Abstract
This article focuses on the (functional) anatomy and biomechanics of the pelvic girdle and specifically the sacroiliac joints (SIJs). The SIJs are essential for effective load transfer between the spine and legs. The sacrum, pelvis and spine, and the connections to the arms, legs and head, are functionally interrelated through muscular, fascial and ligamentous interconnections. A historical overview is presented on pelvic and especially SIJ research, followed by a general functional anatomical overview of the pelvis. In specific sections, the development and maturation of the SIJ is discussed, and a description of the bony anatomy and sexual morphism of the pelvis and SIJ is debated. The literature on the SIJ ligaments and innervation is discussed, followed by a section on the pathology of the SIJ. Pelvic movement studies are investigated and biomechanical models for SIJ stability analyzed, including examples of insufficient versus excessive sacroiliac force closure.
Keywords: ankylosing spondylitis, pelvic girdle pain, pelvis, sacroiliac joint, sacrum, thoracolumbar fascia
Introduction
This article focuses on the anatomy and biomechanics of the pelvic girdle and, specifically, the sacroiliac joints (SIJs). The SIJs are essential for effectively transferring loads between the spine and legs.
Although topographical classifications such as ‘sacroiliac’, ‘pelvis’ and ‘spine’ serve a crucial didactic purpose, they can impede our understanding of normal and altered functional mechanisms.
Topographical anatomy helps us to understand the constituents of our body. However, no anatomical structure functions in isolation, and the mechanical load encountered anywhere in the body is distributed through a continuous network of fascia, ligaments and muscles supporting the entire skeleton.
Therefore, the sacrum, pelvis and spine, and the connections to the arms, legs and head are functionally interrelated through muscular, fascial and ligamentous interconnections. Likewise, efficient motor control does not provide a solution for individual joints, but orchestrates efficient reaction forces to integrate and stabilize the kinematics of our body. Focusing on singular anatomical structures to comprehend lumbopelvic pain, rather than considering the spine and pelvis as an integrated, interdependent and dynamic biological structure, might ‘blind’ the observer to the larger picture (Vora et al. 2010).
Functional anatomical and biomechanical models are required to analyze a puzzle as complex as low back pain (LBP) and pelvic girdle pain (PGP). Such an approach can help us understand that seemingly different structures are functionally related. In this respect, we quote Radin, who stated, “Functional analysis, be it biological, mechanical or both, of a single tissue, will fail as in all complex constructs, the interaction between the various components is a critical part of their behaviour” (Radin, 1990).
Unlike standard topographic anatomy schemata, functional anatomy should present the necessary information to comprehend the complex interrelationships between muscle, its internal fascial skeleton and the surrounding external fascial network into which it is integrated. Such an approach can be easily missed in traditional anatomical dissection, yet can be achieved by dissecting the continuity of connective tissue as an integrating matrix (Van der Wal, 2009). Insight into intra- and extra-muscular myofascial force transmission in the locomotor system may be an essential component when studying the functionality of the locomotor system (Huijing & Baan, 2003). In this overview of the SIJ, the literature will be analyzed from both a topographic and functional perspective and the relevant clinical implications presented.
Historical overview of SIJ research
Over many centuries scientists have shown an interest in the structure and function of the SIJ in relation to movement and pain. These historical studies include many important facts as well as various misconceptions. One of the most contentious issues in SIJ research has been the mobility of the joint.
From Hippocrates (460–377 BC) to Vaesalius (1514–1564) and until Pare (Vaesalius, 1543; Pare, 1634; Lynch, 1920), it was suggested that the SIJs are mobile only during pregnancy. Nonetheless, studies in the early 18th century show that SIJs are usually mobile in both in women and men (Diemerbroeck, 1689). Following Diemerbroeck's work, Albinus (1697–1770, as cited in Lynch, 1920) observed that the SIJ has a synovial membrane, confirming its mobility, and Zaglas (as cited in Weisl, 1955), in the mid-19th century, demonstrated that most of the sacral movement takes place around a transverse axis, situated at the level of the second sacral vertebra. Iliac rotation relative to the sacrum (i.e. rotation occurring mainly around a transverse axis) was named ‘nutation’ (forward nodding) and ‘counternutation’ (backward nodding). Other studies followed, and Duncan (1854) concluded that the generalized pivot of the SIJ must be localized at the level of the iliac tuberosity. This tuberosity is a bony structure located dorsal to the auricular part of the SIJ (Fig. 1A,B; Duncan, 1854). Indeed, its location was confirmed by additional investigations by Meyer (1878). Von Luschka (1864) described the joint as a real diarthrosis, i.e. a mobile joint with a joint cavity between two bony surfaces. Using a specific staining technique, Albee (1909) validated the synovial nature of the SIJ, thereby confirming that the joint is mobile to some extent. The belief that the SIJ is a true diathrosis was strengthened by Sashin (1930) after investigating 257 young adult specimens. Meanwhile, Walcher (1889), Forthergill (1896), Pinzani (1899) and Jarcho (1929) used various methodologies with living subjects and embalmed corpses to determine the pelvic conjugata vera (anterior–posterior diameter) and the conjugata diagonalis (oblique diameter). These authors determined that the conjugata vera becomes smaller (1–1.3 cm) when movement takes place from a supine position, into maximal hip and trunk extension, and back to a normal supine position. Lessening the conjugata vera is a result of nutation and leads to enlarging the caudal pelvic aperture (i.e. a small pelvic inlet implies a large pelvic outlet). Similarly, Von Schubert (1929) described a SIJ movement study, based on X-ray analysis of the conjugata vera. Also this study shows a reduction of the conjugate vera of 0.5–0.7 cm, when changing from a supine position to standing upright.
Fig. 1.
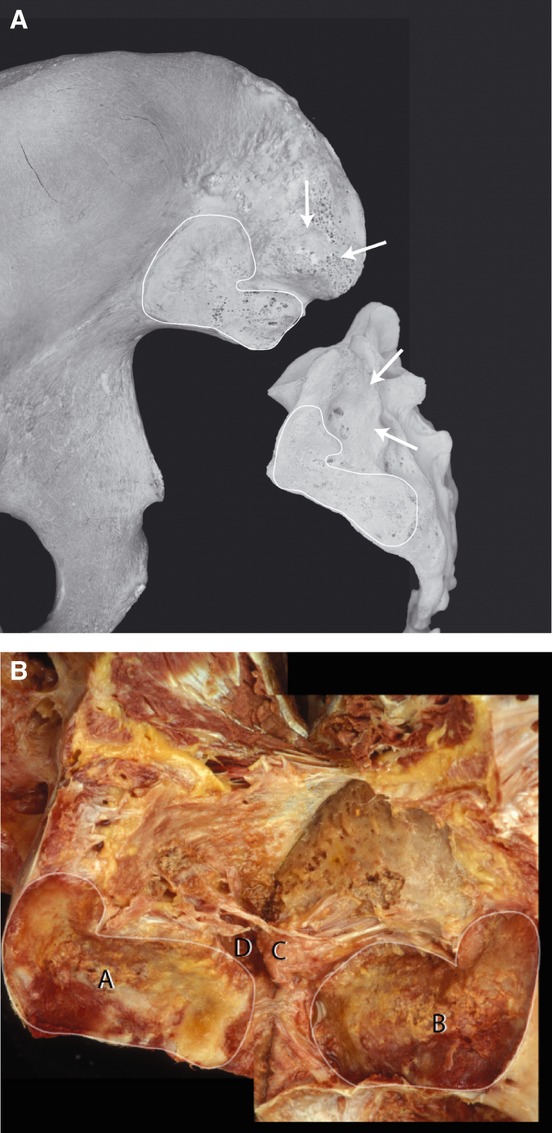
(A) Unpaired sacrum and ilium showing the interindividual variation of the auricular part of the sacrum and ilium (articulated line). On the iliac side a more C-like form of the auricular SIJ is visible. Contrary, on the sacrum a L-form is present. The arrows indicate the position of the sacral concavity and the iliac tuberosity located dorsal to the auricular part of the SIJ (axial joint). (With permission from the Willard Carreiro collection.) (B) Paired sacrum and ilium. The SIJ is folded open posteriorly by dissecting all major ligaments. Notice (A) the concavity of the sacral auricular part, and (B) the corresponding auricular iliac part. (C) The iliac tuberosity of the axial joint and (D) the sacral concavity of the axial joint covered with rough cartilage. Notice parts of the interosseous ligaments (arrows).
Given evidence of a small amount of SIJ movement, Kopsch (1940) suggested that the SIJ is an intermediate joint between a synarthrosis and a diathrosis, and Gray proposed the term ‘amphiarthrosis’, thus implying the SIJ permits only minimal movement (Gray, 1938). In 1949, Testut & Latarjet further modified the description by concluding that the SIJ actually contains a freely mobile ventral aspect and an ossified dorsal aspect. They dubbed the SIJ a ‘diarthro-amphiarthrosis’, i.e. a joint that has the characteristics of both a freely mobile joint (diarthrosis) and an ossified joint (synarthrosis).
In light of the well-developed ligaments and the irregular form of the articular surfaces, it was concluded that movement is not (or is hardly) possible, except in the case of pregnancy (Solonen, 1957). Chamberlain (1930) reported that the considerable intra-pelvic movement occurring at childbirth may lead to SIJ lesions. These lesions are supposedly caused by increased laxity of the SIJ ligaments, due to hormonal changes, and may also occur during menstruation. In the context of movement restriction, Gray (1938) proposed that pain may be caused by minor movements that result in collisions between the complementary ridges and grooves of the SIJ.
Throughout the 18th, 19th and early 20th centuries, older men and women were thought to have decreased SIJ mobility due to ankylosis, as reported by Solonen (1957). However, this concept became questionable when Smith & Jones initiated archeological anatomical research on several hundreds of skeletons from the site of the first Egyptian Aswan Dam, and only nine of them showed signs of SIJ ankylosis (Smith & Jones, 1910).
In the early 20th century, the SIJ was a major focus of research on LBP and PGP. At that time, mortality during pregnancy and labor was not exceptional, post mortem studies showed increased mobility of the SIJ, and an increased amount of synovial SIJ and symphyseal fluid in pregnant women (Brooke, 1924). During this era, before the hazards of roentgen screening of pregnant women were fully realized, increased mobility and widening of the symphysis in relation to PGP were even well documented using X-ray analysis (Abramson et al. 1934). Abramson et al. described the changes that occur in the SIJ of pregnant women before and after childbirth. Notably, among their various techniques, the researchers use radiographic images to analyze pelvic mobility in women who were 8 months pregnant. The authors made a distinction between symptoms related to the pubic joint pain vs. those derived from the SIJ, or a combination of both structures. In their schema, symptoms related to the SIJ presented as most caudal (pain predominantly over the pelvic area), and were associated with localized pain and tenderness in the SIJ region. Furthermore, they noted a waddling gait and a positive Trendelenburg sign (inability to stabilize the pelvis in the horizontal plane), which were associated with SIJ dysfunction.
In a landmark study, Mixter & Barr (1934) demonstrated that ischialgia is the result of rupturing the intervertebral disc. The fact that bulging discs could lead to nerve entrapment and radiating pain resulted in a major shift in the attention of the medical world away from the SIJ as the primary cause of ischialgia. Additionally, after surveying 6895 patients with LBP, Solonen reported that only 2–4% of them have real SIJ pathology (Solonen, 1957). Interestingly, Solonen did not discuss possible kinematic relationships between SIJ pathology and disc problems. Solonen reports that in view of the strongly developed SIJ ligaments and the supposed irregular form of the articular surfaces, joint mobility is not possible, or at best only minimal, except in case of pregnancy.
Historically, the period of the 1950s in which Solonen finalized his thesis on the SIJ, is significant for spine research. In the preceding decades, spine research and treatment had focused on the role of the SIJ as a source of LBP. From the mid-1930s following the study of Mixter & Barr, until approximately the 1980s, abberent SIJ movement fell out of favor as a scientific explanation for lumbopelvic pain, and the SIJs were mainly considered as immobile joints. This view was partially initiated by the unsubstantiated opinion of Ghormley, who declared that the SIJs were immobile and LBP could not result from SIJ pathology (Ghormley, 1944). Nonetheless, based on a general investigation of joints, Gardner (1950) concluded that movement is necessary for the development and self-maintenance of the SIJ. Solonen stated that underestimation of the significance of SIJ lesions, due to traumatic or structural causes, has gone too far in the decades before his study. Solonen argues that the SIJ embodies all the usual elements of an articulation, and should be considered as a source of subjective symptoms and objective signs.
Due to the discrepancies outlined above, it is apparent that one must exercise caution when reviewing historical research on the SIJ. For example, Weisl (1955) described the SIJ as consisting of two condyles, forming what may schematically be regarded as a sellar (or saddle) joint. Reliance on the model of Weisl initially influenced Solonen's research (Solonen, 1957). Solonen described the articular surface of the SIJ as ‘nodular and pitted’. Despite earlier reports (Brooke, 1924; Schuncke, 1938) that had shown congruence to be rare in the SIJ, Solonen regarded his findings to be pathological, as he tried to understand SIJ function by envisioning the sacrum as a simple wedge between the iliac bones (e.g. the Weisl model).
Anatomy and development of the SIJ complex and associated pelvic structures
The SIJs are highly specialized joints that permit stable (yet flexible) support to the upper body. In bipeds, the pelvis serves as a basic platform with three large levers acting on it (the spine and two legs). Both the tightness of the well-developed fibrous apparatus and the specific architecture of the SIJ result in limited mobility. Sacral movement involves the SIJ, and also directly influences the discs and most likely the higher lumbar joints as well. For example, forward and backward tilting of the sacrum between the iliac bones affects the joints between L5–S1, as well as most likely influencing joints at the higher spinal levels (Vleeming & Stoeckart, 2007).
Besides small internal pelvic motions of the SIJ and symphysis, substantial motion of the external pelvic platform takes place. Movement of the pelvic platform upon the hip joints relative to the femur, such as flexion and extension (pelvic ante- and retroversion), and rotation and abduction/adduction, strongly influences lumbar and spine movement. Coupled pelvic and hip, flexion and extension have a key role in establishing lordosis and kyphosis in the lower spine (Vleeming & Stoeckart, 2007). Because fascial, ligamentous and muscular tissue span (non)-adjacent vertebrae and sacrum, movement between sacrum and adjacent vertebrae, or movement resulting in external pelvic (tilt) motion, can affect each other. Considerable forces are exerted in the area of the caudal intervertebral discs. The ventrally directed angle between L5 and the sacrum tends to become more acute when loaded as the sacrum will undergo enhanced nutation. Accordingly, the thick anterior longitudinal ligament spans the ventral aspect of L5 and S1, buttressing against excessive extension (Vleeming & Stoeckart, 2007).
It has been postulated that the SIJs act as important stress relievers in the ‘force–motion’ relationships between the trunk and lower limb. These joints ensure that the pelvic girdle is not a solid ring of bone that could easily fracture under the great forces to which it might be subject, either from trauma or its many bipedal functions (Lovejoy, 1988). Analysis of gait mechanics demonstrates that the SIJs provide sufficient flexibility for the intra-pelvic forces to be transferred effectively to and from the lumbar spine and lower extremities (Lee & Vleeming, 2007). More recently, finite element modeling estimates that a leg-length discrepancy as small as 1 cm increases the load across the SIJ fivefold (Kiapour et al. 2012).
To allow bipedal gait in humans, evolutionary adaptations of the pelvis have been necessary. For example, the ilia flare outward in the sagittal plane to provide a more optimal lateral attachment site for the gluteus medius (an important muscle for hip pelvic stability). Also, a dramatically increased attachment site for the gluteus maximus muscle has changed this muscle – a relatively minor muscle in the chimpanzee – into one of the largest muscles of the human body. Thus, the bipedal human pelvis has evolved quite differently to that of the quadrapedal chimpanzee (Lovejoy, 1988, 2007).
Additional evolutionary changes in humans are the muscular and ligamentous connections between the sacrum and ilia. Examples include: (i) muscles such as the lower lumbar multifidi, that insert into the sacrum and also into the medial cranial aspects of the ilium; (ii) changes in the position of the coccygeus and the piriformis muscles, and of the gluteus maximus muscle such that they originate in part from the sacrum and sacrotuberous ligaments (STLs); (iii) extensive fibrous connections adapted to the typical anatomy of the SIJ, such as the interosseous ligaments, surrounding an iliac protrusion that inserts into a dorsal sacral cavity, just behind the auricular surfaces of the SIJ; (iv) ventral and dorsal SIJ ligaments, STLs and sacrospinous ligaments (SSLs) between sacrum and lumbar spine (anterior longitudinal ligaments); (v) direct fibrous connections like the iliolumbar ligaments (ILs) exist between the iliac bone and L4 and L5 (Lovejoy, 1988, 2007; Vleeming & Stoeckart, 2007).
SIJ development and maturation
At about week 8 of intra-uterine development, a three-layered structure develops in the pelvic mesenchyme, the layers are: first, sacral cartilage; second, iliac cartilage; and third, the interposed zone of mesenchyme, containing a slit, which forms the early articular cavity. The SIJ will develop from this structure (Schuncke, 1938). In week 10, cavities emerge centrally as well as peripherally, whereas in other diarthroses only a central cavity is formed. The minor movements of a joint that occur in utero are reported to influence the formation of the central cavity in the SIJ (Gardner, 1950), as neonatal paralysis of the lower body coincides with anomalies in both the sacrum and the SIJ, as discussed by Brochner (1962).
Fibrous septa protrude into the cavity of the joint, both from its sacral and iliac sides, the latter ones gradually developing into a delicate transverse ridge on the auricular aspect of the ilium. Essentially, this ridge divides the cartilage into cranial and caudal parts. On the auricular cartilage of the sacrum, the septa remain separate. It is suggested that these septa usually disappear during the first postnatal year, remaining present only in exceptional cases (Schuncke, 1938; Drachman & Sokoloff, 1966). Schuncke (1938) report-ed that the SIJ can be recognized as a typical joint from the second month in utero onwards, and that the development of the joint cavity is completed by months 7–8. Examination of 200 preparations revealed that the bony surfaces of the joint are smooth until puberty. At a later age, different combinations of bony ridges and grooves occur. The most frequent location of the ridges appears to be on the ilium. Schuncke (1938) did not classify these bony irregularities as ‘arthrosis’.
Normally, the SIJ cavity is near full development in the eighth month. At that time, the general contour of the joint can clearly be recognized, and the joint has acquired the potential to move (Bowen & Cassidy, 1981). Shortly before birth the synovial membrane of the SIJ develops out of the mesenchyme surrounding the edge of the primordial central cavity. A similar late development also takes place in the temporo-mandibular joint (Moffett, 1957), another joint that hardly moves before birth. The sacrum as a fused entity does not exist during the intra-uterine period. The coalescence of the five separate vertebrae starts after birth, not to be finished until the age of 25–30 years (Tondury, 1970).
During intra-uterine development, conspicuous differences can be seen between the auricular cartilage of the ilium and that of the sacrum. The sacral cartilage is glossy and white, whereas the iliac cartilage is dull and striped (partly due to irregularities in the underlying bone tissue). These features, especially at the iliac side, were initially misinterpreted as degenerative arthrosis, as shown by Sashin (1930) and Bowen & Cassidy (1981). Sacral cartilage is two–three times thicker than iliac cartilage (Sashin, 1930; Bowen & Cassidy, 1981). Microscopically, iliac cartilage is usually characterized as fibrocartilage (Bowen & Cassidy, 1981), and sacral cartilage as hyaline cartilage. However, histological and biochemical analysis appeared to contradict this distinction. Paquin et al. (1983) concluded that iliac cartilage represents a special form of hyaline cartilage. Furthermore, Kampen & Tillmann (1998) reported that the iliac joint surface is ‘fibrocartilaginous’ only in early childhood, becoming more hyaline with maturation.
The iliac auricular cartilage is rougher compared with the sacral cartilage, and this roughened pattern is already present before birth (Bowen & Cassidy, 1981). Although the sacral surface will also start to roughen, it will continue to lag behind development of the ilium in this respect (Brooke, 1924; Sashin, 1930; Schuncke, 1938; Dar & Hershkovitz, 2006). In the adult, the cartilage on the sacral surface of the joint can reach 4 mm in thickness, but does not exceed 1–2 mm on the iliac surface (Bowen & Cassidy, 1981; Kampen & Tillmann, 1998); however, the iliac cartilage has a greater cell density (McLauchlan & Gardner, 2002). The subchondral plate supporting the iliac articular cartilage is thicker than on the opposing sacral aspect (Kampen & Tillmann, 1998). The plate is most dense towards the cranial and caudal ends of the joint, and least dense near the center of the auricular surfaces (Putz & Muller-Gerbl, 1992). The underlying cancellous bone is also denser on the iliac side (McLauchlan & Gardner, 2002).
It has been suggested that clefts in the thin iliac cartilage allow for easier penetration by osteophytes (Resnick et al. 1975). Possible support for this contention can be found in the observation that the initial lesions of ankylosing spondylitis (AS) tend to occur earlier on the iliac side (Dihlmann, 1962; Brower, 1989; Muche et al. 2003), which might be more susceptible to exaggerated compressive stresses than the sacral side with its thicker cartilage.
