Abstract
Movement and stability of the lumbosacral region is contingent on the balance of forces distributed through the myofascial planes associated with the thoracolumbar fascia (TLF). This structure is located at the common intersection of several extremity muscles (e.g. latissimus dorsi and gluteus maximus), as well as hypaxial (e.g. ventral trunk muscles) and epaxial (paraspinal) muscles. The mechanical properties of the fascial constituents establish the parameters guiding the dynamic interaction of muscle groups that stabilize the lumbosacral spine. Understanding the construction of this complex myofascial junction is fundamental to biomechanical analysis and implementation of effective rehabilitation in individuals with low back and pelvic girdle pain. Therefore, the main objectives of this study were to describe the anatomy of the lateral margin of the TLF, and specifically the interface between the fascial sheath surrounding the paraspinal muscles and the aponeurosis of the transversus abdominis (TA) and internal oblique (IO) muscles. The lateral margin of the TLF was exposed via serial reduction dissections from anterior and posterior approaches. Axial sections (cadaveric and magnetic resonance imaging) were examined to characterize the region between the TA and IO aponeurosis and the paraspinal muscles. It is confirmed that the paraspinal muscles are enveloped by a continuous paraspinal retinacular sheath (PRS), formed by the deep lamina of the posterior layer of the TLF. The PRS extends from the spinous process to transverse process, and is distinct from both the superficial lamina of the posterior layer and middle layer of the TLF. As the aponeurosis approaches the lateral border of the PRS, it appears to separate into two distinct laminae, which join the anterior and posterior walls of the PRS. This configuration creates a previously undescribed fat-filled lumbar interfascial triangle situated along the lateral border of the paraspinal muscles from the 12th rib to the iliac crest. This triangle results in the unification of different fascial sheaths along the lateral border of the TLF, creating a ridged-union of dense connective tissue that has been termed the lateral raphe (Spine, 9,1984, 163). This triangle may function in the distribution of laterally mediated tension to balance different viscoelastic moduli, along either the middle or posterior layers of the TLF.
Keywords: lateral raphe, lumbar interfascial triangle, lumbar spine, paraspinal retinacular sheath, pelvis, thoracolumbar fascia, transversus abdominis
Introduction
Effective movement and stability of the lumbosacral region is contingent on the balance of forces distributed through the myofascial planes associated with the thoracolumbar fascia (TLF). The mechanical properties of the fascial constituents of the TLF influence the dynamic interaction of muscle groups stabilizing the lumbosacral spine. Understanding the anatomy of this complex myofascial junction is fundamental to functional anatomical and biomechanical analysis and, hence, the design of effective rehabilitation in individuals with low back and pelvic girdle pain.
The TLF envelops the back muscles from the sacral region, through the thoracic region, and is comprised of anterior (ALF), middle (MLF), and posterior (PLF) layers. Of these, the PLF consists of superficial and deep laminae. The superficial lamina of the PLF is continuous with the latissimus dorsi (LD), and partially continuous with the gluteus maximus, external abdominal oblique (EO) and trapezius and contribution from the serratus posterior inferior (SPI). The deep lamina of the PLF has contributions from the SPI, lumbosacral attachments to interspinous ligaments, the long dorsal sacroiliac ligament, and the iliac crest and cranial attachments extending into the cervical paraspinal region (Gracovetsky et al. 1981; Bogduk & MacIntosh, 1984; Gracovetsky, 1985, 1986; Macintosh & Bogduk, 1987; Vleeming et al. 1995; Barker & Briggs, 1999; Barker et al. 2004, 2007). The lateral margin of the TLF is located at the common intersection of several extremity muscles, such as the LD, as well as the intersection of the hypaxial (e.g. ventral trunk muscles) and epaxial (paraspinal) muscles.
The arrangement of fascial compartments in the lumbar spine, created by a fascial sheath encapsulating the paraspinal muscles, has been noted or illustrated by numerous authors (Spalteholz, 1923; Schaeffer, 1953; Hollinshead, 1969; Grant, 1972; plate 481; Bogduk & MacIntosh, 1984; Clemente, 1985; Tesh et al. 1987; Barker & Briggs, 1999; Gatton et al. 2010). Of special note is its designation as an osteofascial compartment (Standring, 2008), as the anteromedial portion is made up by the lumbar vertebrae and the remainder by a fascial sheet. This encapsulating sheath was named by Gray the ‘lumbar aponeurosis’; however, no attempt to separate deep from superficial layers of the PLF was made (Gray, 1870). Many authors cited above utilize the ‘deep lamina of the PLF’ to describe the posterior wall of this encapsulating sheath and the ‘MLF’ to describe the anterior wall. However, most of these descriptions are based on the assumption that the deep lamina of the PLF is a longitudinally oriented, flat fascial sheath. The present article has examined the shape of the deep lamina of the PLF in the lumbar region, confirming that it does form a sheath surrounding the paraspinal muscles. In the present article, this enveloping structure has been termed the paraspinal retinacular sheath (PRS; Fig. 1).
Fig. 1.
