Abstract
Within the nasal cavity of mammals is a complex scaffold of paper-thin bones that function in respiration and olfaction. Known as turbinals, the bones greatly enlarge the surface area available for conditioning inspired air, reducing water loss, and improving olfaction. Given their functional significance, the relative development of turbinal bones might be expected to differ among species with distinct olfactory, thermoregulatory and/or water conservation requirements. Here we explore the surface area of olfactory and respiratory turbinals relative to latitude and diet in terrestrial Caniformia, a group that includes the canid and arctoid carnivorans (mustelids, ursids, procyonids, mephitids, ailurids). Using high-resolution computed tomography x-ray scans, we estimated respiratory and olfactory turbinal surface area and nasal chamber volume from three-dimensional virtual models of skulls. Across the Caniformia, respiratory surface area scaled isometrically with estimates of body size and there was no significant association with climate, as estimated by latitude. Nevertheless, one-on-one comparisons of sister taxa suggest that arctic species may have expanded respiratory turbinals. Olfactory surface area scaled isometrically among arctoids, but showed positive allometry in canids, reflecting the fact that larger canids, all of which are carnivorous, had relatively greater olfactory surface areas. In addition, among the arctoids, large carnivorous species such as the polar bear (Ursus maritimus) and wolverine (Gulo gulo) also displayed enlarged olfactory turbinals. More omnivorous caniform species that feed on substantial quantities of non-vertebrate foods had less expansive olfactory turbinals. Because large carnivorous species hunt widely dispersed prey, an expanded olfactory turbinal surface area may improve a carnivore's ability to detect prey over great distances using olfactory cues.
Keywords: Arctoidea, Canidae, Carnivora, nasal turbinals, olfaction, respiration
Introduction
The nasal cavity of mammals is a complicated structure filled with an extensive framework of delicate bones called turbinals (or turbinates). Covered in mucosa, the turbinals are involved in multiple functions, including olfaction, respiration, water conservation, and thermoregulation. Given this, the size and complexity of the turbinals is expected to reflect functional demands. For example, semi-aquatic carnivorans such as seals have both a reduced need for olfaction and an enhanced need for water and heat conservation. Consequently, they have greatly reduced olfactory turbinals and enlarged respiratory turbinals relative to their terrestrial relatives (Van Valkenburgh et al. 2011). Among terrestrial carnivorans, more subtle differences in olfactory and respiratory demands might be reflected in the relative dimensions of the turbinals, but this has been little explored until now. Here we report the first quantitative assessment of skeletal turbinal dimensions within a broad group of terrestrial carnivorans, the Caniformia, a clade that includes the family Canidae and its sister group, the Arctoidea (ursids, mustelids, mephitids, procyonids, pinnipeds).
Traditionally, the turbinals have been divided into three regions, each named by the primary, but not necessarily only, cranial structure to which they are attached in the adult: maxilloturbinals to the maxillary bone, nasoturbinals to the nasal bones, and ethmoturbinals to the ethmoid (Moore, 1981), a structure that is now known to be a composite of several turbinal elements and the cribriform plate (Rowe et al. 2005; Macrini, 2012; Fig. 1). Recent detailed anatomical studies of mammalian turbinals have revealed a greater complexity of attachments and interrelationships among the three regions, and the terminology is evolving (Rowe et al. 2005; Smith et al. 2007a,b; Macrini, 2012). For our comparisons of functional differences, the simplified tripartite division is sufficient.
Fig. 1.
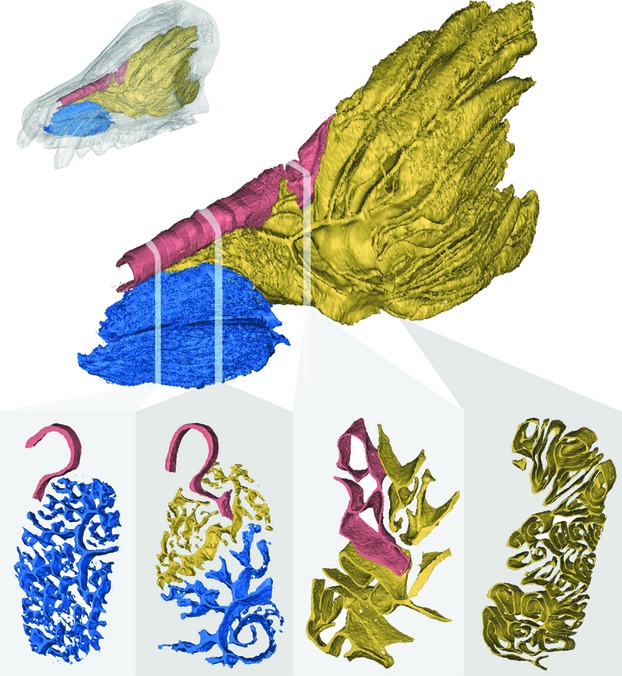
(Top) Three-dimensional reconstruction of the maxilloturbinals (blue), ethmoturbinals (yellow) and nasoturbinals (pink) of an arctic fox (UCLA15161) based on high-resolution CT scans shown within the skull (small image) and extracted from the skull (large image). Below are four slices taken at the positions indicated along the skull from rostral (left) to caudal (right).
The maxilloturbinals are referred to as respiratory turbinals because they function to warm and humidify inspired air before it enters the lungs. When cool air from the environment is inhaled, it passes over the moist, body-temperature mucosa of the maxilloturbinals and is warmed and saturated with water, allowing for more efficient gas exchange in the lungs. Upon exhalation, the air passes over the now cooler and drier mucosa, allowing a substantial quantity of water and heat to be recovered (Schmidt-Nielsen et al. 1970; Hillenius, 1992).
The olfactory turbinals include both the ethmo- and nasoturbinals and are defined by being covered in part with sensory epithelium. As inhaled air (or, especially, air that is sniffed) is directed toward the olfactory turbinals, it is moved dorsally and posteriorly into the olfactory recess of the nasal chamber and toward the olfactory bulb (Craven et al. 2007, 2010). In at least one species, the domestic dog, airflow modeling shows slower and more laminar flow in the olfactory recess (Craven et al. 2010), allowing more time for odorants to bind to receptors found on the sensory neurons in the olfactory epithelium.
Because of their different functions, respiratory and olfactory turbinals likely are subject to somewhat different selective pressures. Selection relating to latitude would be expected to act on respiratory turbinals more so than olfactory turbinals. For example, the demands of heat and water retention might be greater for caniform species living at high latitudes with cold, dry environments (e.g. tundra) than those living at lower latitudes with more temperate, mesic environments. Consequently, high latitude species might be expected to have expanded respiratory surface areas relative to low latitude species. Latitude has been used as a proxy for climate in previous studies of Bergmann's rule (Meiri et al. 2004; Huston & Wolverton, 2011), and a similar relationship between nasal morphology and climate has been claimed for humans (Yokley, 2009; Noback et al. 2011).
Diet would be expected to influence the olfactory turbinals more so than the respiratory turbinals. For example, because omnivorous species have a varied diet and thus must discriminate among a greater variety of food resources than highly carnivorous species, omnivores might show increased olfactory turbinal area to accommodate a more diverse array of olfactory receptors. However, carnivorous species have larger home range sizes (Gittleman & Harvey, 1982) and probably need to detect more widely dispersed resources. This might also favor enhanced olfactory sensitivity and an enlarged olfactory area. Gittleman (1991) found a positive association between olfactory bulb size and home range size, and postulated that carnivorans with larger ranges rely more heavily on olfactory cues to keep track of territorial scent marks and other indicators of spatial location.
Here, we use high-resolution computed tomography (HRCT) scans of skulls to quantify the olfactory and respiratory turbinal surface area of 10 species within the family Canidae and 10 species within the superfamily Arctoidea (three ursids, two procyonids, four mustelids, one mephitid; Table 1). To establish the general allometric relationship, we first looked at how turbinal surface area scales to various measures of body size in canids and arctoids, as well as how scaling in canids compares with that in arctoids. Given the exceptional olfactory ability of dogs, we hypothesized that canids might have relatively larger olfactory turbinals than arctoids. We also examined the possible effects of diet on relative olfactory turbinal surface area across all species studied. If a greater relative olfactory turbinal surface area allows for better detection of widely dispersed resources, we expect to see greater olfactory turbinal surface area in animals that eat more vertebrate foods. Alternatively, if olfactory anatomy functions more to detect a diversity of food resources, we expect species with omnivorous diets (more non-vertebrate foods) to have greater amounts of olfactory turbinal surface area. Finally, we analyzed the effect of latitude on relative respiratory turbinal surface area across all species. Given that respiratory turbinals assist in heat and water retention, we predicted that species living at higher latitudes (in colder climates) would have relatively greater respiratory turbinal surface area than those living at lower latitudes.
Table 1.
