Abstract
Context
Previous studies have found that few chronically depressed patients remit with antidepressant medications alone.
Objective
To determine the role of adjunctive psychotherapy in the treatment of chronically depressed patients with less than complete response to an initial medication trial.
Design
This trial compared 12 weeks of (1) continued pharmacotherapy and augmentation with cognitive behavioral analysis system of psychotherapy (CBASP), (2) continued pharmacotherapy and augmentation with brief supportive psychotherapy (BSP), and (3) continued optimized pharmacotherapy (MEDS) alone. We hypothesized that adding CBASP would produce higher rates of response and remission than adding BSP or continuing MEDS alone.
Setting
Eight academic sites.
Participants
Chronically depressed patients with a current DSM-IV–defined major depressive episode and persistent depressive symptoms for more than 2 years.
Interventions
Phase 1 consisted of open-label, algorithm-guided treatment for 12 weeks based on a history of antidepressant response. Patients not achieving remission received next-step pharmacotherapy options with or without adjunctive psychotherapy (phase 2). Individuals undergoing psychotherapy were randomized to receive either CBASP or BSP stratified by phase 1 response, ie, as nonresponders (NRs) or partial responders (PRs).
Main Outcome Measures
Proportions of remitters, PRs, and NRs and change on Hamilton Scale for Depression (HAM-D) scores.
Results
In all, 808 participants entered phase 1, of which 491 were classified as NRs or PRs and entered phase 2 (200 received CBASP and MEDS, 195 received BSP and MEDS, and 96 received MEDS only). Mean HAM-D scores dropped from 25.9 to 17.7 in NRs and from 15.2 to 9.9 in PRs. No statistically significant differences emerged among the 3 treatment groups in the proportions of phase 2 remission (15.0%), partial response (22.5%), and non-response (62.5%) or in changes on HAM-D scores.
Conclusions
Although 37.5% of the participants experienced partial response or remitted in phase 2, neither form of adjunctive psychotherapy significantly improved outcomes over that of a flexible, individualized pharmacotherapy regimen alone. A longitudinal assessment of later-emerging benefits is ongoing.
Trial Registration
clinicaltrials.gov Identifier: NCT00057551
The past decade has witnessed significant advances in the treatment of chronic depression. Several antidepressant medications1 and forms of psychotherapy2,3 have shown efficacy. Still, approximately 50% of chronically depressed patients in intent-to-treat analyses fail to respond to an adequate trial of antidepressant medication or psychotherapy, and an additional 20% do not obtain complete remission.1,4–6 This is important because residual symptoms are associated with significant functional impairment7 and increased risks of relapse and recurrence.8–10
When patients do not respond adequately to antidepressant medications, clinicians generally adopt one of 3 strategies: switching medication, augmentation with adjunctive medication, or adding psychotherapy. There is a growing literature on the first two strategies,11–14 including work specific to chronic depression. 15 Few data address the third option, even though it may be among the most common. Although most studies have failed to demonstrate decisive advantages for combined medication and psychotherapy over monotherapy in acute major depressive disorder (MDD)16–19 or dysthymic disorder,20 one large study found combination treatment significantly more efficacious than either medication or psychotherapy alone in chronic depression.2 Combination treatment is more expensive, at least acutely, than medication alone. Therefore, the most fiscally conservative strategy may be a stepwise approach: initially treat chronic depression with antidepressant medication, and add adjunctive psychotherapy for patients who have a poor or partial response. Recent data21,22 suggest that this may be a useful alternative to further courses of pharmacotherapy for MDD. The value of combining both strategies (ie, changing pharmacotherapy and adding psychotherapy) has not been studied. We therefore formulated the present study believing that augmentation with psychotherapy might play an important role in treating chronically depressed patients because chronic depression is less likely to respond to medication than is acute MDD,1 suggesting a greater need for adjunctive treatment strategies, and because patients with chronic depression exhibit greater psychosocial and interpersonal deficits than do patients with acute MDD,23,24 problems that psychotherapy may address. Because a previous study by our group found that the cognitive behavioral analysis system of psychotherapy (CBASP)25 had a significant additive effect on chronic depression when combined with the antidepressant nefazodone hydrochloride2 and appeared to be an effective switch option when nefazodone alone was ineffective,26 we chose this psychotherapy for the present trial.
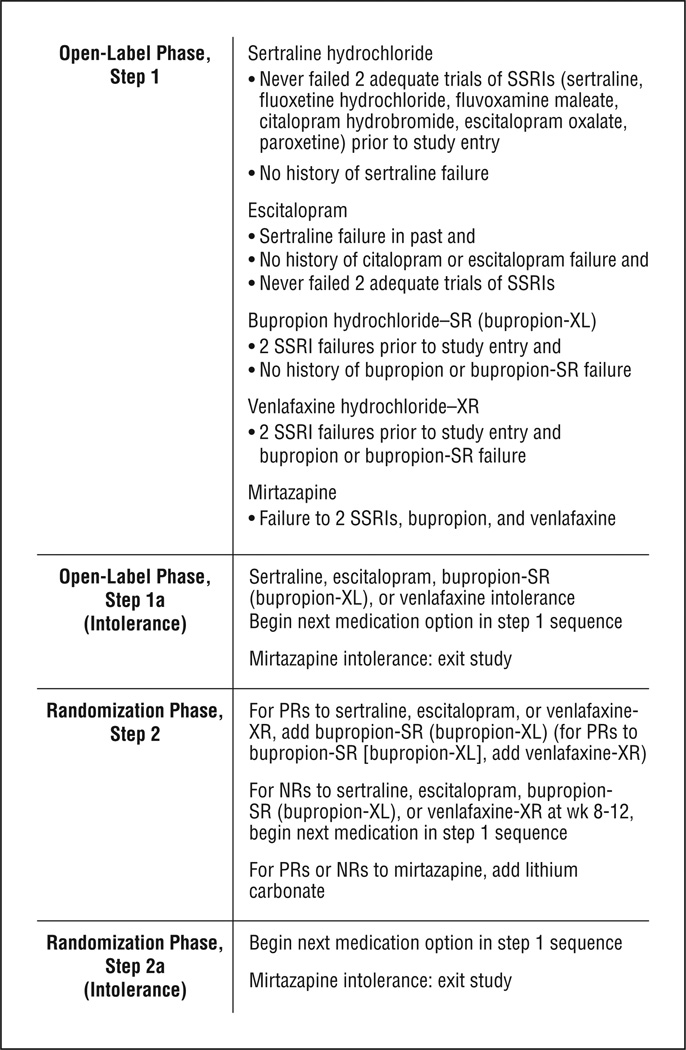
This study was designed to address 2 aims. The first was to compare the efficacy of adding psychotherapy to continued treatment with a medication algorithm relative to continued medication algorithm alone in outpatients with chronic MDD who were either nonresponders (NRs) or partial responders (PRs) to an initial trial of an antidepressant medication algorithm. A medication algorithm was chosen to optimize the pharmacological treatment and increase generalizability to clinical practice. The medication algorithm (Figure 1) was based on the Texas Medication Algorithm Project and the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study.27,28 Nonresponders and PRs to an open-label phase of the study were changed to a new medication or medication combination (per the algorithm) at the start of the randomized phase of the trial. This design tested the hypothesis that, among NRs and PRs to medication, adding psychotherapy would yield higher rates of response and remission than continuing optimized pharmacotherapy (MEDS) alone.
Figure 1.
Pharmacotherapy algorithm for chronic depression. NRs indicates nonresponders; PRs, partial responders; SR, sustained release; SSRI, selective serotonin reuptake inhibitor; and XL, extended release.
The second aim was to examine the specific efficacy of CBASP as augmentation by comparing it against augmentation with brief supportive psychotherapy (BSP). The CBASP is a highly structured, manual-guided therapy developed specifically to treat chronic depression and has shown value for this disorder when combined with medication. 2 However, it was unclear whether CBASP had specific efficacy for chronic depression or whether other psychotherapies would be equally efficacious. To test the specific efficacy of CBASP, we compared CBASP with manual-guided BSP that contained the nonspecific therapeutic factors most forms of psychotherapy share (eg, empathy, understanding, therapeutic optimism, and acknowledgment of patients’ assets) but lacked the structure and specific direct interventions of CBASP or other empirically validated psychotherapies for depression. We hypothesized that adding CBASP for NRs and PRs would produce higher rates of response and remission than would adding BSP or continuing MEDS alone.
