Abstract
Studies on gene therapy for hemophilia B (HB) using adeno-associated viral (AAV) vectors showed that the safety of a given strategy is directly related to the vector dose. To overcome this limitation, we sought to test the efficacy and the risk of immunogenicity of a novel factor IX (FIX) R338L associated with ∼ 8-fold increased specific activity. Muscle-directed expression of canine FIX-R338L by AAV vectors was carried out in HB dogs. Therapeutic levels of circulating canine FIX activity (3.5%-8%) showed 8- to 9-fold increased specific activity, similar to humans with FIX-R338L. Phenotypic improvement was documented by the lack of bleeding episodes for a cumulative 5-year observation. No antibody formation and T-cell responses to FIX-R338L were observed, even on challenges with FIX wild-type protein. Moreover, no adverse vascular thrombotic complications were noted. Thus, FIX-R338L provides an attractive strategy to safely enhance the efficacy of gene therapy for HB.
Introduction
Hemophilia B (HB) is an X-linked bleeding disorder resulting from a deficiency of coagulation factor IX (FIX). Gene therapy is an attractive strategy for the treatment of the disease because continuous maintenance of FIX levels as low as 1%-5% of normal have been shown to substantially ameliorate the bleeding phenotype in both preclinical and clinical models.1–6
Studies using adeno-associated viral (AAV) vectors showed that the safety profile is vector dose-dependent.3,4 In a liver-directed approach, immune responses to AAV-capsid proteins at the highest dose tested (2 × 1012 vg/kg) required transient immunosuppression for sustained transgene expression.3,4 In a study on direct intramuscular AAV-FIX, the safety profile was excellent2 and the local transgene expression of FIX in the injected muscle persisted for 3.7 and 10 years in 2 human subjects tested.7,8 However, all doses tested in the intramuscular study resulted in subtherapeutic circulating FIX levels.2 The use of FIX variants with gain of function offers the opportunity to enhance the efficacy of gene-based approaches for HB without increasing the vector doses. In an early study, we demonstrated that replacement of arginine 338 by alanine (R338A) was associated with an ∼ 3-fold increase in the protein specific activity in murine models of HB receiving AAV-FIX-R338A9 as later confirmed in other models.10,11 Recently, we described a naturally occurring gain-of-function mutation in humans characterized by leucine at position 338 (R338L), which exhibits normal antigen levels, but an ∼ 8-fold higher specific activity.12 Notably, the arginine at position 338 in FIX is conserved among mammals, and this region of the enzyme appears to be part of the substrate exosite for factor X.13 Here we report, for the first time, the use of the homologous FIX-R338L in a large and immunocompetent canine model of severe HB (< 1% of normal).
Methods
AAV vector production and administration
Recombinant AAV6 vectors were produced by a triple transfection protocol as previously described using an expression cassette containing canine FIX-R338L (cFIX Padua) under control of a cytomegalovirus promoter.1,2 The R338L mutation was generated using a QuickChange II-XL site-directed mutagenesis kit (Stratagene). All animal experiments were approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia and the University of North Carolina at Chapel Hill. Three adult male HB dogs were administered between 2.5 and 3 × 1012 vg/kg of AAV6-cFIX-R338L by peripheral transvenular delivery to the skeletal muscle as previously described.1
Systemic and local toxicity
Hematologic and comprehensive biochemical analyses of blood and serum samples for liver and kidney function tests were performed as previously described.1,14 Thrombin/antithrombin complex (TAT) levels were measure at baseline and at day 50, 100, and > 400 after AAV injection using Enzygnost TAT kit (Siemens Healthcare Diagnostic).
Normal plasma challenges
Two cFIX-R338L–expressing dogs (N07 and M59) were treated 4 times/wk with 100 mL of normal canine plasma intravenously. Plasma samples were collected before and after administration of the pooled normal plasma, and PBMCs were collected after the fourth challenge.