In the first decade the joint capsule has two layers. The outer, fibrous layer consists of firm connective tissue containing many fibroblasts, blood vessels and collagenous fibers. The inner synovial membrane, a so-called ‘intima’, has two–three cellular layers. Synovial villi may reach deep into the joint. Immediately after birth, the general orientation of the human SIJ is very similar to that of quadrupeds. The articular surfaces have the same orientation as the zygapophyseal joints of the lumbar vertebrae. Change begins as soon as the child starts to locomote (Solonen, 1957). The sacrum enlarges laterally, and the articular surfaces modify to a more complex adult curvature, resulting in the surfaces profiles of the joint bearing a resemblance to a propeller-like shape (Solonen, 1957). Comparative anatomy and paleontological research indicate that these changes are brought about by mechanical factors, such as the supine position, body weight, load on the femur, and strain on the pubic symphysis (Solonen, 1957). It has been suggested that the most important process in the development of the SIJ is the torsion between the ilia and the sacrum (Solonen, 1957). Evidence for the mobility of the joint can be demonstrated in manual examination of specimens taken from the first decade of life (Brooke, 1924; Sashin, 1930; Schuncke, 1938; Bowen & Cassidy, 1981).
Although in general pelvic gender differences become recognizable as early as the fourth month in utero (Schuncke, 1938), SIJ gender dimorphisms do not emerge until puberty. Male SIJ development seems to be a functional adaptation in order to cope with major forces. According to Schuncke (1938), this results in a thickening of the ligaments and decreased mobility. Initially, female SIJ development also reveals a restriction of mobility (at about 14 years); however, it begins to increase again in the latter part of the second decade (Brooke, 1924). It should be noted that this finding was the result of studying intra-pelvic movement post mortem. Due to sampling problems inherent to the mobility investigation, the data can only be used as a general guide.
Finally, recent research reveals that the pelvis does not stop expanding after skeletal maturation and cessation of longitudinal growth. There is a strong correlation between increasing age and the width of both the L4 vertebra and the pelvis, after the second decade. The bony pelvis widens more than 20 mm over the course of a lifetime (Berger et al. 2011).
Bony anatomy of the pelvis and SIJ
Typically, the SIJ is formed within sacral segments S1, S2 and S3, although inclusion of the complete S3 segment in the SIJ is not common for females (Vleeming & Stoeckart, 2007). In general, fusion of the sacral vertebra begins early in the second decade (Scheuer & Black, 2000). The bony anatomy is highly variable in size, shape and contour among individuals (Schuncke, 1938), and the shape of the joint changes markedly from infancy to adulthood (Bowen & Cassidy, 1981). The sacral auricular part is generally concave; however, often an intra-articular bony tubercle is present ventrally, in the middle aspect of the auricular surface of the sacrum. The iliac part is predominantly convex. Large variations of the auricular surfaces exist (Figs 1 and 2).
Fig. 2.
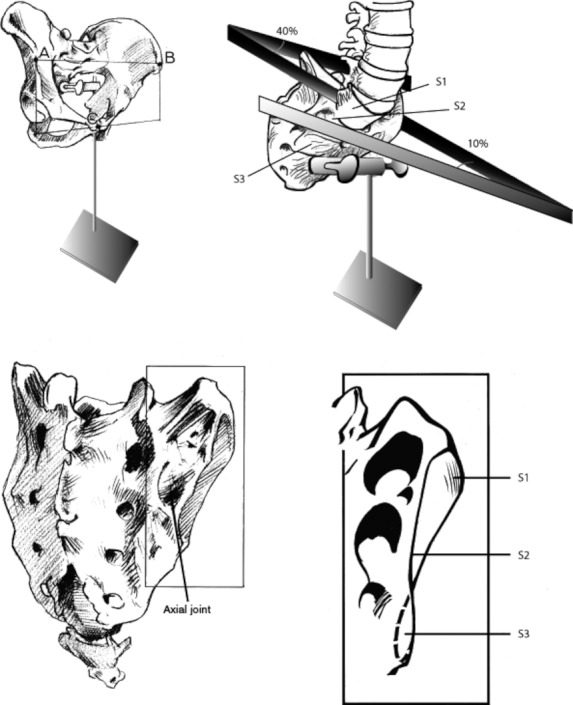
(Top left) Pelvis in erect posture. View of the pelvis from the ventrolateral side. (Bottom left) Dorsolateral view of the sacrum. The position of the axial joint is indicated, made up from the smaller cavity of the sacrum, corresponding with a generally larger iliac tubercle. (Top right) Showing the different angles of S1-S3 between left and right sacral articular surface. (Bottom right) Sacral articular surface at the right side. The different angles reflect the propeller-like shape of an adult SIJ. (With permission from Vleeming collection.)
Assimilation (sacralization) of the fifth lumbar vertebrae into the body of the sacrum occurs in 6% of American adults, based on the Hamann-Todd and Terry skeletal collections (Tague, 2009). The L5 and S1 vertebra can fuse at one or more locations, such as between transverse processes, vertebral bodies or facet joints. Females with sacralization have a narrower sacral angulation compared with males, as well as shorter posterior sagittal diameter of the pelvic outlet and a narrower sacrum compared with males.
Fusion of the sacrum to the coccyx can occur after birth (Tague, 2011). Results show that the sexes do not differ markedly in prevalence of sacro-coccygeal fusion up to the fourth decade (24% in females, 30% in males). The range of prevalence in the age group 50–79 years in women is about 44% and for males is 52%. In more than half of women with coccyx fusion, a contracted posterior sagittal diameter is present. The combination of conjoined anatomies, such as a shorter posterior sagittal outlet diameter and a narrow sub-pubic arch, may obstruct childbirth (Tague, 2011).
As previously inferred, in the adult, the SIJ has an auricular or C-shaped, L-shaped configuration (Fig. 1). The SIJ has a short cranial and longer caudal limb. The lower portion of the cranial limb and the caudal limb are synovial in construction, whereas the upper part of the cranial limb is more fibrous (Cole et al. 1996). The SIJs lie obliquely at an angle to the sagittal plane (Solonen, 1957; Bowen & Cassidy, 1981; Vleeming et al. 1990a). In the standing position, the S1 part of the joint lies mainly vertical, and its surface runs obliquely and sagittally from craniolateral to slightly caudomedial (Dijkstra et al. 1989).
The surface of the SIJ can be divided into three parts, roughly corresponding to the three sacral elements (S1, S2, S3) that participate in the (sacral) auricular surface. These three parts, of which the S1 part is the largest and the S3 part the smallest, are generally designated as the cranial, middle and caudal, respectively. However, in the erect posture terms like ventral, middle and dorsal would be more appropriate as, in this position, the sacrum is tilted forwards. The mean angle of the auricular surfaces of 10 sacra of older specimen (Dijkstra et al. 1989) is 40 ° at S1, 25 ° at S2 and −10 ° at S3 (Solonen, 1957; Vleeming et al. 1992b). This implicates that, after puberty, the SIJ gets a sinusoidal or propeller-shaped form, with the dorsal portion of the joint S3 predominantly oriented in the sagittal plane (Fig. 3; Dijkstra et al. 1989).
Fig. 3.
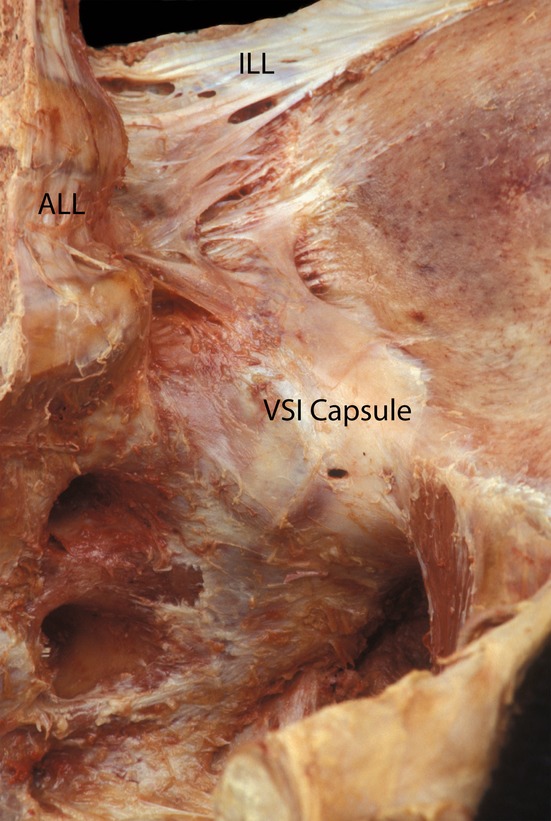
Ventral view of the thin anterior capsule of the SIJ (VSI). The iliolumbar ligaments are clearly visible (ILL), also the anterior long spinal ligament (ALL). (With permission from the Willard Carreiro collection.)
The interdigitating symmetrical grooves and ridges (Bowen & Cassidy, 1981; Vleeming et al. 1990a,b) of the SIJ articular surfaces contribute to the highest coefficient of friction of any diarthrodial joint. This property enhances the stability of the joint against shearing (Vleeming et al. 1990a, 1992b). The ‘keystone-like’ bony anatomy of the sacrum further contributes to stability within the pelvic ring. At its base, the sacrum is wider superiorly than inferiorly, it is also wider anteriorly than posteriorly, permitting the sacrum to become ‘wedged’ cranially and dorsally into the ilia within the pelvic ring (Vleeming et al. 1990a). This anatomical structure of the sacrum in humans is adapted to resist shearing from vertical compression (e.g. gravity) and anteriorly directed forces on the spine (Abitbol, 1987b; Lovejoy, 1988, 2007; Aiello & Dean, 1990).
Sexual dimorphism of the pelvis
Sexual dimorphism is evident in the pelvis. The gender-related divergence of the pelvic dimensions are especially prominent at about the 22nd month, with the male pelvis being larger. This distinction decreases in the later years of childhood. Overall pelvic dimensions such as inter-cristal measurement are greater in males. In males, the articular facet on the sacral base for the fifth lumbar vertebra occupies more than a third of the width of the sacral base, but less than a third in the female, whose sacrum is relatively wider.
The female sacrum is more uneven, less curved and more backward tilted than the male sacrum. The male pelvis is relatively long and narrow, and the iliac crest reaches higher. Generally, the pelvic cavity is longer and more conical in males, whereas the female pelvic cavity is shorter and more cylindrical (Gray, 1973).
The internal aspect of the joint can have several sexually dimorphic topographic features. Brooke showed that in 88% of 55 male preparations of diverse ages, an intra-articular bony tubercle is present ventrally in the middle aspect of the auricular surface of the sacrum. This small tubercle, covered with cartilage, is present as early as 14 years old. In 15% of 95 female preparations, only a rather small tubercle could be shown. In females, a second tubercle directly dorsal to the first may be present. If no tubercle is present, the whole auricular surface of the sacrum is concave. Women in the second decade also develop a groove in the iliac bone, the paraglenoidal sulcus, which is usually absent in men. This small but conspicuous groove can be found ventrocaudally to the iliac articular surface. Often, a pronounced bony edge is present at the ventral end of the groove. Part of the anterior joint capsule is connected to this edge (Brooke, 1924). Gender-related differences in SIJ development can lead to a higher rate of SIJ misalignment in young females. Stoev et al. (2012) report that of patients aged 10–20 years (median 15.7 years) who presented with LBP resulting from SIJ misalignment, 77% were female.
Limited data indicate that SIJ surface area is somewhat greater in adult males than females (Ebraheim & Biyani, 2003), and this presumably reflects increased biomechanical loading in males. In females, the average auricular surface area is reported to range from 10.7 to 14.2 cm2 (Miller et al. 1987; Ebraheim & Biyani, 2003) or up to 18 cm2 (Sashin, 1930) compared with a ligamentous area of 22.3 cm2 in males (Miller et al. 1987). Moreover, Fischer et al. (1976) and Bakland & Hansen (1984) demonstrated significant intra-individual variation (left vs. right) in SIJ size as well as considerable interindividual SIJ size variability. Lumbar isometric strength is also almost twice as great in males as in females (Graves et al. 1990). Thus, greater load transfers are required through the SIJs of males, an observation that is consistent with the threefold greater occurrence of AS in males (Masi, 1992; Masi & Walsh, 2003).
Braune & Fischer (1892) related the position of the center of gravity in the trunk to SIJ function and gender. They assumed that a change in the localization of the center of gravity is related to altered SIJ function. The larger the distance between the SIJs and the vertical line through the center of gravity, the less stable the joint, as rotational torque increases as a function of the lever arm.
From a functional point of view, it is realistic to assume that the large distance between the assumed rotational pivot of the SIJ and the vertical line through the center of gravity is a major influence on the development of specific SIJ form. Several authors have indicated the existence of a gender difference in position of the center of gravity. In women, a vertical line through the center of gravity is supposed to pass directly in front of or through the SIJ, whereas in men its position is more ventral (Braune & Fischer, 1892; Tischauer et al. 1973; Bellamy et al. 1983). This would imply a greater lever arm in men than in women, resulting in higher loads on the joints. As a result, the male SIJ would become stronger, with restricted mobility, if indeed the surmised difference in the localization of the center of gravity exists. More research is needed before this can be decided as, at the moment, the whole discussion is based on empirical estimates. Apart from the above-outlined differences between men and women, the load-carrying surface of the female SIJ is usually smaller, and the position of the sacrum is usually more horizontal (Derry, 1912; Brooke, 1924; Sashin, 1930; Schuncke, 1938; Solonen, 1957).
In both sexes, SIJ mobility decreases from birth to puberty but then, according to Brooke, increases transiently in adult females to a peak at about 25 years old (Brooke, 1924), while in males, joint mobility remains low, especially in middle- and old-aged men. More recent studies partially contradict the age-related findings on decreasing SIJ mobility. Pelvic motion of males and females was investigated by roentgen stereophotogrammetric motion analysis (RSA). RSA is a technique for measuring small movements, and is regarded as the gold standard for determining mobility in orthopedics (Kibsgård et al. 2012). In several studies, Sturesson et al. (1989, 1999, 2000a,b) applied this technique to measure the mean SIJ mobility around the sagittal axis in patients with PGP. As expected, the average mobility for men is about 40% less than for women. However, with age, there was no detectable decrease in total mobility in either gender in patients (up to 50 years old). In fact, there was a significant increase of mobility with age for both ‘supine to sitting’ and ‘standing to prone with hyperextension’ tests in both sexes. It should be noted that the latter studies analyzed mainly patients under the age of 50 years, possibly influencing the results.
Gender differences of symphyseal motion were analyzed in a group of 45 asymptomatic individuals. In this study, the Chamberlain ‘standing on one leg’ method was used to examine both men and women (Chamberlain, 1930). In men, the average movement was 1.4 mm, and in nulliparous women 1.6 mm. However, in multiparous women, motion increases to 3.1 mm (Garras et al. 2008). The increased SIJ mobility in females over males has possible anatomical correlates. The curvature of the SIJ surfaces is usually less pronounced in women to allow for higher mobility. Also, the pubic angle differs between men and women. An average angle of 50–82 ° is typical for males, compared with an average of 90 ° for women (Bertino, 2000). The increase in mobility of the pelvic ring seen in the post-pubescent female pelvis is functional in allowing passage for the child during labor.
During pregnancy, the SIJ fibrous apparatus loosens under the influence of relaxin and relative symphysiolysis seems to occur, both factors resulting in an increase in SIJ mobility. Increased mobility may also lead to complaints of pelvic pain (Bonnaire & Bue, 1899; Brooke, 1924; Hisaw, 1925; Von Schubert, 1929; Chamberlain, 1930; Borell & Fernstrom, 1957). The latter studies used various research methods, among them post mortem movement analysis and X-ray studies. Detailed information about the methodology for movement analysis in these studies is often lacking. Separation of the joint surfaces was measured manually after applying traction to the pelvis or by analyzing changes in the conjugate vera, measuring increased SIJ nutation.
A more recent study by Damen et al. (2001) describes the relation between female SIJ asymmetric laxity and the severity of complaints. Women with asymmetric laxity of the SIJ during pregnancy have a threefold higher risk of developing moderate to severe PGP, persisting into the post partum period, compared with women with symmetric laxity during pregnancy. A European guideline has concluded that PGP is a specific form of LBP (Vleeming et al. 2008). Generally, comparing sex differences, it seems obvious that women run the biggest risk in developing PGP. This form of pain generally arises in relation to pregnancy, trauma, arthritis and/or osteoarthritis. The point prevalence of pregnant women suffering from PGP is about 20%. Risk factors for developing PGP during pregnancy are most probably a history of previous LBP, and previous trauma to the pelvis (Vleeming et al. 2008).
Ligaments supporting the SIJ
The SIJ capsule closely follows its articular margins. In addition, the associated core ligaments are numerous and strong (Palastanga et al. 1998), including the ventral, dorsal and interosseous ligaments (Soames, 1995). Short and long dorsal sacroiliac ligaments complement the interosseous ligaments. The long dorsal ligament (LDL), which originates from the posterior superior iliac spine (PSIS), is the most superficially and dorsally located of the SIJ ligaments (Vleeming et al. 1996). The STLs, SSLs and ILs are strong accessory SIJ ligaments (Palastanga et al. 1998). Furthermore, the ILs are connected to both the dorsal and ventral sacroiliac transverse ligaments (Pool-Goudzwaard et al. 2001). These ligaments play important roles in increasing joint stability via force closure (discussed later).
The ventral part of the joint
The superior aspect of the joint capsule appears to be a caudalward extension of the IL, specifically the lumbosacral band of the ligament. The anterior aspect of this capsule (also termed the anterior sacroiliac ligament, ASL) is composed of a smooth sheet of dense connective tissue stretched between the ventral surface of the sacral alar and that of the ilium (Fig. 4). The caudal border of the ventral sacroiliac capsule blends with the cranial edge of the SSL. In the area between the psoas major and the cranial insertion of the obturator internus, the ventral SIJ ligament relates closely to the lumbosacral trunk (fibers from L4–L5) and the nerve bundle of the obturator nerve. The psoas major is immediately anterior to the SIJ, and major blood vessels (e.g. iliac artery and vein) can be found nearby.
Fig. 4.
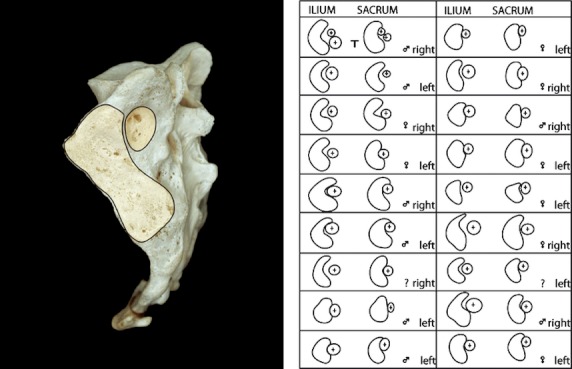
Differences in the geometry of the auricular and axial areas. Corresponding numbers depict both the right and the left SIJ. Note the large intra- and interindividual SIJ differences (reprinted with permission from Bakland & Hansen, 1984).
The ventral SIJ capsule is relatively thin and frequently has defects that allow fluid substances in the joint space to leak out onto surrounding structures. In 61% of 76 joints examined by injection and imaging, leakage of injected contrast was reported (Fortin et al. 1999a). Notably, contrast leaked into the ventral region in close juxtaposition with the lumbosacral plexus and into the dorsal sacral foramina, where it could be in contact with the dorsal sacral plexus (Fortin et al. 1999b). These findings may help explain the results of Indahl et al. (1999) who, using pigs, revealed that stimulation with bipolar wire electrodes in the ventral SIJ capsule initiated a muscular response of the gluteus maximus and the quadratus lumborum muscles. The close association of the dorsal sacral rami to the interosseous ligaments (Willard et al. 1998; McGrath & Zhang, 2005) may explain why stimulation directly dorsal of the SIJ capsule provoked a response in the deep medial multifidus fascicles lateral to the L5 spinous process.
Interosseous ligaments and accessory SIJs
Accessory SIJs are described as extracapsular fibrocartilaginous articulations for biomechanical enhancement. Trotter examined 958 human skeletons, and concluded that in these skeletons one or more accessory SIJs were present (Trotter, 1964). About 50% of these ‘accessory joints’ are found bilaterally, at the level of the second sacral foramen. It remains unclear what exactly is meant by ‘accessory’, and, per exclusionem, what are the anatomic features used to name these structures ‘joints’. In that study, no information is given as to whether these accessory joints are covered with cartilage.
Although many investigators reported extra-articular SIJs, Bakland & Hansen (1984) were the first to offer a detailed description. They describe the main accessory SIJ as the interosseous part of the SIJ. It is surrounded by the prominent interosseous ligaments, which lie dorsal to the main auricular-shaped surface of the synovial SIJ. The articulation was named the ‘axial part of the SIJ’, because it is the supposed location of the SIJ axis for tilting movements (nutation and counternutation). The axial joint has a larger convex iliac tuberosity on its iliac side compared with the smaller concavity on its sacral side, with very little congruence. In most of the preparations, the convexity of the llium is too large for the concavity of the sacrum, but congruity is most likely improved somewhat by an iliac plate of fibrocartilage (Figs 3; Bakland & Hansen, 1984). Using a system of coordinates, Bakland & Hansen classified intra- and interindividual differences (Fig. 2). Because they observed the presence of cartilage, they concluded that movement can occur in the axial joint as well. However, if the axis for SIJ tilting is located in the axial joint, the presence of cartilage could likewise be explained as diminishing friction at this location.
