Abstract
Therapeutic monoclonal antibodies (mAbs) that target the CD20 antigen on B cells are successfully used in the clinic for the depletion of B cells to treat various forms of cancer and autoimmune diseases. The first CD20 mAb, approved by the FDA in 1998, was rituximab (RTX) and since then it has been widely used to treat more than one million patients thus far. The success of RTX has led to a general interest in the mechanism of action of CD20 mAbs. CD20 mAbs can induce tumor killing via various mechanisms, such as direct induction of apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent lysis (CDC). Although we now understand these mechanisms better, it is still unclear which of these mechanisms is the most important for in vivo RTX action. Not every patient respond to RTX treatment and eventually the overwhelming majority will experience a relapse. Therefore, there is an urgent need to improve the efficacy of CD20 mAbs. This review aims to summarize our current understanding on the mechanism of action of CD20 mAbs.
Keywords: Antibodies, CD20, effector mechanisms, Fc receptors, complement, complement receptors, apoptosis
CD20 and CD20 antibodies
CD20 is a general B cell marker that is expressed during B cell differentiation from the pro-B cell phase until the plasma cell stadium. The physiological ligand and exact biological function of CD20 is currently unknown. In vitro studies propose a role for CD20 as a store operated Ca++ channel [1]. Initial analysis of CD20 deficient mice showed no immune-deficient phenotype [2,3], however, more recently CD20 deficient mice and humans were found to exhibit decreased T-independent immune responses [4]. Largely fuelled by the interest in CD20 as a tumor target, research on CD20 biology has intensified in the recent years. The current knowledge on CD20 biology is summarized in an excellent review by Cragg et al [5].
The chimeric mAb rituximab (RTX, Roche / Genentech) was the first FDA-approved CD20 mAb. It is approved to treat CD20 positive B cell malignancies; e.g. non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL), and for some autoimmune diseases, including rheumatoid arthritis. RTX is also being used in several other autoimmune diseases, such as systemic lupus erythematosus (SLE) or multiple sclerosis [6]. In oncology indications, RTX is used in combination with chemotherapy [7]. The fully human CD20 mAb ofatumumab (OFA, Genmab / GSK) was approved by the FDA in 2009 for the treatment of CLL patients resistant for both alemtuzumab and fludarabine [8]. Next to the two unconjugated CD20 mAbs there are two radio-conjugated CD20 mAbs on the market used as part of radio-immunotherapy. Ibritumomab tiuxetan (Zevalin) is a CD20 mAb coupled with the radioactive isotope yttrium-90 or indium-111. I-131 Tositumumab (Bexxar) is a iodine-131-labeled CD20 mAb, based on the mouse CD20 mAb B1 and is used to treat follicular lymphoma (FL) patients [9,10]. This review will focus on the mechanisms of action of unconjugated CD20 mAbs.
Mechanism of action of CD20 mAbs
Unconjugated CD20 mAbs can exert anti-tumor effect via Fab-mediated and Fc-mediated effects that involve the activation of immune effector mechanisms Figure 1. CD20 mAbs can be grouped into type I and type II based on their ability to induce the reorganization of CD20 molecules into lipid rafts upon binding [11]. Type I CD20 mAbs induce the reorganization of CD20 molecules into lipid rafts and efficiently activate the classical pathway of the complement system. In contrast, type II CD20 mAbs poorly activate complement, but are capable to directly induce cell death upon binding to CD20 without cross-linking by secondary Abs. Both types are capable of inducing antibody dependent cell-mediated cytotoxicy (ADCC) in the presence of effector cells.
Figure 1.
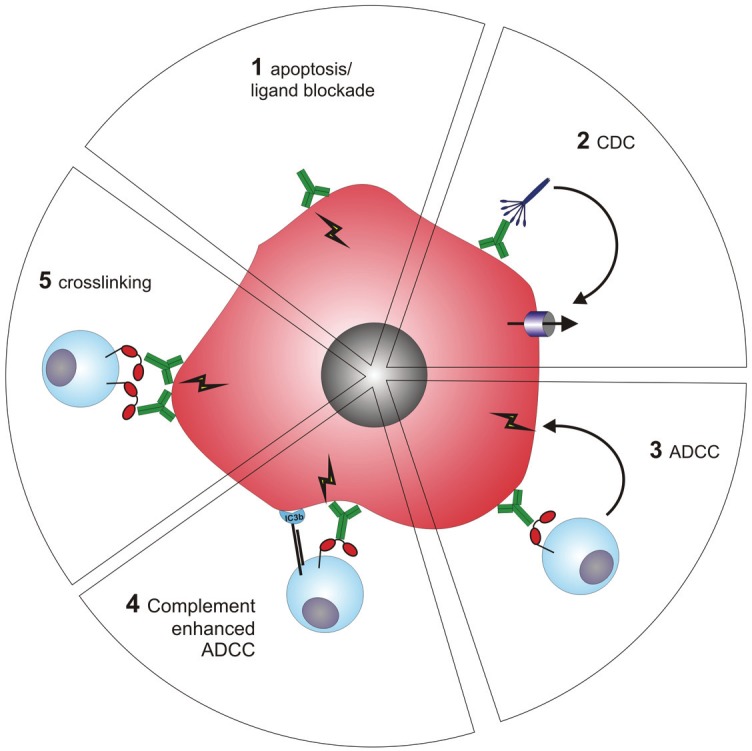
Mechanisms of action of therapeutic CD20 mAbs. CD20 mAbs can induce tumor killing in several ways. A. Direct binding of CD20 mAbs initiate the crosslinking of multiple CD20 molecules, resulting in cell-death via induction of non-classical apoptosis; B. Activation of complement result in complement-dependent cytotoxicity; C. recognition of opsonized tumor cells by FcγRs expressed on immune effector cells initiate antibody dependent cell-mediated cytotoxicity; D. FcγR may only serve as crosslinking platform and thereby enhance antigen signaling in the tumor cells; E. Ab-initiated complement activation yields to deposition of complement cleavage fragments, which may enhance tumor killing through recognition by complement receptors (CRs) in a process called complement-enhanced ADCC.
Antibody dependent cell-mediated cytotoxicity
A large body of evidence, both from pre-clinical and clinical studies, supports a role for Fc receptors during RTX therapy suggesting that ADCC is an important mechanism of action of RTX.
Fcγ receptors (FcγRs) are surface receptors for IgG and are broadly expressed on leukocytes and varying affinity to the different IgG subclasses (Table 1). Functionally they can be grouped into activating and inhibiting receptors depending on the nature of the signal they induce after receptor crosslinking by IgG. Most activating FcγRs associate with the signal transducing γ-chain (FcRγ-chain) [12]. FcRγ-chain is also required for surface expression, illustrated by the lack of surface expression of all activating FcγRs in FcRγ-chain knockout (KO) mice [13]. Cellular activation upon crosslinking of the receptors by IgG-IC is induced by the immunoreceptor tyrosine-based activation motifs (ITAM) that are located on the intracellular part of the FcRγ-chain. The inhibitory FcγRIIb is a single chain molecule that inhibits cellular activation when co-engaged with activating FcγRs. The inhibitory signal is transmitted through the immunoreceptor tyrosine-based inhibiton (ITIM) motif that is located on the intracellular part of the receptor [12].
