Abstract
In this overview, new and existent material on the organization and composition of the thoracolumbar fascia (TLF) will be evaluated in respect to its anatomy, innervation biomechanics and clinical relevance. The integration of the passive connective tissues of the TLF and active muscular structures surrounding this structure are discussed, and the relevance of their mutual interactions in relation to low back and pelvic pain reviewed. The TLF is a girdling structure consisting of several aponeurotic and fascial layers that separates the paraspinal muscles from the muscles of the posterior abdominal wall. The superficial lamina of the posterior layer of the TLF (PLF) is dominated by the aponeuroses of the latissimus dorsi and the serratus posterior inferior. The deeper lamina of the PLF forms an encapsulating retinacular sheath around the paraspinal muscles. The middle layer of the TLF (MLF) appears to derive from an intermuscular septum that developmentally separates the epaxial from the hypaxial musculature. This septum forms during the fifth and sixth weeks of gestation. The paraspinal retinacular sheath (PRS) is in a key position to act as a ‘hydraulic amplifier’, assisting the paraspinal muscles in supporting the lumbosacral spine. This sheath forms a lumbar interfascial triangle (LIFT) with the MLF and PLF. Along the lateral border of the PRS, a raphe forms where the sheath meets the aponeurosis of the transversus abdominis. This lateral raphe is a thickened complex of dense connective tissue marked by the presence of the LIFT, and represents the junction of the hypaxial myofascial compartment (the abdominal muscles) with the paraspinal sheath of the epaxial muscles. The lateral raphe is in a position to distribute tension from the surrounding hypaxial and extremity muscles into the layers of the TLF. At the base of the lumbar spine all of the layers of the TLF fuse together into a thick composite that attaches firmly to the posterior superior iliac spine and the sacrotuberous ligament. This thoracolumbar composite (TLC) is in a position to assist in maintaining the integrity of the lower lumbar spine and the sacroiliac joint. The three-dimensional structure of the TLF and its caudally positioned composite will be analyzed in light of recent studies concerning the cellular organization of fascia, as well as its innervation. Finally, the concept of a TLC will be used to reassess biomechanical models of lumbopelvic stability, static posture and movement.
Keywords: abdominal muscles, fascia, lumbar spine, lumbar vertebrae, sacrum, spine, thoracolumbar fascia, transversus abdominis muscle
Introduction
The lumbosacral spine plays a central role in sustaining the postural stability of the body; however, the lumbar spine alone is not capable of sustaining the normal loads that it carries daily (Crisco et al. 1992). To stabilize the lumbar vertebrae on the sacral base requires the assistance of a complex myofascial and aponeurotic girdle surrounding the torso (Bergmark, 1989; Cholewicki et al. 1997; Willard, 2007). On the posterior body wall, the central point of this girdling structure is the thoracolumbar fascia (TLF), a blending of aponeurotic and fascial planes that forms the retinaculum around the paraspinal muscles of the lower back and sacral region (Singer, 1935; Romanes, 1981; Clemente, 1985; Vleeming & Willard, 2010; Schuenke et al. 2012). This complex composite of fascia and aponeurotic tissue is continuous with paraspinal fascia in the thoracic and cervical regions, eventually fusing to the cranial base. Numerous trunk and extremity muscles with a wide range of thicknesses and geometries insert into the connective tissue planes of the TLF, and can play a role in modulating the tension and stiffness of this structure (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999; Vleeming & Willard, 2010; Crommert et al. 2011; Schuenke et al. 2012).
| Abbreviations | Structures |
|---|---|
| GM | Gluteus Maximus |
| GMed | Gluteus Medius |
| IAP | Intra-abdominal Pressure |
| LD | Latissimus Dorsi |
| LIFT | Lumbar Interfascial Triangle |
| LR | Lateral Raphe |
| MLF | Middle Layer of Thoracolumbar Fascia |
| IO | Internal oblique |
| EO | External oblique |
| PLF | Posterior Layer of Thoracolumbar Fascia |
| PRS | Paraspinal Retinacular Sheath |
| QL | Quadratus Lumborum |
| SIJ | Sacroiliac Joint |
| slPL | Superficial Lamina of Posterior Layer |
| SPI | Serratus Posterior Inferior |
| STL | Sacrotuberous Ligament |
| TLC | Thoracolumbar Composite |
| TLF | Thoracolumbar Fascia |
| TrA | Transversus Abdominis |
This article will focus on the integration of the passive connective tissues and active muscular structures of the lumbopelvic area, and the relevance of their mutual interactions in relation to low back and pelvic pain. Muscular forces are transmitted through associated endo- and epimysial connective tissue matrices into the surrounding skeletal system via ligaments, tendons and aponeuroses. Moments and reaction forces generated by muscles and their associated passive structures combine to provide equilibrium at the multiple degrees of freedom of the lumbar spine and sacroiliac joints. The passive structures also interact with the muscular system through their role as sensory organs, thereby adding a component of feedback control to the system (Solomonow, 2010; Vleeming & Willard, 2010).
The TLF is a critical part of a myofascial girdle that surrounds the lower portion of the torso, playing an important role in posture, load transfer and respiration (Bogduk & Macintosh, 1984; Mier et al. 1985; Tesh et al. 1987; De Troyer et al. 1990; Vleeming et al. 1995; Hodges, 1999; Barker et al. 2004; Gatton et al. 2010). What is traditionally labeled as TLF is in reality a complex arrangement of multilayered fascial planes and aponeurotic sheets (Benetazzo et al. 2011). Portions of this dense connective tissue structure were described as a ‘functional composite’ of structures (Vleeming & Willard, 2010). This complex structure becomes especially notable at the caudal end of the lumbar spine where multiple layers of aponeurotic tissue unite and blend to form a thickened brace between the two posterior superior iliac spines (PSIS) and extending caudalward to reach the ischial tuberosities. Various myofascial structures with differing elastic moduli contribute to the formation of this thoracolumbar composite (TLC). Describing the arrangement, physical properties and functions of these tissues is a necessary prerequisite to understanding the role of this multilayered structure in supporting the lower back during static and dynamic postures, as well as in breathing movements.
Currently, several models of this TLF exist, and various authors tend to use somewhat different nomenclature, resulting in confusion that hampers the interpretation of biomechanical studies (for a discussion, see Goss, 1973). In this overview, new and existent material on the fascial organization and composition of the TLF will be reviewed, and a geometric structure of the TLF will be proposed. This three-dimensional structure will then be evaluated in light of recent advances concerning the cellular organization of fascia, as well as its innervation. Finally, the concept of a TLC will be used to reconsider models of lumbopelvic stability, both static posture and movement.
Definition of fascia
Before considering the anatomy of the TLF and associated structures, it is necessary to address the definition of fascia as an organ system. Fascia is an important and often misunderstood concept in medicine. As such, definitions of fascia can vary from one text to another as well as from one country to another (Singer, 1935; Wendell-Smith, 1997). A clear definition and concept of fascia is important when attempting to relate anatomical and biomechanical studies.
A consistent theme in the established anatomical literature concerning the definition of fascia is epitomized in the English and American versions of Henry Gray’s historical anatomy text. Essentially, fascia is generally defined by these resources as connective tissue composed of irregularly arranged collagen fibers, distinctly unlike the regularly arranged collagen fibers seen in tendons, ligaments or aponeurotic sheets (Clemente, 1985; Standring, 2008). The irregular arrangement of collagen fibers allows fascia to fulfill a role as packing tissue and resist tensional forces universally. Conversely, tendons, ligaments and aponeuroses have a pronounced regular arrangement of collagen fibers thus specializing the tissue to resist maximal force in a limited number of planes, while rendering them vulnerable to tensional or shear forces in other directions. Thus, aponeurotic tissue differs from that of fascia in the sense that it represents a flattened tendon composed of collagenous fiber bundles with a regular distribution. This distinction of aponeuroses from fascial tissues is also congruent with the Terminologia Anatomica of the Federative Committee on Anatomical Terminology (1998). Thus, fascia, as so defined, with its irregular weave of collagenous fibers is best suited to withstand stress in multiple directions (reviewed in Willard et al. 2011), whereas retinaculum means ‘retaining band or ligament’ (Stedman’s Medical Dictionary, 2000), and has also been described as ‘strap-like thickening of dense connective tissue’ (Benjamin, 2009). Those bands that lack regularly arranged collagenous fibers should, most likely, be termed fascia, while those that have a regular arrangement of collagenous fibers, such as are present around the ankle (Benjamin, 2009), should be classified as ligaments.
The subject of this article, the TLF, is composed of both aponeurotic structures and fascial sheets. However, this multilayered structure has traditionally been categorized as ‘fascia’. To avoid unnecessary confusion in this article, we will continue to refer to the TLF using its traditional terminology as a fascia.
Classification of fascia
Using a generalized system of classification, the fascial system contains four fundamental types. First is pannicular or superficial (Lancerotto et al. 2011) fascia that surrounds the body; and second is deep or investing fascia surrounding the musculoskeletal system. This latter tissue has also been termed axial or appendicular fascia based on its location (Willard, 2012). Third is meningeal fascia investing the central nervous system; and fourth is visceral or splanchnic fascia investing the body cavities and their contained organs. These fundamental fascial layers can be envisioned as existing in a series of concentric tubes (Willard et al. 2011). Conversely, other more regionalized systems of classifications have been used for fascia, such as that presented in Benjamin (2009).
The old term for the areolar tissue or subcutaneous fat and fascia was the panniculus (panniculus adiposus; Romanes, 1981). Recently, two studies analyzed this layer and describe it as the superficial layer and confirm that it can be subdivided into three sublayers (Chopra et al. 2011; Lancerotto et al. 2011).The superficial fascia consists of a superficial adipose layer and a deep adipose layer, the fascia itself separating them. This division in sublayers of the superficial fascia is proposed as a general description of the subcutaneous tissue throughout the body (Lancerotto et al. 2011).
Deep to the superficial layer lies what is often termed the investing fascia or deep fascia of the musculoskeletal system. It is a thicker, denser fascia, often bluish-white in color, typically devoid of fat and often described as ‘felt-like’ in composition and texture. This layer of fascia surrounds all bones, cartilages, muscles, tendons, ligaments and aponeuroses. The investing fascia blends seamlessly into the periosteum of bone, epimysium of skeletal muscle and the peritenon of tendons and ligaments (Singer, 1935; Schaeffer, 1953). Though not named as such, this investing layer of fascia also extends from muscle to any associated aponeuroses. On an aponeurosis, the investing fascia represents the irregular, translucent layer that has to be removed, usually by meticulous dissection, to reveal the underlying regularly arranged collagen fibers in the aponeurosis (as noted in Bogduk & Macintosh, 1984).
The investing (or deep) fascia can be divided into two forms based on location, that which surrounds muscles of the trunk or torso (axial investing fascia) and that which surrounds muscles of the extremity (appendicular investing fascia; Fig. 1). Axial investing fascia is divided regionally into hypaxial fascia investing those muscles that develop anterior to the transverse processes of the vertebrae and, as such, are innervated by the anterior or ventral primary ramus; while epaxial fascia surrounds those muscles that develop posterior to the transverse processes and receive their innervation by branches of the posterior or dorsal primary ramus. Referring to the terminology used commonly for the TLF, the epaxial fascia is the same as what is typically termed the deep lamina of the posterior layer of the TLF (PLF). The hypaxial and epaxial fasciae fuse together as they approach the transverse processes, creating an intermuscular septum that attaches to the transverse process of the vertebrae (Fig. 2). Hypaxial investing fascia forms one large cylinder investing the muscles of the thoracoabdominopelvic cavity. Epaxial investing fascia is divided into two longitudinal cylinders by the spinous processes of the vertebrae.
Fig. 1.
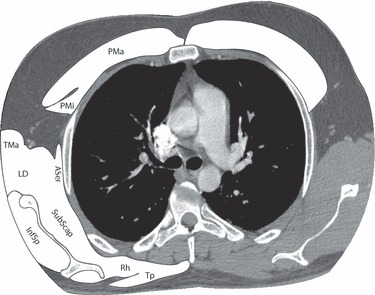
This is an axial plane CT with contrast taken through the chest at the level of the pulmonary trunk. The bridging muscles (muscles that cross between upper extremity and torso) have been shaded white. These muscles are in a common fascial sheath that extends from the extremity medially to surround the upper portion of the torso. This sheath reaches as far caudalward as the sternum anteriorly and the sacrum posteriorly. Inside the sheath are the hypaxial and epaxial muscle compartments of the thorax and abdomen, each surrounded by its own fascial sheath. ASer, anterior serratus; InfSp, infraspinatus; LD, latissimus dorsi; PMa, pectoralis major; PMi, pectoralis minor; Rh, rhomboid; SubScap, subscapularis; TMa, teres major; Tp, trapezius.
Fig. 2.
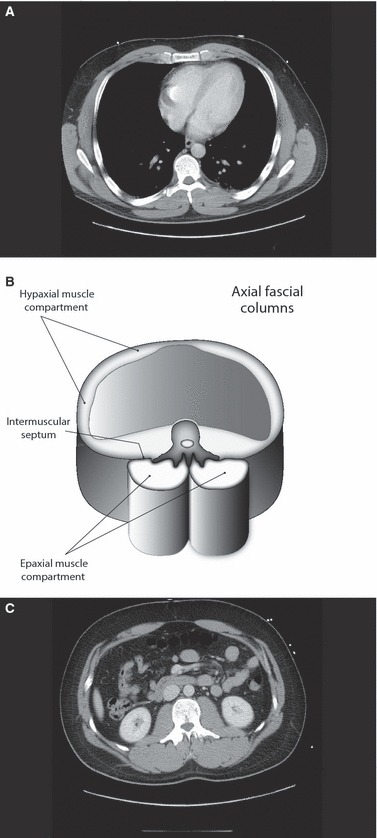
The hypaxial and epaxial myofascial compartments of the torso. (A and C) Axial plane CT scans taken through the thorax at the level T8 (A) and through the abdomen at the level of L1 (C). (B) A schematic drawing of the hypaxial cylinder separated from the twin epaxial cylinders by the vertebral column; it is derived from approximation between the two levels shown in (A and C). It illustrates the hypaxial myofascial compartment anteriorly surrounding the body cavity and the epaxial myofascial compartment posteriorly. The epaxial compartment is divided into two subcompartments by the spinous process of the vertebra. The hypaxial and epaxial compartments are separated by an intermuscular septum that medially attaches to the transverse processes of the vertebra. In the lumbar region, this septum forms the middle layer of the TLF.
Another way to conceive of this relationship is that the muscles spanning from extremity to torso (bridging muscles), such as the pectoralis major and minor, rhomboid major and minor, trapezius, latissimus dorsi (LD), serratus anterior and serratus posterior muscles are embedded in a common blanket of fascia that extends from the limb to wrap around the torso. This blanket reaches from the first rib down to the xiphoid process anteriorly and from the cranial base to the sacrum posteriorly (Sato & Hashimoto, 1984; as cited in Stecco et al. 2009).
A common feature of these upper extremity-bridging muscles lies in their embryology; each of these muscles arises from the limb bud mesenchyme and grows onto, but not into, the somatic portion of the body forming a broad expansion that ensheaths the torso. This appendicular fascial sheath is shaped like an inverted cone, which fits over the tapering walls of the thorax to support the upper extremity (Willard, 2012). Each muscle in the proximal portion of the extremity has to find an attachment to the torso, but cannot penetrate through axial muscles (Clemente, 1985). Thus, the pectoral muscles and the serratus anterior form attachments to the ribs and associated hypaxial fascial membranes covering the hypaxial muscles. The trapezius and rhomboid muscles extend to the midline. The LD wraps around the body to reach the midline in the thoracolumbar region and then extends on a diagonal line attached to the investing fascia of the epaxial muscles all the way to the iliac crest in some individuals (Clemente, 1985; Yahia & Vacher, 2011).
Based on the embryology of the musculoskeletal system as described in Bailey & Miller (1916), it is expected that the paraspinal (epaxial) muscles would be located in an intact fascial sheath (retinaculum) and that this sheath should pass from the spinous processes and supraspinous ligament around the lateral border of the muscles to reach the tip of the transverse processes. Furthermore, it would be expected that this sheath should extend, uninterrupted, from the cranial base to the sacrum providing a retinaculum for the paraspinal muscles and that bridging muscles from the extremity will attach to the sheath but not penetrate into it. Finally, based on the development of the upper extremity, it would be expected that the bridging muscles should form an external layer (superficial lamina of the PLF) covering the paraspinal retinaculum.
The TLF
The TLF is a complex of several layers that separates the paraspinal muscles from the muscles of the posterior abdominal wall, quadratus lumborum (QL) and psoas major. Numerous descriptions of this structure have presented either a two-layered model or a three-layered model (Goss, 1973). Both models will be summarized, and a consensus approach will be attempted. Figure 3 presents a summary diagram illustrating the two- vs. three-layered model of the TLF.
Fig. 3.
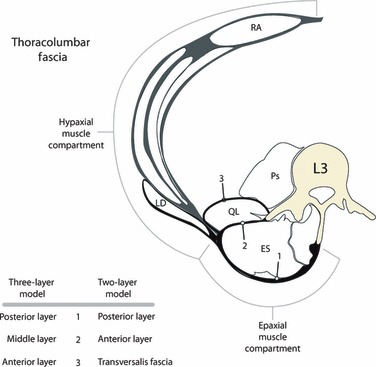
This is a tracing of the hypaxial and epaxial myofascial compartments, illustrating the comparison between the two-layered and three-layered models of the TLF. The latissimus dorsi (LD) is seen lying on the external wall of the hypaxial compartment and extending over the epaxial compartment to reach its attachments on the midline. In doing so, the aponeurosis of the LD contributes to the superficial lamina of the PLF.
The two-layer model
The two-layered model of TLF recognizes a posterior layer surrounding the posterior aspect of the paraspinal muscles and an anterior layer lying between the paraspinal muscles and the QL (Fig. 3). The two-layered model has been presented in the early English versions of Henry Gray’s work (Gray, 1923) and from the first American edition (Gray, 1870) to the 30th (Clemente, 1985). Other proponents of the two-layered model of TLF include such authorities as Spalteholz (1923), Schaeffer (1953), Hollinshead (1969) and Clemente (1985).