The vast interosseous sacroiliac ligament (ISL) is the strongest of the SIJ-supporting ligaments, and encloses the axial joint as well as filling the spaces dorsal and cephalad to the synovial portion of the joint; it provides for major multidirectional structural stability. It also has the most extensive bony origin and overall volume of all SIJ ligaments, regardless of gender (Steinke et al. 2010). The ISL is larger in females than in males, whereas the ASL and posterior sacroiliac ligaments (PSL) are larger in males (Steinke et al. 2010). The PSL imposes the most influence on SIJ mobility, whereas the ASL has very little effect on mobility of the SIJ (Vrahas et al. 1995). The axial joint can become partly or completely obliterated, and this has been classified as either amphiarthroses (Gerlach & Lierse, 1992), symphyses (Puhakka et al. 2004) or syndesmoses (Soames, 1995).
Ehara et al. (1988), using computed tomography (CT) scanning, observed the axial joint in no more than 13 of 100 test persons. They reported that the interosseous joint may be present at birth, just as a true diarthrosis, but that it can also appear postnatally, in which case the joint is purported to be fibrocartilaginous. However, the interosseous SIJ area is especially complicated to visualize with CT (Dijkstra et al. 1989). It is difficult to assess the ‘axial’ joint, due to the irregular bony contours, the extensive fibrous apparatus and the large interindividual variations. Between the ilium and sacrum lies a funnel-shaped complex of the interosseous ligaments, the apex of which is connected to the sacrum. The rough cartilage from the iliac side of the ‘axial’ joint is localized in the middle of the funnel. Because of the difficulty of precisely establishing the border between fibrous apparatus and cartilage, confirmation of the anatomical coordinates of Bakland & Hansen is not entirely possible. Nonetheless, their morphological data were generally validated (Vleeming, 1990). Although the axial joint requires additional research, the axis of SIJ rotation in current kinematic studies seems to be located near or within the boundaries of the interosseous ligament (Egund et al. 1978; Sturesson et al. 1989).
The dorsal part of the joint
Directly under the skin in the lumbopelvic area lies the superficial and deep lamina of the posterior layer of the thoracolumbar fascia (TLF) (PLF; Vleeming et al. 1995). This fascia is strongly fused to the aponeurosis of both the erector spinae and multifidi muscles at the sacral levels. The caudal parts of the erector spinae (combined portions of iliocostalis lumborum and longissimus thoracis), termed the sacropinalis muscle, and the deeper multifidus lumborum lie directly under this aponeurosis. Contractions of these muscles will cause tension in this ‘composite’ of both the superficial and deep lamina of the PLF, loose connective tissue and tendinous aponeurosis (Fig. 5; Vleeming & Willard, 2010). The superficial part of the PLF is partly continuous with the fascia glutea, covering the gluteus muscles, and fuses with cranial muscle fibers of the gluteus maximus. This tight composite of tissues covers the sacrum between the median sacral crest and the lateral border (Fig. 6A–C; Johnston & Whillis, 1944).
Fig. 5.
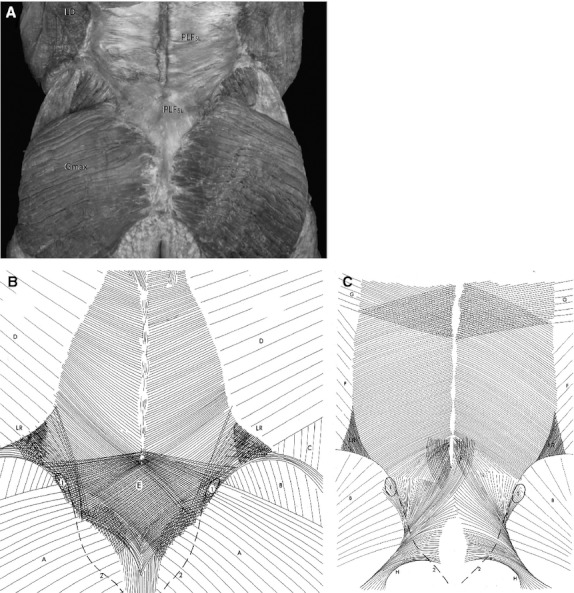
(A) Dorsal overview of the lumbopelvic area. The investing superficial fasciae over the muscles are removed. The superficial lamina of the posterior lumbar fascia is indicated as PLFsl. Note the thickness of the fascia over the sacrum and its geometry, forming part of the composite of the TLF over the sacrum. Gmax, gluteus maximus; PLFsl, superficial lamina of the posterior lumbar fascia. (Figure used with permission from the Willard Carreiro collection.) (B) The superficial lamina of the PLF. Notice the increased and specific density patterns of the superficial lamina over L4-L5 and sacrum. A, fascia of the gluteus maximus; B, fascia of the gluteus medius; C, fascia of external oblique; D, fascia of latissimus dorsi; 1, increased density of the lamina over the posterior superior iliac spine (PSIS); 2, sacral crest; LR, multidirectional thickening of the lamina over the lateral raphe. The connections of the mm. transverses abdominus and obliquus internus to the lateral raphe are located under the latissimus muscle. (Reproduced from Vleeming et al. 1995, with permission from Spine.) (C) The deep lamina of the PLF. Notice the overall fiber direction of the deep lamina in relation to the superficial fascia (A). The deep lamina. B, Fascia of the gluteus medius; E, connections between the deep lamina and the underlying aponeurosis of the erector spinae and multifidi muscles. Notice the increased and specific density patterns of the deep lamina over L4-L5, especially covering lower lumbar multifidi and sacrum. The more caudal part of the deep lamina fuses with the STL; F, fascia of the internal oblique; G, fascia of the serratus posterior inferior; H, STL; 1, the posterior superior iliac spine (PSIS); 2, sacral crest; LR, lateral raphe formed by the aponeurosis of both the internal oblique and the transversus muscle connecting to the deep lamina. (Reproduced from Vleeming et al. 1995, with permission from Spine.)
Fig. 6.
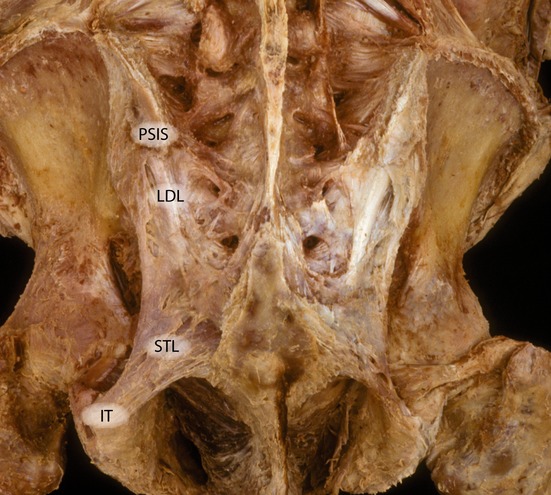
Dorsal overview of the lumbosacral spine. The multifidus are removed. The posterior superior spine is indicated (PSIS). The long dorsal ligament is indicated (LDL). The ischial tuberosity is visible (IT) and the sacrotuberous diverges craniomedially. (With permission from the Willard Carreiro collection.)
While palpating the upper part of the sacrum lateral of the spinous processes, this composite of structures can give the impression of feeling hard bone. This could mistakenly suggest that it is the sacrum itself that can be directly felt, instead of the tight fascial and tendinous composite enclosing the multifidus and sacrospinalis muscles.
The erector spinae and gluteus maximus are functionally interdependent as controlling forces that are mutually exerted on the ilium and the sacrum. The erector spinae and multifidi muscles assist in pulling the sacrum into nutation, while parts of these muscles also attach to the medial iliac crest. The gluteus maximus with attachments to the sacrum pulls the sacrum laterally into the ilium (Fig. 6; Vleeming, 1990). The gluteus maximus is also strongly connected and fused to the STLs and SSLs. Only after removing the muscle do these ligaments become visible (Vleeming et al. 1989a).
The dorsal ligamentous area of the SIJ is much more complex compared with its anterior part, and is composed of two groups of superficial and deep ligament layers. The dorsal SIJ ligaments extend from the median and lateral sacral crests, diagonally in a superior direction across the sacral gutter, and attach to the PSIS. When all dorsally located ligaments can be viewed at one time, a multidirectional cross-hatched fiber direction can be identified, suitable for compressing the sacrum to the ilia.
It has been shown that the thin dorsal fascia of the piriformis is continuous with the STL (Fig. 7; Vleeming et al. 1989a). In this study, in two out of 23 cases examined, it was found that the dorsal fascia of the piriformis was bilaterally aponeurotic and not connected to the STL. Of the 23 specimen examined, the biceps femoris of five females and one male bilaterally radiated out into the STL without being connected directly to the ischial tuberosity. In five other preparations, the biceps femoris was partially connected to the corresponding STL and ischial tuberosity unilaterally. In these cases the biceps femoris is able to stretch the ligament thereby tightening the SIJ (Vleeming et al. 1989a). Barker et al. (2004) confirmed these findings and indicated that, in unembalmed specimens, the semi-membranosus and semitendinosus muscles could have a similar effect.
Fig. 7.
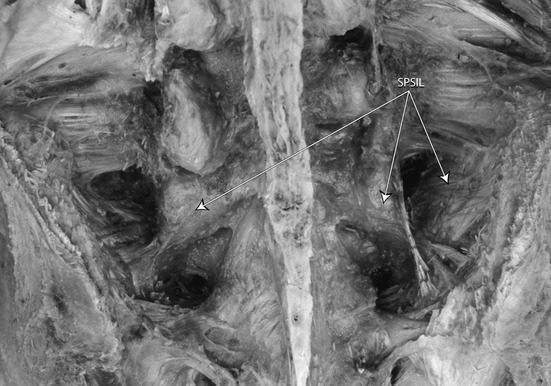
A dorsal overview of the deep dorsal SIJ ligaments after removing fascia and muscles and the STLs. The short posterior sacroiliac ligaments are indicated (SPSIL). (With permission from the Willard Carreiro collection.)
In order to assist in the balanced transfer of mechanical energy from one region of the body to another, normal joints and their associated entheses are constructed of tissue of varying viscoelastic moduli (Biermann, 1957; Knese & Biermann, 1958). Importantly, these enthesial sites often involve a bony tuberosity featuring the conjoint attachment of multiple muscles, such as those seen with the ischial tuberosity and STL, which receive the long head of the biceps femoris, semitendinosus and semimembranosus from below and the gluteus maximus, piriformis and lumbar multifidus from above (Soames, 1995). Such conjoint attachments contribute to a kinetic chain, which directly assists in load transfers and mechanoforce transmission from the spine and sacrum to the lower limbs (Vleeming et al. 1996). In this above example, the strong STL and its associated SSL join with the LDL to stabilize the SIJ. The STL and SSL resist nutation of the joint (Sashin, 1930; Vleeming et al. 1989b), while the LDL resists counternutation (Vleeming et al. 1996). The kinetic chain of muscles attached to the ischial tuberosity and STL can then influence the balance between these two opposing movements. This contention has been supported by the studies examining the effects of contraction of the muscles of this kinematic chain on the stiffness of the SIJ (van Wingerden et al. 2004).
The LDL can be palpated in the area directly caudal to the PSIS, and upon palpation feels like a bone-hard structure (Fig. 7). The ligament is of special interest, as women who complain of lumbopelvic back pain during pregnancy frequently experience pain within the boundaries of this ligament (Fortin et al. 1994a; Vleeming et al. 2002 1996; Ronchetti et al. 2008). Pain localized to this area is also common in men. The ligament is the most superficially located SIJ ligament, and is therefore well suited to mirror asymmetric stress of the SIJ. Cranially, the LDL is attached to the PSIS and the adjacent part of the iliac bone, and caudally it is attached to the lateral crest and transverse tubercle of the third and fourth sacral segments (Vleeming et al. 1996; Moore et al. 2010). The lateral expansion of the LDL, directly caudal to the PSIS, ranges from 15 to 30 mm. The length, measured between the PSIS and the third and fourth sacral segments, ranges from 42 to 75 mm. The LDL is pierced by lateral branches from the dorsal rami of S2 (96%), S3 (100%) and S4 (59%) and, very rarely, by S1 (4%; Willard et al. 1998; McGrath & Zhang, 2005). The lateral part of the LDL is continuous with fibers of the STL, passing between the ischial tuberosity and the iliac bone. There is a wide-ranging variation among fibers of the LDL, being connected to the deep lamina of the PLF, to the aponeurosis of the erector spinae muscle and multifidus muscle. The ligament is tensed when the SIJs are counternutated and slackened when nutated (Vleeming et al. 1996). In a finite element analysis study of the SIJ ligaments, creating a 3D reconstruction of CT scans, it was shown that during SIJ counternutation the LDL is one of the major constraints. The authors propose that increased laxity of the SIJ could lead to counternutation and increased pain in the LDL (Eichenseer et al. 2011).
SIJ nutation decreases during flattening of the lumbar lordosis. Lordosing the spine creates the opposite pattern (Weisl, 1955; Egund et al. 1978; Lavignolle et al. 1983; Walheim, 1984; Sturesson et al. 1989; Vleeming et al. 1992b). Flattening and lordosing the spine are mainly initiated by external motion of the pelvis on the hip joints (Vleeming & Stoeckart, 2007). Slackening of the LDL can be counterbalanced by both the STL and the erector spinae muscle. Pain localized within the boundaries of the LDL could indicate, among other disorders, a condition with sustained counternutation of the SIJ. In diagnosing patients with specific LBP, and especially PGP, the LDL should not be neglected (Vleeming et al. 1996). Even in cases of arthrodesis of the SIJ, tension in the LDL can still be altered by different structures (Vleeming et al. 1996). The STL, in contrast to the LDL, is tensed during nutation and also subjected to increased tension of the biceps femoris and/or gluteus maximus muscle (Fig. 7). After freeing the erector spinae and its fibrous sheath from the ligaments connected to the muscle, numerous discontinuous interwoven bands of dense connective tissue become visible on the medial side of the LDL. These short posterior SIJ ligaments arise on the intermediate and lateral sacral crest and attach to the rough sacropelvic surface of the ilium (Fig. 8). The intertransverse ligaments, usually depicted only in the lumbar region, can hardly be distinguished from the short posterior ligaments but do exist lumbosacrally as well.
Fig. 8.
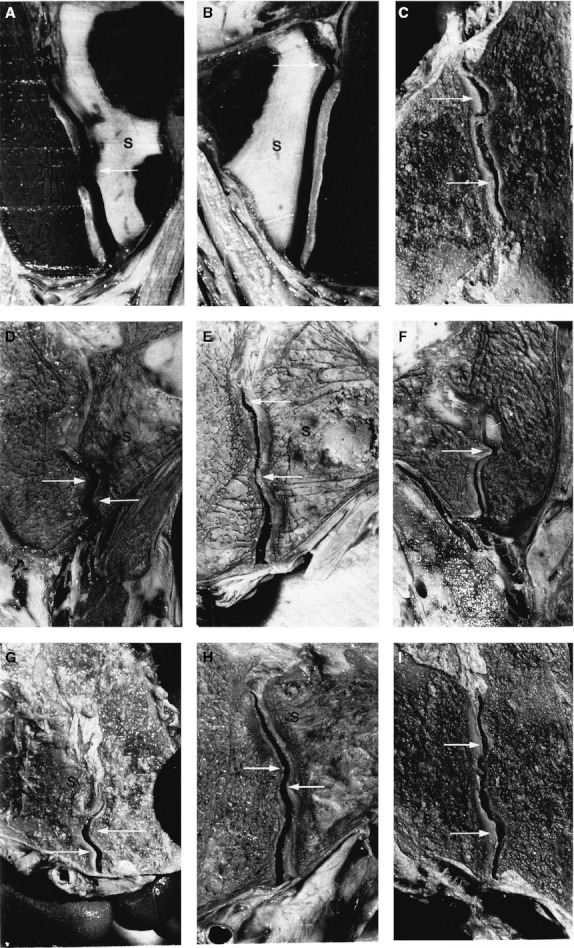
Frontal sections of the sacroiliac joint (SIJ) of embalmed male specimen. S indicates the sacral side of the SIJ. (A) and (B) concern a 12-year-old boy; (C)-(I) concern a specimen older than 60 years. Arrows are directed at complementary ridges and depressions. They are covered by intact cartilage, as was confirmed by opening the joints afterwards. (With permission from the Vleeming collection.)
The Iliolumbar Ligament
A large fan-shaped, complex ligament extends laterally from the transverse processes of the lower two lumbar vertebrae and reaches the iliac crest and the SIJ capsule (Fig. 4). Conventionally, this structure is termed the Iliolumbar Ligament (IL), but components of this structure have been renamed as the lumbosacral ligament (Pool-Goudzwaard et al. 2003), and the entire structure has also been referred to as the lumbo-ilio-sacral ligament (Hanson & Sonesson, 1994; Hanson et al. 1998; Hanson & Sorensen, 2000). The IL has previously been described as developing out of the inferior border of the quadratus lumborum muscle in the second decade of life (Luk et al. 1986). However, this was refuted with the observation that the ligament is present in the fetus as early as 11–15 weeks gestational age (Uhthoff, 1993; Hanson & Sonesson, 1994).
Extensive variation exists in the anatomical description of the IL. Kapandji (1974) describes superior and inferior bands with an occasional sacral band below the inferior band. O'Rahilly (Gardner et al. 1975) describes anterior, superior and inferior bands, whereas Bogduk & Twomey (1991) describe anterior, posterior, superior, inferior and vertical iliolumbar divisions. A study based on 100 specimens reported only two parts to the ligament: the anterior and the posterior part (Hanson & Sonesson, 1994), which was corroborated by Hammer et al. (2010), and one study described a dorsal band, ventral band, sacroiliac part and a lumbosacral band (Pool-Goudzwaard et al. 2003).
These individual fascial bands are highly variable in both number and form, but consistently blend superiorly with the intertransverse ligaments of the lumbar vertebrae, and inferiorly with both the posterior and anterior aspects of the SIJ capsule, and attach laterally to the iliac crest (Willard, 2007).
In some individuals, the IL arises from the transverse processes of L4 and L5 (Pool-Goudzwaard et al. 2003; Standing, 2005; Willard, 2007); this is contrary to observations that the ligament attaches only to L5 (Hanson & Sonesson, 1994). The taut bands of the IL form hoods over the L4 and L5 nerve roots. These hoods could be capable of compressing the associated nerve roots (Briggs & Chandraraj, 1995). The IL is subject to fatty degeneration after the first decade of life, and has been reported to ossify on occasion (Lapadula et al. 1991).
The major function of the IL is to restrict motion at the lumbosacral junction, particularly that of side bending (Leong et al. 1987; Chow et al. 1989; Yamamoto et al. 1990). It is also strained during slouching, but this strain is reduced upon contraction of the multifidus or erector spinae muscles (Snijders et al. 2008). After bilateral transection of the IL, rotation about the vertebral axis is increased by 18%, extension by 20%, flexion by 23% and lateral bending by 29% (Yamamoto et al. 1990). Thus, one function of the ligament is to stabilize the lumbar vertebrae on the sacral base. Other studies have described the influence of the IL on SIJ stability. Transection experiments revealed that the ligaments, especially the ventral part, could restrict sagittal movement of the SIJ (Pool-Goudzwaard et al. 2003). This can only be understood in light of the vast insertion of these ligaments to the ilia.
Due to the above-mentioned muscular and ligamentous connections, movement of the sacrum with respect to the iliac bones, or vice versa, affects the joints between L5 and S1 and between the higher lumbar levels. Thus, anatomical and functional disturbances of the pelvis or lumbar region influence each other.
Innervation of the SIJ
Although the SIJ is known (clinically) to be involved in lumbopelvic pain (Wyke & Jayson, 1976; Beal, 1982; Bernard & Kirkaldy-Willis, 1987; Kirkaldy-Willis & Burton, 1992; Fortin et al. 1994a,b, 1999a; Bogduk, 1995; Daum, 1995; Schwarzer et al. 1995; Borenstein, 1996), few studies have examined its innervation. Innervation by branches from the ventral lumbopelvic rami has been reported (Ikeda, 1991); however, it has not been verified. Conversely, innervation of the SIJ by small branches from the posterior rami has been reported by numerous authors. Bradley found fine fibers innervating the joint from L5 to S3 (Bradley, 1974), while Grob et al. (1995) reported branches to the joint from posterior rami S1–S4. Willard et al. (1998) were able to trace small branches from a communicating branch of L5, as well as from S1 and S2, into the edge of the joint, and McGrath & Zhang (2005) reported fine fibers from S2 to S4 and rarely S1 in the ligaments near the joint. In 2012, Patel et al. reported successful attenutation of SIJ pain using neurotomy of the L5 dorsal primary ramus and lateral branches of the dorsal sacral rami from S1 to S3 (Patel et al. 2012). In rats, double-labeling techniques have demonstrated the presence of dichotomizing axons from neurons of the dorsal root ganglion at levels L1–L6 (Umimura et al. 2012). These axons sent branches to both the SIJ and the multifidus muscle, suggesting a possible mechanism for referred pain in the lower back.
