Abstract
The response of the ascorbate-glutathione cycle was investigated in roots of young wheat (Triticum aestivum L.) seedlings that were deprived of oxygen either by subjecting them to root hypoxia or to entire plant anoxia and then re-aerated. Although higher total levels of ascorbate and glutathione were observed under hypoxia, only the total amount of ascorbate was increased under anoxia. Under both treatments a significant increase in the reduced form of ascorbate and glutathione was found, resulting in increased reduction states. Upon the onset of re-aeration the ratios started to decline rapidly, indicating oxidative stress. Hypoxia caused higher activity of ascorbate peroxidase, whereas activities of monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase were diminished or only slightly influenced. Under anoxia, activities of ascorbate peroxidase and glutathione reductase decreased significantly to 39 and 62%, respectively. However, after re-aeration of hypoxically or anoxically pretreated roots, activity of enzymes approached the control levels. This corresponds with the restoration of the high reduction state of ascorbate and glutathione within 16 to 96 h of re-aeration, depending on the previous duration of anoxia. Apparently, anoxia followed by re-aeration more severely impairs entire plant metabolism compared with hypoxia, thus leading to decreased viability.
One of the major biological consequences of soil flooding is oxygen deficiency. Underground plant organs such as roots or rhizomes suffer especially from the periodical or prolonged absence of oxygen. Oxygen deprivation, either complete (anoxia) or partial (hypoxia), interferes with respiration at the level of electron transport. The lack of a suitable electron acceptor leads to saturated redox chains, accumulation of NAD(P)H, and declined generation of ATP (for review, see Kennedy et al., 1992; Perata and Alpi, 1993; Crawford and Brändle, 1996).
Additionally, it has been shown that re-exposure to air after a period of oxygen deprivation can cause serious injury and is possibly even more detrimental in some species or plant organs than oxygen deficiency itself (Monk et al., 1987; Crawford, 1992). The phenomenon of postanoxic or posthypoxic injury is brought about by generation of reactive oxygen radicals and toxic oxidative products such as acetaldehyde (Crawford, 1992). Conditions favoring the formation of reactive oxygen species, such as low energy charge, high levels of reducing equivalents, and saturated electron transport chains, usually prevail in plant tissues when oxygen supply is restricted (VanToai and Bolles, 1991).
The increased production of toxic oxygen species is a feature commonly observed under certain stress conditions (Foyer et al., 1994), when the equilibrium of formation and detoxification of active oxygen species can no longer be maintained. To counter the hazardous effects of oxygen radicals, all aerobic organisms have evolved a complex antioxidative defense system composed of both enzymatic constituents and free radical scavengers such as ascorbate and glutathione. Ascorbate, glutathione, and NAD(P)H are the substrates of a detoxification cycle first proposed by Foyer and Halliwell (1976). A higher level of antioxidants and an increase in the activity of antioxidative enzymes are assumed to be advantageous in overcoming certain stress situations, as has been shown in many physiological studies (Walker and McKersie, 1993; Foyer et al., 1995; Mishra et al., 1995).
There are only a few reports of investigations of changes in activities of components of the antioxidative system in response to anoxic or hypoxic conditions followed by re-aeration. Monk et al. (1987) and VanToai and Bolles (1991) demonstrated that high SOD activity may contribute to flooding tolerance by improving detoxification of superoxide upon re-admission of oxygen. Changes in the activity of respective enzymes and levels of antioxidant substrates involved in the removal of reactive oxygen species in rice seedlings (Oryza sativa) in response to varying oxygen availability were studied by Ushimaro et al. (1992). There was a gradual increase in enzyme activity and ascorbate and glutathione contents when anoxically pretreated plants were returned to air. Roots of wheat (Triticum aestivum) seedlings could cope with the deleterious effects of oxygen radical generation due to posthypoxia by increasing GR activity and the content of glutathione (Albrecht and Wiedenroth, 1994).
In this paper we report the response of the ascorbate-glutathione cycle in roots in response to hypoxia and anoxia and to subsequent re-aeration. Our major aims were (a) to investigate the impact of hypoxia and posthypoxia on the antioxidative defense system of roots of wheat seedlings and (b) to examine whether these effects were more pronounced under anoxic conditions. Therefore, the content of ascorbate and glutathione and the activities of enzymes involved in the ascorbate-glutathione cycle were determined in hypoxically or anoxically grown and subsequently re-aerated roots and in roots of continuously aerated control plants.