Sampled species with associated body size, turbinal chamber volume and surface area measurements
| Code | Family | Species | Sex | Diet | Body mass (kg) | Skull length (mm) | Chamber volume (mm3) | TTSA (mm2) | RTSA (mm2) | OTSA (mm2) | OTSA/RTSA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VLA | Canidae | Vulpes lagopus | M | V | 5.75 | 139.7 | 23 396.8 | 42 420.5 | 9237.5 | 33 183 | 3.59 |
| F | 5.75 | 119.2 | 19 990.9 | 35 399.2 | 7736.5 | 27 662.7 | 3.58 | ||||
| CLA | Canidae | Canis latrans | M | V/NV | 13 | 178.5 | 49 723.3 | 61 537.4 | 15117.1 | 46 420.3 | 3.07 |
| F | 13 | 177.5 | 46 113 | 56 998.2 | 16926.7 | 40 071.5 | 2.37 | ||||
| CLUa | Canidae | Canis lupus arctos | M | V | 44.5 | 238.9 | 16 4810 | 14 8159.6 | 31911 | 11 6248.7 | 3.64 |
| CLUb | Canidae | Canis lupus baileyi | M | V | 38 | 236.2 | 10 2307 | 87 789 | 18621.1 | 69 167.9 | 3.71 |
| F | 38 | 236 | 10 0632.7 | 11 2706.7 | 22361 | 90 345.8 | 4.04 | ||||
| LPI | Canidae | Lycaon pictus | F | V | 19.8 | 194.4 | 11 4255.2 | 89 481 | 19089.3 | 70 391.7 | 3.69 |
| NPR | Canidae | Nyctereutes procyonoides | M | NV | 7 | 127.3 | 15 929.4 | 23 548.4 | 7183.5 | 16 364.9 | 2.28 |
| F | 7 | 124.1 | 16 438.88 | 26 034.5 | 8099.7 | 17 934.8 | 2.21 | ||||
| OME | Canidae | Otocyon megalotis | M | NV | 3.75 | 124.6 | 12 304.93 | 15 617 | 4945.5 | 10 671.5 | 2.16 |
| F | 3.75 | 124 | 12 360.1 | 19 578.7 | 6183.9 | 13 394.8 | 2.17 | ||||
| SVE | Canidae | Speothos venaticus | M | V | 9 | 130 | 19 974.4 | 25 307.9 | 5639.8 | 19 668.1 | 3.49 |
| UCI | Canidae | Urocyon cinereoargenteus | M | NV | 4.4 | 110.2 | 8504.5 | 12 979.4 | 2970.6 | 10 008.8 | 3.37 |
| F | 4.4 | 114.6 | 11 175.9 | 18 466.9 | 4611 | 13 855.9 | 3 | ||||
| VVU | Canidae | Vulpes vulpes | M | V/NV | 7.3 | 145 | 28 356.9 | 43 588.8 | 7748.5 | 35 840.3 | 4.63 |
| F | 7.3 | 130.9 | 21 364.7 | 39 608.3 | 6344 | 33 264.3 | 5.24 | ||||
| VMA | Canidae | Vulpes macrotis | M | V/NV | 2.6 | 109 | 7874.8 | 16 448.4 | 3624 | 12 824.3 | 3.54 |
| F | 2.6 | 108.89 | 8942.2 | 18 901.7 | 4842 | 14 059.8 | 2.9 | ||||
| MME | Mephitidae | Mephitis mephitis | M | NV | 3.5 | 91.1 | 8615.2 | 19 526.8 | 6152 | 13 374.8 | 2.17 |
| F | 3.1 | 75.8 | 5645 | 10 248 | 3094 | 7153.8 | 2.31 | ||||
| GGU | Mustelidae | Gulo gulo | M | V | 22.4 | 173.4 | 39 380.9 | 87 727 | 16084.5 | 71 642.3 | 4.45 |
| F | 16.6 | 152.5 | 32 360.9 | 64 286.8 | 11736.4 | 52 550.4 | 4.48 | ||||
| MFR | Mustelidae | Mustela frenata | M | V | 0.31 | 49.8 | 798.2 | 4017.9 | 999.7 | 3018.2 | 3.02 |
| F | 0.17 | 40.3 | 431.8 | 2054.4 | 359.4 | 1695.1 | 4.72 | ||||
| NVI | Mustelidae | Neovison vison | M | V/NV | 1.2 | 75.2 | 1954.9 | 7057 | 2343.1 | 4714 | 2.01 |
| TTA | Mustelidae | Taxidea taxus | M | V/NV | 8 | 121.8 | 15 723.6 | 31 447.2 | 8200.2 | 23 247 | 2.83 |
| F | 6 | 129 | 19 595.5 | 32 045.6 | 9090.4 | 22 955.1 | 2.53 | ||||
| PFL | Procyonidae | Potos flavus | M | NV | 2.5 | 88.7 | 7998.2 | 16561.8 | 3707.5 | 12 854.3 | 3.47 |
| F | 2.5 | 87.5 | 7146.1 | 15 121.1 | 3736 | 11 385.1 | 3.05 | ||||
| PLO | Procyonidae | Procyon lotor | M | NV | 6.3 | 121 | 16 686.7 | 28 500.2 | 9100.9 | 19 399.3 | 2.13 |
| F | 5.7 | 122.6 | 17 768.8 | 34 954 | 12596.9 | 22 357.2 | 1.77 | ||||
| UAM | Ursidae | Ursus americanus | M | NV | 155 | 30 1335.4 | 13 6851.7 | 64168.8 | 72 682.8 | 1.13 | |
| F | 90 | 261.7 | 16 2129.4 | 12 1404.8 | 50820.6 | 70 584.2 | 1.39 | ||||
| UAR | Ursidae | Ursus arctos | M | NV | 240 | 565.6 | 73 5532 | 32 9930.9 | 165290 | 16 4640.7 | 1 |
| F | 120 | 416.3 | 48 1331 | 26 4457.7 | 120728 | 14 3929.5 | 1.19 | ||||
| UMA | Ursidae | Ursus maritimus | M | V | 550 | 414.2 | 61 4321 | 40 1611.3 | 146820 | 25 4792 | 1.74 |
| U | 550 | 518.9 | 80 7961.6 | 47 6030.8 | 203666 | 27 2364.1 | 1.34 |
Code refers to abbreviation used to identify points in Figs 4; sex is M, male, F, female, U, unknown; diet is V, vertebrate, V/NV, vertebrate/non-vertebrate, and NV, non-vertebrate. TTSA, total turbinal surface area; RTSA and OTSA are the estimated surface areas available for respiratory epithelia and olfactory epithelia, respectively (see text). Estimated body masses are from Smith et al. (2003) and Wilson et al. (2010); skull lengths are condylobasal and were taken from digital images of the skulls using imagej. This was not possible for the male Ursus americanus as the scale bar was not visible.
Methods
Specimens
A total of 38 skulls were scanned that spanned 20 species (listed in Supporting Information). Species were chosen to span a range of body sizes, diets, and latitudes, as well as to represent taxonomic breadth. In most cases, each species was represented by two adult skulls, one male and one female. Sample sizes were constrained by the substantial expense and labor involved in producing and analyzing the scans. In the case of the gray wolf, we also included individuals from distinct habitats, the arctic subspecies Canis lupus arctos and Mexican subspecies Canis lupus baileyi. We included two species that we considered ‘semi-terrestrial’, the American mink (Neovison vison) and the polar bear (Ursus maritimus), while we excluded ‘semi-aquatic’ species, such as the river otter (Lontra canadensis). Although N. vison and U. maritimus swim frequently, they feed and spend an extensive amount of time on land. By contrast, L. canadensis was excluded from our study because it forages almost exclusively in water and our previous study (Van Valkenburgh et al. 2011) showed that it had markedly expanded respiratory turbinals relative to terrestrial caniforms. Skulls were selected if they were wild-caught adults with well preserved turbinals as viewed from the external nares. We obtained high-resolution computed tomography (HRCT) scans of skulls with slice number ranging from approximately 500–2000 slices. All scans were done by the University of Texas HCRT Facility (http://www.ctlab.geo.utexas.edu/) and are accessible at http://www.digimorph.org.
To test for differences associated with diet, we classified species into one of three categories according to the relative proportion of vertebrate and non-vertebrate (plants, invertebrates) foods in their diets: (i) vertebrate – > 70% vertebrates in their diet; (ii) vertebrate/non-vertebrate – 50–70% vertebrates with the balance non-vertebrate foods; or (iii) non-vertebrate – < 50% vertebrate with non-vertebrate foods predominating. Classification was based on information provided in Wilson et al. (2009) as well as previously published analyses (Van Valkenburgh, 1988). We recognize that these are fairly broad categories, but more specific categories (e.g. insectivorous, small vs. large prey specialists) would have resulted in very small sample sizes. In addition, use of a continuous measure, such as the percent of vertebrates in the diet, is unrealistic given levels of intraspecific dietary variation within omnivores. Moreover, these or similar categories have proved useful in prior analyses of dietary morphology in mammals (Van Valkenburgh, 1988; 1991, Evans et al. 2007).
