Abstract
The intervertebral disc (IVD) is a moderately moving joint that is located between the bony vertebrae and provides flexibility and load transmission throughout the spinal column. The disc is composed of different but interrelated tissues, including the central highly hydrated nucleus pulposus (NP), the surrounding elastic and fibrous annulus fibrosus (AF), and the cartilaginous endplate (CEP), which provides the connection to the vertebral bodies. Each of these tissues has a different function and consists of a specific matrix structure that is maintained by a cell population with distinct phenotype. Although the healthy IVD is able to balance the slow matrix turnover of synthesis and degradation, this balance is often disturbed, leading to degenerative disorders. Successful therapeutic management of IVD degeneration requires a profound understanding of the cellular and molecular characteristics of the functional IVD. Hence, the phenotype of IVD cells has been of significant interest from multiple perspectives, including development, growth, remodelling, degeneration and repair. One major challenge that complicates our understanding of the disc cells is that both the cellular phenotype and the extracellular matrix strongly depend on disc maturity and health and as a consequence are continuously evolving. This review delineates the diversity of the cell types found in the intervertebral disc, with emphasis on human, but with reference to other species. The cells of the NP appear rounded and express a proteoglycan-rich matrix, whereas the more elongated AF cells are embedded in a collagen fibre matrix and the CEPs represent a layer of cartilage. Even though all disc cells have often been referred to as ‘intervertebral disc chondrocytes’, distinct phenotypical differences in comparison with articular chondrocytes exist and have been reported recently. The availability of more specific markers has also improved our understanding of progenitor cell differentiation towards an IVD cell phenotype. Ultimately, new cell- and tissue-engineering approaches to regenerative therapies will only be successful if the specific characteristics of the individual tissues and their context in the function of the whole organ, are taken into consideration.
Keywords: annulus fibrosus, cartilaginous endplate, disc cell phenotype, intervertebral disc function, notochordal cells, nucleus pulposus
Introduction
The intervertebral disc (IVD) is a moderately moving joint that separates the vertebrae of the spine. The main role of the IVD is to provide flexibility to the otherwise rigid spine, allowing for a wide range of movements and transmission of mechanical load. IVDs occupy one-third of the total human spine length and represent the largest avascular structure of the body. There are 25 IVDs in the human adult and they are subdivided according to the anatomical region into cervical (7), thoracic (12), lumbar (5) and sacral (1). With the exception of the first cervical and the sacral IVD, all the other IVDs have a similar structure (Moore, 2000). Distinct anatomical regions can be distinguished within the IVD. These include the central gel-like nucleus pulposus (NP), which is peripherally contained by the fibrous annulus fibrosus (AF), and the cartilaginous endplates (CEP) that anchor the discs to the adjacent vertebral bodies (Figs 1 and 2). Each of these tissues has a different function and consists of a specific matrix structure that is maintained by distinct cell populations (Fig. 3). In spite of a low cell density, the healthy IVD is able to balance the slow matrix turnover of synthesis and degradation.
Fig. 1.
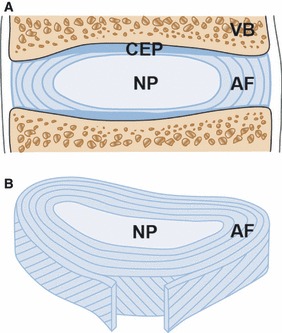
Schematic representation of an intervertebral disc (IVD). The cartilaginous endplate (CEP) and the adjacent vertebrae (VB) are visualized in the sagittal section of the disc (A). The annulus fibrosus (AF) is formed by structured lamellae which encapsulate the central nucleus pulposus (NP) (B).
Fig. 2.
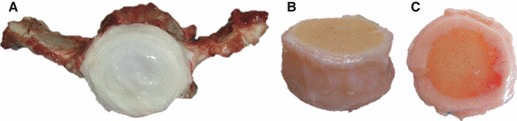
Gross morphology of a bovine caudal IVD from a 6-month-old calf, from different viewpoints. The concentric rings of the annulus fibrosus and central gelatinous core of the nucleus pulposus can be visualized in the transversal section of the IVD (A). The shape of the disc and the calcified portion of the endplates can be appreciated from a lateral view of the disc (B). Blood vessels are present in the outer part of the cartilaginous endplate, as observed in the top view of the IVD (C).
Fig. 3.
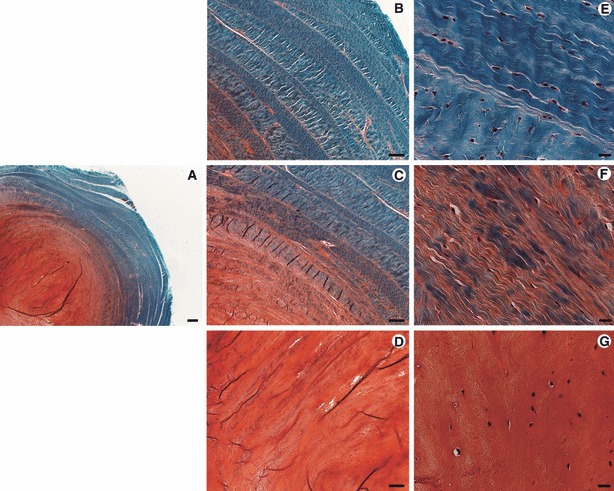
Safranin O-Fast-green staining of a caudal IVD from a 6-month-old bovine calf showing the distribution of sulphated glycosaminoglycans and collagen across the disc. An outer AF region rich in collagen and a central NP region rich in glycosaminoglycans are observed in the overview of the disc (A). The AF is formed by concentric lamellae (B) and its cells follow the orientation of these lamellae (E). The transition zone between AF and NP is characterized by a gradual decrease in collagen, increase in glycosaminoglycans and loss of the lamellar structure (C); cells of the AF are generally elongated (F). The NP matrix is mainly composed of glycosaminoglycans (D) and cells of the NP generally have a rounded morphology (G). Scale bars: 500 μm (A), 200 μm (B–D), 20 μm (E–G).
Degenerative disorders of the IVD have been associated with neck and low back pain and are commonly encountered in Western societies; the fact that the majority of the population is affected at some point during their lives accounts for significant health care and related costs (Juniper et al. 2009; Hoy et al. 2010). Surgical intervention is warranted when conservative therapy is not successful; although various surgical options are available, ranging from the standard procedures of discectomy and spinal fusion to the more recently introduced disc arthroplasty, the mechanical function of the native disc is not recapitulated by any of these methods and the rate of complication is considerable (Hanley, Jr et al. 2010). Therefore, there is a need for new treatments that aim to restore the IVD structure and function by addressing the specific biological and biochemical features of the tissues. As alterations in the extracellular matrix composition during development and ageing can at least partially be attributed to changing function and vitality of the disc cells (Zhao et al. 2007), supplementations of cell populations and endogenous cell activation have been considered as preventative and therapeutic means for degenerative and traumatic IVD disorders (Masuda & An, 2006; Yoon & Patel, 2006; Sakai, 2008; Grad et al. 2010).
Successful therapeutic management also requires a profound understanding of the cellular and molecular characteristics of the functional IVD. Hence, the phenotype of the IVD cells has been of significant interest from multiple perspectives, including development, growth, remodelling, degeneration and repair. One major challenge that complicates our understanding of the disc cells is that their reference state is a changing target. Indeed, both the cellular phenotype and the extracellular matrix depend strongly on disc maturity and health and as a consequence are continuously evolving (Antoniou et al. 1996a, b; Boos et al. 2002; Roughley, 2004; Chen et al. 2006; Rutges et al. 2010; Weiler et al. 2010). Moreover, several intrinsic and extrinsic factors influence the cellular and molecular state of the IVD, including ageing (Antoniou et al. 1996a, b), genetics (Chan et al. 2006), transport of nutrients and metabolic products (Grunhagen et al. 2006; Rastogi et al. 2009), and the mechanical environment (Iatridis et al. 2006; Guehring et al. 2010). This makes it difficult to distinguish the vital cell type to target for therapeutic measures.
A further difficulty in IVD research is the restricted availability of human cells and intact tissues that would enable more comprehensive analyses. Therefore, animal models are indispensable for both basic and applied investigations in spite of their limitations, which refer to the differences in cellular development, disc size, endplate characteristics and biomechanical environment (Alini et al. 2008). Comparative studies have shown that with reference to anatomical and biomechanical features, both small and large animals could be suitable models of the human IVD (O’Connell et al. 2007; Beckstein et al. 2008). It is suggested that animal models are also appropriate to study the basic molecular and cellular pathways that maintain the functional disc in order to answer specific biochemical, biological, or genetic questions. Nevertheless, recent investigations have shown that there are considerable inter-species variations in the phenotypical characteristics of the IVD cells (Sakai et al. 2009; Minogue et al. 2010b; Rutges et al. 2010). These variations may partially be related to the morphological changes of the NP cells from a notochord-like to the mature NP cell phenotype, given that the rate at which these changes occur, strongly depends on the species (Hunter et al. 2004; Miyazaki et al. 2009).
Despite this complexity, our knowledge in IVD biology has been expanded significantly in the last decade. Cells of the IVD, which had generally been referred to as ‘chondrocyte-like’ cells or ‘IVD chondrocytes’, have been profiled and characterised in detail. Although phenotypical similarities exist between NP cells and articular chondrocytes, distinct differences have been identified (Mwale et al. 2004; Lee et al. 2007; Sakai et al. 2009; Minogue et al. 2010a, b; Rutges et al. 2010; Power et al. 2011). This is of crucial importance for the success of regenerative and repair strategies, considering the structural and mechanical distinctions between IVD and cartilage tissues. Similarly, AF cells have often been related to ‘fibroblast-like’ cells, although they generate an extracellular matrix that differs from fibrous cartilage, ligament and tendon. The CEP has often been described as a tissue very similar to articular cartilage, despite important differences in the cell arrangement, organisation of the extracellular matrix and function. This review article describes the phenotypical characteristics of the IVD cells in relation to the tissue function, thereby addressing the NP, AF and CEP separately but in an inter-related context. Implications for cellular and molecular strategies to repair or regenerate damaged or diseased tissue are discussed.
