Summary
The debate is still ongoing about the long term effects of the mininvasive vertebral augmentation techniques and their usefulness in treating more complex cases where a bone inducing effect more than a merely bone substitution would be suitable, such as the vertebral fractures in young patients. We previously developed a clinically relevant gene therapy approach using modified dermal fibroblasts for inducing bone healing and bone formation in different animal models. The aim of this study is to show the feasibility of a minimally invasive percutaneous intrasomatic ex vivo gene therapy approach to treat thoracolumbar vertebral fractures and anterior column bone defects in a goat model.
Introduction
Thoracolumbar fractures are more frequent in men (2/3) than in women (1/3) and peak between the ages of 20 and 40 years. (1) Approximately, 160 000 patients/year sustain an injury of the spinal column in United States. (2) The most common mechanism of injury of the thoracolumbar spine involves flexion/distraction forces causing eccentric compression of the vertebral bodies and discs resulting in anterior wedge compression fractures. (1) Even if non-operative treatment is still a viable and effective treatment for a good number of patients, many patients still need to be treated surgically in order to restore a correct spinal sagittal alignment. The reduction of vertebral compression fractures leaves residual bone defects that pose a significant challenge to the practicing spinal surgeon. (3) Bone-grafting procedures often allow filling of bone defects, but the materials commonly used in everyday practice have many limitations. (4) (5) These limitations include morbidity of donor sites, limited availability of graft materials for autografts, risks of disease transmission, and complications such as infection, fracture, and nonunion of allografts. Calcium phosphate cement (CPC) is one alternate bone substitute and has been used to treat compression fractures in hips, radii, and vertebrae. (3) (6)
Osteoinductive cytokines possess the potential to stimulate fracture healing. The most extensively studied osteoinductive cytokines are bone morphogenetic protein-2 (BMP-2) and BMP-7. The U.S. Food and Drug Administration (FDA) has approved recombinant human BMP-2 and BMP-7 for restricted use in open tibial fractures and spinal fusion. In two clinical trials, large doses of BMP were required to induce adequate bone repair, suggesting that the mode of BMP delivery still requires further optimization. (7) (8) BMP-2 gene transfer might be a useful way to stimulate fracture healing. We previously developed a clinically relevant gene therapy approach using adenoviral transduced dermal fibroblasts for inducing bone healing and bone formation in different animal models. (9) The aim of this study was to show the feasibility of a minimally invasive spinal percutaneous intrasomatic ex vivo gene therapy approach to treat thoracolumbar critical anterior column bone defects in a goat model.
Materials and Methods
Construction of Recombinant Adenoviruses
E1- and E3-deleted adenoviral vectors expressing human BMP-2 (Ad-hBMP2) and enhanced green fluorescent protein (Ad-eGFP) were constructed for this study, as previously described. (10) An E1/E3-substituted recombinant adenovirus containing the cDNA for enhanced green fluorescent protein (Ad-eGFP) was also used as a negative control. Titers were determined by optical density at 260 nm (OD260) and a standard plaque assay. (11)
Isolation and culture of Autologous Dermal Fibroblasts
Primary dermal fibroblast cultures were established as previously described. (9) Primary dermal fibroblasts cultures were established using a 0.5 cm diameter punch biopsy of shaved skin obtained from the abdomen of each goat under general anesthesia and in aseptic conditions. The tissue was washed in sterile PBS, mashed into small fragments and left for 10 min in an incubator at 37°C with 5% CO2 and 85% humidity to enhance their adhesion to the plastic surface. Complete culture medium (Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 5% horse serum, 100 U/ml penicillin and 100 μg/ml streptomycin) was then carefully added. Tissue cultures were incubated at 37°C in a humidified incubator with 5% CO2 until confluent fibroblast layers were obtained. Tissue fragments were then removed and cells were expanded in culture for at least six passages, plated at a density of 5 × 104 cells/cm2 and grown to 80% confluence for adenoviral infection.