We explored the association between latitude and turbinal size using the location of capture recorded on the specimen tags (Supporting Information Table S2). We also did a more fine-scale analysis of the influence of climate by comparing relative turbinal surface area between three pairs of sister taxa that were included because they exist in distinct habitats: arctic fox (Vulpes lagopus) vs. kit fox (Vulpes macrotis), Mexican (Canis lupus baileyi) vs. arctic (C. l. arctos) gray wolf, and polar bear (Ursus maritimus) vs. grizzly bear (U. arctos).
Turbinal measurements
Data on turbinal size for the arctoid species are taken from Van Valkenburgh et al. (2011). For the canids, we followed the same protocol as described there but used mimics 14.0 (Materialise, Inc.) 3-dimensional visualization software rather than amira (Visage Imaging). To verify that mimics and amira produce equivalent estimates of surface area, we measured the surface area and volume of a disc of known dimensions with each software program. The difference between the programs was minimal, and thus we use both program measurements interchangeably.
Because turbinal bones are extremely thin, it is often difficult to distinguish them from background levels of ‘noise’ in CT scans, which makes quantification extremely difficult. As in Van Valkenburgh et al. (2011), we first applied contrast limited adaptive histogram equalization (CLAHE; Jain, 1989) to improve the level of contrast between turbinals and background noise, while maintaining contrast integrity in other parts of the scans. The improved images were then imported to mimics for quantification (see Supporting Information). As in Van Valkenburgh et al. (2011) we measured the total volume of the chamber housing the turbinals (total chamber volume, TCV) and total turbinal surface area (TTSA). The total surface area of the turbinals was then divided into the portions assigned to either respiratory or olfactory function, respectively. Olfactory turbinal surface area (OTSA) included the summed area of the ethmoturbinals and nasoturbinals, whereas respiratory turbinal surface area (RTSA) included only the maxilloturbinals. It is important to note that total ethmoturbinal surface area may overestimate the extent of olfactory epithelium. Histological studies of selected mammals have shown that the extent of coverage of the ethmoturbinals by sensory mucosa varies among species (Rowe et al. 2005; Smith et al. 2007a,b, 2011, 2012). However, this is mitigated in part by the fact that we did not include the nasal septum, which is often partially covered in sensory epithelium (Smith et al. 2007a,b). Since this study focuses on comparative analyses and not absolute measurements, we assume that the overestimation of surface area is similar across all species and thus does not seriously affect the validity of cross-species comparisons. In some groups, the ethmoturbinals extend anteriorly and likely contribute to conditioning inspired air (e.g. primates, Smith et al. 2007a,b; and bats, Smith et al. 2012). However, in our study of caniforms, we found a clear spatial separation between the ethmoturbinals and maxilloturbinals that reassures us that our measures reflect functional separation. Ultimately, the determination of absolute measurements of olfactory vs. respiratory turbinal surface area requires histological analysis of the nasal chamber, a task that was beyond the scope of the current project.
Allometric effects
To control for the effects of body size, we examined the scaling of turbinal surface area with body mass, skull length, and turbinal chamber volume using log-transformed linear regressions. Body mass is often preferred for studies of how organs scale with increasing size because it is a more direct reflection of total metabolic demands than a linear measure of the skeleton. However, body composition, such as the proportion of fat to muscle, can vary greatly among species and obscure this relationship. Moreover, we had to use species mean body masses taken from the literature (Table 1) because the body mass of the individuals used in this study were unknown. We did have specimen-specific skull length data, and skull length avoids the problem of large fat reserves increasing mass without having an effect on skeletal dimensions. However, there may be a bias due to differences in facial proportions among species, such as having a long as opposed to a short snout. Finally, chamber volume may have a more direct relation to turbinal surface area. Because the nasal chamber contains the turbinals (and little else), measurements of chamber volume should be most highly correlated with surface area measurements. On the other hand, examining scaling relationships using chamber volume as a proxy for body size is not ideal because volume may vary for skulls of similar length or bodies of similar mass. Because each of the three proxies provides a different perspective on scaling, we included all of them in our scaling analyses.
We explored the scaling relationship between turbinal surface area (total, respiratory and olfactory) and estimates of body size (skull length, chamber volume, mass) with reduced major axis and least squares linear regressions (Warton et al. 2006) using the smatr package in r 2.15.0 (R Development Core Team 2012). Results did not differ significantly between the two regression methods and the least squares analyses are presented here. In all cases, we used all the data rather than species means because we felt that a species mean based on two individuals is not a valid representation of the mean.
We first tested for the presence of isometric scaling in canids and arctoids, and for differences in scaling between the two groups. We then examined the distribution of individuals by diet and latitude relative to the combined regression lines calculated for the entire sample of Caniformia. Our assumption is that species that fall on or near the regression line display the generalized and possibly plesiomorphic condition for the size of the turbinals relative to body size, and that species lying well above or below this line represent a derived condition that is presumably a specialization. Thus, the associations between turbinal surface area and diet and latitude, respectively, were explored using regression residuals. In the case of diet, the significance of differences in mean residual value between dietary groups was assessed with Mann–Whitney U-tests because residuals were not normally distributed. In the case of latitude, the residuals from the regression of respiratory turbinal surface area on body size were regressed against latitude. In sum, we calculated: (i) least squares scaling regressions of turbinal surface areas against body size measurements for canids and arctoids; (ii) Mann–Whitney U-test comparisons of the residuals by dietary type produced by regressions of olfactory turbinal surface area against body size measures; and (iii) a regression of respiratory turbinal surface area residuals against latitude. All regressions and subsequent statistical tests (slope comparisons, Mann–Whitney U) were done using r v.2.15.0 (R Development Core Team, 2012).
Phylogenetic comparative analysis
To account for the effects of similarity due to shared ancestry (Felsenstein, 1985), we re-performed regressions using phylogenetic generalized least squares (PGLS; Martins & Hansen, 1997). We used the caniform phylogeny of Slater et al. (2012) but pruned it so that only our sampled species were represented. We performed PGLS analyses with the ape package in r v.2.15.0 (Paradis et al. 2004; R Development Core Team, 2012), using a Brownian motion model when generating the phylogenetic variance-covariance matrix. In all cases, PGLS yielded slopes that did not differ significantly from those produced by our non-phylogenetically informed analyses, and so we present the latter here. The phylogenetic tree and full results of PGLS analyses are provided in the Supporting Information.
Results
Although we created all possible regression plots of the three turbinal surface area measurements against the three body size indices and against each other, we present a subset of the plots that illustrate our results to avoid redundancy.
Total turbinal surface area
Within the arctoids, total turbinal surface area (TTSA) increases isometrically with body mass, skull length, and chamber volume, respectively. That is, the slopes do not differ significantly from what is expected under geometric similarity (0.67 in the case of mass and chamber volume, or 2 in the case of skull length; Table 2). Canid TTSA also scales isometrically with respect to body mass and chamber volume, but scales positively with skull length (slope = 2.5, Table 2). Positive allometry in the canids reflects the fact that, relative to skull length, larger canids tend to have greater turbinal surface areas than smaller canids (Fig. 2A).
Table 2.