The two-layered model presents a posterior layer that attaches to the tips of the spinous processes of the lumbar vertebrae as well as the supraspinous ligament, and wraps around the paraspinal muscles reaching a raphe on their lateral border. The posterior layer is typically described as being composed of two sheets, a deep lamina that invests the paraspinal muscles and a superficial lamina that joins the deep lamina in the lower lumbar region. The superficial lamina is derived in large part from the aponeurosis of the LD. The serratus posterior inferior (SPI) and its very thin, aponeurosis inserts, when it is present, between the aponeurosis of the LD and the deep lamina (Fig. 4). This latter structure fuses to the outer surface of the deep lamina more so than it does to the superficial lamina.
Fig. 4.
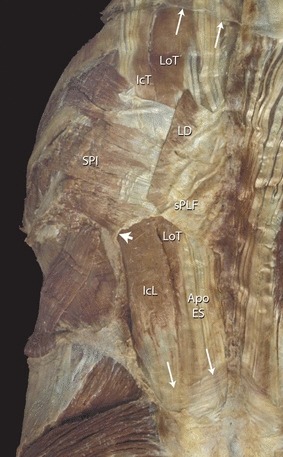
This is a posterior view of the lower thoracic and lumbar spine illustrating the construction of the superficial and deep lamina of the PLF. The LD has been sectioned to expose the underlying SPI. The aponeuroses of these two muscles combine to form the sPLF. The sPLF attaches to the deep lamina of PLF; both of these laminae have been removed over the lumbar region to expose the erector spinae muscles. The short arrow points to the curvature of the deep lamina as it wraps around the erector spinae muscles laterally forming the epaxial myofascial compartment. The paired long arrows, top and bottom, point to the sectioned edge of the deep lamina. Note that the deep lamina is thick and aponeurotic in nature at the lower lumbar level, but thin and fascial in nature in the upper thoracic region. Apo ES, aponeurosis of the erector spinae; IcL, iliocostalis lumborum; IcT, iliocostalis thoracis; LD, latissimus dorsi; LoT, longissimus thoracis; sPLF, superficial lamina of posterior layer of thoracolumbar fascia; SPI, serratus posterior inferior.
In a cranial direction, the deep lamina of the posterior layer continues cranially along the thoracic paraspinal muscles. However, here it is thin and can easily be missed; its lateral attachments reach out to the angle of the ribs, and its medial attachments are to the spinous processes and interspinous ligaments. Finally in the cervical region, the deep lamina of the posterior layer continues to cover the paraspinal muscles (all the muscles innervated by the posterior primary ramus) including the splenius capitis, as it blends with surrounding cervical fascias; eventually this paraspinal fascial sheath fuses to the cranial base (Wood Jones, 1946).
The lumbar region of the posterior layer, including both superficial and deep lamina, was originally termed the ‘lumbar aponeurosis’, while the thoracic and cervical portions, containing only a deep lamina, were termed the ‘vertebral aponeurosis’ (Gray, 1870). Recent authors have termed the entire deep lamina as the vertebral aponeurosis (Loukas et al. 2008).
The trapezius, LD and rhomboid muscles (all derived from limb buds) are positioned external to the posterior layer and contained in their own envelope of epimysial fascia (see Fig. 1; Stecco et al. 2009). These bridging extremity muscles pass external to the paraspinal muscles, eventually reaching their attachments on midline structures, such as the spinous processes and supraspinous ligament, or they attach to the outer portion of the investing layer of epaxial fascia surrounding the paraspinal muscles. In the lumbar region, the aponeurosis of the LD crosses diagonally over the deep layer, thus creating the superficial lamina.
In the two-layered model, what is termed the anterior layer of TLF is a thick band of regularly arranged collagen bundles separating the paraspinal muscles from the QL (Fig. 3). Thus, this layer really represents an aponeurosis. It is attached medially to the tips of the transverse processes of the lumbar vertebrae, and laterally it joins the posterior layer along a thickened seam, termed the lateral raphe. Note that this anterior layer is described in most current textbooks that use the three-layered model as the middle layer (MLF) of TLF. Finally, in the two-layered model, the fascia on the anterior aspect of the QL has been depicted to be an extension of the transversalis fascia from the abdominal wall (Fig. 3; Hollinshead, 1969).
The three-layer model
The three-layered model has been endorsed by numerous authors (Testut, 1899; Huber, 1930; Singer, 1935; Green, 1937; Wood Jones, 1946; Anson & Maddock, 1958), including some of the recent authors (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 2007; Standring, 2008). Of interest, Grant’s Atlas of Anatomy presented the three-layered model of TLF at least up to the 2nd edition, then changed to a two-layered model by the 6th edition (Grant, 1972).
The three-layered model has strong similarities with the previously described model containing two layers (Fig. 3). The posterior layer consists of two laminae: superficial (the aponeurosis of the LD); and deep lamina. In between these laminae above the L4 level, the aponeurosis of the SPI is present. The MLF is the fascial band that passes between the paraspinal muscles and the QL. The anterior layer is defined as passing anterior to the QL and ending by turning posterior to pass between the QL and the psoas. The anterior layer has been described as being an extension of the transversalis fascia. As previously stated, typically authors using the two-layered model refer to the fascia anterior to the QL simply as transversalis fascia and exclude it from the model.
The three-layered model is the most commonly used model in most research studies (Bogduk & Macintosh, 1984; Vleeming et al. 1995; reviewed in Barker & Briggs, 2007). This review will use the terminology of the three-layered model with the understanding that the anterior layer may be little more than a thin transversalis fascia and as such may not be able to transmit tension from the abdominal muscles to the thoracolumbar spine.
Compartmentalization of the paraspinal muscles
In both models of the TLF, the paraspinal muscles are depicted as being contained in a fascial compartment (Fig. 4); however, terminology and descriptions concerning the layers of this compartment vary considerably. Some authors consider the compartment to be a continuous sheet of fascia wrapping around the paraspinal muscles and attaching to the spinous process posteromedially and transverse process anterolaterally (Spalteholz, 1923; Schaeffer, 1953; Hollinshead, 1969; Bogduk & Macintosh, 1984; Clemente, 1985; Tesh et al. 1987; Gatton et al. 2010). Others conceive of the anterior and posterior walls of the compartment as arising from a split of the aponeurosis of the transversus abdominis (TrA; Anson & Maddock, 1958; Barker & Briggs, 1999). Regardless of the approach, it is clear that the paraspinal muscles are contained in a sealed osteofibrous compartment attached to the spinous processes on the midline and the transverse processes anterolaterally (see Standring, 2008, pp. 708–709). On the lateral extreme of the compartment, it is joined by the thick aponeurosis of the TrA; this junction point is termed the lateral raphe (Bogduk & Macintosh, 1984). A number of texts and reports described or illustrated the aponeurosis of the TrA as simply joining the lateral border of the compartment of the paraspinal muscles (Spalteholz, 1923; Tesh et al. 1987; Gatton et al. 2010) or as continuing medially to form the anterior wall (MLF) of the compartment (Romanes, 1981). However, a new study confirms that the fascia covering the paraspinal muscles forms a continuous sheath to which the aponeurosis of the TrA contributes laterally (Schuenke et al. 2012).
Although the aponeurosis of the TrA appears to contribute to both the posterior and anterior walls of the para-spinal compartment, based on the increased thickness of the anterior wall (or MLF) it is likely that most of the aponeurosis joins the anterior wall. This dual arrangement supports the work of Tesh et al. (1987) who described the MLF as having two layers. Thus, in the most common terminology, the compartment is made up of the deep lamina of the PLF (Bogduk & Macintosh, 1984) that extends continuously from the spinous processes to the transverse processes. When opened, this compartment presents a smooth, curved lateral boundary with no indication of a seam or split.
Proposed model of the TLF
The TLF is a structural composite built out of aponeurotic and fascial planes that unite together to surround the paraspinal muscles and stabilize the lumbosacral spine. Approaching this composite from the posterior aspect finds the aponeurotic attachments of two muscles: the LD and the SPI combining to form a superficial lamina of the PLF (Fig. 5). However, the central component of the TLF is not the superficial lamina of the posterior layer, but the deep lamina of the PLF forming a fascial sheath, coined the paraspinal retinacular sheath (PRS), which lies directly beneath it (Schuenke et al. 2012). The anterior wall, blended to this retinaculum, has been termed the MLF. The compartment arrangement, created by this retinaculum, has been noted or illustrated by numerous authors (Spalteholz, 1923; Schaeffer, 1953; Hollinshead, 1969; Grant, 1972; plate 481; Bogduk & Macintosh, 1984; Clemente, 1985; Tesh et al. 1987; Barker & Briggs, 1999; Gatton et al. 2010). Of special note is its designation as an osteofascial compartment (Standring, 2008), as the anteromedial portion is made up by the lumbar vertebrae and the remainder by a fascial sheet. Further research is needed to analyze the fiber direction of the PRS.
Fig. 5.
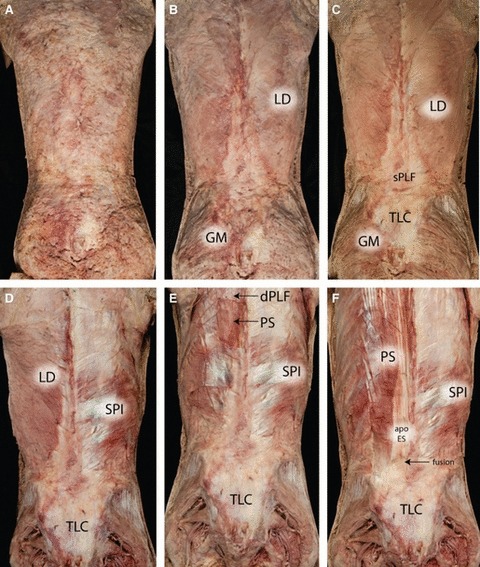
This is a series of photographs illustrating a superficial-to-deep dissection of the lower thoracic and lumbar region. (A) The panniculus of fascia following removal of the skin. (B) The panniculus has been removed to display the underlying epimysium of the latissimus dorsi (LD) and the gluteus maximus (GM). (C) The epimysium of the LD has been removed to display the underlying muscles and the aponeurotic attachments of the LD forming in part the superficial lamina of the PLF (sPLF). (D) The LD has been removed for the right side to reveal the underlying serratus posterior inferior (SPI) and its aponeurosis. (E) The LD and rhomboid muscles have been removed bilaterally and a window placed in the aponeurosis of the serratus posterior superior to expose the underlying paraspinal muscles (PS) and their investing fascia. Note the thin sheet of the deep lamina (dPLF) seen above the window. Finally, (F) the posterior serratus muscles remain on the right side, whilst the left side has had the dorsal aspect of the PRS removed to expose the paraspinal muscles and the aponeurosis of the erector spinae (apo ES) caudally. The apo ES first fuses with the overlying deep and then with the superficial laminae of the PLF to form a tough composite of dense connective tissue that extends over the sacrum and to which the GM is attached. The thoracolumbar composite (TLC) is seen in the last four photographs (C–F).
The description of the PRS is best approached from inside out; thus, beginning with the muscles contained in the compartment. Three large paraspinal muscles of the lumbosacral region are present in the compartment in the lumbar region, from lateral to medial: iliocostalis; longissimus; and multifidus (Bogduk, 1980; Macintosh et al. 1986; Macintosh & Bogduk, 1987; Bogduk & Twomey, 1991; Fig. 6). In the older literature, the two lateral-most muscles of the erector spinae group are often fused in the lower lumbar and sacral levels, where they are termed the sacrospinalis muscle (Gray, 1870). Medial to the erector spinae muscles lies the lumbar multifidus, a member of the transverso-spinalis group. This pyramidal shaped, multi-layered muscle begins at L1 and expands caudalward to occupy most of the sacral gutter on the posterior aspect of the sacrum (the region that lies between the lateral and medial sacral crests; Macintosh et al. 1986; Bogduk et al. 1992).
Fig. 6.
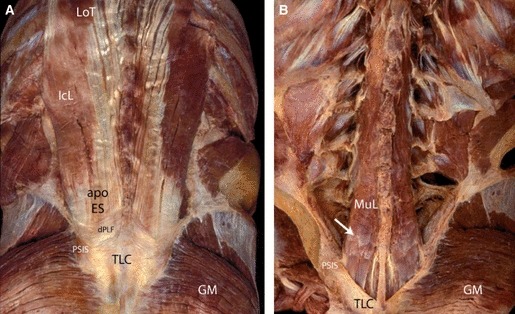
These are posterior views of the paraspinal muscles in the lumbosacral region. (A) The PLF has been removed to expose the iliocostalis lumborum (IcL) and the longissimus thoracis (LoT), as well as the aponeurosis of these two muscles (apo ES). A narrow rim of the deep lamina (dPLF) is seen at the point where the apo ES and the overlying TLF fuse to form the thoracolumbar composite (TLC). This fusion occurs at or slightly above the level of the posterior superior iliac spine (PSIS). (B) Erector spinae muscles of the lumbar region, IcL and LoT, have been removed to expose the more medially positioned multifidus lumborum (MuL). The opaque white bands (arrow) on the posterior surface of the MuL represent regions where the muscle bands fused with the inner aspect of the overlying apo ES. GM, gluteus maximus.
In the lower lumbar region, the paraspinal muscles are completely covered by the dense erector spinae aponeurosis (Fig. 5F). Laterally, this aponeurotic band extends upward to approximately the inferior border of L3, while medially the aponeurosis extends cranially well into the thoracic region. Thus, the lumbar multifidus is completely covered by this structure (Macintosh & Bogduk, 1987). Although this band of regular dense connective tissue is named the aponeurosis of the erector spinae, the lumbar multifidus as well as both of the erector spinae muscles in the lumbar region have strong attachments to its inner surface, making it a common aponeurosis for these three muscles.
Beginning at approximately L5 and below, the aponeurosis of the erector spinae muscles and all of the more superficial layers overlying it fuse tightly together making one very thick aponeurotic structure, which attaches laterally to the iliac crest at PSIS (Fig. 7). It then spreads caudolaterally to join the gluteus maximus and finally ends by covering the sacrotuberous ligament (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999). This combined structure also can receive an attachment from the biceps femoris (Vleeming et al. 1989; Barker & Briggs, 1999), and semimembranosus and semitendinosus muscles (Barker & Briggs, 1999). It is this combined structure with its multiple sheets of aponeurotic tissue to which the term ‘TLC’ has been applied (Vleeming & Willard, 2010).
Fig. 7.
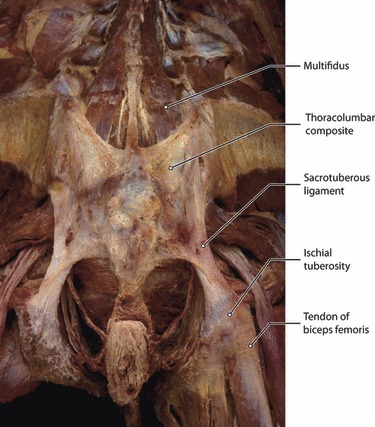
This is a posterior view of the lumbosacral region following removal of the gluteus maximus and the erector spinae muscles. Multifidus lumborum is seen inserting into the TLC. The composite extends caudally to cover the sacrotuberous ligaments and reach the ischial tuberosities.
The PRS is made of dense connective tissue reinforced on the anteromedial wall by the transverse and spinous processes of the lumbar vertebrae (Standring, 2008; Schuenke et al. 2012). Older names for this retinaculum include the lumbar aponeurosis (Gray, 1870). More recent terminology utilizes the deep lamina of the PLF to describe the posterior wall of the retinaculum and the MLF to describe the anterior wall. However, these descriptions are based on the assumption that the deep layer is a longitudinally oriented, flat fascial sheath, instead of a circular fascia encapsuling the paraspinal muscles. For that reason, Schuenke et al. (2012) recently described the deep layer as PRS. Laterally, this ring-like retinaculum creates a triangular structure where it meets the anterior and posterior laminae of the TrA aponeurosis (Fig. 8). This triangulum is named the lumbar interfascial triangle (LIFT).
Fig. 8.
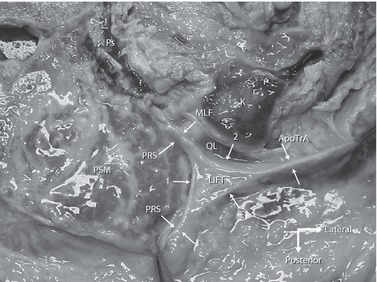
This is a photograph of a transverse section taken approximately at level L3 and illustrating the fascial structures lateral to the paraspinal muscles. The specimen was embalmed using the Thiel method. This method maintains the non-linear load-deformation characteristics of biological tissue (Wilke et al. 2011). LIFT, lumbar interfascial triangle (Schuenke et al. 2012). The deep lamina of the PLF actually forms an encapsulating sheath around the multifidi and paraspinal muscles (PSM), this is the paraspinal retinacular sheath (PRS). In this image, the LIFT is under tension from forceps pulling laterally (far right side of the picture) and posteriorly (bottom of the picture). The aponeurosis of the transversus abdominis (ApoTrA) is seen to divide into a posterior (3) and anterior (2) layer before joining the PRS. The sheath is seen to form a continuous layer wrapping around the paraspinal muscles (1). This arrangement strongly suggests that the aponeurosis of the TrA and IO does not solely form the PLF and MLF, but splits to contribute to these layers by joining the PRS (a more detailed description of the composition of the fascial layers can be found in Fig. 10). Note in this specimen that the quadratus lumborum (QL) and the psoas muscle (Ps) are both strongly atrophied. Anteriorly of the QL a small part of the kidney (K) can be seen (specimen kindly supplied by the Medical faculty Ghent Belgium, Department of Anatomy).
Posteriorly, on the midline, the PRS is attached to the lumbar spinous processes and the associated supraspinal ligament. This cylindrical sheath then passes laterally around the border of the paraspinal muscles, coursing between these muscles and the QL to reach the tips of the transverse processes of the lumbar vertebrae L2–L4. As the PRS enters the space between the QL and the paraspinal muscles, it is joined by the aponeurosis of the TrA; in addition, these two thickened bands (PRS and aponeurosis of TrA) fuse with the posterior epimysium of the QL. Thus, the structure termed the MLF, in actuality is derived of three separate layers of connective tissue, at least two of which are aponeurotic in nature. These observations are in keeping with the suggestion of Tesh et al. (1987) that the MLF is multilayered.
Anteromedially, the PRS ends on the transverse processes of the lumbar vertebrae (see illustrations in Spalteholz, 1923; also see description in Hollinshead, 1969; Grant, 1972; plate 481; Bogduk & Macintosh, 1984; Tesh et al. 1987). Superiorly, the anterior wall of the PRS (at this point fused with the middle layer of fascia) ascends cranially only so far as the 12th rib where it attaches firmly. Above the 12th rib, the anterior wall of the PRS is composed of the posterior aspect of the ribs and associated fascia, to which the para-spinal muscles attach.
