Abstract
FDA approval of new therapies in 2011 has greatly expanded the treatment options for metastatic melanoma. Patients with V600 mutant v-raf murine sarcoma viral oncogene homolog B1 (B-RAF) positive metastatic melanoma are now treated with the RAF inhibitor, vemurafenib (Zelboraf) as a first line therapy. Vemurafenib decreases tumor size by at least 30% in approximately 50% of patients and increases progression-free survival and overall patient survival compared to the previous standard-of-care, dacarbazine. However, some patients treated with vemurafenib fail to show significant tumor shrinkage, and most patients who initially respond to the drug eventually show disease progression. Therefore, there is a clinical need to improve efficacy and prevent resistance to vemurafenib. It has been previously shown that cell death resulting from RAF/mitogen-activated protein kinase kinase (MEK) inhibition is largely dependent on increased expression of pro-apoptotic, Bcl-2 homology domain (BH3)-only proteins, such as Bcl-2-like 11 (Bim-EL) and Bcl-2 modifying factor (Bmf). Here, we show that contrary to expression of Bim-EL and Bmf, the pro-apoptotic, BH3-only protein, phorbol-12-myristate-13-acetate-induced protein 1 (Noxa), is strongly downregulated after RAF/MEK inhibition. This downregulation occurs at both the protein and mRNA level of expression and is associated with the inhibition of cell cycle progression. Restoring expression of Noxa in combination with RAF/MEK inhibition enhances cell death. Co-expression of the pro-survival, B-cell CLL/lymphoma 2 (Bcl-2) family member, myeloid cell leukemia sequence 1 (Mcl-1), with Noxa fully mitigates the enhanced cell death associated with increased Noxa expression. These data indicate that manipulating the Noxa/Mcl-1 axis may enhance the efficacy of RAF/MEK inhibitors.
Keywords: Melanoma, B-RAF, Noxa, RAF/MEK inhibition
Introduction
Prior to 2011, treatment options for metastatic melanoma were limited. Standard of care for the disease usually involved systemic therapies such as IL-2 or dacarbazine. These therapies were often associated with high levels of toxicity and low success rates [1]. However, recent FDA approval of new treatments for metastatic melanoma has brought hope to this struggling field. Ipilimumab (Yervoy) was the first of the recently approved therapeutic interventions. This human monoclonal antibody binds to cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and stimulates the immune response [2]. It has been approved to treat melanoma that cannot be cured by surgical resection and is not dependent on the mutational status of the patient’s disease. A second recently approved therapeutic is the small molecule RAF inhibitor, vemurafenib (Zelboraf). Vemurafenib inhibits B-RAF signaling in mutant B-RAF melanoma cells and is specifically used in patients with metastatic melanoma harboring a V600 mutation [3-5].
B-RAF is a serine/threonine kinase that is mutated in approximately 50% of melanomas [6]. The most common mutation in B-RAF is a V600E substitution in the activation domain that causes constitutive kinase activity and downstream phosphorylation/activation of the MEK-extracellular signal-regulated kinase (ERK)1/2 cascade [6]. Because mutant B-RAF is known to act as a driver mutation in melanoma, the development and clinical efficacy of vemurafenib has been an important step forward in treating this disease. In clinical trials, the inhibitor has shown tremendous success and has yielded a significant reduction in tumor burden in approximately 50% of patients with V600 mutant B-RAF melanoma [3,4]. Additionally, progression-free survival and overall survival were significantly improved compared to dacarbazine treatment [3].
Despite the initial success of vemurafenib, most patients experience tumor relapse while on this therapy [4,7]. Several mechanisms accounting for resistance to vemurafenib have been identified [8-13]. These mechanisms usually involve either reactivation of ERK1/2 signaling or increased activity of other compensatory pathways [14,15]. Although some mechanisms of resistance have already been identified, it is critical to further understand why some patients do not respond to vemurafenib in order to maximize the cytotoxic effects of RAF inhibitors.
