumab was given intravenously every 2 weeks at doses of 12 mg/kg for children whose body weight was less than 30 kg or 8 mg/kg for children with a body weight of more than 30 kg. To be eligible for enrollment, participants had to have had active SJIA for at least 6 months and demonstrate an intolerance or inadequate response to previous treatments. Patients were allowed to continue on stable doses of methotrexate, nonsteroidal anti-inflammatory drugs, or corticosteroids (maximum dosage of 0.5 mg/kg/day). A significant percentage of the patients studied had failed to respond to prior anti-TNF and anti-IL-1 therapy. The average age of the patients studied was 9.5 years with an average disease duration of 5 years. Approximately half of the children had a fever and an average of 19 swollen joints. However, at 12 weeks, 85% of the participants receiving tocilizumab met the primary endpoint compared with 24% of the children receiving placebo. As the secondary endpoint, 85% of the children in the tocilizumab group achieved an ACR Pedi 50% response and 71% an ACR Pedi 70% response by week 12 compared with corresponding values of only 11% and 8% of the children who received placebo. Patients who had previously been treated with anti-IL-1 receptor antagonist, anakinra or TNF inhibitors, responded well to tocilizumab. At 12 weeks, 85% of the patients with previous anakinra use had attained ACR Pedi 30% plus absence of fever, 71% an ACR Pedi 70% response and 39% an ACR Pedi 90% response, while the corresponding percentages were 82%, 64%, and 35% for patients who had previously received a TNF inhibitor. The most frequently occurring AEs in the study were upper respiratory infections, headaches, nasopharyngitis, and diarrhea. The most commonly reported infections were pneumonia, gastroenteritis, chickenpox, and ear infections, which were more frequently seen in patients receiving tocilizumab. Infusion reactions were detected in 16% of the patients in the tocilizumab group and in 5% of those in the placebo group, including two separate anaphylactic reactions in one patient receiving tocilizumab treatment, who eventually was withdrawn from the study. Five additional serious AEs consisting of pneumonia, septic arthritis, chickenpox, dehydration, and MAS were observed in patients receiving tocilizumab. Grade 3 (less than 1.0 × 109/L) neutropenia was identified in five children receiving tocilizumab, grade 2 (2.5–5 × upper normal limit) ALT elevation in another five children receiving tocilizumab, and four showed an elevation in total cholesterol of at least 240 mg/dl compared with only one patient in the placebo group. In addition, 1-year results of the TENDER study showed that 88% of patients receiving tocilizumab attained ACR Pedi 30% plus absence of fever, 89% an ACR Pedi 70% response, and 65% an ACR Pedi 90% response [De Benedetti et al. 2011].
On the basis of its outstanding efficacy and tolerable safety profile, the US Food and Drug Administration (FDA) approved tocilizumab in April 2011 for the treatment of active SJIA in patients 2 years of age and older. This makes tocilizumab the first FDA-approved drug for the treatment of SJIA. The recommended tocilizumab dosing for patients with SJIA weighing less than 30 kg is 12 mg/kg and 8 mg/kg for those weighing more than 30 kg. It is administered intravenously once every 2 weeks. Tocilizumab should be avoided for children with active acute or chronic infections, a known history of elevated liver or lipid levels, or neutropenia. Special caution should be taken for children with a history of serious infusion reactions to other biological agents. The FDA labeling recommendations for laboratory monitoring of patients with SJIA indicate focusing on neutrophils, platelets, and liver enzymes at the time of the second infusion, and then every 2–4 weeks. The recommended lipid monitoring for patients with SJIA is the same as for rheumatoid arthritis, that is, every 4–8 weeks following initiation of therapy, and then at approximately 24-week intervals.
The summary of the efficacy of tocilizumab in phase II and III trials for SJIA is shown in Table 1. A total of 128 patients with SJIA who completed the MRA011JP and MRA316JP studies as well as another 61 patients were enrolled to evaluate the tolerability of tocilizumab, which was administered at 8 mg/kg every 2 weeks for a median duration of 78 weeks [Yokota et al. 2008b]. In total, 14 patients were withdrawn from the study. Eight were due to AEs including MAS, anaphylactoid reaction, cardiac amyloidosis, duodenal perforation, gastrointestinal hemorrhage, and infusion reaction, five due to the development of anti-tocilizumab antibodies, and one due to unsatisfactory efficacy.