MATERIALS AND METHODS
Plant Growth
Plants were cultivated in a growth chamber with a 16-h, 22°C, 380-μmol quanta m−2 s−1 day/8-h, 17°C night cycle. Caryopses of wheat (Triticum aestivum L. cv Alcedo) were germinated on moist filter paper for 2 d, and 70 plants were grown on vessels containing 5 L of Knop nutrient solution (Schropp, 1951) flushed with either air or pure nitrogen. Further treatments were as follows: control, nutrient solution flushed with air (concentration of O2 < 8.3 mg L−1); hypoxia, oxygen deficiency in the root environment (concentration of O2 < 0.1 mg L−1), nutrient solution flushed with pure nitrogen during the growth period of 8 d; anoxia, shoots and roots of 6-d-old aerated plants were then flushed with pure nitrogen for 2, 4, or 8 d; re-aeration, hypoxically or anoxically treated plants were re-aerated for 20 min, 2 h, and 16 h (only 8-d anoxically treated plants were additionally re-aerated for 96 h).
For anoxic treatment, plants were sealed off from the environment by 3-L glass containers that were flushed with nitrogen and placed upside down on the growth vessels. Although we are aware of the possible side effects caused by this treatment (e.g. the greenhouse effect, impairment of photosynthesis in shoots), we considered it to be the most appropriate approach to produce anoxic conditions in roots. Plants were harvested at 8 to 14 d and immediately frozen in liquid nitrogen.
Enzyme Activity Assay
All enzyme extracts were kept at 4°C. Enzymes were extracted from 100 to 200 mg of powdered root tissue in 1.5 mL of the following buffers: APX (EC 1.11.1.11) and GR (EC 1.6.4.2) in 50 mm K2PO4, pH 7.8, containing 5 mm ASA, 5 mm DTT, 100 mm NaCl, 5 mm EDTA, and 2% PVP (Aono et al., 1995); MDAR (EC 1.6.5.4) and DHAR (EC 1.8.5.1) in 50 mm K2PO4, pH 7.8, containing 0.2 mm EDTA, 10 mm β-mercaptoethanol, and 2.5% PVP (Moran et al., 1994). The extracts were centrifuged at 15,000g for 15 min, and the supernatants were used for the different assays. APX activity was measured by following the decrease in A290 for 20 s due to ASA oxidation (Nakano and Asada, 1981). DHAR activity was determined by monitoring the formation of ASA at 265 nm (Hossain et al., 1984). MDAR and GR activity were assayed by following the oxidation of NADH or NADPH at 340 nm (Foyer et al., 1989; Aono et al., 1991). Correction was made by subtracting values obtained in the absence of either substrate or enzymatic extracts. Protein content of the enzyme extracts was measured according to the method of Bradford (1976).
Determination of Ascorbate and Glutathione
Aliquots of 150 mg of frozen root powder were homogenized in 1.5 mL of 10% perchloric acid containing 1 mm bathophenanthroline disulfonic acid and centrifuged at 15,000g for 15 min at 4°C. Total ascorbate and ASA were quantified spectrophotometrically according to the method of Law et al. (1983). GSH and GSSG were determined by HLPC using the method described by Siller-Cepeda et al. (1991). The method is based on carboxymethylation with iodoacetic acid followed by derivatization with 2,4-dinitro-1-fluorobenzene. Dinitrophenol derivatives were separated by HPLC using a 3-aminopropyl-Spherisorb column (VDS, Berlin, Germany), and absorbency was monitored at 365 nm. Standard curves were obtained for each compound within the range 0.125 to 2.5 nmol per 100 μL injected. Each determination was verified by adding γ-glutamyl-glutamate as an internal standard.
Statistics
Sample variability is given as the sd. Student's t test was applied to determine the significance of results between different treatments.
RESULTS
Plant Growth under Hypoxia and Anoxia
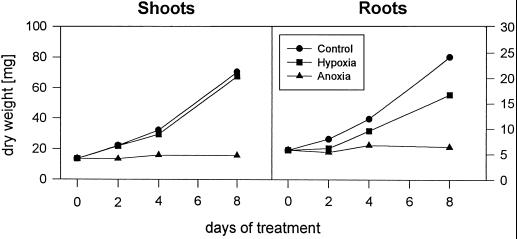
The differences in growth under hypoxic and anoxic conditions compared with aerated controls are summarized in Figure 1. Flushing only the nutrient solution with nitrogen (root hypoxia) led to retarded root growth (by approximately 30%), whereas shoots were hardly affected, causing an increased shoot/root ratio. Under anoxia of both roots and shoots, growth virtually stopped and dry weight remained nearly constant during the investigation period. The shoots appeared to be much more affected by this treatment and wilted, presumably because of deregulation of the stomatal function. This effect seemed to be enhanced when plants were re-aerated. Additionally, their dry weight was decreased (data not shown). Most of the shoots died from desiccation with increasing duration of anoxia. However, even after 8 d of anoxia about 20% of the plants were able to survive and form new shoots.
Figure 1.
Effect of hypoxic or anoxic conditions on growth of wheat shoots and roots. The data are given as mean dry weights per plant and are from at least three independent experiments, each with 10 seedlings. sds are (in the range of) ≤10%.