Canine FIX antigen, activity, and antibody assays
The whole blood clotting time, cFIX antigen and activity levels, neutralizing antibodies to cFIX (Bethesda assay), and non-neutralizing antibodies against cFIX were measured as previously described.1,14
ELISpot analysis
One-color ELISpot assays were used to measure IFN-γ T-cell responses to AAV capsid, cFIX protein, or overlapping peptides spanning the 338 region of the cFIX protein (RATCLR/LSTKFTIYNM, LKVPVDRATCLR/LST). All proteins and peptides were used at a final concentration of 10 μg/mL as previously described.14 Concanavalin stimulated PBMCs were used as a positive control, and media alone was the negative control. PBMCs collected after the fourth challenge with canine plasma were used for analysis.
Results and discussion
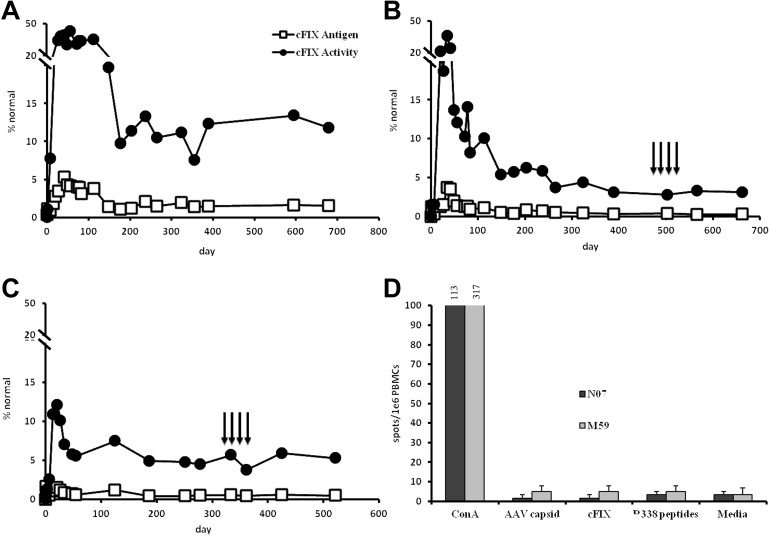
Skeletal muscle is an ideal target for the expression of therapeutic transgenes in genetic diseases that are associated with primary or iatrogenic underlying liver disease. In adults with hemophilia, the high rates of viral hepatitis resulting from blood transfusion preclude the enrollment of many patients in liver-directed gene therapy. We sought to test whether cFIX-R338L could be safely expressed in HB dogs by regional transvenular delivery of AAV vector to skeletal muscle of a single limb. We generated an AAV-6 vector encoding cFIX-338L and delivered it to 3 adult HB dogs. We chose to use an AAV-6 vector given the excellent tropism for systemic transduction of striated muscle15 and our own experience using AAV-6 for muscle transduction in HB dogs.1 There was a continuous increase in the circulating cFIX levels that reached plateau levels after day 100 (Figure 1). The kinetics and magnitude of expression in cFIX-338L dogs were similar to those observed in our earlier studies in dogs using cFIX wild-type (WT) via transvenular route.1 In a series of previous studies in HB dogs after liver, intramuscular, or intravascular delivery to skeletal muscle of AAV vectors of distinct serotypes expressing cFIX-WT, there was a linear correlation between cFIX antigen and activity levels (ration, 0.8-1.1).1,3,5,16 Here the ratio of antigen and activity clearly demonstrated that cFIX-338L exhibits an 8- to 9-fold higher specific activity of the protein (Table 1), as observed in humans with R338L.12 The expression of cFIX-338L was accompanied by normalization of the whole blood clotting time 1 week after vector delivery; this was stable throughout the follow-up (Table 1) and comparable with values observed in hemostatically normal dogs.17 Moreover, no spontaneous bleeding episodes requiring treatment occurred for a cumulative period of ∼ 5 years after vector delivery (ongoing observations). The frequency of spontaneous bleeding episodes in untreated HB dogs is 5.5 bleeds per dog/year18; thus, the phenotypic improvement supported by cFIX-338L is clinically evident.