METHODS
DESIGN
Conducted between 2002 and 2006, the study had two 12-week phases. During phase 1, patients were assigned to receive an antidepressant medication according to a pharmacotherapy algorithm and response was evaluated. Patients achieving less than remission (≥60% reduction in Hamilton Scale for Depression [HAM-D] score, a 24-item HAM-D total score less than 8, and no longer meeting DSM-IV criteria for MDD for 2 consecutive visits during weeks 6 through 12) were randomized into phase 2. Our definitions of response and remission differed from standard definitions29 because many patients with a 50% reduction in HAM-D score continue to have residual symptoms that could benefit from augmentation.
Phase 2 subjects all received the next-step treatment in the pharmacotherapy algorithm and were randomly assigned to one of 3 treatment cells in a 2:2:1 ratio: to have CBASP or BSP added to their pharmacotherapy or to receive MEDS alone. The randomization was stratified according to whether patients achieved remission vs partial response in phase 1. The 12-week duration mirrored those of the previous study by our group2,26 and the STAR*D study.22
SUBJECTS
Eight academic centers were clinical sites. Recruitment involved outreach to clinicians and advertising. Patients had a current major depressive episode, defined by DSM-IV and assessed on the Structured Clinical Interview for DSM-IV Axis-I Disorders, Patient Edition,30 for at least 4 weeks and depressive symptoms for more than 2 years without remission. Subjects met criteria for double depression (ie, current major depression with antecedent dysthymic disorder), chronic major depression, or recurrent major depression with incomplete recovery between episodes. Patients were between 18 and 75 years old, had scored at least 20 on the 24-item HAM-D31,32 at baseline, were fluent in English, understood the nature of the study, and had provided signed informed consent.
Exclusion criteria were pregnancy; current diagnosis of any psychotic disorder; history of bipolar disorder; dementia; a principal diagnosis of posttraumatic stress disorder, anorexia, bulimia nervosa, or obsessive-compulsive disorder; antisocial, schizotypal, or severe borderline personality disorder; and current alcohol or other substance-related dependence disorder (excepting nicotine dependence) requiring detoxification. Patients with substance abuse disorders were permitted to enroll if they agreed to participate in Alcoholics Anonymous or chemical dependence counseling and to implement a sobriety plan in conjunction with study treatment.
Also excluded were patients who previously had been treated with CBASP, who had already failed at least 4 of the treatment steps in the pharmacotherapy algorithm, who were unwilling to terminate other forms of psychiatric treatment, and who had serious unstable or terminal medical illness that would compromise study participation.
PHARMACOTHERAPY
The pharmacotherapy algorithm (Figure 1) was based on empirically derived algorithms33–35 such as the Texas Medication Algorithm Project27 and other expert approaches36 and closely paralleled those in the STAR*D study.28 The sequence included 2 selective serotonin reuptake inhibitors (SSRIs), sertraline hydrochloride and escitalopram oxalate, and newer alternatives to SSRIs. Sertraline has documented efficacy in chronic depression. 4,6,7 Bupropion hydrochloride (in both the sustained release and extended release formulations) was intended for patients who reported no response to 2 adequate SSRI trials or to augment treatment in those who responded only partially to an SSRI. Patients who had not benefited from these medications were offered more options, including venlafaxine hydrochloride extended release, mirtazapine, and lithium carbonate augmentation. Protocols specified minimum and maximum doses, speed of dosage escalation, and trial lengths.
Patients were evaluated every 2 weeks. To minimize attrition, a patient intolerant to a medication during the first 4 weeks could be moved to the next level of the sequence (from receiving sertraline to receiving escitalopram, from escitalopram to bupropion sustained or extended release, from bupropion sustained or extended release to venlafaxine extended release, or from venlafaxine extended release to mirtazapine). Zolpidem tartrate and zaleplon were allowed for insomnia. No other psychotropic medications were permitted. Biweekly pharmacotherapy visits continued until a patient met remission criteria for 2 consecutive visits. Remitters were then followed up monthly and remained on the medication through week 24.
Pharmacotherapists followed the manual by Fawcett et al37,38 from the National Institute of Mental Health Treatment of Depression Collaborative Research Program study,39 with minimal psychotherapeutic intervention. During the randomized phase of the study, sessions were audiotaped and reviewed for adherence to guidelines. Bimonthly supervision by senior pharmacotherapists helped to ensure adherence.
Patients were given packets of pills containing their daily dose for the interval between visits. At each visit, pharmacotherapists asked patients about treatment adherence and to return unused pills.
COGNITIVE BEHAVIORAL ANALYSIS SYSTEM OF PSYCHOTHERAPY
The CBASP is highly structured therapy that falls squarely within the family of cognitive and behavioral therapies. It differs from cognitive therapy in its focus on a structured interpersonal problem-solving algorithm and by viewing maladaptive cognitions more in terms of their contribution to desired outcomes in interpersonal situations than their validity. It is similar to interpersonal psychotherapy in its focus on interpersonal problems, but it is much more highly structured, emphasizes teaching a specific approach to interpersonal problem-solving, and makes extensive use of structured homework assignments. Comprehensive descriptions of CBASP and therapist and patient manuals have been published.25,40,41 In our study, CBASP was administered twice weekly during weeks 1 through 4 and weekly during weeks 5 through 12. Therapists and supervisors were trained and certified in CBASP by James McCullough, PhD, who developed the therapy. Therapists were required to have at least 2 years of clinical experience after their psychiatric residency or to have completed a PhD program, or to have had 5 years of experience after completing a masters in social work degree. Therapists met with site supervisors weekly. Therapy sessions were videotaped, and the integrity of the therapists’ adherence to protocol and performance was monitored by McCullough and the site supervisors using a CBASP Therapist Adherence Rating Scale that is based on the manual41 and probes specific behaviors unique to the delivery of CBASP as well as behaviors foreign to CBASP methods.
BRIEF SUPPORTIVE PSYCHOTHERAPY
As defined in an unpublished treatment manual (J.C.M. and Michael H. Sacks, MD, 2002), BSP emphasizes the nonspecific or “common” factors assumed to be important ingredients across psychotherapies,42,43 including reflective listening, empathy, evoking affect, therapeutic optimism, and acknowledgment of patients’ assets. Specific interpersonal, cognitive, behavioral, and psychodynamic interventions were strictly proscribed. Paralleling the CBASP condition, 16 to 20 BSP sessions were scheduled during the 12 weeks of treatment. The BSP therapists’ professional degrees, amount of clinical experience, training, and supervision were comparable to those of the CBASP therapists. The certification and training procedures were led by one of us (J.C.M.).44
ADHERENCE MONITORING
During the randomized phase of the study, all treatments (including pharmacotherapy) were monitored to ensure therapist adherence to protocol. All psychotherapy sessions were videotaped and all pharmacotherapy sessions were audiotaped. For each psychotherapy therapist-patient dyad, 1 or 2 tapes were randomly selected—1 early (sessions 2-6) and 1 from later in treatment (session 8 or later)—and rated in their entirety using the Collaborative Study Psychotherapy Rating Scale from the National Institute of Mental Health Treatment of Depression Collaborative Research Program study39 and the Therapist Adherence Rating Scale. Together, these 2 scales assess adherence to BSP, pharmacotherapy, and CBASP guidelines. Selected pharmacotherapy audiotapes were also monitored using the same scales. Adherence ratings were conducted at the Cornell site by 2 trained raters of established reliability.
RANDOMIZATION
Randomization was done centrally at the Pittsburgh data-coordinating center stratified by site, phase 1 response status (ie, no response or partial response), and medication history (failure to respond to <3 adequate medication trials, including the trials given in this study, vs failure to respond to ≥3 adequate medication trials).
OUTCOME MEASURES
The primary outcome measure during the randomized phase 2 portion was the categorical response status as an NR, PR, or remitter at each visit. Being a remitter was defined as having a HAM-D score of less than 8 that had decreased by at least 50% from baseline and having a Clinical Global Improvement (CGI) score of 1 or 2 for 2 consecutive visits. Being a PR was defined as having a HAM-D score of 8 to 16 that had decreased by at least 50% from baseline and having a CGI score of 3 or less or as having a HAM-D score of less than 8 and a CGI score of 1 or 2 for 1 week but not for 2 consecutive weeks. Such a participant was classified as a PR for that 1 week. Being an NR was defined as not meeting the criteria for being either a remitter or a PR. The HAM-D and CGI ratings were performed every 2 weeks.