The Response of the Antioxidative System to Hypoxia and Re-Aeration
Hypoxic treatment of roots caused an increase in the content of total ascorbate (Table I) and glutathione (Table II). Whereas the concentration of the oxidized forms (DHA, GSSG) remained nearly constant, there was a significant increase in their reduced forms (ASA, GSH), resulting in an increased reduction state. After the onset of re-aeration the ratio of reduced to oxidized forms started to decline rapidly. This can mainly be attributed to the increasing content of DHA and GSSG. The total amount of glutathione increased during the 16 h of re-aeration. In contrast, after a short period of increase, the total content of ascorbate decreased 2 h posthypoxia. Eventually, after 16 h of re-aeration, roots of wheat seedlings were able to restore the usual high reduction state of both antioxidant pools.
Table I.
Effect of re-aeration on levels of ASA and DHA in roots of hypoxically (H) or anoxically (A) pretreated wheat seedlings compared with aerated control (C)
| Treatment | ASA + DHA | ASA | DHA | ASA/DHA | ASA |
|---|---|---|---|---|---|
| μmol g−1 fresh wt | ratio | % | |||
| C | 2.64 (0.47) | 1.5 (0.37) | 1.14 | 1.32 | 56.8 |
| H | 3.4 (0.48)* | 2.1 (0.9)* | 1.29 | 1.64 | 62.1 |
| H + 20 min C | 3.79 (0.33) | 1.87 (0.22) | 1.92 | 0.97 | 49.3 |
| H + 2 h C | 3.53 (0.36) | 2.2 (0.32) | 1.33 | 1.65 | 62.3 |
| H + 16 h C | 3.23 (0.45) | 2.25 (0.21) | 0.98 | 2.30 | 69.7 |
| C | 2.31 (0.37) | 1.2 (0.24) | 1.11 | 1.08 | 51.9 |
| C + 2 d A | 2.02 (0.19) | 1.6 (0.28)* | 0.42 | 3.81 | 79.2 |
| C + 2 d A + 20 min C | 2.52 (0.26)* | 1.51 (0.13) | 1.01 | 1.50 | 59.9 |
| C + 2 d A + 2 h C | 1.86 (0.25) | 1.41 (0.24) | 0.45 | 3.13 | 75.8 |
| C + 2 d A + 16 h C | 2.14 (0.34) | 1.39 (0.28) | 0.75 | 1.85 | 65.0 |
| C | 3.45 (0.43) | 1.74 (0.21) | 1.71 | 1.02 | 50.4 |
| C + 4 d A | 5.55 (0.43)* | 3.07 (0.37)* | 2.48 | 1.24 | 55.3 |
| C + 4 d A + 20 min C | 5.01 (0.48)* | 2.46 (0.32)* | 2.55 | 0.96 | 49.1 |
| C + 4 d A + 2 h C | 4.52 (0.53)* | 2.51 (0.20)* | 2.01 | 1.25 | 55.5 |
| C + 4 d A + 16 h C | 4.47 (0.62)* | 2.43 (0.13)* | 2.04 | 1.19 | 54.4 |
| C | 5.31 (0.49) | 2.46 (0.15) | 2.85 | 0.86 | 46.3 |
| C + 8 d A | 8.58 (0.96)* | 4.98 (0.08)* | 3.6 | 1.38 | 58.0 |
| C + 8 d A + 16 h | 7.85 (0.44) | 3.34 (0.07)* | 4.51 | 0.74 | 42.5 |
| C + 8 d A + 96 h C | 9.46 (0.35) | 2.16 (0.09)* | 7.3 | 0.30 | 22.8 |
Each value represents the mean of at least five independent experiments. sd values are shown in parentheses. Asterisks indicate significant differences at the 5% level between values obtained under control and hypoxia or anoxia and following re-aeration after hypoxia or anoxia.
Table II.
Effect of re-aeration on levels of GSH and GSSG in roots of hypoxically (H) or anoxically (A) pretreated wheat seedlings compared with aerated control (C)
| Treatment | GSSG + GSH | GSH | GSSG | GSH/GSSG | GSH |
|---|---|---|---|---|---|
| nmol g−1 fresh wt | ratio | % | |||
| C | 576 | 488 (68) | 88 (18) | 5.5 | 84.7 |
| H | 657 | 579 (35)* | 78 (10) | 7.4 | 88.1 |
| H + 20 min C | 668 | 564 (24) | 104 (18)* | 5.4 | 84.5 |
| H + 2 min h C | 726 | 653 (83) | 74 (15) | 8.8 | 89.9 |
| H + 16 h C | 956 | 910 (164)* | 46 (7) | 19.8 | 95.2 |
| C | 595 | 509 (47) | 85 (14) | 5.9 | 85.7 |
| C + 2 d A | 455 | 400 (91) | 55 (12)* | 7.3 | 88.0 |
| C + 2 d A + 20 min C | 441 | 369 (36) | 72 (19) | 5.1 | 83.7 |
| C + 2 d A + 2 h C | 478 | 408 (10) | 69 (14) | 5.9 | 85.5 |
| C + 2 d A 16 h C | 594 | 533 (85) | 62 (9) | 8.6 | 89.7 |
| C | 521 | 443 (4) | 77 (20) | 5.8 | 85.2 |
| C + 4 d A | 333 | 304 (8)* | 29 (11)* | 10.5 | 91.2 |
| C + 4 d A + 20 min C | 373 | 326 (40) | 47 (6)* | 6.9 | 87.3 |
| C + 4 d A + 20 min C | 392 | 354 (47) | 37 (9) | 9.6 | 90.5 |
| C + 4 d A + 16 h C | 518 | 485 (34)* | 33 (3) | 14.6 | 93.6 |
Each value represents the mean of at least five independent experiments. sd values are shown in parentheses. Asterisks indicate significant differences at the 5% level between values obtained under control and hypoxia or anoxia, and following re-aeration after hypoxia or anoxia. For statistical analysis, see Table I.