The origin of the intervertebral disc from the notochord
The notochord provides the template for the development of the spinal segments of the disc (Peacock, 1951; Adams et al. 1990; Aguiar et al. 1999; Fleming et al. 2001; Hunter et al. 2004). The course of notochordal development towards mature disc cells represents a multi-stage mechanism involving various mechanical and chemical events. A detailed description of this process is available in the literature, such as the recent review by Risbud et al. (2010). Briefly, the notochord is derived from the process of gastrulation, whereby the embryo develops into three layers (ectoderm, endoderm and mesoderm). The early notochord is a rod-like, axial structure derived from the mesoderm that induces differentiation of the ventral somatic derivatives into the sclerotome (Pourquie et al. 1993; Aszodi et al. 1998). Sclerotomal cells migrate toward the notochord, where they form a continuous and initially unsegmented perichordal tube or peri-notochordal sheath. Initially, cells within notochord synthesize proteoglycans that increase the osmotic pressure within their cell vacuoles. The swelling of the cell vacuoles creates an osmotic pressure that increases the internal pressure within the notochord causing it to be elongated and straightened, forming the basis of the vertebral column (Adams et al. 1990; Stemple, 2005). The notochord and surrounding sheath are subjected to various developmental signals [e.g. Sonic Hedgehog (Shh) and brachyury] that enable the formation of the IVD (Choi et al. 2008; Risbud et al. 2010; Choi & Harfe, 2011).
The sclerotomally derived mesenchymal cells condense within the peri-notochordal sheath and form a repeating annular condensed area that gives rise to the AF (Christ & Wilting, 1992; Aszodi et al. 1998). The noncondensed regions of the sheath form the cartilaginous primordial of the vertebral bodies with a soft CEP forming on each side of the developing vertebral bodies (Theiler, 1988; Christ & Wilting, 1992; Aszodi et al. 1998). Following the condensation of the sheath, the notochord surrounded by the AF begins to condense and leads to the formation of the NP (Choi et al. 2008; Risbud et al. 2010; Choi & Harfe, 2011; Hayes et al. 2011a). It has been shown that embryonic spine development may be interrupted via deletion of transcription factors such as SOX-5 and SOX-6, leading to a loss of the notochord and affecting NP formation (Smits & Lefebvre, 2003; Choi & Harfe, 2011). As the notochord differentiates intervertebrally to form the NP, it pushes against surrounding annular condensations, triggering the creation of an inner and outer AF. The outer AF is composed of laminae of type I collagen fibres aligned with cells, whereas the inner section contains more widely spaced layers and greater amounts of glycosaminoglycans and type II collagen compared with the outer section (Peacock, 1951, 1952; Eyre & Muir, 1976, 1977; Rufai et al. 1995; Hayes et al. 1999, 2011a, b; Hayes & Ralphs, 2011). The central nucleus pulposus is predominantly composed of glycosaminoglycans and type II collagen (Peacock, 1951, 1952). A swelling pressure is created by the presence of glycosaminoglycans in the NP that results in circumferential tensile stresses being applied to the outer annulus which are maximal at the periphery, whereas the inner annulus is subjected to maximal compressive stresses (Adams et al. 2009; O’Connell et al. 2011; Michalek et al. 2012).
The CEP and inner AF are interconnected by organised collagen fibre bundles that form a cage-like structure enclosing the NP (Humzah & Soames, 1988; Hayes et al. 2011b), whereas the collagen bundles in the outer AF are strongly anchored to the vertebral bodies. This fibrillar organisation is present at birth, before the vertebrae undergo calcification (Peacock, 1951, 1952). The CEP is loosely attached to the growing trabecular structure of the vertebral bodies by a thin layer of calcified material and benefits from a relatively high and deep vasculature. The CEP adhesion to the underlying vertebrae becomes stronger as the vertebral trabecular structure matures (Humzah & Soames, 1988).
The nucleus pulposus
Extracellular matrix of nucleus pulposus: composition, structure and function
The NP is a heterogeneous structure with large fibrillar spaces within a 3D mesh of collagen fibrils that contain proteoglycans (Humzah & Soames, 1988). It is predominantly made up of water (70–90% depending on the age), collagen type II (ca. 20% of dry weight) and proteoglycans (ca. 50% of dry weight) (Buckwalter, 1995). However, NP tissue is distinctly different from hyaline cartilage due to the fact that the ratio between proteoglycan and collagen (measured as GAG to hydroxyproline ratio) within the NP is 27 : 1, whereas in CEP this ratio is 2 : 1 (Mwale et al. 2004). Variations in aggrecan to collagen ratios have therefore been used to potentially distinguish between chondrogenic and ‘IVD-like’ differentiation of MSCs (Gantenbein-Ritter et al. 2011; Stoyanov et al. 2011). Studies have also shown that NP cells are characterised by a significantly higher expression for aggrecan and collagen type II and greater proteoglycan synthesis compared with other cells within the disc (Chelberg et al. 1995; Poiraudeau et al. 1999; Horner et al. 2002). Aggrecan is a large proteoglycan that forms aggregates (molecular weight range: 3 × 105–7 × 106 Dalton) with hyaluronan through an interaction that is stabilised by a link protein (Sztrolovics et al. 1997). The presence of proteoglycans with negatively charged side chains enables the disc to remain highly hydrated and to undergo reversible deformation, especially under compression. In particular, the proteoglycans, aggrecan and biglycan have been found to be highly expressed within the NP; decorin and versican are present but at lower levels compared with the AF (Melrose et al. 2001; Sztrolovics et al. 2002; Singh et al. 2009; Hayes et al. 2011b). However, the presence of proteoglycans and retention of water within the NP decreases with age. This leads to a reduction in pressure within the NP core and results in increased compressive stress being applied to the inner annulus (Adams & Hutton, 1985; Adams et al. 2009).
The main function of the NP is to equalise the compressive stress on the vertebral EP. In vertebrates, IVDs are subjected to complex loading mechanisms involving compression, tension, torsion, shear and fluid flow (Skaggs et al. 1994; Ebara et al. 1996; Iatridis et al. 2006). However, there are different responses to each of these mechanical signals depending on the disc cell type (Iatridis et al. 1998, 2006; Li et al. 2008, 2011; Korecki et al. 2009). For instance, Li et al. (2008) demonstrated that there were distinct variations in the arrangement of cytoskeletal elements within bovine NP and AF cells. In particular, β-tubulin and vimentin were highly expressed within NP compared with AF cells. The organisation of these cytoskeletal elements within the NP was similar to that described for articular chondrocytes. The expression of these particular elements within the NP suggests that these cells are loaded in compression, whereas the stronger expression of β -actin within the AF cells was hypothesised to be associated with tensile loading. However, it should be noted that in vivo, the presence of a hydrostatic pressure may also influence the expression of cytoskeletal elements (Nachemson, 1981; Adams & Hutton, 1985, Setton & Chen, 2006). This was clarified in a recent study by the same authors, whereby tensile loading did not induce changes in bovine NP cells compared with AF in cytoskeleton remodelling or expression of anabolic and catabolic genes (Li et al. 2011). In vitro and in vivo models have shown the up-regulation of various matrix-associated genes (such as type II collagen, aggrecan, matrix metalloproteinase 3 and 13) and cytokines, e.g. interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α), when NP cells were subjected to compressive loading (Wang et al. 2007; Korecki et al. 2009; Wuertz et al. 2009). Furthermore, the greater amount of proteoglycans within the NP compared with articular cartilage may be associated with the predominantly compressive loading of the tissue compared with cartilage that is influenced both by compressive and shear loading. In vivo NP cells may undergo shear strain, although this is likely to be negligible compared with the shear strain experienced by chondrocytes in cartilage (Schätti et al. 2011; Egli et al. 2011; Grad et al. 2011).
In evaluating these in vitro and in vivo models, it should be noted that load regimes are different in these situations, thus there may be differing responses in cell metabolism. In general, cell culture and in vitro models may refer to deformation in the form of compression, whereas in in vivo, a hydrostatic pressure is applied without cell deformation (Nachemson, 1981; Adams & Hutton, 1985, Setton & Chen, 2006). Studies have evaluated the effects of hydrostatic pressure on in vitro cultured NP cells using various loading systems. In summary, these studies showed that application of a hydrostatic pressure below 3 MPa and at a range of frequencies induced proteoglycan synthesis, whereas pressures greater than 3 MPa induced nitric oxide production and inhibited matrix synthesis (Ishihara et al. 1996; Kasra et al. 2003; Gokorsch et al., 2004; Le Maitre et al. 2008; Neidlinger-Wilke et al. 2012).
The phenotype and function of nucleus pulposus cells
Phenotype and function of notochordal cells
Notochordal cells of the postnatal NP are large (30–40 μm in diameter), commonly appear in clusters and contain intracellular vacuoles making up at least 25% of the cell area (Hunter et al. 2003, 2004). Notochordal cells have been generally described as being lost with age in humans, although many animal species retain the presence of these cells (Peacock, 1952; Hunter et al. 2003, 2004).