Myelinated and unmyelinated fibers along with encapsulated endings have been found in the joint (Grob et al. 1995). Many axons in the nerves to the SIJ were approximately 0.2–2.5 mm in diameter, placing them well within the range of the group IV (C-fibers) and possibly within the smaller end of the group III (A-delta) fiber range (Ikeda, 1991). Electrophysiological recordings from axons innervating the cat SIJ revealed that most fibers are high-threshold, group III in nature (Sakamoto et al. 2001). Small fibers positive for substance P and calcitonin gene-related polypeptide have been observed in the cartilage on both sides of the joint and surrounding ligaments, but were not present in substantial amounts in the subchondral bone (Szadek et al. 2008, 2010). Axons of this size and with these physiological properties have been associated with nociception in other areas and most likely are involved in the perception of pain from the SIJ.
A study using gross dissection and fluoroscopic imaging of small metal wires placed on the lateral branches of the dorsal sacral plexus demonstrated small fibers entering the SIJ along its medial and inferior boundaries, and provided evidence that these fibers can be related to pain patterns seen in selected patients with LBP (Yin et al. 2003). From these studies it is clear that the outer border of the joint receives innervation, at least from the posterior primary rami of the lower lumbar and upper sacral segments.
Pathology of the SIJ
Ankylosis has been long considered a significant problem in the SIJ. Brooke (1924) studied ankylosis in males of late middle or of advanced age. At about 50 years old, para-articular osteophytes usually arise at several places around the SIJ. In men, they typically localized around the cranial iliac aspect of the joint, while in women they occur around its ventrocaudal aspect. Brooke concluded that SIJ ankylosis is more often a male than a female affliction, male SIJ often showing extra-articular tubercles. In male preparations of diverse ages, 37% of 105 male preparations revealed complete extra-articular ankylosis. Among 105 female preparations of different ages, hardly any signs of ankylosis were found. According to Brooke, progressive cartilaginous degeneration may present itself with local intercartilaginous connections. However, later research reported much lower incidences of ankylosis in older men (Stewart, 1984; Dar & Hershkovitz, 2006). It may be of relevance that because Brooke investigated people who lived before 1925, lifestyle and environment may have influenced their skeletons. It remains unclear in how far the alleged ankylosis in males was caused by para- or intra-articular pathology.
In 38 preparations from Brooke's study, it was demonstrated that SIJ ankylosis directly affects lumbosacral mobility. Of these 38 cases with SIJ ankylosis, 81% had increased mobility between L5 and sacrum; on the other hand, 19% presented also with lumbosacral ankylosis (Brooke, 1924). Based on this research, it can be concluded that SIJ ankylosis seldom occurs in either women or men aged 50 years or younger. Finally, ankylosis of the SIJ may also occur as a result of AS (< 1% of adults) with accompanying involvement of the spine (Brooke, 1924).
Another study by Sashin (1930) examined 51 male and female preparations (> 60 years); it was observed that 82% of SIJ in men and 30% of SIJ in women showed local or global osteophytic pathology. Similarly, McDonald & Hunt (1952) observed real intra-articular ankylosis in no more than two of 59 preparations (3%), and analysis of 88 radiographs of people (≤ 50 years) without LBP showed that 6% had articular and subarticular SIJ erosion (Cohen et al. 1967). Kajava, as cited in Solonen (1957), analysed patients who did not (or hardly) load their hip joints for a considerable amount of time; it was concluded that the incidence of SIJ ankylosis was directly related to the decreased loading of the joint especially when the periods of unloading occurred during adolescence.
During routine autopsies, Resnick et al. (1975) investigated (radiologically and pathologically) the SIJ in 46 males and females, discriminating between para- and intra-articular arthrosis. Para-articular arthrosis was found in only one person (2%) below the age of 50 years. In four preparations (9%), real intra-articular ankylosis, resulting from the pathological degeneration of AS (Bechterew's disease) was observed. Para-articular ankylosis was observed in 24% of the cases. In the elderly, changes of cartilage are mostly due to osteoarthrosis, and the joint cleft narrows at later ages. In people aged 50–70 years, the cleft is usually 0.1–0.2 cm, and in those aged 70+ years the cleft is generally 0–0.1 cm (Resnick et al. 1975). Note that there is a tendency to underestimate the width of the cleft when applying plain X-ray techniques, due to the overprojection of the sinusoidal form of the SIJ (Dijkstra et al. 1989).
Differences in the experimental data concerning osteophythosis, arthrosis and ankylosis arise in part from the lack of clearly defined criteria in these studies. It is suggested to systematically discriminate between different aspects of the pathological process leading to ankylosis, i.e. para-/intra-osteophytosis and/or the presence of osteophytes type II, namely, osteophytes that completely bridge the joint, thereby causing real fusion (Resnick et al. 1975). Degenerative para-articular osteophytosis might immobilize SIJ and needs to be differentiated from the uncommon intra-articular ankylosis of AS. Intra-articular ankylosis of SIJ would be an exceptional finding in osteoarthritis, even in old age and with radiologically marked osteoarthrosis (Resnick et al. 1975).
Partial ankylosis, i.e. collagenous ankylosis rather than ossification of the SIJ, is a separate entity (Resnick et al. 1975). Persistently excessive compressional forces and stiffness of the spine, sacrum and SIJ in AS could contribute to the earliest sacroiliac changes of osteitis (Dihlmann, 1980; Ahlstrom et al. 1990; Jurriaans & Friedman, 1997; Masi et al. 2007), as well as progression to its latest stage, i.e. lesion of intra-articular bony fusion (Resnick et al. 1975; Dihlmann, 1980; Jurriaans & Friedman, 1997). Concurrently, such altered kinetic forces could exert greater tensional strains on ligamentous and tendinous attachments at various bony sites on the sacrum and spine, leading to the characteristic enthesopathy lesions (Ball, 1971; Benjamin & McGonagle, 2001). Exaggerated spinal–pelvic force transfers could secondarily lead to excessive impacts and stresses being transmitted to lower extremity joints, and contribute to those typical involvements of AS (Masi et al. 2007).
Stewart (1984) investigated racial and gender differences in pelvic structure using 1986 pelves. Particular attention was given to distinguishing between para- and intra-articular ankylosis. Following former researchers (Brooke, 1924; Sashin, 1930; Schuncke, 1938), Stewart observed that the most important age-related SIJ changes occur at the ilium, which has since been confirmed by others (Dar & Hershkovitz, 2006). In addition, Stewart found that the formation of both para- and intra-articular osteophytes is more rare in women than in men. In men, the osteophytic process occurs especially at the cranial aspect of the joint. Also, enthesopathic calcification or osteophytes follow tensional stresses, as occur in syndesmophytes in AS (Benjamin & McGonagle, 2001).
Concerning female ankylosis, the following quantitative data are presented, without distinguishing between para- and intra-articular causes (Stewart, 1984). Examination of 227 pelves of Caucasian women revealed that 4% were ankylotic, two of which were unilateral only; out of 267 American and African Negroid female pelves, 3% were ankylotic, all of them unilateral. Stewart states that the (rare) occurrence of osteophytes (the possible cause of female ankylosis) may be due to intra-articular hemorrhage during pregnancy precipitated by the stretching of the ligaments. Concerning male ankylosis, an examination of 347 American Caucasian pelves found 11.2% to be ankylotic, 7.2% of which were unilateral, and the remaining 4% bilateral. In 241 African American males, 24.9% were ankylotic, 15.8% of which were unilateral and 9.1% bilateral. In marked contrast, an examination of 335 African males found only 8.6% of the SIJs ankylotic, of which 3.6% were unilateral and 5% bilateral (Stewart, 1984).
Based on the cited studies above, it is obvious that the incidence of ankylosis is much lower than formerly thought, and intra-articular ankylosis seldom occurs (Brooke, 1924; Sashin, 1930; Stewart, 1984). This conclusion is further supported by the findings of Dar & Hershkovitz (2006) who analysed an extensive and diverse collection of pelves. SIJ bridging (osteophytosis) was present in 12.27% of males and in 1.83% of females. In 97.5% of the males the bridging was extra-articular, whereas in females it was intra-articular. In addition, CT images of 81 in vivo individuals were examined for the same phenomenon. It was shown that out of 38 living males, 34.2% had SIJ bridging, in contrast to 2.3% of the females. The authors comment that the high rate of bridging is not surprising because of the high average age of the males (average 69.6 years; Dar & Hershkovitz, 2006). These studies lead to the conclusion that for reliable sexing of skeletal pelves, bony spurs in the ilium creating a partial or full articular SIJ bridge are indicative of a male skeleton (Dar & Hershkovitz, 2006). The studies of Stewart & Dar provide convincing support that extra-articular ankylosis normally commences from the ilium, and that ankylosis is rare in women and not typical at high age in men (Vleeming et al. 1992b). Additionally, when SIJ bridging does occur in females, the area adjacent to the arcuate line is most typically involved.
Another study investigated 24 female and 13 male preparations of different ages, under embalmed and non-embalmed conditions (Vleeming et al. 1992b). Ankylosis was found in only two of the male preparations, one of which intra-articularly; both these preparations were over 60 years old (Vleeming et al. 1990a, 1992b). It was also shown that even at advanced age (> 72 years), the combined movement of nutation and counternutation can amount to 4 °; normally, movements of this joint are < 2 °. The SIJ with the lowest mobility showed radiologically marked arthrosis. Thus, intra-articular ankylosis of the SIJ was found to be an exception, even at advanced age (Vleeming et al. 1992b).
Motion may play a large part in maintaining the health of the joint. Narrowing or obliteration of SIJs has been commonly reported in paraplegics. However, complete bony fusion, as found in AS, is not well documented (Wright et al. 1965; Khan & Kushner, 1979). Trunk mobility was proposed to be essential for maintaining SIJ integrity, and its absence in many paraplegics might compromise the structural integrity of the joint (Khan & Kushner, 1979).
Possible mechanisms of arthrosis and ankylosis of the SIJ
Without being validated, terms such as ‘arthrotic processes’ are used in the literature to describe the SIJ. However, it is possible that a textbook statement such as: “…the SIJ synovial joint rather regularly shows pathological changes in adults, and in many males more than 30 years of age, and in most males after the age of 50, the joint becomes ankylosed” (Hollinshead, 1969), is based on incorrect interpretation of anatomical data. If indeed the described macroscopic alterations at higher ages are at least partially adaptive, then the question remains – do individuals of advanced age still retain mobility of the SIJ?
A series of anatomical and biomechanical studies raised the question as to whether certain macroscopic changes of the auricular surfaces have to be viewed as pathological or as functional (as described above) adaptations (Vleeming et al. 1990a,b). Frontal sections of embalmed specimen were used in these studies. Different aspects of roughening of the auricular part of the SIJ were considered, such as the texture of the cartilage and the feature of complementary ridges and depressions. In all preparations ridges can be seen on the iliac or sacral side, which involve both bone and cartilage. At the site of the ridge a complementary depression exists in the other part of the joint. As a consequence of the size and irregular shapes of the ridges and depressions, large differences in the shape of the SIJ exist between the front and the back of the slice, even at a thickness of 7 mm. It is noteworthy that ridges and depressions are already visible at a young age, as was apparent (albeit very small) in a specimen of a boy aged 12 years (Fig. 9). In general, less pronounced ridges and grooves appeared in younger female specimens than was apparent in frontal SIJ sections from relatively older males and females. Features such as coarse texture, ridges and depressions, enhance friction and, consequently, the stability of the SIJ (Vleeming et al. 1990a,b).
Fig. 9.
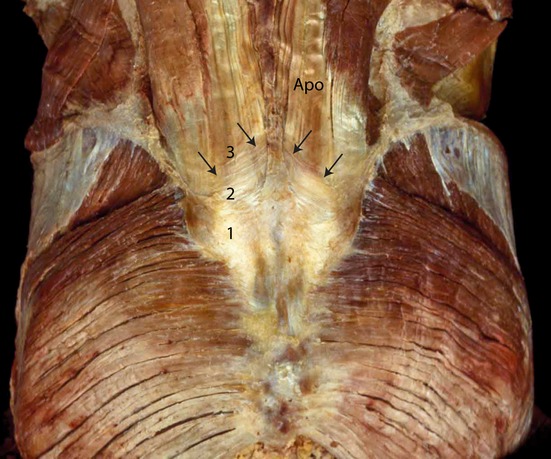
Components of the composite over the lumbosacral spine. The superficial lamina of the PLF (1), which is dissected at (2) where the deep lamina of the PLF becomes visible. The deep lamina (2) is dissected and the aponeurosis tendon of the erector trunci and multifidi becomes visible (APO). (With permission from the Willard Carreiro collection.)
Another related investigation showed that the friction coefficients of the auricular joint faces are especially high in specimens with large complementary ridges and depressions. Biomechanical calculations showed that both a higher friction coefficient and a greater wedge angle of the sacrum influence the stability of the SIJ (Vleeming et al. 1990a,b). Three large lever arms, the trunk and both legs, act directly on the pelvis and SIJ. It was suggested that, during growth, these lever arms generate an increasing force until full body weight is reached. As a consequence, the SIJ will be dynamically modified by changes in the direction and the strength of the forces acting on it (Vleeming et al. 1990b).
The alteration of forces during pubertal growth results in roughening of the SIJ articular surfaces. This roughening is characterized by curvatures of the articular surfaces, by increased texture of the cartilage and by symmetrical ridges and depressions, which as a rule are covered with the typical cartilage as already described prenatally. Macroscopic changes of the SIJ, such as coarse texture and the appearance of complementary grooves and ridges, have already commenced during the intra-uterine period and become more pronounced later in life (Bowen & Cassidy, 1981). These articular changes reflect functional adaptations to stability, possibly promoted by the increase of weight, especially after puberty. All these features are expected to reflect adaptation to human bipedality, contributing to a high coefficient of friction and enhancing the stability of the joint against shear. As a consequence, less muscle and ligament force is required to bear the upper part of the body (Vleeming et al. 1990a).
An anatomical and radiological study utilized plain and complex tomography to examine the pathological changes seen in SIJ structure (Dijkstra et al. 1989). The authors concluded that when diagnosing presumed structural defects of the SIJ, it is essential to have detailed knowledge of the individual configuration of the joint. Because of its complex sinusoidal form, the dorsal portion of the joint has to be tomographed in the frontal projection, and the middle and ventral portion in oblique projection. In 56 patients referred to a specialized rheumatology department for diagnosing AS, 72 SIJs were investigated. Based on plain radiography, only six joints appeared normal, and when examined using frontal tomography, five joints were diagnosed as normal. However, based on oblique tailored tomography, 31 joints in these patients were diagnosed as normal. Additionally, a pelvic clay model was used to simulate artificial erosion of the SIJ. Plain radiography cannot demonstrate the erosion, whereas the frontal tomogram shows a vague light spot. Only oblique tomography clearly delineates the small 2-mm-deep erosion. The study showed that detailed analysis of the SIJ is only possible with oblique tomography (CT or magnetic resonance imaging; Dijkstra et al. 1989; Puhakka et al. 2003).
Age-related structural changes in the SIJ and their effects on joint mobility were examined in a study utilizing embalmed specimens of elderly people (Vleeming et al. 1992b). The data were correlated with a radiological survey. In the sagittal plane, both ventral rotation and dorsal rotation of the sacrum relative to the ilium was confirmed. These data support the notion that small movements in the SIJ, even at older ages, are possible and that ankylosis in this age group is not the normal situation (Bakland & Hansen, 1984; Vleeming et al. 1992b). The latter study also showed that intra-individual differences occur in the mobility of the SIJ. The observation supports the idea that asymmetry in form and function of the SIJ is normal (Vleeming et al. 1992b). Furthermore, a relation is suggested between mobility and certain radiographic features. Arthrosis of the SIJ or of the nearby intervertebral joints, combined with pronounced grooves and ridges on the SIJ surfaces, appears to be associated with limited SIJ movement.
It is possible that AS is not fully explained by inflammatory processes alone (Masi et al. 2011). Clinical, epidemiological and genetic factors as well as the natural history of disease indicate that additional host-related risk processes and predispositions are present in the etiology of AS. Collectively, the pattern of strong predisposition to onset in adolescent and young adult ages, coupled with male preponderance, is unique among inflammatory rheumatic diseases. However, this pattern could reflect biomechanical and structural differences between the sexes interacting with naturally occurring musculoskeletal changes over the life cycle. All of those host factors may be interacting on a background of genetic polymorphism in myofascial stabilization of the spine present in the population. During juvenile development, the body is more flexible than during adolescent maturation and young adulthood. The concept of an innate axial myofascial hypertonicity, presenting as chronically excessive increased force closure, especially in the lumbopelvic area, reflects basic mechanobiological principles in human function, tissue reactivity and pathology. In this scenario, these proposed physical mechanisms interact with recognized immunobiological pathways. In fact, it is possible that the structural biomechanical processes and tissue reactions could precede initiation of other AS-related pathways. However, these processes have been little studied and require much critical testing. Research in the combined structural mechanobiological and immunobiological processes promises to improve understanding of the initiation and perpetuation of AS. The combined processes may lead to a better explanation of the characteristic enthesopathic and inflammatory processes in AS (Masi et al. 2011).
Movement studies of the SIJ
In bipedal gait, the SIJ are the ‘hub’ of forces transferred from the trunk to the ground and vice versa (Lovejoy, 1988; Aiello & Dean, 1990). The pelvis requires stabilization for its own coordinated movements on the femurs as well as for controlled SIJ flexibility in various load transfers (Vleeming et al. 1990a,b; Vleeming & Stoeckart, 2007). Numerous researchers have tried to model SIJ function by studying its principal displacement characteristics. A common assumption of these studies is that increased loading on the sacrum leads to tilting the proximal aspect of the sacrum ventrally, a process by which most dorsal ligaments are stretched and the dorsal aspects of the two iliac bones are drawn together (Meyer, 1878; Albee, 1909; Kopsch, 1940; Shipp & Haggart, 1950; Solonen, 1957). Indeed, Weisl (1955) demonstrated SIJ mobility in vivo by using lateral roentgenograms. Weisl used the terminology of nutation and counternutation, terms referring to a combined rotation–translation displacement. Nutation implies a tilting of the sacrum relative to the ilia and vice versa, deepening of the lumbar lordosis, whereas counternutation lessens the lordosis. Weisl noted a 6-mm displacement between endpoints, but the error of measurement was calculated to be 3 mm. Similarly, Colachis et al. (1963) performed a study with rods in the iliac bones and reported 5 mm of translation.
Chamberlain (1930) developed a radiological method to diagnose pelvic stability. Anterior–posterior radiograms are taken while the subject is asked to stand on one leg, the contralateral leg hanging free. According to Chamberlain, the criterion for abnormal mobility is vertical pubic movement of more than 2 mm. Steiner et al. (1977) mention a minimum of 4 mm as being pathological. However, because the symphysis is angled on average about 45 ° with respect to the frontal plane, anterior–posterior measurement will therefore tend to underestimate real mobility (Walheim et al. 1984).
Walheim et al. (1984) also measured pubic mobility around the symphysis. Two rods were bilaterally inserted into the symphysis, allowing for continuous electromechanical registration of pubic movements. Tests were performed on four embalmed corpses; under local anesthesia rods were implanted on 14 young volunteers, and six male volunteers aged 21–27 years, six nulliparous women and three multiparous women. Supine subjects were asked to flex the hip 90 ° and then perform maximal abduction. Registrations also took place during walking. This study has shown that the pubic bones translate vertically during walking. Average translation displacement was 1 mm in the male and 1.3 mm in the female, with a maximum of 3.1 mm in one of the multiparous women (Walheim et al. 1984). Rotations of the symphysis around a transverse axis were < 2 °, again with little difference between men and women. The average rotation was < 0.3–0.8 °, much less than the values found by Pitkin (1947) of 5.5 °. In Pitkin's study, the methods of registration lacked precision, whereas the data from Walheim et al. are more reliable. Walheim et al. (1984) report measurement errors of < 0.0005 mm. Rotations in the symphysis around an idealized sagittal axis were found to be 0.4°, without clear differences between the sexes. In the frontal plane, pubic movement was approximately 0.5 mm in adult males, 1 mm in nulliparous women and 2 mm in multiparous women. In multiparous women, if the range of motion was larger than 2 mm, articular pain was always present (Chamberlain, 1930).
The study of Walheim et al. (1984) revealed little difference in symphyseal movement between men and women. However, interpretation of these data is difficult. First, the data were derived from a sample with a limited age distribution. The authors also did not test physiological movements, such as hip extension from the upright position, and the chosen test position (90 ° hip flexion with abduction) is not common. Conversely, Chamberlain's subjects have a wider age spectrum and included a larger number of people, including several patients. Furthermore, Chamberlain explicitly differentiates between the presence or absence of pelvic pain. A more recent study applied Chamberlain's method on 45 asymptomatic individuals to analyze motion of the symphysis in the frontal plane. On average, men show 1.4 mm displacement, nulliparous women 1.6 mm and multiparous women 3.1 mm (Garras et al. 2008). The results of this study confirm the notion that multiparous women have relatively mobile pelves. Other studies come to the same conclusion with different methodologies to measure pelvic motion (Brooke, 1924; Chamberlain, 1930; Bowen & Cassidy, 1981; Sturesson et al. 1989).