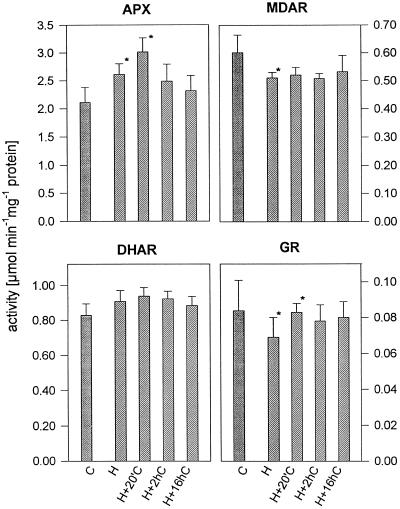
The activity of enzymes involved in the ascorbate-glutathione cycle are shown in Figure 2. Following oxygen shortage in the root environment we found higher activity of APX and, to a lesser extent, of DHAR. In comparison, the activity of MDAR and GR were reduced under hypoxic conditions.
Figure 2.
Activities of APX, MDAR, DHAR, and GR in roots of wheat seedlings under hypoxia (H) followed by re-aeration for 20 min, 2 h, and 16 h (H+20′C, H+2hC, and H+16hC, respectively) compared with aerated control (C). Each data point represents the mean of five separate experiments. Bars, ± sd; asterisks, the significance of differences at the 5% level between control and hypoxia and between hypoxia and re-aeration.
Immediately after hypoxically pretreated roots were returned to air, the activity of APX increased to its highest value, followed by a slow decline to the initial level. ASA is consumed by this reaction. To maintain the ascorbate pool in its highly reduced state, oxidation products have to be regenerated by subsequent reactions. The activity of MDAR showed little effect under varying oxygen availability, whereas the activity of DHAR tended to follow the response pattern of APX. The low activity of GR in wheat roots under hypoxia increased after re-exposure to aerated nutrient solution (Fig. 2.) However, the enhanced activity after 20 min of re-aeration could not prevent the short-term accumulation of GSSG (Table II).
Finally, within 16 h of recovery enzyme activities approached the levels prevailing in controls and the measured short-term imbalance of the ratio of reduced to oxidized forms of ascorbate and glutathione was overcome.
Response of the Antioxidative System to Different Durations of Anoxia and Subsequent Recovery in Air
In addition to the effects observed under hypoxia, we wanted to examine how the antioxidative system of roots is affected by the complete absence of oxygen. To prevent the possible transport of oxygen from shoots to roots, entire plants were kept in a nitrogen-sparged atmosphere. The severe environmental constraints were reflected by an increase of the reduced forms of ascorbate and glutathione in roots compared with the aerated control (Tables I and II). The more reduced redox state of the ascorbate pool under anoxic conditions was paralleled by a significant increase in its total level. Anoxic treatment caused a stronger increase of ASA compared with DHA (Table I). In contrast to the response pattern of the ascorbate pool, the content of GSH and GSSG remained unaltered under aerated conditions and declined with the duration of anoxic stress (Table II). The higher reduction state of glutathione was attributed to the faster decrease of GSSG than of GSH.
The onset of re-aeration led to an accumulation of DHA as well as of GSSG, resulting in decreased ASA/DHA and GSH/GSSG ratios (Tables I and II). The shift of the reduction state in favor of the oxidized forms was reversed during the following recovery phase. After 96 h of re-aeration of wheat seedlings pretreated anoxically for 8 d, we noticed a remarkably enhanced total ascorbate content in roots, which was made up almost exclusively of the oxidized form, indicating the extraordinary stress situation. Contrary to this, investigating the glutathione pool under recovery conditions, we found a gradual increase of GSH and a simultaneous decrease of GSSG, finally approaching the levels in aerated plants.