Previous research has shown that cell death resulting from RAF/MEK inhibition is largely dependent on changes in Bcl-2 family member proteins, such as increased expression of proapoptotic BH3-only proteins, Bim-EL and Bmf, and decreased expression of anti-apoptotic proteins, such as Mcl-1 [16,17]. The action of pro-apoptotic, BH3-only proteins is crucial to neutralizing anti-apoptotic Bcl-2 family members and activating Bcl-2-antagonist/killer1 (BAK) and Bcl-2-associated X protein (BAX) [18,19]. This activation leads to enhanced permeability of the outer membrane of the mitochondria, which is a crucial step for the initiation of cell death [19].
BH3-mimetic compounds, such as ABT-737, have been developed in an effort to therapeutically recapitulate the action of these BH3-only proteins in order to increase tumor cell death [20,21]. However, the use of ABT-737 as a single agent therapy in the clinic has been disappointing in solid cancers, largely as a result of strong Mcl-1 activity, which is not targeted by this compound [21]. Despite this difficulty, the concept of modulating the balance of Bcl-2 family member proteins in combination with other therapeutic interventions is presently an area of intense investigation.
In this study, we show that the Mcl-1-antagonizing, BH3-only protein, Noxa, is strongly downregulated after treatment with RAF/MEK inhibitors in melanoma cells. This downregulation is associated with decreased abundance of Noxa mRNA. PLX4720-induced alterations in cell cycle proteins correlate with decreased Noxa expression, and direct inhibition of cell cycle progression using a cyclin dependent kinase (CDK)4/6 inhibitor results in a downregulation of Noxa mRNA and protein. Restoration of Noxa expression increases cell death resulting from treatment with RAF/MEK inhibitors. Co-expression of Mcl-1 with Noxa fully mitigates the enhanced cell death observed in RAF inhibitor treated cells expressing Noxa alone.
Materials and methods
Cell culture
Human melanoma cell lines, WM115 and 1205Lu, were kindly donated by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA). A375 and SK-MEL28 cells were purchased from the American Type Culture Collection (Manassas, VA). WM115, 1205Lu and SK-MEL28 cells were cultured in MCDB 153 medium containing 20% Leibovitz L-15 medium, 2% fetal bovine serum (FBS), 0.2% sodium bicarbonate and 5μg/mL insulin. A375 cells were cultured in DMEM with 10% FBS. All cell lines have been verified by DNA sequencing for their B-RAF mutational status.
Inhibitors
PLX4720 was kindly provided by Dr. Gideon Bollag (Plexxikon, Berkeley, CA). U0126 was purchased from Cell Signaling Technology (Beverly, MA). PD-0332991 was purchased from Selleck Chemicals (Houston, TX).
Western blotting
Western blotting was done as previously described [22]. The following antibodies were utilized: anti-B-RAF (#SC-5284, Santa-Cruz Biotech, Santa Cruz, CA); anti-Noxa (#OP180, Calbiochem, Billerica, MA); anti-phosphoERK1/2 Thr202/Tyr204 (#4377, Cell Signaling Tech anti-ERK2 (#SC-154, Santa-Cruz Biotech); anti-actin (#A2066, Sigma-Aldrich, St Louis, MO); anti-E2F1 (#SC-251, Santa-Cruz Biotech); anti-phosphoRb Ser780 (#9307, Cell Signaling Tech); anti-Rb (#9309, Cell Signaling Tech); anti-Cyclin A1 (#SC-751, Santa-Cruz Biotech); anti-phosphoRb Ser801/807 (#9308, Cell Signaling Tech); anti-PARP (#9542, Cell Signaling Tech); anti-Mcl-1 (#559027, Becton Dickinson Biosciences, San Jose, CA). Signal was detected using peroxidase-conjugated secondary antibody followed by development using chemiluminescence substrate (Pierce, Rockford, IL) and a Versadoc Imaging system equipped with Quantity-One software (Bio-Rad, Hercules, CA).
siRNA transfections
Cells were transfected with siRNAs at a final concentration of 25nmol/L using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA). Non-targeting control (5’-UGGUUUACAUGUCGACUAA-3’) and B-RAF (5’-ACAGAGACCUCAAGAGUAAUU-3’) siRNAs were purchased from Dharmacon (Lafayette, CO).