The posterior wall of the PRS becomes markedly thinner as it enters the thoracic region and is termed the vertebral aponeurosis (Gray, 1870; Spalteholz, 1923; Anson & Maddock, 1958). The thinness of this layer of fascia in the lower thoracic region has led some authors to report it as absent (Bogduk & Macintosh, 1984), only to describe its reappearance in the cervical region; however, the continuity of this portion of the retinaculum has been demonstrated by its careful isolation and removal as a single entity (Barker & Briggs, 1999). As the posterior layer of the PRS (deep layer of TLF) extends into the cervical region, it becomes the investing fascia of the cervical paraspinal muscles (Gray, 1870; Wood Jones, 1946), including the splenius muscles as noted by Barker & Briggs (1999). In essence, the PRS, including that portion of which is termed the deep layer of the PLF, represents the original epaxial fascial sheath into which the paraspinal muscles formed during embryogenesis.
The inferior border of the PRS is more complicated (Fig. 9). The anterior wall of the sheath (blended with the aponeurosis of the TrA in the MLF) terminates by fusing with the iliolumbar ligament at the level of the iliac crest. Below this level, the anterior wall of the PRS is replaced by the iliolumbar ligament and the sacroiliac joint capsule. The posterior wall of the PRS (deep lamina of PLF) attaches to the PSIS then descends over the sacrum, blending laterally with the attachments of the gluteus maximus and inferiorly with the sacrotuberous ligament (Gray, 1870; Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999). Attachment of the paraspinal muscles to the inside wall of the sheath is accomplished through very loose connective tissue fascia posteriorly. Below the level of L5, the erector spinae aponeurosis (the common tendon of the erector and multifidi muscles) fuses with the PRS (synonymous with the deep lamina of the PLF; Fig. 10) and the superficial lamina of the PLF to form one, very thick, aponeurotic composite covering the sacrum, termed the ‘TLC’.
Fig. 9.
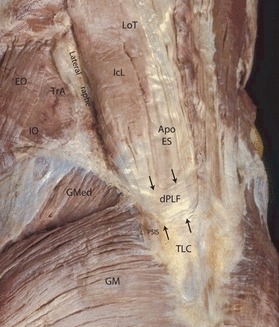
A posterior oblique view of the lumbosacral region illustrating the aponeurosis of the erector spinae muscles (Apo ES), the deep lamina (dPLF) and the thoracolumbar composite (TLC). The Apo ES and dPLF fuse with the overlying posterior lamina (not shown) to form the TLC. Laterally, the dPLF will wrap around the border of iliocostalis lumborum (IcL) forming the PRS. This sheath creates a strong fascial compartment around the paraspinal muscles. On the lateral border of the IcL the dl is joined by the aponeurosis of the transversus abdominis (TrA) to form the lateral raphe. Also attached to the raphe in this specimen is the internal oblique (IO); the external oblique (EO) in this specimen did not reach the lateral raphe. The gluteus maximus (GM) attaches to the TLC beginning around the level of the posterior superior iliac spine (PSIS) and below. The gluteus medius (GMed) does not make an attachment to the TLC.
Fig. 10.
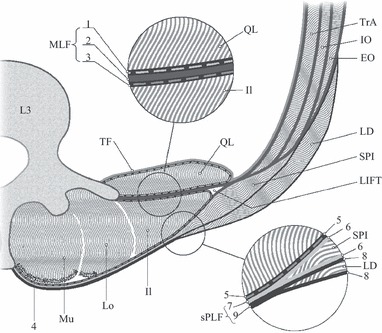
This is a transverse section of the posterior (PLF) and middle layer (MLF) of the TLF and related muscles at the L3 level. Fascial structures are represented such that individual layers are visible, but not necessarily presented to scale. Please note that the serratus posterior inferior (SPI) often is not present caudal to the L3 level. The transversus abdominis (TrA) muscle is covered with a dashed line on the peritoneal surface illustrating the transversalis fascia (TF). This fascia continues medially covering the anterior side of the investing fascia of the quadratus lumborum (QL). Anteriorly and medially, the TF also fuses with the psoas muscle fascia (not drawn). The internal (IO) and external obliques (EO) are seen external to TrA. SPI is highly variable in thickness and, more often than not, absent on the L4 level. Latissimus dorsi (LD) forms the superficial lamina of the PLF together with the SPI, when present. The three paraspinal muscles, multifidus (Mu), longissimus (Lo) and iliocostalis (Il) are contained within the PRS. The aponeurosis (tendon) of the paraspinal muscles (4) is indicated by stippling. Please note that the epimysium of the individual spinal muscles is very thin and follows the contours of each separate muscle within the PRS. The epimysium is not indicated in the present figure but lies anteriorly to the aponeurosis (4). The upper circle shows a magnified view of the different fascial layers contributing to the MLF. The picture shows that MLF is made up of three different structures: (1) this dashed line depicts the investing fascia of QL; (3) this dashed line represents the PRS, also termed the deep lamina of the PLF encapsulating the paraspinal muscles; (2) the thick dark line between the two dashed lines 1 and 2 represents the aponeurosis of the abdominal muscles especially deriving from TrA. Numbers 1, 2 and 3 form the MLF. The lower circle shows a magnified view of the different fascial layers constituting the PLF. The picture shows that on the L3 level the PLF is also made up of three layers, as the fascia of SPI is normally present on this level. (5) This dashed line depicts the PRS or deep lamina of the PLF encapsulating the paraspinal muscles; (6) the investing fascia of SPI is seen blending medially into the gray line marked (7) and representing the aponeurosis of SPI – posteriorly to the PRS; (8) this dark line represents the investing fascia of LD blending medially into the black line representing the LD aponeurosis (9) posteriorly to the SPI aponeurosis. Numbers 5, 7 and 9 form the PLF. Numbers 7 and 9 form the superficial lamina of the posterior layer (sPLF). LIFT is the lumbar interfascial triangle, as described by Schuenke et al. (2012). As indicated, the PRS encapsulates the paraspinal muscles; together with PLF and MLF and the lateral border of PRS, a triangle is formed normally also visible on axial lumbar MRIs. For further specification, see Fig. 8.
The PRS receives the aponeurotic attachments of several muscle groups. Superficially, the aponeurosis of the LD lies across the retinaculum passing from craniolateral to caudomedial in a broad flat fan-shaped aponeurosis (Fig. 11). Laterally, above the L4/L5 levels, the PRS and the aponeurosis of the LD are separated by the SPI and its thin aponeurotic attachments. From approximately L5 and below, the PRS and the LD aponeurosis begin fusing together. The attachment of the aponeurosis of the SPI begins on the lateral border of the posterior wall of the PRS (deep lamina of the posterior TLF) and extends medially to reach the spinous processes and supraspinal ligament of the lumbar vertebrae. Whilst the lateral-most connections of the SPI to the PRS can be separated bluntly, those of the medial two-thirds of the sheath cannot be broken by blunt dissection (Fig. 12).
Fig. 11.
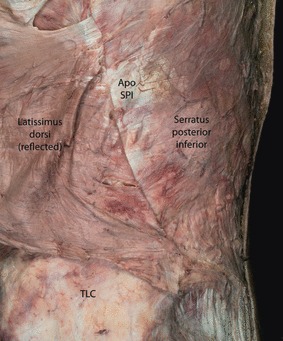
A posterior oblique view of the right lumbar region illustrating the removal of the LD to expose the serratus posterior inferior (SPI) and its associated aponeurosis (ApoSPI). Although the LD is firmly adhered to the SPI, it can be separated by careful dissection. These two aponeurotic structures combine to form the PLF. In this specimen, muscle fibers of the LD reach caudalward to the crest of the ilium. TLC, thoracolumbar composite.
Fig. 12.
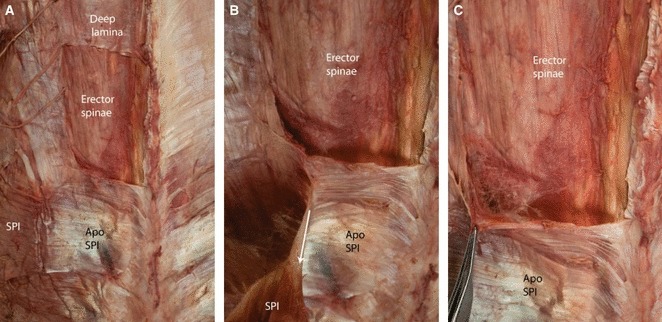
A posterior view of the left thoracolumbar region illustrating the relationship of the serratus posterior inferior (SPI) and the deep lamina of the PLF covering the paraspinal muscles. (Note that the deep lamina represents the posterior wall of the PRS.) The bridging muscles from the extremity, such as the LD, trapezius and rhomboids have been removed in this specimen. (A) A window has been opened in the deep lamina to expose the erector spinae muscles. (B) The SPI has been elevated laterally and is being tensioned on the medial attachment of its aponeurosis. (C) The deep lamina (PRS) and aponeurosis of the SPI (ApoSPI) are being elevated with forceps to illustrate the loose connective tissue located between the paraspinal muscles and the surrounding PRS.
The major lateral attachment to the PRS arises from the abdominal muscles. Most prominent amongst these is the TrA aponeurosis, which joins the border of the PRS at the lateral raphe (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Schuenke et al. 2012) and then continues medially, fused to the retinaculum, to reach the tips of the transverse processes (Fig. 10; Tesh et al. 1987; Barker et al. 2007). This combined layer has an unusual medial border. Between the transverse processes, this layer is relatively free from attachment giving it a dentate appearance; through this arrangement, the posterior primary rami pass as they depart the spinal nerve and gain access to their epaxial muscular targets in the PRS.
Cranially, there is another specialization involving the middle layer. Because the fibers of the TrA are horizontally oriented and pass inferior to the subcostal margin to reach the vertebral transverse processes, this leaves a small region superior to the aponeurosis of the TrA and inferior to the arch of the 12th rib that would not be covered by thickened aponeurotic tissue. This area is reinforced by thickened bands of collagen fibers derived from the transverse processes of L1 and L2 and extending to the inferior border of the 12th rib. These bands form the lumbocostal ligament (Testut, 1899; Spalteholz, 1923; Anson & Maddock, 1958; Clemente, 1985).
What emerges from this discussion is an osteofibrous retinacular sheath surrounding the large paraspinal muscles of the lumbosacral region. The medial wall of the cylinder is made up of the posterior arch elements of the cervical, thoracic and lumbar vertebrae as well as the ribs in the thoracic region, while its base is composed of the sacrum and the ligaments supporting the sacroiliac joint. The posterior, lateral and anterior walls are composed of the PRS. Attached to this structure are several muscles that can influence the tension in the sheath. Given this construction, it is necessary to examine the possible role of the PRS in the stability and movement of the lumbosacral spine. In the following sections, we will examine the details of the construction of specific parts of the TLF and then consider their biomechanical properties.
The PLF
Superficial lamina of the PLF
The superficial lamina of the PLF ‘itself’ divide into sublayers (Benetazzo et al. 2011). In this study, the authors show that the superficial layer of the PLF (680 μm in their specimen) can be divided into three sublayers based on the organization of collagenous fiber bundles. The superficial sublayer has a mean thickness of 75 μm with parallel undulating collagen fibers and with few elastic fibers. This layer derives of the thin epimysium of the LD. The intermediate sublayer (152 μm) is made of packed straight collagen bundles, disposed in the same direction without elastic fibers, deriving from the aponeurosis of the LD. The deepest sublayer is made of loose connective tissue (450 μm) separating the superficial lamina of the PLF from the deep lamina of the PLF or, on higher lumbar levels, the aponeurosis of the SPI lying between the superficial and deep lamina of the PLF. This deepest sublayer allows for gliding between the superficial and deep lamina of the PLF. The three sublayers form the superficial lamina of the PLF as a multidirectional construct with the same characteristics as the crural fascia also studied by the same authors (Benetazzo et al. 2011).
Disposition of the LD
The LD is a broad, fan-shaped muscle, the aponeurosis of which contributes to the superficial lamina of the PLF (Fig. 13). The aponeurosis of the LD has been divided into several regions based on its distal attachments (Bogduk et al. 1998). The upper border (or thoracic attachment) involves the lower six thoracic spinous processes and supraspinous ligaments. Next are the ‘transitional’ fibers that reach the first and second lumbar spinous processes and supraspinous ligaments. This is followed by the ‘raphe’ fibers of the aponeurosis that attach to the lateral raphe and then continue on to reach the third–fifth spinous processes and intraspinous ligaments. Finally, the ‘iliac’ fibers attach to the iliac crest, and the lower border (or costal fibers) attach to a variable number of the lower first–third ribs. Although much of the aponeurosis is fused to the underlying structures, such as the lateral raphe and the SPI, it can be separated from the sheath by blunt dissection.
Fig. 13.
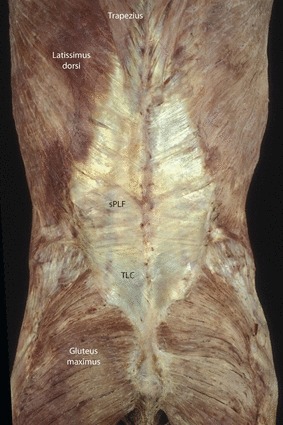
A posterior view of the back illustrating the attachments of the LD, trapezius and gluteus maximus to the TLF and thoracolumbar composite (TLC). The LD is the major component of the superficial lamina of the PLF (sPLF).
Fiber orientation for the LD
Bogduk & Macintosh (1984) were the first to carefully analyze the orientation of collagenous fibers within the laminae of the posterior layer. Since then, the trajectory of collagenous fibers has been examined by several authors with relatively good agreement (Barker & Briggs, 1999, 2007; Fig. 14). Fiber angles are described as varying from horizontal superiorly to approximately 20–40 º sloping craniolateral-to-caudomedial and progressing from a shallower angle superiorly to a steeper angle inferiorly. These collagen fiber angles should not be confused with the angles described for the muscles fibers that are attached to the TLF. The thickness of this aponeurosis was found to be approximately 0.52–0.55 mm in the lumbar region (Barker & Briggs, 1999), but to become significantly thinner in the thoracic portion.
Fig. 14.
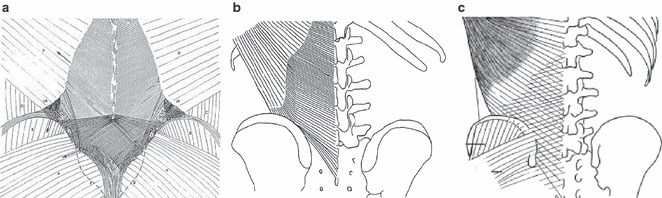
A comparison between drawings of three studies of the superficial lamina of the PLF: (a) Vleeming et al.; (b) Bogduk et al.; (c) Barker et al. (a–c) The same fiber direction of the superficial lamina. (a and c) A crosshatched appearance and the connections to the gluteus maximus fascia. (a) Along with variation in fiber direction there are changes in fiber density in the superficial lamina as well. Where the abdominal muscles join the paraspinal muscles, the orientation of the fibers change and they become denser. From the level of L4 to the lower part of the sacrum, the fiber density markedly increases. This density change corresponds to the area where the different fascial layers fuse to form the TLC.
Bogduk and Vleeming separately describe the extension of fibers from the superficial lamina across the midline at the L4–L5 levels and below (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Bogduk et al. 1998). Finally, it was noted that the superficial lamina attaches firmly to the lateral raphe only near the iliac crest. Above this level, the SPI intervenes and the attachment of the LD is less firm and in some cases may not exist at all (Bogduk & Macintosh, 1984).
Attachments of the SPI
The SPI normally consists of four thin rectangular sheets of muscle attached to the inferolateral margin of the ninth–12th ribs (Fig. 5). Medially, this muscle gives way to a thin aponeurosis that reaches deep to the aponeurosis of the LD to attach to the lower two thoracic and upper two or three lumbar spinous processes and associated interspinous ligaments. Bogduk & Macintosh (1984) found that the SPI attached to the aponeurosis of the LD, but Vleeming et al. (1995) found some attachments only to the deep lamina.
Superior and inferior borders of the superficial lamina
The existent descriptions of the superior and inferior borders of the superficial lamina have significant variation. Wood Jones (1946), who did not distinguish superficial or deep laminae in his text, described the posterior layer as extending upward to cover the splenius capitis in the cervical region. Bogduk & Macintosh (1984) found that superiorly the superficial lamina of the posterior layer passes under the trapezius and rhomboids. Inferiorly, it attaches to PSIS, fusing with the underlying aponeurosis of the SPI and with the origin of the gluteus maximus. Vleeming et al. (1995) describe the posterior layer as extending upward to the fascia nuchae. Barker & Briggs (1999) also commented on the extension of the superior layer to fuse with that of the trapezius and rhomboids while the deep layer reaches the splenius muscles. Barker also noted that the superficial lamina of the posterior TLF (LD fascia) is continuous with that surrounding the rhomboids.
Unifying theory of the superficial lamina
Both the LD and the SPI are innervated by branches from the ventral rami; thus, neither of these muscles are epaxial in origin. Specifically, the LD is innervated by thoracodorsal nerve (C6, 7 and 8), which arises from cords of the brachial plexus (Clemente, 1985). The SPI is innervated from branches of the thoracoabdominal intercostals nerves (T9–T12). Therefore, both of these muscles, the aponeuroses of which contribute to the superficial lamina, are not original components of the back but have to migrate posteriorly during development to achieve this position. In this sense, these two muscles are part of a group of bridging muscles that extend from the upper extremity to the torso. This arrangement (LD positioned superficially and SPI positioned deep in the posterior layer) can be understood from embryological principals. Based on innervations patterns, the SPI is most closely related to the thoracic hypaxial muscles and has to migrate over the intermuscular septum to attach to the outer side of the epaxial compartment containing the paraspinal muscles. The LD, a bridging extremity muscle, then has to migrate over the serratus (a hypaxial muscle) to gain its attachment on the iliac crest and lumbar spine. In a related fashion, the superior extension of the superficial lamina to involve the rhomboid muscles and trapezius is consistent from a developmental standpoint as these two muscles share a similar origin as with the LD. Thus, the superficial lamina can be seen as part of a continuous sheet of fascia containing several muscles that bridge the junction between the extremity and the torso (Sato & Hashimoto, 1984; Stecco et al. 2009).