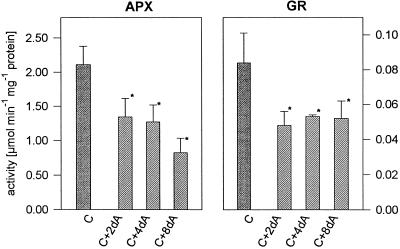
Whereas the activity of APX was elevated under hypoxic conditions, it was lower under anoxia compared with aerated control plants (Fig. 3). After 8 d of anoxic treatment it was decreased to nearly one-third of the activity observed under control conditions. GR activity was also reduced under anoxia (Fig. 3). The activity of both enzymes was unchanged in aerated control plants.
Figure 3.
Activity of APX and GR after 2, 4, and 8 d of anoxia (C+2dA, C+4dA, and C+8dA, respectively) compared with aerated controls (C). Each data point represents the mean of three or 10 (for control) separate experiments. Bars, ± sd; asterisks, the significance of differences at 5% level between control and anoxically grown plants.
In plants anoxically treated for 2 or 4 d, the activity of APX and GR gradually increased after the start of re-aeration, reaching the same level after 16 h as in control plants (Table III). After 8 d of anoxia the restoration of the activity of both protective enzymes was delayed after plants had been re-aerated, correlating with the findings for the ascorbate and glutathione pools. However, after 96 h postanoxia, there was a more than doubled APX activity compared with that of controls.
Table III.
Effect of re-aeration on levels of APX and GR in roots of anoxically treated (A) and control (C) plants
| Treatment | Enzyme Level
|
|
|---|---|---|
| APX | GR | |
| μmol ASA min−1 mg−1 protein | μmol NADPH min−1 mg−1 protein | |
| C | 2.11 ± 0.27 | 0.084 ± 0.017 |
| C + 2 d A | 1.04 (0.18) | 0.046 (0.004) |
| C + 2 d A + 20 min C | 1.45 (0.36) | 0.041 (0.007) |
| C + 2 d A + 2 h C | 1.31 (0.21) | 0.059 (0.005)* |
| C + 2 d A + 16 h C | 1.96 (0.30)* | 0.090 (0.003)* |
| C + 4 d A | 1.27 (0.25) | 0.053 (0.001) |
| C + 4 d A + 20 min C | 1.30 (0.34) | 0.069 (0.005)* |
| C + 4 d A + 2 h C | 1.56 (0.19) | 0.074 (0.005)* |
| C + 4 d A + 16 h C | 2.04 (0.22)* | 0.087 (0.007)* |
| C + 8 d A | 0.82 (0.21) | 0.052 (0.01) |
| C + 8 d A + 16 h C | 1.14 (0.18) | 0.060 (0.009) |
| C + 8 d A + 96 h C | 5.62 (0.11)* | 0.095 (0.009)* |
Each value represents the mean of three independent experiments. SD values are shown in parentheses. Asterisks indicate significant differences at the 5% level between values obtained under control and the respective anoxically pretreated plants (P ≤ 0.05).
To exclude the possibility that the alterations in enzymatic activities were solely a consequence of general changes in protein content, we examined the concentration of soluble protein (Table IV). There was no marked difference under hypoxia and 2 or 4 d of anoxia or following re-aeration. After 8 d of anoxia a slightly decreased protein content was measured and further reduction was observed with prolonged time of postanoxic recovery. This might indicate degradative processes and may partly explain the high values of enzyme activities based on protein content under these conditions.
Table IV.
Effect of re-aeration on level of root soluble protein in roots of hypoxically (H) and anoxically (A) pretreated wheat seedlings compared with aerated control (C)
| Treatment | Root Soluble Protein |
|---|---|
| mg g−1 fresh wt | |
| C | 7.53 (1.49) |
| H | 7.33 (1.06) |
| H + 20 min C | 6.54 (0.83) |
| H + 2 h C | 7.47 (1.47) |
| H + 16 h C | 7.56 (2.14) |
| C + 2 d A | 7.83 (1.38) |
| C + 2 d A + 20 min C | 8.80 (1.48) |
| C + 2 d A + 2 h C | 8.43 (1.10) |
| C + 2 d A + 16 h C | 7.37 (0.29) |
| C + 4 d A | 7.99 (1.24) |
| C + 4 d A + 20 min C | 7.55 (1.33) |
| C + 4 d A + 2 h C | 6.82 (0.81) |
| C + 4 d A + 16 h C | 7.06 (1.02) |
| C + 8 d A | 6.74 (0.35) |
| C + 8 d A + 16 h C | 4.10 (0.37)* |
| C + 8 d A + 96 h C | 3.51 (0.29)* |
Each value represents the mean of five to ten independent replications. sd values are shown in parentheses. Asterisks indicate significant differences at the 5% level between values obtained under control and hypoxia or anoxia and following re-aeration after hy-poxia or anoxia.
DISCUSSION
The importance of the ascorbate-glutathione system for the detoxification of hydrogen peroxide is well characterized in leaf chloroplasts (Foyer and Halliwell, 1976; Gillham and Dodge, 1986; Asada, 1992). However, this cycle also operates in the cytosol (Dalton et al., 1986; Asada, 1992; Cakmak et al., 1993), where it is presumably more effective than catalase, the latter being mainly associated with the removal of hydrogen peroxide in peroxisomes.