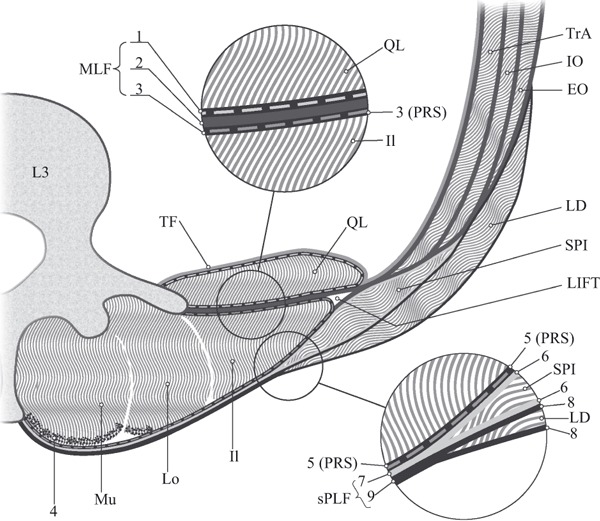
Modified with permission from Willard Vleeming Schuenke: fig. 9 in: The thoracolumbar fascia: anatomy function and clinical considerations. Submitted to the Journal of Anatomy, 2012. This is a transverse section of the posterior (PLF) and middle layer (MLF) of the thoracolumbar fascia (TLF) and related muscles at the L3 level. Fascial structures are represented such that individual layers are visible, but not necessarily presented to scale. Please note that the serratus posterior inferior (SPI) often is not present caudal to the L3 level. The transversus abdominis (TrA) muscle is covered with a dashed line on the peritoneal surface illustrating the transversalis fascia (TF). This fascia continues medially covering the anterior side of the investing fascia of the QL. Anteriorly and medially, the transversalis fascia (TF) also fuses with the psoas muscle fascia (not drawn). The internal (IO) and external oblique (EO) are seen external to TrA. SPI is highly variable in thickness and, more often than not, absent on the L4 level. Latissimus dorsi (LD) forms the superficial lamina of the PLF together with the SPI, when present. The three paraspinal muscles, multifidus (Mu), longissimus (Lo) and iliocostalis (Il) are contained within the paraspinal retinacular sheath (PRS). The aponeurosis (tendon) of the paraspinal muscles (4) is indicated by stippling. Please note that the epimysium of the individual spinal muscles is very thin and follows the contours of each separate muscle within the PRS. The epimysium is not indicated in the present figure but lies anteriorly to the aponeurosis (4). The upper circle shows a magnified view of the different fascial layers contributing to the MLF. The picture shows that MLF is made up of three different structures: (1) this dashed line depicts the investing fascia of QL; (3) this dashed line represents the PRS, also termed the deep lamina of the PLF encapsulating the paraspinal muscles; (2) the thick dark line between the two dashed lines 1 and 3 represents the aponeurosis of the abdominal muscles especially deriving from TrA. Numbers 1, 2 and 3 form the MLF. The lower circle shows a magnified view of the different fascial layers constituting the PLF. The picture shows that on the L3 level the PLF is also made up of three layers, as the fascia of SPI is normally present on this level. (5) This dashed line depicts the PRS or deep lamina of the PLF encapsulating the paraspinal muscles; (6) the investing fascia of SPI is seen blending medially into the gray line marked (7) and representing the aponeurosis of SPI- posteriorly to the PRS; (8) this dark line represents the investing fascia of LD blending medially into the black line representing the LD aponeurosis (9) posteriorly to the SPI aponeurosis. Numbers 5, 7 and 9 form the PLF. Numbers 7 and 9 form the superficial lamina of the posterior layer (sPLF).
Along the lateral border of the PRS, a complex interaction occurs between the attachments of the abdominal muscles. The blending of the aponeurotic sheaths of the transversus abdominis (TA) and internal oblique (IO) muscles along with the lateral margin of the TLF gives rise to a ridged-union of dense connective tissue. This area of fascial fusion exists just lateral to the paraspinal muscles through much of the lumbar region, and was coined the lateral raphe (LR; Bogduk & MacIntosh, 1984). The LR extends from the iliac crest caudally to the 12th rib cranially. Thus, the raphe is formed at the location where abdominal myofascial structures join the fascial structures surrounding the paraspinal muscles. Since Bogduk and MacIntosh’s original use of the phrase ‘lateral raphe’, several articles (e.g. Vleeming et al. 1995; Bogduk et al. 1998; Barker et al. 2001, 2004, 2007, 2010; Jemmett et al. 2004; Urquhart et al. 2005) make reference to the LR. Yet, to the authors’ knowledge, its boundaries and attachments have not been fully characterized.
To date, some of the muscle attachments to the LR have been described. Bogduk & MacIntosh (1984) note that the TA and, in some specimens, the IO arise from the LR. This arrangement has since been corroborated in several studies (Tesh et al. 1987; Vleeming et al. 1995; Bogduk et al. 1998; Barker et al. 2004, 2007, 2010), and additional attachments proposed: LD (Bogduk et al. 1998) and the EO (Barker et al. 2004). It is important to note that this description of the LR assumes there are ALF, MLF and PLF layers to the TLF (as described in Bogduk & MacIntosh, 1984; Tesh et al. 1987; Vleeming et al. 1995; Barker et al. 2004, 2006, 2007, 2010; Standring, 2008). According to some reports, the ALF is comprised of the internal abdominal wall fascia, known as the transversalis fascia (specified as ‘TF’ in Fig. 1), covering the anterior surface of the quadratus lumborum (QL; Moore & Dalley, 2006; Vleeming et al. 2007). Therefore, some texts (e.g. Rosse & Gaddum-Rosse, 1997) describe only an ALF, that lines the anterior side of the paraspinal muscles (described by most as MLF), and a PLF.
Mechanical tensioning studies suggest that the region of the LR may be important for force transmission from the abdominal muscles to the lumbosacral spine. The TA and IO muscles attach to the lateral margin of the TLF via an aponeurosis. (Bogduk & MacIntosh, 1984). That these anterolateral abdominal muscles are involved in spinal stabilization has been implicated in a number of studies (Hodges et al. 1996, 2003; Hodges & Richardson, 1997; Hides et al. 2011), and altered activation of these muscles is correlated with lumbopelvic pain (Hodges & Richardson, 1996; Hungerford et al. 2003). Using strain gages and raster photography on unembalmed cadavers, Barker et al. (2001, 2004) demonstrate that the TA and IO are capable of producing tension in the TLF. These articles indicate that much of the tension of these muscles passes through the LR, and they provide valuable insights into the mechanical relationships that govern load transfer between muscles and the TLF.