Studies have assessed the notochordal cell phenotype for the purpose of understanding the initial states of nucleus pulposus cells prior to their maturation. Chen et al. (2006) used a porcine model system to describe the characteristics of an immature nucleus pulposus phenotype within the disc cell population. These cells were found to have a reduced gene expression of specific matrix molecules such as collagen type I and biglycan and the metalloproteinase inhibitor known as tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) compared to mature porcine NP cells. However, no differences in the expression of chondrogenic genes (SOX-9, collagen type II and aggrecan) between notochordal and mature porcine NP cells were noted, although these ‘notochordal-like’ cells did express higher levels of integrins α1 and α6, which are involved in interactions with collagen and laminins during tissue development (Chen et al. 2006). Furthermore, the same group recently demonstrated that laminins may also be used to differentiate between notochordal and mature porcine NP cells within the disc (Chen et al. 2009).
A recent investigation demonstrated that a cell population expressing phenotypic markers of notochordal cells is also present within adult human discs (Weiler et al. 2010). The study examined distinct notochordal markers including cytokeratin-8, -18, -19 and galectin-3. The latter gene is involved in regulating cell differentiation and mediating inflammation, while cytokeratins are involved within the cell ultrastructure (Goetz et al. 1995, 1997). The study demonstrated the expression of these markers in young human discs (subject age range: 0–17 years), although their expression was reduced with age of the disc, particularly in elderly subjects (> 60 years). It was concluded that expression of these notochordal markers in mature discs (> 18 years), particularly galectin-3, may lead to the onset of disc degeneration. However, this is somewhat contradictory to previous conclusions regarding notochordal cell function within the disc, as it has been extensively described that this cell type helps in regeneration of the tissue and that the loss of these cells increases the likelihood of degeneration (Cappello et al. 2006; Henriksson et al. 2009; Kim et al. 2009a; Sakai et al. 2009; Blanco et al. 2010; Minogue et al. 2010a; Risbud et al. 2010). It may be concluded that cells with a notochordal phenotype, indicating a population of notochordal origin, are present within human NP tissue, although the presence or loss of these cells with age within human subjects remains speculative (Erwin, 2010; Risbud et al. 2010).
Using in vitro culture systems, studies on several species have shown the beneficial effects of notochordal cells, particularly in co-culture with NP or mesenchymal stem cells (MSCs) (Aguiar et al. 1999; Erwin & Inman, 2006; Kim et al. 2009a; Korecki et al. 2010; Purmessur et al. 2011). Aguiar et al. (1999) demonstrated in a 3D in vitro co-culture model that the interaction between NP cells (from fetal or mature bovines) and notochordal cells (from non-chondrodystrophoid canines) induced a high proteoglycan synthesis due to the secretion of soluble factors by the notochordal cells. This concept has been applied in recent investigations, in which porcine notochordal cell-conditioned medium was used to culture human MSCs (Korecki et al. 2010; Purmessur et al. 2011). It was concluded that the conditioned medium had the potential to differentiate MSCs towards a young NP phenotype. A comprehensive analysis of the composition of the notochordal cell-conditioned medium has not been reported yet, although connective tissue growth factor (CTGF) has been suggested to play a role (Erwin & Inman, 2006). Together, these studies, alongside those previously described, show that the notochordal cell phenotype and function have a pivotal role in NP development, although their presence and purpose in the mature human NP remains a matter of debate. In addition, as noted earlier for NP cells, in vitro experiments may not reflect the in vivo situation with respect to hydrostatic pressure, transportation of metabolites, cell metabolism and cell–tissue interaction, which may also influence the notochordal cell behaviour within the developing NP tissue.
There is increasing evidence that mature NP cells are derived from the notochordal cells that are functional in the development of the disc during embryogenesis (Choi et al. 2008; Risbud et al. 2010). The evidence for this may be derived partly from phenotype expression, surface marker and genome-wide microarray studies (Poiraudeau et al. 1999; Sive et al. 2002; Fujita et al. 2005; Lee et al. 2007; Sakai et al. 2009; Gilson et al. 2010; Minogue et al. 2010a, b; Power et al. 2011). It has been found that cytokeratin-8, -18, -19 and brachyury or T-Box gene are commonly expressed by nucleus pulposus and notochordal cells (Lee et al. 2007; Sakai et al. 2009; Minogue et al. 2010b; Rutges et al. 2010). Brachyury expression is required for notochordal cell differentiation during development and formation from the mesoderm, although misexpression could lead to ectopic notochord formation (Herrmann & Kispert, 1994; Takahashi et al. 1999). However, there remains considerable debate regarding the presence of notochordal cells and how this relates to degeneration and regeneration (Yang et al. 2009). Risbud et al. (2010) comprehensively reviewed information regarding the derivation of nucleus pulposus cells and hypothesised that NP cells are derived from notochordal cells and that the variations in morphology and size of these cells relates to their maturation and function. Further evidence regarding the derivation of NP from notochordal cells may be related to the signalling pathway used in notochordal development, particularly the utilisation of Sonic Hedgehog (Shh) signalling (Choi & Harfe, 2011). Choi et al. (2008) showed that notochordal cells expressing Shh genes formed the NP and that this pathway was essential for the NP, although not necessarily for AF and CEP development.
Phenotype of mature nucleus pulposus cells
Compared to the notochordal cells, mature NP cells are smaller (about 10 μm in diameter) and lack intracellular vacuoles (Hunter et al. 2004). Their morphology appears rounded (Fig. 3G), and their density in the NP is approximately 4 × 106 cells cm−3 (Maroudas et al. 1975; Roughley, 2004).
Recent microarray studies on the transcriptome of the nucleus pulposus cells have contributed significantly to identify NP specific markers (Table 1), although clear on-off markers have not been discovered. These markers not only provide insight into the NP development and metabolism, but could also be utilised to monitor the phenotype of MSCs during differentiation into the NP-like phenotype (Lee et al. 2007; Sakai et al. 2009; Minogue et al. 2010b; Rutges et al. 2010; Power et al. 2011; Stoyanov et al. 2011). Initial investigations assessing the phenotypic expression of NP cells demonstrated that within both healthy and degenerative discs, chondrocyte markers like SOX-9 and collagen type II were expressed (Sive et al. 2002; Clouet et al. 2009). Lee et al. (2007) used a rat tail model to identify specific genes associated with NP cells. They showed that the expression of cytokeratin-19 and glypican-3 was higher in NP compared with AF cells and articular chondrocytes. A possible limitation of the study is that rats have a mixed population of cells in the NP region comprising both notochordal and NP cell population.
Table 1.
Selection of human and animal studies of the phenotype of IVD cells
| Origin | Cell type* | Characterisation | References |
|---|---|---|---|
| Human, bovine | NC, NP, AF, AC | Microarray, RT-PCR | Minogue et al. (2010a, b) |
| Human | NP, AF, AC | RT-PCR, Immunohistochemistry | Rutges et al. (2010) |
| NP, AF, AC | Microarray, RT-PCR, Immunohistochemistry | Power et al. (2011) | |
| NP, AF | RT-PCR | Kluba et al. (2005) | |
| NP, AF | In-situ hybridisation, Immunohistochemistry | Sive et al. (2002) | |
| NC, NP, AF | Immunohistochemistry | Weiler et al. (2010) | |
| NP, AF, CEP | Immunoassays | Johnstone et al. (1993) | |
| NP, AF | Immunoassays | Antoniou et al. (1996a) | |
| AF | Microarray, RT-PCR | Gruber et al. (2010a, b, 2011) | |
| CEP | Immunoassay | Antoniou et al. (1996b) | |
| Human, bovine, ovine, rat | NP, AF, CEP | Immunohistochemistry | Roberts et al. (1994) |
| Human, canine | NP, AF, CEP | Immunohistochemistry | Inkinen et al. (1999) |
| Bovine | NP, AF, AC | Immunohistochemistry, proteomics | Gilson et al. (2010) |
| Porcine, rat | NC, NP, AF | Flow cytometry, RT-PCR | Chen et al. (2006) |
| Canine | NP, AF, AC | Microarray, RT-PCR, Immunohistochemistry | Sakai et al. (2009) |
| Leporid | NP, AF, AC | PCR, matrix quantification | Poiraudeau et al. (1999) |
| NP, AF, AC | RT-PCR | Clouet et al. (2009) | |
| Rat | NP, AF, AC | Microarray, RT-PCR, Immunohistochemistry | Lee et al. (2007) |
Cell type: NC, notochordal; NP, nucleus pulposus; AF, annulus fibrosus; CEP, cartilaginous endplate; AC, articular chondrocytes.
Similarly, Sakai et al. (2009) used a beagle dog model because of its comparability to human with respect to the drastic loss of notochordal cells after birth (Alini et al. 2008). In contrast to the rat model, glypican-3 was found to be expressed within the AF rather than the NP tissue, indicating considerable inter-species differences. However, in terms of a model from a non-notochordal species, genes highly expressed by NP cells compared with AF cells included cytokeratin-18, CD56 (also called neural cell adhesion molecule-1 or NCAM1), α-2 macroglobulin and desmocollin-2. Recent investigations comparing human and bovine tissue have clarified that there are also significant differences between human and animal samples (Minogue et al. 2010b; Rutges et al. 2010).
Fujita et al. (2005) identified CD24 as a specific marker on NP cells derived from rats. However, recent studies utilising human tissue have not identified CD24 as a pure NP cell marker (Minogue et al. 2010a; Rutges et al. 2010; Power et al. 2011). Rutges et al. (2010) and Minogue et al. (2010a) demonstrated cytokeratin-19 to be a marker for human NP cells, although its expression was decreased with increasing age or degree of degeneration, which is also reflected at the protein level (Fig. 4A–C). As cytokeratin-19 is associated with notochordal cells (Fig. 4A), its expression within mature NP provides further evidence of the notochordal origin of these cells (Rutges et al. 2010). Interestingly, cytokeratin-19 labelling could also be localised in regions of pathological degenerative IVD tissue (Fig. 4C), in accordance with previous studies (Weiler et al. 2010). Analysis of degenerative human NP also showed a gene up-regulation of fibulin, whose function is to regulate the aggrecanase ADAMTS-1, a marker expressed in degenerated IVDs (Lee et al. 2005; Pockert et al. 2009). It was speculated that up-regulation in this gene may be associated with changes in the extracellular matrix of the disc.