Scaling regressions of turbinate surface area on proxies of body size
| Linear regression | n | Group | Slope (b) | y-intercept | r2 | 95% CI for slope |
|---|---|---|---|---|---|---|
| Total turbinal surface area (TTSA) | ||||||
| Body mass | 38 | All | 0.66 | 3.92 | 0.95 | 0.61–0.72 |
| 19 | Canids | 0.77 | 3.85 | 0.86 | 0.61–0.93 | |
| 19 | Arctoids | 0.65 | 3.91 | 0.97 | 0.60–0.71 | |
| Skull length | 38 | All | 2.05 | 0.15 | 0.95 | 1.88–2.21 |
| 19 | Canids | 2.51* | −0.88 | 0.89 | 2.06–2.96 | |
| 19 | Arctoids | 1.99 | 0.29 | 0.97 | 1.81–2.17 | |
| Chamber volume | 38 | All | 0.69 | 1.53 | 0.97 | 0.65–0.73 |
| 19 | Canids | 0.75 | 1.27 | 0.96 | 0.66–0.83 | |
| 19 | Arctoids | 0.68 | 1.59 | 0.98 | 0.63–0.73 | |
| Respiratory turbinal surface area (RTSA) | ||||||
| Body mass | 38 | All | 0.74* | 3.27 | 0.96 | 0.69–0.80 |
| 19 | Canids | 0.70 | 3.29 | 0.84 | 0.54–0.85 | |
| 19 | Arctoids | 0.75* | 3.29 | 0.97 | 0.68–0.81 | |
| Skull length | 38 | All | 2.27* | −0.90 | 0.96 | 2.10–2.43 |
| 19 | Canids | 2.31 | −1.07 | 0.90 | 1.91–2.71 | |
| 19 | Arctoids | 2.27* | −0.83 | 0.98 | 2.10–2.43 | |
| Chamber volume | 38 | All | 0.74* | 0.75 | 0.92 | 0.67–0.82 |
| 19 | Canids | 0.67 | 0.96 | 0.93 | 0.58–0.77 | |
| 19 | Arctoids | 0.78* | 0.64 | 0.99 | 0.74–0.82 | |
| Olfactory turbinal surface area (OTSA) | ||||||
| Body mass | 38 | All | 0.62 | 3.82 | 0.92 | 0.56–0.69 |
| 19 | Canids | 0.80 | 3.71 | 0.84 | 0.62–0.97 | |
| 19 | Arctoids | 0.61 | 3.79 | 0.96 | 0.54–0.67 | |
| Skull length | 38 | All | 1.93 | 0.26 | 0.92 | 1.73–2.13 |
| 19 | Canids | 2.58* | −1.14 | 0.87 | 2.07–3.10 | |
| 19 | Arctoids | 1.85 | 0.43 | 0.95 | 1.62–2.08 | |
| Chamber volume | 38 | All | 0.65 | 1.55 | 0.95 | 0.60–0.70 |
| 19 | Canids | 0.77* | 1.05 | 0.94 | 0.67–0.87 | |
| 19 | Arctoids | 0.63 | 1.64 | 0.96 | 0.57–0.70 | |
Indicates significant difference (P < 0.05) in slope from expected values of 0.67 for body mass and chamber volume, and 2.0 for skull length, respectively.
Fig. 2.
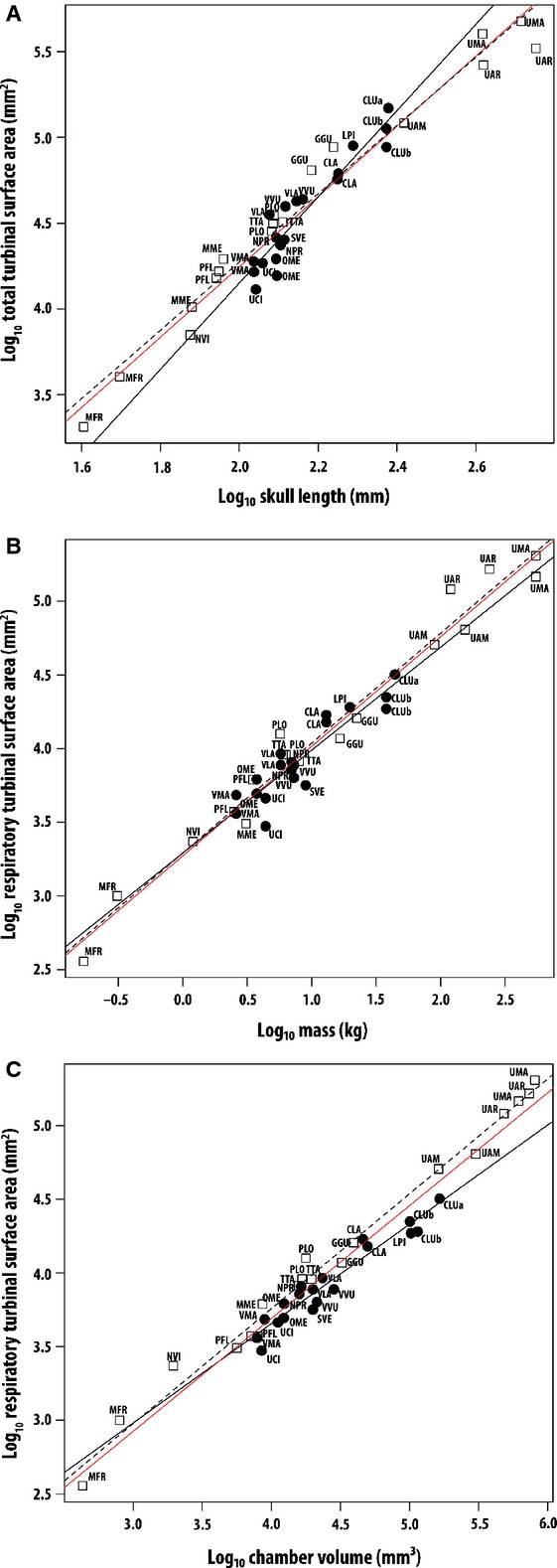
Log10/log10 plot of (A) total turbinal surface area (TTSA) against skull length, (B) respiratory surface area (RTSA) against body mass, and (C) respiratory surface area (RTSA) against chamber volume. Open squares, arctoids; solid circles, canids. Least-squares regression lines are shown for the total sample (red), arctoids (dashed) and canids (solid). See Table 1 for species codes and Table 2 for line equations and regression statistics.
Respiratory surface area
Respiratory turbinal surface area (RTSA) scales positively and similarly with all three estimates of body size in canids and arctoids (Fig. 2B,C; Table 2), but only among the arctoids are the slopes significantly greater than isometry. In the plot of RTSA against chamber volume (Fig. 2C), canids differ from arctoids in tending to fall on or below the common regression line and this difference is significant (P < 0.05, Table 3). Thus, canids tend to have less respiratory surface area than arctoids for a given chamber volume.
Table 3.
Results of Mann–Whitney U-tests on residuals of selected regressions. ‘Carnivore’ includes species within the vertebrate and vertebrate/non-vertebrate dietary groups. ‘Omnivore’ includes species within non-vertebrate group. The difference between the ‘carnivores’ and ‘omnivores’ was also significant when the vertebrate/non-vertebrate group was combined with the non-vertebrate group
| Regression | Comparison | Mean residual value | Mean residual value | P |
|---|---|---|---|---|
| RTSA vs. chamber volume | Canids vs. arctoids | Canids | Arctoids | |
| −0.073 | 0.073 | < 0.01 | ||
| OTSA vs. mass | Carnivore vs. omnivore | Carnivore | Omnivore | |
| 0.067 | −0.092 | < 0.01 | ||
| OTSA vs. skull length | Carnivore vs. omnivore | 0.054 | −0.072 | 0.01 |
| OTSA vs. chamber volume | Carnivore vs. omnivore | 0.063 | −0.086 | < 0.01 |
Olfactory turbinal surface area
Canids exhibit greater positive allometry than arctoids in olfactory surface area (OTSA) relative to all three body size estimates, and differ significantly from isometry in the relationships to skull length and chamber volume (Fig. 3; Table 2). Whereas canids tended to fall below the common regression line on some of the respiratory surface area plots, the opposite is the case with OTSA, at least for the larger canids (body mass > 12 kg). Larger canids tend to have greater olfactory surface areas than similarly sized arctoids. Two exceptions to this are the wolverine (Gulo gulo) and polar bear (Ursus maritimus), both of which also have unusually large olfactory turbinals for their body mass, skull length and chamber volumes (GGU and UMA in Fig. 3).
Fig. 3.
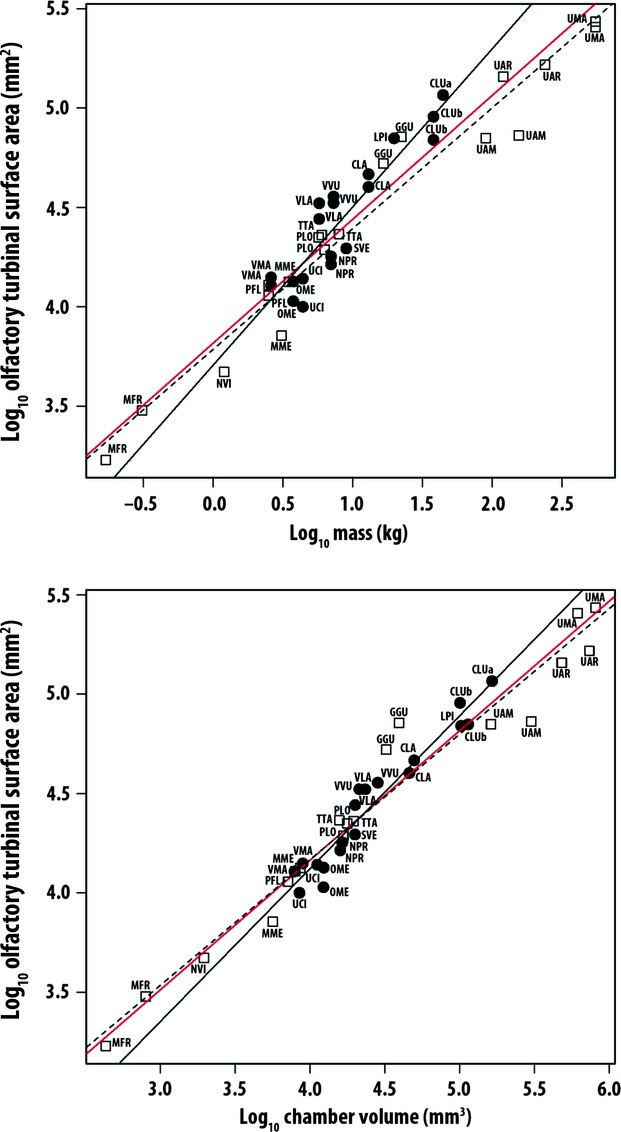
Log10/log10 plot of olfactory surface area (OTSA) against body mass (top) and chamber volume (bottom). Symbols and species codes as in Fig. 2. See Table 2 for line equations and regression statistics.