Recent research has revealed the complexity of connective tissue matrices as they function in load transfer (reviewed in Huijing, 2007). Loose connective tissue is embedded in a larger myofascial support matrix, such as is apparent in the lumbopelvic region, including tendons, aponeurotic sheaths and ligaments (Benetazzo et al. 2011). Forces of muscles are not only transmitted longitudinally (tensile), but also passed to adjacent fibers (shearing transmission) via endomysial connections (Huijing, 2007; Guimberteau et al. 2010). Force also can be passed to antagonistic muscles across interosseus membranes or through fascial compartments. This transfascial transmission of force emphasizes the importance of muscular convergence sites, such as the lateral margin of the TLF.
In light of these findings, to better understand the dynamic stability in vertebrates under constantly changing conditions, fascial structures like the TLF, encapsulating spinal muscles and connecting to the fascia of the abdominal muscles, cannot be viewed as distinct structures. Muscular forces are transmitted to the skeletal system through passive connective tissue structures, such as tendons and aponeurosis. The mechanical properties of these tissues thus co-determine the dynamic effects of muscle action. The TLF is itself a composite (Vleeming & Willard, 2010), comprised by layers of fascia, ligaments, loose connective tissue and muscle (Benetazzo et al. 2011). Without a comprehensive understanding of the complexity of the anatomy of the lateral margins of the TLF it becomes difficult to grasp effective load transfers between tissues or to infer the functional implications of the LR. Therefore, the main objective of this study is to describe the boundaries of, and muscles contributing to form, the lateral margin of the TLF.
Materials and methods
Sample characteristics
Twelve embalmed human specimens (four male, eight female; 84.1 ± 11.9 years) were examined, of which 11 were embalmed with a standard formaldehyde method (70% isopropyl alcohol, 2% phenol, 1% formaldehyde), and one female specimen embalmed using the Thiel method (17% ethylene glycol, 11% ammonium nitrate, 3% chlorate kersol, 2% formalin). None had evidence of lumbosacral pathology or surgical procedures in the lumbar region, but one female cadaver exhibited a slight scoliosis.
Objectives
(i) To test the hypothesis that a single continuous fascia (described in this manuscript as PRS) encloses the paraspinal muscles from the spinous process to the transverse process; (ii) to characterize the relationship between the PRS and the anterior and posterior bifurcating lamina of the TA and IO aponeurosis; (iii) to describe the tissue residing in between the anterior and posterior bifurcating lamina from the TA and IO aponeurosis; and (iv) to corroborate previous data on the constituents of the LR.
Two formaldehyde-embalmed specimens (70-year-old female; 86-year-old female) and one Thiel-embalmed specimen (82-year-old female) were used to dissect the lateral margin of the TLF. Serial reduction dissections from anterior and posterior approaches were used. A total of 27 axial slabs (2 cm; levels T12-S1) were sectioned from these specimens. Each axial section was examined both bilaterally and cranially and caudally, to study the lateral margin of the TLF. Therefore, one axial section produces four views of the lateral margin. However, due to variability in vertebral height and imprecision in sectioning, the number of axial sections per vertebral level varied. For example, in cadaver 1418, only one axial section was made at the L2 vertebral level, whereas two axial sections were made at the L3 vertebral level (Table 1).
Table 1.
Number of possible and observed LIFTs by vertebral level of three specimens and pooled MRIs
| Vertebral level | |||||||
|---|---|---|---|---|---|---|---|
| Specimen | LIFTs | T12 | L1 | L2 | L3 | L4 | L5 |
| 1418 | Observed | 0 | 5 | 4 | 8 | 2 | 0 |
| Total possible* | 4 | 8 | 4 | 8 | 4 | 4 | |
| 1392 | Observed | 0 | 2 | 5 | 8 | 8 | 0 |
| Total possible | 4 | 8 | 8 | 8 | 8 | 4 | |
| Thiel | Observed | 0 | 7 | 8 | 8 | 4 | 0 |
| Total possible | 4 | 8 | 8 | 8 | 4 | 4 | |
| MRIs (pooled) | Observed | 0 | 28 | 127 | 250 | 257 | 13 |
| Total possible | 27 | 32 | 155 | 302 | 296 | 86 | |
Total possible is calculated as [number of axial sections] × [number of sides (left and right)] × [number of approaches (superior and inferior)]. Most vertebral levels produced two cadaveric axial sections, therefore total possible for these levels is 8 (2 axial sections × 2 sides per section × 2 approaches). However, at some vertebral levels (e.g. L2 of specimen 1418), only one axial section was produced, in which case only 4 LIFTs are possible (1 axial section × 2 sides per section × 2 approaches). Similarly, MRI sequences are two-dimensional, so it is only possible to see a maximum of two LIFTs per sequence (e.g. no inferior approach).
MRI, magnetic resonance imaging.
Findings of the axial-sectioned specimens were compared with photographic images from previous dissections (n = 9; Willard & Carreiro, 2011) that were initiated to characterize the myofascial constituents of the lateral border of the TLF.
Additionally, 37 T1-weighted axial magnetic resonance imaging (MRI) sequences across vertebral levels T12-S1 were examined to compare with the cadaveric findings. These MRIs were made from individuals with no structural evidence of lumbosacral pathology or surgical procedures in the lumbar region. Of the 37 MRI sequences, there were a total of 449 images of the relevant area. Each MRI was examined bilaterally, giving a total of 898 TLF lateral margins of the TLF to be examined. However, not every MRI sequence was through the entire T12-S1 vertebral span, so the number of MRIs per vertebral level varied. For example, there were a total of 302 MRI sequences at the L3 level, but only 155 MRI sequences of the L2 level (Table 1). The MRI sequences were also used to localize the inferior border of the SPI muscle.
To localize the axial level at which the lateral border of the QL widens beyond the LR (crossover point), nine axial plane CTs (five male and four female) were obtained from the imaging library at the Department of Anatomy, University of New England. The lumbar spine was examined to ensure the absence of gross structural abnormalities. The section where the lateral margin of the QL and the LR were paired in the A-P plane was marked and the associated vertebral level determined.