Fig. 4.
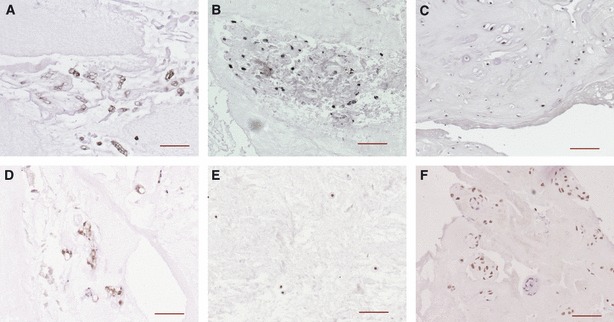
Immunostaining for cytokeratin-19 (A–C) and carbonic anhydrase 12 (CA12) (D–F) in human nucleus pulposus tissue. Notochordal-like cells of a 3-year-old individual stain positive for both markers (A and D). Nucleus pulposus cells of a 14-year-old individual also show immunoreactivity for both markers (B and E), whereas positive staining is hardly observed in normal adult IVD (not shown). However, pathological tissue of degenerated discs (C: 79 years and F: 62 years) can react positive for cytokeratin-19 and CA12. Scale bar: 100 μm. Original references are from Rutges et al. (2010) and Power et al. (2011).
Power et al. (2011) and Minogue et al. (2010b) performed genome-wide arrays of human NP cells from healthy and degenerative tissues. The latter study evaluating human NP cells compared with articular chondrocytes identified paired box-1 (PAX1), forkhead box F1 (FOXF1), ovostatin-2, carbonic anhydrase XII (CA12) and haemoglobin β (HBB) as potential NP markers. PAX1 had been shown to be expressed in the discs of human fetal vertebral columns, encoding the transcription factor that regulates pattern formation during embryogenesis. Its expression is regulated by Shh, which, as discussed, is involved in the development of the nucleus pulposus within the notochord (Smith & Tuan, 1994). Furthermore, Shh has also been shown to regulate and activate FOXF1, which controls the cell growth and differentiation of various tissues, although its function during disc development is yet to be ascertained (Mahlapuu et al. 2001).
HBB and CA12 are associated with the hypoxic environment of NP cells. It has been previously observed in studies examining hypoxia inducible factor-1α expression that this environment is a further determinant of the NP cell phenotype (Rajpurohit et al. 2002; Risbud et al. 2006). HBB is associated with oxygen storage within the cell and provides a mechanism for cell survival under hypoxic conditions (Newton et al. 2006). Power et al. (2011) identified CA12 as an NP-specific cell surface marker and showed that its function within NP cells is likely to regulate intracellular pH, especially under hypoxia. However, age-dependent variations in CA12 expression are evident at both the transcript and protein level (Figs 4D–F and 5).
Fig. 5.
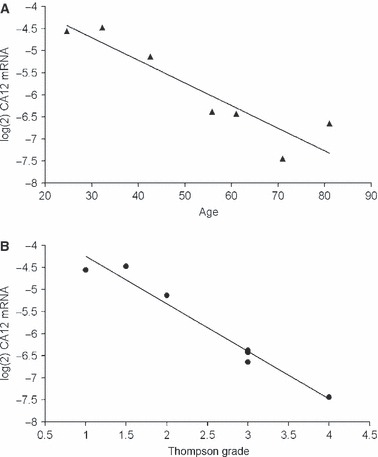
Relative mRNA expression for carbonic anhydrase 12 (CA12) in nucleus pulposus cells of seven individuals between 25 and 81 years of age. Gene expression was normalised to four housekeeping genes. A decrease in the CA12 expression with (A) increasing age and (B) disc degeneration level (Thompson grade) is noted. Original reference is from Power et al. (2011).
During the process of degeneration, NP cells have been shown to up-regulate matrix degrading enzymes, such as aggrecanases and collagenases, leading to down-regulation of aggrecan and collagen type II (Cs-Szabo et al. 2002; Kluba et al. 2005; Le Maitre et al. 2006, 2007b; Cui et al. 2010). This is often associated with an increase in inflammatory mediators such as IL-1β and TNF-α. The process may be accompanied by a reduction in water content and an increase in collagen type II breakdown within the NP of the disc (Antoniou et al. 1996a). In conjunction with this process, the NP has been shown to express senescence markers (e.g. p16) that further contribute towards the progression of degeneration (Le Maitre et al. 2007a). The relationship between matrix disruption and cellular changes may be termed a vicious cycle that results in disc degeneration.
The annulus fibrosus
Extracellular matrix of annulus fibrosus: composition, structure and function
The AF is composed principally of water (60–80%, depending on the region and the age), collagen (50–70% of dry weight), aggregating and nonaggregating proteoglycans (10–20% of dry weight) and noncollagenous proteins (ca. 25% of dry weight) (Bayliss & Johnstone, 1992). The proportion of type I collagen increases from the inner part towards the outer annulus, whereas type II collagen follows a diverse distribution (Bron et al. 2009).
The large aggregating proteoglycans include aggrecan and versican, whereas the small interstitial proteoglycans include biglycan, decorin, fibromodulin and lumican (Singh et al. 2009; Hayes et al. 2011b). Proteoglycans comprise a small proportion of the AF tissue and are substituted with several negatively charged glycosaminoglycans (GAGs) that are responsible for the hydration of the tissue through their water-binding capacity, enabling the tissue to undergo rapid reversible deformation (Sztrolovics et al. 1997). Versican is present in the IVD, especially in the fetal AF tissue, but its function is not clear (Melrose et al. 2001). The small proteoglycans are characterised by a leucine-rich core protein substituted by a few GAG side chains. They bind to collagens, growth factors, and other matrix components and are believed to play important roles in the regulation of matrix assembly and repair after injury or damage. Decorin and biglycan are the two most prominent members of the small proteoglycan family in the IVD, decorin being more abundant in the AF and biglycan in the NP (Singh et al. 2009).
The AF consists of a series of concentric rings, or lamellae, with collagen fibres lying parallel within each lamella (Marchand & Ahmed, 1990; Pezowicz et al. 2005), as shown in Figs 2A and 3A–F. In addition to the collagen network, a network of elastin fibres is present between the lamella and provides an integral part of the AF structure (Yu et al. 2002).
The AF plays a key role in the mechanical function of the IVD. The AF cells and the extracellular matrix synthesised by AF cells are the basis for its complex mechanical behaviour, which is nonlinear, anisotropic (direction-dependent) and viscoelastic (rate-dependent) (Nerurkar et al. 2008). This complex mechanical behaviour can be affected by changes in collagen type within the inner annulus with ageing, as collagen type II is replaced by collagen type I in this region (Peacock, 1952; Schollmeier et al. 2000; Longo et al. 2006). This phenomenon was also observed in rat, where in the outer annulus, type II collagen and the glycosaminoglycans chondroitin 4-sulphate and keratan sulphate only appeared in adult animals, indicating a metaplastic change in the lamellar fibroblasts (Rufai et al. 1995). It has been observed with human discs that there is a decrease in the content of type II collagen and aggrecan within the AF during degeneration (Antoniou et al. 1996a), whereas the elastic modulus of AF increases. This suggests that the load carriage mechanism of AF shifts from fluid pressurisation to elastic deformation within the inner annulus, although the outer annulus remains under tension (Iatridis et al. 1998). With time, this may lead to pain and annular rupture.
Phenotype of annulus fibrosus cells
The AF cells are morphologically similar to fibroblasts, appearing thin and elongated (Fig. 3E,F). The cell density in mature AF is about 9 × 106 cells cm−3 (Maroudas et al. 1975; Roughley, 2004). Additionally, a higher cell density has been found in the outer compared with the inner annulus (Hastreiter et al. 2001).
Similar to investigations into NP cells, recent studies have sought to find specific markers of AF cells (Table 1); however, conclusive phenotypic markers have not yet been identified for AF cells. Clouet et al. (2009) identified that the expression of type V collagen was markedly higher in AF cells compared with NP cells and articular chondrocytes in rabbit, indicating that type V collagen could potentially be a marker for AF cells. However, as interspecies differences in gene expression were evident (Sakai et al. 2009; Rutges et al. 2010), it is important to identify both the cell type and species when defining the AF cell phenotype. A recent study showed that tenomodulin (a member of the small proteoglycan family) may serve as a marker for the AF phenotype, as its gene expression in AF was significantly higher than in NP cells and articular chondrocytes, in both human and bovine species (Minogue et al. 2010b). In addition, tenomodulin gene expression was significantly increased in degenerated compared with normal human AF cells.