Secondary outcome measures included the HAM-D score, HAM-D remission, the Quick Inventory of Depressive Symptoms score, and the Range of Impaired Functioning Tool (LIFE-RIFT). We chose the 24-item version of the HAM-D32 because it contains cognitive items characteristic of chronically depressed patients and had been used in all previous major chronic depression studies. A HAM-D remission at any given visit was defined as having a HAM-D score of less than 8 that had decreased by at least 50% from baseline. The Quick Inventory of Depressive Symptoms, developed by Rush et al,45 includes 16 items that assess the 9 symptom groups in the DSM-IV criteria for MDD and was rated by the treating pharmacotherapist. It has good internal consistency, is highly correlated with more established rating scales for depression, and is sensitive to change. A remission according to the Quick Inventory of Depressive Symptoms was defined as having a total score of less than 6. Treating psychiatrists also determined a CGI32 score at each visit. The Longitudinal Interval Follow-up Evaluation (LIFE) psychosocial assessments46 were rated at baseline and at 4-week intervals to score the LIFE-RIFT,47 a brief measure of functional impairment that examines work, interpersonal relations, satisfaction, and recreation.
Adverse Effects
The adverse effect burden was evaluated with the Frequency, Intensity, and Burden of Side Effects Rating,48 a 3-item assessment of the frequency, intensity, and overall burden of adverse effects in the past 7 days at each pharmacotherapy visit.
Assessment Blinding Procedures
To maintain unbiased estimates of treatment effects, the HAM-D and LIFE-RIFT evaluations were performed by blinded raters. Raters instructed patients at the beginning of each rating session not to mention psychotherapy or their psychotherapist during the interviews. Each site had managed blinding successfully in previous studies by adhering to this procedure and by ensuring physical separation between clinicians and raters.
DATA ANALYSIS
The randomized treatment groups were compared on baseline demographic and clinical variables. Analyses of variance or Kruskal-Wallis tests were used to compare the groups on continuous or ordinal variables. We used χ2 tests for categorical variables. Similar analyses were used to compare dropouts and completers on baseline demographic and clinical variables. Each statistical test in this report had a 2-tailed α level of .05.
The general data analytic strategy for efficacy used mixed-effects models because they are flexible enough to account for different numbers of observations per subject. Furthermore, this modeling procedure can account for the changing symptomatic state of subjects over the course of the trial. To compare the efficacy of augmentation with CBASP and BSP vs MEDS alone on the primary outcome measure, mixed-effects ordinal logistic regression analyses49 were based on the categorical dependent variable response status (NR, PR, or remitter). Mixed-effects linear regression analysis was used for secondary dimensional outcome measures such as the HAMD and the LIFE-RIFT. Each model included 2 random effects (intercept and slope) and fixed effects for treatment, site, time, and response to treatment in phase 1 of the study (NR or PR).
RESULTS
SUBJECTS
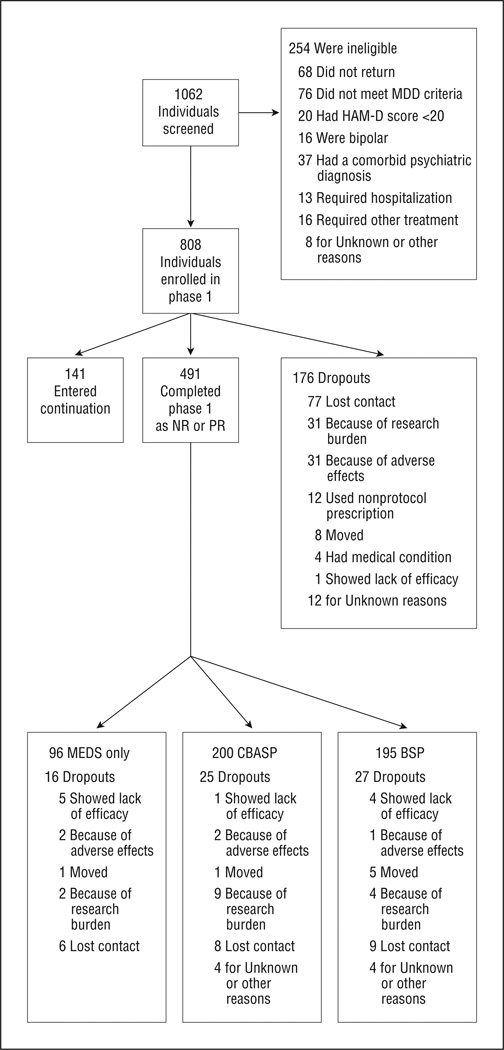
We screened 1062 potential participants and enrolled 808 in phase 1 (Figure 2). Index depressive episodes had lasted longer than 6 years on average, and the mean duration of illness since the first episode of MDD or dysthymic disorder was 20 years. Of the entire sample, 36.0% had chronic MDD, 30.9% had MDD with incomplete interepisode recovery, and 33.1% had double depression, with approximately two-thirds of the last group meeting criteria for both chronic MDD and dysthymic disorder. Lifetime rates of anxiety disorder and alcohol and other drug abuse disorders were similar across the 3 treatment groups. Although 62.7% had educations beyond high school, 32.0% were unemployed at study entry. Only 41.1% were married. Of the participants, 10.2% had made suicide attempts. The mean (SD) lifetime number of depressive episodes was 2.7 (5.9). A moderate level of depression severity was present at study entry, with a mean 24-item HAM-D score of 28. Past treatment varied, but only 32.7% had received an adequate trial of pharmacotherapy. 50 Of the 808 patients who entered the algorithm, 620 received sertraline; 92, escitalopram; 63, bupropion; 30, venlafaxine; and 2, mirtazapine. (The drug was unknown for 1 patient who dropped out at the week 2 visit. Phase 1 was completed by 632 patients (78.2%). Of those who completed phase 1, 141 (22.3%) remitted and 491 (77.7%) were PRs or NRs.
Figure 2.
Research Evaluating the Value of Augmenting Medication With Psychotherapy (REVAMP) flowchart. BSP indicates brief supportive psychotherapy; CBASP, cognitive behavioral analysis system of psychotherapy; HAM-D, Hamilton Scale for Depression; MDD, major depressive disorder; MEDS, optimized pharmacotherapy; NR, nonresponder; and PR, partial responder.
Table 1 provides the clinical and demographic characteristics of the 491 participants enrolled in phase 2 by randomization group. There were no statistically significant differences among the groups randomized to the 3 treatments except for a slightly higher percentage of whites in those randomized to MEDS plus psychotherapy vs MEDS alone.
Table 1.