Quantitative reverse transcription polymerase chain reaction (q-RT-PCR)
q-RT-PCR was performed as previously described [16]. The following primers were used: Noxa-forward, 5’-TGTTCGTGTTCAGCTCGCGTC-3’; Noxa-reverse, 5’-CACACTCGACTTCCAGCTCTGC-3’; actin-forward, 5’ -TACCTCATGAAGATCCTCACC-3’; actin-reverse, 5’-TTTCGTGGATGCCACAGGAC-3’. Relative mRNA levels were calculated using the comparative Ct (ΔCt) method. Quantitation of mRNA levels relative to actin represents data from three independent experiments.
Xenograft assays
Melanoma cells (1x106) were injected intradermally into female athymic mice (NU/J: Jackson) and allowed 10 days to reach appropriate volume. Mice were then fed either AIN-76A chow or AIN-76A with 417 mg/kg PLX4720 chow for 72 hrs and tumors were harvested for RNA using TRIzol (Invitrogen).
Lentiviral construction and transduction
pLenti6/TR was purchased from Invitrogen. Noxa and Mcl-1 were cloned into pENTR/D-TOPO (Invitrogen) and LR recombined into pLentipuro/TO/V5-DEST or pLenti4/TO/V5-DEST, respectively. Expression constructs and packaging plasmids pLP1, pLP2, and pLP/VSVG were cotransfected into HEK293FT cells to generate viral particles. Cells were transduced with particles for 72 hrs and then selected with blasticidin (pLenti6/TR), puromycin (pLentipuro constructs) or zeocin (pLenti4 constructs), as described previously [23,24]. A375TR and SK-MEL28TR cells are clonal isolates selected for high expression of the Tet repressor (TR) and used for sequential transductions. Transgene expression was induced with doxycycline (100ng/mL).
Cell death assays
Analysis of annexin V staining (BD Biosciences, San Jose, CA) was performed as previously described [16]. Staining was measured by flow cytometry on the FACS Calibur (BD Biosciences), and data were analyzed using Flowjo software (Three Star, Inc., Ashland, OR).
Results
Noxa is downregulated by inhibition of RAF-MEK-ERK1/2 signaling
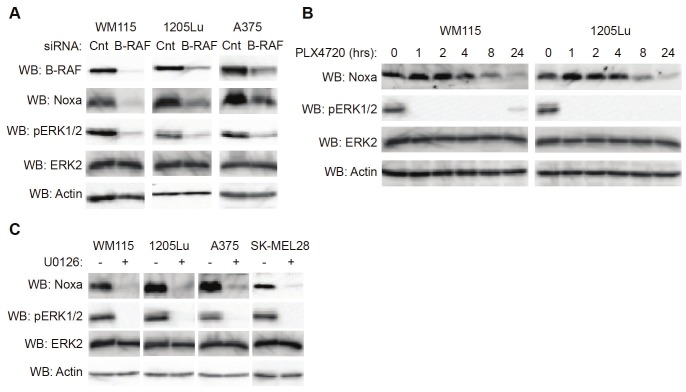
We and others have previously shown that inhibition of the RAF-MEK-ERK1/2 pathway in melanoma cells results in a dramatic increase in the levels of pro-apoptotic proteins, such as Bim-EL and Bmf [16,17]. To explore the effects of ERK1/2 signaling on apoptotic signaling further, B-RAF regulation of additional pro-apoptotic, BH3-only family members was tested. Mutant B-RAF melanoma cells were transfected with either control or B-RAF siRNA. Expression levels of the pro-apoptotic protein, Noxa, were readily detectable in several melanoma cell lines transfected with control siRNA; however, contrary to the pattern of other BH3-only family members, expression of Noxa was dramatically downregulated by B-RAF knockdown (Figure 1A). Noxa levels showed a slight decrease after eight hours of treatment with the RAF inhibitor, PLX4720, and showed a further decrease after 24 hours of treatment (Figure 1B). Downregulation of Noxa in melanoma cells was also detected using the MEK1/2 inhibitor, U0126 (Figure 1C). In summary, inhibition of mutant B-RAF-MEK signaling causes decreased expression of pro-apoptotic Noxa.
Figure 1.