Comprehensive studies addressing the phenotypical characteristics of AF cells from healthy and diseased tissues have only been initiated recently. In one study a laser capture microdissection method was used to selectively harvest senescent and non-senescent cells in paraffin sections of human AF tissue, and their gene expression profiles were subsequently compared by microarray analysis (Gruber et al. 2010a). Major genes were identified which have recognised relationships to cell senescence and may be targeted in regenerative approaches to prevent cellular senescence in the AF, including mitogen-activated protein kinase p38, growth arrest and DNA-damage-inducible β, retinoblastoma (Rb)-associated KRAB repressor gene, discoidin CuB and LCCL domain containing protein 2, gene inhibitor of growth family member 5, somatostatin receptor 3, interferon-induced transmembrane protein 1, sphingosine 1-phosphate receptor 2, nitric oxidase synthase 1, and heat shock 70 kDa protein 6. Human AF cells were also analysed for gene expression profiles related to mitochondrial function (Gruber et al. 2011). The data revealed gene expression patterns consistent with mitochondrial dysfunction in AF cells from degenerated discs, suggesting that mitochondrial-focused approaches may be considered in future AF regenerative therapies. Finally, recent work from the same research group compared gene expression patterns in human AF cells isolated from discs of different levels of degeneration after culture in 3D collagen scaffolds (Gruber et al. 2010b). AF cells from more degenerated discs showed altered gene expression profiles after 3D culture; specifically, variations in the expression of interleukins, cytokines, extracellular matrix components and apoptosis regulators were identified. While these findings contribute to the understanding of cellular changes during AF degradation, the use of passaged cells after in vitro culture may be a limitation of the study. Our previous results have indicated correlations in the expression of certain genes in AF cells with age and degeneration, e.g. levels of pleiotrophin mRNA in AF cells were found to correlate significantly with patient age (Rutges et al. 2010). This may be related to increased vascular in-growth in degenerate AF (Johnson et al. 2007). Moreover, a positive correlation of orosomucoid 1 and negative correlations of spondin 2 and tubulin polymerisation-promoting protein family member three gene expressions were found for human AF cells with age and degeneration. In rat, higher AF levels of glypican 3, cytokeratin-19, matrix gla protein, and pleiotrophin mRNA were found in aged compared with young tissue (Lee et al. 2007).
The cartilaginous endplate
Extracellular matrix of cartilaginous endplate: composition, structure and function
The healthy endplate is comprised of an osseous and a cartilaginous part (Fig. 2B–C); when referring to the latter, the term cartilaginous endplate (CEP) is generally used. Similar to the other IVD tissues (NP and AF) and to articular cartilage, the main component of the CEP is water (close to 80% after birth, but below 70% after 15 years of age), followed by type II collagen and proteoglycans. The ratio between proteoglycans and collagens (measured as GAG to hydroxyproline ratio) within the CEP is 2 : 1, which is similar to that of articular cartilage and much lower than that of NP (Mwale et al. 2004). As for the AF and NP, other smaller types of proteoglycans (e.g. decorin, biglycan) have also been described recently for CEP (Hayes et al. 2011b).
The thickness of the human CEP is 0.5–1 mm at the periphery and diminishes toward the centre. The CEP is organised in a highly hydrated proteoglycan matrix reinforced by collagen fibrils. The orientation of the collagen fibrils changes across the CEP with collagen fibrils orientated parallel to the vertebral bodies in the centre of the CEP (corresponding to the NP location) but are curved closer to the inner AF region, where they merge with the AF collagen fibres (Maroudas et al. 1975; Humzah & Soames, 1988). Elastic fibres run parallel to collagen fibrils in the inner AF region that connects to the CEP, and it has been observed that elastic fibres contribute to anchor the NP to the adjacent CEPs (Yu et al. 2002).
The CEP has a structural, semi-permeable barrier and load-bearing functions. The structural function of the CEP is to separate the intervertebral disc from the adjacent vertebrae and to contain the NP tissue. Concerning the barrier function, it has to be noted that although a blood supply is present in the outer AF, the healthy NP is avascular. Indeed, during first decade of life, the NP has vascular supply both from the CEP and the AF, but the blood vessels recede with age and thus are not present within the adult NP. The diffusion distance from the blood supply to cells in the central portion of the NP can reach 8 mm (Benneker et al. 2005). Therefore, the CEP represents the main route by which small solutes diffuse into the NP. The exchange of solutes is ensured by capillaries found in the calcified part of the CEP that form buds in the proximity of the CEP. The central zone of the end-plate allows for the highest diffusion of small molecules (Maroudas et al. 1975). The outer AF also allows for small molecule diffusion, but the inner AF (region of interconnection between AF and CEP collagen fibres) is almost impermeable. However, it should be noted that the diffusion process is not only influenced by the CEP permeability but also by the molecule size and ionic charge (Urban et al. 1977). Indeed, small molecules such as glucose and oxygen can easily migrate through the IVD, whereas larger molecules (e.g. enzymes) or charged molecules have difficulties migrating through the disc. In terms of mechanical perspective, the CEP contributes towards evenly distributing the compressive load originating from the IVD on to the vertebral body (Broberg, 1983). The ability of the CEP to bear load is governed by the balance between collagen, proteoglycan and water content, in addition to the structural integrity of the matrix (Antoniou et al. 1996b).
Changes in the endplate with ageing have been summarised by Moore (2006) and are often due to or accompanied by changes in the NP and AF tissues. During IVD degeneration, the cartilage endplate becomes thinner, and fissures and sclerosis of the subchondral bone may be observed (Roberts et al. 2006). The first defects found in endplate are transversal fissures, which may be accompanied by blood vessel invasion and endplate ossification. Several studies, both theoretical (Natarajan et al. 1994) and experimental (Tanaka et al. 1993; Moore et al. 1996), have shown that the point of failure of the endplate is generally located in the vicinity of the subchondral bone. Additionally, disc protrusion in the vertebral body through a small opening in the CEP (also called ‘Schmorl’s nodes’) is commonly observed, both in younger and older spines (Moore, 2000). This defect causes reduction in disc height and eventually formation of cartilage and new bone around the prolapsed region. It has been hypothesised that, in the absence of trauma or disease, these defects may originate in highly vascularised regions, as the scar tissue formed after the closure of the blood channels is weaker than the rest of the CEP matrix (Moore, 2006).
The permeability of the CEP has been shown to decrease with IVD degeneration (Humzah & Soames, 1988; Roberts et al. 1996). The vascular supply to the CEP diminishes and calcification of the CEP increases, starting from the second decade of life, concomitantly with the onset of NP breakdown. At later stages, the calcified part is completely replaced by bone and nutrients canals are partially to completely occluded (Lee et al. 2001). Benneker et al. (2005) found a strong correlation between opening density in the range of 20–50 μm in the human endplate and grade of disc degeneration. In an in vivo ovine model, Van der Werf et al. (2007) showed that inhibiting perfusion through the endplate resulted in a nine-fold decrease in transport rate. Similar results were also obtained in a canine model, where the blockage of the endplate affected solute diffusion to the NP more than blood vessel disruption within the AF (Ogata & Whiteside, 1981). However, recent in vitro and in vivo studies using magnetic resonance imaging (MRI) and X-ray microtomography techniques have demonstrated that endplate permeability and porosity increased with age (Rajasekaran et al. 2004; Rodriguez et al. 2011, 2012). Therefore, changes in cell function and capillary density may be the primary reason for disc degeneration rather than inhibited disc nutrition via the endplate (Rajasekaran et al. 2004; Rodriguez et al. 2012).
At the cellular level, higher degrees of senescence and matrix metalloproteinase production were observed in CEP cells from herniated discs and those affected by spondylolisthesis had very long processes. In the case of other spinal disorders such as scoliosis, ectopic calcification in the CEP, and even in the IVD, has been observed (Roberts et al. 2006). In a mouse model, Ariga et al. (2001) found that cell apoptosis increased with ageing. Apoptotic cells were found in the NP, AF and CEP, although most of the apoptotic cells were localised in the CEP. Endplate ossification followed this apoptotic process and preceded IVD degeneration (Ariga et al. 2001). Abnormal mechanical stress has been suggested as a possible cause of CEP cell apoptosis but other factors linked to ageing are likely involved (Roberts et al. 2006).
Phenotype of cartilaginous endplate cells
Cells of the CEP have a rounded morphology, similarly to articular chondrocytes. While slight local variations in the cell distribution of the CEP are found, no distinct layers are observed as in articular cartilage (Moore, 2000). Compared with the AF and NP, the CEP is a region with a higher cell density of approximately 15 × 106 cells cm−3 (Maroudas et al. 1975; Roughley, 2004); a similar cell density is also found in articular cartilage.
Several studies have addressed the phenotype of NP and AF cells and have suggested markers to distinguish them from articular chondrocytes. However, few studies have investigated the phenotype of CEP cells and potential changes with ageing, and most of the available literature on CEP phenotype is based on immunostaining of matrix proteins.
Antoniou et al. (1996b) investigated the endplates from 121 human lumbar segments of different age and grades of degeneration. Based on extensive extracellular matrix investigations, they have identified three distinct phases of matrix turnover. In the first phase (‘growth’), an active synthesis of matrix molecules and active denaturation of type II collagen take place. In the second phase (‘aging and maturation’), a drop in synthetic activity and reduction in denaturation of type II collagen was observed. In the third phase (‘degenerative’), an increase in type II collagen denaturation and type I procollagen synthesis was detected. Aggrecan and collagen are the main components of both the CEP and the other disc tissues, but their synthetic profiles are very different. The epitope levels of type I and II procollagen in the younger groups (< 5 years of age) are two to three times lower in CEP than in NP and AF, whereas aggrecan chondroitin sulphate 846 epitope (CS-846) levels (indicating aggrecan synthesis) are two to three times higher in CEP (Antoniou et al. 1996b). In another study, the expression of type X collagen was investigated, a well known marker for chondrocyte hypertrophy. It was found that type X collagen content in the CEP of beagle dogs increased with age, although it was also present in some dogs within the AF and NP (Lammi et al. 1998).
Progenitor cells in the intervertebral disc
Recent studies have demonstrated the presence of cell niches and progenitor cells in the tissues of the IVD (Risbud et al. 2007; Henriksson et al. 2009; Blanco et al. 2010; Feng et al. 2010; Liu et al. 2011). Henriksson et al. (2009) showed the presence of a slow-cycling cell or stem cell population within the perichondium or outer AF region of the human disc that may be activated upon disc degeneration. The presence of a stem cell niche environment was detected using primitive stem cell markers (STRO-1, Ki67). Further to this study, Risbud et al. (2007) and Blanco et al. (2010) isolated a stem cell-like population from degenerated human IVDs. In both cases, the cells could be differentiated towards each of the mesodermal lineages and, in the case of the former, showed antigen markers similar to bone marrow-derived MSCs.