Baseline Characteristics
| Characteristic | MEDS Only (n=96) |
MEDS+Psychotherapy (n=395) |
P Value | BSP (n=195) |
CBASP (n=200) |
P Value |
|---|---|---|---|---|---|---|
| Categorical Characteristicsa | ||||||
| Race, No. (%) | ||||||
| White | 82 (85.4) | 354 (89.6) | .03 | 174 (89.2) | 180 (90.0) | .72 |
| Black | 1 (1.0) | 16 (4.1) | 7 (3.6) | 9 (4.5) | ||
| Other | 13 (13.5) | 25 (6.3) | 14 (7.2) | 11 (5.5) | ||
| Hispanic ethnicity, No. (%) | ||||||
| Yes | 9 (9.5) | 29 (7.5) | .51 | 14 (7.2) | 15 (7.7) | .86 |
| No | 86 (90.5) | 360 (92.5) | 180 (92.8) | 180 (92.3) | ||
| Sex, No. (%) | ||||||
| Male | 49 (51.0) | 170 (43.0) | .16 | 82 (42.1) | 88 (44.0) | .70 |
| Female | 47 (49.0) | 225 (57.0) | 113 (57.9) | 112 (56.0) | ||
| Employment status, No. (%) | ||||||
| Employed | 56 (58.3) | 242 (61.6) | .28 | 122 (62.9) | 120 (60.3) | .84 |
| Unemployed | 36 (37.5) | 121 (30.8) | 57 (29.4) | 64 (32.2) | ||
| Retired | 4 (4.2) | 30 (7.6) | 15 (7.7) | 15 (7.5) | ||
| Education, No. (%) | ||||||
| <High school | 0 | 7 (1.8) | .23 | 4 (2.1) | 3 (1.5) | .06 |
| High school graduate | 40 (41.7) | 135 (34.3) | 77 (39.7) | 58 (29.0) | ||
| ≥High school | 56 (58.3) | 252 (64.0) | 113 (58.2) | 139 (69.5) | ||
| Recurrence, No. (%) | ||||||
| Yes | 58 (60.4) | 264 (67.0) | .22 | 128 (65.6) | 136 (68.3) | .57 |
| No | 38 (39.6) | 130 (33.0) | 67 (34.4) | 63 (31.7) | ||
| Attempted suicide, No. (%) | ||||||
| Yes | 12 (12.8) | 38 (10.2) | .46 | 19 (10.4) | 19 (9.9) | .89 |
| No | 82 (87.2) | 336 (89.8) | 164 (89.6) | 172 (90.1) | ||
| Marital status, No. (%) | ||||||
| Married | 41 (42.7) | 161 (40.9) | .31 | 89 (45.6) | 72 (36.2) | .14 |
| Never | 34 (35.4) | 122 (31.0) | 51 (26.2) | 71 (35.7) | ||
| Divorced | 17 (17.7) | 101 (25.6) | 51 (26.2) | 50 (25.1) | ||
| Widowed | 4 (4.2) | 10 (2.5) | 4 (2.1) | 6 (3.0) | ||
| Continuous Characteristics | ||||||
| Age, y | (n=96) | (n=393) | (n=194) | (n=199) | ||
| Mean (SD) | 43.2 (13.4) | 45.9 (11.8) | .05 | 46.4 (11.7) | 45.3 (11.9) | .33 |
| Age at initial onset of MDD, y | (n=92) | (n=380) | (n=190) | (n=190) | ||
| Mean (SD) | 25.0 (11.9) | 26.7 (13.5) | .26 | 26.6 (13.9) | 26.8 (13.2) | .91 |
| Length of index episode of MDD, mo | (n=96) | (n=388) | (n=193) | (n=195) | ||
| Mean (SD) | 91.1 (106.1) | 92.4 (116.0) | .92 | 92.5 (113.2) | 92.4 (119.1) | .99 |
| Duration of MDD, y | (n=92) | (n=378) | (n=189) | (n=189) | ||
| Mean (SD) | 17.7 (12.2) | 19.0 (13.8) | .41 | 19.6 (14.0) | 18.3 (13.6) | .34 |
| No. of episodes of MDD | (n=77) | (n=348) | (n=171) | (n=177) | ||
| Mean (SD) | 2.8 (6.4) | 2.5 (2.2) | .72 | 2.4 (2.0) | 2.6 (2.3) | .59 |
Abbreviations: BSP, brief supportive psychotherapy; CBASP, cognitive behavioral analysis system of psychotherapy; MDD, major depressive disorder; MEDS, optimized pharmacotherapy.
Information was missing for some patients. Percentages are based on the category total and, because of rounding, may not total 100.
RANDOMIZED PHASE TREATMENT DELIVERY AND ADHERENCE
Patients assigned to BSP attended a mean (SD) of 13.1 (7.0) therapy sessions, and patients assigned to CBASP attended 12.5 (6.6) sessions. The mean (SD) numbers of pharmacotherapy visits were 5.4 (1.4), 5.3 (1.5), and 5.2 (1.5) in the CBASP, BSP, and MEDS only groups, respectively. Adherence ratings were conducted on 84 BSP, 68 CBASP, and 52 pharmacotherapy sessions. Only 1 CBASP session was rated as having inadequate adherence to protocol.
TREATMENT OUTCOMES
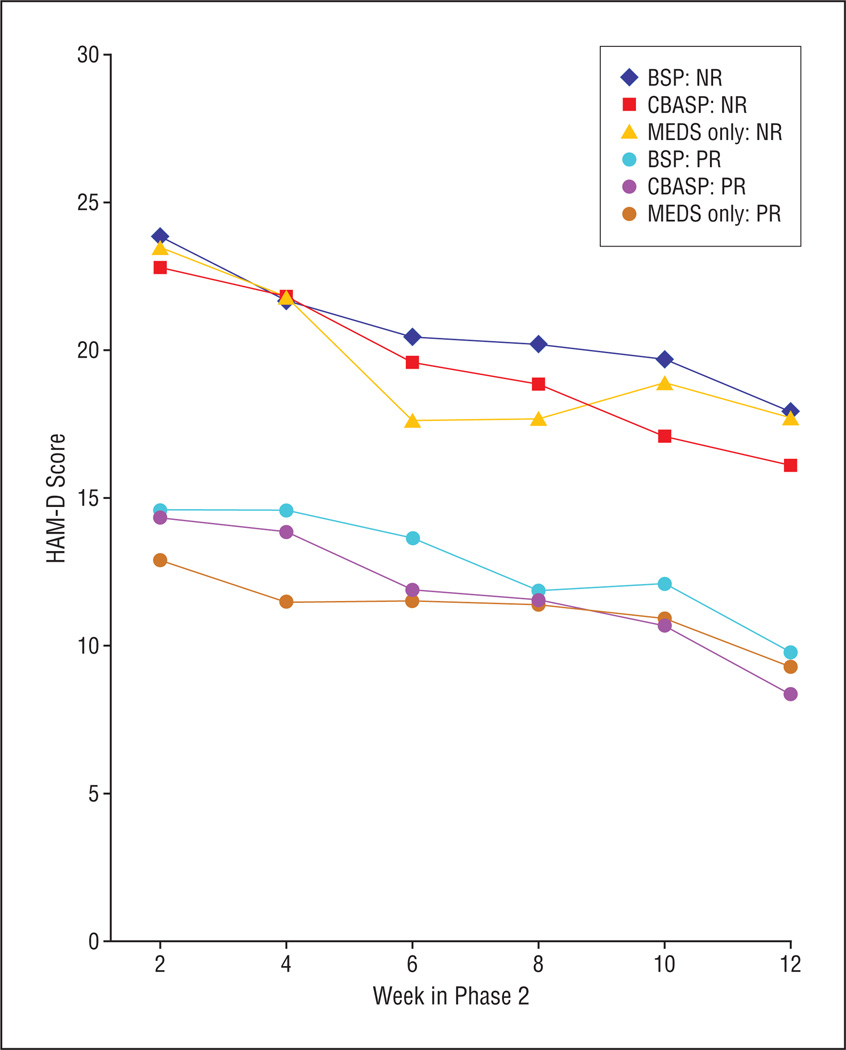
Remission rates and rating scale scores are summarized in Table 2 and Table 3. Mean HAM-D scores dropped from 25.9 to 17.7 in NRs and from 15.2 to 9.9 in PRs (Figure 3). No statistically significant differences emerged among the 3 treatment groups in the proportions of phase 2 remission (15.0%), partial response (22.5%), and no response (62.5%) or in changes on HAM-D scores. All models were fitted with a site main effect and a treatment × site interaction, neither of which was statistically significant in any model, and these were removed from the final models. Mixed-effects ordinal logistic regression analyses conducted on categorical outcomes showed no significant main effect of medication vs psychotherapy (F1,372=0.73, P=.39) or CBASP vs BSP (F1,187=3.72, P=.06). There was a small yet significant treatment × time interaction for the comparison of MEDS only with MEDS plus psychotherapy (F1,2083=4.55, P=.03) but not for the comparison of CBASP with BSP (F1,1683=0.07, P=.79). The significant time × treatment interaction indicates that early in the treatment course those assigned to the MEDS plus psychotherapy arms were somewhat less likely to respond but, by the time treatment had been completed, they were more likely to respond. The response rates at all time points are shown in Table 2. The differences do not appear to be clinically meaningful.
Table 2.