Table 1.
Properties of human FcγRs
| FcγRI | FcγRIIa | FcγRIIb | FcγRIIc | FcγRIIIa | FcγRIIIb | |
|---|---|---|---|---|---|---|
| Gene | FCGR1A | FCGR2A | FCGR2B | FCGR2C | FCGR3A | FCGR3B |
| SNPs1 | H131R | I232T | V158F | NA1, NA2, SH | ||
| CNV | No | No | No | Yes | Yes | Yes |
| Affinity | High | Low to medium | Low to medium | Low to medium | Low to medium | Low to medium |
| Ligand preference | IgG1 = IgG3 > IgG4 | H131: IgG1 > IgG2 = IgG4 R131: IgG1 > IgG3 > IgG4 > IgG2 | IgG4 = IgG3 = IgG1 > IgG2 | IgG4 = IgG3 = IgG1 > IgG2 | V158: IgG3 > IgG1 > IgG4 > IgG2 F158: IgG3 > IgG1 > IgG4 > IgG2 | NA1/ NA2/ SH: IgG3 > IgG1 |
| Expression pattern | Macrophages, monocytes, PMNs, eosinophils, dendritic cells | Macrophages, PMNs, eosinophils, dendritic cells, platelets | B cells, mast cells, basophils, macrophages, eosinophils, PMNs, dendritic cells | Monocytes, PMNs, NK cells | Macrophages, monocytes, NK cells, mast cells, eosinophils, dendritic cells | PMNs, eosinophils |
| Type | Activating | Activating | Inhibiting | Activating | Activating | Activating (GPI-linked)2 |
| Signaling | FcRγ-chain ITAM | Own ITAM | Own ITIM | Own ITAM | FcRγ-chain ITAM | Via FcγRIIa or CR3 |
Only those SNPs are listed that directly influence IgG binding. In case of FCGR2C, the FCGR2C-ORF genotype is due to a G > C mutation at position -386 that results in functional expression of FcγRIIc.
FcγRIIIb is a GPI-linked membrane protein that is shown to signal via FcγRIIa and / or CR3.
There is both pre-clinical and clinical evidence that the interaction of the Ab Fc part with cellular receptors is important for the activity of therapeutic CD20 mAbs. Studies in mice demonstrated that CD20 mAb therapy is no longer effective in FcRγ-chain KO mice [14-18]. In contrast to FcRγ-chain KO mice, CD20 mAb therapy was enhanced in mice lacking the inhibitory FcγRIIb in both a xenograft and a syngenic tumor model [14,16,17]. In other studies, however, using different tumor models, the inhibitory role of FcγRIIb was not confirmed [18]. This discrepancy most likely caused by the different characteristics of the animal models and the number of FcγRIIb+ effector cells at the tumor site. In mouse tumor models, the IgG2a isotype was found to be the most potent in depleting both normal and malignant B cells. IgG2a efficiently engages both activating FcγRI and FcγRIV [15], and has the most favorable A/I ratio [19]. Expression of the intermediate affinity FcγRIV alone was sufficient for the elimination of low tumor burden, whereas elimination of high tumor burden required the presence of all activating FcγR: FcγRI, FcγRIII and FcγRIV [17].
The role of FcγR in the RTX therapy is further supported by clinical association studies performed in RTX-treated individuals. Several single nucleotide polymorphisms (SNP) are described in the genes encoding the low affinity FcγR (FCGR2A, FCGR2B, FCGR2C, FCGR3A, FCGR3B). These SNPs either directly influence ligand binding or are located in the promoter region and alter receptor expression [20]. The SNP in FCGR2C results in the expression of functional FcγRIIc in 18% of healthy individuals [21]. More recently copy number variation (CNV) have also been described in FCGR2C, FCGR3A and FCGR3B [20,22]. However, genetic association studies in the complex FcγR region are complicated by the high homology of the FcγR genes.
The different FcγRs have different affinity to IgG subclasses (Table 1). In humans, for instance, both IgG1 and IgG3 interact well with all FcγRs and therefore these are potent subclasses capable of efficiently inducing effector functions. IgG2, on the other hand, binds predominantly to FcγRIIa with a lower affinity [23].
Polymorphisms in FcγRs genes can influence the affinity for IgG. FcγRIIIa has two allelic variant that results either in a valine (V) or a phenylalanine (F) at position 158. The V158 allotype binds IgG1 and IgG3 with an increased affinity compared to the F158 allotype [24,25]. The presence of a homozygous V158 allele in RTX-treated NHL patients was shown to associate with longer progression free survival [26,27]. An SNP in FCGR2A results in an amino acid substitution at position 131 arginine (R) to histidine (H). The H131 variant is able to bind IgG2 [28]. In certain studies this SNP is also associated with RTX therapy [27], which is likely caused by linkage disequilibrium between the FCGR3A and FCGR2A genes. In CLL however, FcγR polymorphisms did not associate with response with RTX treatment alone [29] or in combination with chemotherapy [30]. The polymorphisms I232T in FCGR2B encoding the inhibitory FcγRIIb influence signaling capability through exclusion of FcγRIIb from lipid rafts [31]. This polymorphism is convincingly shown to be associated with SLE and with parasite infections in humans [32]. Interestingly, FCGR2B I232T polymorphisms does not associate with RTX therapy in FL [33]. Although several reports have shown that CNV in FcγRs associate with inflammatory autoimmune diseases [21,22,34-36], no CNV in FcγR genes were correlated with outcome of RTX treatment so far.
The nature of the effector cells mediating ADCC
FcγR are expressed on several immune effector cell populations. In ex vivo ADCC assays using isolated human FcγR positive effector cells are all able to induce the lysis or phagocytosis of CD20 positive tumor cells in the presence of CD20 mAbs [26,37,38]. It is however less clear which effector cell population contributes to in vivo efficacy of CD20 mAbs.
Natural killer (NK) cells are potent effector cell population and able to induce tumor cell lysis at low (1:1) effector-to-target (E:T) ratios [39]. The strong association of RTX efficacy with polymorphism in FcγRIIIa, the only FcγR expressed on NK cells, implies a role for this effector cell population [27]. In line with this, stimulating NK cells with an anti-CD137 mAbs in vitro and in vivo results in enhanced RTX-dependent cytotoxicity [40]. Mouse studies, however, have not found evidence for the role of NK cells [15,41,42]. This may be due to the low expression of FcγRIII on mouse NK cells [43]. The observation in mice that FcγRIIb modulates RTX activity in vivo suggest that the effector cell type coexpresses both activating and inhibiting FcγR, which questions the role of NK cells. It is also important to note that in vitro ADCC assays using human NK cells represent an allogeneic setting and therefore likely overestimate the cytotoxic capacity of NK cells [44].