Downregulation of Noxa occurs after inhibition of RAF/MEK signaling in melanoma cells. A: Mutant B-RAF harboring WM115, 1205Lu and A375 cells were transfected with non-targeting control siRNA or B-RAF siRNA. After 72 hrs, cells were lysed and analyzed by Western blotting, as indicated. B: WM115 and 1205Lu cells were treated with PLX4720 (5μM) for the indicated times. Cells were then lysed and analyzed by Western blotting, as indicated. C: WM115, 1205Lu, A375 and SK-MEL28 cells were treated with DMSO (-) or U0126 (10μM). After 24 hrs, cells were lysed and analyzed by Western blotting, as indicated.
Noxa downregulation occurs at the mRNA level
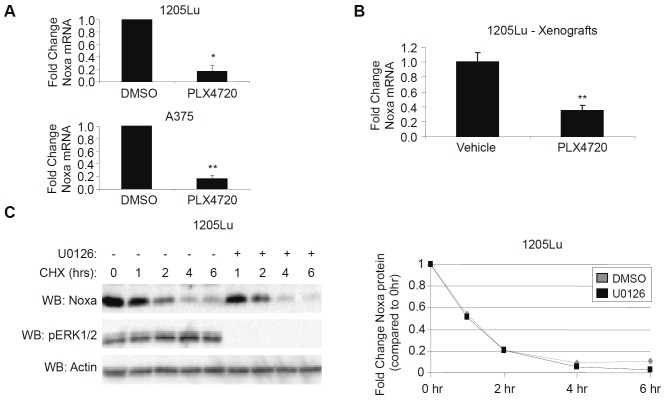
Bcl-2 family members are often regulated by multiple mechanisms, including transcription and post-translational modifications [25,26]; thus, we sought to clarify the mechanism by which Noxa expression decreased after RAF/MEK inhibition. Noxa mRNA abundance, as determined by q-RT-PCR, was significantly decreased in cells treated with PLX4720 in vitro (Figure 2A). In order to explore this result further, we injected 1205Lu cells intradermally into nude mice, allowed cells to establish tumors and exposed them to PLX4720 for five days. Similar to in vitro studies, Noxa mRNA was also decreased in tumors harvested from xenograft samples treated with PLX4720 compared to tumors harvested from vehicle-treated mice (Figure 2B). To exclude the possibility that Noxa protein was downregulated after RAF/MEK inhibition through increased protein turnover, cells were exposed to the translational inhibitor, cycloheximide in the absence and presence of the MEK inhibitor, U0126. Although Noxa protein rapidly decreased in a matter of hours, the turnover of protein was not influenced by MEK-ERK1/2 signaling (Figure 2C). These data suggest that Noxa downregulation after RAF/MEK inhibition is dependent on decreased mRNA abundance.
Figure 2.
Downregulation of Noxa after RAF/MEK inhibition is associated with decreased mRNA. A: 1205Lu and A375 cells were treated with DMSO or PLX4720 (5μM). After 24hrs, cells were harvested for total RNA isolation and analyzed by qRT-PCR for Noxa and actin (control). Quantitation of data from three independent experiments is represented as the mean relative mRNA level of Noxa in each condition. Error bars represent standard error. *p-value<0.05 and **p-value<0.01 comparing DMSO and PLX4720 conditions based on unpaired Student t-test. B: Athymic, nude mice harboring 1205Lu intradermal xenograft tumors were fed either vehicle chow (N=4) or PLX4720-containing chow (N=3). After 72hrs, tumors were harvested for total RNA isolation and analyzed by qRT-PCR for Noxa and actin (control). Quantitation of data is represented as the mean relative mRNA level of Noxa. Error bars represent standard error. **p-value<0.01 comparing vehicle and PLX4720 conditions based on unpaired Student t-test. C: 1205Lu cells that were treated with either DMSO (-) or U0126 (10μM) were treated with 10μg/mL cycloheximide (CHX). After the indicated time, cells were lysed and analyzed by Western blotting, as indicated. Graphed is quantition of Noxa protein normalized to actin.