Outcomes of MEDS vs MEDS Plus Psychotherapy for Chronic Depression
| Week | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | |
| MEDS Only | |||||||
| HAM-D score | (n=94) | (n=92) | (n=85) | (n=80) | (n=84) | (n=79) | (n=76) |
| Mean (SD) | 18.37 (8.00) | 16.82 (9.21) | 15.27 (9.46) | 13.74 (7.97) | 13.71 (8.54) | 13.66 (8.52) | 12.28 (8.44) |
| QIDS score | (n=89) | (n=88) | (n=81) | (n=75) | (n=82) | (n=77) | (n=73) |
| Mean (SD) | 10.16 (4.50) | 9.10 (5.19) | 8.49 (5.51) | 7.69 (4.56) | 7.95 (5.12) | 7.60 (4.61) | 7.49 (5.24) |
| LIFE-RIFT score | (n=77) | (n=81) | (n=80) | (n=75) | |||
| Mean (SD) | 12.64 (3.01) | ND | 12.07 (3.54) | ND | 11.15 (3.33) | ND | 10.96 (3.63) |
| Remission, No. (%) | |||||||
| HAM-D | 8 (8.5) | 17 (18.5) | 24 (28.2) | 23 (28.8) | 23 (27.4) | 21 (26.6) | 30 (39.5) |
| QIDS | 15 (16.9) | 24 (27.3) | 29 (35.8) | 27 (36.0) | 28 (34.1) | 32 (41.6) | 32 (43.8) |
| Response, No. (%) | (n=92) | (n=85) | (n=80) | (n=83) | (n=79) | (n=75) | |
| None | … | 79 (85.9) | 66 (77.6) | 59 (73.8) | 61 (73.5) | 58 (73.4) | 48 (64.0) |
| Partial | … | 6 (6.5) | 9 (10.6) | 8 (10.0) | 9 (10.8) | 8 (10.1) | 16 (21.3) |
| Full | … | 7 (7.6) | 10 (11.8) | 13 (16.2) | 13 (15.7) | 13 (16.5) | 11 (14.7) |
| MEDS+Psychotherapy | |||||||
| HAM-D score | (n=384) | (n=370) | (n=359) | (n=346) | (n=341) | (n=333) | (n=342) |
| Mean (SD) | 19.48 (8.27) | 17.87 (8.55) | 17.09 (8.49) | 15.55 (8.65) | 14.74 (8.45) | 14.04 (8.90) | 12.02 (8.39) |
| QIDS score | (n=365) | (n=347) | (n=351) | (n=336) | (n=326) | (n=323) | (n=330) |
| Mean (SD) | 10.85 (4.77) | 9.90 (4.64) | 9.27 (4.65) | 8.81 (4.76) | 8.27 (4.60) | 7.62 (4.83) | 6.96 (4.59) |
| LIFE-RIFT score | (n=306) | (n=342) | (n=326) | (n=334) | |||
| Mean (SD) | 12.70 (3.05) | ND | 11.96 (3.15) | ND | 11.50 (3.29) | ND | 10.48 (3.36) |
| Remission, No. (%) | |||||||
| HAM-D | 29 (7.6) | 46 (12.4) | 50 (13.9) | 71 (20.5) | 76 (22.3) | 89 (26.7) | 119 (34.8) |
| QIDS | 57 (15.6) | 64 (18.4) | 94 (26.8) | 93 (27.7) | 101 (31.0) | 126 (39.0) | 152 (46.1) |
| Response, No. (%)a | (n=367) | (n=358) | (n=344) | (n=339) | (n=330) | (n=340) | |
| None | … | 325 (88.6) | 305 (85.2) | 265 (77.0) | 241 (71.1) | 220 (66.7) | 199 (58.5) |
| Partial | … | 33 (9.0) | 31 (8.7) | 48 (14.0) | 51 (15.0) | 53 (16.1) | 89 (26.2) |
| Full | … | 9 (2.5) | 22 (6.1) | 31 (9.0) | 47 (13.9) | 57 (17.3) | 52 (15.3) |
Abbreviations: Ellipses, not applicable; HAM-D, Hamilton Scale for Depression; LIFE-RIFT, Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool; MEDS, optimized pharmacotherapy; ND, not done; QIDS, Quick Inventory of Depressive Symptoms.
Because of rounding, percentages may not total 100.
Table 3.
Outcomes of BSP vs CBASP for Chronic Depression
| Week | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | |
| BSP | |||||||
| HAM-D score | (n=189) | (n=181) | (n=176) | (n=170) | (n=168) | (n=163) | (n=168) |
| Mean (SD) | 19.44 (8.31) | 18.14 (8.99) | 17.24 (8.04) | 16.28 (8.70) | 15.08 (8.26) | 14.94 (9.38) | 12.77 (8.45) |
| QIDS score | (n=179) | (n=170) | (n=171) | (n=166) | (n=161) | (n=154) | (n=162) |
| Mean (SD) | 10.89 (4.79) | 10.20 (4.88) | 9.50 (4.65) | 9.25 (4.97) | 8.47 (4.59) | 8.03 (4.89) | 7.30 (4.41) |
| LIFE-RIFT score | (n=154) | (n=171) | (n=162) | (n=162) | |||
| Mean (SD) | 12.71 (3.14) | ND | 12.13 (3.15) | ND | 11.76 (3.28) | ND | 10.73 (3.46) |
| Remission, No. (%) | |||||||
| HAM-D | 15 (7.9) | 26 (14.4) | 21 (11.9) | 30 (17.6) | 33 (19.6) | 39 (23.9) | 52 (31.0) |
| QIDS | 31 (17.3) | 32 (18.8) | 45 (26.3) | 43 (25.9) | 47 (29.2) | 56 (36.4) | 67 (41.4) |
| CBASP | |||||||
| HAM-D score | (n=195) | (n=189) | (n=183) | (n=176) | (n=173) | (n=170) | (n=174) |
| Mean (SD) | 19.52 (8.26) | 17.61 (8.13) | 16.94 (8.92) | 14.85 (8.57) | 14.42 (8.65) | 13.18 (8.36) | 11.29 (8.30) |
| QIDS score | (n=186) | (n=177) | (n=180) | (n=170) | (n=165) | (n=169) | (n=168) |
| Mean (SD) | 10.82 (4.76) | 9.60 (4.39) | 9.04 (4.66) | 8.38 (4.52) | 8.08 (4.60) | 7.25 (4.77) | 6.63 (4.76) |
| LIFE-RIFT score | (n=152) | (n=171) | (n=164) | (n=172) | |||
| Mean (SD) | 12.69 (2.96) | ND | 11.78 (3.14) | ND | 11.25 (3.30) | ND | 10.24 (3.25) |
| Remission, No. (%) | |||||||
| HAM-D | 14 (7.2) | 20 (10.6) | 29 (15.8) | 41 (23.3) | 43 (24.9) | 50 (29.4) | 67 (38.5) |
| QIDS | 26 (14.0) | 32 (18.1) | 49 (27.2) | 50 (29.4) | 54 (32.7) | 70 (41.4) | 85 (50.6) |
Abbreviations: BSP, brief supportive psychotherapy; CBASP, cognitive behavioral analysis system of psychotherapy; ellipses, not applicable; HAM-D, Hamilton Scale for Depression; LIFE-RIFT, Longitudinal Interval Follow-up Evaluation Range of Impaired Functioning Tool; ND, not done; QIDS, Quick Inventory of Depressive Symptoms.
Figure 3.
Phase 2 Hamilton Scale for Depression (HAM-D) scores according to phase 1 response status. BSP indicates brief supportive psychotherapy; CBASP, cognitive behavioral analysis system of psychotherapy; MEDS, optimized pharmacotherapy; NR, nonresponder; and PR, partial responder.
Mixed-effects linear regression analysis of secondary dimensional outcome measures, the HAM-D and the LIFERIFT, also showed no significant differences between patients assigned to MEDS only vs MEDS plus psychotherapy (HAM-D: F1,469=0.18, P=.67, and LIFE-RIFT: F1,454=1.03, P=.31). There was a statistically significant difference in HAM-D scores for those assigned to CBASP vs BSP (HAM-D: F1,375=4.16, P=.04) but not for the LIFERIFT (F1,364=2.85, P=.09). Specifically, for the HAM-D, those in the CBASP arm had on average a HAM-D score that was 1.59 points lower than those in the BSP arm, independent of time and severity of depression at presentation. No statistically significant interaction between treatment and time was identified for the comparison of MEDS only with MEDS plus psychotherapy for the HAM-D (F1,469=3.70, P=.06) or the LIFE-RIFT (F1,454=1.22, P=.27), nor was it identified for the comparison of CBASP with BSP for the HAM-D (F1,375=2.53, P=.11) or the LIFE-RIFT (F1,364=0.41, P=.52).