Deep lamina of the PLF
Posterior presentation
Removal of the LD exposes the SPI and its thin aponeurosis, this latter structure is closely applied to an underlying sheet of fascia (the PRS) and the two cannot be separated by blunt dissection (Fig. 9). Numerous studies have examined this deep fascial structure, grouping it with the posterior layer and giving it the term ‘deep lamina of the PLF’ (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999).
Bogduk described the deep lamina as having alternating bands of fibers based on density; fibers run at an angle of 20–30 ° below horizontal and are best seen in the lower lumbar levels, becoming scant in the upper lumbar region (Bogduk & Macintosh, 1984). The authors termed these bands accessory ligaments and state that the deep lamina is most likely the crossed fibers of the aponeurosis of the LD. Vleeming et al. (1995) and Barker & Briggs (1999) found the same fascial orientation, typically characterizing the deep lamina of the PLF, but no differentiated accessory ligaments. Figure 15 is a comparison of the fiber orientation as depicted by the two major groups studying this area; the similarity in fiber trajectory is obvious in the three diagrams, although there is a discrepancy in the clustering of fibers as illustrated in the Bogduk and Macintosh diagram.
Fig. 15.
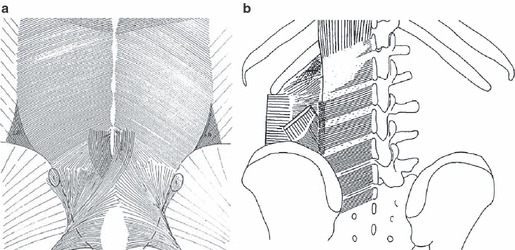
A comparison between drawings of two studies of the deep lamina of the PLF: (a) Vleeming et al.; (b) Bogduk et al. (a and b) The same fiber direction; however, in (b) the dense parts of the deep lamina are coined as accessory ligaments. (b) The lateral raphe is indicated as a dotted vertical line, indicating the area where the abdominal muscles join the paraspinal muscles. (a) An increase of density in the same area. More caudally, it can be noticed that the sacrotuberous ligament partially fuses to the deep lamina. The fiber characteristics show increased density and an altered pattern in the region over the sacrum. This pattern is another indication that the various layers of the TLF and aponeurosis fuse into the TLC, as referred to in the text.
The aponeurosis of the SPI fuses with both the LD (Bogduk & Macintosh, 1984) and the posterior surface of the deep lamina (Vleeming et al. 1995) as it projects toward the midline of the back. This fusion with the deep lamina occurs approximately half the distance across the lateral-to-medial expanse of the deep lamina. Lateral to the fusion with the aponeurosis of the SPI, the deep lamina curves around the lateral border of the paraspinal muscles to form the PRS. For embryological reasons, the deep lamina cannot represent a posterior extension of fascia derived from either the LD, PSI or any abdominal muscle; as a component of the PRS, developmentally the deep lamina is completely separated from the abdominal (hypaxial) muscle fascia by an intermuscular septum (Bailey & Miller, 1916; Fig. 16).
Fig. 16.
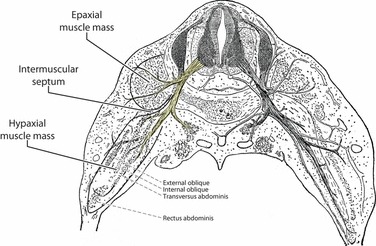
A schematic diagram of a human embryo demonstrating the epaxial and hypaxial myofascial compartments. The spinal nerve is seen dividing into its dorsal and ventral ramus. The dorsal ramus innervates the epaxial compartment, whilst the ventral ramus innervates the hypaxial compartment. Between the two compartments lies the intermuscular septum of connective tissue from which the middle layer of the TLF will develop (figure modified from: Bailey & Miller, 1916).
Inferior border of the deep lamina
The inferior border of the TLF was succinctly described by Henry Gray in 1870 as blending with the ‘greater sacrosciatic’ (sacrotuberous) ligament; more recent authors have elaborated on these arrangements. At L5–S1 level, Bogduk found the superficial lamina of the TLF to fuse inseparably with the underlying aponeurosis of the paraspinal muscles and continuing caudalward to blend with the gluteal fascia (Bogduk & Macintosh, 1984). This being the case, the deep lamina would be trapped between these two thick aponeurotic sheets as they fuse. A similar observation was made by Vleeming who found the superficial and deep laminae fusing with the aponeurosis of the erector spinae and the combined structure (TLC) attaching laterally to the PSIS and progressing caudally, to become continuous with the sacrotuberous ligament (Vleeming et al. 1995).
The relationship with the gluteal fascia is complex. Laterally, the deep lamina fuses over the iliac crest with the aponeurosis of the gluteus medius. More medially, the deep and superficial laminae fuse together at the level of PSIS. Below PSIS, this combined aponeurotic structure extends laterally to create an intermuscular septum to which the gluteus maximus attaches in a bipennate arrangement (reviewed in Willard, 1995).
Superior border deep lamina
Wood Jones (1946) described the PLF (what he terms the layer deep to the attachments of the LD and SPI) as becoming very thin and passing upward under the serratus posterior superior to eventually blend with the fascia surrounding the splenius muscles in the cervical region; thus, he seems to be describing what is currently termed the deep lamina of the PLF. Bogduk found the upper portions of the deep lamina to be poorly developed. In fact, they lost the deep lamina transiently above the superior border of the SPI, only to see it return at higher levels as a thin membrane (Bogduk & Macintosh, 1984). Vleeming traced the deep lamina upward into the thoracic region where it thinned significantly and was joined by the aponeurosis of the SPI (Vleeming et al. 1995). Barker was able to trace the superior border of the deep lamina cranially to where it blended with the border of the splenius cervicis and capitis muscles (Barker & Briggs, 1999). Taken together, these descriptions suggest that the deep lamina extends from the sacrum cranially to the splenius capitis and eventually fuses to the cranial base at the nuchal line with the cervical fascia. This would be the expected arrangement of the investing fascia (PRS) surrounding the paraspinal muscles.
Lateral border of the deep lamina
The lateral border of the deep lamina lies along the lateral raphe and has been described by numerous authors (Schaeffer, 1953; Bogduk & Macintosh, 1984; Tesh et al. 1987; Vleeming et al. 1995; Barker & Briggs, 2007). Spalteholz (1923) clearly illustrates the lateral border as curving continuously around the lateral margin of the paraspinal muscles to participate in the formation of the middle layer separating paraspinal muscles from QL. Schaeffer (1953) described the TLF as being continuous around the lateral margin of the erector spinae muscles and forming the ventral or deep layer that creates an intermuscular septum separating QL from the sacrospinalis muscles. Tesh et al. (1987) illustrate the lateral border of the TLF as having a deep lamina that continues uninterrupted around the lateral border of the erector spinae muscles to become what they describe as an inner lamina of the middle layer. In addition, Carr et al. (1985) verified the presence of a retinacular sheath surrounding the paraspinal muscles both anatomically and physiologically using dissection and intracompartmental pressure recordings in various postures. Thus, the PRS is formed by the deep lamina creating a compartment for the paraspinal muscles in the lumbar region. It is along the lateral border of this compartment that the aponeurosis of the TrA joins forming the lateral raphe (Bogduk & Macintosh, 1984; Vleeming et al. 1995; Barker & Briggs, 1999). These findings have recently been confirmed with regard to the compartmental construction of the PRS and its relationship to the lateral raphe (Schuenke et al. 2012). Along with demonstrating that the aponeurosis of the TrA joins the deep lamina to form the MLF, Schuenke et al. describe a slip of aponeurotic tissue stretched between the aponeurosis posteriorly as it approaches the deep lamina and blending with the posterior aspect of the deep lamina (Fig. 8). Thus, a LIFT is created by the division of the aponeurosis. This triangle has been previously illustrated but not described in Grant’s Atlas of Anatomy (Grant, 1972).
The MLF
The MLF is situated between the QL and the paraspinal muscles. This aponeurotic structure has been suggested as being the primary link between the tension generated in the abdominal muscle band and the lumbar spine (Barker et al. 2004, 2007). This layer is viewed by many authors as a medial continuation of the aponeurosis of the TrA (Romanes, 1981; Clemente, 1985; Standring, 2008) or, alternatively, a lateral continuation of the intertransverse ligaments (Bogduk, 2005). In their study of this layer, Bogduk & Macintosh (1984) found it to be a thick, strong aponeurotic structure arising from the tips of the transverse processes. The upper border of the middle layer of fascia is the 12th rib. However, between T12 and the first two lumbar transverse processes the middle layer is re-enforced by arcuate collagenous bands termed the lumbocostal ligament. From L2 caudally, the MLF is described as giving rise to the aponeurosis of the TrA laterally. The lower border of the MLF is the iliolumbar ligament and the iliac crest.
The abdominal muscles form the primary attachment to the MLF, but their arrangement has proven to be somewhat contentious (Urquhart & Hodges, 2007). The TrA and the internal oblique connect in an aponeurosis that becomes the MLF as it passes internal to the lateral border of the erector spinae muscles (Fig. 10). In the area where the aponeurosis joins the deep lamina of the posterior layer (PRS) on the lateral border of the erector spinae, a thickening in the tissue forms that is termed the lateral raphe (Fig. 9; Bogduk & Macintosh, 1984). The TrA attachment to the PRS extends from the iliac crest to the 12th rib, whilst the attachment of the internal oblique is much more variable and occurs principally in the inferior portion of the lateral raphe (Bogduk & Macintosh, 1984; Tesh et al. 1987; Barker et al. 2007). Typically, the lateral-most slips of the external oblique muscle form an attachment to the 12th rib; however, this muscle has been reported to also gain access to the upper boundary of the aponeurosis of the TrA (Barker et al. 2007).
Barker et al. (2007) demonstrated that the precise attachment of the MLF is to the lateral margins of the transverse processes; it was noted that measuring the MLF as it approached the tip of the transverse process yields a thickness of approximately 0.62 mm, but elsewhere varied from 0.11 to 1.34 mm. Because the average thickness of the superficial lamina of the PLF near the spinous processes was reported to be 0.56 mm (Barker & Briggs, 1999), it appears that the MLF is thicker than the PLF. In marked contrast, the anterior layer of TLF is thin (0.10 mm, range 0.06–0.14 mm; Barker & Briggs, 1999) and membranous; it extends from the lateral raphe, passing anterior to the QL to attach towards the distal end of each transverse process between the attachments of the psoas and QL.
The attachment of the MLF to the transverse process is quite strong. This was demonstrated in older specimens by applying elevated tension (average: 82 N in the transverse plane and 47 N in the anterior–posterior plane) to the transverse process, which typically fractured before the MLF or its osseous attachment failed (Barker & Briggs, 2007).
Most of the collagenous fibers in the middle layer are oriented slightly caudolaterally (10–25 ° below the horizontal) until they reach the transverse processes (Barker & Briggs, 2007). As they approach the lumbar spine, the collagen bundles focus on the tips of the transverse processes, leaving a less well organized zone between each transverse process (Tesh et al. 1987). It is through this intertransverse region that the posterior primary ramus gains access to the compartment of the PRS (Fig. 17).
Fig. 17.
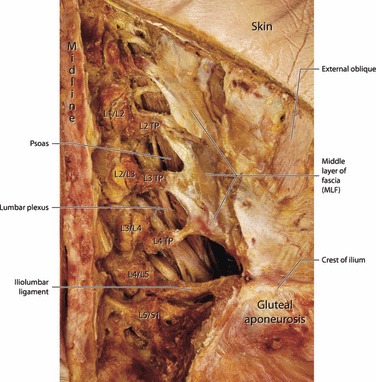
This is a posterior view of a deep dissection of the middle layer (MLF) of the TLF. The erector spinae muscles and the multifidus have been complete removed to expose the facet joints, transverse processes (TP) and the MLF. The MLF is composed of the aponeurosis of the TrA and the PRS, as well as the epimysium of the QL. The ventral rami of the lumbar plexus and the psoas muscle can be seen deep to the arches of the MLF. It is through these arches that the dorsal ramus gains access to the paraspinal muscles in the epaxial compartment.
The middle layer appears to derive from an intermuscular septum that separates the epaxial from the hypaxial musculature. This septum develops during the fifth and sixth weeks of gestation (Hamilton et al. 1972). The intermuscular septum represents a consolidation of mesenchyme that not only separates the two components of the myotome but also participates in forming the investing fascia that surrounds both of these muscle masses. Thus, it is speculated that from this mesenchymal wrapping, the PRS and the MLF are formed. Furthermore, the authors would like to pose that the middle layer itself, due to the dual origin, is most likely composed of at least two sublayers of separate embryologic origin – the most posterior sublayer deriving from the epaxial mesenchyme, whilst the more anterior sublayer deriving from the hypaxial mesenchyme. The presence of at least two-layered in the MLF was previously suggested by Tesh et al. (1987) based on histological preparations. In this case, the aponeurosis of the TrA would be representing the hypaxial muscle investment, and the posterior wall of the PRS would represent the epaxial muscle investment. In addition, Schuenke et al. (2012) observed that the epimysial fascia of the QL represents a third component of the MLF (Figs 8 and 10).
The lateral raphe
The lateral raphe (in fact a ridged union) deserves special attention as it lies at the junction of the fasciae of the hypaxial and epaxial muscles. The raphe represents a thickened complex of dense connective tissue at the lateral border of the PRS (deep lamina) from the iliac crest inferiorly to the 12th rib superiorly (Bogduk & Macintosh, 1984). It marks the junction of the aponeurosis of the TrA (hypaxial muscle) with the paraspinal sheath of the epaxial muscles. Thus, the raphe is formed at the location where abdominal myofascial structures join the retinaculum surrounding the paraspinal muscles (Schuenke et al. 2012; see Figs 8–10). The blending of the aponeurotic sheaths of the TrA and IO muscles along with the lateral margin of the paraspinal sheath creates a ridged union of dense connective tissue. Close inspection of this region finds that as the aponeurosis of the TrA curves inward, it joins the inner aspect of the PRS, most likely creating a double layer as described by Tesh et al. (1987). At this point, an additional lamina of connective tissue appears between the aponeurosis and the outer border of the PRS as well. This configuration creates a triangle-shaped structure at the lateral raphe termed the LIFT (Schuenke et al. 2012; see Fig. 8). It is through this connective tissue complex that the raphe appears to dissipate the tension generated by the abdominal myofascial girdle to the anterior and posterior aspects of the PRS.
Innervation of the TLF
The innervation of the PLF
While most neuroanatomical studies of the lumbar region explored the discs, facet joints and spinal ligaments, there is a comparable lack of histological studies and related knowledge about the innervation of the TLC. Such studies as exist currently in the literature tend to indicate a significant innervation of the PLF (Table 1). Only one study failed to find nerve endings in this layer; however, this study explored tissues from a selected group of chronic back pain patients only (Bednar et al. 1995). The density of nerves fibers in the PLF appears to be even higher than that of the underlying muscle (Tesarz et al. 2011).
Table 1.
Histological studies exploring the superficial layer of the PLF
| Study | Tissue source | Method | Nerve endings found | Remarks |
|---|---|---|---|---|
| Stilwell (1957) | Macaca mulatta (n = 17), rabbit (n = 4) | Methylene blue | Rich supply by FNE. Groups of large Pacinian corpuscles at penetration points of dorsal rami through TLF. Also small Pacinian-like and Golgi-Mazzoni corpuscles* | Study included human tissues too, but no nerve type analysis was performed on those |
| Hirsch (1963) | Human (n = ?) | Methylene blue | FNE, ‘complex unencapsulated endings’* | Number of donors not mentioned. Also found: unmyelinated nerve fiber network associated with blood vessels |
| Yahia et al. (1992) | Human (n = 7) | IH: Neurofilament protein and S-1 00 protein | FNE, Ruffini, Pacini* | |
| Bednar et al. (1995) | Human (n = 12) | IH: neuron-specific enolase | No terminal nerves found* | Study performed with CLBP patients only. Found: small peripheral nerve bundles at the margins and in association with small vessels |
| Corey et al. (2011) | Rats (n = 5) | 3-D reconstructions of thick (30–80 μm) tissue sections IH: PGP9.5, CGRP, fast blue | CGRP positive FNE. | Also found: some non-terminating CGRP-labeled fibers along blood vessels |
| Tesarz et al. (2011) | Rat (n = 8) Human (n = 3) | IH: PGP 9.5, TH, CGRP, SP | Rich innervation with terminal nerves. Most nerve fibers located in the outer layer and in the SCT | Also found: rich supply with transient nerves |
| Benetazzo et al. (in press) | Human (n = 2) | 3D reconstruction of serial sections. IH: S100 | Study did not investigate nerve terminations | Small nerves (mean diameter 15 μm) found, flowing from the superficial sublayer into the adjacent subcutaneous loose connective tissue. No nerves visible in intermediate and deep sublayers |
FNE, free nerve endings; IH, immunohistochemical analysis.
Method of identification of termination of small nerves not mentioned. Not included in this table are studies on supraspinous, interspinous or iliolumbar ligaments.
A presence of visible nerves does not necessarily imply that these nerves are actually innervating the fascia. Some nerves may just transit through the PLF on their way to the muscle or skin. A common weakness, particularly of the older innervation studies in Table 1, is that little clarification is provided regarding the terminal morphology of any of the identified smaller nerves. The most notable and convincing exception in this respect is the recent examination by Corey et al. (2011), which used three-dimensional reconstructions of thick (30–80 μm) tissue sections and confirmed the widespread termination of small sensory neurons in rat lumbar fascia. In addition, the recent study by Tesarz et al. (2011), although using two-dimensional sections only, identified numerous small nerves in both rat as well as human lumbar fascia that expressed a chain of at least three varicosities and can therefore be identified as terminal endings of unmyelinated nerves. Both of these studies confirm that the posterior layer contains sensory nerves terminating in this tissue.
‘Fasciotomes’ as segmental innervation zones?
Several studies performed on rats suggest that the PLF is innervated by the dorsal rami of the spinal nerves (Bove & Light, 1995; Budgell et al. 1997). Taguchi et al. (2008) report that the sensory endings project to spinal cord areas that are located in the dorsal horn two–three segments cranially relative to the location of the terminal endings. This innervation pattern appears to be congruent with the underlying musculature. The similarity in the innervation pathways of the fascia corresponding with the segmental innervation of these underlying muscles (‘myotomes’) suggests that the overlaying fascia may also contain a segmentally related pattern of innervation. In reference to the posterior layer of the TLF, Tesarz et al. (2011) have suggested the term ‘fasciotomes’ for such segmental innervation fields. Verification of a clear segmental innervation could have implications for the potential role of the lumbar fascia in low back pain (Schleip et al. 2007). However, current histological evidence is still insufficient to support the validity of such a segmental innervation concept. In the light of the strong associations of the TLF with other muscles such as the gluteus maximus or the LD, it cannot be excluded that fascial fields with multi-segmental innervation patterns may exist.