Adenoviral-Mediated Transduction
Subconfluent dermal fibroblasts were infected with either Ad-hBMP2 or Ad-eGFP using a MOI of 100 p.f.u. per cell. Ad-eGFP was used as a vector control for testing transduction efficiency, whereas untransduced cells served as additional negative controls. The efficiency of adenovirus-mediated transduction was calculated by both evaluating the number of Ad-eGFP transduced cells under fluorescence microscopy and measuring transgene expression in Ad-hBMP-2 transduced cells by RT-PCR and qPCR. For animal treatments, transduced cells were harvested 24 h after viral infection, washed twice in PBS, resuspended in sterile PBS (2 × 104 cells/μl) and adsorbed onto an injectable bone paste made by nano-particles of hydroxyapatite (Ha) magnesium-enriched (Mg-Ha) in PBS solution (SINTlife® paste- Fin-Ceramica Faenza S.p.A). (12) (13)
Real-Time Polymerase Chain Reaction
Transgenic BMP-2 expression was quantitatively assessed by real-time polymerase chain reaction (PCR) on RNA isolated from dermal fibroblasts after 0, 2, 7, and 14 days of adenoviral-mediated transduction, using Ad-eGFP transduced cells at the same time points as negative controls. Three independent biological replicated for each tested time point were carried out. Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) in accord with the manufacturer’s instructions, and digested with amplification-grade DNase I (Qiagen). The yield of RNA isolation was determined using spectrophotometry (Beckman DU800; Beckman Coulter, Fullerton, CA), and the quality and integrity of total RNA were assessed by electrophoresis on 1% agarose gel in RNase-free conditions. Purified RNA (1 μg) was then reverse-transcribed (RT) using the SuperScript First-Strand Synthesis System with random hexamers (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. To rule our genomic DNA contamination, no-reverse controls (ie, RT reactions carried out in the absence of the reverse-transcriptase enzyme) were added for each sample. Thereafter, the single stranded cDNA was used as a template for real-time PCR, performed using the SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and hBMP-2 sequence-specific primers. Target genes were normalized to the reference housekeeping 18S ribosomal RNA gene. The analysis was performed on an ABI Prism 7900 Sequence Detection System (Applied Biosystems).
Animal Model and Surgical Procedure
All procedures and experiments on animals were approved by the Animal Care and Use Committee of the Catholic University of Rome. Ten adult Dutch milk goats were obtained from a professional stockbreeder at least 4 months prior to surgery. On the day of the surgery, aliquots of Ad-hBMP-2 and Ad-ψ5 transduced and non-transduced cells were transported to the operating room. In each goat L4 vertebral body was injected with absorbed Ad-hBMP-2 transduced cells, whereas L5 vertebral body was injected with absorbed non-transduced cells, L6 vertebral body was injected with non absorbed non-transduced cells and the L3 vertebral body was left untreated. Before surgery the animals were sedated by an intravenous injection of detomidine, and general inhalation anesthesia was provided by a halothane gas mixture. The vertebral body access was realized using custom designed surgical instruments similar to the instruments normally used for vertebral augmentation techniques. Briefly, a small incision was made in the lumbar region through which, using fluoroscopy, a trocar was inserted through the pedicle into the vertebral body. The trocar was then removed and a K-wire was placed along its path, the K-wire was finally substituted by a working cannula through which a curette was inserted and used to create an intrasomatic bone defect. The trabecular intrasomatic bone defect was 5 mm in diameter and 10 mm in length. In the meanwhile cells were mixed with the SINTlife biomaterial using a two-syringe system. The absorbed cells were then injected into the vertebral body bone defect through the cannula previously inserted. This procedure was repeated for each treated vertebral level in each animal. Postoperative pain relief was achieved by buprenorphine; antibiotic prophylactics consisted of cephazolin subcutaneously administered for 3 days postoperatively (doses 0.5 g per day). After surgery all animal were returned to their cages without any movement restraint. The animals were euthanized using an injection of 20 mL of 400 mg Na-pentobarbital/mL into the jugular vein at 4 and 12 weeks post-implantation. The lumbar spines were dissected for further analysis.
Radiological Analysis
The goat spines were evaluated by x-rays and by computed tomography scans. An electron-beam CT scanner was used (C300 EBT; GE Imatron, San Francisco, CA, USA). We used a standardized imaging protocol that included the following parameters: collimation 3 mm, field-of-view 9 cm, table increment 3 mm resulting in a pitch of 1124 mAs, and 130 kV. All animals were examined following the standardized imaging protocol, and a volume-rendering technique was used to reconstruct the images after scanning. Vertebral bodies were also dissected and embedded for histological analysis. Von Kossa and Alizarin Red stainings were performed to assess the formation of a mineralized matrix.
Results
Dermal Fibroblasts were efficiently transduced using Adenoviral Vectors
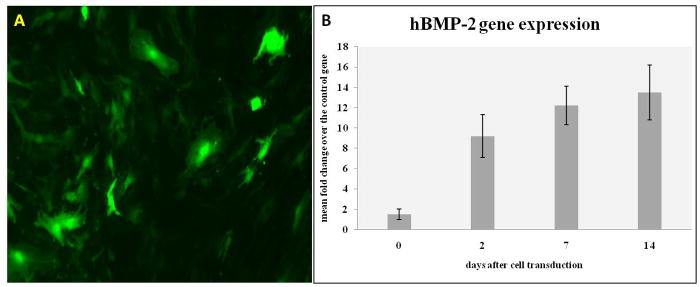
Dermal fibroblasts were isolated in primary culture from skin biopsies obtained from each goat. Spindle-shaped fibroblastoid cells appeared in culture as soon as 2 to 3 days after tissue plating. Fibroblasts were cultured for few passages and then transduced with either Ad-hBMP-2 or Ad-eGFP. To determine whether primary dermal fibroblasts could be efficiently transduced with adenoviral vectors, cells were infected with Ad-eGFP using an MOI of 100 pfu per cell. eGFP-positive cells were then evaluated by fluorescence microscopy, which showed significant and time-related transgene expression, in that the number of fluorescent cells approached 50% and 90%, 24 and 48 hours, respectively, after transduction (Figure 1A). hBMP-2 expression in transduced cells was also tested by means of QT-PCR analysis. Gene expression analysis was performed on multiple experiments and at different timepoints (Figure 1B).