Polymorphonuclear granulocytes (PMNs) are the most populous FcR positive effector cell type in the blood and their role in mAb therapy is often overlooked. Human PMNs are able to induce efficient cytotoxicity in vitro by IgG1 or by IgG2 anti-tumor Abs [45,46]. PMNs were shown to be contribute in vivo to RTX action against B cell lymphoma cells in SCID mice [47]. However, in a mouse model of B cell depletion using human CD20 transgenic mice no role was found for PMNs [41]. This was confirmed by an endogenous B cell depletion model using anti-mouse CD20 Abs in wild type mice [48]. In contrast to mouse PMNs, human PMNs express FcγRIIa, which may result in underestimation of the role of PMNs in mouse studies.
Monocytes / macrophages have been shown to be important for B cell depletion in various mouse model [17,41]. Our results obtained with a short term peritoneal tumor model, confirmed the predominant role for macrophages [18]. It is still not fully clear in which organ the phagocytic cells reside that mediate depletion of B cells. Interestingly, not splenectomy but compromised blood flow through the liver affected CD20 mAb efficacy [41,48]. Recently, an important role has been suggested for resident (non-classical) LyC6low monocytes in B cell depletion [48]. Furthermore, tumor cells were found to be associated in vivo with macrophages and PMNs during mAb therapy in mice [42].
Most likely the contribution of phagocytic cells to B cell depletion in the different organs is strongly influences by the nature of the tumor or the subset of the B cells that is targeted. Circulatory dynamics of normal - and most likely also of malignant - B cells is also important since circulating B cells are more sensitive to depletion, whereas MZ B cells or peritoneal B1 B cells are more resistant for depletion [41]. When they are mobilized by anti-integrin antibodies they are also efficiently depleted, showing that there is a need to access the circulation [41]. In line with this, blocking CD47 function using an anti-CD47 Ab in a human NHL xenograft model was shown to synergize with RTX action and this was dependent on macrophages and not on NK cells [49]. Macrophages are also found recruited to the tumor site, however, whether they beneficial or not is still a matter of debate [50,51].
Taken together, there is convincing evidence from both preclinical and clinical association studies for a role for FcγR during RTX therapy. Based on mouse studies, FcγR-expressing monocytes and / or macrophages seem to be most important effector cells. However, it is clear that FcγR-mediated effector mechanisms do not always explain clinical RTX efficacy, indicating a role for other effector mechanisms.
Complement activation
The role of complement in RTX therapy is rather controversial with available data showing beneficial, neutral and even detrimental effects [52]. RTX efficiently binds C1q, activates complement in vitro and induce complement-dependent cytotoxicity (CDC) in lymphoma cell lines and primary tumor cells [11,53].
A number of studies suggest a beneficial role for complement during RTX therapy, often based on the detection of tumor evasion mechanisms from complement-mediated killing. Complement-regulatory proteins (CRP) are frequently observed on circulating tumor cells and complement resistance from complement-mediated lysis of these tumor cells in vitro was dependent on the expression of CD55 and CD59 [54]. Overexpression of the CRPs CD55 and CD59 is shown to correlate with resistance in B cell lymphoma cell lines in vitro [55-57]. In vitro and in vivo neutralization of CRPs by Abs increases complement mediated tumor kill and overcomes complement resistance [58,59]. Furthermore, complement is rapidly depleted after RTX infusion in CLL patients [60] or in mouse models [17]. In line with this, injection of complement-active fresh frozen plasma enhanced B cell depletion [61,62]. In a mouse study using a syngeneic tumor model expressing human CD20, the first component of the classical complement pathway, C1q, was essential for RTX therapy [63]. Using the same tumor cells we have found that type I CD20 mAbs require complement for elimination of both low and large tumor burden [18].
Other studies, however, question the involvement the complement. In a pre-clinical model for normal or malignant B cell depletion using cobra venom factor (CVF)-depleted mice, or mice deficient for complement factors C1q, C3 or C4 no role for complement was found [15,17]. Interestingly, in a model using human CD20 transgenic mice CVF treatment affected the depletion of MZ B cells but not circulating B cells [41]. It appears that complement has no role in fully syngeneic mouse models, where the CD20 mAbs used do not cross-react with the normal host B cell reservoir [64].
In humans, expression of CRPs on tumor cells did not predict clinical outcome in FL [65] or in CLL [66]. Furthermore, it was proposed that that complement activation inhibits ADCC by NK cells as a results of deposited iC3b on tumor cells that blocks binding of IgG to FcγRIIIa [67]. This was also confirmed in an in vivo model [68]. In line with this, a polymorphism in C1qA associated with low C1q levels correlated with better response to RTX therapy in FL [69]. The most often observed adverse effects during mAb therapy is infusion reactions (fever and / or chill and skin rashes etc), which is most pronounced at the first infusion may be attributed to complement activation [70].
Next to inducing direct tumor lysis by CDC, complement may play additional roles during RTX therapy. Deposited complement fragments (C3b(i)) can be detected upon incubation of CLL cells with RTX ex vivo [71] and also on tumor cells in mouse models [18]. These fragments can serve as ligands for complement receptors (CRs) expressed on effector cells, such as macrophages. Binding of iC3b to CR3 alone can result in direct complement-dependent cellular cytotoxicity (CDCC). This mechanism requires CR3 to be preactivated and therefore unlikely to contribute to the mAb therapy under normal circumstances [72].
There is interplay between FcγR and complement. During complement activation small cleavage fragments C3a and C5a are generated that function as anaphylatoxins to further recruit effector cells to the site of the tumor. Binding of C5a to C5aR on local macrophages shifts the balance of activating and inhibitory FcγR expression resulting in activated state [73]. Moreover, CR3 binding to iC3b ligands can also serve to amplify ADCC. mAb therapy of large tumor burden by type I CD20 mAbs was dependent on CR3 expression in a peritoneal tumor model. In line with this, we have found that in in vitro macrophage-mediated killing assays iC3b-CR3 interactions tipped the balance of killing at suboptimal E:T ratios [18].
The second FDA-approved unconjugated CD20 mAb OFA activates complement more efficiently than RTX, presumably because it binds a unique, membrane proximal epitope compared to RTX [55,74]. OFA is able to lyse even RTX-resistant Raji cell line expressing low levels of CD20 [74], primary tumor cell lines resistant to RTX in vitro [55] or in a mouse model of human lymphoma [75]. Whether this better complement activation translates into superior clinical efficacy in head-to-head trials with RTX remains to be seen.
Taken together, the role of complement is currently controversial and requires further investigations. It is likely that next to CD20 expression levels and CRP expression levels on the tumor cells, circulatory dynamics of normal or malignant B cells, their anatomical location or tumor burden, together influence the involvement of complement in CD20 therapy.