“DOSE” OF PSYCHOTHERAPY
The mean, SD, and range of the number of sessions attended were, respectively, 12.6, 6.7, and 0 to 19 for CBASP and 13.2, 7.0, and 0 to 21 for BSP. Correlations between the number of sessions attended and HAM-D change and final scores were small and not statistically significant. The association of the number of sessions attended with the probability of remission was calculated. The overall odds ratio was 1.01 (P=.40), the BSP odds ratio was 1.01 (P=.62), and the CBASP odds ratio was 1.02 (P=.43).
ADVERSE EFFECTS
The Frequency, Intensity, and Burden of Side Effects Rating form was used to collect information on the adverse effects of the antidepressant medication at each pharmacotherapy visit. Adverse effects of at least moderate intensity were reported by 27.0%, 26.2%, and 17.7% of patients receiving BSP, CBASP, and MEDS only, respectively. Adverse effects of at least moderate burden were reported by 11.8%, 14.0%, and 8.3% of patients receiving BSP, CBASP, and MEDs only, respectively. Dropout owing to adverse effects occurred in 1, 2, and 2 cases in the 3 treatment arms, respectively.
COMMENT
This study found that, among chronically depressed patients who did not fully respond to 12 weeks of MEDS, approximately40%later remitted with 12 more weeks of treatment. Surprisingly, the addition of either of 2 forms of psychotherapy to the pharmacotherapy protocol did not produce significant differences in outcome compared with pharmacotherapy alone. Furthermore, the form of therapy specifically developed for chronic depression—CBASP—added no value over BSP. Our hypothesis that CBASP would produce higher response and remission rates, when compared with adding BSP or continuing MEDS alone, was not supported. These results contradict common clinical practice reflected in the American Psychiatric Association guideline for the treatment of major depression,36 and they also contradict predictions based on previous studies with chronic depression by our group.2,26
What explains these discrepancies? First, sample characteristics may vary across studies. Perhaps there are moderators of response to different psychotherapies, which will be examined in secondary analyses. Second, pharmacotherapy in our previous study2 may not have been optimal. The addition of psychotherapy may have greater benefit in real-world clinical practice. Third, a potentially important difference between this study and that of Keller et al2 was a difference in the mean number of CBASP therapy sessions (16.0 vs 12.5 in the present study). Finally, it is possible that the continued switch/augment strategies used in REVAMP had psychological effects. Our patients may have been less receptive to psychotherapy than were samples in some other studies. The design of the trial ensured that all patients would receive pharmacotherapy throughout, whereas provision of psychotherapy was not guaranteed—a design that may have selected for patients more interested in pharmacotherapy than in psychotherapy. Alternatively, it is conceivable that patients may have become less motivated to engage in psychotherapy after completing 1 or more courses of pharmacotherapy. Another anecdotal impression was that many patients randomized to CBASP and BSP required considerable convincing that psychotherapy was important.47 Future analyses of this sample will examine the effects of patient treatment preferences and the therapeutic alliance. In addition, few studies have examined whether the sequence in which switching/augmentation occurs (ie, pharmacotherapy before psychotherapy vs the opposite) influences treatment outcome.
To our knowledge, the only comparable study of psychotherapy augmentation for patients with major depression is the STAR*D study, which compared the effectiveness of cognitive therapy and pharmacotherapy as second-step strategies for outpatients who had received inadequate benefit from an initial trial of citalopramm hydrobromide.22 Both studies were relatively inclusive, and both used antidepressant pharmacotherapy as the initial treatment. In the STAR*D study, patients who entered a medication algorithm trial were reluctant to consider randomization to psychotherapy. But REVAMP focused specifically on chronic depression, whereas the STAR*D study had a mixed sample. In addition, the STAR*D study offered alternate randomization options for patients who did not wish to receive cognitive therapy. In the STAR*D study, cognitive therapy, both singly and in combination with citalopram, was compared with medication augmentation and switch strategies. Among those who were willing to consider cognitive therapy as a second step, those who received cognitive therapy (alone or in combination with citalopram) had similar response and remission rates to those assigned to medication strategies. Specifically, among patients receiving augmentation with cognitive therapy (n = 65) and pharmacotherapy (n=117), the HAM-D remission rates were 23.1% and 33.3%, respectively. For those who switched to cognitive therapy (n=36) and pharmacotherapy (n=86), the HAM-D remission rates were 30.6% and 26.7%, respectively. For comparison purposes, the last-observation-carried-forward HAM-D remission rates for the 3 treatment groups in REVAMP were 43.0%, 37.9%, and 40.6% for CBASP, BSP, and MEDS, respectively. Overall, neither the STAR*D study nor REVAMP found an advantage for psychotherapy augmentation over MEDS in short-term outcomes.
Taken together with the STAR*D study results, the present findings question the value of psychotherapy augmentation compared with pharmacotherapy augmentation/switch alone. Alternative designs might be more attractive to patients who prefer psychotherapy or might include a component to enhance motivation at the psychotherapy augmentation phase. Perhaps there are subpopulations, such as pregnant or nursing women, individuals who greatly prefer psychotherapy,51 or patients with a history of early adversity,52 for whom psychotherapy augmentation may be an appropriate option. We are currently testing some of these possibilities in moderator analyses using these data.
Limitations of this study include that patients were from academic centers, that the nature of the design and sequencing of interventions may have reduced responsiveness and/or motivation for psychotherapy, and that the duration of psychotherapy may have been too brief for this sample of patients with chronic depression and comorbidities. Like the STAR*D study, the present study used a pragmatic drug treatment design, with broad inclusion criteria and an algorithm of medications. As with the STAR*D study and some other large treatment trials with pragmatic designs,53 the findings have been essentially negative. A speculative point worth investigating is that such designs make differences between treatments hard to demonstrate.
Proponents of cognitive therapy believe that psychotherapy has enduring effects, arguing that patients are taught new skills that they continue to use after the termination of treatment.54,55 We are conducting a follow-up study on the patients in REVAMP to determine whether the combination of CBASP plus MEDS and/or the combination of BSP plus MEDS might have long-term benefits that were not apparent in the short run.
Acknowledgments
Funding/Support: The REVAMP study was sponsored by the NIMH. All medications for this study were donated by Forest Laboratories, GlaxoSmithKline, Organon Pharmaceuticals Inc, Pfizer Inc, and Wyeth Pharmaceuticals.
Role of the Sponsor: The NIMH had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
REVAMP Investigators: The following investigators participated in the design and execution of the study. Weill Medical College of Cornell University, New York, New York (National Institute of Mental Health [NIMH] grant UO1 MH62475): James H. Kocsis, MD (lead principal investigator), John C. Markowitz, MD, Andrew C. Leon, PhD, and Richard A. Friedman, MD. University of Pittsburgh, Pittsburgh, Pennsylvania (UO1 MH61587): Michael E. Thase, MD (co–lead principal investigator), Edward S. Friedman, MD, Robert H. Howland, MD, Stephen R. Wisniewski, PhD (director of the Data Coordinating Center), and Jennifer Barkin, MS. State University of New York, Stony Brook (UO1 MH62546): Daniel N. Klein, PhD, Dina Vivian, PhD, Frank Dowling, MD, and Thomas D’Zurilla, PhD. The University of Texas Southwestern Medical Center, Dallas (UO1 MH61562): Madhukar H. Trivedi, MD, Prabha Sunderajan, MD, David Morris, PhD, and Beverly Kleiber, PhD. Emory University School of Medicine, Atlanta, Georgia (UO1 MH63481): Barbara O. Rothbaum, PhD, Boadie Dunlop, MD, Philip T. Ninan, MD, and Steven J. Garlow, MD, PhD. University of Arizona, Tucson (U01 MH62465): Alan J. Gelenberg, MD, and John Misiaszek, MD. Brown Medical School, Providence, Rhode Island (UO1 MH61590): Martin B. Keller, MD, Ivan Miller, MD, Gabor Keitner, MD, and Susan Raffa, PhD. Stanford University, Stanford, California (UO1 MH61504): Alan F. Schatzberg, MD, Bruce A. Arnow, PhD, Rachel Manber, PhD, and H. Brent Solvason, PhD, MD. Virginia Commonwealth University, Richmond (UO1 MH62491): James McCullough, PhD. NIMH Collaborators: George Niederehe, PhD, Louise Ritz, MBA, and Elizabeth Zachariah, MS.