Figure 1.
Culture and transduction efficiency of skin fibroblasts. (A) skin fibroblasts were transduced using a defective adenovirus carrying the eGFP gene, eGFP expression at 48 hours; (B) qPCR analysis showing efficient expression of hBMP-2 in transduced skin fibroblasts;
Engineered dermal fibroblasts promote bone formation in the rat mandibular bone defect
A critical bone defect (non healing defect) was created in all animal at each treated vertebral level. Autologous Ad-hBMP-2 transduced cells were adsorbed on the SINTlife scaffold and implanted in the intrasomatic bone defect, to evaluate their in vivo osteogenic potential. Naked scaffold and scaffold seeded with untransduced cells served as controls. The occurrence of bone formation at the site of implantation was then evaluated at 1 and 3 months post-operatively by means of histological analysis and CT scans, with 3D reconstruction.
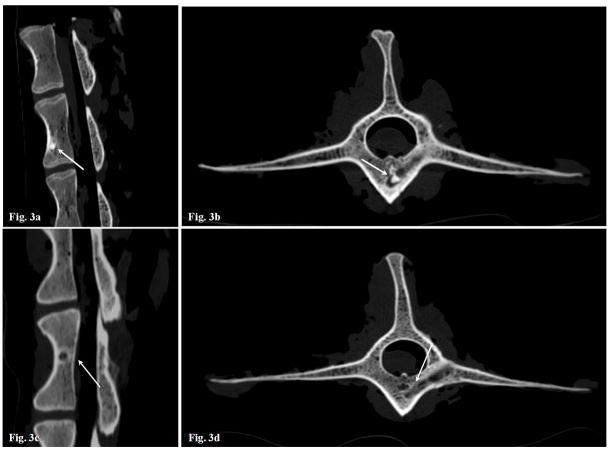
None of the animals showed evidence of systemic or local toxicity. CT analysis revealed an almost complete repair of the defects in all vertebral levels treated with autologous dermal fibroblasts transduced with Ad-BMP-2 (Figure 3) at 12 weeks after implantation, whereas a still not complete repair was observed at 4 weeks timepoints. Control animals, treated with either nontransduced cells (Figure 3) or unseeded scaffold, were negative for bone formation up to 12 weeks after surgical implantation. Histological analysis showed that the newly formed tissue inside the bone defect was constituted by compact bone (data not shown), this confirming the successful bone healing induced by the treatment. No bone tissue could be morphologically detected in all control levels.
Figure 3.
CT sagittal and axial scans. (A) Sagittal scanning, Ad-hBMP-2 transduced cells absorbed on SINTlife biomaterial, a complete healing of the bone defect can be observed at 12 weeks; (B) Axial scanning, Ad-hBMP-2 transduced cells absorbed on SINTlife biomaterial, partial healing can be observed at 4 weeks; (C) Sagittal scanning, untreated vertebral level, no healing can be observed at 12 weeks; (D) Axial scanning, SINTlife biomaterial injected vertebra, only a partial healing can be observed at 12 weeks. The transpedicular surgical access is still visible;
Discussion
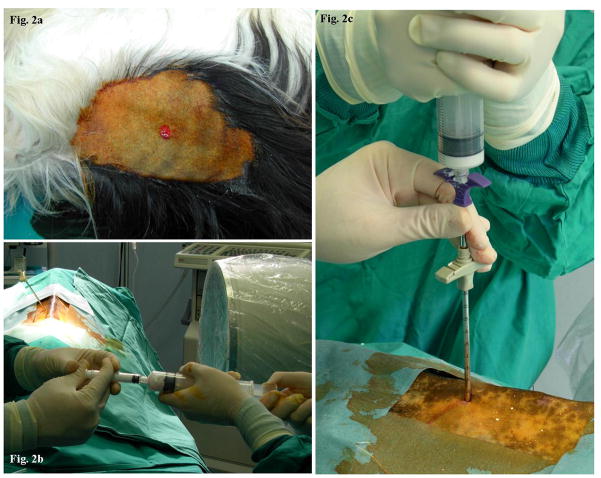
Traumatic vertebral fractures in young people often require an invasive surgical approach or a prolonged conservative treatment. The reduction of vertebral compression fractures leaves residual bone defects that pose a significant challenge to the practicing spinal surgeon. (3) Bone-grafting procedures often allow filling of bone defects, but the materials commonly used in everyday practice have many limitations. (5) (4) Tissue engineering and gene therapy technologies currently represent the most attractive and promising tools for orthopaedic reconstruction surgery. Recombinant human BMP-2 has been approved for clinical use by FDA, however a significant amount of recombinant protein is required to promote osteogenesis. Ex vivo gene therapy using autologous skin fibroblasts offers great advantages for future clinical applications, as we have previously showed in many animal models. (9) The aim of this study was to develop a minimally invasive gene therapy approach to apply to the treatment of anterior column bone defects. We developed designed custom surgical tools for minimally invasive access to the vertebral body (Figure 2). We then created a critical intrasomatic bone defect at different vertebral levels and filled the defect with Ad-BMP-2 transduced autologous skin fibroblasts absorbed on a biological scaffold.