The high density of sympathetic fibers
The presence of a network of sympathetic nerves in human TLF was first reported by Hirsch (1963). More recently, a high density of sympathetic neurons was found in this fascia of both rats and humans (Tesarz et al. 2011). This is consistent with the findings of Staubesand et al. (1997) who documented the presence of an abundance of sympathetic neurons in human crural fascia. In all three studies, it was shown that a significant portion of these sympathetic nerves accompanied blood vessels. This suggests that these nerves have a strong vasomotor component. The presence of a significant number of efferent nerves is also suggested by Tesarz et al. (2011), who found the total number of neuronal fibers being five–six times higher than that of fibers staining positive for either calcitonin gene-related peptide or substance-P, indicating that only a small fraction of the innervation is sensory.
The same study also demonstrated the presence of some sympathetic fibers that terminate away from the blood vessels (Tesarz et al. 2011). These were more commonly found in the superficial lamina of the PLF in the rat. If some of these fibers are ergoreceptors or other mechanosensitive interoceptors, which are sensitive to muscle contraction, it is possible that they could exert a modulating effect on vasomotor activity and sympathovagal balance systemically in response to movement (De Meersman et al. 1998). Stimulation of those vasomotor fine nerve endings could serve as a cause of ischemic pain.
The high density of sympathetic nerves in fascia is certainly intriguing and merits further exploration. Staubesand et al. (1997), as well as Tesarz et al. (2011), proposed that a close relation could exist between the sympathetic nervous system and the pathophysiology of fascial disorders. This could potentially explain why some patients with low back pain report increased intensity of pain when they are under psychological stress (Chou & Shekelle, 2010). Based on this information, it is feasible that the stimulation of intrafascial sympathetic afferents (e.g. via manual medicine therapy) may trigger modifications in global autonomic nervous system tone, as well as in local circulation and matrix hydration (Schleip, 2003).
Potential proprioceptive role
The presence of corpuscular receptors in the PLF, such as Golgi, Pacini and Ruffini endings, is commonly described by the older studies (Stilwell, 1957; Hirsch, 1963; Yahia et al. 1992). Interestingly, the most recent study by Tesarz et al. (2011) failed to find such corpuscular endings, with the exception of a possible Ruffini ending. However, this latter study only included a very small sample size of human specimens.
A recent investigation of the threshold of the spinal facet joint capsules (Lanuzzi et al. 2011) suggests that a stimulation of proprioceptive receptors in the joint capsule only happens in extremely large joint movements (e.g. during a spinal flexion beyond 80% of the totally available range of motion). While earlier concepts viewed the joint receptors as important sources of proprioception during everyday movements, a more recent review suggests that such receptors tend to work mostly as range of motion limit detectors (Proske & Gandevia, 2009). Most likely, this is due to the fact that their close proximity to the joint axis requires large angular movements in order to exert sufficient stretch on these tissues. In contrast, the superficial layer of the TLF is positioned at a much greater distance from the joint axis. Therefore, stretch receptors in this tissue may already experience sufficient stretch stimulation during much smaller joint motion in lumbar flexion (Schleip et al. 2007). While this context would support a proprioceptive function of the PLF in everyday movements, the histological examinations as summarized in Table 1 do not yet allow one to come to a decisive conclusion as to whether this tissue does in fact contain sufficient proprioceptive innervation. Most likely, the cutaneous receptors and muscle spindles in lumbar musculature also play a major role in proprioception of lumbar motion. In fact, the small muscles located closest to the vertebral column, such as the rotatores and the inner most layers of the multifidus have notably higher density of muscle spindles, suggesting that their primary role may be proprioceptive rather than initiating prime movement (Bakker & Richmond, 1982; Richmond & Bakker, 1982; Nitz & Peck, 1986).
If one expands the concept of the TLF to include surrounding structures, such as the supraspinous, interspinous and iliolumbar ligaments, then a proprioceptive function is more strongly indicated for this combined structure. Yahia et al. (1992) reported the presence of both Ruffini and Pacini endings in the supraspinous and interspinous ligaments, and a recent examination of the iliolumbar ligament found this tissue to be abundantly innervated by Pacinian receptors and Ruffini endings as well as a few Golgi tendon organs (Kiter et al. 2010). It is also possible that some of the band-like portions within this ligament might express a more developed proprioceptive innervation and function than other areas, similar to the dense proprioceptive innervation of the wrist flexor retinaculum in comparison to other regions of the antebrachial fascia (Stecco et al. 2007).
Clarification of the potential proprioceptive role of the TLF could have important implications, not only for surgery but also for clinical rehabilitation of patients with low back pain. Several studies have supported the concept of a mutually antagonistic relationship between low back pain and lumbar proprioception. The presence of low back pain tends to associate with reduced lumbar proprioception (Leinonen et al. 2003; O’Sullivan et al. 2003), and inhibition of proprioceptive signaling induces a strong augmentation of pain sensitivity (Lambertz et al. 2006). Such mutually antagonistic influences could occur via polymodal ‘wide dynamic range neurons’ in the dorsal horn of the spinal cord. Various treatments, such as manual therapy or exercise programs, have been proposed for increasing lumbar proprioception in patients with low back pain. An improved understanding of the proprioceptive capacity of human TLF could possibly increase their effectiveness. The inter-relationship between low back pain and abnormal proprioceptive sensory information has recently been reviewed by Brumagne et al. (2010).
Potential nociceptive role
Because the majority of low back pain cases is idiopathic, Panjabi (2006) proposed a new concept in which micro-injuries in lumbar connective tissues may result in impaired function of embedded mechanoreceptors, leading to muscle control dysfunction and subsequent biomechanical impairments. While this model considered paraspinal connective tissues, other authors suggested that the PLF should also be involved as a candidate for similar microinjuries (Schleip et al. 2007; Langevin et al. 2011).
Can abnormalities in the TLF contribute to the etiology of low back pain, and how much support is there for this concept? Dittrich (1963), as well as Bednar et al. (1995), examined pieces of the TLF taken from patients with low back pain during lumbar surgery. The authors report on frequent signs of injury and inflammation (Dittrich, 1963; Bednar et al. 1995). However, because the frequency of similar fascial changes is not tested for age-matched healthy people, it is possible that such degenerative changes may be very common among adults. For example, there are many cases of disc anomalies present in people who are asymptomatic for back pain (Jensen et al. 1994).
Despite the paucity of fine nerve endings reported for the PLF by Bednar et al. (1995), a more detailed examination of the various sublayers of this tissue by Tesarz’s group found such nerve endings not embedded in the dense layer of the fascia itself but on its superficial surface, as well as in the adjacent subcutaneous loose connective tissue (Tesarz et al. 2011). It remains to be seen whether or not the findings of Bednar will be replicated. In that case, comparison of tissue samples from healthy individuals is essential.
Taken together, these investigations indicate that the TLF contains nociceptive nerve endings, and that injury or irritation to these nerves may be able to elicit low back pain. While injuries or microinjuries to the TLF can occur in everyday life, the above studies cannot confirm whether or not the described tissue dynamics are in fact a common source of low back pain.
A recent examination by Langevin et al. (2011) investigated for the first time the PLF of patients with low back pain compared with a group of age-matched controls. Using ultrasound cine-recording, the shear-motion within the posterior layer of the TLC was examined during passive lumbar flexion. A significant reduction in the shear-strain was found in the low back pain group. In addition, the patients in this study showed increased thickness in the PLF, although this increase in thickness was only found in male patients. The reduction in shear-strain could be due to tissue adhesions induced by previous injury or inflammation, and could then be consistent with the proposed etiology suggested by Dittrich (1963) and Bednar et al. (1995). However, as the authors of this recent study emphasize themselves, it is also possible that the observed tissue changes are merely the result of a reduction (immobility) in everyday lumbar movements related to low back pain. In this case, the fascial changes would be the effect of low back pain rather than a cause.
Several studies have explored eliciting nociceptive responses by stimulating the TLF in vivo. Pedersen et al. (1956) pinched the TLF of decerebrated cats and could elicit spastic contractions in the back muscles (mostly ipsilateral), and also in the hamstring and gluteal muscles of the same leg. These contractions were much stronger in response to pinching the TLF than pinching the underlying muscle tissues. The authors interpreted this as evidence that low back pain in humans, with or without radiating symptoms, could be caused by nociceptive impulses originating in the TLF. This finding is in contrast to the extensive exploration done by Kuslich et al. (1991) of patients with low back pain during disc surgery performed under progressive local anesthesia. While mechanical stimulation of the nerve root led to strong and often radiating low back pain symptoms, the same stimulation on the TLF failed to elicit similar responses in the majority of patients. The more recent examination by Taguchi et al. (2008), on the other hand, demonstrated that pinching the TLF of rats as well as applying hypertonic saline to it with a cotton ball elicited responses in a significant number of neurons of the dorsal horn of the spinal cord. Because application of hypertonic saline is known to be the most effective stimulus for type IV afferents, this response suggests a nociceptive functional capacity of the TLF.
The same study demonstrated that inducing a chronic inflammation in the local musculature (via injection of Freund Adjuvans solution) lead to a threefold increase in the number of dorsal horn neurons, which are responsive to TLF stimulation. This strong sensitization of the TLF is reminiscent of the recent study of Gibson et al. (2009), which reported that hypertonic saline strongly increased pain when injected into the epimysium of a muscle exposed to delayed onset soreness after eccentric exercise, whereas no comparable response was observed when the substance was injected into the actual muscle itself or into the non-exercised muscle in the contralateral leg.
To summarize, the sensory innervation of the TLF suggests at least three different mechanisms for fascia-based low back pain sensation: (1) microinjuries and resulting irritation of nociceptive nerve endings in the TLF may lead directly to back pain; (2) tissue deformations due to injury, immobility or excessive loading could also impair proprioceptive signaling, which by itself could lead to an increase in pain sensitivity via an activity-dependent sensitization of wide dynamic range neurons; and finally (3) irritation in other tissues innervated by the same spinal segment could lead to increased sensitivity of the TLF, which would then respond with nociceptive signaling, even to gentle stimulation. Whether or not each of these scenarios (or various combinations of them) manifest in low back pain, or how often they occur remains to be examined more closely in the future. Clarification of these questions could provide useful contributions for the treatment and prevention of back pain.
Distribution of nerve terminals in the TLF
Unfortunately, the earlier studies in Table 1 did not distinguish between various sublayers within the PLF. Such a differentiated analysis was only performed in the most recent studies. Among these, Tesarz et al. (2011) included the most differentiated analysis of the sensory innervation of the various sublayers. They reported that the outer sublayer of TLF in the rat and the adjacent subcutaneous tissue showed a particularly dense innervation with sensory fibers. In addition, Substance P-positive fibers – which are assumed to be nociceptive – were exclusively found in these superficial layers. The authors did not relate their sublayers to the established layers in the TLF of the human, such as the superficial and deep laminae of the PLF. Despite this, their findings may lead to an increased exploration of manipulative treatments targeted at the more superficial layers of the TLF, such as acupuncture, cupping therapy or skin taping.
Recently, a second study has addressed the density of nerves in the sublayers of the superficial lamina of the PLF using human tissue (Benetazzo et al. 2011). Of the three sublayers, they found only the superficial sublayer to have any appreciable evidence of innervation. Employing S100 immunohistochemical staining, a plexus of small nerves (15 μm diameter) was visualized in the superficial sublayer and extending into the overlying subcutaneous loose connective tissue. No stainable fibers were detected in the intermediate and deep sublayers. Because myelinating and non-myelinating Schwann cells can express S100 protein (Gonzalez-Martinez et al. 2003), it is not possible to catalog the type of nerve fibers present in the tissue with S100 protein immunohistochemistry; however, the diameter of the fibers reported by Benetazzo et al. (2011) places these axons in the range of encapsulated cutaneous touch receptors and proprioceptors.
Tonus/stiffness regulation of lumbar fasciae
An in vitro examination of samples of human TLF by Yahia et al. (1993) documented the contractile ability of this tissue. Based on their results, the authors concluded that a histological examination for contractile cells within this tissue should be undertaken. Using α-smooth muscle actin as an immunohistochemical marker for the stress fiber bundles in smooth muscle-like cells, Schleip (2003) conducted a preliminary analysis of the superficial lamina of the PLF from 25 human cadavers (age 17–91 years, mean 47 years). Myofibroblasts were identified in all tissues examined, although they were present at varying densities. Myofibroblasts are connective tissue cells with an increased contractile force, and are responsible for wound closure (Grinnell, 1994) as well as pathological fascial contractures such as morbus Dupuytren contracture (Shih & Bayat, 2010) or frozen shoulder (Bunker & Anthony, 1995; Ko & Wang, 2011). While the short-term contractile ability of myofibroblasts is considerably weaker compared with skeletal muscle fibers, an incremental summation of their cellular contractions together with remodeling of the surrounding collagenous matrix could lead to a strong tissue ‘contracture’ over time (Tomasek et al. 2002).
Immunohistochemical examination of samples from the TLF in two cases of patients with low back pain demonstrated a myofibroblast density comparable to that found in frozen shoulders (see Fig. 18). It is an intriguing thought that some cases of low back pain may be associated with a similar stiffening of the TLF, in which case such a condition could be described as ‘frozen lumbars’.
Fig. 18.
Example of density of myofibroblasts in TLF comparable to frozen shoulder or Dupuytren contracture. Stress fiber bundles that are positively marked for α-smooth muscle actin (here in dark red) are used to identify myofibroblasts. Arrows indicate clearly visible myofibroblasts.
The question arises as to which factors could influence the proliferation and activity of lumbar myofibroblasts? Increased mechanical strain, such as hypertonicity, as well as biochemical changes have been described as stimulatory conditions (Tomasek et al. 2002). One of the strongest physiological agents for stimulating myofibroblast activity is the cytokine tumor growth factor (TGF)-β1 (reviewed in Willard et al. 2011). Because high sympathetic activity tends to go along with increased TGF-β1 expression (Bhowmick et al. 2009), it is possible that it might also be a contributing factor for stiffening and loss of elasticity in the TLF. Other contributory factors could be genetic makeup, the presence of inflammatory cytokines and the presence of frequent micro-injuries.
Biomechanical studies
The lumbosacral region can be conceptualized as the union of three large levers: the spine and the two legs. These movement arms are united by the TLC of fascia and aponeuroses. This fascial and aponeurotic composite is affected through the attachments of several large groups of muscles: the bridging muscles from the upper extremity; muscles of the lower extremity; and the torso muscles including both epaxial and hypaxial components. As has been presented in this overview, the bridging muscles of the upper extremity are those muscles arising from the limb buds of the lateral mesodermal, such as the trapezius and LD that grow centrifugally on to the torso bridging the gap between the extremity and the TLF. This also implies that the lumbosacral region is biomechanically coupled to the arms. The muscles of the lower extremity that can reach the TLF include the gluteus maximus and biceps femoris. Finally, the torso muscles involved in influencing this fascial and aponeurotic composite include the lumbar epaxial muscles and hypaxial muscles such as the TrA and the internal oblique, and possibly a small part of the external oblique. An understanding of the physical properties of the TLF and the influence that attached muscle groups have on this composite is a critical gateway for clinical application.
Biomechanical properties of the TLF
As a composite fabric, the TLF appears to be very strong. Using tensile tests on small samples, Tesh et al. (1987) estimated that the posterior layer of this fascia can resist up to 335 N vertical stress. This value has been suggested to be an underestimate by Adams & Dolan (2007), based on the fact that sectioning collagenous bundles to obtain tissue samples significantly reduces the total tensile strength of the sampled layer. In subsequent studies, it has been calculated that the tensile strength of the TLF, including the connections to the supraspinous ligament, may exceed 1 kN (Adams & Dolan, 2007).
Along with tensile strength, the distensible nature of the TLF is important. The viscoelastic properties of fascia from human specimens have been studied by Yahia et al. (1993). These researchers found that successive stretches produced an increase in the stiffness of the fascia, which showed signs of recovery towards baseline within a rest period of 1 h. It was also demonstrated that isometrically stabilized stress on the tissue resulted in gradual tightening of the tissue. The tightening was deduced from the observation that it required an increased load to maintain the initial strain on the tissue. This last finding is fascinating as it suggests that in vitro isolated connective tissue samples somehow change their own physical properties without the help of the normally attached muscles. Following attempts to eliminate the obvious sources of the tightening, such as changes in hydration, ionic balance and temperature, the authors propose the presence of muscle fibers in the fascia inducing this effect in vitro. However, their existence was not verified (Yahia et al. 1993). Additional insight into the issue of movement-dependent changes in the viscoelastic properties of fascia has been provided in a study by Schleip et al. (2012). These authors found an increased stiffness, termed strain hardening, occurred in fascial tissue subsequent to a stretch and rest paradigm. With sufficient rest time, ‘super-compensation’ occurred in the tissue, with analysis demonstrating a matrix hydration that was higher than the initial levels. This hardening occurred even in tissue that had been previously frozen, suggesting cellular contraction was not responsible. These findings could have significant implications for practitioners specialized in the treatment of the locomotor system, particularly those using techniques such as manual medicine (Schleip et al. 2012).
When the spine is placed in full flexion, the TLF increases in length from the neutral position by about 30% (Gracovetsky et al. 1981). The expansion in length of this tissue is accomplished by a tightening in width. This deformation places ‘strain-energy’ into the tissue, which should be recoverable in the form of reduced muscle work when the spine moves back in extension (Adams & Dolan, 2007). As Adams points out, this type of strain-energy is used by grazing animals to reduce the level of muscle contraction required to lift the head. Thus, it would be very useful for lifting large loads if some of the energy invested in the connective tissue of the thoracolumbar region could be reclaimed by assisting the extensor muscles.