Results
PRS and lumbar interfascial triangle (LIFT)
A primary finding of the present study is confirming the existence of a continuous fascial sheath, from the spinous process to the transverse process, that envelops the paraspinal muscles, referred to as the PRS (Fig. 1). The PRS is evident in both cadaveric (Fig. 2) and MRI (Fig. 3) axial views, and extends from the spinous process dorsomedially to the transverse process ventrolaterally. It varies considerably in thickness and is made up exclusively of the deep lamina of the PLF. It is distinct from both the superficial lamina of the posterior layer and the middle layer of the TLF. The epimysium of the individual spinal muscles is very thin and follows the contours of each separate muscle within the PRS. The epimysium is not indicated in Fig. 1, but lies anteriorly to the erector spinae aponeurosis (indicated as 4).
Fig. 2.
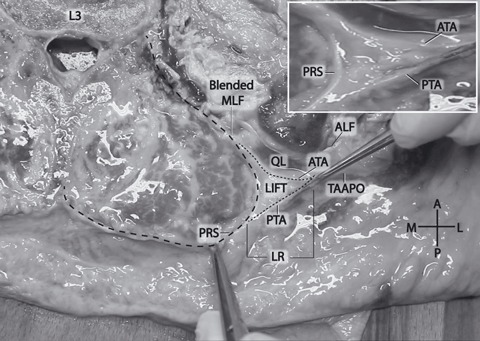
A comparatively large lumbar interfascial triangle (LIFT) at the L3 vertebral level. Note the fatty composition of the LIFT. The right pincer is pulling the junction of the anterior and posterior laminae of the TA aponeurosis (small dashed outline). The left pincer is pulling on the PRS (large dashed outline). Inset: magnified view of LIFT without dashed lines. ALF, anterior layer of thoracolumbar fascia (transversalis fascia); ATA, anterior lamina of TA aponeurosis; LIFT, lumbar interfascial triangle; LR, lateral raphe; MLF, middle layer of thoracolumbar fascia; PRS, paraspinal retinacular sheath; PTA, posterior lamina of TA aponeurosis; QL, quadratus lumborum; TAAPO, transversus abdominis aponeurosis.
Fig. 3.
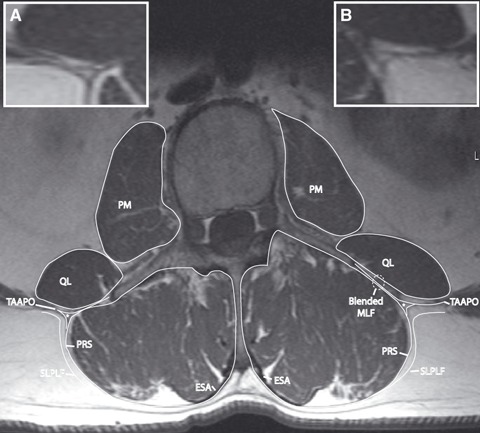
T1 MRI tracing demonstrating the relationship of the paraspinal retinacular sheath (PRS) and the aponeurosis of the transversus abdominis (TAAPO). TAAPO splits into anterior and posterior laminae, which separately join the PRS. The lumbar interfascial triangle (LIFT, indicated by ‘*’) resides in between the laminae of the TAAPO. The anterior lamina of the TAAPO blends with the investing fascia of quadratus lumborum (QL) and the PRS to form a thickened middle layer of thoracolumbar fascia (MLF). (Inset A) Magnified view of LIFT from the left side of image, without labels. (Inset B) Magnified view of LIFT from the right side of image, without labels. ESA, erector spinae aponeurosis; PM, psoas major; SLPLF, superficial lamina of posterior layer of thoracolumbar fascia.
As the aponeurosis of the TA and IO approaches the lateral border of the PRS, it appears to bifurcate into two distinct laminae, which merge externally to the anterior and posterior walls of the PRS. These two laminae and the portion of the PRS that spans between them create the boundaries of a previously undescribed adipose-filled LIFT, situated along the lateral margin of the paraspinal muscles from the 12th rib cranially to the crest of the ilium caudally (Fig. 1).
The regional distribution of the LIFT in the caudo-cranial axis was examined using axial-plane MRIs and cadaveric sections of the lumbar spine. Of the 27 cadaveric axial sections, 69 LIFTs were identified (out of a possible 108; 63.9%). However, when five axial sections from levels T12 and L5 were excluded from the data set, due to the presence of a rib or ilium, 69 LIFTs were now identified out of a possible 88 (78.4%). Of the 449 MRI images, 675 LIFTs were identified (out of 898 possible; 75.2%). When 113 MRI sequences from levels T12 and L5 were excluded from the data set due to the presence of a rib or ilium, 662 LIFTs were observed out of a possible 785 (84.3%). Table 1 describes the regional distribution of the LIFT as identified via cadaveric and MRI axial sections. This LIFT results in the unification of several different fascial sheaths along the lateral border of the TLF, creating a ridged-union of thickened dense connective tissue, which has been termed in previous studies the LR (Bogduk & MacIntosh, 1984; Fig. 4). The LIFT is positioned at the core of the LR.
Fig. 4.