In contrast to numerous reports in which the function of the ascorbate-glutathione cycle in above-ground parts of plants was described, there is little detailed information available about its action in roots deprived of oxygen. Our aim was to elucidate this pathway in wheat roots in response to hypoxia or anoxia and after re-aeration.
We interpret higher total levels of ascorbate (30%) and glutathione (15%) as an acclimatization of roots of wheat seedlings to hypoxia. Under hypoxic conditions the respiratory chain is not fully inhibited (Pradet and Bomsel, 1978); therefore, plants can partially maintain their energy charge by residual respiration (Pfister-Sieber and Brändle, 1995). Flushing only the root environment with nitrogen resulted in acclimatization of young wheat plants, including anatomical and biochemical changes. According to Erdmann et al. (1986) and He et al. (1994), the transport of oxygen from shoots to roots is improved by enlargement of the intercellular space. Thus, roots were able to resume their elongation under hypoxia. In contrast, we observed no further root growth under anoxic conditions. When entire plants are flushed with nitrogen, preventing transport of oxygen from shoots to roots, the resulting reducing conditions will lead to electron-saturated redox chains, eventually causing a lack of ATP.
Greater amounts of ascorbate under anoxia could apparently not prevent metabolic malfunction. In agreement with our results, Pfister-Sieber and Brändle (1995) and Sieber and Brändle (1991) demonstrated an improved viability of potato tubers under hypoxia compared with anoxia and attributed this to the ability of maintaining the energy charge at an intermediate level under hypoxia, whereas anoxic tubers revealed the complete breakdown of energy metabolism.
The highly reducing conditions prevailing under hy-poxia and anoxia in our experiments were reflected by increasing levels of ASA and GSH, leading to increased reduction states. The onset of re-aeration of hypoxically or anoxically pretreated plants caused enhanced oxidation of the reduced fractions, resulting in decreased ASA/DHA and GSH/GSSG ratios. The shift in the ratios of reduced to oxidized forms of ascorbate and glutathione might indicate the increased generation of reactive oxygen species. Impairment of the reduction state of both antioxidants is considered to be a strong indicator for oxidative stress, as has been also observed under the influence of several other environmental constraints such as chilling (Walker and McKersie, 1993) or herbicide application (Knörzer et al., 1996).
The process of lipid peroxidation, which was found to be accelerated after re-exposure to air in a previous study (Albrecht and Wiedenroth, 1994), might be taken as further sign of oxidative stress. However, after a 16-h recovery phase, hypoxically and 2- or 4-d anoxically treated plants were able to restore the normal high reduction states. Prolonged anoxia (8 d) followed by 96 h of re-aeration resulted in almost a doubling of the amount of oxidized ascorbate, which emphasizes the extraordinary stress situation. Although it seems to be clear that most of the damage to plant growth is directly due to anoxia, re-oxygenation also contributes to decreased viability (VanToai and Bolles, 1991). The ability to survive after re-aeration declined with increasing duration of anoxic pretreatment, and whereas most of the shoots died from desiccation, roots remained viable and plants were capable of forming new shoots.
The enhanced activity of APX under hypoxia further increased immediately upon exposure to air, coinciding with an accumulation of oxidized ascorbate. The ability to keep the antioxidants in their physiologically active, reduced form was proposed to be more important than their pool size for survival of plant cells under severe stress (Knörzer et al., 1996). Because of that, the pool of ASA has to be permanently regenerated. This is achieved by the activity of MDAR, which consumes NADH (Hossain et al., 1984), or by a sequence of reactions coupling the reduction of DHA with the oxidation of NADPH via DHAR, glutathione, and GR (Foyer and Halliwell, 1976). The only slightly altered MDAR activity implies that ascorbate reduction under our conditions is mainly brought about by the GSH-dependent DHAR and GR branch of the pathway. Inconsistent results have been reported in this context.
Mishra et al. (1995) found declined or unaffected activity of DHAR, supporting the assumption of Asada (1994) that the major route of ascorbate regeneration in chloroplasts can be assigned to the activity of MDAR. Our present data suggest that GSH-mediated ascorbate reduction makes an important contribution to maintaining a highly reduced state of the ascorbate pool in roots of wheat seedlings, as has also been shown previously for leaf chloroplasts (Foyer and Halliwell, 1976; Foyer et al., 1995). Although the activity of GR, which was found to be diminished under hypoxic conditions, was enhanced after 20 min of re-aeration, increasing oxidation of the glutathione pool could not be prevented. However, increasing GR activity allowed the restoration of the usual high reduction state of glutathione and ascorbate within 16 h posthypoxia.