Based on its physical properties, the deformation of the TLF offers an interesting mechanism for increasing efficacy of the extensor muscles of the back and stabilizing the spine. Any muscle or group of muscles that can resist the narrowing of the TLF by applying laterally directed traction force to the lateral margins of this structure is essentially applying an extensor force to the lumbar spine (Fairbank & O’Brien, 1980; Gracovetsky et al. 1981, 1985). The magnitude of the extensor force generated by the abdominal muscles was seriously questioned Macintosh et al. (1987) on theoretical grounds. An experimental test of the role of lateral forces on the TLF was undertaken by Tesh et al. (1987). The findings supported a smaller than expected role for lateral forces in sagittal plane motion, but a larger than anticipated role in preventing lateral flexion. However, part of these findings could be the effect of testing cadavers in full spinal flexion and having their arms folded behind the head in this study. The TLF is strongest in a relative straight back posture while flattening the lumbar lordosis (Adams & Dolan, 2007).
The TLF is arranged in a position to generate large extensor moments with low compression forces on the vertebral column (Adams & Dolan, 2007). In the extended state, with the paraspinal muscles contracting, the plane of the TLF is displaced at least 50 mm posterior to the center of the intervertebral discs, and at the lumbosacral level this distance averaged 62 mm (Tracy et al. 1989). This distance provides a reasonably long moment arm for resisting extension around the center of motion, which would lie in the vertebral body; however, this distance was derived from supine subjects in an MRI gantry and may be an underestimate. Thus, the TLF is capable of creating a sizable extensor moment, which is especially important because passive (non-muscular) tissue develops less compressive strain on the intervertebral than active contraction of muscles (Adams & Dolan, 2007).
An estimate of the magnitude of the extensor moment of the TLF was derived experimentally (Dolan et al. 1994). With subjects attempting repeated isometric lifting exercises in a stooped (flexed) posture, the EMG activity of the back muscles was compared with the extensor moments generated, and a value in the range of 80 Nm extensor moment was attributable to the passive structures such as the TLF. It is now reasonable to ask how the numerous muscles, both surrounding and inside this fascial composite, could influence tension and increase its extensor moment as well.
The abdominal torso muscles
One of the initial studies to suggest a role for the muscular connections of the TLF analyzed the abdominal muscles during lifting (Fairbank & O’Brien, 1980; Fairbank et al. 1980). The results suggested that patients with low back pain, exacerbated by lifts, experienced an abnormal rise in intra-abdominal pressure (IAP) during the pain-evoking lifts. An elaborate mathematical model of the lumbar spine during lifting was developed (Gracovetsky et al. 1981) and adapted to explain how contraction of the abdominal muscles and the resulting increased IAP could place stress on the intervertebral joint during lifting (Gracovetsky et al. 1985). This model was based on the assumption that lateral pull by the aponeurosis of the TrA would resist longitudinal expansion of the TLF in the vertical plane and thereby provide a significant extensor force to the lumbar spine; however, the theoretical lifting models were not verified experimentally. Subsequent calculations, based on corrected assumptions and anatomy, demonstrated the forces generated to be smaller than initially predicted (Macintosh et al. 1987).
An intriguing question was addressed by Tesh et al. (1987); could contraction of the abdominal muscles, and the resulting increase in IAP, influence the postural stability of the lumbar spine by acting through their attachments to the TLF? By tensioning the lumbar spinous processes in a craniocaudal direction and the MLF in a mediolateral direction they demonstrated sufficient stabilizing forces in the posterior layer of the TLF. Significantly, in this experiment the PRS was expanded by replacing the paraspinal muscles with plastic foam dowels, thereby mimicking the role of the contracted erector spinae and multifidus muscles. These studies were followed by research utilizing balloon inflation in the abdominal cavity of cadaveric specimens to investigate the influence of increased IAP. Significant resistance to flexion was observed with increased IAP. In addition, inflating the balloons between 60 and 120 mm Hg also produced significant resistance to lateral flexion. Their model utilized the attachments of the MLF on the tips of the transverse processes to demonstrate a compressive force emerging between the TPs when the MLF is tensioned in the mediolateral direction such as would occur with increased IAP. Tesh et al. (1987) concluded that the MLF could contribute as much as 40% of restriction in the total flexion moment utilized to support the lumbar spine in extreme lateral bending. Barker et al. (2007) tested this model by applying a symmetrical force of 20 N tension to the TrA aponeurosis. With this strain applied to the TrA–MLF complex, a 44% increase in resistance to flexion moments at all lumbar segments was detectable. Hodges et al. (2003), using a porcine model, reported that electrical-stimulated contraction of TrA results in tension across the MLF, increasing resistance to flexion at the L3/L4 level. All of these results are in line with the outcome of previous studies that have examined the application of a lateral constraint to a uniaxially loaded muscle (Aspden, 1990). In this case, the constraint is a moderate force through the TrA aponeurosis affecting the erector muscles within the fascial sheath, restricting lumbar flexion. However, in a cadaver study that applied direct tension to the aponeurosis of the TrA (Barker et al. 2007), the erector spinae muscles can not be tensed within the PRS, to resist pull on the TrA–MLF complex as compared with in vivo situations. Therefore, currently it is difficult to ultimately determine how the tension applied to the TrA would divide between the MLF and the PLF in the face of normal intra-compartmental pressure in the PRS. However, the above-reported results may at least give insight into the pathological condition of muscle atrophy that frequently occurs in the erector spinae compartment (Danneels, 2007; Kalichman et al. 2010).
The TrA muscle, based on its anatomy and function, has the greatest potential for influencing the stability of the lumbar spine (Hodges et al. 2003; Barker et al. 2007). Its role in respiratory movement has been reviewed by De Troyer et al. (1990). De Troyer (2005), and its role in postural stability has been reviewed by Hodges (1999, 2008). Contraction of the TrA reduces abdominal circumference and increases IAP. All of the abdominal muscles are active during flexion; only TrA continues activity during extension. Of the abdominal muscles, only TrA is incapable of producing significant trunk torque due to its horizontal disposition. These observations support a generalized role for TrA in postural support of the lumbosacral spine. During rapid limb movement, the onset of TrA activity in healthy individuals precedes that of all other torso muscles, and TrA activity was independent of the direction of limb movement. The TrA generated a rise in IAP, and was demonstrated to be fast and of sufficient magnitude to increase the stability of the lumbar spine prior to the contraction of torque-producing muscles. The response of TrA was linked to the magnitude and speed of the perturbing force; small or slow movements did not engage the TrA (Hodges, 1999, 2008).
Increased postural demand, regardless of direction, activated TrA and the EMG activity co-varied with the extent of the postural demand (Crommert et al. 2011). Thus, TrA presets the torso preparatory strategy prior to the contraction of the torque-producing muscles, such as the erector spinae, suggesting a major role for TrA on the stability of the spine. Support for these theories can be seen in the delayed activity in TrA in patients with low back pain. Finally, complete absence of the abdominal muscles, such as can occur in the ‘prune-belly syndrome’, has been associated with decomposition of the spinal column, suggesting a role for the abdominal muscles in maintaining the postural stability of the lumbosacral spine (Lam & Mehdian, 1999).
How could contraction of the TrA as well as other abdominal muscles function in postural homeostasis? Connective tissues such as the endo- and epimysium of the abdominal wall muscles and their associated fascial sheaths are arranged in such a way that contraction of the abdominal muscles has an effect on the posterior spine as well as on the rectus abdominis muscle and its sheath. It has been demonstrated that active muscles will transfer forces outward as well as longitudinally and therefore deform their associated connective tissues, depending on the magnitude and direction of forces generated (Brown & McGill, 2009). Huijing (2003) and Aspden (1990) have shown that muscle force transmission is not exclusively a serial process from muscles fibers to tendon, but that an additional lateral component of transmission occurs through the surrounding connective tissue connections. Brown & McGill (2009) assessed rat abdominal muscles in vivo. Even when the TrA aponeurosis was sectioned, there is still lateral transmission of forces during contraction of the TrA through surrounding connective tissue to other muscles. In a separate study, the same authors utilized ultrasound imaging to demonstrate that the rectus abdominis muscle and sheath expanded in multiple planes when the TrA was contracted. Based on these data, it was suggested that the linea alba is especially capable of accommodating forces transferred from one side of the abdominal wall to the other without intersecting skeletal endpoints.
The primary conclusion from this body of data is that the TrA plays an important role in stabilizing the lumbar spine against forces attempting to perturb balance. It is also worth noting that this large body of information has recently been challenged by the observation that the TrA does not co-contract prior to rapid postural challenge such as rapid arm movements (Allison & Morris, 2008; Morris et al. 2012). The investigators’ hypothesis is that the TrA is asymmetrically contracting to balance the rotational torque generated by the rapid arm movement. However, Hodges (2008) responds that former studies have already described that the TrA is active asymmetrically during trunk rotation. In fact, the TrA is active with both directions of rotation, but greater when rotating the thorax towards the side of the muscle, and the muscle has a trivial moment arm to generate rotation torque (Urquhart & Hodges, 2005). The contribution of the muscle to rotation may relate to control of the linea alba, while the contralateral obliquus externus (OE) and ipsilateral obliquus internus (OI) contribute most to the torque (Urquhart et al. 2005).
The paraspinal torso muscles
The anatomy of the paraspinal muscles has been well documented (Bogduk, 1980; Macintosh & Bogduk, 1987, 1991). These muscles are confined within a thick-walled connective tissue compartment, the PRS. This latter structure is formed out of the expansion of the deep lamina of the TLF continuing anteromedially around the lateral border of the paraspinal muscles to reach the tips of the transverse processes.
The paraspinal muscles, like any biological material with a mechanical function, are often subject to forces that are primarily uniaxial. Analysis of uniaxial strain in the paraspinal muscles leads to induced strain in perpendicular directions. The ratio of the induced strain in the TLF relative to the uniaxial strain in muscles is called the Poisson ratio. This ratio is defined for a body subjected only to uniaxial stress, all other directions in the body being unconstrained (Aspden, 1990).
Constraining muscular expansion by surrounding it with a strong connective tissue like the TLF connected to other fascia sheets and muscles could therefore considerably increase the strength and stiffness of the muscles within the sheet. Because of the relatively high stiffness of connective tissue, the stresses produced could have a significant effect on muscle stress. Connective tissues are also viscoelastic, which means that their stiffness increases as the rate of straining increases. Thus, rapid contraction of a muscle would be expected to generate a greater strengthening and stiffening effect by the fascia (Aspden, 1990).
Contraction of the paraspinal muscles can increase intra-compartmental pressure in the PRS (Tesh et al. 1987). Hukins et al. (1990) developed a model demonstrating that, in the face of active contraction of the erector spinae muscles, the TLF can increase axial stress by restricting the muscles’ radial expansion (bulging). This stress was calculated both for the radius of the abdominal cavity and the IAP generated during lifting (measured from MRIs and values of IAP obtained from literature). The results indicate that restriction of radial expansion of the erector spinae muscles within the TLF may increase the axial stress generated during their contraction, by up to about 30%.
The efficacy of the erector spinae and multifidi muscles is dependent on posture. Increasing the lordosis accentuates the extensor lever arm for the erector spinae and multifidi and the aponeurosis, whilst kyphosis shortens the length of the lever arm to both the erector spinae and multifidi muscles. However, lumbar flexion increases tension in the TLF (Tveit et al. 1994). Co-activation of the lumbar erector spinae/multifidi- and trunk and hip flexor muscles results in forward pelvic tilt, which can balance the neutral spinal posture (Cholewicki et al. 1997).
Extremity muscles
The influence of the extremity muscles on the TLC has been examined by a number of authors (Bogduk & Macintosh, 1984; Vleeming et al. 1989, 1995; Bogduk et al. 1998; Barker & Briggs, 1999; Barker et al. 2004, 2007). Typically, the studies are approached by isolating groups of muscle fibers, applying a quantified amount of tension to the fibers and measuring the displacement seen in the TLF. The results of the traction studies varied considerably depending on the site of the traction (Vleeming et al. 1995).
In examining the upper extremity muscles, it was concluded that traction to the cranial fascia and muscle fibers of the LD showed limited displacement of the superficial lamina (homolaterally up to 2–4 cm), while traction to the caudal part of the LD caused displacement up to the midline. The specific midline area involved was located as much as 8–10 cm away from the site of traction. In addition, it was found that between L4–L5 and S1–S2 levels, the displacement of the superficial lamina of the PLF from this traction actually spread to the contralateral side. The extent of the influence that the LD has on the TLF reflects the wide distribution of the aponeurosis of this muscle across the low back region. In all preparations, traction to the trapezius muscle resulted in a relatively small effect (up to 2 cm); this is in keeping with the relatively small attachment of the muscle to the TLF (Vleeming et al. 1995).
Studies examining lower extremity muscles also revealed significant effects of muscle traction to the TLF. Traction to the gluteus maximus caused displacement across the midline and into the contralateral side. The distance between the site of traction and visible displacement varied from 4 to 7 cm (Vleeming et al. 1995).
Traction to the biceps femoris tendon (BT), applied in a lateral direction, resulted in displacement of the deep lamina up to the level L5–S1. Obviously, this load transfer is conducted to the sacrotuberous ligament. In two specimens, displacement occurred at the contralateral side, 1–2 cm away from the midline. Traction to the BT directed medially, showed homolateral displacement in the deep lamina, up to the median sacral crest (Vleeming et al. 1989). As shown by the traction tests, the tension of the PLF can be influenced by contraction or stretch of a variety of muscles. It is noteworthy that especially muscles such as the LD and gluteus maximus are capable of exerting a contralateral effect especially to the lower lumbar spine and pelvis. This implies that the ipsilateral gluteus maximus and contra-lateral LD both can tension the PLF. Hence, parts of these muscles provide a pathway for mechanical transmission between pelvis and trunk. One could argue that the partial lack of connection between the superficial lamina of the PLF and the supraspinous ligaments in the lumbar region is a disadvantage for stability. However, it would be disadvantageous only in case strength, coordination and effective coupling of the gluteus maximus and the caudal part of the contralateral LD are diminished. It can be expected that increased strength of these mentioned muscles, among others, accomplished by torsional training could influence the quality of the PLF. Following this line of thinking, the PLF could play an integrating role in rotation of the trunk and in load transfer, and hence stability of the lower lumbar spine and pelvis (Vleeming & Stoeckart, 2007).
Barker & Briggs (1999) make the interesting comment that the PLF is ideally positioned to receive feedback from multiple structures involved in lumbar movements, and may regulate ligamentous tension via its extensive muscular attachments to both deep stabilizing and more superficial muscles. They report that the fascia displays visco-elastic properties and thus is capable of altering its structure to adapt to the stresses placed on it. The PLF has been reported to stiffen with successive loading, and adaptive fascial thickening is possible. Barker & Briggs (1999) comment that when adaptive strengthening of the posterior layer takes place, one might expect to facilitate this by using exercises that strengthen its attaching muscles, both deep and superficial. Adaptive strengthening therefore would be expected to occur with exercises using contralateral limbs, such as swimming and walking and torsional training. It also might occur with recovery of muscle bulk and function (erector spinae/multifidus) during lumbopelvic stabilization exercises (Mooney et al. 2001; Vleeming & Stoeckart, 2007).
Bogduk et al. (1998) did not agree with the concept that the LD has a role in rotating the spine, and comment that the muscle is designed to move the upper limb and its possible contribution to bracing the sacroiliac joints via the TLF is trivial. In contrast to this, Kumar et al. (1996) show that axial rotation of the trunk involves agonistic activity of the contralateral external obliques, and ipsilateral erector spinae and LD as agonistic muscles to rotate the trunk. Mooney et al. (2001) used the anatomical relation of the LD and the gluteus maximus to study their coupled effect during axial rotation exercises and walking. They concluded that in normal individuals, walking a treadmill, the functional relationship between the mentioned muscles could be confirmed. It was apparent that the right gluteus maximus had, on average, a lower signal amplitude compared with the left (n = 15; 12 right-handed). This reciprocal relationship of muscles correlates with normal reverse rotation of shoulders vs. the pelvis in normal gait. The authors showed that during right rotation of the trunk, the right LD muscle is significantly more active than the left, and the left gluteus maximus muscle is more active than the right.
In the same study, patients with sacroiliac joint dysfunction had a strikingly different pattern. On the symptomatic side, the gluteus maximus was far more active compared with healthy subjects. The reciprocal relation between LD and gluteus maximus, however, was still present. After an intense rotational trunk-strengthening training program, the patients showed a marked increase of LD strength and diminished activity of the gluteus maximus on the symptomatic side. The importance of these findings could be that rotational trunk muscle training is important particularly for stabilizing the sacroiliac joint and lower spine. These studies support a possible role for the LD in movement and stability of the lumbosacral spine.
It is noteworthy that, as shown in this study (Mooney et al. 2001), the coupled function of the gluteus maximus and the contralateral LD muscles creates a force perpendicular to the sacroiliac joint and lumbar facet joints. Attention is drawn to a possible role of the erector muscle and multi-fidus in load transfer. Between the lateral raphe and the interspinous ligaments, the deep lamina encloses the erector muscle and multifidus. It can be expected that contraction of the erector/multifidus will longitudinally increase the tension in the deep lamina. In addition, the whole posterior layer of the TLF will be ‘inflated’ (hydraulic amplifier effect; Gracovetsky et al. 1977) by contraction of the erector spinae/multifidus (Vleeming et al. 1995). Consequently, it can be assumed that training of muscles such as the gluteus maximus, LD and erector spinae, and the multifidus can assist in increasing force-closure also by strengthening the posterior layer of the TLF (Vleeming & Stoeckart, 2007).
As in quadrupeds, the arms and legs of bipeds move rhythmically (Vleeming & Stoeckart, 2007). In normal gait, before right-sided heel strike, the trunk is already showing counter-rotation (left arm forwards). This is especially distinct in energetic movements such as running and jumping. The counter-rotation of the trunk and anteflexion of the left arm assist in passively tensing the LD, and consequently the TLF, in combination with the action of the right-sided gluteus maximus. Also, the simultaneous alternating pelvic tilt in the frontal and transversal plane can help to strain the TLF. On the contralateral side, the right arm extends, activated among others by the LD.
In this scenario, a large oblique dorsal muscle-fascia sling is tensed, which partially crosses the spine. Strain energy stored in each tissue is equal to the area under its force-deformation curve and proportional to the maximum stretching of the structure, multiplied by the maximum tension acting on it (Adams & Dolan, 2007). Energy is accumulated during gait (Margaria, 1968). Margaria described that the work done by muscles in active or passive tension is partly stored as elastic energy. It is concluded that this energy can be utilized if the muscle is allowed to shorten immediately afterwards. Using this model, trunk torsion during walking, involving left and right trunk muscles connecting to the TLF, could function as a large spring. Energy is stored that can be used to minimize muscle action (Vleeming & Stoeckart, 2007).