Apoptosis induced via cross-linking of RTX by FcR-expressing cells
It has been suggested that RTX induce apoptosis of malignant B cells, based on increased apoptosis in tumor cells from CLL patients treated with RTX compared to untreated patients [76]. This was supported by in vitro studies; where RTX was cross-linked by a secondary Ab or by FcR-expressing cells [77,78]. It is therefore possible that in vivo FcR-expressing cells would provide cross-linking stimulus similar to secondary Abs in vitro inducing cell death in the tumor cell. It has been shown that the in vivo activity of the agonistic CD40 mAb depends on cross-linking provided by FcγRIIb demonstrating that cross-linking by FcγRs can be an important mechanism of action of mAb therapy [79,80]. For CD20 mAb therapy, expression of FcγRs are needed as shown by FcRγ-chain KO mice, but whether this is required for active signaling or whether FcγR are purely serve as a scaffold to provide cross-linking was unclear. To investigate if cross-linking by FcγR in vivo possibly contributes to the mechanism of action of CD20 mAb, a novel transgenic mouse strain (NOTAM) was generated. NOTAM mice carry a mutation in the ITAM of the FcRγ-chain and have normal surface expression of activating FcγR [81]. Using the NOTAM mice we demonstrated that cross-linking by FcR does not contribute to in vivo action of type I CD20 mAbs [81]. Our finding was confirmed in a different model using BJAB tumor cells and mice that only express FcγRIIb [82]. Therefore current data suggest that cross-linking by FcγR does not substantially contribute to the in vivo mechanism of action of CD20 mAb.
Fab-mediated direct induction of cell death
In contrast to type I, type II CD20 mAb or tositumumab-like reagents are able to induce direct cell death upon binding to CD20 [83,84]. This is independent of the Fc part of the Ab but requires F(ab)2 fragments and the binding of at least two CD20 molecules [85,86]. The induction of programmed cell death (PCD) follows a mechanism that is independent of BCL-2 and caspases but requires lysosomal enzymes [83,87]. More recently it was shown that cell-death evoked by type II CD20 mAbs requires a novel reactive oxygen species-dependent pathway [88].
The prototypic type II CD20 mAb B1 is shown to exert superior effects when compared with type I CD20 mAbs in preclinical model of B cell depletion [37]. This was confirmed with GA101 (obinutuzumab), another clinical candidate type II CD20 mAb in a xenograft tumor model as monotherapy [89] or in combination with chemotherapy [90]. GA101’s ability to induce apoptosis and its Fc-independent mode of action has potentially important advantage over RTX in circumventing tumor cell resistance to apoptosis or avoid influence of exhaustion of FcR-bearing effector cells.
Induction of long-term anti-tumor immunity
Next to the direct anti-tumor effects, CD20 mAb may induce long-term anti-tumor immunity, which could be the underlying reason why some patients experience durable tumor regression [91]. Ab-opsonized tumor cells may be phagocytosed by tissue resident dendritic cells (DCs) that can induce the generation of tumor-specific cytotoxic T cells [92]. This idea is supported by the observation that in patients, clinical tumor shrinkage is only visible after one week after the first infusion. Pre-clinical evidence shows that CD20 mAbs induce protective anti-tumor responses against human CD20-expressing tumors in immunocompetent mice [93]. Immune complexes (ICs) that are formed during CD20 mAb therapy are potent activators of DCs and could induce anti-tumor immunity [94-96]. ICs are effectively taken up by DCs via activating FcγRs, which leads to potent stimulation of DCs culminating in cross-presentation of tumor-derived antigens [96]. Therefore it cannot be excluded that the association between FcγR polymorphisms in RTX-treated patients reflects a role for FcγR on DCs may be involved in inducing anti-tumor immunity [92]. Currently, chemotherapy is given together with CD20 mAbs. Preclinical studies with herceptin showed that chemotherapy may impair the developing tumor-specific T cell response [97]. This may also be true for CD20 mAb therapy, suggesting that altering the treatment schedule could allow the generation of adaptive anti-tumor responses.
Synergy of effector mechanisms
It is likely that in vivo a combination of effector mechanism is involved in anti-tumor action of CD20 mAbs. The dominant effector mechanism may change during the course of the therapy and perhaps also differ between different anatomical locations. For instance, circulating tumor cells can be eliminated by complement in blood or via the phagocytic cells of the liver or spleen. Killing of a non-circulating tumor cell in an anatomical compartment, where complement levels are relatively low, may rely on other mechanisms.
Tumor burden may also impact effector mechanisms of CD20 mAbs. We showed in a short peritoneal tumor model that complement activation is sufficient for the elimination of low tumor burden. However, elimination of high tumor burden by CD20 mAb requires a concerted action of all effector mechanisms, because lack of FcγR, complement or CR3 expression fully abrogated therapy [18].
RTX and chemotherapy
RTX works synergistically with chemotherapy in the clinic [98]. The mechanism likely underlying this synergy is that RTX sensitizes lymphoma cells for chemotherapy by downregulating antiapoptotic proteins [99-101]. Using a type II CD20 mAb GA101, it was shown in vitro that CD40 stimulation of peripheral CLL cells sensitizes tumor cells for direct cell death induction [102]. These results suggest that combination of cytotoxic drugs with CD20 mAb can be beneficial.
Resistance to RTX therapy
The majority of the cancer patients eventually become refractory to RTX treatment. There are several mechanisms by which this can happen, including increased Ab catabolism, selection of tumor cells with low-levels of surface CD20 expression, resistance to mAb-effector mechanisms or impaired immune cell function [103]. An important escape mechanism is the loss of CD20 from tumor cells after RTX treatment [104]. It was recently shown that type I CD20 mAbs induce internalization of CD20 by tumor cells. The degree of internalization is higher with type I than with type II CD20 mAbs [105]. The internalization requires the binding of the Fc tail of RTX to FcγRIIb on the same tumor cell. In support of this, FcγRIIb expression on different forms of B cell malignancies was correlated with CD20 internalization and with resistance to RTX therapy [106].
CD20 can also be removed from the surface of the tumor cells in a process initially described as shaving [60,107]. Shaving of CD20 follows the cellular mechanisms of trogocytosis. Trogocytosis was first described during antigen presentation between T cells and DCs and involves transfer of cell membrane between two cells during immunological synapse formation. Similarly, cytotoxic synapses are formed during ADCC and CD20 molecules are transferred from the surface of the tumor cells to the effector cells and subsequently internalized. Trogocytosis of CD20 results in CD20-negative B cells, which are therefore resistant to CD20 mAb therapy. Trogocytosis of CD20 requires Fc receptors, such as human FcγRI [107]. We showed in a mouse model that all mouse FcγR, both activating and inhibiting FcγR are able to mediate trogocytosis of CD20 [108]. Whether internalization or trogocytosis of CD20 is more relevant in vivo mechanism for CD20 mAb therapy will need to be further investigated. A recent report suggest, however, that trogocytosis occurs faster then internalization [109].