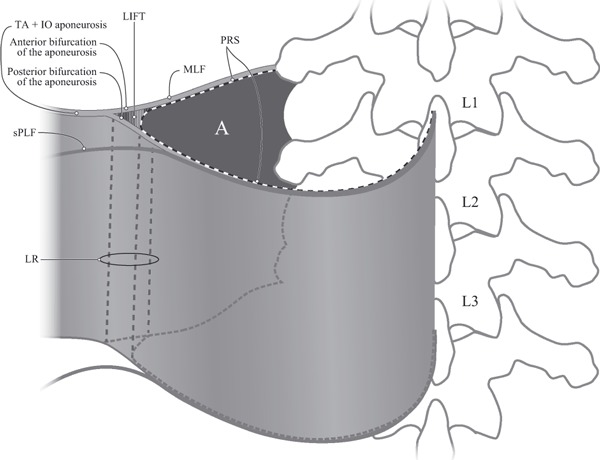
A schematic and simplified view of the bifurcation of the TA and IO aponeurosis and the paraspinal retinacular sheath (PRS), creating the lumbar interfascial triangle (LIFT). A represents the empty space normally occupied by the paraspinal muscles and enclosed by the PRS. The aponeurosis of the transversus abdominis (TA) and internal oblique (IO) bifurcates into anterior and posterior laminae. The anterior lamina contributes to the middle layer of the thoracolumbar fascia (MLF). The posterior lamina contributes to the deep lamina of the posterior layer of the thoracolumbar fascia (PLF). The lateral raphe (LR) represents a thickened complex of dense connective tissue at the lateral border of the PRS, from the iliac crest caudally to the 12th rib cranially. The junction of the TA aponeuroses with the PRS creates the LIFT, which is at the core of the LR. Thus, the raphe is formed at the location where abdominal myofascial structures join the PRS surrounding the paraspinal muscles. sPLF, superficial lamina of PLF.
Components of the lateral border of the TLF
A second objective of the present study was to corroborate and extend previous findings on the anatomical constituents of the LR. Significant muscles interacting with the LR include the LD, SPI, TA, IO and QL.
The LD forms a broad, flat aponeurosis that approaches the spine from a craniolateral position. Medially, the aponeurosis attaches to the lower six spines of the thoracic vertebrae and the spines of the lumbar vertebrae. Laterally, the muscle attachment has a variable arrangement; in all 10 of our specimens, the LD or its aponeurosis attached to the iliac crest, typically from the iliac tubercle posteriorly to the posterior superior iliac spine. In seven out of 10 specimens, the LD had become aponeurotic more than 5 cm cranial to the lateral-most attachment to the iliac crest. In two out of 10 specimens, the LD became aponeurotic < 5 cm cranial to the iliac crest, and in one specimen the LD muscle fibers reached the crest at its lateral-most attachment. In this latter arrangement, the LD lies over the LR posteriorly.
The SPI is a series of four thin rectangular sheets of muscle attached to the caudolateral margin of the ninth–12th ribs. Medially, the aponeurosis of the SPI inserts between the superficial and deep laminae of the PLF, terminating by attaching to both laminae. Those laminae continue medially to attach to the spinous processes. The inferior border of the SPI is of significance as it will determine how much of the LD and its aponeurosis can contribute to the LR. In two out of four dissected specimens where the caudal border of SPI could be accurately determined, it was found at approximately the L3 level, in one specimen it was at the L4 level and in another at the L2 level. Using axial MRI sequences, the caudal border of the SPI was found to be located at the L3 vertebral level in 57% of specimens, whereas in 30% of specimens, SPI ended at the level of L2. In another 10% of the specimens, the caudal border of the SPI was at the level of the intervertebral disc between L2 and L3, and in the remaining 3%, the caudal border of SPI was located at the level of the L4 vertebra. Therefore, the LD is separated from the LR by the SPI for most of its superior extent (Fig. 1).
The middle fibers of the TA arise from the rectus sheath anterior and form a thick aponeurosis laterally. This aponeurosis is joined by fibers from the lateral aspect of the IO, and wraps laterally around the torso to reach the PRS between the T12 and L4 levels. Between the L2/L3 intervertebral disc level to the L4 vertebral level, as this aponeurosis approaches the PRS, it appears to separate into two layers (Figs 1 and 2) that contribute to the formation of the LIFT, as previously described.
In no specimen was the EO seen to directly communicate with the lateral margin of the TLF. The caudolateral attachment of the EO on the iliac crest terminates anteriorly to the TLF, and the cranial border of the EO attaches to the 12th rib. However, the thin, investing fascia (epimysium) of the EO is seen attaching to the aponeurosis of the TA and IO.
The QL resides anterior to the MLF extending from the iliac crest to the 12th rib. Like all muscles, the QL is surrounded by a thin investing fascia (histologically termed the epimysium; Fig. 1). The anterior surface of the QL is also covered by a thin, velvety layer of TF. Moving from cranial to caudal, the lateral border of the QL expands laterally as it approaches the crest of the ilium. The location at which the lateral border of the QL becomes lateral to the lateral border of the paraspinal muscles (crossover point) varies. In the series of nine abdominal CT scans, the lateral border of the QL typically passes lateral to the paraspinal muscles at the level of the L2, being seen as high as the L1/L2 intervertebral disc in two scans (one male and one female) and as low as the caudal border of L2 in two scans (one male and one female). Cranial to the crossover point, the TF and the MLF merge at the anterolateral border of the QL, thereby contributing to the anterior border of the LIFT and the LR (Fig. 1).
Discussion
The purpose of the present study is to confirm the presence of a retinacular sheath surrounding the paraspinal muscles, and to characterize the boundaries and contents of the lateral margin of the TLF.
Components of the lateral border of the paraspinal muscles and their relationship with the LR
The present dissections confirm that the so-called TA aponeurosis is actually created by the TA and IO. These findings support most existing literature on the relationship between the TA and the IO (Bogduk & MacIntosh, 1984; Tesh et al. 1987; Vleeming et al. 1995; Barker et al. 2004). The findings are in contrast to Jemmett et al. (2004) who described the TA aponeurosis as bypassing the LR and as contributing solely to the ALF. However, their data are derived from a single dissection, and the authors acknowledged that their results may well have been indicative of simple variation in the normal anatomy.
In the present study, no direct contribution of the EO aponeurosis to the lateral border of the TLF was found. This is in contrast to the findings of Barker et al. (2004), who report a connection of the EO to the LR superior to the L3 vertebral level. The loose attachment of the muscle to the underlying aponeurosis of the TA does not support a major role of the EO in load transfer to the lumbar spine.
