Abstract
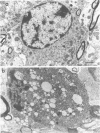
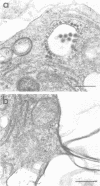
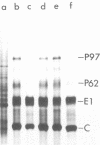
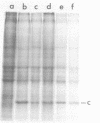
We have analyzed the pathogenicity and host range properties of four neurovirulence mutants of Semliki Forest virus which, unlike the wild type (WT), allow the survival of weanling mice injected intraperitoneally with 102 PFU. The mutant M9 showed a sustained multiplication in the brains of infected mice. It produced paralysis in 35%, and 8% died. Demyelination occurred in 94% of the surviving mice and was associated with the destruction of oligodendrocytes. All of the mutants showed a restricted ability to multiply in BHK, C1300 (neuroblastoma), and G26-24 (oligodendroglioma) cells as compared with the WT, and this was not associated with differential interferon production or action. C1300 cells infected with the mutants survived, whereas WT-infected cells were killed. In G26-24 cells all of the mutants and the WT produced a rapid cytopathic effect which was inhibited by pretreatment with 10 U of mouse interferon. Extensive RNA synthesis was detected for all of the mutants and the WT in BHK and C1300 cells, but it was only detectable in G26-24 cells in small amounts early in the infection. The mutant M4 had a defect in the nucleocapsid assembly, whereas M9 had a defect in total RNA synthesis. M136 was defective in the synthesis of 26S RNA, and M103 showed defective synthesis of viral core protein in C1300 cells. It is concluded that C1300 cells can tolerate viral RNA synthesis by