Novel CD20 mAbs
Second generation CD20 mAbs carry a humanized or fully human and are expected to be less immunogenic than the chimeric Ab RTX [110]. Examples are ofatumumab, the humanized ocrelizumab [111] and veltuzumab [112]. Ofatumumab (OFA, Arzerra) was the second unconjugated CD20 mAb that has been approved by the FDA [113]. OFA is a fully human CD20 mAb recognizes an overlapping epitope on the small and big extracellular loop of CD20, which results in better complement activation than RTX [55,114]. Veltuzumab (IMMU-106, hA20) is a humanized CD20 mAb that carry similar variable sequences to RTX but expected to induce stronger CDC than RTX [110]. Ocrelizumab (Genentech / Roche) is a humanized CD20 mAb with increased binding affinity for the low-affinity variants of the FcγRIIIa receptor [110].
Third generation CD20 mAb have improved effector functions by glyco- or protein-engineering and include obinutuzumab, TRU-015, AME133V and Pro131921. Obinutuzumab (GA101, GlycArt / Roche) is the most advanced of these reagents. In contrast to all other CD20 mAbs that are in clinical development, obinutuzumab is unique in that it is a type II CD20 mAb. Pre-clinical studies suggest, that type II CD20 mAb may outperform type I CD20 mAbs both in xenograft tumor studies [85,90] and in depletion of normal B cells [37]. This may partially be due to the fact that type II CD20 mAbs induce less CD20 internalization than type I CD20 mAbs [105]. Obinutuzumab was selected for its superior ability to induce cell death in tumor cells [89]. In addition to its improved ability to induce cell death, the Fc region of obinutuzumab is glycoengineered to provide better binding to FcγRIIIa independent of its allotype [115]. It will be difficult to separate the advantage provided by these two modifications, however, preclinical studies using a non-glycoenginered version of obinutuzumab suggest that its type II characteristics are more important [116]. Obinutuzumab was well tolerated in Phase 1 clinical studies [117,118]. AME-133v (LY2469298) and PRO131921 are both humanized type I CD20 mAb with protein-engineered Fc part that provide enhanced affinity for FcγRIIIa and therefore induce better ADCC activity compared to RTX [110]. TRU-015 is a smaller CD20 mAb with retained Fc-mediated effector functions and is currently in development for RA [110].
Future directions
Despite intense research, the mechanisms of action of CD20 mAb are still incompletely understood [119]. There are several novel, next generation CD20 mAb currently in development or under clinical testing phase [120]. The results and efficacy data from these studies will inevitably learn us about the mechanisms of CD20 mAbs in patients.
Exhaustion of effector mechanisms during CD20 mAbs therapy can be a problem [121]. Lack of available active complement can be circumvented by injection of fresh frozen plasma. It was shown that much lower dose of RTX that is currently given may also been effective in eliminating tumor cells [61]. To prevent exhaustion of immune effector mechanisms the CD20 mAb dosing should be revisited. Altered timing of chemotherapy and mAb therapy may allow the generation of long-term protective antitumor immunity.
Research in the recent years has identified several mutations of combinations of mutations that modify IgG affinity to different FcγR [122]. In addition, our understanding on how glycose side-chains influence IgG properties have also increased [123]. Mutations introduced in the CH3 domain of IgG1 affecting binding to FcRn, which confer to longer serum half-life, will possibly allow less frequent administration of drugs [124]. Protein- and / or glyco-engineering of therapeutic Abs promise to be a powerful strategy to improve their efficacy.
We need to better understand the impact of FcγR polymorphisms on CD20 mAb therapy, how SNPs and CNVs influence therapeutic efficacy in different B cell malignancies. Patients may be pre-screened in the future for FcγR polymorphisms and expression of FcγRIIb on tumor cells may be determined. Patients with low FcγRIIb expression may be treated with type I CD20 mAbs, whereas patients with high FcγRIIb expression may benefit from the use of antibody drug-conjugates (ADCs), where internalization of the target is desirable. Protein- and / or glyco-engineering of Fc part of the Abs can influence the binding for FcγRs. Type I CD20 mAbs engineered to bind activating FcγR more efficiently and / or FcγRIIb less efficiently may provide better therapy. Better insight into the mechanisms of action of CD20 mAbs together with novel screening methods will allow the selection of the optimal treatment regimen.
In addition, combination of RTX with CD47 can enhance phagocytosis [49]. Double targeting or of CD20 and CD47 with bispecific Abs was shown to induce efficient apoptosis in primary malignant cells [49,125].
Conclusions
Currently, several novel CD20 mAbs, improved in different ways are in clinical testing phase or close to obtain regulatory approval. The coming years will learn us whether these improvements will also translate to increased clinical efficacy. The success or failure of these novel Abs will inevitably generate better insight into the mechanism of action of CD20 therapeutic mAbs.
Acknowledgement
P.B. was supported by a grant from AICR.
Disclose of potential conflict of interest
None.
References
- 1.Putney JW Jr. TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci U S A. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Keefe TL, Williams GT, Davies SL, Neuberger MS. Mice carrying a CD20 gene disruption. Immunogenetics. 1998;48:125–132. doi: 10.1007/s002510050412. [DOI] [PubMed] [Google Scholar]
- 3.Uchida J, Lee Y, Hasegawa M, Liang Y, Bradney A, Oliver JA, Bowen K, Steeber DA, Haas KM, Poe JC, Tedder TF. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 4.Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, Beaumont T, Tedder TF, van Noesel CJ, Eldering E, van Lier RA. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 7.Cheung MC, Haynes AE, Meyer RM, Stevens A, Imrie KR. Rituximab in lymphoma: a systematic review and consensus practice guideline from Cancer Care Ontario. Cancer Treat Rev. 2007;33:161–176. doi: 10.1016/j.ctrv.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, Robak T, Furman RR, Hillmen P, Trneny M, Dyer MJ, Padmanabhan S, Piotrowska M, Kozak T, Chan G, Davis R, Losic N, Wilms J, Russell CA, Osterborg A. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, Lister TA, Stagg RJ, Tidmarsh GF, Kroll S, Wahl RL, Knox SJ, Vose JM. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J. Clin. Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 10.Horning SJ, Younes A, Jain V, Kroll S, Lucas J, Podoloff D, Goris M. Efficacy and safety of tositumomab and iodine-131 tositumomab (Bexxar) in B-cell lymphoma, progressive after rituximab. J. Clin. Oncol. 2005;23:712–719. doi: 10.1200/JCO.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 11.Cragg MS, Morgan SM, Chan HT, Morgan BP, Filatov AV, Johnson PW, French RR, Glennie MJ. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101:1045–1052. doi: 10.1182/blood-2002-06-1761. [DOI] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 13.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 14.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 15.