Inhibition of cell cycle progression downregulates Noxa expression
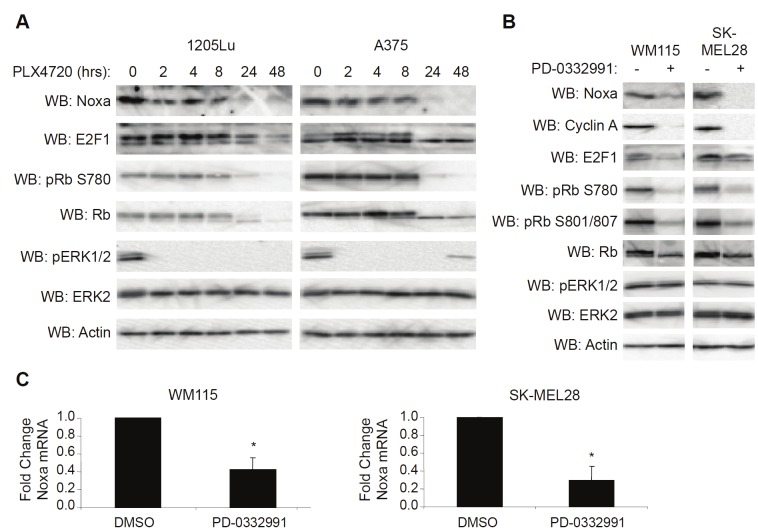
Treatment of mutant B-RAF melanoma cells with RAF inhibitors elicits an overall growth arrest accompanied by alterations in cell cycle proteins [27,28]. Because Noxa expression has been associated with cell cycle progression [29,30] in addition to its role in apoptosis, we hypothesized that downregulation of Noxa by RAF/MEK inhibition may be due to changes in cell cycle kinetics. Western blot analysis revealed that decreased Noxa expression correlated with PLX4720-induced changes in cell cycle regulators such as Rb and E2F1 (Figure 3A). In order to determine if altering cell cycle progression independent of RAF/MEK inhibition would result in Noxa downregulation, we treated mutant B-RAF melanoma cell lines with the CDK4/6 inhibitor, PD-0332991. Efficacy of this compound was confirmed by Rb dephosphorylation and E2F1 and cyclin A downregulation (Figure 3B). Noxa expression was severely attenuated by PD-0332991 treatment despite any change in ERK1/2 phosphorylation (Figure 3B). Noxa downregulation by PD-0332991 was also associated with decreased mRNA abundance as determined by q-RT-PCR (Figure 3C). These data indicate that Noxa expression is associated with cell cycle progression in mutant B-RAF melanoma cells.
Figure 3.
Noxa downregulation occurs in response to cell cycle inhibition. A: 1205Lu and A375 cells were treated with PLX4720 (5μM) for the indicated times. Cells were then lysed and analyzed by Western blotting, as indicated. B: WM115 and SK-MEL28 cells were treated with DMSO (-) or PD-0332991 (500nM). After 24 hrs, cells were lysed and analyzed by Western blotting, as indicated. C: Same as B, except that cells were harvested for total RNA isolation and analyzed by qRT-PCR for Noxa and actin (control). Quantitation of data from three independent experiments is represented as the mean relative mRNA level of Noxa in each condition. Error bars represent standard error. *p-value<0.05 comparing DMSO and PD-0332991 conditions based on unpaired Student t-test.
Restoration of Noxa expression enhances cell death caused by RAF/MEK inhibitors
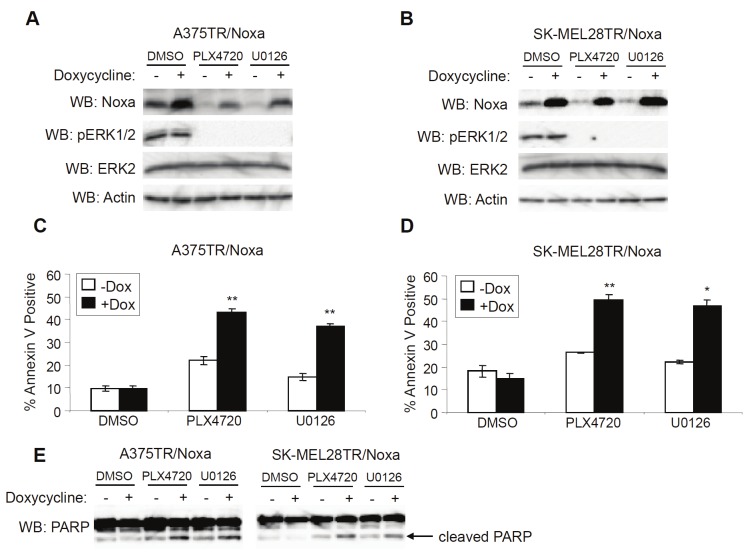
We hypothesized that one way to improve the cytotoxic actions of RAF inhibitors would be to restore expression of Noxa. To test this hypothesis, we utilized doxycycline-regulated cell lines to induce Noxa expression in the absence of ERK1/2 signaling (Figure 4A and 4B). Although Noxa expression alone had no effect on cell death, the expression of Noxa in the presence of PLX4720 or U0126 resulted in a dramatic increase in cell death based on annexin V staining (Figure 4C and 4D). Additionally, the increase in annexin V staining was accompanied by increased cleavage of PARP, another marker of apoptosis (Figure 4E). In summary, Noxa re-expression enhances RAF/MEK inhibitor-induced cell death.