Using RSA (see description in Current in vivo movement studies section), it becomes possible to measure movements in three dimensions (Selvik, 1974, 1990). Using RSA with implanted markers, Egund et al. (1978) demonstrated that different SIJ movements take place in transitions from one body position to another. In the prone patient, manual pressure on the apex of the sacrum leads to counternutation. The upright position normally leads to nutation, and maximizing lordosis increases nutational tilt. Lavignolle et al. also used RSA, but without implantation of markers (instead using bone landmarks with much lower accuracy). They observed large SIJ mobility in the supine position with hip flexion (Lavignolle et al. 1983). Importantly, this implies that the passive straight leg raise test performed with more than 60 ° flexion, applied for detecting ischial neuropathy, could mobilize and provoke the SIJ, which may influence other parts of the spinal column (Vleeming & Stoeckart, 2007).
In most individuals, standing upright appears to lead to a close packed position in the SIJ. Nutation is increased in load-bearing situations (e.g. standing and sitting). In lying prone, nutation is also increased compared with supine positions (Weisl, 1955; Egund et al. 1978; Sturesson et al. 1989, 2000a). Post mortem studies have shown that even at advanced age (> 72 years) the combined movement of nutation and counternutation can amount to 4 ° but, normally, movements are < 2 ° (Vleeming et al. 1992b) In this study, the SIJ with the lowest mobility showed radiologically marked arthrosis.
Miller et al. showed (on elder specimens) that full sacral nutation relative to the ilia results in 6 ° rotation. However, the study may have been confounded by the fact that the intervertebral discs and a large part of the SIJ ligamentous apparatus were removed to be able to fix the tested specimen, enabling isolated mobility testing of the SIJ (Miller et al. 1987).
Current in vivo movement studies
Four different techniques for analyzing in vivo SIJ movements have been applied. The first technique involved RSA, a procedure that is widely used to measure small movements. Several studies of the SIJ have independently used the RSA technique (Egund et al. 1978; Sturesson et al. 1989, 2000a,b; Selvik, 1990; Tullberg et al. 1998). Tantalum beads with a diameter of 0.8 mm are implanted into the pelvic bones. At least three, but usually four–six, beads are placed geometrically well spread into each ilium and into the sacrum. In various studies, Sturesson et al. (1989, 2000a,b) revealed that nutation occurs when patients loaded their spine by means of rising from a supine towards a sitting or standing position. The authors demonstrated that movements do not differ between symptomatic and asymptomatic side. Also, SIJ movements were reduced as additional loads were placed on the joint. The largest movement occurred when changing from standing to lying prone with hyperextension of a leg. SIJ mobility in men averaged 30–40% less than in women.
The same authors describe that movement in most patients can be reduced by applying an external Hoffman Slatis frame, which generally reduces the pain (Sturesson et al. 1999). This finding is in agreement with studies using pelvic belts to normalize SIJ movement (Vleeming et al. 1992a; Mens et al. 2006 1999). Tullberg et al. studied the effect of successful manipulation of the SIJ. The patients (n = 10) were examined both clinically and with RSA, before and after manipulation. Manipulation did not alter the position of the sacrum in relation to the ilium. The result seems to indicate that effective manipulation is not dependent on positional change of the joints (Tullberg et al. 1998).
The second in vivo technique used to measure SIJ movements involved the placement of surgical Kirscher rods in both the ilia and the sacrum of healthy volunteers (Kissling et al. 1990). Subsequently, measurements were made in standing, anteflexion and retroflexion of the lumbar spine. The study showed an average total rotation in the SIJ between standing erect on both feet and one-legged stance of about 2 ° (range 0.4–4.3 °), and no significant differences with regard to sex, age or parturition.
Surprisingly, Smidt et al. (1995), using a third technique, demonstrate large SIJ motion with an external skeletal measurement system in the reciprocal straddle position. They registered movement far larger than those in any other study. For mean sagittal SIJ motion, occurring between alternating right/left straddle position, 9 ° of motion was registered. The greatest error of measurement with this technique is the calculation of bony landmarks lying under the skin markers. Sturesson et al. re-evaluated these results using RSA with internal markers and demonstrated that a reciprocal movement indeed takes place. However, the movements are 10-fold smaller than those reported by Smidt et al. (1995).
A conclusion derived from the various studies cited above suggests that SIJ mobility depends on positioning and the distribution of load. There are reported differences as to the degree and the direction of the possible rotational and translational movements. Various investigators have described rotational, gliding, anteroposterior and vertical movements of the SIJ, but rotation of the sacrum (nutation and counternutation) around its transverse axis at S2 is considered to be the main movement (Egund et al. 1978; Bellamy et al. 1983; Bakland & Hansen, 1984; Sturesson et al. 1989; Vleeming et al. 1992b; Sturesson, 2007). Mobility of the SIJ normally takes place in all three major planes, normally limited to about ± 2 ° (Weisl, 1955; Sturesson et al. 1989, 2000a,b; Vleeming et al. 1992a). Counternutation normally takes place in unloaded situations, such as lying. While lying, maximal flexion in the hips, using the legs as levers to posteriorly rotate the iliac bones relative to the sacrum, creates nutation. This is also called the ‘labor position’, because it creates a larger posterior sagittal diameter of the pelvic outlet through which the child can be delivered (Weisl, 1955).
One may want to apply these SIJ displacement research data in testing SIJ movement in patients. It appears, however, that large interindividual variability exists in the anatomy and movement of the SIJ (Weisl, 1955; Solonen, 1957; Bakland & Hansen, 1984; Dijkstra et al. 1989; Vleeming et al. 1990a).
Clinical manual movement tests are unreliable for the SIJ (Vleeming et al. 2008). A commonly used diagnostic test for SIJ pain is the standing flexion test, or ‘Gillet test’. Unfortunately, the test has low reliability (Potter & Rothstein, 1985) and low reproducibility (McCombe et al. 1989). Sturesson et al. applied this test to 22 patients with severe SIJ pain, using RSA. The results show minimal change of movements during the test, and no differences between symptomatic and asymptomatic sides. When the pelvis is loaded in a one-leg standing position while flexing the contralateral leg, patients are physically challenged, leading to bilateral increased force closure of the SIJ (Sturesson et al. 2000a; O'Sullivan et al. 2002; Beales et al. 2009). Assumed SIJ motion during this test does not occur. The authors conclude that movement of the external pelvis relative to the hips gives the (manual) illusion that the SIJ are repositioned (Sturesson et al. 2000a).
The fourth in vivo technique discussed here was developed by Buyruk et al. (1995a,b), who applied unilateral oscillations to the anterior superior iliac spine to assess laxity of the pelvic joints in specimens. Using Doppler imaging of vibrations (DIV), they measured the stiffness/laxity ratio of artificially unstabilized SIJ in comparison with stabilized pelves. This new method proved objective and repeatable. In vivo studies followed in healthy subjects (Damen et al. 2001, 2002a). It was shown that pelvic belts are effective to alter the laxity of the SIJ, with an applied force of the pelvic belt of maximally 50 N. Subsequently, the authors show that the laxity values of the SIJ decrease after application of a pelvic belt in patients with PGP (Vleeming et al. 1992a; Damen et al. 2001, 2002a; Mens et al. 2006). Patients with asymmetric SIJ laxity report significantly more pain during pregnancy compared with patients with symmetric laxity (Damen et al. 2002a). Pregnant women with moderate or severe pelvic pain have the same SIJ laxity as pregnant women with no or mild pain. According to these studies, the ‘asymmetry’ of laxity correlates with the symptoms within an individual. These findings seem to contradict the RSA studies. However, the RSA methodology analyzed patients during the flexion forward test in a loaded standing position. The DIV studies typically analyze unloaded patients in a supine position. The outcome of the DIV study appears to show that asymmetry can only be noticed when patients are relaxed and lying in a supine position. Another DIV study focused on the role of the transversus abdominis and multifidus muscles in stabilizing the SIJ, and confirmed increased stiffness and force closure of the SIJ when these muscles are activated (Richardson et al. 2002). Additional research using DIV reports that voluntary isometric contractions of muscles crossing the pelvis (e.g. the erector spinae, gluteus maximus and biceps femoris) also increase SIJ stiffness and force closure of the SIJ (van Wingerden et al. 2004). Nevertheless, the DIV methodology is demanding and therefore has been used only in a small subset of women with severe PGP, who also had sufficiently high baseline SIJ laxity to detect variations in the oscillations (de Groot et al. 2004). De Groot et al. (2004) comment that the DIV method is not reliable in patients with lower baseline SIJ laxity.
The conclusion of these latter studies is that a relation exists between pelvic asymmetric laxity and the severity of complaints (Buyruk et al. 1999; Damen et al. 2002a). Damen et al. state that subjects with asymmetric laxity of the SIJ during pregnancy have a threefold higher risk of moderate to severe pelvic pain persisting into the post partum period, compared with subjects with symmetric laxity during pregnancy, and that pelvic belt application can diminish the laxity and stiffen the pelvis. Based on the above-mentioned studies, a dysfunctional SIJ is normally not related to a subluxated position of the joint, but to increased or decreased compression/force closure due to asymmetric forces acting on the joint.
SIJ joint stability: a model based on form and force closure
Compared with quadripedal existence, bipedal posture in humans requires greater resistance to gravity. In the upright posture increased lumbopelvic compressional forces are necessary for stability, at the expense of mobility (Abitbol, 1987a,b, 1988; Aiello & Dean, 1990; Lovejoy, 2007). The principal areas of osteo-ligamentous support for the pelvis are the SIJ and the pubic symphysis. The pelvis can be regarded as a closed ring of variable stiffness (Vleeming et al. 1990a,b; Buyruk et al. 1995a, 1999; Mens et al. 2001). Biomechanical analysis shows that the major part of load transfer is through its cortical shell, and myofascial forces have a stabilizing effect on pelvic load transfer (Dalstra et al. 1993).
A model on myofascial–ligamentous force closing des-cribes efficient transfer of load that could not be sustained by pelvic structures alone (Vleeming, 1990; Vleeming et al. 1990a,b; Snijders et al. 1993a,b; Vleeming & Stoeckart, 2007). To illustrate the importance of stabilization in the SIJ, the principles of form and force closure were introduced (Vleeming, 1990; Vleeming et al. 1990a,b). Form closure refers to a theoretical stable situation in a joint with closely fitting surfaces, where no extra forces are needed to maintain the state of the system, given the actual load situation. For example, if the sacrum would fit in the pelvis with perfect form closure, no lateral compressional forces would be needed. However, such a construction would make mobility practically impossible. With force closure (leading to joint compression), both a lateral force and friction are needed to withstand vertical load.
The structural features that contribute to SIJ stability via ‘form closure’ include: (i) the configuration of the interfacing joint surfaces, along with dorsocranial ‘wedging’ of the sacrum into the ilia; (ii) the complementary ridges and grooves of the articular surfaces of the SIJs (Fig. 9) and resultant high coefficient of friction (Snijders et al. 1993a,b; Vleeming, 1990; Vleeming et al. 1990a,b); and (iii) the integrity of the binding ligaments, which are among the strongest in the body (Vleeming, 1990).
Shear in the SIJ is the result of a combination of specific anatomical features; form and force closure leading to effective tailored joint accommodation, and balancing friction/compression in the joint. Force closure is the result of altered joint reaction forces by tensing ligaments, fasciae, muscles and ground reaction forces (Vleeming, 1990; Vleeming et al. 1990a,b; Snijders et al. 1993a,b). Force closure ideally generates a perpendicular compressional reaction force to the SIJ to overcome the forces of gravity (Vleeming et al. 1990b).
In force closing the pelvis, nutation of the sacrum is essential. This movement can be regarded as anticipation for joint loading. Hodges et al. (2003) use the terminology ‘preparatory motion’ for a comparable phenomenon in the lumbar spine. Nutation represents a movement that tightens most of the SIJ ligaments, among which are the vast interosseous and dorsal sacroiliac ligaments (except the LDL), thereby preparing the pelvis for increased loading (Vrahas et al. 1995; Steinke et al. 2010). As a consequence, the posterior parts of the iliac bones are pressed together, increasing compression of the SIJ.
Importantly, stability and flexibility modes are simultaneously counter-opposing states in the SIJ and place conflicting demands on its joint construction. Ligaments are tensed when bones move in directions that lengthen them and/or the attached muscles contract. When the joint is force closed, friction increases (Vleeming et al. 1990b) and consequently augments, what is coined self-bracing of the joint (Vleeming, 1990; Vleeming et al. 1990a,b; Snijders et al. 1993a,b). Force closure reduces the joint's ‘neutral’ zone and increases the stiffness value of the joint. Shear forces are thereby controlled, facilitating stabilization of the joint.
The combination of regional and local ligaments, muscles, fascial systems and gravity contributes to force closure of the SIJ; thus, force closure is not the exclusive property of the deep stabilizing muscles (Stevens et al. 2007). When this mechanism works efficiently in the pelvis, the shear forces between the iliac bones and sacrum are adequately controlled, and loads can be effectively transferred between the trunk, pelvis and legs (Snijders et al. 1993a,b).
Moment-to-moment efficient neuromotor control of force closure is required to reinforce the normal postural myofascial tone that helps counteract gravity forces (Hodges & Richardson, 1996, 1997a,b; Moseley et al. 2002; Richardson et al. 2002; van Wingerden et al. 2004). Both normal postural tone and force closure are needed to provide an adequately tailored compression of the joints for movements and positioning, at efficient energy costs (Lee & Vleeming, 2007; Masi et al. 2007; Vleeming et al. 2008).
Motor control studies illustrate that a ‘rigid’ motor strategy is used for handling high loads and tasks with low predictability (O'Sullivan et al. 2002; Cholewicki et al. 2003; van Dieen et al. 2003a; Hodges et al. 2003; Beales et al. 2009, 2010). This strategy is often adopted in chronic lumbopelvic patients when there is insufficiency of the passive system and/or motor control as well as high levels of stress, and increased or perceived risk of pain and/or poor awareness of position and demand. Rigid strategies typically are based on maladaptive compensatory patterns in patients, leading to excessive force closure/compression, characterized by bracing and splinting of muscles. When larger levers are applied and/or coordination time becomes less, the general effect in the locomotor system will be closure or reduction of the degrees of freedom of the kinematic chain. Such responses lead to a reduction of the chain's mobility or a gain of stability by increasing force closure (Huson, 2007). ‘Control’ strategies are typical for efficient awareness of position and demand, and when there is a low real/or perceived risk for pain and stress. Thus, control strategies are suitable for handling low loads with high predictability (Cholewicki et al. 2003; Hodges & Cholewicki, 2007).
Disturbed or excessive force transfers through the SIJ can cause exaggerated compressional or torsional stresses on these joints. Such altered transmission to the spine and lower limbs can result in tissue effects with deleterious consequences (De Rosa & Porterflield, 2007; Masi et al. 2007). In contrast to excessive SIJ force closure, a counter-opposing condition of insufficient stability occurs in the syndrome of pregnancy-related PGP (Vleeming et al. 2008). Insufficient and asymmetric compression of the SIJs were shown to occur in PGP (Vleeming et al. 1992a; Damen et al. 2002a,b; Mens et al. 2006). Non-optimal load transfers and clinical effects would be expected to occur from either the suspected excessive pelvic and SIJ stiffness (Masi et al. 2007 2003; Lee & Vleeming, 2007) or the documented insufficient pelvic girdle stability of PGP (Mens et al. 1999). Sufficient compression of the SIJs can be defined as the amount needed to provide the necessary stability or stiffness for the particular demands of static or dynamic load transfer, at optimal utilization of energy (Vleeming et al. 2008 1990b; van Wingerden et al. 2004). Thus, stability per se is an instantaneous phenomenon, and is antagonistic to flexibility (McGill et al. 2003). Neither too little nor too much SIJ stability from either mechanical stiffness properties or force closure/compression is optimal.
>Self-bracing the pelvis
Various muscles are involved in force closure of the SIJ. Even muscles, such as the rectus femoris, sartorius, iliacus, gluteus maximus and hamstrings have adequate lever arms to influence movement in the SIJ. The effect of these muscles is dependent on open or closed kinematic movements, and whether the pelvis is sufficiently braced (Vleeming & Stoeckart, 2007).
Strain on the SIJ might result from asymmetric forces acting on the joint, such as increased unilateral pull through the erector/multifidus muscles and quadratus lumborum, or through the hamstrings (Vleeming & Stoeckart, 2007). Studies conclude that SIJ stiffness increases even with slight muscle activity, supporting the notion that effective load transfer from spine to legs is possible when muscle forces actively compress the SIJ, preventing shear (Richardson et al. 2002; van Wingerden et al. 2004). This is in agreement with studies from Cholewicki et al. (2000a,b), showing that in healthy people sufficient stability of the spine is achieved with modest levels of co-activation of the paraspinal and abdominal wall muscles. Altered motor control of deeper muscles, like the transversus abdominis, internal oblique, multifidus, diaphragmatic and pelvic floor muscles, plays an important role in lumbopelvic pain and support. These muscles exhibit anticipatory stabilizing ability, being activated just before gross movements with relatively higher predictability and lower loads (van Dieen et al. 2003b; Hodges et al. 2007). Deeper muscles are closer to the centers of rotation of the spinal elements and SIJs, compared with more superficial and external oblique slings, and hence exert higher compressive forces (Adams & Dolan, 2007). These muscles have a biomechanical advantage in providing truncal stabilization via increased force closure of spinal elements and the SIJs (Vleeming & Stoeckart, 2007). Along with the large muscles overlying the joint, contraction of other muscles such as the transversus abdominis, the pelvic floor muscles and the diaphragm can affect the stiffness of the spine and SIJ (Richardson et al. 2002; O'Sullivan et al. 2002; Hodges et al. 2003; Beales et al. 2009, 2010).
Recent studies have suggested that the SIJs are suboptimally braced when pelvic floor function fails. During increase of intra-abdominal pressure, the pelvic floor requires effective feedforward timing and muscle recruitment (O'Sullivan et al. 2002; Beales et al. 2009, 2010). Lumbopelvic muscle recruitment is also altered in the presence of LBP or SIJ pain (Hungerford et al. 2003; Hungerford & Gilleard, 2007). The latter study reveals a functional relationship between the biceps femoris, gluteus maximus, latissimus dorsi and erector spinae/multifidus muscles. This widespread muscle recruitment pattern was found to be altered in patients with PGP.
The erector spinae/multifidus is the pivotal muscle group that loads and extends the spine and pelvis. The sacral connections of the erector spinae/multifidus complex induce nutation in the SIJ, tensing ligaments such as the interosseous, STL and SSL. These muscles have a double function because their iliac connections pull the posterior sides of the iliac bones towards each other, constraining nutation. This implies that during nutation, due to action of the erector spinae/multifidus, the cranial side of the SIJ tends to be compressed whereas the caudal side has a tendency to widen or gape (Solonen, 1957). This process is restricted by the STL, tensed by nutation and with direct fascial connections to the erector spinae (Vleeming et al. 1995).
Owing to its perpendicular orientation to the SIJ, the gluteus maximus can force close the joints directly and also indirectly by its vast muscular connections with the STL. Additionally, cross-bracing occurs in the more superficial oblique back muscles, for example, latissimus dorsi and contralateral gluteus maximus (Vleeming et al. 1995; Mooney et al. 2001). The tension of the PLF can be influenced by these muscles as demonstrated by traction tests. Each of these coupled, cross-bracing structures allows for both load transfers and synergistic self-bracing between trunk, pelvis and contralateral thighs. Mooney et al. (2001) used the anatomical relation of the latissimus dorsi and the contralateral gluteus maximus muscles to study their coupled effect during axial rotation exercises and walking. They concluded that in normal individuals, walking a treadmill, the functional relationship between the mentioned muscles could be confirmed. In patients with SIJ problems a strikingly different pattern was noticed. On the symptomatic side the gluteus maximus was far more active compared with the healthy subjects. The reciprocal relation between latissimus and gluteus maximus muscles, however, was still present (Mooney et al. 2001). Despite the increased activation of gluteus maximus, patients with SIJ dysfunction demonstrate significant weakness in the muscle (Massoud Arab et al. 2011).
Comparable to the erector muscle, a double function of the hamstrings (including the biceps femoris muscle) can be described. Particularly in stooped positions and in sitting upright with straight legs, the hamstrings are well positioned to tilt the pelvis backwards. Additionally, the iliac bones are rotated posteriorly relative to the sacrum by tension or contraction of the biceps femoris and semimembranosus with connections to the STL (Vleeming et al. 1989a; van Wingerden et al. 1993; Barker et al. 2004). Nutation in stooped positions can help to avoid excessive loading of the posterior part of the lumbar discs.
The active straight leg raise (ASLR) test; analyzing load transfer in the pelvis
Mens et al. have developed a new diagnostic test for SIJ dysfunction. They studied the relationship between the ASLR test and mobility of the pelvic joints with and without the application of a pelvic belt. The sensitivity and specificity of the test proved to be high for patients with PGP. The test is suitable to discriminate between patients with PGP and healthy individuals (Mens et al. 2001 2002 1999). By means of X-rays taken after pregnancy, the authors demonstrated that the pubic bone rotates downwards on the symptomatic side, relative to the contralateral side when the symptomatic leg is freely hanging down in a standing position. This procedure differs from the classical Chamberlain X-ray method, which screens the symptomatic loaded side. The authors conclude that this symphyseal displacement on the symptomatic side could indicate an anterior rotation of the iliac bone relative to the sacrum (counternutation; Mens et al., 1999).