The QL is a hypaxial muscle, located anteriorly to the paraspinal muscles. It extends from the 12th rib cranially to the crest of the ilium caudally. Its medial border attaches to the transverse processes of the lumbar vertebrae, and its lateral border is free. Typically this border begins cranially from a point medial to the lateral border of the paraspinal muscle and angles from craniomedial to caudolateral as it descends towards the ilium. At some point along this line, the free border of the QL widens lateral to the lateral margin of the paraspinal muscles. In this study, this crossover point has been determined to occur approximately at L2. Thus, below L2, the structures forming the lateral margin of the paraspinal muscles (TLF, LR and LIFT) are reinforced internally by the presence of the QL and its associated fascia (Fig. 1); above L2 the lateral margin of the paraspinal muscles is reinforced only by the TF.
PRS
The present study demonstrates that the paraspinal muscles are invested by a PRS with variable thickness. This PRS is distinct from and external to the very thin epimysial layer covering the individual lumbar muscles. The PRS is distinct and can be separated from the bifurcating laminae of the TA aponeurosis. Based on embryological observations (Bailey & Miller, 1916), the muscles of epaxial origin, such as the paraspinal muscles, develop encased in an intact fascial sheath (the PRS) from the spinous processes to the transverse processes. Furthermore, it would be expected that hypaxial muscles, such as the TA, will attach to the PRS but not penetrate into it (Willard et al. in press).
At this time, to effectively conceptualize the PRS, is to view it as the deep lamina of PLF extending around the lateral border of the paraspinal muscles and joining the MLF to reach the transverse processes of the lumbar vertebrae. Barker et al. (2007) report a significant thickening of the MLF as it approaches the transverse processes medially. Based on the present data, we interpret this medial thickening as resulting from blending initially between the anterior lamina of the aponeurosis of the TA and IO and the posterior investing fascia of the QL; subsequent addition of the PRS to this combined layer creates the medial thickening (Fig. 1). In this interpretation, the thinner, more lateral part of the MLF is comprised of the anterior lamina of the TA and IO aponeurosis and the QL fascia, while the thicker, more medial portion is composed of three fascial sheets. Further, the present dissections indicate that the anterior surface of the QL (commonly referred to as the ALF) is lined by TF, in agreement with Hollingshead (Rosse & Gaddum-Rosse, 1997), and Barker and Briggs (Barker et al. 2007; Vleeming et al. 2007).
If the paraspinal muscles were only enveloped by the MLF and PLF, rather than one continuous PRS, lateral expansion of the paraspinal muscles could cause a split all the way to the lateral edge of the TA and IO aponeurosis. Because the PRS is a complete fibro-osseous ring, there is no such point of weakness. Tesh et al. (1987) did not describe such a separation during inflation of intra-paraspinal balloons. Furthermore, without a PRS, it is unlikely that a paraspinal compartment syndrome could develop (Carr et al. 1985).
The PRS could play a vital role in spinal stabilization via the hydraulic amplifier mechanism (Gracovetsky et al. 1981; Bogduk & MacIntosh, 1984; Gracovetsky, 1985, 1986, 2008). In brief, as paraspinal muscles contract, the PRS and the associated fascial layers connected to it, such as the MLF and the superficial layer of the PLF, are tensed in all directions, thereby compressing the sheath against the paraspinal muscles and creating a hydraulic effect that aids in erecting the spine from a flexed position.
Formation of the LIFT along the lateral border of the paraspinal muscles
The anterior and posterior laminae of the TA and IO aponeurosis diverge approximately 0.5–2 cm lateral to the PRS, and become continuous with the anterior and posterior margins of the PRS, respectively. The two laminae of the TA and IO aponeurosis and the PRS form the boundaries of an adipose-filled region that in this article is called the LIFT. To the authors’ knowledge, the LIFT has not been discussed in previous literature. However, the LIFT has appeared in previous diagrams (fig. 2 in Carr et al. 1985; figs 1 and 5 in Tesh et al. 1987), but was not addressed or specified in the text of these articles.
The existence of the LIFT explains why a ridged-union of connective tissue is formed, called the LR. This region of increased fascial density receives direct and indirect contributions from several muscles associated with the anterolateral abdominal wall, superficial and deep back, and the gluteal region. Huijing (2007) describes force transmission of muscles across the extracellular matrix to neighboring muscles, both synergists and antagonists. Similarly, the LIFT may function in the distribution of laterally mediated tension to balance different visco-elastic moduli, along either the MLF or PLF. The converse may also be true: tension in the anterior and posterior laminae of the TA aponeurosis is likely dependent on contraction of muscles within the PRS. In this scenario, the function of the LIFT may be to reduce friction of adjacent fascias under high tension (Theobald et al. 2007). Alternatively, the LIFT may accommodate lateral expansion of the paraspinal muscles during contraction. Nevertheless, the present study has made no attempt to determine the effect of tension on the PRS or LIFT, and these should be areas of future research.
Relationship of the LIFT to the LR
Based on the present findings, the following updated definition of the LR is proposed.
The LR (Bogduk & MacIntosh, 1984) represents a thickened complex of dense connective tissue at the lateral border of the PRS, from the iliac crest caudally to the 12th rib cranially (Fig. 1). It marks the junction of the aponeurosis of the TA and IO with the PRS. This junction creates the formation of the LIFT. The blending of the bifurcating anterior and posterior laminae from the TA and IO aponeurosis to the PRS forming the LIFT gives rise to a ridged-union of dense connective tissue, which has been named the LR. It is through this dense connective tissue complex (raphe) that the tension, generated by the abdominal myofascial girdle, dissipates across the paraspinal sheath.