Table 1.
Summary of phase II/III clinical trials of tocilizumab.
| Study | Patients enrolled | Study design | Patient number | Treatment arm | Endpoint | Reference |
|---|---|---|---|---|---|---|
| Phase II MRA011JP (Japan) |
High dose of corticosteroid IR | Three TCZ infusions every 2 weeks | 11 | Dose escalating (2, 4 or 8 mg/kg TCZ) | ACR Pedi 30%, 91% ACR Pedi 50%, 91% ACR Pedi 70%, 64% | Yokota et al. [2005] |
| Phase II LRO320 (UK and France) |
High disease activity with receiving more than 0.2 mg/kg/day prednisolone | One injection of TCZ | 15 | TCZ (2, 4 or 8 mg/kg) | ACR Pedi 30%, 73% ACR Pedi 50%, 53% | Woo et al. [2005] |
| Phase III MRA316JP (Japan) |
Conventional treatment IR | Open-label lead-in phase (6 weeks, 8 mg/kg TCZ every 2 weeks) DBPC phase (12 weeks, PBO or 8 mg/kg TCZ every 2 weeks) Open-label extension phase (48 weeks, 8 mg/kg TCZ every 2 weeks) |
56 43 PBO 23 TCZ 20 48 |
TCZ (8 mg/kg) PBO or TCZ (8 mg/kg) TCZ (8 mg/kg) |
ACR Pedi 30%, 91% ACR Pedi 50%, 86% ACR Pedi 70%, 68% ACR Pedi 30% PBO 17% versus TCZ 80% ACR Pedi 30%, 98% ACR Pedi 50%, 94% ACR Pedi 70%, 90% |
Yokota et al. [2008b] |
| Phase III TENDER WA18221 (multi-national) |
Previous treatment IR | DBPC (12 wks, PBO or TCZ every 2 weeks) | 112 PBO 37 TCZ 75 |
PBO or TCZ (8 mg/kg for patients over 30 kg or 12 mg/kg for patients less than 30 kg) | ACR Pedi 30% + absence of fever PBO 24% versus TCZ 85% ACR Pedi 50% PBO 11% versus TCZ 85% ACR Pedi 70% PBO 8% versus TCZ 71% In patients with previous anakinra use treated with TCZ: ACR Pedi 30% + absence of fever, 85%, ACR Pedi 70%, 71% In patients with previous TNF inhibitor use treated with TCZ: ACR Pedi 30% + absence of fever, 82%, ACR Pedi 70%, 64% |
De Benedetti et al. [2010] |
ACR Pedi, American College of Rheumatology Pediatric; DBPC, double blind, placebo controlled; IR, inadequate response; PBO, placebo; TCZ, tocilizumab; TNF, tumor necrosis factor.
Macrophage activation syndrome in patients treated with tocilizumab
For around 7% of patients with SJIA, MAS develops in the course of conventional therapies, which include corticosteroids and nonsteroidal anti-inflammatory drugs. As previously reported in patients treated with etanercept [Ramanan and Schneider, 2003] and anakinra [Lurati et al. 2005], MAS was also reported in patients with SJIA under the treatment of tocilizumab [De Benedetti et al. 2010]. In the phase II/III of the trials for tocilizumab in Japan, no patients had the complication of MAS. However, the true incidence of MAS in patients treated with tocilizumab is still difficult to estimate because of the lack of definitive diagnostic criteria. Therefore, the differences in clinical symptoms, signs, and laboratory findings between patients with MAS under the treatment of conventional drugs and biological response modifiers should be further investigated.