In another study, Feng et al. (2010) isolated a progenitor cell population from the AF of non-degenerative human discs (13–16 years old) that demonstrated similar expression for CD markers compared with bone marrow-derived mesenchymal stem cells (MSCs) and had the ability to differentiate towards each of the mesodermal lineages. Furthermore, a recent study has described the presence of a progenitor population within the degenerative CEP that showed similar properties to MSCs (Liu et al. 2011). The identification of progenitor cells and cells with a notochordal-like phenotype within human discs indicates that natural repair mechanisms exist within the disc and may be activated for regeneration, although the mechanisms and function of these cells need to be elucidated (Kim et al. 2009b).
Conclusions
This review has described the phenotypic expression and functions of the cells within the IVD, namely the notochordal, mature NP, AF and CEP cells and has pointed out differences between NP, AF and CEP (Table 2). The differences in expression with respect to matrix genes are reflected in the surrounding structures within each section of the IVD. However, these tissues interact during IVD degeneration, resulting in changes in phenotypic gene expression. Furthermore, the cell function is compromised due to this loss in phenotype.
Table 2.
Summary of characteristics of human IVD cells
| NP cells | AF cells | CEP cells | |
|---|---|---|---|
| Morphology | Rounded | Elongated | Rounded |
| Gene expression | Type II collagen, Cytokeratin-19, FOX-F1, CA12, PAX-1, Brachyury. | Type I collagen, type V collagen, tenomodulin | – |
| ECM proteins | Collagen type II, Aggrecan, hyaluronan | Type I collagen, Type II collagen, aggrecan, elastin | Type II collagen, aggrecan, hyaluronan |
Identification of specific markers within the cell types of the disc will help to define the phenotype of each disc cell for regenerative strategies. Specifically, NP cell markers have recently been described in the literature, with FOXF1, CA12, cytokeratin-19 and PAX1 being prime candidates. AF cell markers include tenomodulin and decorin, although specific cell markers for these and CEP cells have not been found in the literature. The latter cell type is an area of research that requires substantial investigation, particularly in the context of the onset and progression of IVD degeneration.
Several investigations have described the differentiation of MSCs towards the nucleus pulposus phenotype, although in many of these studies, only chondrogenic rather than specific NP cell markers were used. Recently, the availability of more specific markers has resulted in improved analysis of ‘IVD-like’ differentiation studies (Minogue et al. 2010a; Gantenbein-Ritter et al. 2011; Stoyanov et al. 2011). Tapp et al. (2008) described the differentiation of adipose-derived MSCs towards the AF phenotype, although few studies have been published concerning MSC differentiation towards this phenotype. An attractive approach in this context is the use of co-culture systems of disc cells in conjunction with MSCs (Strassburg et al. 2010; Watanabe et al. 2010). In all cases, the use of specific markers would enable more detailed characterisation of cell and tissue development. This also applies to tissue-engineering strategies involving cell-seeded scaffolds and hydrogels to support functional tissue formation (Grad et al. 2010; Peroglio et al. 2011).
In the context of these regenerative approaches, greater focus could be directed towards investigating the signalling pathways involved during the process of disc development. Particularly, Shh signalling is a key component during this process and has been shown recently to be involved in both NP and AF development (Dahia et al. 2009, 2011). Recent studies have investigated the Notch and Wnt/β-catenin pathways amongst NP cells and their effect on disc degeneration and regeneration (Hiyama et al. 2011a, b). Furthermore, the presence of resident stem cells within distinct niches of the IVD may also facilitate a method of disc regeneration through the process of cell recruitment and activation (Risbud et al. 2007; Henriksson et al. 2009; Blanco et al. 2010; Feng et al. 2010; Liu et al. 2011). The approach for disc regeneration may require it to be tailored to the affected tissue, although the question regarding the resident progenitor cell populations is whether they originate from the same source or are different sub-populations.
In conclusion, the disc contains a variety of cells with specific phenotypes and functions that enable the IVD to perform under various loading and biochemical conditions. Knowledge of the cellular phenotype enables us to understand the in vivo function and track progenitor cell differentiation towards a specific cell type in vitro. Novel cell-based regenerative and repair strategies will be more successful if the specific disc cell phenotypes and functions are taken into consideration.
Acknowledgments
The authors would like to gratefully acknowledge N.M. Goudsouzian and M. Bluvol for their assistance with histological preparations and P. Schmid for the preparation of the scheme of the intervertebral disc structure. Studies were partially supported by the Swiss National Science Foundation (SNF Grant #3320000-116818) and a Research Grant from the North American Spine Society (NASS).
References
- Adams MA, Hutton WC. The effect of posture on the lumbar spine. J Bone Joint Surg Br. 1985;67:625–629. doi: 10.1302/0301-620X.67B4.4030863. [DOI] [PubMed] [Google Scholar]
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- Adams MA, Dolan P, McNally DS. The internal mechanical functioning of intervertebral discs and articular cartilage, and its relevance to matrix biology. Matrix Biol. 2009;28:384–389. doi: 10.1016/j.matbio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996a;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, et al. The human lumbar endplate: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. Spine (Phila Pa 1976) 1996b;21:1153–1161. doi: 10.1097/00007632-199605150-00006. [DOI] [PubMed] [Google Scholar]
- Ariga K, Miyamoto S, Nakase T, et al. The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine (Phila Pa 1976) 2001;26:2414–2420. doi: 10.1097/00007632-200111150-00004. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Chan D, Hunziker E, et al. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss MT, Johnstone B. Biochemistry of the intervertebral disc. In: Jayson MI, Dixon ASJ, editors. The Lumbar Spine and Back Pain. Edinburgh: Churchill Liningstone; 1992. pp. 111–131. [Google Scholar]
- Beckstein JC, Sen S, Schaer TP, et al. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine (Phila Pa 1976) 2008;33:E166–E173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- Benneker LM, Heini PF, Alini M, et al. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine (Phila Pa 1976) 2005;30:167–173. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- Blanco JF, Graciani IF, Sanchez-Guijo FM, et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- Broberg KB. On the mechanical behaviour of intervertebral discs. Spine (Phila Pa 1976) 1983;8:151–165. doi: 10.1097/00007632-198303000-00006. [DOI] [PubMed] [Google Scholar]
- Bron JL, Helder MN, Meisel HJ, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301–313. doi: 10.1007/s00586-008-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Cappello R, Bird JL, Pfeiffer D, et al. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine (Phila Pa 1976) 2006;31:873–882. doi: 10.1097/01.brs.0000209302.00820.fd. [DOI] [PubMed] [Google Scholar]
- Chan D, Song Y, Sham P, et al. Genetics of disc degeneration. Eur Spine J. 2006;15(Suppl 3):S317–S325. doi: 10.1007/s00586-006-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelberg MK, Banks GM, Geiger DF, et al. Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat. 1995;186(Pt 1):43–53. [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–S311. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Jing L, Gilchrist CL, et al. Expression of laminin isoforms, receptors, and binding proteins unique to nucleus pulposus cells of immature intervertebral disc. Connect Tissue Res. 2009;50:294–306. [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Harfe BD. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci U S A. 2011;108:9484–9489. doi: 10.1073/pnas.1007566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ B, Wilting J. From somites to vertebral column. Ann Anat. 1992;174:23–32. doi: 10.1016/s0940-9602(11)80337-7. [DOI] [PubMed] [Google Scholar]
- Clouet J, Grimandi G, Pot-Vaucel M, et al. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology (Oxford) 2009;48:1447–1450. doi: 10.1093/rheumatology/kep262. [DOI] [PubMed] [Google Scholar]
- Cs-Szabo G, Ragasa-San JD, Turumella V, et al. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine (Phila Pa 1976) 2002;27:2212–2219. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- Cui Y, Yu J, Urban JP, et al. Differential gene expression profiling of metalloproteinases and their inhibitors: a comparison between bovine intervertebral disc nucleus pulposus cells and articular chondrocytes. Spine (Phila Pa 1976) 2010;35:1101–1108. doi: 10.1097/BRS.0b013e3181c0c727. [DOI] [PubMed] [Google Scholar]
- Dahia CL, Mahoney EJ, Durrani AA, et al. Intercellular signaling pathways active during intervertebral disc growth, differentiation, and aging. Spine (Phila Pa 1976) 2009;34:456–462. doi: 10.1097/BRS.0b013e3181913e98. [DOI] [PubMed] [Google Scholar]
- Dahia CL, Mahoney EJ, Durrani AA, et al. Intercellular signaling pathways active during and after growth and differentiation of the lumbar vertebral growth plate. Spine (Phila Pa 1976) 2011;36:1071–1080. doi: 10.1097/BRS.0b013e3181f7a3ca. [DOI] [PubMed] [Google Scholar]
- Ebara S, Iatridis JC, Setton LA, et al. Tensile properties of nondegenerate human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1996;21:452–461. doi: 10.1097/00007632-199602150-00009. [DOI] [PubMed] [Google Scholar]
- Egli RJ, Wernike E, Grad S, et al. Physiological cartilage tissue engineering effect of oxygen and biomechanics. Int Rev Cell Mol Biol. 2011;289:37–87. doi: 10.1016/B978-0-12-386039-2.00002-X. [DOI] [PubMed] [Google Scholar]
- Erwin WM. The enigma that is the nucleus pulposus cell: the search goes on. Arthritis Res Ther. 2010;12:118. doi: 10.1186/ar3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin WM, Inman RD. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine (Phila Pa 1976) 2006;31:1094–1099. doi: 10.1097/01.brs.0000216593.97157.dd. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Feng G, Yang X, Shang H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am. 2010;92:675–685. doi: 10.2106/JBJS.H.01672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Keynes RJ, Tannahill D. The role of the notochord in vertebral column formation. J Anat. 2001;199:177–180. doi: 10.1046/j.1469-7580.2001.19910177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Miyamoto T, Imai J, et al. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem Biophys Res Commun. 2005;338:1890–1896. doi: 10.1016/j.bbrc.2005.10.166. [DOI] [PubMed] [Google Scholar]