Hungerford et al. (2003) drew the same conclusion employing a different approach. These researchers used an external motion analysis system, using skin markers and a videosystem. They studied (three-dimensionally) the angular and translational displacements in patients with SIJ problems and in healthy persons. Externally placed skin markers can also be displaced due to skin and superficial fascial movements and, as such, are challenging to effectively analyze when attempting to evaluate minimal SIJ motion. However, Hungerford et al. showed full reversed movement patterns between healthy individuals and patients. The authors concluded that posterior rotation of the iliac bones relative to the sacrum (nutation) occurs in healthy persons on the weight-bearing side. Conversely, the iliac bones rotated anteriorly relative to the sacrum (counternutation) in the patient group. The same conclusion followed for the standing flexion test in patients – only on the loaded (standing) symptomatic side did anterior rotation of the iliac bone take place. It is suggested that these findings may also be due to reduced tonicity in the erector spinae, gluteus maximus, biceps femoris and external oblique muscles during ASLR test of patients with SIJ pain (Shadmehr et al. 2012). Shadmehr et al. also demonstrated a delayed activation of the adductor longus at the initation of the ASLR test.
Pregnant women with pregnancy-related low back and pelvic pain (PLBP) developed significantly higher muscle activity in the rectus femoris, psoas major and external oblique during the ASLR test than pregnant women with no PLBP (de Groot et al. 2008). Despite this increase in muscle activity, women with PLBP produced significantly less muscle force than non-PLBP women during the ASLR test. Again, these data suggest a link between SIJ pain and impaired load transfer across the SIJ.
Motor control strategies observed during the ASLR test appear to vary significantly when examined under various loading conditions in pain-free and nulliparous patients, and female patients with chronic PGP (O'Sullivan et al. 2002; Beales et al. 2009, 2010). In patients with PGP, diaphragmatic splinting occurs during the ASLR test while the pelvic floor is depressed. Applying manual support, mimicking the function of a pelvic belt, reverses the splinting motor strategy. This finding is consistent with a pain disorder associated with an impaired force closure mechanism. During the ASLR test, pain-free subjects adopt predominantly a motor pattern on the ipsilateral leg. Chronic patients adopt a bilateral bracing/splinting motor control pattern during the ASLR test. The authors note that the aberrant motor patterns in patients with chronic PGP may be considered as maladaptive.
A small elastic belt can mimic the failed self-bracing mechanisms of the pelvic musculature. In a loading experiment on embalmed human preparations, nutation of the sacrum decreased by about 20% when applying a belt force of only 50 N (Vleeming et al. 1992a). Such a belt is applied with a small force, similar to the force involved in lacing a pair of shoes. This will be sufficient to generate a self-bracing effect in the SIJs under heavy load (Vleeming et al. 1992a; Snijders et al. 1993a,b; Mens et al. 2006). The belt should normally be positioned just cranial to the greater trochanters; it crosses the SIJ over the middle part of the sacrum, and assists in preventing gapping of the SIJ. However, only in severe cases do patients need to wear belts for short periods. Continuous use of the belt could hamper effective rehabilitation; the belt diminishes normal activation patterns of the transversus and internal oblique muscles (Vleeming et al. 2008; Hu et al. 2010), as well as dorsal hip muscles such as the piriformis, gluteus medius, gluteus minimis (Pel et al. 2008a) and pelvic floor muscles (Pel et al. 2008b).
Pelvic belts enhance pelvic stability by reducing SIJ laxity (Damen et al. 2001, 2002a; Mens et al. 2006). Mens et al. suggest that, after initial provocative pelvic tests like the ASLR test, these tests should be repeated with application of a pelvic belt to assess possible differences. In these studies, only small tension (50 N) was applied to the belt, just above the greater trochanter. Larger forces did not yield better results, as predicted in a biomechanical study (Vleeming et al. 1992a). The efficacy of the pelvic belt was primarily dependent on the location of the belt, and the effects were explained by increased compression of the SIJ.
Hu et al. (2010) studied force closure mechanisms by examining the ASLR test. In healthy nulligravidae (N = 17) the ASLR test was performed, as they walked on a treadmill at increasing speeds, with and without a pelvic belt. Fine-wire electromyography (EMG) was used to record the activity of the psoas, iliacus and transversus abdominis muscles, while other hip and trunk muscles were recorded with surface EMG. During the ASLR test, all muscles were active. In both tasks, walking and the ASLR test, the transverse and oblique abdominal muscles became less active with the application of the belt. Hu et al. (2010) reported that hip flexors normally exert a forward rotating torque on the ilium. These researchers conclude that, apparently, the abdominal wall was active to prevent such a forward rotation. The fact that transverse and oblique abdominal muscles become less active in healthy persons when using a belt, suggests that the belt provides force closure in the SIJ, similar to the role of abdominal muscles as described in the literature (Hu et al. 2010). Application of the external Hoffman Slatis fixator in patients with severe PGP especially generates an anterior compression on the ilia, also leading to effective force closure (Sturesson et al. 1999). Hu et al. (2010) also determined that the psoas muscle is bilaterally active in normal healthy individuals when performing the ASLR test. From this observation, they conclude that the bilateral recruitment of the psoas muscle is necessary to stabilize the lumbosacral spine.
A proposed biomechanical model for stabilizing the pelvic joints in the transverse plane
Several studies have demonstrated that, among other muscles, contraction of the internal oblique and transversus results in force closing of the pelvic ring (Hungerford & Gilleard, 2007; Beales et al. 2009, 2010; Hu et al. 2010). Conversely, in patients with PGP, a maladaptive compensatory pattern occurs characterized by diminished activity of these muscles, and a subsequent failing to brace the pelvis (Hu et al. 2010).
A model adapted after Snijders et al. (1995) shows the biomechanical effect of pelvic stabilization by anterior abdominal muscles, and the impact of both the pelvic belt and external fixator (Fig. 10; Sturesson et al. 1999). Muscles, ligaments and fascia with appropriate force direction are indicated. A special effect of the internal oblique and transverse muscles can be noted (Fo). Hip flexors like the iliacus, sartorius and rectus femoris can partially contribute to this reaction force (Fo), dependent on leg position relative to the hip joint.
The lever arm of the anterior muscles (Fo; Fig. 0) provokes a gapping force to the stiff interosseous and dorsal SIJ ligaments and the dense composite of the fascia muscle complex (Fi); a large joint reaction force (Fj) is the result. Altered anterior abdominal muscle function (Fo), with or without combined loss of strength in the dorsal composite and SIJ ligaments, could destabilize the pelvis (Hu et al. 2010; note in Fig. 10, nr 8: a clamp representing the composite). In that case, hip flexor muscles like the iliacus acting within an open kinematic chain, without sufficient bracing of the pelvis, could selectively initiate anterior movement of the ilium relative to the sacrum (counternutation), instead of raising the leg during the ASLR test (Hu et al. 2010). Counternutation of the SIJ is less guarded by ligaments, and depends especially on the integrity of the LDL and probably the anterior part of the strong interosseous ligament (Vleeming et al. 2002). This magnification of anterior abdominal force in this biomechanical model (Fig. 10) resembles the mechanism of a nutcracker (Snijders et al. 1995).
Fig. 10.
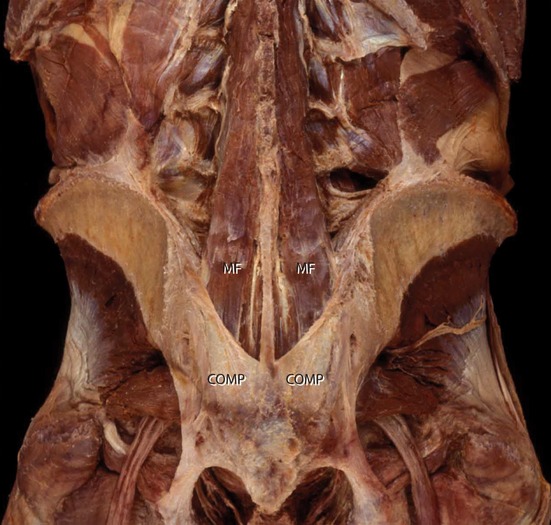
The erector muscles are dissected and the multifidus muscle becomes clearly visible after removing the superficial and deep lamina of the PLF and aponeurose over the multifidus. COMP, indicates the remaining blended parts of the strong fascial composite and aponeurosis over the sacrum. (With permission from the Willard Carreiro collection.)
Finally, tensioning of the anterior muscles compresses the anterior aspect of the SIJ, but would be expected to open the posterior aspect of the joint unless it was braced with a strong ‘clamp’. To this end, a ‘composite’ of structures overlies the lower dorsal vertebral column and the SIJ (Vleeming & Willard, 2010). A previous study has demonstrated that the TLF becomes thicker especially over the L5 and S1, S2 (Fig. 6; Vleeming et al. 1995). Directly anterior to the superficial and deep lamina of the PLF lies the strong erector spinae aponeurosis, overlying both the multifidus and sacrospinal parts of the erector muscles (Fig. 11). The lumbar multifidus blends superficially to the aponeurosis on the dorsal side of the sacrum. At the level of the PSIS the superficial and deep layers of the TLF and the aponeurosis of the erector spinae fuse together. Subsequently, this composite fuses to the ilia and blends into the STL inferiorly (Vleeming & Willard, 2010; reviewed in Willard et al. 2012). Contraction of both the erector muscle and multifidus (Fig. 5) would increase the tension across these blended layers of fascia, by pulling and dilating the composite layers. Tension within this composite of TLF (Fig. 6) will have the effect of drawing PSIS towards the midline and thereby tightening the posterior aspect of the SIJ.
Fig. 11.
Cross-section of the SIJ on the level of S1. Force application indicated, mainly by the transverse and internal oblique muscles (Fo), producing tension dorsally both to the SIJ ligaments and the composite of the TLF; (Fi); a larger reaction force ensues (Fj). Lateral tuberculum sacrum (1), ventral part of the ilium (2), auricular part of the SIJ (3), dorsal SIJ ligaments (4, 6), anterior SIJ ligaments (5), LDL (7), composite of TLF with aponeurosis and muscles visualized as a clamp (8). (Adapted after Snijders et al. 1995.)
Altered motor function of the deep abdominal muscles in patients with PGP leads to insufficient bracing of the pelvis. If a large lever is applied to the pelvis, like rasing a straight leg while lying supine, the pelvic joints are strongly perturbed. Former studies showed that patients with exceptionally severe PGP, who have failed interdisciplinary rehabilitative care and are unable to walk, can reverse their clinical symptoms after application of a surgically fitted external fixator frame (Sturesson et al. 1999). Directly after the operation, anterior compression is applied to the frame, simulating force closure and enabling most patients to walk again. If successful application of the external frame is verified after several weeks, the frame can be removed followed by surgical arthrodesis of the SIJ.
The proposed biomechanical model shows how (deep) abdominal muscles, a pelvic belt or an external pelvic fixation frame can provoke a dorsal gapping force to the pelvis with a resultant force closing reaction of the SIJ joint surfaces. Without appropriate bracing of the deep abdominal muscles, flexor hip muscles like the iliacus can pull the ilium anteriorly, creating a counternutated position in the SIJ. Counternutation is typical for unloaded situations such as encountered when lying supine. Mens et al. (1999), as well as Hungerford et al. (2003) and Hungerford & Gilleard (2007), have demonstrated that counternutation is typical for patients with PGP. Although these initial studies are encouraging, further verification of this model is required.
Summary
Over the past 200 years, descriptions and concepts on the many integrated components of the human SIJs and pelvic girdle systems have emerged. In particular, the concept of motion in the SIJ has undergone a slow, convoluted evolution. Although initial writings suggested that motion existed normally between the ilia and sacrum, the idea fell out of favor in the mid-20th century. Current research, however, now supports the existence of a limited motion in the average range of 2 ° in all three planes of this joint.
The tenet that form follows function and adapts to biomechanical demands can help in interpreting the development, maturation, bony architecture, passive ligamentous supports and active myofascial stabilization of the amphiarthrotic SIJs. Enlargement of the joint is initially seen at 2 months gestation, and evidence suggests that early movement in utero may play a role in the normal development of the joint. Full development of the joint occurs by 8 months gestation; however, the surface of the sacral part of the joint remains smooth until after puberty, when roughening begins to occur. Based on studies of diplegic individuals, movement in the adult joint is also thought to influence the maintenance of its normal adult morphology.
The emergence of a pronounced sexual dimorphism in joint structure occurs after puberty. These changes can progress into age-related degenerative processes, more so in males than females, involving osteophyte formation and anklylosis. However, such events are nowhere near as common as initially proposed in the literature. Recent work has questioned the degree of degeneration normally present in the aging SIJ, suggesting that both sexes maintain a normal range of motion through the sixth decade with a slight reduction of movement occurring in males thereafter.
The SIJ is unique in having elements of a combined synarthrosis and a diarthrosis – hence the term amphiarthrosis. The main portion of the joint is surrounded by a complex capsule and lined with cartilage (diarthrosis). Its shape is auricular, and ‘opens’ posteriorly. The sacrum and ilia have an extracapsular, dorsally located articulation (synarthrosis), which is augmented by the vast ISL that provides considerable internal stability. Essentially, the SIJ is encased in a capsule that has a smooth anterior wall and irregular bands comprising the posterior wall. The capsule is innervated, at least, from the dorsal lumbosacral rami and is surrounded by several strong ligaments, such as the STLs, SSLs, long dorsal sacroiliac and ILs, which influence its range of motion. In turn, these ligaments are related to a complex TLF composite derived from the aponeuroses of several large muscles that surround the joint at a distance. Included in these muscle groups are the paraspinal muscles and the abdominal muscles, as well as some of the muscles from the posterior compartment of the upper and lower extremities.
The SIJ and bony pelvis has been modeled as a closed ring onto which three large levers – the spine and the two lower extremities – are attached. The pelvis has a large external movement, mainly through the hips, and a small internal movement through the SIJ and symphysis. The wedged shape of the sacrum and the lack of significant interdigitation with the ilium means that the SIJ has to depend to a greater extent on force closure of the joint space rather than form closure. The reported increased friction of the SIJ supports this notion. This balance of forces across the lower spine and SIJ is dependent on adequate motor control patterns. Partially coupled muscle groups, such as the latissimus dorsi and gluteus maximus; the erector, the multifidus and hamstrings partially connected to the STL; the pelvic floor muscles, the transverse and internal oblique muscles connected to the TLF and the ilia (in)directly span and can influence the SIJ.
Degenerative damage to the joint may result from trauma or microtrauma secondary to having either too much laxity or excessive compressional stiffness in the joint, either of which can be derived of structural or neuromuscular control etiology. It has been demonstrated that insufficient and/or asymmetric compression of the SIJs were shown to occur in patients suffering from PGP. PGP is felt between the posterior iliac crest and the gluteal fold, generally over the region of the SIJs. Typically, PGP is associated with a current or past pregnancy, but may also arise secondary to trauma or reactive arthritis. Maladaptive, compensatory patterns of motor control are described in PGP, suggesting that the body attempts to compensate for a changed laxity or firmness of the joint. This altered motor function in patients with PGP leads to insufficient bracing of the pelvis. The resulting perturbation of joint mechanics may represent a predisposing factor to the development of degenerative processes in the joint. Understanding of these mechanisms of pelvic instability has led to the development of treatment strategies that have demonstrated promise in helping to stabilize the upright pelvis.