a defective virus without showing a cytopathic effect, but the fully virulent WT virus is cytopathic. G26-24 cells are sensitive to small amounts of viral RNA synthesis. These properties of the WT and mutant viruses correlate with changes produced in the neurons and oligodendrocytes of the central nervous system: the virulence of the WT is due to its ability to destroy both neurons and oligodendrocytes, whereas the demyelination produced by the mutants M9 and M136 is due to the destruction of oligodendrocytes alone.
Full text
PDF
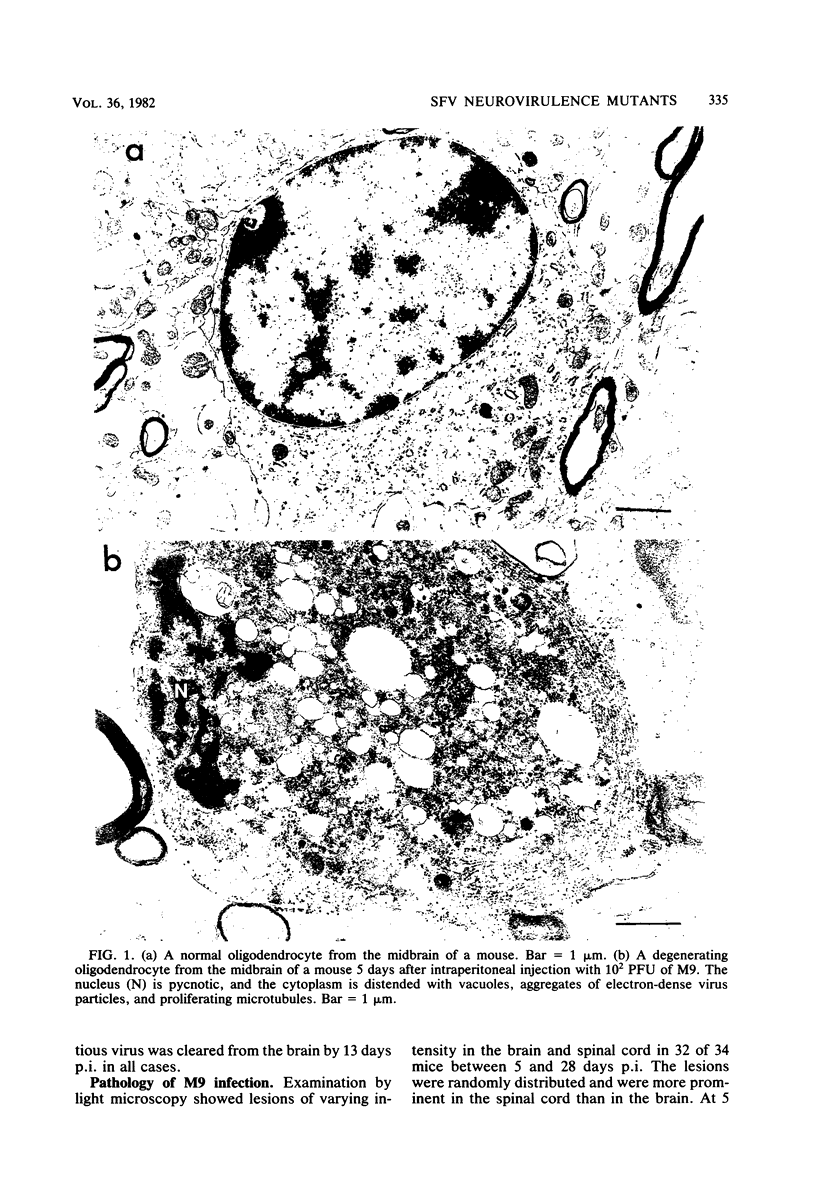
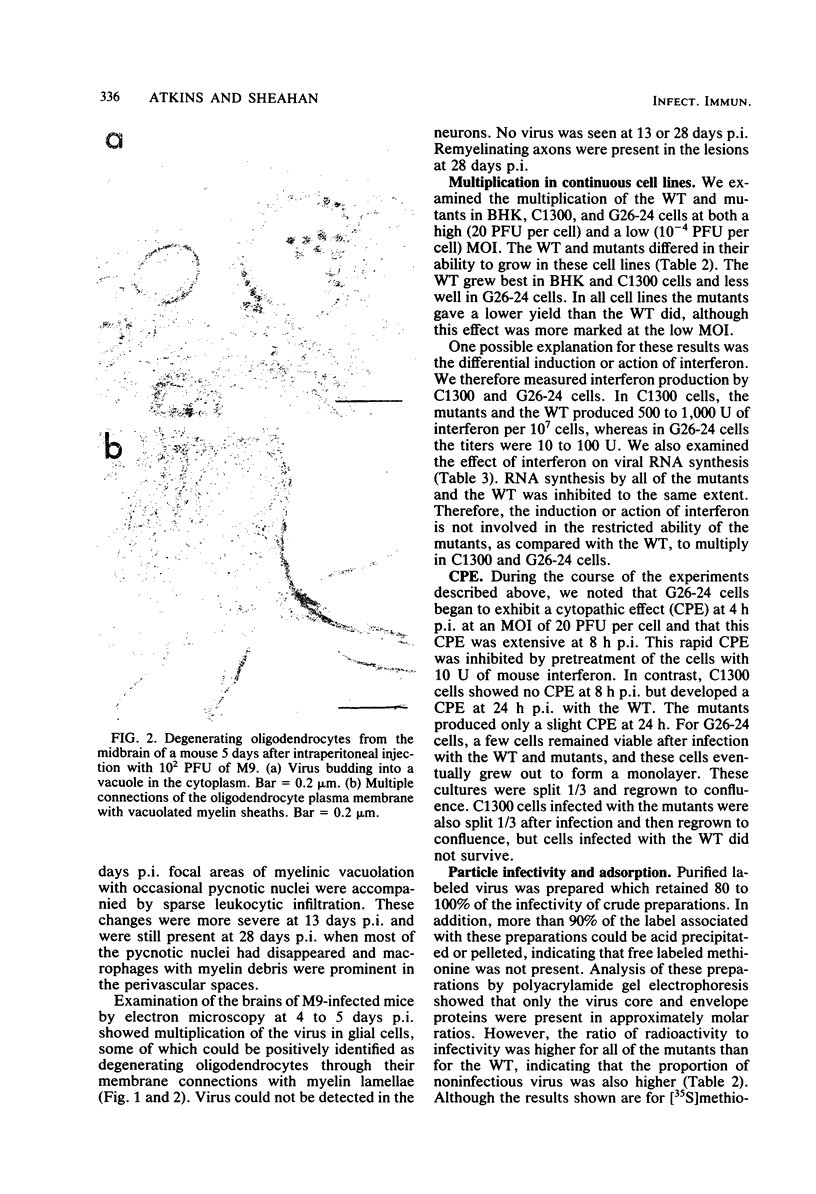
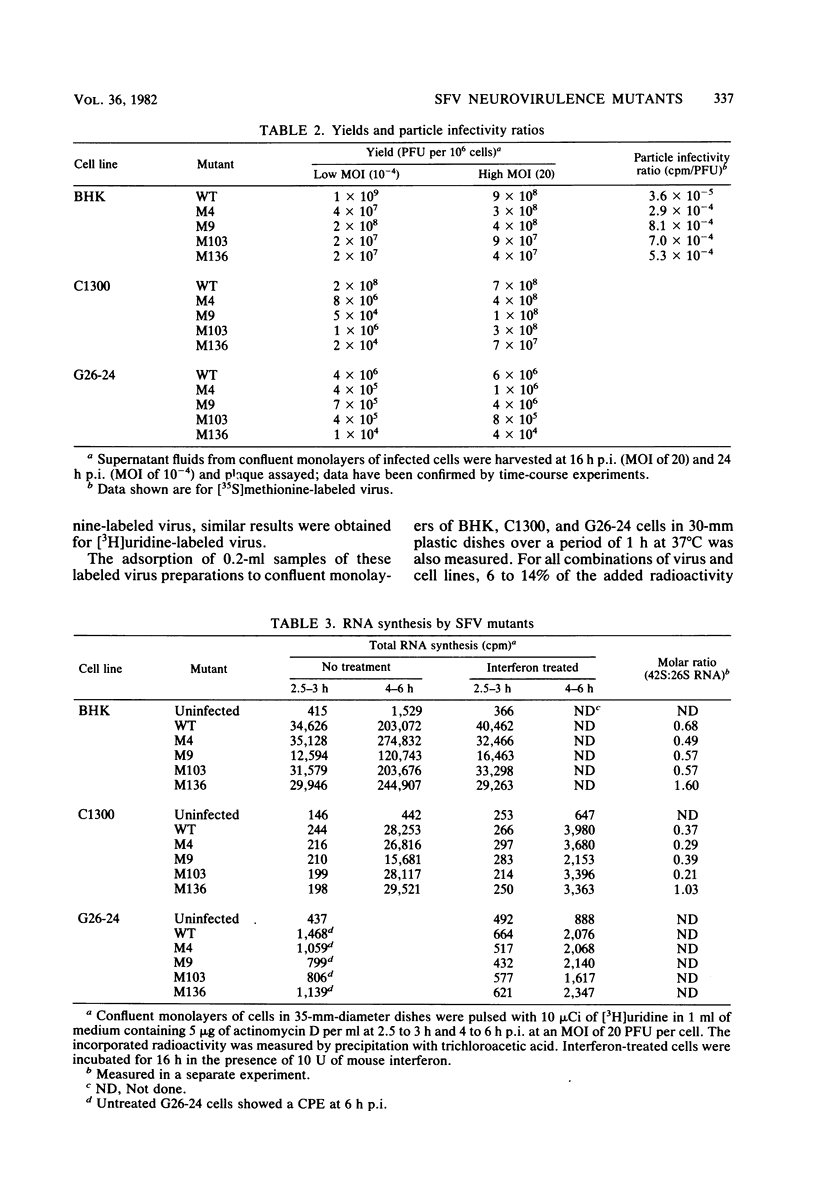
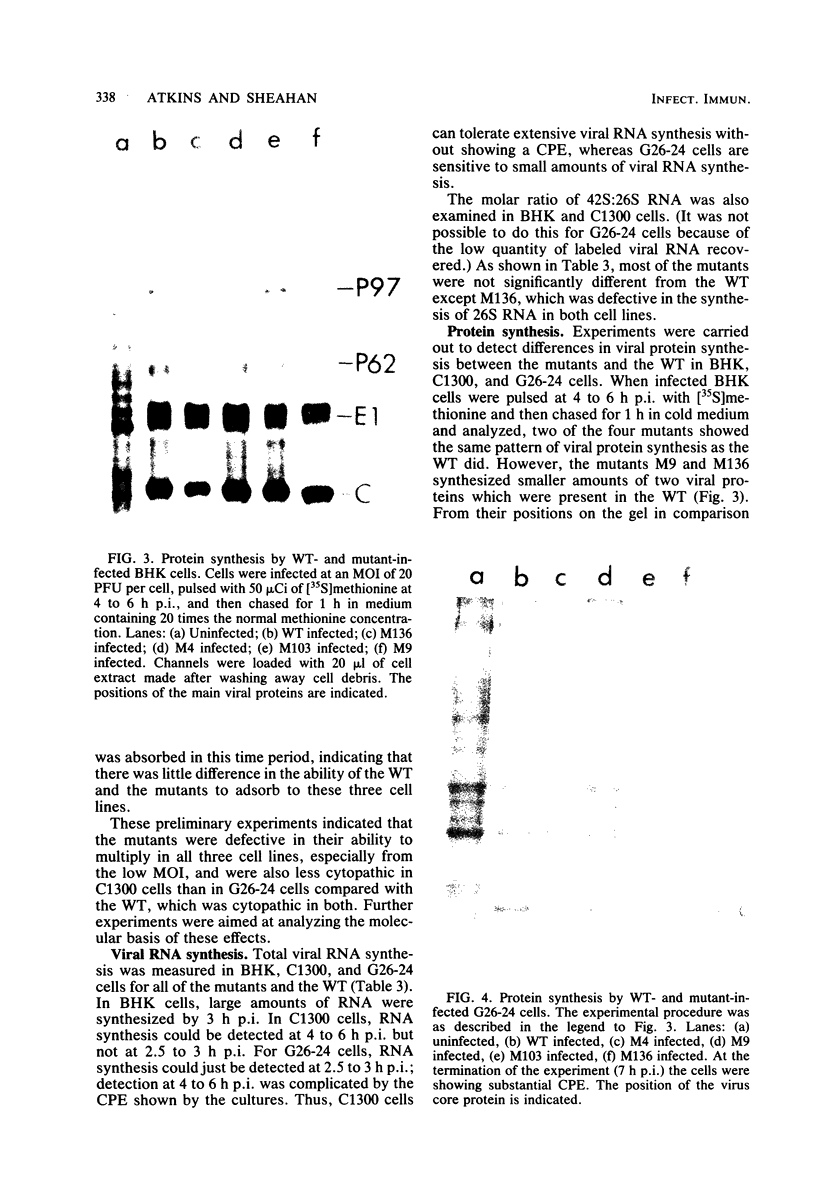
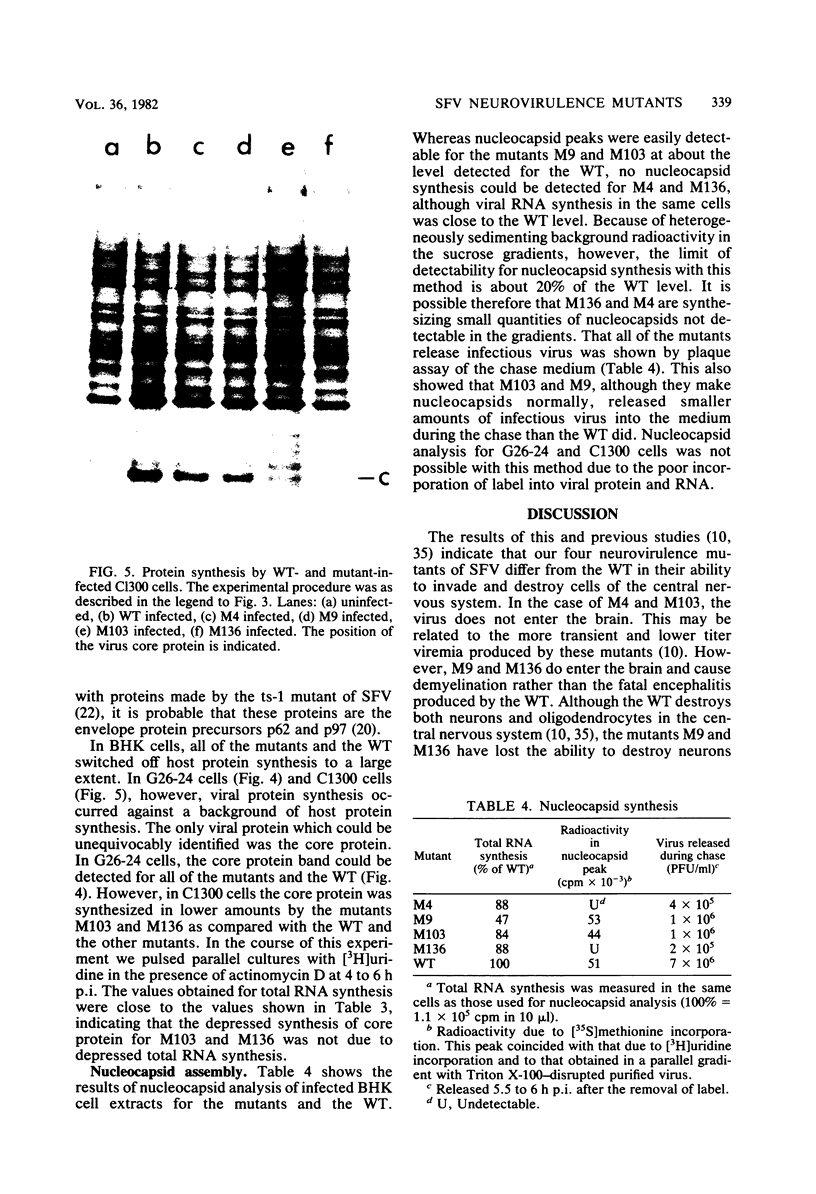


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G. J. Establishment of persistent infection in BHK-21 cells by temperature-sensitive mutants of Sindbis virus. J Gen Virol. 1979 Oct;45(1):201–207. doi: 10.1099/0022-1317-45-1-201. [DOI] [PubMed] [Google Scholar]
- Atkins G. J., Johnston M. D., Westmacott L. M., Burke D. C. Department of Biological Sciences, University of Warwick, Coventry, CV47AL, England. J Gen Virol. 1974 Dec;25(3):381–390. doi: 10.1099/0022-1317-25-3-381. [DOI] [PubMed] [Google Scholar]
- Atkins G. J., Samuels J., Kennedy S. I. Isolation and preliminary characterization of temperature-sensitive mutants of Sindbis virus strain AR339. J Gen Virol. 1974 Dec;25(3):371–380. doi: 10.1099/0022-1317-25-3-371. [DOI] [PubMed] [Google Scholar]