In conclusion, the PLF could play an important role in transferring forces between spine, pelvis and legs, especially in rotation of the trunk and stabilization of lower lumbar spine and sacroiliac joint (Fig. 19). The gluteus maximus and the LD merit special attention because they can conduct forces contralaterally via the posterior layer of the TLF. Because of the coupling between the gluteus maximus and the contralateral LD via the PLF, one must be cautious when categorizing certain muscles as arm, spine or leg muscles. Rotation of the trunk is mainly a function of the abdominal muscles. However, a counter-muscle sling in the back, in contrast to the abdominal sling, helps to preclude deformation of the spine. Rotation against increased resistance will activate the posterior oblique sling of LD and gluteus maximus (Vleeming & Stoeckart, 2007). However, these muscles have a higher threshold value compared with the abdominal muscles (Mooney et al. 2001).
Fig. 19.
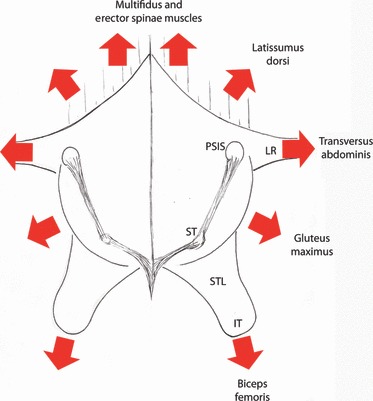
Model of the TLF and its associated muscles and aponeuroses. This is a posterior view of the sacral region. The TLF and its associated aponeuroses have been dissected off the pelvis and flattened in the schematic diagram. The central region of the diagram represents the combined region of the aponeuroses, and has been termed the TLC. This region is the thickest and best positioned to resist lateral movements of the posterior superior iliac spine (PSIS). The PSIS and the lateral sacral tubercle (ST) are connected via the long dorsal sacroiliac ligament. The aponeurosis of the TrA joins the structure at the lateral raphe (LR), and the sacrotuberous ligament (STL), covered by the TLC, is seen ending on the ischial tuberosity (IT).
The TLC and pelvic stabilization
Based on biomechanical studies, the TrA and associated oblique muscles have been described as a contractile bandage, pulling the anterior portions of the blade of the ilium (ASIS) toward each other, thus increasing the pressure in the two surfaces of the sacroiliac joint and thereby stabilizing the pelvis in upright posture (Vleeming et al. 1992; Snijders et al. 1997; Pel et al. 2008). This force-closure mechanism could only work if another force prevents the posterior aspect of the innominate bones from moving laterally as the anterior crest moves inward. An unchecked lateral movement of the posterior ilium would allow an outward rotation of PSIS, thereby opening the posterior aspect of the sacroiliac joint, stressing the interosseous and dorsal sacroiliac joint ligaments and destabilizing the pelvis (Vleeming & Willard, 2010).
The TLC, defined as the blending of the superficial and deep laminae of the PLF to the aponeurosis of the erector/multifidi muscles, starts to thicken especially over the lower part of L5 and the sacrum (Bogduk & Macintosh, 1984; Vleeming et al. 1995). The TLC together with dorsal sacroiliac joint ligaments is best positioned to accomplish the task of resisting lateral movement of the PSIS. With the PSIS of the ilia anchored in place by the TLC, contraction of the TrA and, to a lesser degree the IO, would be able to compress the sacroiliac joint. As seen in the modified CT scan of the pelvis (Fig. 20), inward movement of the ASIS (arrow over the abdominal muscles) would displace the PSIS laterally. Tensioning of the TLC (arrows overlying the TLC) would resist this motion, allowing the anterior medial force to translate into increased compression to the sacroiliac joint (arrows at the sacroiliac joint). Interestingly, this adapted biomechanical model places great emphasis on the condition of the multifidus muscle over the sacrum (Snijders et al. 1997). In the sacral region, the multifidus occupies the space bounded anteriorly by the sacrum, laterally by the ilia and posteriorly by the TLC (Fig. 20). Weakening or fatty involution of this muscle would diminish the hydraulic amplifier mechanism, as proposed by Gracovetsky et al. (1977). The resultant decrease in tension of the TLC would allow more lateral displacement of the ilia, resulting in destabilization of the sacroiliac joint. Conversely, contraction of the muscles in the surrounding aponeurotic sheets (Fig. 20) would be expected to enhance the tension on the TLF, further stabilizing the joint as seen in biomechanical studies (Vleeming et al. 1995) and on EMG studies (van Wingerden et al. 2004). Conversely, hypertonicity of the same muscles (Masi et al. 2007; Vleeming & Stoeckart, 2007), especially in a sitting posture in full lordosis, could overactivate these muscles in such a way that a compartment syndrome could be the result. This proposed model of TLC function needs to be thoroughly investigated through biomechanical experimentation; however, several anatomical and biomechanical studies support this model (Vleeming et al. 1992; Mens et al. 2006; Pel et al. 2008; Hu et al. 2010).
Fig. 20.
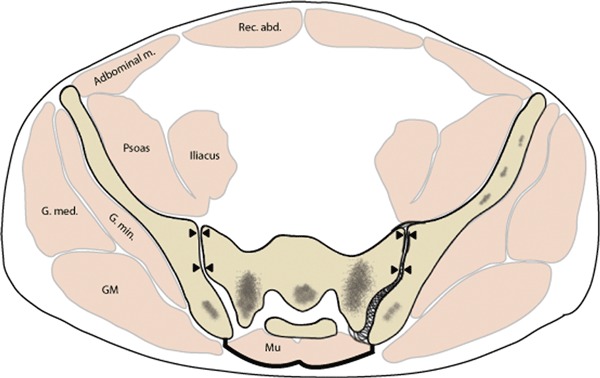
An axial plane CT scan of a male pelvis approximately at the level of PSIS. The sacroiliac joint is indicated by the two opposing black arrows. The body of the multifidus muscle is seen in the sacral groove between the two ilia. The layer covering the multifidus at this level is the TLC and is indicated by the double curved line. At this level, the TLC is composed of the fused aponeuroses of the erector spinae and the LD (see text for further explanation). GM, gluteus maximus, G.med., gluteus medius; G.min., gluteus minimus; Mu, multifidus; Rec. abd., rectus abdominis. Adapted after Snijders et al. (1995).
Summary
The most common terminology for the TLF is derived from the three-layer model of the TLF; however, the two-layered model most likely resembles reality, as the transversalis fascia covering anteriorly the QL and psoas muscle is thin compared with the PLF and MLF. The posterior layer of TLF is divided into superficial and deep laminae. The superficial lamina is derived from the union of two aponeuroses from the LD and the SPI, whilst the deep lamina of the PLF actually is a retinacular sheath surrounding the paraspinal muscles. This latter structure has been termed the PRS. The posterior aspect of the PRS extends from the sacrum to the cranial base; the anterior aspect extends from the iliolumbar ligament to the 12th rib. Above this line the ribs and the transverse processes of the cervical vertebrae form the anterior wall. Laterally the PRS passes around the paraspinal muscles; a thickening in the connective tissue at the lateral extreme of this sheath, termed the lateral raphe, represents the point where the PRS is joined in a triangulation primarily with the aponeurosis of the TrA; the connective tissue triangle thus created is termed the LIFT.
The PRS surrounds the three large lumbar muscles, iliocostalis, longissimus and multifidus. Towards the lower lumber region, the aponeurosis of the erector spinae and multifidi muscles becomes thicker. Below the level of L4–L5, the aponeurosis of the erector spinae fuses to the posterior overlying deep lamina and the superficial lamina to form an inseparable composite, termed the TLC. The TLC adheres tightly to the PSIS and the border of the sacrum. It then covers the sacrotuberous ligaments eventually reaching the ischial tuberosities.
The TLF receives both a proprioceptive and nociceptive innervation. Although large myelinated fibers with encapsulated endings and small unmyelinated fibers have been visualized in certain layers of this structure, it is not clear at this time what role they give the TLF as a sensory organ. Neurophysiological studies as discussed here point towards a role of the TLF in evoking back pain.
Several muscles of various dimensions attach to the TLF and its caudal composite. Examples include the LD, gluteus maximus and the abdominal muscles, primarily the TrA. Biomechanical studies have supported the concept that tension applied by the surrounding muscles, especially the TrA, can be transmitted through the TLF to stiffen the lumbar spine and increase the force-closure of the sacroiliac joint. Flexion of the spine stretches the TLF, diminishing its lateral dimensions. Resistance to lateral retraction of the TLF by the abdominal muscles, acting through some combination of the MLF and PLF, will stiffen this tissue and increase resistance to flexion as well as enhance the extensor moment of the lumbar region. Within the PRS, contraction of the paraspinal muscles has been demonstrated to increase the intracompartmental pressure and thereby contribute to the hydraulic amplifier effect supporting the lumbar spine.
Finally, increased tone in the lumbar multifidus muscle should act to increase the tension created by the TLC between PSIS bilaterally. This increased medially directed tension would lead to force-closure of the sacroiliac joint, thus stabilizing the pelvis.
Acknowledgments
The authors would like to express appreciation to Mr. Oran Suta and Ms. Dawn Whalen for their assistance and artistic expertise on several figures.
References
- Adams MA, Dolan P. How to use the spine, pelvis and legs effectively in lifting. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain. 2nd edn. Edinburgh: Churchill Livingstone Elsevier; 2007. pp. 167–183. [Google Scholar]
- Allison GT, Morris SL. Transversus abdominis and core stability: has the pendulum swung? Br J Sports Med. 2008;42:930–931. doi: 10.1136/bjsm.2008.048637. [DOI] [PubMed] [Google Scholar]
- Anson BJ, Maddock WG. Callander’s Surgical Anatomy. 4th edn. Philadelphia: W.B. Saunders; 1958. [Google Scholar]
- Aspden RM. Constraining the lateral dimensions of uniaxially loaded materials increases the calculated strength and stiffness: application to muscle and bone. J Mater Sci Mater M. 1990;1:100–104. [Google Scholar]
- Bailey FR, Miller AM. Textbook of Embryology. 3rd edn. New York: William Wood; 1916. [Google Scholar]
- Bakker DA, Richmond FJR. Muscle spindle complexes in muscles around upper cervical vertebrae in the cat. J Neurophysiol. 1982;48:62–74. doi: 10.1152/jn.1982.48.1.62. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Briggs CA. Attachments of the posterior layer of the lumbar fascia. Spine. 1999;24:1757–1764. doi: 10.1097/00007632-199909010-00002. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Briggs CA. Anatomy and biomechanics of the lumbar fascia: implications for lumbopelvic control and clinical practice. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain: Integration of Research and Therapy. 2nd edn. Edinburgh: Elsevier; 2007. pp. 63–73. [Google Scholar]
- Barker PJ, Briggs CA, Bogeski G. Tensile transmission across the lumbar fasciae in unembalmed cadavers: effects of tension to various muscular attachments. Spine. 2004;29:129–138. doi: 10.1097/01.BRS.0000107005.62513.32. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Urquhart DM, Story IH, et al. The middle layer of lumbar fascia and attachments to lumbar transverse processes: implications for segmental control and fracture. Eur Spine J. 2007;16:2232–2237. doi: 10.1007/s00586-007-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar DA, Orr FW, Simon GT. Observations on the pathomorphology of the thoracolumbar fascia in chronic mechanical back pain: a microscopic study. Spine. 1995;20:1161–1164. doi: 10.1097/00007632-199505150-00010. [DOI] [PubMed] [Google Scholar]
- Benetazzo L, Bizzego A, Caro RDe, et al. 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat. 2011;33:855–862. doi: 10.1007/s00276-010-0757-7. [DOI] [PubMed] [Google Scholar]
- Benjamin M. The fascia of the limbs and back – a review. J Anat. 2009;214:1–18. doi: 10.1111/j.1469-7580.2008.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmark A. Stability of the lumbar spine: a study in mechanical engineering. Acta Orthop Scand Suppl. 1989;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Bhowmick S, Singh A, Flavell RA, et al. The sympathetic nervous system modulates CD4(+)FoxP3(+) regulatory T cells via a TGF-beta-dependent mechanism. J Leukoc Biol. 2009;86:1275–1283. doi: 10.1189/jlb.0209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. A reappraisal of the anatomy of the human lumbar erector spinae. J Anat. 1980;131:525–540. [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. Clinical Anatomy of the Lumbar Spine. 4th edn. Edinburgh: Elsevier Churchill Livingston; 2005. [Google Scholar]
- Bogduk N, Macintosh JE. The applied anatomy of the thoacolumbar fascia. Spine. 1984;9:164–170. doi: 10.1097/00007632-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Twomey LT. Clinical Anatomy of the Lumbar Spine. 2nd edn. Melbourne: Churchill Livingstone; 1991. p. 1. [Google Scholar]
- Bogduk N, Macintosh JE, Pearcy MJ. A universal model of the lumbar back muscles in the upright position. Spine. 1992;17:897–913. doi: 10.1097/00007632-199208000-00007. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Johnson G, Spalding D. The morphology and biomechanics of latissimus dorsi. Clin Biomech (Bristol, Avon) 1998;13:377–385. doi: 10.1016/s0268-0033(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Bove GM, Light AR. Unmyelinated nociceptors of rat paraspinal tissues. J Neurophysiol. 1995;73:1752–1762. doi: 10.1152/jn.1995.73.5.1752. [DOI] [PubMed] [Google Scholar]
- Brown SH, McGill SM. Transmission of muscularly generated force and stiffness between layers of the rat abdominal wall. Spine (Phila Pa 1976) 2009;34:E70–E75. doi: 10.1097/BRS.0b013e31818bd6b1. [DOI] [PubMed] [Google Scholar]
- Brumagne S, Dolan P, Picker J. Complexity of sensory function in spinal control and low back pain. In: Vleeming A, editor. 7th Interdisciplinary World Congress on Low Back & Pelvic Pain. Los Angeles: Worldcongress LBP Foundation; 2010. pp. 408–413. [Google Scholar]
- Budgell B, Noda K, Sato A. Innervation of posterior structures in the lumbar spine of the rat. J Manipulative Physiol Ther. 1997;20:359–368. [PubMed] [Google Scholar]
- Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br. 1995;77:677–683. [PubMed] [Google Scholar]
- Carr D, Gilbertson L, Frymoyer J, et al. Lumbar paraspinal compartment syndrome. A case report with physiologic and anatomic studies. Spine (Phila Pa 1976) 1985;10:816–820. [PubMed] [Google Scholar]
- Cholewicki J, Panjabi MM, Khachatryan A. Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine. 1997;22:2207–2212. doi: 10.1097/00007632-199710010-00003. [DOI] [PubMed] [Google Scholar]
- Chopra J, Rani A, Rani A, et al. Re-evaluation of superficial fascia of anterior abdominal wall: a computed tomographic study. Surg Radiol Anat. 2011;33:843–849. doi: 10.1007/s00276-011-0801-2. [DOI] [PubMed] [Google Scholar]
- Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303:1295–1302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- Clemente CD. Gray’s Anatomy of the Human Body. Philadelphia: Lea & Febiger; 1985. [Google Scholar]
- Corey SM, Vizzard MA, Badger GJ, et al. Sensory innervation of the nonspecialized connective tissues in the low back of the rat. Cells Tissues Organs. 2011;194:521–530. doi: 10.1159/000323875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisco JJ, Panjabi MM, Yamamoto I, et al. Euler stability of the human ligamentous lumbar spine. Part II Experiment. Clin Biomech. 1992;7:27–32. doi: 10.1016/0268-0033(92)90004-N. [DOI] [PubMed] [Google Scholar]
- Crommert ME, Ekblom MM, Thorstensson A. Activation of transversus abdominis varies with postural demand in standing. Gait Posture. 2011;33:473–477. doi: 10.1016/j.gaitpost.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Danneels LA. Clincial anatomy of the lumbar multifidus. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain: Integration of Research and Therapy. 2nd edn. Edinburgh: Elsevier; 2007. pp. 86–94. [Google Scholar]
- De Meersman RE, Zion AS, Weir JP, et al. Mechanoreceptors and autonomic responses to movement in humans. Clin Auton Res. 1998;8:201–205. doi: 10.1007/BF02267782. [DOI] [PubMed] [Google Scholar]
- De Troyer A. Interaction between the canine diaphragm and intercostal muscles in lung expansion. J Appl Physiol. 2005;98:795–803. doi: 10.1152/japplphysiol.00632.2004. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Estenne M, Ninane V, et al. Transversus abdominis muscle function in humans. J Appl Physiol. 1990;68:1010–1016. doi: 10.1152/jappl.1990.68.3.1010. [DOI] [PubMed] [Google Scholar]
- Dittrich RJ. Lumbodorsal fascia and related structures as factors in disability. J Lancet. 1963;83:393–398. [PubMed] [Google Scholar]
- Dolan P, Mannion AF, Adams MA. Passive tissues help the back muscles to generate extensor moments during lifting. J Biomech. 1994;27:1077–1085. doi: 10.1016/0021-9290(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Fairbank JCT, O’Brien JP. The Abdominal Cavity and Thoraco-Lumbar Fascia as Stabilisers of the Lumbar Spine in Patients with Low Back Pain. Vol 2, Engineering Aspects of the Spine. London: Mechanical Engineering Publications; 1980. pp. 83–88. [Google Scholar]
- Fairbank JCT, O’Brien JP, Davis PR. Intraabdominal pressure rise during weight lifting as an objective measure of low-back pain. Spine. 1980;5:179–184. doi: 10.1097/00007632-198003000-00013. [DOI] [PubMed] [Google Scholar]
- Federative Committee on Anatomical Terminology. Terminologia Anatomica – International Anatomical Terminology. Stuttgart: Thieme; 1998. [Google Scholar]
- Gatton ML, Pearcy MJ, Pettet GJ, et al. A three-dimensional mathematical model of the thoracolumbar fascia and an estimate of its biomechanical effect. J Biomech. 2010;43:2792–2797. doi: 10.1016/j.jbiomech.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Gibson W, Arendt-Nielsen L, Taguchi T, et al. Increased pain from muscle fascia following eccentric exercise: animal and human findings. Exp Brain Res. 2009;194:299–308. doi: 10.1007/s00221-008-1699-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Perez-Pinera P, az-Esnal B, et al. S-100 proteins in the human peripheral nervous system. Microsc Res Tech. 2003;60:633–638. doi: 10.1002/jemt.10304. [DOI] [PubMed] [Google Scholar]