During the course of a tocilizumab postmarketing study in Japan, we had two patients who developed MAS. Clinically, the patients with MAS receiving tocilizumab treatment were afebrile and seemed to be in good spirits rather than being ill. This suggests that the clinical features such as fever and sickness behaviors related to IL-6 function were masked by tocilizumab. The findings can be contrasted with MAS in patients under conventional therapy. However, laboratory investigation revealed that platelet counts were abruptly decreased, and serum levels of fibrin/fibrinogen degradation products (FDP, D-dimer) were increased and accompanied by the increased excretion of urinary β2 microglobulin. Early intervention with corticosteroids and cyclosporine was effectively administered. Thus, it seemed that the laboratory findings of patients with MAS treated with tocilizumab were similar to those receiving conventional treatment.
Though the triggering factors of MAS still remain unknown, patients with MAS were successfully treated with corticosteroids to stabilize activated macrophages and endothelial cells, cyclosporine to block the apoptosis pathway by TNFα, and finally plasma exchange to remove overproduced proinflammatory cytokines from circulation. Overall, early diagnosis and treatment are important in saving children with MAS. Taken together, patients with SJIA receiving tocilizumab should be carefully monitored. Also, blood examinations are essential at periodic intervals.
Conclusion
Tocilizumab has been approved as the first biological drug for the treatment of SJIA in Japan, India, the USA, and the EU due to its outstanding efficacy and tolerable safety profile. According to the 2011 ACR recommendations, anakinra is recommended as a first-line biological for the treatment of patients with systemic arthritis and active systemic features, who meet the ILAR criteria for systemic arthritis, and who have an active fever due to SJIA with or without other systemic features, but not active arthritis [Beukelman et al. 2011]. However, the clinical efficacy of anakinra was observed in less than half of the patients with SJIA, with 10 patients showing a dramatic response to anakinra but 11 an incomplete response or no response [Gattorno et al. 2008]. A more recent report of an open-label study of anakinra for 46 children from an international multicenter series indicated that fever and rash resolved within 1 month in over 95% of children, while the CRP and ferritin normalized within this interval in over 80% of patients [Nigrovic et al. 2011]. In view of the response pattern to anti-IL-1 therapy, it has been proposed that SJIA should be stratified into two subgroups [Gattorno et al. 2008; Prakken et al. 2011]. In the TENDER trial tocilizumab had an excellent ameliorative effect for patients who had been intolerant to anakinra [De Benedetti et al. 2010] so tocilizumab is a great potential treatment for SJIA. TNF inhibitors have been found to have a significant effect on the polyarticular form of JIA in randomized controlled trials, but patients with the systemic form show a much less favorable response to anti-TNF treatment. Although the effect of tocilizumab on polyarticular JIA is beyond the scope of this review, it should be noted that it has been approved for the treatment of polyarticular JIA in Japan [Imagawa et al. 2011]. It can be expected that further clinical evaluation of the efficacy, safety, and tolerability of tocilizumab in actual clinical settings or through clinical trials will bring tocilizumab into worldwide use as an innovative drug for the treatment of SJIA. Certainly, it will be urgent to investigate the causes of MAS and to find the biomarkers for prompt diagnosis of this severe complication.
Footnotes
Funding: Supported by the grant of the Program of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation.
Conflict of Interest statement: Shumpei Yokota and Tadamitsu Kishimoto holds a patent for tocilizumab and receives royalties for Actemra. Toshio Tanaka declares that there is no conflict of interest.
Contributor Information
Shumpei Yokota, Department of Paediatrics, Yokohama City University School of Medicine, Yokohama, Japan.
Toshio Tanaka, Department of Respiratory Medicine, Allergy and Rheumatic Diseases, Osaka University Graduate School of Medicine, Osaka, Japan.
Tadamitsu Kishimoto, Laboratory of Immunoregulation, Immunology Frontier Research Center, Osaka University, 8F IFReC Building, 3-1 Yamada-oka, Suita City, Osaka 565-0871, Japan.