Figure 4.
Restoration of Noxa expression enhances cell death by RAF/MEK inhibitors. A: A375TR cells harboring doxycycline-inducible Noxa were treated with DMSO, PLX4720 (5μM) or U0126 (10μM) and -/+ 100ng/mL doxycycline. After 24 hrs, cells were lysed and analyzed by Western blotting, as indicated. B: Same as A, except that SK-MEL28TR cells harboring doxycycline-inducible Noxa were used. C: Same as A, except that cells were harvested and stained with annexin V-APC for cell death analysis after 48 hrs of treatment. Quantitation of data from three independent experiments is represented by the mean percentage of cells staining positive for annexin V-APC. Error bars represent standard error. ** p-value<0.01 comparing cells in the absence and presence of doxycycline based on unpaired Student t-test. D: Same as C, except that SK-MEL28TR cells harboring doxycycline-inducible Noxa were used. *p-value<0.05 and **p-value<0.01 comparing cells in the absence and presence of doxycycline based on unpaired Student t-test. E: Lysates from A and B were analyzed by Western blotting for cleaved PARP.
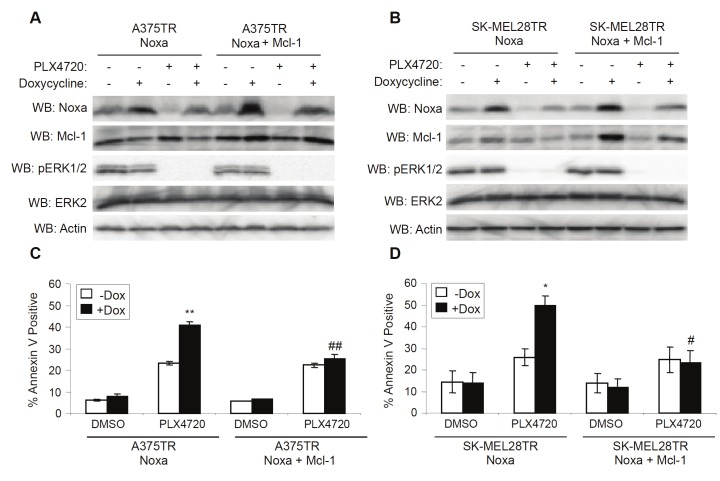
Mcl-1 expression mitigates enhanced PLX4720-induced cell death in Noxa-expressing cells
The efficacy of BH3-mimetics is often stymied by high levels of anti-apoptotic proteins, most notably Mcl-1 [21]. Since Noxa is a direct antagonist of Mcl-1, we sought to determine whether the enhanced efficacy of RAF and MEK inhibition resulting from increased Noxa expression would be reduced by high levels of Mcl-1. In order to address this, we used mutant B-RAF, melanoma cell lines that ectopically expressed Noxa and Mcl-1 simultaneously upon exposure to doxycycline. Noxa expression in the cell lines expressing both Noxa and Mcl-1 was comparable to the level of Noxa expression in the original Noxa-expressing cell lines (Figure 5A and 5B). As expected, restoration of Noxa alone enhanced the cytotoxicity of PLX4720; however, cells that expressed Noxa and Mcl-1 simultaneously had levels of PLX4720-induced cell death that were comparable to non-induced cell lines (Figure 5C and 5D). This demonstrates that the increased cell death caused by restoring Noxa expression can be fully blocked by Mcl-1 expression.
Figure 5.