Financial Disclosure: Dr Kocsis reports receiving research support from AstraZeneca, Burroughs Wellcome Trust, CNS Response, Inc, Forest Pharmaceuticals, the National Institute on Drug Abuse, the NIMH, Pritzker Consortium, and Sanofi Aventis; participating on the speakers bureau for AstraZeneca, Pfizer Inc, and Wyeth; and acting as a consultant to Wyeth. Dr Rothbaum reports receiving grant support from Janssen Pharmaceutical Products LP and Wyeth and owning stock in or being a partial owner in Virtually Better, Inc. Dr Klein reports receiving grant support from Bristol-Myers Squibb Company and acting as a consultant to Wyeth. Dr Trivedi reports receiving research support from Bristol-Myers Squibb Company, Cephalon, Inc, Corcept Therapeutics, Inc, Cyberonics, Inc, Eli Lilly & Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutical Products LP, Merck, the National Alliance for Research in Schizophrenia and Depression, NIMH, Novartis, Pfizer Inc, Pharmacia&Upjohn, Predix Pharmaceuticals, Solvay Pharmaceuticals, Inc, and Wyeth-Ayerst Laboratories; participating on the speakers bureau for Abdi Brahim, Akzo (Organon Pharmaceuticals Inc), Bristol-Myers Squibb Company, Cephalon, Inc, Cyberonics, Inc, Eli Lilly & Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutical Products LP, Pharmacia & Upjohn, Solvay Pharmaceuticals, Inc, and Wyeth-Ayerst Laboratories; and acting as an advisor or consultant to Abbott Laboratories, Inc, Akzo (Organon Pharmaceuticals Inc), AstraZeneca, Bayer, Bristol-Myers Squibb Company, Cephalon, Inc, Cyberonics, Inc, Eli Lilly & Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutical Products LP, Johnson&Johnson PRD, Meade Johnson, Neuronetics, Inc, Parke-Davis Pharmaceuticals, Inc, Pfizer Inc, Pharmacia & Upjohn, Sepracor, Inc, Solvay Pharmaceuticals, Inc, VantagePoint, and Wyeth-Ayerst Laboratories. Dr Manber reports receiving grant support from Forest Laboratories. Dr Keller reports receiving grant or research support from Eli Lilly&Company, Janssen Pharmaceutical Products LP, Pfizer Inc, and Wyeth; acting as a consultant to or receiving honoraria from CENEREX, Cephalon, Inc, Cypress Bioscience, Eli Lilly & Company, Forest Laboratories, Janssen Pharmaceutical Products LP, JDS, Merck, Mitsubishi, Novartis, Organon Pharmaceuticals Inc, Pfizer Inc, Pharmacia, Sanofi-Syntholab, Scirex, Sepracor, Inc, Vela Pharma, and Wyeth; participating on the advisory board for Abbott Laboratories, Inc, Bristol-Myers Squibb Company, CENEREX, Cephalon, Inc, Cyberonics, Inc, Cypress Biosciences, Eli Lilly&Company, Forest Laboratories, Janssen Pharmaceutical Products LP, Merck, Mitsubishi, Novartis, Organon Pharmaceuticals Inc, Pharmacia, Pfizer Inc, Sanofi-Syntholab, Scirex, Sepracor, Inc, and Vela Pharm. Dr Leon reports participating on the data and safety monitoring boards for Dainippon Sumitomo Pharma America, Neuronetics, Inc, Organon Pharmaceuticals Inc, Pfizer Inc, and Vanda; and acting as a consultant to Cyberonics, Inc, MedAvante, and the NIMH. Dr Wisniewski reports acting as a consultant to Cyberonics, Inc (2005–2006), ImaRx Therapeutics, Inc (2006), and Bristol-Myers Squibb Company (2007). Dr Thase reports receiving research support from the NIMH; participating on the speakers bureau for AstraZeneca, Bristol-Myers Squibb Company, Cyberonics, Inc, Eli Lilly&Company, GlaxoSmithKline, Organon Pharmaceuticals Inc, Sanofi Aventis, and Wyeth Pharmaceuticals; acting as an advisor or consultant to AstraZeneca, Bristol-Myers Squibb Company, Cephalon, Inc, Cyberonics, Inc, Eli Lilly & Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutical Products LP, MedAvante, Inc, Neuronetics, Inc, Novartis, Organon Pharmaceuticals Inc, Pfizer Inc, Sepracor, Inc, Shire US Inc, Transcept, and Wyeth Pharmaceuticals; providing expert testimony for Jones Day (Wyeth Pharmaceuticals litigation) and Phillips Lytle (GlaxoSmithKline litigation); having equity holdings (excluding mutual funds/blinded trusts) in MedAvante, Inc; and receiving royalty/patent or other income from American Psychiatric Publishing, Inc, Guilford Publications, and Herald House. Dr Thase reports receiving no pharmaceutical research support during the course of the trial; since 2007, he has received support from Eli Lilly&Company, GlaxoSmithKline, and Sepracor, Inc.
Previous Presentations: This study was presented in part at the 47th New Clinical Drug Evaluation Unit meeting; June 12, 2007; Boca Raton, Florida.
REFERENCES
- 1.Harrison WM, Stewart JW. Pharmacotherapy of dysthymic disorder. In: Kocsis JH, Klein DN, editors. Diagnosis and Treatment of Chronic Depression. New York, NY: Guilford Press; 1995. pp. 124–145. [Google Scholar]
- 2.Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, Markowitz J, Nemeroff CB, Russell JM, Thase ME, Trivedi M, Zajecka J. A comparison of nefazodone, the cognitive behavioral analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342(20):1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz JC. Psychotherapy of dysthymia. Am J Psychiatry. 1994;151(8):1114–1121. doi: 10.1176/ajp.151.8.1114. [DOI] [PubMed] [Google Scholar]
- 4.Keller MB, Gelenberg AJ, Hirschfeld RMA, Rush AJ, Thase ME, Kocsis JH, Markowitz JC, Fawcett JA, Koran LM, Klein DN, Russell JM, Kornstein SG, McCullough JP, Davis SM, Harrison W. The treatment of chronic depression, part 2: a double-blind, randomized trial of sertraline and imipramine. J Clin Psychiatry. 1998;59(11):598–607. doi: 10.4088/jcp.v59n1107. [DOI] [PubMed] [Google Scholar]
- 5.Kocsis JH, Frances AJ, Voss C, Mann JJ, Mason BJ, Sweeney J. Imipramine treatment for chronic depression. Arch Gen Psychiatry. 1988;45(3):253–257. doi: 10.1001/archpsyc.1988.01800270071008. [DOI] [PubMed] [Google Scholar]
- 6.Thase ME, Fava M, Halbreich U, Kocsis JH, Koran L, Davidson J, Rosenbaum J, Harrison W. A placebo-controlled, randomized clinical trial comparing sertraline and imipramine for the treatment of dysthymia. Arch Gen Psychiatry. 1996;53(9):777–784. doi: 10.1001/archpsyc.1996.01830090023004. [DOI] [PubMed] [Google Scholar]
- 7.Miller IW, Keitner GI, Schatzberg AF, Klein DN, Thase ME, Rush AJ, Markowitz JC, Schlager DS, Kornstein SG, Davis SM, Harrison WM, Keller MB. The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59(11):608–619. doi: 10.4088/jcp.v59n1108. [DOI] [PubMed] [Google Scholar]
- 8.Judd LL, Akiskal HS, Paulus MP. The role and clinical significance of subsyndromal depressive symptoms (SSD) in unipolar major depressive disorder. J Affect Disord. 1997;45(1–2):5–18. doi: 10.1016/s0165-0327(97)00055-4. [DOI] [PubMed] [Google Scholar]
- 9.Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- 10.Cornwall PL, Scott J. Partial remission in depressive disorders. Acta Psychiatr Scand. 1997;95(4):265–271. doi: 10.1111/j.1600-0447.1997.tb09630.x. [DOI] [PubMed] [Google Scholar]
- 11.Thase ME, Rush AJ. Treatment-resistant depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press; 1995. pp. 1081–1097. [Google Scholar]
- 12.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 13.Fava M, Rush AJ, Wisniewski SR, Nierenberg AA, Alpert JE, McGrath PJ, Thase ME, Warden D, Biggs M, Luther JF, Niederehe G, Ritz L, Trivedi MH. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163(7):1161–1172. doi: 10.1176/ajp.2006.163.7.1161. [DOI] [PubMed] [Google Scholar]
- 14.Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, Alpert JE, Warden D, Luther JF, Niederehe G, Lebowitz B, Shores-Wilson K, Rush AJ STAR*D Study Team. A comparison of lithium and T3 augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519–1530. doi: 10.1176/ajp.2006.163.9.1519. quiz 1665. [DOI] [PubMed] [Google Scholar]
- 15.Thase ME, Rush AJ, Howland RH, Kornstein SG, Kocsis JH, Gelenberg AJ, Schatzberg AF, Koran LM, Keller MB, Russell JM, Hirschfeld RMA, LaVange LM, Klein DN, Fawcett J, Harrison W. Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry. 2002;59(3):233–239. doi: 10.1001/archpsyc.59.3.233. [DOI] [PubMed] [Google Scholar]