References
- Al-Shanti N., Stewart C. (2011) Inhibitory effects of IL-6 on IGF-1 activity in skeletal myoblasts could be mediated by the activation of SOCS-3. J Cell Biochem 113: 923–933 [DOI] [PubMed] [Google Scholar]
- Beukelman T., Patkar N., Saag K., Tolleson-Rinehart S., Cron R., DeWitt E., et al. (2011) 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res 63: 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger M., Chow D., Brevnova E., Garcia K. (2003) Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science 300: 2101–2104 [DOI] [PubMed] [Google Scholar]
- Bradham C., Qian T., Streetz K., Trautwein C., Brenner D., Lemasters J. (1998) The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol 18: 6353–6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti F., Alonzi T., Moretta A., Lazzaro D., Costa P., Poli V., et al. (1997a) Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-1: a model for stunted growth in children with chronic inflammation. J Clin Invest 99: 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti F., Brunner H., Ruperto N., Calvo N., Cuttica I., Malattia R., et al. (2010) Tocilizumab in patients with systemic juvenile idiopathic arthritis: efficacy data from the placebo-controlled 12-week part of the phase 3 TENDER trial. Arthritis Rheum 62(Suppl. 10): 1434 [Google Scholar]
- De Benedetti F., Brunner H., Ruperto N., Schneider R., Woo P., Burgos-Vargas R., et al. (2011) Efficacy and safety of tocilizumab (TCZ) in patients with systemic juvenile idiopathic arthritis (SJIA): tender 52-week data. Pediat Rheumatol 9(Suppl. 1): P164 [Google Scholar]
- De Benedetti F., Martini A. (2005) Targeting the interleukin-6 receptor: a new treatment for systemic juvenile idiopathic arthritis? Arthritis Rheum 52: 687–693 [DOI] [PubMed] [Google Scholar]
- De Benedetti F., Massa M., Pignatti P., Albani S., Novick D., Martini A. (1994) Serum soluble interleukin 6 (IL-6) receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile idiopathic arthritis. J Clin Invest 93: 2114–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti F., Massa M., Robbioni P., Ravelli A., Burgio G., Martini A. (1991) Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum 34: 1158–1163 [DOI] [PubMed] [Google Scholar]
- De Benedetti F., Pignatti P., Gerloni V., Massa M., Sartirana P., Caporali R., et al. (1997b) Differences in synovial fluid cytokine levels between juvenile and adult rheumatoid arthritis. J Rheumatol 24: 1403–1409 [PubMed] [Google Scholar]
- Fishman D., Faulds G., Jeffery R., Mohamed-Ali V., Yudkin J., Humphries S., et al. (1998) The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 102: 1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Nemeth E. (2011) Hepcidin and disorders of iron metabolism. Annu Rev Med 62: 347–360 [DOI] [PubMed] [Google Scholar]
- Gattorno M., Piccini A., Lasiglie D., Tassi S., Brisca G., Carta S., et al. (2008) The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 58: 1505–1515 [DOI] [PubMed] [Google Scholar]
- Giannini E., Ruperto N., Ravelli A., Lovell D., Felson D., Martini A. (1997) Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 40: 1202–1209 [DOI] [PubMed] [Google Scholar]
- Grom A., Mellins E. (2010) Macrophage activation syndrome: advances towards understanding pathogenesis. Curr Opin Rheumatol 22: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara K., Nishikawa T., Sugamata Y., Song J., Isobe T., Taga T., et al. (2005) Essential role of STAT3 in cytokine-driven NF-kappaB-mediated serum amyloid A gene expression. Genes Cells 10: 1051–1063 [DOI] [PubMed] [Google Scholar]
- Heinrich P., Castell V., Andus T. (1990) Interleukin-6 and the acute phase response. Biochem J 265: 621–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlin T. (2009) Tocilizumab: the evidence for its place in the treatment of juvenile idiopathic arthritis. Core Evid 4: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. (1990) Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63: 1149–1157 [DOI] [PubMed] [Google Scholar]