- Atkins G. J. The effect of infection with Sindbis virus and its temperature-sensitive mutants on cellular protein and DNA synthesis. Virology. 1976 Jun;71(2):593–597. doi: 10.1016/0042-6822(76)90384-6. [DOI] [PubMed] [Google Scholar]
- Augusti-Tocco G., Sato G. Establishment of functional clonal lines of neurons from mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):311–315. doi: 10.1073/pnas.64.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P. N., Atkins G. J. Establishment of persistent infection in mouse cells by Sindbis virus and its temperature-sensitive mutants. J Gen Virol. 1981 May;54(Pt 1):57–65. doi: 10.1099/0022-1317-54-1-57. [DOI] [PubMed] [Google Scholar]
- Barrett P. N., Atkins G. J. Virulence of temperature-sensitive mutants of Sindbis virus in neonatal mice. Infect Immun. 1979 Dec;26(3):848–852. doi: 10.1128/iai.26.3.848-852.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P. N., Sheahan B. J., Atkins G. J. Isolation and preliminary characterization of Semliki Forest virus mutants with altered virulence. J Gen Virol. 1980 Jul;49(1):141–147. doi: 10.1099/0022-1317-49-1-141. [DOI] [PubMed] [Google Scholar]
- Berger M. L. Humoral and cell-mediated immune mechanisms in the production of pathology in avirulent Semliki Forest virus encephalitis. Infect Immun. 1980 Oct;30(1):244–253. doi: 10.1128/iai.30.1.244-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. Infection, interaction and the expression of virulence by defined strains of Semliki forest virus. J Gen Virol. 