Diet and olfactory turbinal size
When species are plotted by dietary category rather than phylogenetic classification, it is apparent that species within the vertebrate and vertebrate/non-vertebrate dietary groups tend to have larger olfactory turbinals than species that rely more heavily on non-vertebrate foods. All regressions of OTSA against body size showed significantly higher residual values in carnivores when compared with omnivores (P < 0.01, Table 3). This is apparent within and across families in a plot of OTSA vs. chamber volume (Fig. 4A) as well as in sagittal sections taken from the CT scans (Fig. 5). To test whether differences among dietary groups were significant, we ran two comparisons, one in which we lumped the vertebrate/non-vertebrate and vertebrate dietary groups due to small sample size in the former, and a second in which we lumped the vertebrate/non-vertebrate with the non-vertebrate dietary group. In both cases, Mann–Whitney comparisons of the regression residuals for OTSA against chamber volume revealed that the vertebrate dietary group, with or without the vertebrate-non/vertebrate group, had more positive residuals (P ≤ 0.01, Table 3) than the alternative group. Moreover, the effect of diet is best expressed among the largest species in both arctoids and canids. For example, the wolverine is the largest mustelid and falls well above the regression line, with relatively large olfactory turbinals. Similarly, the polar bear (U. maritimus) is the most carnivorous of the three sampled bears and also has the most positive residuals. Among the canids, the largest species, gray wolf (Canis lupus), African wild dog (Lycaon pictus) and coyote (Canis latrans), are all classified as carnivorous and fall above the line, while smaller more omnivorous species such as raccoon dog (Nyctereutes procyonoides), gray fox (Urocyon cinereoargenteus), kit fox (V. macrotis), and the insectivorous bat-eared fox (Otocyon megalotis) fall on or below the line.
Fig. 4.
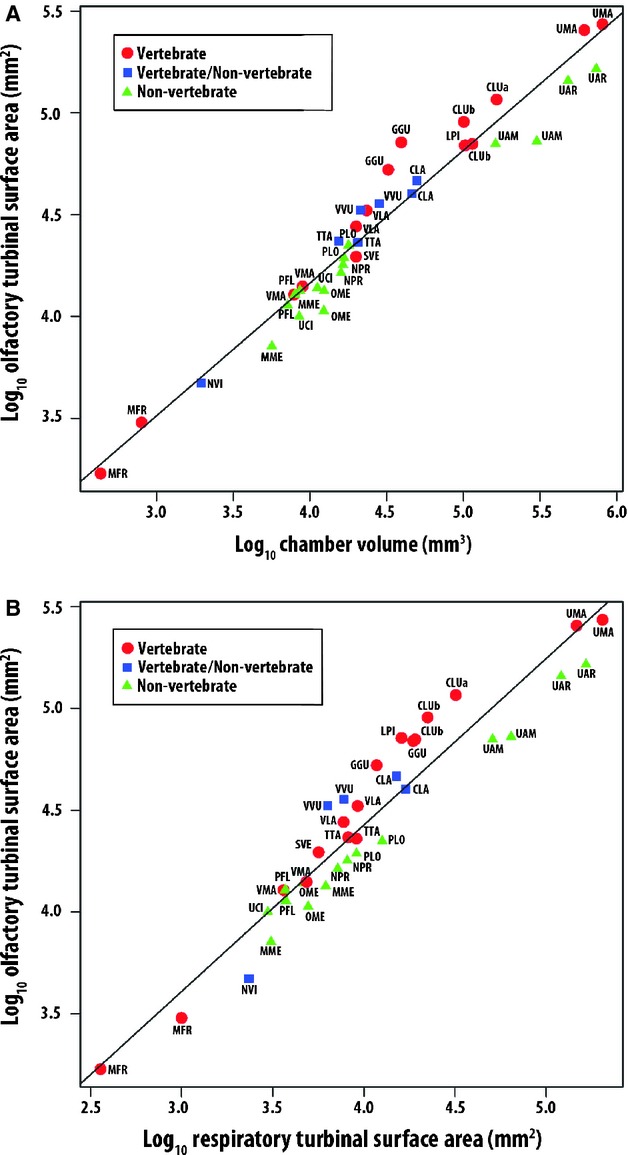
Log10/log10 plot of olfactory surface area (OTSA) against chamber volume (A) and respiratory surface area (RTSA) (B). Species are color-coded by diet, vertebrate (red circles), vertebrate/non-vertebrate (blue squares), and non-vertebrate (green circles). Species abbreviations as in Fig. 2. See Table 2 for line equations and regression statistics.
Fig. 5.
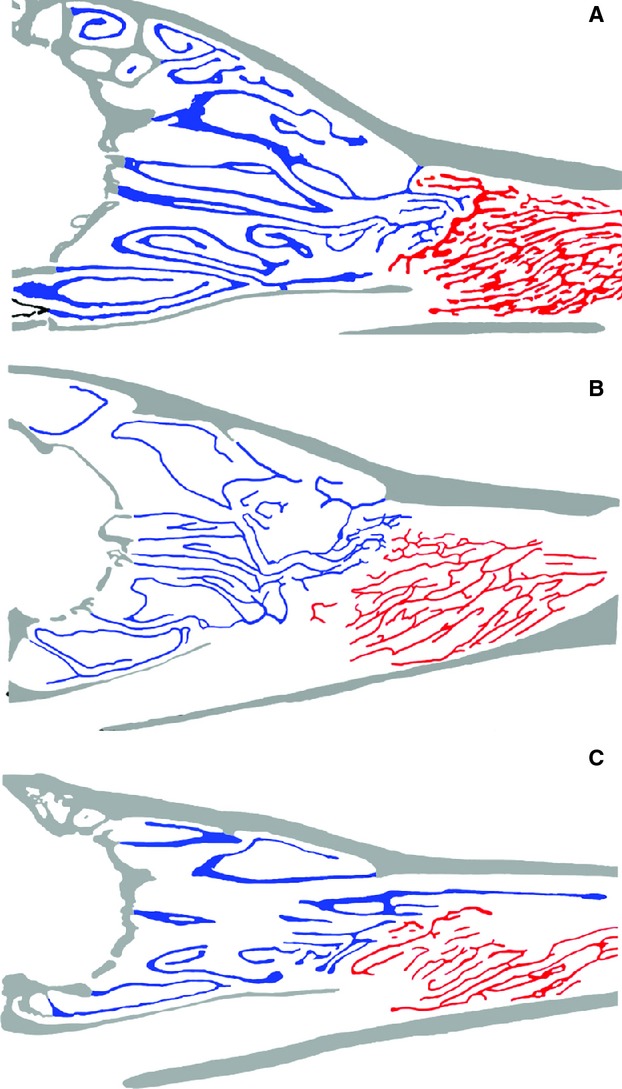
Sagittal sections taken near the midline of the skulls of (A) arctic fox (UCLA15161), (B) raccoon dog (USNM255530), and (C) bat-eared fox (USNM429129). Respiratory turbinals (red); olfactory turbinals (blue). Note the reduced development of olfactory and respiratory turbinals in the omnivorous raccoon dog and insectivorous bat-eared fox relative to the carnivorous arctic fox.
The difference in morphology between the two dietary types is more apparent when OTSA is plotted against RTSA (Fig. 4B). Interestingly, the two smallest vertebrate and vertebrate/non-vertebrate species in this plot (mink, Neovison vison, long-tailed weasel, Mustela frenata) fall below the line, unlike the larger species within their dietary groups. This suggests that factors selecting for olfactory turbinal dimensions may vary with body size.