Several authors have supported the assumption that stress tolerance may be improved by increased antioxidant capacity. Enhanced activities of antioxidative enzymes are thought to be an acclimative response to elevated amounts of reactive oxygen species generated at higher light intensities (Mishra et al., 1995). Mehlhorn et al. (1986) reported a strong increase of ascorbate and glutathione in conifers in relation to increasing concentrations of air pollutants. Monk et al. (1987) compared rhizomes of the anoxia-tolerant species Iris pseudacorus with those of the intolerant species Iris germanica, proposing that high SOD activity may contribute to tolerance against postanoxic stress. In fact, after anoxia we observed the appearance of an additional band of SOD activity in root samples separated by nondenaturing PAGE (S. Biemelt, U. Keetman, and H.-P. Mock, unpublished data).
Many recent attempts to improve stress tolerance in plants have made use of genetic engineering by introducing and expressing genes encoding enzymes involved in the antioxidative defense system. Overexpressing either SOD (Sen Gupta et al., 1993) or GR (Aono et al., 1991; Foyer et al., 1995) in plants resulted in better protection against oxidative stress.
The diminished activities of APX and GR observed under anoxic conditions correlate with the general inhibition of metabolism as indicated by, for example, stunted growth. As shown above, roots of anoxically pretreated wheat seedlings could oppose postanoxic stress by the gradual increase of the activities of both enzymes. A similar response in shoots of submerged rice seedlings was described by Ushimaro et al. (1992), who found that the low activities of antioxidative enzymes following oxygen deprivation increased after exposure of seedlings to air, eventually reaching the same level or exceeding the level of activities observed in aerobically grown control plants after 24 h. The ability to recover from the deleterious effects postanoxia appeared to be delayed with longer duration of anoxia.
Postanoxic and posthypoxic damage are thought to be mainly caused by the generation of reactive oxygen species. Albrecht and Wiedenroth (1994) showed an increase in oxygen uptake following re-aeration as a response to the high energy demands of the re-activated metabolism. This process is accompanied by fast consumption of previously accumulated sugars and rapid resumption of root elongation (Albrecht et al., 1993). However, according to Elstner (1990), accelerated mitochondrial electron transport toward its final acceptor, oxygen, is associated with an increasing potential of concomitant production of oxygen radicals and hydrogen peroxide as possible causes of postanoxic injury.
Our results show that roots of young wheat plants were able to cope with the deleterious effects of oxygen radical generation by means of their antioxidative defense system. Although the viability was decreased under anoxia compared with hypoxia, roots were able to survive up to the investigated period of 8 d of anoxia. The overall capacity to scavenge radicals was found to be sufficient for counteracting the oxidative stress induced by re-aeration. Elevated activities of enzymes of the ascorbate-glutathione cycle enabled the restoration of the essential, highly reduced state of the antioxidants ascorbate and glutathione.
ACKNOWLEDGMENTS
We thank Professor M.C. Drew for critical reading of the manuscript and Professor E.-M. Wiedenroth for helpful discussion. We are grateful to Dr. B. Grimm for the opportunity to carry out experiments in his laboratory at the Institut für Pflanzengenetik und Kulturpflanzenforschung Gatersleben.
Abbreviations:
- APX
ascorbate peroxidase
- ASA
reduced ascorbate
- DHA
dehydroascorbate
- DHAR
dehydroascorbate reductase
- GR
glutathione reductase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- MDAR
monodehydroascorbate reductase
- SOD
superoxide dismutase
Footnotes
This work was supported by a grant (Nafög) to S.B. from the state of Berlin.