- Imagawa T., Yokota S., Mori M., Miyamae T., Takei S., Imanaka H., et al. (2011) Safety and efficacy of tocilizumab, an anti-IL-6-receptor monoclonal antibody, in patients with polyarticular-course juvenile idiopathic arthritis. Mod Rheumatol 22: 109–115 [DOI] [PubMed] [Google Scholar]
- Inaba Y., Ozawa R., Imagawa T., Mori M., Hara Y., Miyamae T., et al. (2011) Radiographic improvement of damaged large joints in children with systemic juvenile idiopathic arthritis following tocilizumab treatment. Ann Rheum Dis 70: 1693–1695 [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Kimura H., Shikama Y., Uchida T., Kariyone S., Hirano T., et al. (1989) Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood 74: 1241–1244 [PubMed] [Google Scholar]
- Kimura A., Kishimoto T. (2010) IL-6: regulator of Treg/Th17 balance. Eur J Immunol 40: 1830–1835 [DOI] [PubMed] [Google Scholar]
- Kishimoto T. (1989) The biology of interleukin-6. Blood 74: 1–10 [PubMed] [Google Scholar]
- Kishimoto T. (2005) Interleukin-6: from basic science to medicine-40 years in immunology. Annu Rev Immunol 23: 1–21 [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. (1994) Cytokine signal transduction. Cell 76: 253–262 [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. (2009) IL-17 and Th17 cells. Annu Rev Immunol 27: 485–517 [DOI] [PubMed] [Google Scholar]
- Kotake S., Sato K., Kim K., Takahashi N., Udagawa N., Nakamura I., et al. (1996) Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res 11: 88–95 [DOI] [PubMed] [Google Scholar]
- Lang B., Schneider R., Reilly B., Silverman E., Laxer R. (1995) Radiographic features of systemic onset juvenile idiopathic arthritis. J Rheumatol 22: 168–173 [PubMed] [Google Scholar]
- Levi M., Keller T., van Gorp E., ten Cate H. (2003) Infection and inflammation and the coagulation system. Cardiovasc Res 60:26–39 [DOI] [PubMed] [Google Scholar]
- Lin Y., Wang C., Gershwin M., Chiang B. (2011) The pathogenesis of oligoarticular/polyarticular vs systemic juvenile idiopathic arthritis. Autoimmun Rev 10: 482–489 [DOI] [PubMed] [Google Scholar]
- Lurati A., Teruzzi B., Salmaso A., et al. (2005) Macrophage activation syndromeduring anti-IL-1 receptor therapy (anakinra) in a patient affected by systemic onset idiopathic juvenile arthritis. Paed Rheumatol Online J 3: 79–85 [Google Scholar]
- Mellins E., Macaubas C., Grom A. (2011) Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more question. Nat Rev Rheumatol 7: 416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K., Herner E., Stagg A., Bendtzen K., Woo P. (1998) Inflammatory cytokines and cytokine antagonists in whole blood cultures of patients with systemic juvenile chronic arthritis. Rheumatology (Oxford) 37: 562–569 [DOI] [PubMed] [Google Scholar]
- Murakami M., Hibi M., Nakagawa N., Nakagawa T., Yasukawa K., Yamanishi K., et al. (1993) IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260: 1808–1810 [DOI] [PubMed] [Google Scholar]
- Nakajima S., Naruto T., Miyamae T., Imagawa T., Mori M., Nishimaki S., et al. (2009a) Interleukin-6 inhibits early differentiation of ATDC5 chondrogenic progenitor cells. Cytokine 47: 91–97 [DOI] [PubMed] [Google Scholar]
- Nakajima S., Naruto T., Miyamae T., Imagawa T., Mori M., Nishimaki S., et al. (2009b) Improvement of reduced serum cartilage oligomeric matrix protein levels in systemic juvenile idiopathic arthritis patients treated with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Mod Rheumatol 19: 42–46 [DOI] [PubMed] [Google Scholar]
- Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B., et al. (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic P., Mannion M., Prince F., Zeft A., Ravinovich C., van Rossum M., et al. (2011) Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis-report of forty-six patients from an international multicenter series. Arthritis Rheum 63: 545–555 [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Hagihara K., Serada S., Isobe T., Matsumura A., Song J., et al. (2008) Transcriptional complex formation of c-Fos, STAT3, and hepatocyte NF-1 alpha is essential for cytokine-driven C-reactive protein gene expression. J Immunol 180: 3492–3501 [DOI] [PubMed] [Google Scholar]
- Nistala K., Wedderburn L. (2009) Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology 48: 602–606 [DOI] [PubMed] [Google Scholar]