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaguchi Y, Xiu Y, Komura K, Nimmerjahn F, Tedder TF. Antibody isotype-specific engagement of Fcgamma receptors regulates B lymphocyte depletion during CD20 immunotherapy. J Exp Med. 2006;203:743–753. doi: 10.1084/jem.20052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minard-Colin V, Xiu Y, Poe JC, Horikawa M, Magro CM, Hamaguchi Y, Haas KM, Tedder TF. Lymphoma depletion during CD20 immunotherapy in mice is mediated by macrophage FcgammaRI, FcgammaRIII, and FcgammaRIV. Blood. 2008;112:1205–1213. doi: 10.1182/blood-2008-01-135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boross P, Jansen JH, de Haij S, Beurskens FJ, van der Poel CE, Bevaart L, Nederend M, Golay J, van de Winkel JG, Parren PW, Leusen JH. The in vivo mechanism of action of CD20 monoclonal antibodies depends on local tumor burden. Haematologica. 2011 doi: 10.3324/haematol.2011.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 20.Bournazos S, Woof JM, Hart SP, Dransfield I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin Exp Immunol. 2009;157:244–254. doi: 10.1111/j.1365-2249.2009.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breunis WB, van ME, Bruin M, Geissler J, de BM, Peters M, Roos D, de HM, Koene HR, Kuijpers TW. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood. 2008;111:1029–1038. doi: 10.1182/blood-2007-03-079913. [DOI] [PubMed] [Google Scholar]
- 22.Breunis WB, van ME, Geissler J, Laddach N, Wolbink G, van der SE, de HM, de BM, Roos D, Kuijpers TW. Copy number variation at the FCGR locus includes FCGR3A, FCGR2C and FCGR3B but not FCGR2A and FCGR2B. Hum Mutat. 2009;30:E640–E650. doi: 10.1002/humu.20997. [DOI] [PubMed] [Google Scholar]
- 23.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 24.Koene HR, Kleijer M, Algra J, Roos D, Von dem Borne AE, de HM. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–1114. [PubMed] [Google Scholar]
- 25.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 27.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Warmerdam PA, van de Winkel JG, Gosselin EJ, Capel PJ. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J Exp Med. 1990;172:19–25. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farag SS, Flinn IW, Modali R, Lehman TA, Young D, Byrd JC. Fc gamma RIIIa and Fc gamma RIIa polymorphisms do not predict response to rituximab in B-cell chronic lymphocytic leukemia. Blood. 2004;103:1472–1474. doi: 10.1182/blood-2003-07-2548. [DOI] [PubMed] [Google Scholar]
- 30.Dornan D, Spleiss O, Yeh RF, Duchateau-Nguyen G, Dufour A, Zhi J, Robak T, Moiseev SI, Dmoszynska A, Solal-Celigny P, Warzocha K, Loscertales J, Catalano J, Afanasiev BV, Larratt L, Rossiev VA, ce-Bruckler I, Geisler CH, Montillo M, Wenger MK, Weisser M. Effect of FCGR2A and FCGR3A variants on CLL outcome. Blood. 2010;116:4212–4222. doi: 10.1182/blood-2010-03-272765. [DOI] [PubMed] [Google Scholar]
- 31.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, Lehner PJ, Ouwehand WH, Allen JM, Watkins NA, Smith KG. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 32.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng WK, Weng WK, Levy R. Immunoglobulin G Fc receptor polymorphisms do not correlate with response to chemotherapy or clinical course in patients with follicular lymphoma. Leuk Lymphoma. 2009;50:1494–1500. doi: 10.1080/10428190903128660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature. 2006;439:851–855. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- 35.Fanciulli M, Vyse TJ, Aitman TJ. Copy number variation of Fc gamma receptor genes and disease predisposition. Cytogenet Genome Res. 2008;123:161–168. doi: 10.1159/000184704. [DOI] [PubMed] [Google Scholar]
- 36.Schaschl H, Aitman TJ, Vyse TJ. Copy number variation in the human genome and its implication in autoimmunity. Clin Exp Immunol. 2009;156:12–16. doi: 10.1111/j.1365-2249.2008.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beers SA, Chan CH, James S, French RR, Attfield KE, Brennan CM, Ahuja A, Shlomchik MJ, Cragg MS, Glennie MJ. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112:4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K, Satoh M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Exp Hematol. 2009;37:309–321. doi: 10.1016/j.exphem.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Pross HF, Maroun JA. The standardization of NK cell assays for use in studies of biological response modifiers. J Immunol Methods. 1984;68:235–249. doi: 10.1016/0022-1759(84)90154-6. [DOI] [PubMed] [Google Scholar]
- 40.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Muller A, Pachynski R, Czerwinski D, Coutre S, Chao MP, Chen L, Tedder TF, Levy R. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 42.Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011;71:5134–5143. doi: 10.1158/0008-5472.CAN-10-4222. [DOI] [PubMed] [Google Scholar]
- 43.Biburger M, Nimmerjahn F. Low level of FcgammaRIII expression on murine natural killer cells. Immunol Lett. 2012 doi: 10.1016/j.imlet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Peipp M, van de Winkel JG, Valerius T. Molecular engineering to improve antibodies’ anti-lymphoma activity. Best Pract Res Clin Haematol. 2011;24:217–229. doi: 10.1016/j.beha.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, Derer S, Beyer T, Lohse S, Bleeker WK, Peipp M, Parren PW, van de Winkel JG, Valerius T, Dechant M. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–520. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 46.Stockmeyer B, Beyer T, Neuhuber W, Repp R, Kalden JR, Valerius T, Herrmann M. Polymorphonuclear granulocytes induce antibody-dependent apoptosis in human breast cancer cells. J Immunol. 2003;171:5124–5129. doi: 10.4049/jimmunol.171.10.5124. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, Czuczman MS. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9:5866–5873. [PubMed] [Google Scholar]
- 48.Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity. 2011;35:932–944. doi: 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, Park CY, Zhao F, Kohrt HE, Malumbres R, Briones J, Gascoyne RD, Lossos IS, Levy R, Weissman IL, Majeti R. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppa S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13:5784–5789. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 51.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, Lamy T, Sonet A, Rousselet MC, Foussard C, Xerri L. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J. Clin. Oncol. 2008;26:440–446. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 52.Beers SA, Cragg MS, Glennie MJ. Complement: help or hindrance? Blood. 2009;114:5567–5568. doi: 10.1182/blood-2009-10-249466. [DOI] [PubMed] [Google Scholar]
- 53.Beum PV, Lindorfer MA, Beurskens F, Stukenberg PT, Lokhorst HM, Pawluczkowycz AW, Parren PW, van de Winkel JG, Taylor RP. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181:822–832. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 54.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, Tedesco F, Rambaldi A, Introna M. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–3908. [PubMed] [Google Scholar]