Figure 2.
(A) skin punch biopsy for dermal fibroblast extraction; (B) 2 syringe system for transduced cell absorbing on scaffold biomaterial; (C) intrasomatic transduced skin fibroblasts and biomaterial injection;
Our radiological and histological results show a complete healing of the bone defect after 12 weeks using Ad-BMP-2 transduced cells absorbed on SINTlife scaffold. Moreover no animal reported any toxicity or complication from the surgical approach. Thus, our findings suggest the feasibility of using a minimally invasive percutaneous approach to deliver gene therapy engineered cells in the intrasomatic bone. The main advantage of this kind of approach over the standard vertebral augmentation techniques is to promote bone healing of complex fractures and anterior column bone defects through a biological way. Dermal fibroblasts present diverse advantages over other cell types for tissue engineering, considering their resistance to enzymatic digestion, the less invasive sampling procedure along with the easy isolation, and in vitro culture procedures. Moreover, the potential plasticity of dermal fibroblasts has been recently demonstrated, which allows a switch from a somatic differentiated phenotype to multipotency upon selected stimulations. Moreover, the use of autologous dermal fibroblast prevents the risk of an autoimmune reaction.
Several other experiments are ongoing as part of this study, mainly we are focused on determining the fate of the injected dermal fibroblasts during the osteogenic process.
References
- 1.Gertzbein SD Scoliosis Research Society. Multicenter spine fracture study. Spine. 1992;17:528–40. doi: 10.1097/00007632-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Hauser CJ, Visvikis G, Hinrichs C, Eber CD, Cho K, Lavery RF, Livingston DH. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma. 2003;55:228–34. doi: 10.1097/01.TA.0000076622.19246.CF. [DOI] [PubMed] [Google Scholar]
- 3.Lindner T, Kanakaris NK, Marx B, Cockbain A, Kontakis G, Giannoudis PV. Fractures of the hip and osteoporosis: the role of bone substitutes. J Bone Joint Surg Br. 2009;91:294–303. doi: 10.1302/0301-620X.91B3.21273. [DOI] [PubMed] [Google Scholar]
- 4.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Nemzek JA, Arnoczky SP, Swenson CL. Retroviral transmission in bone allotransplantation: the effects of tissue processing. Clin Orthop Relat Res. 1996;324:275–282. [PubMed] [Google Scholar]
- 6.Nakano M, Hirano N, Ishihara H, Kawaguchi Y, Watanabe H, Matsuura K. Calcium phosphate cement-based vertebroplasty compared with conservative treatment for osteoporotic compression fractures: a matched case-control study. J Neurosurg Spine. 2006;4:110–117. doi: 10.3171/spi.2006.4.2.110. [DOI] [PubMed] [Google Scholar]
- 7.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, Laforte AJ, Yin S. Osteogenic protein-1 in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 8.Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospetive, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Lattanzi W, Parrilla C, Fetoni A, Logroscino G, Straface G, Pecorini G, Stigliano E, Tampieri A, Bedini R, Pecci R, Michetti F, Gamboto A, Robbins PD, Pola E. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models. Gene Ther. 2008;15:1330–1343. doi: 10.1038/gt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- 11.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landi E, Logroscino G, Proietti L, Tampieri A, Sandri M, Sprio S. Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J Mater Sci Mater Med. 2008;19:239–247. doi: 10.1007/s10856-006-0032-y. [DOI] [PubMed] [Google Scholar]
- 13.Landi E, Tampieri A, Mattioli-Belmonte M, Celotti G. Biomimetic Mg- and Mg, CO3-substituted hydroxyapatites: synthesis characterization and in vitro behaviour. Journal of the European Ceramic Society. 2006;26:2593–2601. [Google Scholar]
- 14.Graham FL, van der Ed Aj. A new technique for assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–7. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]