LITERATURE CITED
- Albrecht G, Kammerer S, Praznik W, Wiedenroth EM. Fructan content of wheat seedlings (Triticum aestivum L.) under hypoxia and following re-aeration. New Phytol. 1993;123:471–476. doi: 10.1111/j.1469-8137.1993.tb03758.x. [DOI] [PubMed] [Google Scholar]
- Albrecht G, Wiedenroth EM. Protection against activated oxygen following re-aeration of hypoxically pre-treated wheat roots. The response of the glutathione system. J Exp Bot. 1994;45:449–455. [Google Scholar]
- Aono M, Kubo A, Saji H, Tanaka K, Kondo N. Resistance to activated oxygen toxicity of transgenic Nicotiana tabacum that expresses the gene for glutathione reductase from Escherichia coli. Plant Cell Physiol. 1991;32:691–697. [Google Scholar]
- Aono M, Saji H, Fujiyama K, Sugita M, Kondo N, Tanaka K. Decrease in activity of glutathione reductase enhances paraquat sensitivity in transgenic Nicotiana tabacum. Plant Physiol. 1995;107:645–648. doi: 10.1104/pp.107.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85:235–241. [Google Scholar]
- Asada K. Production and action of active oxygen species in photosynthetic tissue. In: Foyer CH, Mullineaux PM, editors. Causes of Oxidative Stress and Amelioration of Defence Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 77–104. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Strbac D, Marschner H. Activities of hydrogen peroxide-scavenging enzymes in germinated wheat seeds. J Exp Bot. 1993;44:127–132. [Google Scholar]
- Crawford RMM. Oxygen availability as an ecological limit to plant distribution. Adv Ecol Res. 1992;23:93–185. [Google Scholar]
- Crawford RMM, Brändle R. Oxygen deprivation stress in a changing environment. J Exp Bot. 1996;47:145–159. [Google Scholar]
- Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA. 1986;83:3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner EF (1990) Der Sauerstoff: Biochemie, Biologie und Medizin. BI-Wissenschaftsverlag, Mannheim, Germany, pp 55–157
- Erdmann B, Hoffmann P, Wiedenroth EM. Changes in the root system of wheat seedling following root anaerobiosis. 1. Anatomy and respiration in Triticum aestivum L. Ann Bot. 1986;58:597–605. [Google Scholar]
- Foyer CH, Descourvieres P, Kunert KJ. Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. [Google Scholar]
- Foyer CH, Dujardin M, Lemoine Y. Responses of photosynthesis and the xanthophyll and ascorbate-glutathione cycles to changes in irradiance, photoinhibition and recovery. Plant Physiol Biochem. 1989;27:751–760. [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jounanin L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham DJ, Dodge AD. Hydrogen-peroxide-scavenging system within pea chloroplasts. A quantitative study. Planta. 1986;167:246–251. doi: 10.1007/BF00391422. [DOI] [PubMed] [Google Scholar]
- He CJ, Drew MC, Morgan PW. Induction of enzymes associated with lysogenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen starvation. Plant Physiol. 1994;105:861–865. doi: 10.1104/pp.105.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. [Google Scholar]
- Kennedy RA, Rumpho ME, Fox TC. Anaerobic metabolism in plants. Plant Physiol. 1992;100:1–6. doi: 10.1104/pp.100.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knörzer OC, Durner J, Böger P. Alteration in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol Plant. 1996;97:388–396. [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplast. The effect of hydrogen peroxide and of paraquat. Biochem J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H, Seufert G, Schmidt A, Kunert KJ. Effect of SO2 and O3 on production of antioxidants in conifers. Plant Physiol. 1986;82:336–338. doi: 10.1104/pp.82.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PN, Fatma T, Singhal GS. Development of antioxidative defence system of wheat seedlings in response to high light. Physiol Plant. 1995;95:72–82. [Google Scholar]
- Monk LS, Fagerstedt KV, Crawford RMM. Superoxide dismutase as an anaerobic polypeptide. A key factor in recovery from oxygen deprivation in Iris pseudacorus? Plant Physiol. 1987;85:1016–1020. doi: 10.1104/pp.85.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JF, Becana M, Iturbe-Ormatexe I, Frechilla S, Klucas RV, Aparicio-Teja P. Drought induces oxidative stress in pea plants. Planta. 1994;194:346–352. [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Perata P, Alpi A. Plant responses to anaerobiosis. Plant Sci. 1993;93:1–17. [Google Scholar]
- Pfister-Sieber M, Brändle R. Response of potato tubers to hypoxia followed by re-aeration. Potato Res. 1995;38:231–239. [Google Scholar]
- Pradet A, Bomsel JL (1978) Energy metabolism in plants under hypoxia and anoxia. In DD Hook, RMM Crawford, eds, Plant Life in Anaerobic Environments. Ann Arbor Science Publishers, Ann Arbor, MI, pp 89–118
- Schropp W (1951) Der Vegetationsversuch. 1. Die Methodik der Wasserkultur höherer Pflanzen. Neuman Verlag, Berlin, p 132
- Sen Gupta A, Webb RP, Holaday AS, Allen RD. Overexpression of superoxide dismutase protects plants from oxidative stress. Induction of ascorbate peroxidase in superoxide dismutase overexpressing plants. Plant Physiol. 1993;103:1067–1073. doi: 10.1104/pp.103.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber M, Brändle R. Energy metabolism in rhizomes of Acorus calamus and in tubers of Solanum tuberosus with regard to their anoxia tolerance. Bot Acta. 1991;104:279–282. [Google Scholar]
- Siller-Cepada JH, Chen THH, Fuchigami LH. High performance liquid chromatography analysis of reduced and oxidized glutathione in woody plant tissue. Plant Cell Physiol. 1991;32:1179–1185. [Google Scholar]
- Ushimaro T, Shibasaka M, Tsuji H. Development of O2− detoxification system during adaptation to air of submerged rice seedlings. Plant Cell Physiol. 1992;33:1065–1071. [Google Scholar]
- VanToai TT, Bolles CS. Postanoxic injury in soybean (Glycine max) seedlings. Plant Physiol. 1991;97:588–592. doi: 10.1104/pp.97.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MA, McKersie BD. Role of ascorbate-glutathione antioxidant system in chilling resistance of tomato. J Plant Physiol. 1993;141:234–239. [Google Scholar]