1972 Sep;16(3):359–372. doi: 10.1099/0022-1317-16-3-359. [DOI] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Bradish C. J., Allner K. The early responses of mice to respiratory or intraperitoneal infection by defined virulent and avirulent strains of Semliki forest virus. J Gen Virol. 1972 Jun;15(3):205–218. doi: 10.1099/0022-1317-15-3-205. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Dawson G. Regulation of glycosphingolipid metabolism in mouse neuroblastoma and glioma cell lines. Comparison of glioma (oligodendroglioma-like) with neutroblastoma cell lines. J Biol Chem. 1979 Jan 10;254(1):155–162. [PubMed] [Google Scholar]
- Dawson G., Sundarraj N., Pfeiffer S. E. Synthesis of myelin glycosphingolipids (galactosylceramide and galactosyl(3-O-sulfate)ceramide (sulfatide)) by cloned cell lines derived from mouse neurotumors. J Biol Chem. 1977 Apr 25;252(8):2777–2779. [PubMed] [Google Scholar]
- Fleming P. Age-dependent and strain-related differences of virulence of Semliki Forest virus in mice. J Gen Virol. 1977 Oct;37(1):93–105. doi: 10.1099/0022-1317-37-1-93. [DOI] [PubMed] [Google Scholar]
- Jagelman S., Suckling A. J., Webb H. E., Bowen F. T. The pathogenesis of avirulent Semliki Forest virus infections in athymic nude mice. J Gen Virol. 1978 Dec;41(3):599–607. doi: 10.1099/0022-1317-41-3-599. [DOI] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. doi: 10.1128/jvi.32.1.19-29.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keränen S., Käriäinen L. Isolation and basic characterization of temperature-sensitive mutants from Semliki Forest virus;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):810–820. doi: 10.1111/j.1699-0463.1974.