- 16.Manning DW, Markowitz JC, Frances AJ. A review of combined psychotherapy and pharmacotherapy in the treatment of depression. J Psychother Pract Res. 1992;1(2):103–116. [PMC free article] [PubMed] [Google Scholar]
- 17.Roth A, Fonagy P. What Works for Whom? A Critical Review of Psychotherapy Research. New York, NY: Guilford Press; 1996. [Google Scholar]
- 18.Rush AJ, Thase ME. Psychotherapies for depressive disorders: a review. In: Maj M, Sartorius N, editors. Depressive Disorders. Chichester, England: John Wiley & Sons; 1999. pp. 161–206. WPA Series in Evidence and Experience in Psychiatry; vol 1. [Google Scholar]
- 19.Blom MB, Jonker K, Dusseldorp E, Spinhoven P, Hoencamp E, Haffmans J, van Dyck R. Combination treatment for acute depression is superior only when psychotherapy is added to medication. Psychother Psychosom. 2007;76(5):289–297. doi: 10.1159/000104705. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz JC, Kocsis JH, Bleiberg KL, Christos PJ, Sacks MH. A comparative trial of psychotherapy and pharmacotherapy for “pure” dysthymic patients. J Affect Disord. 2005;89(1–3):167–175. doi: 10.1016/j.jad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, Jenaway A, Cornwall PL, Hayhurst H, Abbott R, Pope M. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- 22.Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, Fava M, Nierenberg AA, McGrath PJ, Warden D, Niederehe G, Hollon SD, Rush AJ. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739–752. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 23.Friedman RA. Social and occupational adjustment in chronic depression. In: Kocsis JH, Klein DN, editors. Diagnosis and Treatment of Chronic Depression. New York, NY: Guilford Press; 1995. pp. 89–102. [Google Scholar]
- 24.Klein DN, Taylor EB, Harding K, Dickstein S. Double depression and episodic major depression: demographic, clinical, familial, personality, and socioenvironmental characteristics and short-term outcome. Am J Psychiatry. 1988;145(10):1226–1231. doi: 10.1176/ajp.145.10.1226. [DOI] [PubMed] [Google Scholar]
- 25.McCullough JP. Treatment for Chronic Depression: Cognitive Behavioral Analysis System of Psychotherapy. New York, NY: Guilford Press; 2000. [DOI] [PubMed] [Google Scholar]
- 26.Schatzberg AF, Rush AJ, Arnow BA, Banks P, Blalock JA, Borian FE, Howland R, Klein DN, Kocsis JH, Kornstein SG, Manber R, Markowitz JC, Miller I, Ninan PT, Rothbaum BO, Thase ME, Trivedi MH, Keller MB. Chronic depression: medication (nefazodone) or psychotherapy (CBASP) is effective when the other is not. Arch Gen Psychiatry. 2005;62(5):513–520. doi: 10.1001/archpsyc.62.5.513. [DOI] [PubMed] [Google Scholar]
- 27.Crismon ML, Trivedi MH, Pigott TA, Rush AJ, Hirschfeld RMA, Kahn DA, DeBattista C, Nelson CJ, Nierenberg AA, Sackeim HA, Thase ME. The Texas Medication Algorithm Project: report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. J Clin Psychiatry. 1999;60(3):142–156. [PubMed] [Google Scholar]
- 28.Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457–494. x. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 29.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis-I Disorders, Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Dept, New York State Psychiatric Institute; 1995. [Google Scholar]
- 31.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 32.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: Dept of Health, Education, and Welfare; 1976. Publication No. (ADM) 76-338. [Google Scholar]
- 33.Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23–29. [PubMed] [Google Scholar]
- 34.Rush AJ, Thase ME. Strategies and tactics in the treatment of chronic depression. J Clin Psychiatry. 1997;58(suppl 13):14–22. [PubMed] [Google Scholar]
- 35.Clinical Practice Guideline No. 5: Depression in Primary Care. Vol 2. Rockville, MD: US Dept of Health and Human Services, Agency for Health Care Policy and Research; 1993. Depression Guideline Panel. AHCPR Publication No. 93-0551. [Google Scholar]
- 36.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2000;157(suppl)(4):1–45. [PubMed] [Google Scholar]
- 37.Epstein PS, Fawcett J. Addendum to Clinical Management—Imipramine/Placebo Administration Manual: NIMH Treatment of Depression Collaborative Research Program. Chicago, IL: Rush Presbyterian-St Lukes Medical Center; 1982. [Google Scholar]
- 38.Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH NIMH Treatment of Depression Collaborative Research Program. Clinical management—imipramine/placebo administration manual. Psychopharmacol Bull. 1987;23(2):309–324. [PubMed] [Google Scholar]
- 39.Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, Glass DR, Pilkonis PA, Leber WR, Docherty JP, Fiester SJ, Parloff MB. National Institute of Mental Health Treatment of Depression Collaborative Research Program: general effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971–983. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- 40.McCullough JP. Patient Manual for the Cognitive Behavioral Analysis System of Psychotherapy. New York, NY: Guilford Press; 2002. [Google Scholar]
- 41.McCullough JP. Training Manual for Diagnosing and Treating Chronic Depression: Cognitive Behavioral Analysis System of Psychotherapy. New York, NY: Guilford Press; 2001. [Google Scholar]
- 42.Rogers CR. Client-Centered Therapy. Boston, MA: Houghton Mifflin; 1951. [Google Scholar]
- 43.Frank JD, Eleventh Emil A. Gutheil memorial conference: therapeutic factors in psychotherapy. Am J Psychother. 1971;25(3):350–361. doi: 10.1176/appi.psychotherapy.1971.25.3.350. [DOI] [PubMed] [Google Scholar]
- 44.Markowitz JC, Manber R, Rosen P. Therapists’ responses to training in brief supportive psychotherapy. Am J Psychother. 2008;62(1):67–81. doi: 10.1176/appi.psychotherapy.2008.62.1.67. [DOI] [PubMed] [Google Scholar]
- 45.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 46.Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 47.Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE-RIFT): a brief measure of functional impairment. Psychol Med. 1999;29(4):869–878. doi: 10.1017/s0033291799008570. [DOI] [PubMed] [Google Scholar]
- 48.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA for the STAR*D Investigators. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12(2):71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Hedeker D, Gibbons RD. A random-effects ordinal regression model for multilevel analysis. Biometrics. 1994;50(4):933–944. [PubMed] [Google Scholar]
- 50.Kocsis JH, Gelenberg AJ, Rothbaum B, Klein DN, Trivedi MH, Manber R, Keller MB, Howland R, Thase ME. Chronic forms of major depression are still under-treated in the 21st century: systematic assessment of 759 patients presenting for treatment. J Affect Disord. 2008;110(1–2):55–61. doi: 10.1016/j.jad.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kocsis JH, Leon AC, Markowitz JC, Manber R, Arnow B, Klein DN, Thase ME. Patient preference as a moderator of outcome for chronic forms of major depressive disorder treated with nefazodone, cognitive behavioral analysis system of psychotherapy, or their combination. J Clin Psychiatry. 2009;70(3):354–361. doi: 10.4088/jcp.08m04371. [DOI] [PubMed] [Google Scholar]
- 52.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Jr, Weiss P, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100(24):14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164(2):201–204. doi: 10.1176/ajp.2007.164.2.201. [DOI] [PubMed] [Google Scholar]
- 54.Hollon SD. Does cognitive therapy have an enduring effect? Cognit Ther Res. 2003;27(1):71–75. [Google Scholar]
- 55.Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, Lovett ML, Young PR, Haman KL, Freeman BB, Gallop R. Prevention of relapse following cognitive therapy versus medication in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]