References
- Abitbol MM. Evolution of the lumbosacral angle. Am J Phys Anthropol. 1987a;72:361–372. doi: 10.1002/ajpa.1330720309. [DOI] [PubMed] [Google Scholar]
- Abitbol MM. Evolution of the sacrum in hominoids. Am J Phys Anthropol. 1987b;74:65–81. doi: 10.1002/ajpa.1330740107. [DOI] [PubMed] [Google Scholar]
- Abitbol MM. Evolution of the ischial spine and of the pelvic floor in the Hominoidea. Am J Phys Anthropol. 1988;75:53–67. doi: 10.1002/ajpa.1330750107. [DOI] [PubMed] [Google Scholar]
- Abramson D, Roberts SM, Wilson PD. Relaxation of the pelvic joints in pregnancy. Surg Gynecol Obstet. 1934;58:595–613. [Google Scholar]
- Adams MA, Dolan P. How to use the spine, pelvis, and legs effectively in lifting. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone Elsevier; 2007. pp. 167–183. [Google Scholar]
- Ahlstrom H, Feltelius N, Nyman R, et al. Magnetic resonance imaging of sacroiliac joint inflammation. Arthritis Rheum. 1990;33:1763–1769. doi: 10.1002/art.1780331202. [DOI] [PubMed] [Google Scholar]
- Aiello L, Dean C. An Introduction to Human Evolutionary Anatomy. London: Academic Press; 1990. Bipedal locomotion and the postcranial skeleton; pp. 244–274. [Google Scholar]
- Albee FH. A study of the anatomy and the clinical importance of the sacroiliac joint. JAMA. 1909;16:1273–1276. [Google Scholar]
- Bakland O, Hansen JH. The “axial sacroiliac joint”. Anat Clin. 1984;6:29–36. doi: 10.1007/BF01811211. [DOI] [PubMed] [Google Scholar]
- Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213–223. doi: 10.1136/ard.30.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PJ, Briggs CA, Bogeski G. Tensile transmission across the lumbar fasciae in unembalmed cadavers: effects of tension to various muscular attachments. Spine (Phila Pa 1976) 2004;29:129–138. doi: 10.1097/01.BRS.0000107005.62513.32. [DOI] [PubMed] [Google Scholar]
- Beal MC. The sacroiliac problem: review of anatomy, mechanics, and diagnosis. J Am Osteopath Assoc. 1982;81:667–679. [PubMed] [Google Scholar]
- Beales DJ, O'Sullivan PB, Briffa NK. Motor control patterns during an active straight leg raise in chronic pelvic girdle pain subjects. Spine (Phila Pa 1976) 2009;34:861–870. doi: 10.1097/BRS.0b013e318198d212. [DOI] [PubMed] [Google Scholar]
- Beales DJ, O'Sullivan PB, Briffa NK. The effects of manual pelvic compression on trunk motor control during an active straight leg raise in chronic pelvic girdle pain subjects. Man Ther. 2010;15:190–199. doi: 10.1016/j.math.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Bellamy N, Parl W, Rooney PJ. What do we know about the sacroiliac joint? Semin Arthritis Rheum. 1983;12:282–313. doi: 10.1016/0049-0172(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AA, May R, Renner JB, et al. Surprising evidence of pelvic growth (widening) after skeletal maturity. J Orthop Res. 2011;29:1719–1723. doi: 10.1002/jor.21469. [DOI] [PubMed] [Google Scholar]
- Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;217:266–280. [PubMed] [Google Scholar]
- Bertino AJ. Forensic Science – Fundamentals and Investigations. Mason, OH: South-Western Cengage Learning; 2000. p. 368. [Google Scholar]
- Biermann H. Die knochenbildung im bereich periostaler-diaphysarer sehnen- und bandanstaze. [Ossification in the region of periosteal–diaphysial tendon and ligament insertion] Z Zellforsch Mikrosk Anat. 1957;46:635–671. [PubMed] [Google Scholar]
- Bogduk N. The anatomical basis for spinal pain syndromes. J Manipulative Physiol Ther. 1995;18:603–605. [PubMed] [Google Scholar]
- Bogduk N, Twomey LC. Clinical Anatomy of the Lumbar Spine. Edinburgh: Churchill Livingstone; 1991. [Google Scholar]
- Bonnaire E, Bue V. Influence de la position sur la forme et les dimensions du bassin. Ann Gynecol Obstet. 1899;52:296. [Google Scholar]
- Borell U, Fernstrom I. The movements at the sacroiliac joints and their importance to changes in the pelvic dimensions during parturation. Acta Obstet Gynecol Scand. 1957;36:42–57. doi: 10.3109/00016345709158023. [DOI] [PubMed] [Google Scholar]
- Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 1996;8:124–129. doi: 10.1097/00002281-199603000-00007. [DOI] [PubMed] [Google Scholar]
- Bowen V, Cassidy JD. Macroscopic and microscopic anatomy of the sacroiliac joint from embryonic life to the the eighth decade. Spine (Phila Pa 1976) 1981;6:620–628. doi: 10.1097/00007632-198111000-00015. [DOI] [PubMed] [Google Scholar]
- Bradley KC. The anatomy of backache. Aust N Z J Surg. 1974;41:227–232. doi: 10.1111/j.1445-2197.1974.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Braune CW, Fischer O. Bestimmung der Tragheitsmomente des menschlichen Korpers und seine Glieder. Abhandl Math Phys Kl Sachs Ges Wiss. 1892;18:409. [Google Scholar]
- Briggs CA, Chandraraj S. Variations in the lumbosacral ligament and associated changes in the lumbosacral region resulting in compression of the fifth dorsal root ganglion and spinal nerve. Clin Anat. 1995;8:339–346. doi: 10.1002/ca.980080506. [DOI] [PubMed] [Google Scholar]
- Brochner JEW. Die Wirbelsaulenleiden und ihre differentialdiagnose. Stuttgart: Thieme; 1962. [PubMed] [Google Scholar]
- Brooke R. The sacro-iliac joint. J Anat. 1924;58:299–305. [PMC free article] [PubMed] [Google Scholar]
- Brower AC. Disorders of the sacroiliac joint. Surgical Rounds for Orthopedics. 1989;13:47–54. [Google Scholar]
- Buyruk HM, Snijders CJ, Vleeming A, et al. The measurements of sacroiliac joint stiffness with colour Doppler imaging: a study on healthy subjects. Eur J Radiol. 1995a;21:117–121. doi: 10.1016/0720-048x(95)00716-4. [DOI] [PubMed] [Google Scholar]
- Buyruk HM, Stam HJ, Snijders CJ, et al. The use of color Doppler imaging for the assessment of sacroiliac joint stiffness: a study on embalmed human pelvises. Eur J Radiol. 1995b;21:112–116. doi: 10.1016/0720-048x(95)00704-t. [DOI] [PubMed] [Google Scholar]
- Buyruk HM, Stam HJ, Snijders CJ, et al. Measurement of sacroiliac joint stiffness in peripartum pelvic pain patients with Doppler imaging of vibrations (DIV) Eur J Obstet Gynecol Reprod Biol. 1999;83:159–163. doi: 10.1016/s0301-2115(98)00331-5. [DOI] [PubMed] [Google Scholar]
- Chamberlain WE. The symphysis pubis in the Roentgen examination of the sacro-iliac joint. Am J Roentgenol. 1930;24:621–625. [Google Scholar]
- Cholewicki J, Polzhofer GK, Radebold A. Postural control of trunk during unstable sitting. J Biomech. 2000a;33:1733–1737. doi: 10.1016/s0021-9290(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Simons AP, Radebold A. Effects of external trunk loads on lumbar spine stability. J Biomech. 2000b;33:1377–1385. doi: 10.1016/s0021-9290(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, van Dieen JH, Arsenault AB. Muscle function and dysfunction in the spine. J Electromyogr Kinesiol. 2003;13:303–304. doi: 10.1016/s1050-6411(03)00038-5. [DOI] [PubMed] [Google Scholar]
- Chow DH, Luk KDK, Leong JC, et al. Torsional stability of the lumbosacral junction. Significance of the iliolumbar ligament. Spine (Phila Pa 1976) 1989;14:611–615. doi: 10.1097/00007632-198906000-00013. [DOI] [PubMed] [Google Scholar]
- Cohen AS, McNeill JM, Calkins E, et al. The “normal” sacroiliac joint. Analysis of 88 sacroiliac roentgenograms. Am J Roentgenol Radium Ther Nucl Med. 1967;100:559–563. [PubMed] [Google Scholar]
- Colachis SC, Worden RE, Bechtol CO, et al. Movement of the sacroiliac joint in adult male. Arch Phys Med Rehabil. 1963;44:490–498. [PubMed] [Google Scholar]
- Cole JD, Blum DA, Ansel LJ. Outcome after fixation of unstable posterior pelvic ring injuries. Clin Orthop Relat Res. 1996;16:0–79. doi: 10.1097/00003086-199608000-00020. [DOI] [PubMed] [Google Scholar]
- Dalstra M, Huiskes R, Odgaard A, et al. Mechanical and textural properties of pelvic trabecular bone. J Biomech. 1993;26:523–535. doi: 10.1016/0021-9290(93)90014-6. [DOI] [PubMed] [Google Scholar]
- Damen L, Buyruk HM, Guler-Uysal F, et al. Pelvic pain during pregnancy is associated with asymmetric laxity of the sacroiliac joints. Acta Obstet Gynecol Scand. 2001;80:1019–1024. doi: 10.1034/j.1600-0412.2001.801109.x. [DOI] [PubMed] [Google Scholar]
- Damen L, Buyruk HM, Guler-Uysal F, et al. The prognostic value of asymmetric laxity of the sacroiliac joints in pregnancy-related pelvic pain. Spine (Phila Pa 1976) 2002a;27:2820–2824. doi: 10.1097/00007632-200212150-00018. [DOI] [PubMed] [Google Scholar]
- Damen L, Spoor CW, Snijders CJ, et al. Does a pelvic belt influence sacroiliac joint laxity? Clin Biomech. 2002b;17:495–498. doi: 10.1016/s0268-0033(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Dar G, Hershkovitz I. Sacroiliac joint bridging: simple and reliable criteria for sexing the skeleton. J Forensic Sci. 2006;51:480–483. doi: 10.1111/j.1556-4029.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- Daum WJ. The sacroiliac joint: an underappreciated pain generator. Am J Orthop. 1995;24:475–478. [PubMed] [Google Scholar]
- De Rosa C, Porterflield JA. Anatomical linkages and muscle slings of the lumbopelvic region. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 47–62. [Google Scholar]
- Derry DE. The influence of sex on the position and composition of the human sacrum. J Anat Physiol. 1912;46:184–192. [PMC free article] [PubMed] [Google Scholar]
- van Dieen JH, Cholewicki J, Radebold A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine (Phila Pa 1976) 2003a;28:834–841. [PubMed] [Google Scholar]
- van Dieen JH, Kingma I, van der Bug P. Evidence for a role of antagonistic cocontraction in controlling trunk stiffness during lifting. J Biomech. 2003b;36:1829–1836. doi: 10.1016/s0021-9290(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Diemerbroeck I. The Anatomy of Human Bodies. London: Brewster; 1689. [Google Scholar]
- Dihlmann W. [Roentgen diagnostic studies on the sacroiliac joint. I. Degenerative changes] Fortschr Geb Rontgenstr Nuklearmed. 1962;96:812–822. [PubMed] [Google Scholar]
- Dihlmann W. Diagnostic Radiology of the Sacroiliac Joints. New York: Georg Thieme; 1980. [Google Scholar]
- Dijkstra PF, Vleeming A, Stoeckart R. Complex motion tomography of the sacroiliac joint. An anatomical and roentgenological study. Rofo. 1989;150:635–642. doi: 10.1055/s-2008-1047093. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Sokoloff I. The role of movement in embryonic joint development. Dev Biol. 1966;14:401–420. [Google Scholar]
- Duncan JM. The behaviour of the pelvic articulations in the mechanism of parturation. Dublin Quart J Med Sci. 1854;18:60–69. [PMC free article] [PubMed] [Google Scholar]
- Ebraheim NA, Biyani A. Percutaneous computed tomographic stabilization of the pathologic sacroiliac joint. Clin Orthop Relat Res. 2003;408:252–255. doi: 10.1097/00003086-200303000-00033. [DOI] [PubMed] [Google Scholar]
- Egund N, Olsson TH, Schmid H, et al. Movements in the sacroiliac joints demonstrated with roentgen stereophotogrammetry. Acta Radiol Diagn. 1978;19:833–846. doi: 10.1177/028418517801900513. [DOI] [PubMed] [Google Scholar]
- Ehara S, el-Khoury GY, Bergman RA. The accessory sacroiliac joint: a common anatomic variant. Am J Roentgenol. 1988;150:857–859. doi: 10.2214/ajr.150.4.857. [DOI] [PubMed] [Google Scholar]
- Eichenseer PH, Sybert DR, Cotton JR. A finite element analysis of sacroiliac joint ligaments in response to different loading conditions. Spine (Phila Pa 1976) 2011;36:1446–1452. doi: 10.1097/BRS.0b013e31820bc705. [DOI] [PubMed] [Google Scholar]
- Fischer LP, Gonon GP, Carret JP, et al. Biomecanique articulaire. Lyon: Ass. Corp. et Med. Tome 2; 1976. pp. 33–36. [Google Scholar]
- Forthergill WF. Walchers position in parturation. Br Med J. 1896;2:1290–1292. [Google Scholar]
- Fortin JD, Aprill CN, Ponthieux B, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique: part II: clinical evaluation. Spine (Phila Pa 1976) 1994a;19:1483–1489. [PubMed] [Google Scholar]
- Fortin JD, Dwyer AP, West S, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976) 1994b;19:1475–1482. [PubMed] [Google Scholar]
- Fortin JD, Kissling RO, O'Connor BL, et al. Sacroiliac joint innervation and pain. Am J Orthop. 1999a;28:687–690. [PubMed] [Google Scholar]
- Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. Am J Neuroradiol. 1999b;20:1429–1434. [PMC free article] [PubMed] [Google Scholar]
- Gardner E. Physiology of movable joints. Physiol Rev. 1950;30:127–176. doi: 10.1152/physrev.1950.30.2.127. [DOI] [PubMed] [Google Scholar]
- Gardner E, Gray DJ, O'Rahilly R. Anatomy: A Regional Study of Human Structure. Philadelphia: W.B. Saunders; 1975. [Google Scholar]
- Garras DN, Carothers JT, Olson SA. Single-leg-stance (flamingo) radiographs to assess pelvic instability: how much motion is normal? J Bone Joint Surg Am. 2008;90:2114–2118. doi: 10.2106/JBJS.G.00277. [DOI] [PubMed] [Google Scholar]
- Gerlach UJ, Lierse W. Functional construction of the sacroiliac ligamentous apparatus. Acta Anat. 1992;144:97–102. doi: 10.1159/000147291. [DOI] [PubMed] [Google Scholar]
- Ghormley RK. Backache, examination and differential diagnosis. JAMA. 1944;125:412–416. [Google Scholar]
- Graves JE, Pollock ML, Carpenter DM, et al. Quantitative assessment of full range-of-motion isometric lumbar extension strength. Spine (Phila Pa 1976) 1990;15:289–294. doi: 10.1097/00007632-199004000-00008. [DOI] [PubMed] [Google Scholar]
- Gray H. Sacroiliac joint pain: finer anatomy, mobility and axes of rotation. Etiology, diagnosis and treatment by manipulation. Int Clin. 1938;2:54–96. [Google Scholar]
- Gray H. Gray's Anatomy. London: Longman; 1973. [Google Scholar]
- Grob KR, Neuhuber WL, Kissling RO. Innervation of the sacroiliac joint of the human. Z Rheumatol. 1995;54:117–122. [PubMed] [Google Scholar]
- de Groot M, Spoor CW, Snijders CJ. Critical notes on the technique of Doppler imaging of vibrations (DIV) Ultrasound Med Biol. 2004;30:363–367. doi: 10.1016/j.ultrasmedbio.2003.10.025. [DOI] [PubMed] [Google Scholar]
- de Groot M, Pool-Goudzwaard AL, Spoor CW, et al. The active straight leg raising test (ASLR) in pregnant women: differences in muscle activity and force between patients and healthy subjects. Man Ther. 2008;13:68–74. doi: 10.1016/j.math.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Hammer N, Steinke H, Böhme J, et al. Description of the iliolumbar ligament for computer-assisted reconstruction. Ann Anat. 2010;192:162–167. doi: 10.1016/j.aanat.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Hanson P, Sonesson B. The anatomy of the iliolumbar ligament. Arch Phys Med Rehabil. 1994;75:1245–1246. doi: 10.1016/0003-9993(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Hanson P, Sorensen H. The lumbosacral ligament. An autopsy study of young black and white people. Cells Tissues Organs. 2000;166:373–377. doi: 10.1159/000016753. [DOI] [PubMed] [Google Scholar]
- Hanson P, Magnusson SP, Sorensen H, et al. Differences in the iliolumbar ligament and the transverse process of the L5 vertebra in young white and black people. Acta Anat. 1998;163:218–223. doi: 10.1159/000046501. [DOI] [PubMed] [Google Scholar]
- Hisaw FL. The influence of the ovary on the resorption of the pubic bones. J Exp Zool. 1925;23:661. [Google Scholar]
- Hodges PW, Cholewicki J. Functional control of the spine. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement Stability & Lumbopelvic Pain: Integration and research. Edinburgh: Churchill Livingstone; 2007. pp. 489–512. [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine (Phila Pa 1976) 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997a;77:132–142. doi: 10.1093/ptj/77.2.132. discussion 42–44. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Exp Brain Res. 1997b;114:362–370. doi: 10.1007/pl00005644. [DOI] [PubMed] [Google Scholar]
- Hodges P, Kaigle Holm A, Holm S, et al. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine (Phila Pa 1976) 2003;28:2594–2601. doi: 10.1097/01.BRS.0000096676.14323.25. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Sapsford R, Pengel LH. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodyn. 2007;26:362–371. doi: 10.1002/nau.20232. [DOI] [PubMed] [Google Scholar]
- Hollinshead WH. Anatomy for Surgeons. New York: Harper and Row; 1969. [Google Scholar]
- Hu H, Meijer OG, van Dieen JH, et al. Muscle activity during the active straight leg raise (ASLR), and the effects of a pelvic belt on the ASLR and on treadmill walking. J Biomech. 2010;43:532–539. doi: 10.1016/j.jbiomech.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Baan GC. Myofascial force transmission: muscle relative position and length determine agonist and synergist muscle force. J Appl Physiol. 2003;94:1092–1107. doi: 10.1152/japplphysiol.00173.2002. [DOI] [PubMed] [Google Scholar]
- Hungerford B, Gilleard W. The pattern of intrapelvic motion and lumbopelvic muscle recruitment alters in the presence of pelvic girdle pain. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability, and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 361–376. [Google Scholar]
- Hungerford B, Gilleard W, Hodges P. Evidence of altered lumbopelvic muscle recruitment in the presence of sacroiliac joint pain. Spine (Phila Pa 1976) 2003;28:1593–1600. [PubMed] [Google Scholar]
- Huson A. Kinematic models and the human pelvis. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration of Research and Therapy. Edinburgh: Churchill Livingstone; 2007. pp. 159–166. [Google Scholar]
- Ikeda R. Innervation of the sacroiliac joint. Macroscopical and histological studies. Nippon Ika Daigaku Zasshi. 1991;58:587–596. doi: 10.1272/jnms1923.58.587. [DOI] [PubMed] [Google Scholar]
- Indahl A, Kaigle A, Reikeras O, et al. Sacroiliac joint involvement in activation of the porcine spinal and gluteal musculature. J Spinal Disord. 1999;12:325–330. [PubMed] [Google Scholar]
- Jarcho J. Value of Walcher position in contracted pelvis with special reference to its effect on true conjugate diameter. Surg Gynecol Obstet. 1929;49:854–858. [Google Scholar]
- Johnston TB, Whillis J. Gray's Anatomy: Descriptive and Applied. 28th edn. London: Longmans, Green; 1944. p. 1558. [Google Scholar]
- Jurriaans E, Friedman L. CT and MRI of the sacroiliac joints/movement, stability and low back pain: the essential role of the pelvis. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 1997. pp. 347–367. [Google Scholar]
- Kampen WU, Tillmann B. Age-related changes in the articular cartilage of human sacroiliac joint. Anat Embryol. 1998;198:505–513. doi: 10.1007/s004290050200. [DOI] [PubMed] [Google Scholar]
- Kapandji IA. The Physiology of the Joints. Edinburgh: Churchill Livingstone; 1974. [Google Scholar]
- Khan MA, Kushner I. Ankylosing spondylitis and multiple sclerosis: a possible association. Arthritis Rheum. 1979;22:784–786. doi: 10.1002/art.1780220716. [DOI] [PubMed] [Google Scholar]
- Kiapour A, Abdelgawad AA, Goel VK, et al. Relationship between limb length discrepancy and load distribution across the sacroiliac joint – a finite element study. J Orthop Res. 2012;30:1577–1580. doi: 10.1002/jor.22119. [DOI] [PubMed] [Google Scholar]
- Kibsgård TJ, Røise O, Stuge B, et al. Precision and accuracy measurement of radiostereometric analysis applied to movement of the sacroiliac joint. Clin Orthop Relat Res. 2012 doi: 10.1007/s11999-012-2413-5. DOI: 10.1007/s11999-012-2413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkaldy-Willis WH, Burton CV. Managing Low Back Pain. New York: Churchill Livingstone; 1992. Pathology and pathogenesis of low back pain; pp. 49–79. [Google Scholar]
- Kissling R, Brunner C, Jacob HA. [Mobility of the sacroiliac joint in vitro] Z Orthop Ihre Grenzgeb. 1990;128:282–288. doi: 10.1055/s-2008-1039994. [DOI] [PubMed] [Google Scholar]
- Knese KH, Biermann H. Die knochenbildung an sehnen- und bandansatzen im bereich ursprunglich chondraler apophysen. Z Zellforsch Mikrosk Anat. 1958;49:142–187. [PubMed] [Google Scholar]
- Kopsch FR. Rauber-Kopsch: Lehrbuch und Atlas der Anatomie des Menschen. Leipzig: Georg Thieme; 1940. [Google Scholar]
- Lapadula G, Covelli M, Numo R, et al. Iliolumbar ligament ossification as a radiologic feature of reactive arthritis. J Rheumatol. 1991;18:1760–1762. [PubMed] [Google Scholar]
- Lavignolle B, Vital JM, Senegas J, et al. An approach to the functional anatomy of the sacroiliac joints in vivo. Anat Clin. 1983;5:169–176. doi: 10.1007/BF01799002. [DOI] [PubMed] [Google Scholar]
- Lee D, Vleeming A. An integrated therapeutic approach to the treatment of the pelvic girdle. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 621–638. [Google Scholar]
- Leong JC, Luk KD, Chow DH, et al. The biomechanical functions of the iliolumbar ligament in maintaining stability of the lumbosacral junction. Spine (Phila Pa 1976) 1987;12:669–674. doi: 10.1097/00007632-198709000-00005. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO. Evolution of human walking. Sci Am. 1988;259:118–125. doi: 10.1038/scientificamerican1188-118. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO. Evolution of the human lumbopelvic region and its relationship to some clinical deficits of the spine and pelvis. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 141–158. [Google Scholar]
- Luk KDK, Ho HC, Leong JCY. The iliolumbar ligament a study of its anatomy development and clinical significance. J Bone Joint Surg Br. 1986;68:197–200. doi: 10.1302/0301-620X.68B2.3958002. [DOI] [PubMed] [Google Scholar]
- Luschka H. Die Anatomie des Menschen in der Rücksicht auf die Bedürfnisse der praktischen Heilkunde. In: Bender H, editor. Rom und römisches Leben im Alterthum. Tubingen: Verlag der H. Laupp'schen Buchhandlung Laupp & Siebeck; 1864. pp. 94–103. [Google Scholar]
- Lynch FW. The pelvic articulations during pregnancy, labour and puerperium: an X-ray study. Surg Gynecol Obstet. 1920;30:575–580. [Google Scholar]
- Masi AT. Do sex hormones play a role in ankylosing spondylitis? Rheum Dis Clin North Am. 1992;18:153–176. [PubMed] [Google Scholar]
- Masi AT, Walsh EG. Ankylosing spondylitis: integrated clinical and physiological perspectives. Clin Exp Rheumatol. 2003;21:1–8. [PubMed] [Google Scholar]