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Structure and replication of alpha-viruses. Curr Top Microbiol Immunol. 1978;82:15–69. doi: 10.1007/978-3-642-46388-4_2. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando Z., Teitelbaum D., Arnon R. Induction of experimental allergic encephalomyelitis in genetically resistant strains of mice. Nature. 1980 Oct 9;287(5782):551–552. doi: 10.1038/287551a0. [DOI] [PubMed] [Google Scholar]
- Lucas A., Coulter M., Anderson R., Dales S., Flintoff W. In vivo and in vitro models of demyelinating diseases. II. Persistence and host-regulated thermosensitivity in cells of neural derivation infected with mouse hepatitis and measles viruses. Virology. 1978 Jul 15;88(2):325–337. doi: 10.1016/0042-6822(78)90289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Mázló M., Tariska I. Morphological demonstration of the first phase of polyomavirus replication in oligodendroglia cells of human brain in progressive multifocal leukoencephalopathy (PML). Acta Neuropathol. 1980;49(2):133–143. doi: 10.1007/BF00690753. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978 Oct 13;44(1):63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pattyn S. R., De Vleesschauwer L., van der Groen G. Replication of arboviruses in mouse organ cultures. II. Multiplication of virulent and avirulent Semliki Forest and western equine encephalitis viruses in mouse organ cultures. Arch Virol. 1975;49(1):33–37. doi: 10.1007/BF02175593. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Wolinsky J. S. Neuronal and oligodendroglial infection by the WW strain of Theiler's virus. Lab Invest. 1979 Mar;40(3):324–330. [PubMed] [Google Scholar]