- 55.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, van MT, Ebeling S, Vink T, Slootstra JW, Parren PW, Glennie MJ, van de Winkel JG. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 56.Macor P, Tripodo C, Zorzet S, Piovan E, Bossi F, Marzari R, Amadori A, Tedesco F. In vivo targeting of human neutralizing antibodies against CD55 and CD59 to lymphoma cells increases the antitumor activity of rituximab. Cancer Res. 2007;67:10556–10563. doi: 10.1158/0008-5472.CAN-07-1811. [DOI] [PubMed] [Google Scholar]
- 57.Treon SP, Mitsiades C, Mitsiades N, Young G, Doss D, Schlossman R, Anderson KC. Tumor cell expression of CD59 is associated with resistance to CD20 serotherapy in patients with B-cell malignancies. J Immunother. 2001;24:263–271. [PubMed] [Google Scholar]
- 58.Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today. 1999;20:576–582. doi: 10.1016/s0167-5699(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 59.Jurianz K, Ziegler S, Garcia-Schuler H, Kraus S, Bohana-Kashtan O, Fishelson Z, Kirschfink M. Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol. 1999;36:929–939. doi: 10.1016/s0161-5890(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, Williams ME, Taylor RP. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172:3280–3288. doi: 10.4049/jimmunol.172.5.3280. [DOI] [PubMed] [Google Scholar]
- 61.Taylor RP. Use of fresh frozen plasma to enhance the therapeutic action of rituximab. QJM. 2008;101:991–992. doi: 10.1093/qjmed/hcn132. [DOI] [PubMed] [Google Scholar]
- 62.Klepfish A, Rachmilewitz EA, Kotsianidis I, Patchenko P, Schattner A. Adding fresh frozen plasma to rituximab for the treatment of patients with refractory advanced CLL. QJM. 2008;101:737–740. doi: 10.1093/qjmed/hcn085. [DOI] [PubMed] [Google Scholar]
- 63.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, Botto M, Introna M, Golay J. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–1587. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 64.Alduaij W, Illidge TM. The future of anti-CD20 monoclonal antibodies: are we making progress? Blood. 2011;117:2993–3001. doi: 10.1182/blood-2010-07-298356. [DOI] [PubMed] [Google Scholar]
- 65.Weng WK, Levy R. Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. Blood. 2001;98:1352–1357. doi: 10.1182/blood.v98.5.1352. [DOI] [PubMed] [Google Scholar]
- 66.Bannerji R, Kitada S, Flinn IW, Pearson M, Young D, Reed JC, Byrd JC. Apoptotic-regulatory and complement-protecting protein expression in chronic lymphocytic leukemia: relationship to in vivo rituximab resistance. J. Clin. Oncol. 2003;21:1466–1471. doi: 10.1200/JCO.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 67.Wang SY, Racila E, Taylor RP, Weiner GJ. NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood. 2008;111:1456–1463. doi: 10.1182/blood-2007-02-074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang SY, Veeramani S, Racila E, Cagley J, Fritzinger DC, Vogel CW, St JW, Weiner GJ. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood. 2009;114:5322–5330. doi: 10.1182/blood-2009-01-200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Racila E, Link BK, Weng WK, Witzig TE, Ansell S, Maurer MJ, Huang J, Dahle C, Halwani A, Levy R, Weiner GJ. A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma. Clin Cancer Res. 2008;14:6697–6703. doi: 10.1158/1078-0432.CCR-08-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Kolk LE, Grillo-Lopez AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;115:807–811. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 71.Kennedy AD, Solga MD, Schuman TA, Chi AW, Lindorfer MA, Sutherland WM, Foley PL, Taylor RP. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101:1071–1079. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 72.Gelderman KA, Tomlinson S, Ross GD, Gorter A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt RE, Gessner JE. Fc receptors and their interaction with complement in autoimmunity. Immunol Lett. 2005;100:56–67. doi: 10.1016/j.imlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 74.Teeling JL, French RR, Cragg MS, van den BJ, Pluyter M, Huang H, Chan C, Parren PW, Hack CE, Dechant M, Valerius T, van de Winkel JG, Glennie MJ. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 75.Barth MJ, Hernandez-Ilizaliturri FJ, Mavis C, Tsai PC, Gibbs JF, Deeb G, Czuczman MS. Ofatumumab demonstrates activity against rituximab-sensitive and -resistant cell lines, lymphoma xenografts and primary tumour cells from patients with B-cell lymphoma. Br J Haematol. 2012;156:490–498. doi: 10.1111/j.1365-2141.2011.08966.x. [DOI] [PubMed] [Google Scholar]
- 76.Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, Reed JC. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–1043. doi: 10.1182/blood.v99.3.1038. [DOI] [PubMed] [Google Scholar]
- 77.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998;91:1644–1652. [PubMed] [Google Scholar]
- 78.Stolz C, Hess G, Hahnel PS, Grabellus F, Hoffarth S, Schmid KW, Schuler M. Targeting Bcl-2 family proteins modulates the sensitivity of B-cell lymphoma to rituximab-induced apoptosis. Blood. 2008;112:3312–3321. doi: 10.1182/blood-2007-11-124487. [DOI] [PubMed] [Google Scholar]
- 79.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White AL, Chan HT, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, Cragg MS, Beers SA, Glennie MJ. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187:1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- 81.de Haij S, Jansen JH, Boross P, Beurskens FJ, Bakema JE, Bos DL, Martens A, Verbeek JS, Parren PW, van de Winkel JG, Leusen JH. In vivo cytotoxicity of type I CD20 antibodies critically depends on Fc receptor ITAM signaling. Cancer Res. 2010;70:3209–3217. doi: 10.1158/0008-5472.CAN-09-4109. [DOI] [PubMed] [Google Scholar]
- 82.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, Ross S, Vernes JM, Lu Y, Adams C, Offringa R, Kelley B, Hymowitz S, Daniel D, Meng G, Ashkenazi A. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Chan HT, Hughes D, French RR, Tutt AL, Walshe CA, Teeling JL, Glennie MJ, Cragg MS. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 2003;63:5480–5489. [PubMed] [Google Scholar]
- 84.Beers SA, Chan CH, French RR, Cragg MS, Glennie MJ. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin Hematol. 2010;47:107–114. doi: 10.1053/j.seminhematol.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103:2738–2743. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- 86.Cardarelli PM, Quinn M, Buckman D, Fang Y, Colcher D, King DJ, Bebbington C, Yarranton G. Binding to CD20 by anti-B1 antibody or F(ab’)(2) is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51:15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ivanov A, Beers SA, Walshe CA, Honeychurch J, Alduaij W, Cox KL, Potter KN, Murray S, Chan CH, Klymenko T, Erenpreisa J, Glennie MJ, Illidge TM, Cragg MS. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest. 2009;119:2143–2159. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Honeychurch J, Alduaij W, Azizyan M, Cheadle EJ, Pelicano H, Ivanov A, Huang P, Cragg MS, Illidge TM. Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood. 2012;119:3523–3533. doi: 10.1182/blood-2011-12-395541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van PE, Ferrara C, Sondermann P, Jager C, Strein P, Fertig G, Friess T, Schull C, Bauer S, Dal PJ, Del NC, Dabbagh K, Dyer MJ, Poppema S, Klein C, Umana P. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dalle S, Reslan L, Besseyre de HT, Herveau S, Herting F, Plesa A, Friess T, Umana P, Klein C, Dumontet C. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther. 2011;10:178–185. doi: 10.1158/1535-7163.MCT-10-0385. [DOI] [PubMed] [Google Scholar]