- Gantenbein-Ritter B, Benneker LM, Alini M, et al. Differential response of human bone marrow stromal cells to either TGF-beta(1) or rhGDF-5. Eur Spine J. 2011;20:962–971. doi: 10.1007/s00586-010-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson A, Dreger M, Urban JP. Differential expression level of cytokeratin 8 in cells of the bovine nucleus pulposus complicates the search for specific intervertebral disc cell markers. Arthritis Res Ther. 2010;12:R24. doi: 10.1186/ar2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz W, Kasper M, Fischer G, et al. Intermediate filament typing of the human embryonic and fetal notochord. Cell Tissue Res. 1995;280:455–462. doi: 10.1007/BF00307819. [DOI] [PubMed] [Google Scholar]
- Goetz W, Kasper M, Misoge N, et al. Detection and distribution of the carbohydrate binding protein galectin-3 in human notochord, intervertebral disc and chordoma. Differentiation. 1997;62:149–157. doi: 10.1046/j.1432-0436.1997.6230149.x. [DOI] [PubMed] [Google Scholar]
- Gokorsch S, Nehring D, Grottke C, et al. Hydrodynamic stimulation and long term cultivation of nucleus pulposus cells: a new bioreactor system to induce extracellular matrix synthesis by nucleus pulposus cells dependent on intermittent hydrostatic pressure. Int J Artif Organs. 2004;27:962–970. doi: 10.1177/039139880402701109. [DOI] [PubMed] [Google Scholar]
- Grad S, Alini M, Eglin D, et al. Cells and Biomaterials for Intervertebral Disc Regeneration. Milton Keynes: Morgan & Claypool Publishers; 2010. [Google Scholar]
- Grad S, Eglin D, Alini M, et al. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 2011;469:2764–2772. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber HE, Hoelscher GL, Ingram JA, et al. Senescent vs. non-senescent cells in the human annulus in vivo: cell harvest with laser capture microdissection and gene expression studies with microarray analysis. BMC Biotechnol. 2010a;10:5. doi: 10.1186/1472-6750-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber HE, Hoelscher GL, Hanley EN., Jr Annulus cells from more degenerated human discs show modified gene expression in 3D culture compared with expression in cells from healthier discs. Spine J. 2010b;10:721–727. doi: 10.1016/j.spinee.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Watts JA, Hoelscher GL, et al. Mitochondrial gene expression in the human annulus: in vivo data from annulus cells and selectively harvested senescent annulus cells. Spine J. 2011;11:782–791. doi: 10.1016/j.spinee.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Grunhagen T, Wilde G, Soukane DM, et al. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88(Suppl 2):30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- Guehring T, Nerlich A, Kroeber M, et al. Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur Spine J. 2010;19:113–121. doi: 10.1007/s00586-009-1217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley EN, Jr, Herkowitz HN, Kirkpatrick JS, et al. Debating the value of spine surgery. J Bone Joint Surg Am. 2010;92:1293–1304. doi: 10.2106/JBJS.I.01439. [DOI] [PubMed] [Google Scholar]
- Hastreiter D, Ozuna RM, Spector M. Regional variations in certain cellular characteristics in human lumbar intervertebral discs, including the presence of alpha-smooth muscle actin. J Orthop Res. 2001;19:597–604. doi: 10.1016/S0736-0266(00)00069-3. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Ralphs JR. The response of foetal annulus fibrosus cells to growth factors: modulation of matrix synthesis by TGF-beta1 and IGF-1. Histochem Cell Biol. 2011;136:163–175. doi: 10.1007/s00418-011-0835-x. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn. 1999;215:179–189. doi: 10.1002/(SICI)1097-0177(199907)215:3<179::AID-AJA1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Hughes CE, Ralphs JR, et al. Chondroitin sulphate sulphation motif expression in the ontogeny of the intervertebral disc. Eur Cell Mater. 2011a;21:1–14. [PubMed] [Google Scholar]
- Hayes AJ, Isaacs MD, Hughes C, et al. Collagen fibrillogenesis in the development of the annulus fibrosus of the intervertebral disc. Eur Cell Mater. 2011b;22:226–241. doi: 10.22203/ecm.v022a18. [DOI] [PubMed] [Google Scholar]
- Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine (Phila Pa 1976) 2009;34:2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- Herrmann BG, Kispert A. The T genes in embryogenesis. Trends Genet. 1994;10:280–286. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- Hiyama A, Sakai D, Tanaka M, et al. The relationship between the Wnt/beta-catenin and TGF-beta/BMP signals in the intervertebral disc cell. J Cell Physiol. 2011a;226:1139–1148. doi: 10.1002/jcp.22438. [DOI] [PubMed] [Google Scholar]
- Hiyama A, Skubutyte R, Markova D, et al. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: implications in degenerative disc disease. Arthritis Rheum. 2011b;63:1355–1364. doi: 10.1002/art.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner HA, Roberts S, Bielby RC, et al. Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine (Phila Pa 1976) 2002;27:1018–1028. doi: 10.1097/00007632-200205150-00004. [DOI] [PubMed] [Google Scholar]
- Hoy D, Brooks P, Blyth F, et al. The epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24:769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–356. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat. 2003;202:279–291. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatridis JC, Setton LA, Foster RJ, et al. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J Biomech. 1998;31:535–544. doi: 10.1016/s0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Maclean JJ, Roughley PJ, et al. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88(Suppl 2):41–46. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkinen RI, Lammi MJ, Agren U, et al. Hyaluronan distribution in the human and canine intervertebral disc and cartilage endplate. Histochem J. 1999;31:579–587. doi: 10.1023/a:1003898923823. [DOI] [PubMed] [Google Scholar]
- Ishihara H, McNally DS, Urban JP, et al. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol. 1996;80:839–846. doi: 10.1152/jappl.1996.80.3.839. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Patterson AM, Eisenstein SM, et al. The presence of pleiotrophin in the human intervertebral disc is associated with increased vascularization: an immunohistologic study. Spine (Phila Pa 1976) 2007;32:1295–1302. doi: 10.1097/BRS.0b013e31805b835d. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Markopoulos M, Neame P, et al. Identification and characterization of glycanated and non-glycanated forms of biglycan and decorin in the human intervertebral disc. Biochem J. 1993;292:661–666. doi: 10.1042/bj2920661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper M, Le TK, Mladsi D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opin Pharmacother. 2009;10:2581–2592. doi: 10.1517/14656560903304063. [DOI] [PubMed] [Google Scholar]
- Kasra M, Goel V, Martin J, et al. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res. 2003;21:597–603. doi: 10.1016/S0736-0266(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Kim JH, Deasy BM, Seo HY, et al. Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine (Phila Pa 1976) 2009a;34:2486–2493. doi: 10.1097/BRS.0b013e3181b26ed1. [DOI] [PubMed] [Google Scholar]
- Kim KW, Ha KY, Lee JS, et al. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine J. 2009b;9:323–329. doi: 10.1016/j.spinee.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kluba T, Niemeyer T, Gaissmaier C, et al. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine (Phila Pa 1976) 2005;30:2743–2748. doi: 10.1097/01.brs.0000192204.89160.6d. [DOI] [PubMed] [Google Scholar]
- Korecki CL, Kuo CK, Tuan RS, et al. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27:800–806. doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korecki CL, Taboas JM, Tuan RS, et al. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi P, Inkinen RI, von der MK, et al. Localization of type X collagen in the intervertebral disc of mature beagle dogs. Matrix Biol. 1998;17:449–453. doi: 10.1016/s0945-053x(98)90104-4. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. Human disc degeneration is associated with increased MMP 7 expression. Biotech Histochem. 2006;81:125–131. doi: 10.1080/10520290601005298. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007a;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007b;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- Le Maitre CL, Frain J, Fotheringham AP, et al. Human cells derived from degenerate intervertebral discs respond differently to those derived from non-degenerate intervertebral discs following application of dynamic hydrostatic pressure. Biorheology. 2008;45:563–575. [PubMed] [Google Scholar]
- Lee SW, Mathie AG, Jackson JE, et al. Investigation of vertebral ‘end plate sclerosis’. Skeletal Radiol. 2001;30:454–459. doi: 10.1007/s002560100378. [DOI] [PubMed] [Google Scholar]
- Lee NV, Rodriguez-Manzaneque JC, Thai SN, et al. Fibulin-1 acts as a cofactor for the matrix metalloprotease ADAMTS-1. J Biol Chem. 2005;280:34796–34804. doi: 10.1074/jbc.M506980200. [DOI] [PubMed] [Google Scholar]
- Lee CR, Sakai D, Nakai T, et al. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Duance VC, Blain EJ. Zonal variations in cytoskeletal element organization, mRNA and protein expression in the intervertebral disc. J Anat. 2008;213:725–732. doi: 10.1111/j.1469-7580.2008.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jia X, Duance VC, et al. The effects of cyclic tensile strain on the organisation and expression of cytoskeletal elements in bovine intervertebral disc cells: an in vitro study. Eur Cell Mater. 2011;21:508–522. doi: 10.22203/ecm.v021a38. [DOI] [PubMed] [Google Scholar]
- Liu LT, Huang B, Li CQ, et al. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS ONE. 2011;6:e26285. doi: 10.1371/journal.pone.0026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo UG, Ripalda P, Denaro V, et al. Morphologic comparison of cervical, thoracic, lumbar intervertebral discs of cynomolgus monkey (Macaca fascicularis. Eur Spine J. 2006;15:1845–1851. doi: 10.1007/s00586-005-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlapuu M, Ormestad M, Enerback S, et al. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine (Phila Pa 1976) 1990;15:402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J. 2006;15(Suppl 3):S422–S432. doi: 10.1007/s00586-006-0149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek AJ, Gardner-Morse MG, Iatridis JC. Large residual strains are present in the intervertebral disc annulus fibrosus in the unloaded state. J Biomech. 2012;45:1227–1231. doi: 10.1016/j.jbiomech.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, et al. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010a;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, et al. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010b;62:3695–3705. doi: 10.1002/art.27710. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kobayashi S, Takeno K, et al. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng Part A. 2009;15:3835–3846. doi: 10.1089/ten.tea.2009.0250. [DOI] [PubMed] [Google Scholar]