Effect of latitude
Although we predicted that high latitude species should have enhanced respiratory turbinals to aid in heat conservation, correlations between latitude and either relative respiratory or relative olfactory turbinal size were not significant when examined across our entire sample of 20 species. Nevertheless, in our sister-taxa comparisons, the northern taxa did exhibit enlarged respiratory and olfactory turbinals relative to their southern cousins (Table 1). For example, relative to skull length, arctic taxa (V. lagopus, C. l. arctos, U. maritimus) showed greater average RTSA (19.6 ± 3.9% greater RTSA), OTSA (plus 27.7 ± 8.5%), and TTSA (plus 24.2 ± 3.4%) over their respective temperate sister taxa (V. macrotis, C. l. baileyi, U. arctos). Additionally, arctic taxa showed greater average chamber volume when controlled for skull length (plus 14.2 ± 3.1%) over temperate species.
Discussion
The scaling of respiratory turbinal surface area with body size is broadly similar in arctoids and canids, with most relationships exhibiting isometry or slight positive allometry. Under isometric growth, surface areas increase less rapidly than volume, and thus the ratio of surface area to volume declines with larger size. The fact that larger caniforms have relatively less respiratory surface area for their mass probably reflects a decreased need for heat retention and/or water conservation in larger species due to reduced body surface area to volume ratios. We did not find a significant relationship between respiratory turbinal surface area and climate, at least as estimated by latitude. Nevertheless, our three comparisons between arctic and temperate individuals within the same species (arctic vs. Mexican gray wolf) or between closely related sister taxa (arctic vs. kit fox, polar vs. grizzly bear) suggest that ecological parameters may have an impact. In all three examples, arctic individuals had greater relative RTSA than temperate individuals, which could be explained by an increased need for heat and water retention in cold, dry environments. Interestingly, the arctic individuals also had enlarged OTSA relative to temperate individuals, a finding that we did not expect. As discussed below, this may reflect relatively larger home ranges or more carnivorous diets in arctic animals. However, given that we had a sample size of one for each of our three comparisons, these results cannot be considered significant but they do suggest the need to explore this further with larger samples from each taxon.
Unlike respiratory turbinal surface area, olfactory turbinal surface area did appear to scale differently between canids and arctoids, with canids showing positive allometry and arctoids showing isometry. The slopes of the regression lines of olfactory surface area against both skull length (2.6 vs. 2) and chamber volume (0.77 vs. 0.68) were more positive in the canids than in the sampled arctoids, although these differences were not significant at the 0.05 level. Larger canids tend to have expanded olfactory surface areas relative to both smaller canids and all arctoids, with the exception of the wolverine and polar bear. Is the positive scaling observed in the canids simply a derived characteristic of the family with little functional significance, or does it reflect ecological differences between smaller and larger canids? The latter seems more likely. All the larger canids are more carnivorous than most smaller canids, whereas larger arctoids include both carnivorous as well as omnivorous species. Both of the two large carnivorous arctoids, the polar bear and wolverine, are similar to the large canids in having relatively larger olfactory turbinals. Thus, our results suggest that olfactory turbinal surface area scales positively with body size when larger animals also exhibit greater degrees of carnivory.
An alternative explanation for the expanded olfactory surface area in the largest canids might relate to social behavior. Both the gray wolf and African wild dog live in packs, and it is possible that pack-living favors enhanced olfaction to recognize conspecifics. However, the other pack-hunter in our sample, the diminutive bush dog (Speothos venaticus), does not have a large olfactory turbinal surface area. That fact plus some additional observations suggest that differences in group size have little impact on olfactory needs. First, recognition of conspecifics would seem to be equally important to non-pack living species. Carnivores are almost always territorial and use prolific scent-marks to signal their identity, presence, and perhaps sex to conspecifics (Gorman & Trowbridge, 1989). Even somewhat less gregarious canids (kit foxes, Murdoch et al. 2008) and arctoids (e.g. kinkajou, Kays & Gittleman, 2001; raccoons, Prange et al. 2011) that typically exist as singletons or dyads have frequent social interactions with neighboring conspecifics and so need to recognize kin and non-kin. Secondly, among canids, group size is highly variable and not closely tied to body mass. Instead, group size depends on resource levels with larger groups forming when resources are plentiful (Bekoff et al. 1981; Moehlman, 1989; Macdonald et al. 2004). For example, multiple families of arctic foxes have been observed to share a den (Hamilton, 2008), and group size in bat-eared foxes ranged from one to eight in the Kalahari in response to changing rainfall amounts (Nel et al. 1984). It is difficult to be certain but it does not seem likely that pack-living itself would exact greater demands on olfactory ability than other sorts of territorial lifestyles. Finally, the fact that two typically solitary arctoids, the wolverine and polar bear, exhibit large olfactory surface areas also suggests that something other than social behavior is playing a role in determining olfactory demands.
In addition to a highly carnivorous diet, another feature shared by the larger canids, the wolverine, and the polar bear is that they all have fairly large home ranges. Larger home range size is correlated with both carnivory and greater olfactory bulb size (Gittleman & Harvey, 1982; Gittleman, 1991). Gittleman (1991) suggested that the positive association between home range size and olfactory bulb size might reflect increased selection for olfaction due to aspects of maintaining ranges, such as scent marking. In a theoretical paper, Benhamou (1989) suggested that orienting oneself in a large home range might demand greater olfactory abilities to detect gradients in scent strength that reflect the time and location of scent placement. However, this would suggest that the need to use scent marks for orientation might scale with the size of the carnivore relative to its range, such that small and large species might be similar in olfactory requirements. It seems more likely that carnivory drives both range size increase and olfactory turbinal scaling. Although species vary in their reliance on vision, hearing and olfaction to find prey, most or all carnivores will take advantage of scent to find prey, as was recently demonstrated experimentally by Hughes et al. (2010). Vertebrate prey is usually less abundant than either invertebrate or plant food resources, which forces carnivores to forage further for their food. For example, both African wild dogs (L. pictus) and gray wolves (C. lupus) hunt herds of ungulates over great distances in Africa and North America, respectively, and consequently, both have home range sizes that cover hundreds of kilometers or more (Gittleman, 1991). Other carnivores such as the arctic fox (V. lagopus), wolverine (G. gulo), and polar bear (U. maritimus) also may have to search for dispersed food resources over large areas (Hornocker, 1982; Eide et al. 2004). Perhaps their expanded olfactory surface areas aid them in detecting prey or carrion over great distances, thus greatly reducing their searching effort, especially during periods of low prey abundance. Carbone et al. (2011) recently showed that large-bodied carnivores expend relatively more energy in hunting, especially when prey are rare, and this leads to more rapid declines in survival and reproduction. This suggests that selection for the ability to detect prey over great distances, and thereby reduce travel costs, would be strongly favored. This may explain why smaller hypercarnivores such as the bush dog (S. venaticus), weasel (M. frenata) and mink (N. vison) do not show expanded olfactory areas. Although carnivorous, these species hunt more abundant prey such as lagomorphs and rodents and do not need to expand their home ranges to find prey. It seems that an increase in olfactory surface area in caniforms is associated with a diet reliant upon widely dispersed, large prey.
By contrast, omnivores are generalists and thus do not need to search as far as the carnivores do to find food. For example, the procyonids [raccoon (Procyon lotor), kinkajou (Potos flavus)], gray fox (U. cinereoargenteus), raccoon dog (N. procyonoides), skunk (Mephitis mephitis), black and brown bears (U. americanus, U. arctos) are all opportunistic feeders that consume a variety of plant and non-vertebrate foods. The bat-eared fox (Otocyon megalotis) is a specialized insectivore that forages in a zig-zag pattern to maximize encounters with its food (termites, beetles) over small areas of African savannah (Waser, 1980). All of these species fall below the caniform regression line for OTSA against RTSA (Fig. 4B), suggesting they are derived (specialized) among the Caniformia in having reduced OTSA (Macrini et al. 2006). Thus it appears that as distance required to find food decreases, the proportion of olfactory turbinals decreases as well in caniforms.