- 91.Hainsworth JD, Litchy S, Burris HA III, Scullin DC, Jr, Corso SW, Yardley DA, Morrissey L, Greco FA. Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin’s lymphoma. J. Clin. Oncol. 2002;20:4261–4267. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 92.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- 94.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Selenko N, Maidic O, Draxier S, Berer A, Jager U, Knapp W, Stockl J. CD20 antibody (C2B8)-induced apoptosis of lymphoma cells promotes phagocytosis by dendritic cells and cross-priming of CD8+ cytotoxic T cells. Leukemia. 2001;15:1619–1626. doi: 10.1038/sj.leu.2402226. [DOI] [PubMed] [Google Scholar]
- 96.Schuurhuis DH, van MN, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief CJ, Verbeek JS, Ossendorp F. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol. 2006;176:4573–4580. doi: 10.4049/jimmunol.176.8.4573. [DOI] [PubMed] [Google Scholar]
- 97.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, Liu Y, Tang J, Wang S, Fu YX. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin’s lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in downregulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res. 2001;61:5137–5144. [PubMed] [Google Scholar]
- 100.Vega MI, Huerta-Yepaz S, Garban H, Jazirehi A, Emmanouilides C, Bonavida B. Rituximab inhibits p38 MAPK activity in 2F7 B NHL and decreases IL-10 transcription: pivotal role of p38 MAPK in drug resistance. Oncogene. 2004;23:3530–3540. doi: 10.1038/sj.onc.1207336. [DOI] [PubMed] [Google Scholar]
- 101.Jazirehi AR, Huerta-Yepez S, Cheng G, Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-{kappa}B signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65:264–276. [PubMed] [Google Scholar]
- 102.Jak M, van Bochove GG, Reits EA, Kallemeijn WW, Tromp JM, Umana P, Klein C, van Lier RA, van Oers MH, Eldering E. CD40 stimulation sensitizes CLL cells to lysosomal cell death induction by type II anti-CD20 mAb GA101. Blood. 2011;118:5178–5188. doi: 10.1182/blood-2011-01-331702. [DOI] [PubMed] [Google Scholar]
- 103.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 104.Hiraga J, Tomita A, Sugimoto T, Shimada K, Ito M, Nakamura S, Kiyoi H, Kinoshita T, Naoe T. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood. 2009;113:4885–4893. doi: 10.1182/blood-2008-08-175208. [DOI] [PubMed] [Google Scholar]
- 105.Beers SA, French RR, Chan HT, Lim SH, Jarrett TC, Vidal RM, Wijayaweera SS, Dixon SV, Kim H, Cox KL, Kerr JP, Johnston DA, Johnson PW, Verbeek JS, Glennie MJ, Cragg MS. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010;115:5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
- 106.Lim SH, Vaughan AT, shton-Key M, Williams EL, Dixon SV, Chan HT, Beers SA, French RR, Cox KL, Davies AJ, Potter KN, Mockridge CI, Oscier DG, Johnson PW, Cragg MS, Glennie MJ. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118:2530–2540. doi: 10.1182/blood-2011-01-330357. [DOI] [PubMed] [Google Scholar]
- 107.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol. 2006;176:2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- 108.Boross P, Jansen JH, Pastula A, van der Poel CE, Leusen JH. Both activating and inhibitory Fc gamma receptors mediate rituximab-induced trogocytosis of CD20 in mice. Immunol Lett. 2012 doi: 10.1016/j.imlet.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Beum PV, Peek EM, Lindorfer MA, Beurskens FJ, Engelberts PJ, Parren PW, van de Winkel JG, Taylor RP. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. J Immunol. 2011;187:3438–3447. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 110.Lim SH, Beers SA, French RR, Johnson PW, Glennie MJ, Cragg MS. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica. 2010;95:135–143. doi: 10.3324/haematol.2008.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Genovese MC, Kaine JL, Lowenstein MB, Del GJ, Baldassare A, Schechtman J, Fudman E, Kohen M, Gujrathi S, Trapp RG, Sweiss NJ, Spaniolo G, Dummer W. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:2652–2661. doi: 10.1002/art.23732. [DOI] [PubMed] [Google Scholar]
- 112.Goldenberg DM, Rossi EA, Stein R, Cardillo TM, Czuczman MS, Hernandez-Ilizaliturri FJ, Hansen HJ, Chang CH. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood. 2009;113:1062–1070. doi: 10.1182/blood-2008-07-168146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hagenbeek A, Gadeberg O, Johnson P, Pedersen LM, Walewski J, Hellmann A, Link BK, Robak T, Wojtukiewicz M, Pfreundschuh M, Kneba M, Engert A, Sonneveld P, Flensburg M, Petersen J, Losic N, Radford J. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111:5486–5495. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 114.Beum PV, Lindorfer MA, Peek EM, Stukenberg PT, de WM, Beurskens FJ, Parren PW, van de Winkel JG, Taylor RP. Penetration of antibody-opsonized cells by the membrane attack complex of complement promotes Ca(2+) influx and induces streamers. Eur J Immunol. 2011;41:2436–2446. doi: 10.1002/eji.201041204. [DOI] [PubMed] [Google Scholar]
- 115.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, Yokoi H, Nakamura K, Shitara K. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 116.Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, Shimada K, Chan CH, Tutt A, Beers SA, Glennie MJ, Cragg MS, Illidge TM. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117:4519–4529. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sehn LH, Assouline SE, Stewart DA, Mangel J, Gascoyne RD, Fine G, Frances-Lasserre S, Carlile DJ, Crump M. A phase I study of obinutuzumab induction followed by two years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012 doi: 10.1182/blood-2012-02-408773. [DOI] [PubMed] [Google Scholar]
- 118.Salles G, Morschhauser F, Lamy T, Milpied NJ, Thieblemont C, Tilly H, Bieska G, Asikanius E, Carlile D, Birkett J, Pisa P, Cartron G. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012 doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 119.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 120.Czuczman MS, Gregory SA. The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk Lymphoma. 2010;51:983–994. doi: 10.3109/10428191003717746. [DOI] [PubMed] [Google Scholar]
- 121.Taylor RP, Lindorfer MA. Antigenic modulation and rituximab resistance. Semin Hematol. 2010;47:124–132. doi: 10.1053/j.seminhematol.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Derer S, Kellner C, Berger S, Valerius T, Peipp M. Fc engineering: design, expression, and functional characterization of antibody variants with improved effector function. Methods Mol Biol. 2012;907:519–536. doi: 10.1007/978-1-61779-974-7_30. [DOI] [PubMed] [Google Scholar]
- 123.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 2012 doi: 10.1111/j.1749-6632.2011.06305.x. [DOI] [PubMed] [Google Scholar]
- 124.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 125.Gupta P, Goldenberg DM, Rossi EA, Cardillo TM, Byrd JC, Muthusamy N, Furman RR, Chang CH. Dual-targeting immunotherapy of lymphoma: potent cytotoxicity of anti-CD20/CD74 bispecific antibodies in mantle cell and other lymphomas. Blood. 2012;119:3767–3778. doi: 10.1182/blood-2011-09-381988. [DOI] [PubMed] [Google Scholar]