This definition is in general agreement with the original definition proposed by Bogduk and MacIntosh. The contributions of the TA and IO are consistent with the original definition. Also, the proposed definition agrees that the MLF and deep lamina of the PLF contribute to the LR. However, the concept of the MLF and deep lamina of the PLF splitting to create the PRS is implicit in the original definition, whereas the proposed definition in this article suggests that the PRS, formed by the deep lamina of the PLF, is a separate entity that receives contributions from the anterior and posterior bifurcations of the TA aponeurosis (Fig. 1).
The strength of the LR is demonstrated by two structures described in the clinical literature as the superior (also known as Grynfeltt’s) and inferior (also known as Petit’s) lumbar triangles (Guillem et al. 2002; Astarcioglu et al. 2003; Armstrong et al. 2008; Lillie & Deppert, 2010). Petit’s triangle overlaps the caudolateral portion of LR. Grynfeltt’s superior lumbar triangle overlaps the superolateral portion of the LR. These triangles are common sites of visceral herniation in the lumbar region. Herniations typically take the path of least resistance. Thus, passage of the herniation just lateral to the LR may speak toward the robustness of the ridged-union known as the LR. Again, the strength of the LR was not assessed in the study, and should be the focus of future studies.
Conclusion
A significant finding in this study is the confirmation of a PRS surrounding the paraspinal muscles between the spinous and transverse processes. This sheath is a continuation of the deep lamina of the PLF around the lateral border of the paraspinal muscles and its subsequent fusion with the MLF. The lateral margin of the TLF represents the junction between the PRS and the aponeurosis of the TA and IO. It forms a triangular-shaped space, the LIFT, deriving from the anterior and posterior bifurcation of the TA aponeurosis, and the portion of PRS that spans between them. This LIFT may act as a fulcrum distributing laterally mediated tension to balance different viscoelastic moduli, along either the MLF or PLF. It may also serve to reduce friction between those two entities. The LIFT has not been discussed in detail in previous literature. The existence of the LIFT elucidates why a ridged-union of connective tissue is formed, called the LR.
Acknowledgments
The authors would like to express appreciation to Mr Oran Suta for his artistic expertise on Figs 1 and 4.
| Abbreviations | Term |
|---|---|
| ALF | Anterior layer of thoracolumbar fascia |
| EO | External abdominal oblique |
| IO | Internal abdominal oblique |
| LD | Latissimus dorsi |
| LIFT | Lumbar interfascial triangle |
| LR | Lateral raphe |
| MLF | Middle layer of thoracolumbar fascia |
| PLF | Posterior layer of thoracolumbar fascia |
| PRS | Paraspinal retinacular sheath |
| QL | Quadratus lumborum |
| SPI | Serratus posterior inferior |
| TA | Transversus abdominis |
| TLF | Thoracolumbar fascia |
References
- Armstrong O, Hamel A, Grignon B, et al. Lumbar hernia: anatomical basis and clinical aspects. Surg Radiol Anat. 2008;30:533–537. doi: 10.1007/s00276-008-0361-2. discussion 609–510. [DOI] [PubMed] [Google Scholar]
- Astarcioglu H, Sokmen S, Atila K, et al. Incarcerated inferior lumbar (Petit’s) hernia. Hernia. 2003;7:158–160. doi: 10.1007/s10029-003-0128-y. [DOI] [PubMed] [Google Scholar]
- Bailey FR, Miller AM. Textbook of Embryology. 3rd edn. New York: William Wood; 1916. [Google Scholar]
- Barker PJ, Briggs CA. Attachments of the posterior layer of lumbar fascia. Spine. 1999;24:1757–1764. doi: 10.1097/00007632-199909010-00002. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Brigg CA, Bogeski G. Montreal, QC, Canada: 2001. Muscle attachments of the lumbar spine; pp. 238–239. 4th Interdisciplinary World Congress on Low Back and Pelvic Pain. [Google Scholar]
- Barker PJ, Briggs CA, Bogeski G. Tensile transmission across the lumbar fasciae in unembalmed cadavers: effects of tension to various muscular attachments. Spine. 2004;29:129–138. doi: 10.1097/01.BRS.0000107005.62513.32. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Guggenheimer KT, Grkovic I, et al. Effects of tensioning the lumbar fasciae on segmental stiffness during flexion and extension: Young Investigator Award winner. Spine. 2006;31:397–405. doi: 10.1097/01.brs.0000195869.18844.56. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Urquhart DM, Story IH, et al. The middle layer of lumbar fascia and attachments to lumbar transverse processes: implications for segmental control and fracture. Eur Spine J. 2007;16:2232–2237. doi: 10.1007/s00586-007-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PJ, Freeman AD, Urquhart DM, et al. The middle layer of lumbar fascia can transmit tensile forces capable of fracturing the lumbar transverse processes: an experimental study. Clin Biomech. 2010;25:505–509. doi: 10.1016/j.clinbiomech.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Benetazzo L, Bizzego A, DeCaro R, et al. 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat. 2011;33:855–862. doi: 10.1007/s00276-010-0757-7. [DOI] [PubMed] [Google Scholar]
- Bogduk N, MacIntosh JE. The applied anatomy of the thoracolumbar fascia. Spine. 1984;9:164–170. doi: 10.1097/00007632-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Johnson G, Spalding D. The morphology and biomechanics of latissimus dorsi. Clin Biomech. 1998;13:377–385. doi: 10.1016/s0268-0033(98)00102-8. [DOI] [PubMed] [Google Scholar]
- Carr D, Gilbertson L, Frymoyer J, et al. Lumbar paraspinal compartment syndrome. A case report with physiologic and anatomic studies. Spine. 1985;10:816–820. [PubMed] [Google Scholar]