- Masi AT, Dorsch JL, Cholewicki J. Are adolescent idiopathic scoliosis and ankylosing spondylitis counter-opposing conditions? A hypothesis on biomechanical contributions predisposing to these spinal disorders. Clin Exp Rheumatol. 2003;21:573–580. [PubMed] [Google Scholar]
- Masi AT, Benjamin M, Vleeming A. Anatomical, biomechanical, and clinical perspectives on sacroiliac joints: an itegrative sythesis of biodynamic mechanisms related to ankylosing spondylitis. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 205–227. [Google Scholar]
- Masi AT, Nair K, Andonian BJ, et al. Integrative structural biomechanical concepts of ankylosing spondylitis. Arthritis. 2011;2011:205 904. doi: 10.1155/2011/205904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud Arab A, Reza Nourbakhsh M, Mohammadifar A. The relationship between hamstring length and gluteal muscle strength in individuals with sacroiliac joint dysfunction. J Man Manip Ther. 2011;19:5–10. doi: 10.1179/106698110X12804993426848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombe PF, Fairbank JC, Cockersole BC, et al. 1989 Volvo Award in clinical sciences. Reproducibility of physical signs in low-back pain. Spine (Phila Pa 1976) 1989;14:908–918. doi: 10.1097/00007632-198909000-00002. [DOI] [PubMed] [Google Scholar]
- McDonald GR, Hunt TE. Sacro-iliac joints: observations on the gross and histological changes in the various age groups. Can Med Assoc J. 1952;66:1–19. [PMC free article] [PubMed] [Google Scholar]
- McGill SM, Grenier S, Kavcic N, et al. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13:353–359. doi: 10.1016/s1050-6411(03)00043-9. [DOI] [PubMed] [Google Scholar]
- McGrath MC, Zhang M. Lateral branches of dorsal sacral nerve plexus and the long posterior sacroiliac ligament. Surg Radiol Anat. 2005;27:327–330. doi: 10.1007/s00276-005-0331-x. [DOI] [PubMed] [Google Scholar]
- McLauchlan GJ, Gardner DL. Sacral and iliac articular cartilage thickness and cellularity: relationship to subchondral bone end-plate thickness and cancellous bone density. Rheumatology. 2002;41:375–380. doi: 10.1093/rheumatology/41.4.375. [DOI] [PubMed] [Google Scholar]
- Mens JM, Vleeming A, Snijders CJ, et al. The active straight leg raising test and mobility of the pelvic joints. Eur Spine J. 1999;8:468–473. doi: 10.1007/s005860050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mens JM, Vleeming A, Snijders CJ, et al. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine (Phila Pa 1976) 2001;26:1167–1171. doi: 10.1097/00007632-200105150-00015. [DOI] [PubMed] [Google Scholar]
- Mens JM, Vleeming A, Snijders CJ, et al. Validity of the active straight leg raise test for measuring disease severity in patients with posterior pelvic pain after pregnancy. Spine (Phila Pa 1976) 2002;27:196–200. doi: 10.1097/00007632-200201150-00015. [DOI] [PubMed] [Google Scholar]
- Mens JM, Damen L, Snijders CJ, et al. The mechanical effect of a pelvic belt in patients with pregnancy-related pelvic pain. Clin Biomech. 2006;21:122–127. doi: 10.1016/j.clinbiomech.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Meyer CH. Der mechanisms der symphysis sacroiliaca. Arch Anat U Physiol. 1878;1:1–19. [Google Scholar]
- Miller JA, Schultz AB, Andersson GB. Load-displacement behavior of sacroiliac joints. J Orthop Res. 1987;5:92–101. doi: 10.1002/jor.1100050112. [DOI] [PubMed] [Google Scholar]
- Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210–215. [Google Scholar]
- Moffett BC. The prenatal development of the human temporomandibular joint. Contrib Embryol Carnegie Inst. 1957;36:19. [Google Scholar]
- Mooney V, Pozos R, Vleeming A, et al. Exercise treatment for sacroiliac pain. Orthopedics. 2001;24:29–32. doi: 10.3928/0147-7447-20010101-14. [DOI] [PubMed] [Google Scholar]
- Moore AE, Jeffery R, Gray A, et al. An anatomical ultrasound study of the long posterior sacro-iliac ligament. Clin Anat. 2010;23:971–977. doi: 10.1002/ca.21039. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine (Phila Pa 1976) 2002;27:29–36. doi: 10.1097/00007632-200201150-00013. [DOI] [PubMed] [Google Scholar]
- Muche B, Bollow M, Francois RJ, et al. Anatomic structures involved in early- and late-stage sacroiliitis in spondylarthritis: a detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:1374–1384. doi: 10.1002/art.10934. [DOI] [PubMed] [Google Scholar]
- O'Sullivan PB, Beales DJ, Beetham JA, et al. Altered motor control strategies in subjects with sacroiliac joint pain during the active straight-leg-raise test. Spine (Phila Pa 1976) 2002;27:E1–E8. doi: 10.1097/00007632-200201010-00015. [DOI] [PubMed] [Google Scholar]
- Palastanga N, Field D, Soames RW. Anatomy and Human Movement: Structure and Function. Oxford, UK: Butterworth Heinemann; 1998. [Google Scholar]
- Paquin JD, van der Rest M, Marie PJ, et al. Biochemical and morphologic studies of cartilage from the adult human sacroiliac joint. Arthritis Rheum. 1983;26:887–895. doi: 10.1002/art.1780260710. [DOI] [PubMed] [Google Scholar]
- Pare A. The Works of Generation of Man. London: Cotes and Young; 1634. [Google Scholar]
- Patel N, Gross A, Brown L, et al. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med. 2012;13:383–398. doi: 10.1111/j.1526-4637.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- Pel JJ, Spoor CW, Goossens RH, et al. Biomechanical model study of pelvic belt influence on muscle and ligament forces. J Biomech. 2008a;41:1878–1884. doi: 10.1016/j.jbiomech.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Pel JJ, Spoor CW, Pool-Goudzwaard AL, et al. Biomechanical analysis of reducing sacroiliac joint shear load by optimization of pelvic muscle and ligament forces. Ann Biomed Eng. 2008b;36:415–424. doi: 10.1007/s10439-007-9385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani E. Zentralbl Gynakol. 1899;23:1057. [Google Scholar]
- Pitkin HC. Orthopedic causes of pelvic pain. J Am Med Assoc. 1947;134:853–857. doi: 10.1001/jama.1947.02880270013003. [DOI] [PubMed] [Google Scholar]
- Pool-Goudzwaard AL, Kleinrensink GJ, Snijders CJ, et al. The sacroiliac part of the iliolumbar ligament. J Anat. 2001;199:457–463. doi: 10.1046/j.1469-7580.2001.19940457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool-Goudzwaard A, Hoek van Dijke G, Mulder P, et al. The iliolumbar ligament: its influence on stability of the sacroiliac joint. Clin Biomech. 2003;18:99–105. doi: 10.1016/s0268-0033(02)00179-1. [DOI] [PubMed] [Google Scholar]
- Potter N, Rothstein J. Intertester reliability for selected clinical tests of the sacroiliac joint. Phys Ther. 1985;65:1671–1675. doi: 10.1093/ptj/65.11.1671. [DOI] [PubMed] [Google Scholar]
- Puhakka KB, Jurik AG, Egund N, et al. Imaging of sacroiliitis in early seronegative spondylarthropathy. Assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003;44:218–229. doi: 10.1080/j.1600-0455.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Puhakka KB, Melsen F, Jurik AG, et al. MR imaging of the normal sacroiliac joint with correlation to histology. Skeletal Radiol. 2004;33:15–28. doi: 10.1007/s00256-003-0691-4. [DOI] [PubMed] [Google Scholar]
- Putz R, Muller-Gerbl M. [Anatomic characteristics of the pelvic girdle] Unfallchirurg. 1992;95:164–167. [PubMed] [Google Scholar]
- Radin EL. The Joint as an Organ: Physiology and Biomechanics congress. La Jolla, USA: 1990. [Google Scholar]
- Resnick D, Niwayama G, Goergen TG. Degenerative disease of the sacroiliac joint. Invest Radiol. 1975;10:608–621. doi: 10.1097/00004424-197511000-00008. [DOI] [PubMed] [Google Scholar]
- Richardson CA, Snijders CJ, Hides JA, et al. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain. Spine (Phila Pa 1976) 2002;27:399–405. doi: 10.1097/00007632-200202150-00015. [DOI] [PubMed] [Google Scholar]
- Ronchetti I, Vleeming A, van Wingerden JP. Physical characteristics of women with severe pelvic girdle pain after pregnancy: a descriptive cohort study. Spine (Phila Pa 1976) 2008;33:145–151. doi: 10.1097/BRS.0b013e3181657f03. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Yamashita T, Takabayashi EA. An electrophysiologic study of mechanoreceptors in the sacroiliac joint and adjacent tissues. Spine (Phila Pa 1976) 2001;26:E468–E471. doi: 10.1097/00007632-200110150-00008. [DOI] [PubMed] [Google Scholar]
- Sashin D. A critical analysis of the anatomy and the pathological changes of the sacroiliac joints. J Bone Joint Surg. 1930;12:891–910. [Google Scholar]
- Scheuer L, Black S. Developmental Juvenile Osteology. San Diego: Elsevier; 2000. [Google Scholar]
- Schuncke GB. The anatomy and development of the sacroiliac joint in man. Anat Rec. 1938;72:313–331. [Google Scholar]
- Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976) 1995;20:31–37. doi: 10.1097/00007632-199501000-00007. [DOI] [PubMed] [Google Scholar]
- Selvik G. A Roentgen Stereophotogrammetric Method for the Study of the Kinematics of the Skeletal System. Lund, Sweden: Medical University Lund; 1974. [Google Scholar]
- Selvik G. Roentgen stereophotogrammetric analysis. Acta Radiol. 1990;31:113–126. [PubMed] [Google Scholar]
- Shadmehr A, Jafarian Z, Talebian S. Changes in recruitment of pelvic stabilizer muscles in people with and without sacroiliac joint pain during the active straight-leg-raise test. J Back Musculoskelet Rehabil. 2012;25:27–32. doi: 10.3233/BMR-2012-0307. [DOI] [PubMed] [Google Scholar]
- Shipp FL, Haggart GE. Further experience in the management of osteitis condensans ilii. J Bone Joint Surg. 1950;32A:841–847. [PubMed] [Google Scholar]
- Smidt GL, McQuade K, Wei SH, et al. Sacroiliac kinematics for reciprocal straddle positions. Spine (Phila Pa 1976) 1995;20:1047–1054. doi: 10.1097/00007632-199505000-00011. [DOI] [PubMed] [Google Scholar]
- Smith GE, Jones FW. Report on the human remains. Archael Surv Nubia Rep. 1910:1907–1908. [Google Scholar]
- Snijders CJ, Vleeming A, Stoeckart R. Transfer of lumbosacral load to iliac bones and legs. Part I: biomechanics of self-bracing of the sacroiliac joints and its significance for treatment and exercise. Clin Biomech. 1993a;8:285–294. doi: 10.1016/0268-0033(93)90002-Y. [DOI] [PubMed] [Google Scholar]
- Snijders CJ, Vleeming A, Stoeckart R. Transfer of lumbosacral load to iliac bones and legs. Part II: loading of the sacroiliac joints when lifting in a stooped posture. Clin Biomech. 1993b;8:295–301. doi: 10.1016/0268-0033(93)90003-Z. [DOI] [PubMed] [Google Scholar]
- Snijders CJ, Vleeming A, Steockart R. Biomechanical modeling of sacroiliac jount stability in different postures. In: Dorman TA, et al., editors. Spine: State of the Art Reviews. Philadelphia: Hanley & Belfus; 1995. pp. 419–432. [Google Scholar]
- Snijders CJ, Hermans PF, Niesing R, et al. Effects of slouching and muscle contraction on the strain of the iliolumbar ligament. Man Ther. 2008;13:325–333. doi: 10.1016/j.math.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Soames RW. Skeletal system. In: Bannister LH, editor. Gray's Anatomy. Edinburgh: Churchill Livingstone; 1995. pp. 425–900. [Google Scholar]
- Solonen KA. The sacroiliac joint in the light of anatomical, roentgenological and clinical studies. Acta Orthop Scand. 1957;28(Suppl):1–127. [PubMed] [Google Scholar]
- Standing S. Gray's Anatomy, the Anatomical Basis of Clinical Practice. Edinburgh: Churchill Livingstone; 2005. [Google Scholar]
- Steiner H, Beck W, Prestel E, et al. Symphysenschaden: Klinik, Therapie und Prognose. Fortschr Med. 1977;2:2132–2137. [PubMed] [Google Scholar]
- Steinke H, Hammer N, Slowik V, et al. Novel insights into the sacroiliac joint ligaments. Spine (Phila Pa 1976) 2010;35:257–263. doi: 10.1097/BRS.0b013e3181b7c675. [DOI] [PubMed] [Google Scholar]
- Stevens VK, Vleeming A, Bouche KG, et al. Electromyographic activity of trunk and hip muscles during stabilization exercises in four-point kneeling in healthy volunteers. Eur Spine J. 2007;16:711–718. doi: 10.1007/s00586-006-0181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TD. Pathological changes in aging sacroiliac joints. Clin Orthop Relat Res. 1984;183:188–196. [PubMed] [Google Scholar]
- Stoev I, Powers AK, Puglisi JA, et al. Sacroiliac joint pain in the pediatric population. J Neurosurg Pediatr. 2012;9:602–607. doi: 10.3171/2012.2.PEDS11220. [DOI] [PubMed] [Google Scholar]
- Sturesson B. Movement of the sacroiliac joint with special reference to the effect of load. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 343–352. [Google Scholar]
- Sturesson B, Selvik G, Uden A. Movement of the sacroiliac joints: a roentgen stereophotogrammetric analysis. Spine (Phila Pa 1976) 1989;14:162–165. doi: 10.1097/00007632-198902000-00004. [DOI] [PubMed] [Google Scholar]
- Sturesson B, Uden A, Onsten I. Can an external frame fixation reduce the movements in the sacroiliac joint? A radiostereometric analysis of 10 patients. Acta Orthop Scand. 1999;70:42–46. doi: 10.3109/17453679909000956. [DOI] [PubMed] [Google Scholar]
- Sturesson B, Uden A, Vleeming A. A radiostereometric analysis of movements of the sacroiliac joints during the standing hip flexion test. Spine (Phila Pa 1976) 2000a;25:364–368. doi: 10.1097/00007632-200002010-00018. [DOI] [PubMed] [Google Scholar]
- Sturesson B, Uden A, Vleeming A. A radiostereometric analysis of the movements of the sacroiliac joints in the reciprocal straddle position. Spine (Phila Pa 1976) 2000b;25:214–217. doi: 10.1097/00007632-200001150-00012. [DOI] [PubMed] [Google Scholar]
- Szadek KM, Hoogland PV, Zuurmond WW, et al. Nociceptive nerve fibers in the sacroiliac joint in humans. Reg Anesth Pain Med. 2008;33:36–43. doi: 10.1016/j.rapm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Szadek KM, Hoogland PV, Zuurmond WW, et al. Possible nociceptive structures in the sacroiliac joint cartilage: an immunohistochemical study. Clin Anat. 2010;23:192–198. doi: 10.1002/ca.20908. [DOI] [PubMed] [Google Scholar]
- Tague RG. High assimilation of the sacrum in a sample of American skeletons: prevalence, pelvic size, and obstetrical and evolutionary implications. Am J Phys Anthropol. 2009;138:429–438. doi: 10.1002/ajpa.20958. [DOI] [PubMed] [Google Scholar]
- Tague RG. Fusion of coccyx to sacrum in humans: prevalence, correlates, and effect on pelvic size, with obstetrical and evolutionary implications. Am J Phys Anthropol. 2011;145:426–437. doi: 10.1002/ajpa.21518. [DOI] [PubMed] [Google Scholar]
- Testut L, Latarjet A. Traite d'Anatomie Humaine. Paris: G. Doin and Cie; 1949. [Google Scholar]
- Tischauer ER, Miller M, Nathan IM. Lordosimetry: a new technique for the measurement of postural response to materials handling. Am Ind Hyg Assoc J. 1973;1:1–12. doi: 10.1080/0002889738506800. [DOI] [PubMed] [Google Scholar]
- Tondury G. Angewandte und Topografische Anatomie. Stuttgart: Thieme; 1970. [Google Scholar]
- Trotter M. Accessory sacroiliac articulations in East African skeletons. Am J Phys Anthropol. 1964;22:137–141. doi: 10.1002/ajpa.1330220213. [DOI] [PubMed] [Google Scholar]
- Tullberg T, Blomberg S, Branth B, et al. Manipulation does not alter the position of the sacroiliac joint – a roentgen stereophotogrammetric analysis. Spine (Phila Pa 1976) 1998;23:1124–1128. doi: 10.1097/00007632-199805150-00010. [DOI] [PubMed] [Google Scholar]
- Uhthoff HK. Prenatal development of the iliolumbar ligament. J Bone Joint Surg Br. 1993;75:93–95. doi: 10.1302/0301-620X.75B1.8421046. [DOI] [PubMed] [Google Scholar]
- Umimura T, Miyagi M, Ishikawa T, et al. Investigation of dichotomizing sensory nerve fibers projecting to the lumbar multifidus muscles and intervertebral disc or facet joint or sacroiliac joint in rats. Spine (Phila Pa 1976) 2012;37:557–562. doi: 10.1097/BRS.0b013e3182293178. [DOI] [PubMed] [Google Scholar]
- Vaesalius A. De Corpori Humanis Fabrica. Basel: Oporini; 1543. [Google Scholar]
- Van der Wal J. In Fascia Research II. Basic Sciences and Implications for Conventional and Complementary Health Care. London and Edinburgh: Elsevier; 2009. The architecture of the connective tissue in the musculoskeletal system. An overlooked functional parameter as to proprioception in the locomotor apparatus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeming A. The Sacroiliac Joint: A Clinical Anatomical Biomechanical and Radiological Study. Thesis Erasmus University Rotterdam: Rotterdam; 1990. [Google Scholar]
- Vleeming A, Stoeckart R. The role of the pelvic girdle in coupling the spine and the legs: a cinical-anatomical perspective on pelvic stability. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 113–137. [Google Scholar]
- Vleeming A, Willard FH. 2010. pp. 23–31. 7th Interdisciplinary World Congress on Low Back and Pelvic Pain, La Jolla, CA.
- Vleeming A, Stoeckart R, Snijders CJ. The sacrotuberous ligament: a conceptual approach to its dynamic role in stabilizing the sacroiliac joint. Clin Biomech. 1989a;4:201–203. [Google Scholar]
- Vleeming A, van Wingerden JP, Snijders CJ, et al. Load application to the sacrotuberous ligament: influences on sacroiliac joint mechanics. Clin Biomech. 1989b;4:204–209. [Google Scholar]
- Vleeming A, Stoeckart R, Volkers AC, et al. Relation between form and function in the sacroiliac joint. Part I: clinical anatomical aspects. Spine (Phila Pa 1976) 1990a;15:130–132. doi: 10.1097/00007632-199002000-00016. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Volkers AC, Snijders CJ, et al. Relation between form and function in the sacroiliac joint. Part II: biomechanical aspects. Spine (Phila Pa 1976) 1990b;15:133–136. doi: 10.1097/00007632-199002000-00017. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Buyruk HM, Stoeckart R, et al. An integrated therapy for peripartum pelvic instability: a study of the biomechanical effects of pelvic belts. Am J Obstet Gynecol. 1992a;166:1243–1247. doi: 10.1016/s0002-9378(11)90615-2. [DOI] [PubMed] [Google Scholar]
- Vleeming A, van Wingerden JP, Snijders CJ, et al. Mobility in the sacroiliac joints in the elderly: a kinematic and radiological study. Clin Biomech. 1992b;7:170–176. doi: 10.1016/0268-0033(92)90032-Y. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine (Phila Pa 1976) 1995;20:753–758. [PubMed] [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Hammudoghlu D, et al. The function of the long dorsal sacroiliac ligament: its implication for understanding low back pain. Spine (Phila Pa 1976) 1996;21:556–562. doi: 10.1097/00007632-199603010-00005. [DOI] [PubMed] [Google Scholar]
- Vleeming A, de Vries HJ, Mens JM, et al. Possible role of the long dorsal sacroiliac ligament in women with peripartum pelvic pain. Acta Obstet Gynecol Scand. 2002;81:430–436. doi: 10.1034/j.1600-0412.2002.810510.x. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Albert HB, Ostgaard HC, et al. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17:794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Schubert E. Rontgenuntersuchungen des knochernen Beckens im Profilbild: exakte messung der Bekkenneigung bei lebenden. Zentralbl Gynakol. 1929;53:1064–1068. [Google Scholar]
- Vora AJ, Doerr KD, Wolfer LR. Functional anatomy and pathophysiology of axial low back pain: disc, posterior elements, sacroiliac joint, and associated pain generators. Phys Med Rehabil Clin N Am. 2010;21:679–709. doi: 10.1016/j.pmr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Vrahas M, Hern TC, Diangelo D, et al. Ligamentous contributions to pelvic stability. Orthopedics. 1995;18:271–274. doi: 10.3928/0147-7447-19950301-09. [DOI] [PubMed] [Google Scholar]
- Walcher G. Zentralbl Gynakol. 1889;13:892. [Google Scholar]
- Walheim GG. Stabilization of the pelvis with the Hoffmann frame. An aid in diagnosing pelvic instability. Acta Orthop Scand. 1984;55:319–324. doi: 10.3109/17453678408992365. [DOI] [PubMed] [Google Scholar]
- Walheim GG, Olerud S, Ribbe T. Motion of the pubic symphysis in pelvic instability. Scand J Rehabil Med. 1984;16:163–169. [PubMed] [Google Scholar]
- Weisl H. Movements of the sacroiliac joint. Acta Anat. 1955;23:80–91. [PubMed] [Google Scholar]
- Willard FH. The muscular, ligamentous, and neural structure of the lumbosacrum and its relationship to low back pain. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain: Integration and Research. Edinburgh: Churchill Livingstone; 2007. pp. 5–45. [Google Scholar]
- Willard FH, Carreiro JE, Manko W. 1998. pp. 207–209. Third Interdisciplinary World Congress on Low Back and Pelvic Pain, Vienna, Austria.
- Willard FH, Vleeming A, Schuenke MD, et al. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012 doi: 10.1111/j.1469-7580.2012.01511.x. DOI: 10.1111/j.1469-7580.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingerden JP, Vleeming A, Snijders CJ, et al. A functional-anatomical approach to the spine-pelvis mechanism: interaction between the biceps femoris muscle and the sacrotuberous ligament. Eur Spine J. 1993;2:140–144. doi: 10.1007/BF00301411. [DOI] [PubMed] [Google Scholar]
- van Wingerden JP, Vleeming A, Buyruk HM, et al. Stabilization of the sacroiliac joint in vivo: verification of muscular contribution to force closure of the pelvis. Eur Spine J. 2004;13:199–205. doi: 10.1007/s00586-003-0575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright V, Catterall RD, Cook JB. Bone and joint changes in paraplegic men. Ann Rheum Dis. 1965;24:419–431. doi: 10.1136/ard.24.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke BD, Jayson MIV. The Lumbar Spine and Back Pain. London: Sector Publishing; 1976. Neurological aspects of low back pain; pp. 189–256. [Google Scholar]
- Yamamoto I, Panjabi MM, Oxland TR, et al. The role of the iliolumbar ligament in the lumbosacral junction. Spine (Phila Pa 1976) 1990;15:1138–1141. doi: 10.1097/00007632-199011010-00010. [DOI] [PubMed] [Google Scholar]
- Yin W, Willard F, Carreiro J, et al. Sensory stimulation-guided sacroiliac joint radiofrequency neurotomy: technique based on neuroanatomy of the dorsal sacral plexus. Spine (Phila Pa 1976) 2003;28:2419–2425. doi: 10.1097/01.BRS.0000085360.03758.C3. [DOI] [PubMed] [Google Scholar]