Figure 1.
cFIX-R338L antigen and activity levels over time in HB dogs treated with AAV6-cFIX-R338L. Three adult male HB dogs (M55 [A], M59 [B], and N07 [C]) were administered 2.5-3 × 1012 vg/kg of AAV6-cFIX-R338L by peripheral transvenular delivery to the skeletal muscle. cFIX antigen levels were measured over time by ELISA (□), and cFIX activity levels were measured by activated partial thromboplastin time (●). Black arrows indicate weekly challenges with pooled normal canine plasma (100 mL). (D) PBMCs collected from M59 and N07 after the fourth challenge were subjected to IFN-γ ELISpot analysis after stimulation with AAV6 capsid, cFIX protein, or overlapping peptides spanning the 338 region (RATCLR/LSTKFTIYNM, LKVPVDRATCLR/LST).
Table 1.
Summary of results in hemophilia B dogs after skeletal muscle delivery of AAV-canine FIX R338L
| Dog | Age/sex/weight | Plateau cFIX expression |
Ratio activity/antigen* | WBCT,† min | Inhibitors | IFN-γ ELISpot |
|||
|---|---|---|---|---|---|---|---|---|---|
| Activity, % | Antigen, % | AAV | cFIX | 338 peptides | |||||
| M55 | 7 mo/male/23 kg | 8 | 1.5 | 8.58 ± 0.46 | 11.4 ± 0.7 | ND | — | — | — |
| M59 | 7 mo/male/16.5 kg | 3.5 | 0.35 | 9.24 ± 0.44 | 13.7 ± 0.7 | ND | ND | ND | ND |
| N07 | 6 mo/male/13 kg | 5.5 | 0.65 | 9.0 ± 0.37 | 14.4 ± 0.7 | ND | ND | ND | ND |
ND indicates not detected; and —, not done.
Average ratio of activity/antigen ± SE from all time points.
Whole blood clotting time: average values ± SE (normal values, 8-12 minutes).
One important consideration in any novel therapeutic approach for hemophilia is the risk of induction of neutralizing antibodies to the clotting factor. The success of gene therapy strategies to cure disease relies on the control of unwanted immune responses to transgene products, genetically modified cells, and/or to the vector.19 In early studies, we showed that transient immunosuppression (up to 4 weeks) of postskeletal muscle delivery of AAV-cFIX is required to prevent antibodies to the cFIX-WT transgene.1,6,19 One potential risk of introducing FIX with a variant sequence distinct from the WT protein is the immune response to the neotransgene product. The underlying mutation in the HB model from the University of North Carolina is a missense mutation that results in a G379E substitution in cFIX.20 Notably, there was no formation of inhibitory antibodies to cFIX-R338L on multiple challenges with cFIX-WT protein, even > 1 year after stopping immunosuppression (Figure 1B-C). These data were further supported by the lack of IFN-γ secretion by T cells after exposure to FIX-WT protein, peptides spanning the 338 residue with (R or L), and AAV-6 capsid (Figure 1D). Thus, the FIX-R338L variant shows no detectable immunogenicity in this approach. There was also no evidence of local or systemic toxicity, changes in TAT levels (data not shown), or clinically evident thrombotic complications; however, these data should be interpreted cautiously given the small number of animals used in this study. The latter is consistent with limited observation in humans where only patients with supraphysiologic levels of FIX-R338L (> 700% of normal) developed thrombosis12 (and P.S., unpublished data, August 2011).
The expression of FIX-R338L variant has the potential to lower the therapeutic vector dose and is an attractive strategy to overcome limitations in performing critical posttranslational modifications for a fully biologically active transgene in ectopic targets6 as we previously reported in murine models of HB.9 Moreover, the superior specific activity of FIX-R338L was also exhibited after infusion of recombinant FIX-R338L in HB dogs (V.R.A., unpublished data, January 2012). In conclusion, our findings in HB dogs support the use of FIX-338L for clinical translational studies of HB in a variety of therapeutic settings, such as protein replacement or gene-based approaches targeting the liver or ectopic sites.
Acknowledgments
The authors thank Scott Ashley and Nicholas Martin for technical assistance.