Mcl-1 expression mitigates enhanced PLX4720-induced cell death in Noxa-expressing cells. A: A375TR cells harboring doxycycline-inducible Noxa or Noxa/Mcl-1 were treated with DMSO (-) or PLX4720 (5μM) and -/+ 100ng/mL doxycycline. After 24 hrs, cells were lysed and analyzed by Western blotting, as indicated. B: Same as A, except that SK-MEL28TR cells harboring doxycycline-inducible Noxa or Noxa/Mcl-1 were used. C: Same as A, except that cells were harvested and stained with annexin V-APC for cell death analysis after 48 hrs of DMSO or PLX4720. Quantitation of data from three independent experiments is represented by the mean percentage of cells staining positive for Annexin V-APC. Error bars represent standard error. **p-value<0.01 comparing cells in the absence and presence of doxycycline based on unpaired Student t-test. ##p-value<0.01 comparing Noxa and Noxa/Mcl-1 cells based on unpaired Student t-test. D: Same as C, except that SK-MEL28TR cells harboring doxycycline-inducible Noxa or Noxa/Mcl-1 were used. *p-value<0.05 comparing cells in the absence and presence of doxycycline based on unpaired Student t-test. #p-value<0.05 comparing Noxa and Noxa/Mcl-1 cells based on unpaired Student t-test.
Discussion
In order to improve the benefit of RAF inhibitors to treat metastatic mutant B-RAF melanoma, it is necessary to identify mechanisms that decrease the full efficacy of these inhibitors. Our data indicate that the pro-apoptotic, BH3-only protein, Noxa, is dramatically downregulated upon inhibition of RAF/MEK signaling in melanoma cells. This downregulation is opposite of the effect observed with other BH3-only proteins, namely Bim-EL and Bmf, and counteracts the overall pro-apoptotic stimulus resulting from RAF/MEK inhibition. The counteracting effects of Noxa downregulation may explain the low level of cell death that some cell lines show in response to inhibitors of RAF or MEK. Additionally, downregulation of Noxa in patients treated with vemurafenib may decrease the efficacy of this inhibitor and may account for the low level of response seen in some patients.
RAF/MEK inhibition has been shown to attenuate cell cycle progression in mutant B-RAF melanoma cells [27,28]. Our data show that expression of Noxa correlates with PLX4720-induced changes in cell cycle regulators. Additionally, direct inhibition of the cell cycle with a CDK4/6 inhibitor causes a dramatic decrease in Noxa expression. This suggests that growth arrested melanoma cells may have less apoptotic potential in response to RAF inhibitors. In support of this idea, initial drug-tolerance in response to chemotherapeutic treatment is characterized by a subpopulation of growth arrested cells that eventually gain further genetic alterations that permit further cell growth [31]. Therefore, decreased cell cycle progression in response to RAF/MEK inhibition may result in gene expression changes that increase survival.
Although Noxa is downregulated by RAF/MEK inhibition, our data indicate that restoring the expression of Noxa increases the cell death response to these inhibitors. Therefore, increasing Noxa expression through clinical methods may offer benefit to patients treated with vemurafenib. For example, previous data have shown that histone deacetylase (HDAC) inhibitors can enhance expression of pro-apoptotic proteins, including Noxa [32,33]. Additionally, preliminary evidence suggests that using HDAC inhibitors such as vorinostat (suberoylanilide hydroxamic acid - SAHA) can enhance the cytotoxic effect of vemurafenib (data not shown). Current work in our lab is investigating this further in order to determine if this combination treatment may offer benefit in a clinical setting.
Since the cell death resulting from RAF/MEK inhibition in melanoma cells is largely dependent on changes in Bcl-2 family member proteins [16,17], using BH3-mimetics that would augment these changes may benefit patients treated with vemurafenib. However, current BH3-mimetics, such as ABT-737, have shown limited efficacy as single agent therapies due to their insufficient ability to target Mcl-1 [21]. In melanoma cells, resistance to ABT-737 as a single agent therapy has been shown to be dependent on high levels of Mcl-1 expression [34]. Our data support a similar conclusion as the increased cell death of Noxa expression is overcome by simultaneously upregulating Mcl-1 expression. Therefore, BH3-mimetics that are capable of targeting Mcl-1 may prove to be the most beneficial to use in combination with vemurafenib.