- Goss CM. Gray’s Anatomy of the Human Body. Philadelphia: Lea & Febiger; 1973. [Google Scholar]
- Gracovetsky S, Farfan HF, Lamy C. A mathematical model of the lumbar spine using an optimized system to control muscles and ligaments. Orthop Clin North Am. 1977;8:135–153. [PubMed] [Google Scholar]
- Gracovetsky S, Farfan HF, Lamy C. The mechanism of the lumbar spine. Spine. 1981;6:249–262. doi: 10.1097/00007632-198105000-00007. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S, Farfan H, Helleur C. The abdominal mechanism. Spine. 1985;10:317–324. doi: 10.1097/00007632-198505000-00005. [DOI] [PubMed] [Google Scholar]
- Grant JCB. An Atlas of Anatomy. 6th edn. Baltimore: Williams & Wilkins; 1972. [Google Scholar]
- Gray H. Anatomy, Descriptive and Surgical. 5th edn. Philadelphia: Henry C. Lea; 1870. [Google Scholar]
- Gray H. Anatomy Descriptive and Applied. London: Longmans, Green; 1923. [Google Scholar]
- Green RM. Warren’s Handbook of Anatomy. Cambridge: Harvard University Press; 1937. [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WJ, Boyd JD, Mossman HW. Human Embryology. 4th edn. Cambridge: W. Heffer; 1972. [Google Scholar]
- Hirsch C. The anatomical basis for low back pain. Acta Orthop Scand. 1963;33:1. doi: 10.3109/17453676308999829. [DOI] [PubMed] [Google Scholar]
- Hodges PW. Is there a role for transversus abdominis in lumbo-pelvic stability? Man Ther. 1999;4:74–86. doi: 10.1054/math.1999.0169. [DOI] [PubMed] [Google Scholar]
- Hodges P. Transversus abdominis: a different view of the elephant. Br J Sports Med. 2008;42:941–944. doi: 10.1136/bjsm.2008.051037. [DOI] [PubMed] [Google Scholar]
- Hodges P, Kaigle HA, Holm S, et al. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine (Phila Pa 1976) 2003;28:2594–2601. doi: 10.1097/01.BRS.0000096676.14323.25. [DOI] [PubMed] [Google Scholar]
- Hollinshead WH. Anatomy for Surgeons: The Back and Limbs. 2nd edn. New York: Hoeber-Harper; 1969. [Google Scholar]
- Hu H, Meijer OG, van Dieen JH, et al. Muscle activity during the active straight leg raise (ASLR), and the effects of a pelvic belt on the ASLR and on treadmill walking. J Biomech. 2010;43:532–539. doi: 10.1016/j.jbiomech.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Huber GC. Piersol’s Human Anatomy. 9th edn. Philadelphia: J.B. Lippincott; 1930. [Google Scholar]
- Huijing PA. Muscular force transmission necessitates a multilevel integrative approach to the analysis of function of skeletal muscle. Exerc Sport Sci Rev. 2003;31:167–175. doi: 10.1097/00003677-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Hukins DW, Aspden RM, Hickey DS. Thoracolumbar fascia can increase the efficiency of the erector spinae muscles. Clin Biomech. 1990;5:30–34. doi: 10.1016/0268-0033(90)90029-6. [DOI] [PubMed] [Google Scholar]
- Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- Kalichman L, Hodges P, Li L, et al. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur Spine J. 2010;19:1136–1144. doi: 10.1007/s00586-009-1257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiter E, Karaboyun T, Tufan AC, et al. Immunohistochemical demonstration of nerve endings in iliolumbar ligament. Spine (Phila Pa 1976) 2010;35:E101–E104. doi: 10.1097/BRS.0b013e3181ae561d. [DOI] [PubMed] [Google Scholar]
- Ko JY, Wang FS. Rotator cuff lesions with shoulder stiffness: updated pathomechanisms and management. Chang Gung Med J. 2011;34:331–340. [PubMed] [Google Scholar]
- Kumar S, Narayan Y, Zedka M. An electromyographic study of unresisted trunk rotation with normal velocity among healthy subjects. Spine (Phila Pa 1976) 1996;21:1500–1512. doi: 10.1097/00007632-199607010-00003. [DOI] [PubMed] [Google Scholar]
- Kuslich SD, Ulstrom CO, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181–187. [PubMed] [Google Scholar]
- Lam KS, Mehdian H. The importance of an intact abdominal musculature mechanism in maintaining spinal sagittal balance. Case illustration in prune-belly syndrome. Spine (Phila Pa 1976) 1999;24:719–722. doi: 10.1097/00007632-199904010-00022. [DOI] [PubMed] [Google Scholar]
- Lambertz D, Hoheisel U, Mense S. Distribution of synaptic field potentials induced by TTX-resistant skin and muscle afferents in rat spinal segments L4 and L5. Neurosci Lett. 2006;409:14–18. doi: 10.1016/j.neulet.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Lancerotto L, Stecco C, Macchi V, et al. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat. 2011;33:835–842. doi: 10.1007/s00276-010-0772-8. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Fox JR, Koptiuch C, et al. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord. 2011;12:203. doi: 10.1186/1471-2474-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuzzi A, Pickar JG, Khalsa PS. Relationships between joint motion and facet joint capsule strain during cat and human lumbar spinal motions. J Manipulative Physiol Ther. 2011;34:420–431. doi: 10.1016/j.jmpt.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen V, Kankaanpaa M, Luukkonen M, et al. Lumbar paraspinal muscle function, perception of lumbar position, and postural control in disc herniation-related back pain. Spine (Phila Pa 1976) 2003;28:842–848. [PubMed] [Google Scholar]
- Loukas M, Shoja MM, Thurston T, et al. Anatomy and biomechanics of the vertebral aponeurosis part of the posterior layer of the thoracolumbar fascia. Surg Radiol Anat. 2008;30:125–129. doi: 10.1007/s00276-007-0291-4. [DOI] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N. The morphology of the lumbar erector spinae. Spine. 1987;12:658–668. doi: 10.1097/00007632-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N. The attachments of the lumbar erector spinae. Spine. 1991;16:783–792. doi: 10.1097/00007632-199107000-00017. [DOI] [PubMed] [Google Scholar]
- Macintosh JE, Valencia FP, Bogduk N, et al. The morphology of the human lumbar multifidus. Clin Biomech. 1986;1:196–204. doi: 10.1016/0268-0033(86)90146-4. [DOI] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N, Gracovetsky S. The biomechanics of the thoracolumbar fascia. Clin Biomech. 1987;2:78–83. doi: 10.1016/0268-0033(87)90132-X. [DOI] [PubMed] [Google Scholar]
- Margaria R. Positive and negative work performances and their efficiencies in human locomotion. Eur J Appl Physiol. 1968;24:339–351. doi: 10.1007/BF00699624. [DOI] [PubMed] [Google Scholar]
- Masi AT, Benjamin M, Vleeming A. Anatomical, biomechanical, and clinical perspectives on sacroiliac joints: an integrative synthesis of biodynamic mechanisms related to ankylosing spondylitis. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain. 2nd edn. Edinburgh: Churchill Livingstone Elsevier; 2007. pp. 205–227. [Google Scholar]
- Mens JM, Damen L, Snijders CJ, et al. The mechanical effect of a pelvic belt in patients with pregnancy-related pelvic pain. Clin Biomech (Bristol, Avon) 2006;21:122–127. doi: 10.1016/j.clinbiomech.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Mier A, Brophy C, Estenne M, et al. Action of abdominal muscles on rib cage in humans. J Appl Physiol. 1985;58:1438–1443. doi: 10.1152/jappl.1985.58.5.1438. [DOI] [PubMed] [Google Scholar]
- Mooney V, Pozos R, Vleeming A, et al. Exercise treatment for sacroiliac pain. Orthopedics. 2001;24:29–32. doi: 10.3928/0147-7447-20010101-14. [DOI] [PubMed] [Google Scholar]
- Morris SL, Lay B, Allison GT. Corset hypothesis rebutted – transversus abdominis does not co-contract in unison prior to rapid arm movements. Clin Biomech. 2012;27:249–254. doi: 10.1016/j.clinbiomech.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Nitz AJ, Peck D. Comparison of muscle spindle concentrations in large and small human epaxial muscles acting in parallel combinations. Am Surg. 1986;52:273–277. [PubMed] [Google Scholar]
- O’Sullivan PB, Burnett A, Floyd AN, et al. Lumbar repositioning deficit in a specific low back pain population. Spine. 2003;28:1074–1079. doi: 10.1097/01.BRS.0000061990.56113.6F. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction. Eur Spine J. 2006;15:668–676. doi: 10.1007/s00586-005-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen HE, Blunck FJ, Gardner E. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerves (sinu-vertebral nerves) J Bone Joint Surg Am. 1956;38:377–391. [PubMed] [Google Scholar]
- Pel JJ, Spoor CW, Pool-Goudzwaard AL, et al. Biomechanical analysis of reducing sacroiliac joint shear load by optimization of pelvic muscle and ligament forces. Ann Biomed Eng. 2008;36:415–424. doi: 10.1007/s10439-007-9385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. J Physiol. 2009;587(Pt 17):4139–4146. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond FJR, Bakker DA. Anatomical organization and sensory receptor content of soft tissues surrounding upper cervical vertebrae in the cat. J Neurophysiol. 1982;48:49–74. doi: 10.1152/jn.1982.48.1.49. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. Cunningham’s Textbook of Anatomy. 12th edn. Oxford: Oxford University Press; 1981. [Google Scholar]
- Sato T, Hashimoto M. Morphological analysis of the fascial lamination of the trunk. Bull Tokyo Med Dent Univ. 1984;31:21–32. [PubMed] [Google Scholar]
- Schaeffer JP. Morris’s Human Anatomy. 11th edn. New York: McGraw-Hill; 1953. [Google Scholar]
- Schleip R. Fascial plasticity – a new neurobiological explanation: Part 1. J Bodyw Mov Ther. 2003;7:11–19. [Google Scholar]
- Schleip R, Vleeming A, Lehmann-Horn F, et al. Letter to the Editor concerning ‘A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction’ (M. Panjabi) Eur Spine J. 2007;16:1733–1735. doi: 10.1007/s00586-006-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleip R, Duerselen L, Vleeming A, et al. Strain hardening of fascia: static stretching of dense fibrous connective tissues can induce a temporary stiffness increase accompanied by enhanced matrix hydration. J Bodyw Mov Ther. 2012;16:94–100. doi: 10.1016/j.jbmt.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Schuenke MD, Vleeming A, Van Hoof T, et al. A description of the lumbar interfascial triangle and its relation with the lateral raphe: anatomical constituents of load transfer through the lateral margin of the thoracolumbar fascia. J Anat. 2012 doi: 10.1111/j.1469-7580.2012.01517.x. doi: 10.1111/j.1469-7580.2012.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010;6:715–726. doi: 10.1038/nrrheum.2010.180. [DOI] [PubMed] [Google Scholar]
- Singer E. Fascia of the Human Body and Their Relations to the Organs they Envelop. Philadephia: Williams and Wilkins; 1935. [Google Scholar]
- Snijders CJ, Vleeming A, Stoeckart R. Biomechanical modeling of sacroiliac joint stability in different postures. In: Dorman TA, et al., editors. Spine: state of the art reviews. Philadelphia: Hanley & Belfus, Inc; 1995. pp. 419–432. [Google Scholar]
- Snijders CJ, Vleeming A, Stoeckart R, et al. Biomechanics of the interface between spine and pelvis in different postures. In: Vleeming A, Mooney V, Snijder CJ, et al., editors. Movement, Stability and Low Back Pain. Edinburgh: Churchill Livingstone; 1997. pp. 103–113. [Google Scholar]
- Solomonow M. Biomechanics, motor control tissue biology & stability of acute cummulative lumbar disorder. In: Vleeming A, editor. 7th Interdisciplinary World Congress on Low Back & Pelvic Pain. Los Angeles: Worldcongress LBP Foundation; 2010. pp. 14–22. [Google Scholar]
- Spalteholz W. Hand Atlas of Human Anatomy. Philadelphia: J.B. Lippincott; 1923. [Google Scholar]
- Standring S. Gray’s Anatomy, The Anatomical Basis of Clinical Practice. 40th edn. Edinburgh: Elsevier Churchill Livingston; 2008. [Google Scholar]
- Staubesand J, Baumbach KU, Li Y. La structure fine de l’aponévrose jambiére. Phlebologie. 1997;50:105–113. [Google Scholar]
- Stecco C, Gagey O, Belloni A, et al. Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie. 2007;91:38–43. doi: 10.1016/j.morpho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Stecco A, Macchi V, Masiero S, et al. Pectoral and femoral fasciae: common aspects and regional specializations. Surg Radiol Anat. 2009;31:35–42. doi: 10.1007/s00276-008-0395-5. [DOI] [PubMed] [Google Scholar]
- Stedman’s Medical Dictionary. Stedman’s Medical Dictionary. 27th edn. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- Stilwell DL. Regional variations in the innervation of deep fasciae and aponeuroses. Anat Rec. 1957;127:635–648. doi: 10.1002/ar.1091270402. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Hoheisel U, Mense S. Dorsal horn neurons having input from low back structures in rats. Pain. 2008;138:119–129. doi: 10.1016/j.pain.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Tesarz J, Hoheisel U, Wiedenhofer B, et al. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience. 2011;194:302–308. doi: 10.1016/j.neuroscience.2011.07.066. [DOI] [PubMed] [Google Scholar]
- Tesh KM, Dunn JS, Evans JH. The abdominal muscles and vertebral stability. Spine (Phila Pa 1976) 1987;12:501–508. doi: 10.1097/00007632-198706000-00014. [DOI] [PubMed] [Google Scholar]
- Testut L. Traite D’Anatomy Humaine. Paris: Octave Doin; 1899. [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Tracy MF, Gibson MJ, Szypryt EP, et al. The geometry of the muscles of the lumbar spine determined by magnetic resonance imaging. Spine (Phila Pa 1976) 1989;14:186–193. doi: 10.1097/00007632-198902000-00007. [DOI] [PubMed] [Google Scholar]
- Tveit P, Daggfeldt K, Hetland S, et al. Erector spinae lever arm length variations with changes in spinal curvature. Spine. 1994;19:199–204. doi: 10.1097/00007632-199401001-00015. [DOI] [PubMed] [Google Scholar]
- Urquhart DM, Hodges PW. Differential activity of regions of transversus abdominis during trunk rotation. Eur Spine J. 2005;14:393–400. doi: 10.1007/s00586-004-0799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart DM, Hodges PW. Clinical anatomy of the anterolateral abdominal muscles. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain. 2nd edn. Edinburgh: Churchill Livingstone Elsevier; 2007. pp. 76–84. [Google Scholar]
- Urquhart DM, Barker PJ, Hodges PW, et al. Regional morphology of the transversus abdominis and obliquus internus and externus abdominis muscles. Clin Biomech (Bristol, Avon) 2005;20:233–241. doi: 10.1016/j.clinbiomech.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Stoeckart R. The role of the pelvic gridle in coupling the spine and the legs: a clinical-anatomical perspective on pelvic stability. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain. Edinburgh: Churchill Livingstone Elsevier; 2007. pp. 114–137. [Google Scholar]
- Vleeming A, Willard FH. Forceclosure and optimal stability of the lumbopelvic region. In: Vleeming A, editor. 7th Interdisciplinary World Congress on Low Back & Pelvic Pain. Los Angeles: Worldcongress LBP Foundation; 2010. pp. 23–35. [Google Scholar]
- Vleeming A, Stoeckart R, Snijders CJ. The sacrotuberous ligament: a conceptual approach to its dynamic role in stabilizing the sacroiliac joint. Clin Biomech. 1989;4:201–203. [Google Scholar]
- Vleeming A, Buyruk HM, Stoeckart R, et al. An integrated therapy for peripartum pelvic instability: a study of the biomechanical effects of pelvic belts. Am J Obstet Gynecol. 1992;166:1243–1247. doi: 10.1016/s0002-9378(11)90615-2. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia: its function in load transfer from spine to legs. Spine. 1995;20:753–758. [PubMed] [Google Scholar]
- Wendell-Smith CP. Fascia: an illustrative problem in international terminology. Surg Radiol Anat. 1997;19:273–277. doi: 10.1007/BF01637586. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Werner K, Haussler K, et al. Thiel-fixation preserves the non-linear load-deformation characteristic of spinal motion segments, but increases their flexibility. J Mech Behav Biomed Mater. 2011;4:2133–2137. doi: 10.1016/j.jmbbm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Willard FH. The lumbosacral connection: the ligamentous structure of the low back and its relation to pain. In: Vleeming A, Mooney V, Dorman T, et al., editors. Second Interdisciplinary Congress on Low Back Pain. San Diego, CA: University of California; 1995. pp. 31–58. [Google Scholar]
- Willard FH. The muscular, ligamentous, and neural structure of the lumbosacrum and its relationship to low back pain. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability & Lumbopelvic Pain. 2nd edn. Edinburgh: Churchill Livingstone Elsevier; 2007. pp. 5–45. [Google Scholar]
- Willard FH. Somatic fascia. In: Schleip R, Findley TW, Chaitow L, Huijing PA, editors. Fascia: Tensional Network of the Human Body. New York: Elsevier; 2012. pp. 11–17. [Google Scholar]
- Willard FH, Fossum C, Standley P. The fascial system of the body. In: Chila A, editor. Foundations for Osteopathic Medicine. 3rd edn. Philadelphia: Lippincott, Williams & Wilkins; 2011. pp. 74–92. [Google Scholar]
- van Wingerden JP, Vleeming A, Buyruk HM, et al. Stabilization of the sacroiliac joint in vivo: verification of muscular contribution to force closure of the pelvis. Eur Spine J. 2004;13:199–205. doi: 10.1007/s00586-003-0575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood Jones F. Buchanan’s Manual of Anatomy. 7th edn. London: Bailliere, Tindall and Cox; 1946. [Google Scholar]
- Yahia SB, Vacher C. Does the latissimus dorsi insert on the iliac crest in man? Anatomic and ontogenic study. Surg Radiol Anat. 2011;33:751–754. doi: 10.1007/s00276-011-0812-z. [DOI] [PubMed] [Google Scholar]
- Yahia L, Rhalmi S, Newman N, et al. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63:195–197. doi: 10.3109/17453679209154822. [DOI] [PubMed] [Google Scholar]
- Yahia LH, Pigeon P, DesRosiers EA. Viscoelastic properties of the human lumbodorsal fascia. J Biomed Eng. 1993;15:425–429. doi: 10.1016/0141-5425(93)90081-9. [DOI] [PubMed] [Google Scholar]