- Clemente CD. Gray’s Anatomy of the Human Body. Philadelphia, PA: Lea & Febiger; 1985. [Google Scholar]
- Gatton ML, Pearcy MJ, Pettet GJ, et al. A three-dimensional mathematical model of the thoracolumbar fascia and an estimate of its biomechanical effect. J Biomech. 2010;43:2792–2797. doi: 10.1016/j.jbiomech.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S. An hypothesis for the role of the spine in human locomotion: a challenge to current thinking. J Biomed Eng. 1985;7:205–216. doi: 10.1016/0141-5425(85)90021-4. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S. Function of the spine. J Biomed Eng. 1986;8:217–223. doi: 10.1016/0141-5425(86)90087-7. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S. Is the lumbodorsal fascia necessary? J Bodyw Mov Ther. 2008;12:194–197. doi: 10.1016/j.jbmt.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Gracovetsky S, Farfan HF, Lamy C. The mechanism of the lumbar spine. Spine. 1981;6:249–262. doi: 10.1097/00007632-198105000-00007. [DOI] [PubMed] [Google Scholar]
- Grant JCB. An Atlas of Anatomy. 6th edn. Baltimore, MD: Williams & Wilkins; 1972. [Google Scholar]
- Gray H. Anatomy, Descriptive and Surgical. Philadelphia: Henry C Lea; 1870. [Google Scholar]
- Guillem P, Czarnecki E, Duval G, et al. Lumbar hernia: anatomical route assessed by computed tomography. Surg Radiol Anat. 2002;24:53–56. doi: 10.1007/s00276-002-0003-z. [DOI] [PubMed] [Google Scholar]
- Guimberteau JC, Delage JP, McGrouther DA, et al. The microvacuolar system: how connective tissue sliding works. J Hand Surg Eur. 2010;35:614–622. doi: 10.1177/1753193410374412. [DOI] [PubMed] [Google Scholar]
- Hides J, Stanton W, Dilani Mendis M, et al. The relationship of transversus abdominis and lumbar multifidus clinical muscle tests in patients with chronic low back pain. Man Ther. 2011;16:573–577. doi: 10.1016/j.math.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997;77:132–142. doi: 10.1093/ptj/77.2.132. [DOI] [PubMed] [Google Scholar]
- Hodges P, Richardson C, Jull G. Evaluation of the relationship between laboratory and clinical tests of transversus abdominis function. Physiother Res Int. 1996;1:30–40. doi: 10.1002/pri.45. [DOI] [PubMed] [Google Scholar]
- Hodges P, Kaigle Holm A, Holm S, et al. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine. 2003;28:2594–2601. doi: 10.1097/01.BRS.0000096676.14323.25. [DOI] [PubMed] [Google Scholar]
- Hollinshead WH. Anatomy for Surgeons: The Back and Limbs. 2nd edn. New York: Hoeber-Harper; 1969. [Google Scholar]
- Huijing PA. Epimuscular myofascial force transmission between antagonistic and synergistic muscles can explain movement limitation in spastic paresis. J Electromyogr Kinesiol. 2007;17:708–724. doi: 10.1016/j.jelekin.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hungerford B, Gilleard W, Hodges P. Evidence of altered lumbopelvic muscle recruitment in the presence of sacroiliac joint pain. Spine. 2003;28:1593–1600. [PubMed] [Google Scholar]
- Jemmett RS, Macdonald DA, Agur AM. Anatomical relationships between selected segmental muscles of the lumbar spine in the context of multi-planar segmental motion: a preliminary investigation. Man Ther. 2004;9:203–210. doi: 10.1016/j.math.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Lillie GR, Deppert E. Inferior lumbar triangle hernia as a rarely reported cause of low back pain: a report of 4 cases. J Chiropr Med. 2010;9:73–76. doi: 10.1016/j.jcm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N. 1987 Volvo award in basic science. The morphology of the lumbar erector spinae. Spine. 1987;12:658–668. doi: 10.1097/00007632-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Moore KL, Dalley AF., II . Clinically Oriented Anatomy. 5th edn. Baltimore, MD: Lippincott, Williams, & Wilkins; 2006. [Google Scholar]
- Rosse C, Gaddum-Rosse P. Hollinshead’s Textbook of Anatomy. 5th edn. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- Schaeffer JP. Morris’s Human Anatomy. 11th edn. New York: McGraw-Hill; 1953. [Google Scholar]
- Spalteholz W. Hand Atlas of Human Anatomy. Philadelphia, PA: J.B. Lippincott; 1923. [Google Scholar]
- Standring S. Gray’s Anatomy. 14th edn. Philadelphia: Churchill Livingstone; 2008. [Google Scholar]
- Tesh KM, Dunn JS, Evans JH. The abdominal muscles and vertebral stability. Spine. 1987;12:501–508. doi: 10.1097/00007632-198706000-00014. [DOI] [PubMed] [Google Scholar]
- Theobald P, Byrne C, Oldfield SF, et al. Lubrication regime of the contact between fat and bone in bovine tissue. Proc Inst Mech Eng H. 2007;221:351–356. doi: 10.1243/09544119JEIM176. [DOI] [PubMed] [Google Scholar]
- Urquhart DM, Barker PJ, Hodges PW, et al. Regional morphology of the transversus abdominis and obliquus internus and externus abdominis muscles. Clin Biomech. 2005;20:233–241. doi: 10.1016/j.clinbiomech.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine. 1995;20:753–758. [PubMed] [Google Scholar]
- Vleeming A, Mooney V, Stoeckart R, editors. 2nd edn. Edinburgh: Churchill Livingstone Elsevier; 2007. Movement, Stability & Lumbopelvic Pain: Integration of Research and Therapy. [Google Scholar]
- Vleeming A, Willard FH. La Jolla, CA: 2010. 7th Interdisciplinary World Congress on Low Back and Pelvic Pain. [Google Scholar]
- Willard FH, Carreiro JE. Imaging Library. Biddeford, ME: University of New England; 2011. [Google Scholar]
- Willard FH, Vleeming A, Schuenke MD, et al. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. doi: 10.1111/j.1469-7580.2012.01511.x. (in press), in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