Acknowledgements
We thank Dr. Gideon Bollag and Plexxikon Inc. (Berkeley, CA) for providing PLX4720 and Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) for WM melanoma cell lines. This work was supported by National Institutes of Health (GM067893, CA125103) and the Department of Defense (W81XWH-11-1-0448). Kevin Basile was supported in part by a grant from the Joanna M. Nicolay Melanoma Foundation. The Animal and Flow core facilities in the Kimmel Cancer Center are supported by the National Cancer Institute Support Grant IP30CA56036.
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Lens MB, Eisen TG. Systemic chemotherapy in the treatment of malignant melanoma. Expert Opin Pharmacother. 2003;4:2205–2211. doi: 10.1517/14656566.4.12.2205. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, WEber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 7.Smalley KS, Sondak VK. Melanoma--an unlikely poster child for personalized cancer therapy. N Engl J Med. 2010;363:876–878. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- 8.Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, Hatton C, Chopra R, Oberholzer PA, Karpova MB, Macconaill LE, Zhang J, Gray NS, Sellers WR, Dummer R, Garraway LA. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106:20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Coqdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, Salton M, Dahlman KB, Tadi M, Wargo JA, Flaherty KT, Kelley MC, Misteli T, Chapman PB, Sosman JA, Graeber TG, Ribas A, Lo RS, Rosen N, Solit DB. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D’Andrea K, Pushparajan A, Hayden JE, Brown KD, Laguerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aplin AE, Kaplan FM, Shao Y. Mechanisms of resistance to RAF inhibitors in melanoma. J Invest Dermatol. 2011;131:1817–1820. doi: 10.1038/jid.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corcoran RB, Diase-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010;3:1–10. doi: 10.1126/scisignal.2001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670–6681. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;15:4934–4942. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 18.Makin GW, Corfe BM, Griffiths GJ, Thistlethwaite A, Hickman JA, Dive C. Damage-induced Bax N-terminal change, translocation to mitochondria and formation of Bax dimers/complexes occur regardless of cell fate. EMBO J. 2001;20:6306–6315. doi: 10.1093/emboj/20.22.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Thomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 21.van Delft MF, Wei AH, Mason KD, Vanderberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisvert-Adamo K, Aplin AE. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25:4848–4856. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- 23.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–3312. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 24.Spofford LS, Abel EV, Boisvert-Adamo K, Aplin AE. Cyclin D3 expression in melanoma cells is regulated by adhesion-dependent phosphatidylinositol 3-kinase signaling and contributes to G1-S progression. J Biol Chem. 2006;281:25644–25651. doi: 10.1074/jbc.M600197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 2003;63:8330–8337. [PubMed] [Google Scholar]
- 26.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 27.Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, Halilovic E, Persaud Y, Xing F, Viale A, Tsai J, Chapman PB, Bollag G, Solit DB, Rosen N. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci U S A. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2004;279:8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Swetzig WM, Medisetty R, Das GM. Estrogen-mediated upregulation of Noxa is associated with cell cycle progression in estrogen receptor-positive breast cancer cells. PLoS One. 2011;6:e29466. doi: 10.1371/journal.pone.0029466. Epub 2011 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Perarnau A, Coll-Mulet L, Rubio-Patino C, Iglesias-Serret D, Cosialls AM, Gonzalez-Girones DM, de Frias M, de Sevilla AF, de la Banda E, Pons G, Gil J. Analysis of apoptosis regulatory genes altered by histone deacetylase inhibitors in chronic lymphocytic leukemia cells. Epigenetics. 2011;6:1228–1235. doi: 10.4161/epi.6.10.17200. [DOI] [PubMed] [Google Scholar]
- 33.Xargay-Torrent S, Lopez-Guerra M, Saborit-Villarroya I, Rosich L, Campo E, Roue G, Colomer D. Vorinostat-induced apoptosis in mantel cell lymphoma is mediated by acetylation of proapoptotic BH3-only gene promoters. Clin Cancer Res. 2011;17:3956–3968. doi: 10.1158/1078-0432.CCR-10-3412. [DOI] [PubMed] [Google Scholar]
- 34.Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC, Weninger W, Haass NK, Allen JD. Modulation of Noxa and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res. 2012;18:783–795. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]