This work was supported by the National Institutes of Health (P01 HL64190, Project 1, V.R.A.; and HL63098, T.C.N.) and Howard Hughes Medical Institute (K.A.H.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.D.F. designed and executed the experiments and drafted the manuscript; T.C.N., E.P.M., and D.A.B. performed the vector administration, provided care to the animals, and collected laboratory samples; N.S. executed the T-cell assays; S.Z. provided the recombinant AAV vectors used in this study; P.S. and K.A.H. provided insights on experimental design and edited the manuscript; and V.R.A. directed experimental design, conducted data analysis and interpretation, and drafted the manuscript.
Conflict-of-interest disclosure: K.A.H. holds patents related to AAV manufacture and purification. She holds patents related to AAV-FIX, in which she has waived any financial interest. The remaining authors declare no competing financial interests.
Correspondence: Valder R. Arruda, Children's Hospital of Philadelphia, 3501 Civic Center Blvd, 5056 Colket Translational Research Center, Philadelphia, PA 19104; e-mail: arruda@e-mail.chop.edu.
References
- 1.Arruda VR, Stedman HH, Haurigot V, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115(23):4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 3.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 4.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niemeyer GP, Herzog RW, Mount J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113(4):797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.High KA. Gene therapy for haemophilia: a long and winding road. J Thromb Haemost. 2011;9(Suppl 1):2–11. doi: 10.1111/j.1538-7836.2011.04369.x. [DOI] [PubMed] [Google Scholar]
- 7.Buchlis G, Podsakoff GM, Radu A, et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119(13):3038–3041. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Pierce GF, Ozelo MC, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14(3):452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Schuettrumpf J, Herzog RW, Schlachterman A, Kaufhold A, Stafford DW, Arruda VR. Factor IX variants improve gene therapy efficacy for hemophilia B. Blood. 2005;105(6):2316–2323. doi: 10.1182/blood-2004-08-2990. [DOI] [PubMed] [Google Scholar]
- 10.Brunetti-Pierri N, Grove NC, Zuo Y, et al. Bioengineered factor IX molecules with increased catalytic activity improve the therapeutic index of gene therapy vectors for hemophilia B. Hum Gene Ther. 2009;20(5):479–485. doi: 10.1089/hum.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kao CY, Lin CN, Yu IS, et al. FIX-Triple, a gain-of-function factor IX variant, improves haemostasis in mouse models without increased risk of thrombosis. Thromb Haemost. 2010;104(2):355–365. doi: 10.1160/TH09-11-0792. [DOI] [PubMed] [Google Scholar]
- 12.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 2009;361(17):1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 13.Chang J, Jin J, Lollar P, et al. Changing residue 338 in human factor IX from arginine to alanine causes an increase in catalytic activity. J Biol Chem. 1998;273(20):12089–12094. doi: 10.1074/jbc.273.20.12089. [DOI] [PubMed] [Google Scholar]
- 14.Haurigot V, Mingozzi F, Buchlis G, et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther. 2010;18(7):1318–1329. doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregorevic P, Allen JM, Minami E, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12(7):787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arruda VR, Stedman HH, Nichols TC, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105(9):3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols TC, Franck HW, Franck CT, De Friess N, Raymer RA, Merricks EP. Sensitivity of whole blood clotting time and activated partial thromboplastin time for factor IX: relevance to gene therapy and determination of post-transfusion elimination time of canine factor IX in hemophilia B dogs. J Thromb Haemost. 2012;10(3):474–476. doi: 10.1111/j.1538-7836.2011.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols TC, Dillow AM, Franck HW, et al. Protein replacement therapy and gene transfer in canine models of hemophilia A, hemophilia B, von Willebrand disease, and factor VII deficiency. ILAR J. 2009;50(2):144–167. doi: 10.1093/ilar.50.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arruda VR, Favaro P, Finn JD. Strategies to modulate immune responses: a new frontier for gene therapy. Mol Ther. 2009;17(9):1492–1503. doi: 10.1038/mt.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans JP, Brinkhous KM, Brayer GD, Reisner HM, High KA. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A. 1989;86(24):10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]