Like others before us, we have assumed that a larger relative olfactory surface or a larger olfactory organ reflects enhanced olfactory ability (Pihlström et al. 2005; Kavoi et al. 2010; Van Valkenburgh et al. 2011). This assumption follows from the idea that increased olfactory surface area should be accompanied by an increased number of olfactory receptor neurons and that a larger number of these neurons spread over a larger epithelial area leads to a lower concentration threshold for detection (better olfactory sensitivity). However, a recent study of olfactory organ size and sensitivity in fish (elasmobranchs and teleosts) revealed no differences in sensitivity to selected amino acids despite the expanded olfactory organs of the elasmobranchs (Meredith & Kajiura, 2010). The authors suggested that substances other than the chosen acids might be more important, and that alternative parameters such as binding affinities of particular olfactory receptor neurons and/or the number of neurons that converge on a specific glomerulus in the olfactory bulb might also be relevant. Unfortunately, neither of these alternative parameters can be read from cranial morphology. There is some evidence that mammalian olfactory surface area does correspond with olfactory sensitivity (i.e. detection threshold or acuity in the sense of Hardy et al. 2012) from experiments done with dogs and primates, including humans (Marshall et al. 1981). Additionally, semi-aquatic and aquatic mammals (e.g. pinnipeds, platypus, cetaceans) that rarely use olfaction in foraging have greatly reduced olfactory organs and surface areas (Huntley et al. 1984; Folkow et al. 1988; Macrini et al. 2006; Pihlström, 2008; Van Valkenburgh et al. 2011). However, we have very little understanding of the relationship between olfactory surface area or organ size and two other aspects of olfactory ability that are not entirely distinct, being able to distinguish among similar odorants [discrimination as in Hildebrand & Shepherd (1997), or acuity as in Cleland & Narla (2003)] and being able to detect a wide array of odorants [olfactory breadth (our term)]. Both of these would seem likely to be associated with possession of a greater variety of functioning olfactory genes, a parameter that we hope to compare with relative olfactory surface area in future work. Unfortunately, the number of functioning OR genes is not known for any of the species in our sample. The only study to date of caniform OR genes found a slightly greater fraction of pseudogenes in the domestic dog relative to the gray wolf, but the difference was not significant (Zhang et al. 2011). Our ability to explain turbinal dimensions in terms of all these facets of olfactory capacity requires a much better understanding of how olfaction works at the level of the receptor neuron as well as how intra-nasal flow dynamics impact the detection of odorants.
Because olfactory epithelium is similar to brain tissue in being relatively expensive to grow and maintain (Niven & Laughlin, 2008), the expansion of olfactory surface area in large caniform hypercarnivores represents a cost of this dietary specialization. This cost must be added to others, such as a relatively greater energy expenditure for foraging (Carbone et al. 2011), an increased risk of injury due to killing large prey and battles over carcasses (Donadio & Buskirk, 2006), as well as prolonged development of foraging skills relative to species that take smaller prey (Van Valkenburgh, 2007). Despite the added expense of olfactory epithelium, we did not find strong evidence of trade-offs between respiratory and olfactory function in caniforms as was previously found among semi-aquatic arctoids (Van Valkenburgh et al. 2011). Among those taxa, expansion of the respiratory turbinals for water and heat conservation was associated with a marked reduction in olfactory turbinals. Among the caniforms, we observed examples of expansion of both olfactory and respiratory turbinals in arctic taxa. Presumably, the greater reduction in olfactory turbinals observed in the semi-aquatic arctoids was due to the combination of strong positive selection for enlarged orbits and/or expanded vibrissae alongside greatly reduced selection for olfactory ability in aquatic environments (Repenning, 1976). Among large canids, it seems there was strong positive selection for olfactory ability, but no reduced selection for respiratory function.
In sum, we have shown that, unlike arctoids, canids are derived in showing positive allometry in the scaling of total turbinal surface area. This is driven by positive allometry in olfactory turbinal scaling and isometry in the respiratory turbinals. Because of the positive allometry, it is mostly large canids that exhibit expanded olfactory turbinal surface areas, and they are joined by at least two arctoid species (wolverine and polar bear). This pattern of expanded olfactory turbinals in larger canids appears particularly noteworthy, in that sensory organs typically scale to body size with strong negative allometry (Nummela, 1995; Howland et al. 2004). In both canids and terrestrial arctoids, large carnivorous species show an increase in olfactory turbinal surface area that likely is selected for due to the demands of locating large, widely dispersed prey. Notably, our data suggest that the enhanced olfactory abilities of the domestic dog reflect its recent descent from a large carnivorous canid, the gray wolf (Vila et al. 1997). Undoubtedly, humans were motivated to domesticate wolves because of their far superior olfactory sensitivity, which made them both helpful hunting companions and early-warning systems for approaching danger (Shipman, 2010).
Although it appears that large meat-eaters have enhanced olfactory abilities relative to more omnivorous species among the Caniformia, it will be interesting to explore the scaling of olfactory turbinal surface area in felids, a group of hypercarnivores that have reduced olfactory bulbs (Gittleman, 1991) and that are thought to rely less on olfaction and more on vision and hearing for hunting. We doubt that our conclusions can be generalized far beyond the Carnivora, and expect that olfactory needs of herbivorous ungulates and rodents, or omnivorous suids (pigs, peccaries), for example, might differ significantly from those of carnivores. We look forward to exploring the scaling of turbinals in a wider variety of mammals.
Acknowledgments
We thank M. Colbert, R. Ketcham, and J. Maisano of the University of Texas HRCT Facility and Digital Morphology Group (http://DigiMorph.org) for their dedication and skills in producing the CT scans, the multiple curators and collection managers that allowed us to borrow skulls for scanning, and two anonymous reviewers for insightful comments. G. Slater advised on the PIC analyses and provided helpful comments on the manuscript. Funding was provided by NSF IOB-0517748 and NSF IOS-1119768 to B.V.V. and NSF IIS-9874781 and IIS-0208675 to T.R.
Author contributions
P.A.G. and B.V.V. were the primary authors on the manuscript. B.V.V. contributed to study concept and design, as well as data analysis, and interpretation. P.A.G. and B.P. were involved in turbinal data acquisition, analysis, and interpretation and performed the comparative phylogenetic analyses. D.B. and A.C. assisted in turbinal data acquisition and T.R. contributed to data interpretation. All authors participated in the writing of the paper.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Phylogeny of the Caniformia used in the comparative analyses.
Fig. S2. Bivariate PGLS regression plots.
Table S1. Regression statistics for the PGLS analyses.
Table S2. List of specimens scanned and associated latitude data used in the analysis.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Bekoff M, Diamond J, Mitton JD. Life-history patterns and sociality in canids: body size, reproduction, and behavior. Oecologia. 1981;5:386–390. doi: 10.1007/BF00344981. [DOI] [PubMed] [Google Scholar]
- Benhamou S. An olfactory orientation model for mammals' movements in their home ranges. J Theor Biol. 1989;139:379–388. [Google Scholar]
- Carbone C, Pettorelli N, Stephens PA. The bigger they come, the harder they fall: body size and prey abundance influence predator-prey ratios. Biol Lett. 2011;7:312–315. doi: 10.1098/rsbl.2010.0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Narla VA. Intensity modulation of olfactory acuity. Behav Neurosci. 2003;117:1434–1440. doi: 10.1037/0735-7044.117.6.1434. [DOI] [PubMed] [Google Scholar]
- Craven B, Neuberger T, Paterson EG, et al. Reconstruction and morphometric analysis of the nasal airway of the dog (Canis familiaris) and implications regarding olfactory airflow. Anat Rec. 2007;290:1325–1340. doi: 10.1002/ar.20592. [DOI] [PubMed] [Google Scholar]
- Craven BA, Paterson EG, Settles GS. The fluid dynamics of canine olfaction: unique nasal airflow patterns as an explanation of macrosmia. J R Soc Interface. 2010;7:933–943. doi: 10.1098/rsif.2009.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio E, Buskirk SW. Diet, morphology, and interspecific killing in Carnivora. Am Nat. 2006;167:524–553. doi: 10.1086/501033. [DOI] [PubMed] [Google Scholar]
- Eide NE, Jepsen JU, Prestrud P. Spatial organization of reproductive Arctic foxes Alopex lagopus: responses to changes in spatial and temporal availability of prey. J Anim Ecol. 2004;73:1056–1106. [Google Scholar]