- Obici L., Perfetti V., Palladini G., Moratti R., Merlini G. (2005) Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta 1753: 11–22 [DOI] [PubMed] [Google Scholar]
- Ogilvie E., Fife M., Thompson S., Twine N., Moroldo M., Fisher S. et al. (2003) The –174G allele of the interleukin-6 gene confers susceptibility to systemic arthritis in children: a multicenter study using simplex and multiplex juvenile idiopathic arthritis families. Arthritis Rheum 48: 3202–3206 [DOI] [PubMed] [Google Scholar]
- Palmqvist P., Persson E., Conaway H., Lerner U. (2002) IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activation of NF-kappa B in mouse calvariae. J Immunol 169: 3353–3362 [DOI] [PubMed] [Google Scholar]
- Petty R., Southwood T., Manners P., Baum J., Glass D., Goldenberg J., et al. (2004) International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31: 390–392 [PubMed] [Google Scholar]
- Pignatti P., Vivarelli M., Meazza C., Rizzolo M., Martini A., De Benedetti F. (2001) Abnormal regulation of interleukin 6 in systemic juvenile idiopathic arthritis. J Rheumatol 28: 1670–1676 [PubMed] [Google Scholar]
- Prakken B., Albani S., Martini A. (2011) Juvenile idiopathic arthritis. Lancet 377: 2138–2149 [DOI] [PubMed] [Google Scholar]
- Ramanan A., Grom A. (2005) Does systemic-onset juvenile idiopathic arthritis belong under juvenile idiopathic arthritis? Rheumatology (Oxford) 44: 1350–1353 [DOI] [PubMed] [Google Scholar]
- Ramanan A., Schneider R. (2003) Macrophage activation syndrome following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis. J Rheumatol 30:401–403 [PubMed] [Google Scholar]
- Rooney M., David J., Symons J., Di Giovine F., Varsani H., Woo P. (1995) Inflammatory cytokine responses in juvenile chronic arthritis. Br J Rheumatol 34: 454–460 [DOI] [PubMed] [Google Scholar]
- Sato K., Tsuchiya M., Saldanha J., Koishihara Y., Oshugi Y., Kishimoto T., et al. (1993) Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res 53: 851–856 [PubMed] [Google Scholar]
- Sawhney S., Woo P., Murray K. (2001) Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child 85: 421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still G. (1941) On a form of chronic joint disease in children. Arch Dis Child 16: 156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. (2012) Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol 52: 199–219 [DOI] [PubMed] [Google Scholar]
- Tran T., Eubanks S., Schaffer K., Zhou C., Linder M. (1997) Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood 90: 4979–4986 [PubMed] [Google Scholar]
- Woo P., Wilkinson N., Prieur A., Southwood T., Leone V., Livermore P., et al. (2005) Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther 7: R1281–R1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., et al. (1988) Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science 241: 825–828 [DOI] [PubMed] [Google Scholar]
- Yokota S., Miyamae T., Imagawa T., Iwata N., Katakura S., Mori M. (2004) Inflammatory cytokines and systemic-onset juvenile idiopathic arthritis. Mod Rheumatol 14: 12–17 [DOI] [PubMed] [Google Scholar]
- Yokota S., Imagawa T., Miyamae T., Kasai K., Mori M., Nishimoto N., et al. (2008b) Long-term safety and efficacy of tocilizumab in children with systemic juvenile idiopathic arthritis. Pediat Rheumatol 6(Suppl. 1): S1 [Google Scholar]
- Yokota S., Imagawa T., Mori M., Miyamae T., Aihara Y., Takei S., et al. (2008a) Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 371: 998–1006 [DOI] [PubMed] [Google Scholar]
- Yokota S., Imagawa T., Takei S., Murata T., Tomiita M., Itoh Y., et al. (2011) Guideline on using tocilizumab for juvenile idiopathic arthritis. Mod Rheumatol 2011: 20 May (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Yokota S., Kishimoto T. (2010) Tocilizumab: molecular intervention therapy in children with systemic juvenile idiopathic arthritis. Expert Rev Clin Immunol 6: 735–743 [DOI] [PubMed] [Google Scholar]
- Yokota S., Miyamae T., Imagawa T., Iwata N., Katakura S., Mori M., et al. (2005) Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 52: 818–825 [DOI] [PubMed] [Google Scholar]