- Moore RJ. The vertebral end-plate: what do we know? Eur Spine J. 2000;9:92–96. doi: 10.1007/s005860050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J. 2006;15(Suppl 3):S333–S337. doi: 10.1007/s00586-006-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Vernon-Roberts B, Osti OL, et al. Remodeling of vertebral bone after outer anular injury in sheep. Spine (Phila Pa 1976) 1996;21:936–940. doi: 10.1097/00007632-199604150-00006. [DOI] [PubMed] [Google Scholar]
- Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. [DOI] [PubMed] [Google Scholar]
- Nachemson AL. Disc pressure measurements. Spine (Phila Pa 1976) 1981;6:93–97. doi: 10.1097/00007632-198101000-00020. [DOI] [PubMed] [Google Scholar]
- Natarajan RN, Ke JH, Andersson GB. A model to study the disc degeneration process. Spine (Phila Pa 1976) 1994;19:259–265. doi: 10.1097/00007632-199402000-00001. [DOI] [PubMed] [Google Scholar]
- Neidlinger-Wilke C, Mietsch A, Rinkler C, et al. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J Orthop Res. 2012;30:112–121. doi: 10.1002/jor.21481. [DOI] [PubMed] [Google Scholar]
- Nerurkar NL, Mauck RL, Elliott DM. ISSLS prize winner: integrating theoretical and experimental methods for functional tissue engineering of the annulus fibrosus. Spine (Phila Pa 1976) 2008;33:2691–2701. doi: 10.1097/BRS.0b013e31818e61f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton DA, Rao KM, Dluhy RA, et al. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem. 2006;281:5668–5676. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- O’Connell GD, Vresilovic EJ, Elliott DM. Human intervertebral disc internal strain in compression: the effect of disc region, loading position, and degeneration. J Orthop Res. 2011;29:547–555. doi: 10.1002/jor.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Whiteside LA. 1980 Volvo award winner in basic science. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine (Phila Pa 1976) 1981;6:211–216. [PubMed] [Google Scholar]
- Peacock A. Observations on the prenatal development of the intervertebral disc in man. J Anat. 1951;85:260–274. [PMC free article] [PubMed] [Google Scholar]
- Peacock A. Observations on the postnatal structure of the intervertebral disc in man. J Anat. 1952;86:162–179. [PMC free article] [PubMed] [Google Scholar]
- Peroglio M, Grad S, Mortisen D, et al. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur Spine J. 2011 doi: 10.1007/s00586-011-1976-2. doi: 10.1007/s00586-011-1976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezowicz CA, Robertson PA, Broom ND. Intralamellar relationships within the collagenous architecture of the annulus fibrosus imaged in its fully hydrated state. J Anat. 2005;207:299–312. doi: 10.1111/j.1469-7580.2005.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockert AJ, Richardson SM, Le Maitre CL, et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60:482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- Poiraudeau S, Monteiro I, Anract P, et al. Phenotypic characteristics of rabbit intervertebral disc cells. Comparison with cartilage cells from the same animals. Spine (Phila Pa 1976) 1999;24:837–844. doi: 10.1097/00007632-199905010-00002. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Coltey M, Teillet MA, et al. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc Natl Acad Sci U S A. 1993;90:5242–5246. doi: 10.1073/pnas.90.11.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power KA, Grad S, Rutges J, et al. Identification of cell surface specific markers to target human nucleus pulposus cells: expression of carbonic anhydrase 12 varies with age and degeneration. Arthritis Rheum. 2011;63:3876–3886. doi: 10.1002/art.30607. [DOI] [PubMed] [Google Scholar]
- Purmessur D, Schek RM, Abbott RD, et al. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther. 2011;13:R81. doi: 10.1186/ar3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran S, Babu JN, Arun R, et al. ISSLS prize winner: A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion innormal and degenerate discs. Spine (Phila Pa 1976) 2004;29:2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- Rajpurohit R, Risbud MV, Ducheyne P, et al. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- Rastogi A, Thakore P, Leung A, et al. Environmental regulation of notochordal gene expression in nucleus pulposus cells. J Cell Physiol. 2009;220:698–705. doi: 10.1002/jcp.21816. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Stokes DG, et al. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Caterson B, Evans H, et al. Proteoglycan components of the intervertebral disc and cartilage endplate: an immunolocalization study of animal and human tissues. Histochem J. 1994;26:402–411. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- Roberts S, Urban JP, Evans H, et al. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine (Phila Pa 1976) 1996;21:415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- Rodriguez AG, Slichter CK, Acosta FL, et al. Human disc nucleus properties and vertebral endplate permeability. Spine (Phila Pa 1976) 2011;36:512–520. doi: 10.1097/BRS.0b013e3181f72b94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AG, Rodriguez-Soto AE, Burghardt AJ, et al. Morphology of the human vertebral endplate. J Orthop Res. 2012;30:280–287. doi: 10.1002/jor.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol (Berl) 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- Rutges J, Creemers LB, Dhert W, et al. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage. 2010;18:416–423. doi: 10.1016/j.joca.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J. 2008;17:452–458. doi: 10.1007/s00586-008-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D, Nakai T, Mochida J, et al. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine (Phila Pa 1976) 2009;34:1448–1456. doi: 10.1097/BRS.0b013e3181a55705. [DOI] [PubMed] [Google Scholar]
- Schätti O, Grad S, Goldhahn J, et al. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 2011;22:214–225. doi: 10.22203/ecm.v022a17. [DOI] [PubMed] [Google Scholar]
- Schollmeier G, Lahr-Eigen R, Lewandrowski KU. Observations on fiber-forming collagens in the anulus fibrosus. Spine (Phila Pa 1976) 2000;25:2736–2741. doi: 10.1097/00007632-200011010-00004. [DOI] [PubMed] [Google Scholar]
- Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- Singh K, Masuda K, Thonar EJ, et al. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976) 2009;34:10–16. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive JI, Baird P, Jeziorsk M, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs DL, Weidenbaum M, Iatridis JC, et al. Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1994;19:1310–1319. doi: 10.1097/00007632-199406000-00002. [DOI] [PubMed] [Google Scholar]
- Smith CA, Tuan RS. Human PAX gene expression and development of the vertebral column. Clin Orthop Relat Res. 1994;302:241–250. [PubMed] [Google Scholar]
- Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135–1148. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Stoyanov JV, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–547. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
- Strassburg S, Richardson SM, Freemont AJ, et al. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5:701–711. doi: 10.2217/rme.10.59. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R, Alini M, Roughley PJ, et al. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326(Pt 1):235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztrolovics R, Grover J, Cs-Szabo G, et al. The characterization of versican and its message in human articular cartilage and intervertebral disc. J Orthop Res. 2002;20:257–266. doi: 10.1016/S0736-0266(01)00110-3. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hotta K, Erives A, et al. Brachyury downstream notochord differentiation in the ascidian embryo. Genes Dev. 1999;13:1519–1523. doi: 10.1101/gad.13.12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Nakahara S, Inoue H. A pathologic study of discs in the elderly. Separation between the cartilaginous endplate and the vertebral body. Spine (Phila Pa 1976) 1993;18:1456–1462. [PubMed] [Google Scholar]
- Tapp H, Deepe R, Ingram JA, et al. Adipose-derived mesenchymal stem cells from the sand rat: transforming growth factor beta and 3D co-culture with human disc cells stimulate proteoglycan and collagen type I rich extracellular matrix. Arthritis Res Ther. 2008;10:R89. doi: 10.1186/ar2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler K. Vertebral malformations. Adv Anat Embryol Cell Biol. 1988;112:1–99. doi: 10.1007/978-3-642-73775-6. [DOI] [PubMed] [Google Scholar]
- Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop Relat Res. 1977;129:101–114. [PubMed] [Google Scholar]
- Van der Werf M, Lezuo P, Maissen O, et al. Inhibition of vertebral endplate perfusion results in decreased intervertebral disc intranuclear diffusive transport. J Anat. 2007;211:769–774. doi: 10.1111/j.1469-7580.2007.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DL, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine (Phila Pa 1976) 2007;32:2521–2528. doi: 10.1097/BRS.0b013e318158cb61. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sakai D, Yamamoto Y, et al. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28:623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- Weiler C, Nerlich AG, Schaaf R, et al. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J. 2010;19:1761–1770. doi: 10.1007/s00586-010-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuertz K, Godburn K, Maclean JJ, et al. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res. 2009;27:1235–1242. doi: 10.1002/jor.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Leung VY, Luk KD, et al. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009;218:113–121. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- Yoon ST, Patel NM. Molecular therapy of the intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S379–S388. doi: 10.1007/s00586-006-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Winlove PC, Roberts S, et al. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465–475. doi: 10.1046/j.1469-7580.2002.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CQ, Wang LM, Jiang LS, et al. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]