- Evans AR, Wilson GP, Fortelius M, et al. High-level similarity of dentitions in carnivorans and rodents. Nature. 2007;445:78–81. doi: 10.1038/nature05433. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- Folkow LP, Blix AS, Eide TS. Anatomical and functional aspects of the nasal mucosa and ophthalmic retia of phocid seals. J Zool. 1988;216:417–436. [Google Scholar]
- Gittleman JL. Carnivore olfactory bulb size, allometry, phylogeny, and ecology. J Zool. 1991;225:253–272. [Google Scholar]
- Gittleman JL, Harvey PH. Carnivore home-range size, metabolic needs and ecology. Behav Ecol Sociobiol. 1982;10:57–63. [Google Scholar]
- Gorman ML, Trowbridge BJ. The role of odor in the social lives of carnivores. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Ithaca, NY: Cornell University Press; 1989. pp. 57–88. [Google Scholar]
- Hamilton G. Arctic Fox. Buffalo: Firefly Books Inc; 2008. [Google Scholar]
- Hardy C, Rosedale M, Messinger JW, et al. Olfactory acuity is associated with mood and function in a pilot study of stable bipolar disorder patients. Bipolar Disord. 2012;14:109–117. doi: 10.1111/j.1399-5618.2012.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Hillenius WJ. The evolution of nasal turbinals and mammalian endothermy. Paleobiology. 1992;18:17–29. [Google Scholar]
- Hornocker M. Ecology of the wolverine in northwestern Montana. NGS Res Rep. 1982;14:341–350. [Google Scholar]
- Howland HC, Merola S, Basarab JR. The allometry and scaling of the vertebrate eye. Vision Res. 2004;44:2043–2065. doi: 10.1016/j.visres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Hughes NK, Price CJ, Banks PB. Predators are attracted to the olfactory signals of prey. PLoS ONE. 2010;5:e13114. doi: 10.1371/journal.pone.0013114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley AC, Costa DP, Rubin RD. The contribution of nasal counter-current heat exchange to water balance in the Northern elephant seal, Mirounga angustirostris. J Exp Biol. 1984;113:447–454. doi: 10.1242/jeb.113.1.447. [DOI] [PubMed] [Google Scholar]
- Huston MA, Wolverton S. Regulation of animal size by eNPP, Bergmann's rule, and related phenomena. Ecol Monogr. 2011;81:349–405. [Google Scholar]
- Jain AK. Fundamentals of Digital Image Processing. Upper Saddle River, NJ: Prentice-Hall, Inc; 1989. [Google Scholar]
- Kavoi B, Makanya A, Hassanali J, et al. Comparative functional structure of the olfactory mucosa in the domestic dog and sheep. Ann Anat. 2010;192:329–337. doi: 10.1016/j.aanat.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Kays R, Gittleman JL. The social organization of the kinkajou Potos flavus (Procyonidae) J Zool. 2001;253:491–504. [Google Scholar]
- Macdonald DW, Creel S, Mills MGL. Canid society. In: Macdonald DW, Sillero-Zubiri C, editors. Biology and Conservation of Wild Canids. Oxford: Oxford University Press; 2004. pp. 85–106. [Google Scholar]
- Macrini TE, Rowe T, Archer M. Description of a cranial endocast from a fossil platypus, Obdurodon dicksoni (Montremata, Ornithorhynchidae), and the relevance of endocranial characters to monotreme monophyly. J Morphol. 2006;267:1000–1015. doi: 10.1002/jmor.10452. [DOI] [PubMed] [Google Scholar]
- Macrini TE. Comparative morphology of the internal nasal skeleton of adult marsupials based on x-ray computed tomography. Bull Am Mus Nat Hist. 2012;365:1–91. [Google Scholar]
- Marshall DA, Blumer L, Moulton DG. Odor detection curves for n-pentanoic acid in dogs and humans. Chem Senses. 1981;6:445–453. [Google Scholar]
- Martins EP, Hansen TF. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat. 1997;149:646–667. [Google Scholar]
- Meiri S, Dayan T, Simberloff D. Carnivores, biases and Bergmann's rule. Biol J Linn Soc. 2004;81:579–588. [Google Scholar]
- Meredith TL, Kajiura SM. Olfactory morphology and physiology of elasmobranchs. J Exp Biol. 2010;213:3449–3456. doi: 10.1242/jeb.045849. [DOI] [PubMed] [Google Scholar]
- Moehlman PD. Intraspecific variation in canid social systems. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Ithaca, NY: Cornell University Press; 1989. pp. 143–163. [Google Scholar]
- Moore WJ. The Mammalian Skull. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Murdoch JD, Ralls K, Cypher B, et al. Social interactions among San Joaquin kit foxes before, during, and after the mating season. J Mammal. 2008;89:1087–1093. [Google Scholar]
- Nel JAJ, Mills MGL, van Aarde RG. Fluctuating group size in bat-eared foxes (Otocyon m. megalotis) in the south-western Kalahari. J Zool. 1984;203:294–298. [Google Scholar]
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- Noback ML, Harvati K, Spoor F. Climate-related variation of the human nasal cavity. Am J Phys Anthropol. 2011;145:599–614. doi: 10.1002/ajpa.21523. [DOI] [PubMed] [Google Scholar]
- Nummela S. Scaling of the mammalian middle ear. Hear Res. 1995;85:18–30. doi: 10.1016/0378-5955(95)00030-8. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Pihlström H. Comparative anatomy and physiology of chemical senses in aquatic mammals. In: Thewissen JGM, Nummela S, editors. Sensory Evolution on the Threshold. Berkeley: University of California Press; 2008. pp. 95–109. [Google Scholar]
- Pihlström H, Fortelius M, Hemila S, et al. Scaling of mammalian ethmoid bones can predict olfactory organ size and performance. Proc Biol Sci. 2005;272:957–962. doi: 10.1098/rspb.2004.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange S, Gehrt SD, Hauver S. Frequency and duration of social contacts between free-ranging raccoons: uncovering a hidden social system. J Mammal. 2011;92:1331–1342. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. ISBN3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Repenning CA. Adaptive evolution of sea lions and walruses. Syst Zool. 1976;25:375–390. [Google Scholar]
- Rowe T, Eiting TP, Macrini TE, et al. Organization of the olfactory and respiratory skeleton in the nose of the gray short-tailed opossum Monodelphis domestica. J Mamm Evol. 2005;12:303–336. [Google Scholar]
- Schmidt-Nielsen K, Hainsworth FR, Murrish DE. Counter-current heat exchange in the respiratory passages: effect on water and heat balance. Respir Physiol. 1970;9:263–276. doi: 10.1016/0034-5687(70)90075-7. [DOI] [PubMed] [Google Scholar]
- Shipman P. The animal connection and human evolution. Curr Anthropol. 2010;51:519–538. [Google Scholar]
- Slater GJ, Harmon LJ, Alfaro ME. Integrating fossils with molecular phylogenies improves inference of trait evolution. Evolution. 2012 doi: 10.1111/j.1558-5646.2012.01723.x. doi: 10.1111/j.1558-5646.2012.01723.x. [DOI] [PubMed] [Google Scholar]
- Smith FA, Lyons SK, Morgan E, et al. Body mass of late Quaternary mammals. Ecology. 2003;84:3403. [Google Scholar]
- Smith T, Bhatnagar K, Rossie J, et al. Scaling of the first ethmoturbinal in nocturnal strepsirrhines, olfactory and respiratory surfaces. Anat Rec. 2007a;290:215–237. doi: 10.1002/ar.20428. [DOI] [PubMed] [Google Scholar]
- Smith T, Rossie JB, Bhatnagar K. Evolution of the nose and nasal skeleton in primates. Evol Anthropol. 2007b;16:132–146. [Google Scholar]
- Smith T, Eiting TP, Rossie J. Distribution of olfactory and nonolfactory surface area in the nasal fossa of Microcebus murinus: implications for microcomputed tomography and airflow studies. Anat Rec. 2011;294:1217–1225. doi: 10.1002/ar.21411. [DOI] [PubMed] [Google Scholar]
- Smith T, Eiting TP, Bhatnagar K. A quantitative study of olfactory, nonolfactory, and vomeronasal epithelia surface area in the nasal fossa of the bat Megaderma lyra. J Mamm Evol. 2012;19:27–41. [Google Scholar]
- Van Valkenburgh B. Trophic diversity in past and present guilds of large predatory mammals. Paleobiology. 1988;14:155–173. [Google Scholar]
- Van Valkenburgh B. Iterative evolution of hypercarnivory in canids (Mammalia: Carnivora): evolutionary interactions among sympatric predators. Paleobiology. 1991;17:340–362. [Google Scholar]
- Van Valkenburgh B. Deja vu: the evolution of feeding morphologies in the Carnivora. Integr Comp Biol. 2007;47:147–163. doi: 10.1093/icb/icm016. [DOI] [PubMed] [Google Scholar]
- Van Valkenburgh B, Curtis A, Samuels J, et al. Aquatic adaptations in the nose of carnivorans: evidence from the turbinals. J Anat. 2011;218:298–310. doi: 10.1111/j.1469-7580.2010.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila C, Savolainen P, Maldonado JE, et al. Multiple and ancient origins of the domestic dog. Science. 1997;276:1687–1689. doi: 10.1126/science.276.5319.1687. [DOI] [PubMed] [Google Scholar]
- Warton DI, Wright IJ, Falster DS, et al. Bivariate line-fitting methods for allometry. Biol Rev. 2006;81:259–291. doi: 10.1017/S1464793106007007. [DOI] [PubMed] [Google Scholar]
- Waser PM. Small nocturnal carnivores: ecological studies in the Serengeti. Afr J Ecol. 1980;18:167–185. [Google Scholar]
- Wilson DE, Mittermeier RA, editors. Handbook of the Mammals of the World – Volume 1, Carnivores. Barcelona: Lynx Edicions, Conservation International, IUCN, publishers; 2009. [Google Scholar]
- Yokley TR. Ecogeographic variation in human nasal passages. Am J Phys Anthropol. 2009;138:11–22. doi: 10.1002/ajpa.20893. [DOI] [PubMed] [Google Scholar]
- Zhang H-H, Wei Q, Zhang H-S, et al. Comparison of the fraction of olfactory receptor pseudogenes in wolf (Canis lupus) with domestic dog (Canis familiaris. Journal of Forestry Research. 2011;22:275–280. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