- Pusztai R., Gould E. A., Smith H. Infection patterns in mice of an avirulent and virulent strain of Semliki Forest virus. Br J Exp Pathol. 1971 Dec;52(6):669–677. [PMC free article] [PubMed] [Google Scholar]
- Schluter B., Bellomy B., Brown A. Pathogenesis of temperature-sensitive mutants of sindbis virus in the embryonated egg. I. Characterization and kinetics of viral multiplication. Infect Immun. 1974 Jan;9(1):68–75. doi: 10.1128/iai.9.1.68-75.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan B. J., Barrett P. N., Atkins G. J. Demyelination in mice resulting from infection with a mutant of Semliki Forest virus. Acta Neuropathol. 1981;53(2):129–136. doi: 10.1007/BF00689993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundarraj N., Schachner M., Pfeiffer S. E. Biochemically differentiated mouse glial lines carrying a nervous system specific cell surface antigen (NS-1). Proc Natl Acad Sci U S A. 1975 May;72(5):1927–1931. doi: 10.1073/pnas.72.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vänänen P., Käriäinen L. Fusion and haemolysis of erythrocytes caused by three togaviruses: Semliki Forest, Sindbis and rubella. J Gen Virol. 1980 Feb;46(2):467–475. doi: 10.1099/0022-1317-46-2-467. [DOI] [PubMed] [Google Scholar]
- Woodward C. G., Marshall I. D., Smith H. Investigations of reasons for the avirulence of the A7 strain of Semliki Forest virus in adult mice. Br J Exp Pathol. 1977 Dec;58(6):616–624. [PMC free article] [PubMed] [Google Scholar]