Abstract
Oxidative burst constitutes an early response in plant defense reactions toward pathogens, but active oxygen production may also be induced by other stimuli. The oxidative response of suspension-cultured tobacco (Nicotiana tabacum cv Xanthi) cells to hypoosmotic and mechanical stresses was characterized. The oxidase involved in the hypoosmotic stress response showed similarities by its NADPH dependence and its inhibition by iodonium diphenyl with the neutrophil NADPH oxidase. Activation of the oxidative response by hypoosmotic stress needed protein phosphorylation and anion effluxes, as well as opening of Ca2+ channels. Inhibition of the oxidative response impaired Cl− efflux, K+ efflux, and extracellular alkalinization, suggesting that the oxidative burst may play a role in ionic flux regulation. Active oxygen species also induced the cross-linking of a cell wall protein, homologous to a soybean (Glycine max L.) extensin, that may act as part of cell volume and turgor regulation through modification of the physical properties of the cell wall.
Plants have to cope with a large variety of environmental and developmental stimuli. Oxidative burst, which corresponds to a transient production of AOS, such as superoxide anion and H2O2, is a part of the response to pathogen attack in plants. The plant oxidative response plays direct and indirect roles in plant defense (for reviews, see Low and Merida, 1996; Mehdy et al., 1996). The produced AOS can act as an antibiotic toward the pathogen (Mehdy et al., 1996) and reinforce the cell wall by catalyzing cross-linking of cell wall proteins through a peroxidase-dependent reaction (Bradley et al., 1992; Brisson et al., 1994; Tenhaken et al., 1995; Otte and Barz, 1996). AOS are also second messengers that activate downstream defense reactions, such as synthesis of pathogenesis-related proteins (Chen et al., 1993), glutathione S-transferase, glutathione peroxidase, and ubiquitin (Levine et al., 1994), as well as phytoalexin accumulation (Devlin and Gustine, 1992).
Structurally and chemically distinct compounds have been isolated from fungi, bacteria, and plant cell walls that were effective elicitors of oxidative burst in different plant models. In tobacco (Nicotiana tabacum L.) cells, several molecules are able to induce oxidative burst, such as the bacterial protein harpin (Baker et al., 1993), oomycete elicitins (Yu, 1995) including cryptogein (Bottin et al., 1994), and plant cell wall-derived oligogalacturonides (Mathieu et al., 1996). Eliciting compounds seem to be recognized by receptors at the plasma membrane, because specific binding sites have been visualized (Nurnberger et al., 1995; Wendehenne et al., 1995). Transduction pathways appear to differ according to the plant-elicitor model, and only a few steps have been identified. The oxidative burst involves protein phosphorylations (Schwacke and Hager, 1992; Viard et al., 1994; Chandra and Low, 1995; Mathieu et al., 1996). The Ser/Thr kinase encoded by the Pto-resistance gene in tomato induces a specific oxidative burst in response to infection by an avirulent pathogen expressing avrPto (Chandra et al., 1996b). The oxidative burst activation by cryptogein in tobacco cell suspension and by fungal extracts in spruce cells was shown to be Ca2+ dependent (Schwacke and Hager, 1992; Tavernier et al., 1995). GTP-binding protein and inositol trisphosphate-mediated transduction was observed in soybean (Glycine max L.) cells in response to oligogalacturonides (Legendre et al., 1992, 1993b). Phospholipase A involvement was reported in soybean cells elicited by extracts from Verticillium dahliae (Chandra et al., 1996a).
The AOS-producing machinery activated in response to elicitor molecules displays similarities with the neutrophil plasma membrane oxidase involved in phagocytosis (Henderson and Chappell, 1996). The oxidative burst in plants can be inhibited by IDP, an inhibitor of the neutrophil NADPH oxidase (Levine et al., 1994; Dwyer et al., 1996; Rouet-Mayer et al., 1997), and is dependent on NADPH (Pugin et al., 1997). Moreover, there are immunochemical (Dwyer et al., 1996) and functional data (Coffey et al., 1995) that suggest the existence of an analogous enzymatic complex in plants. A cDNA has also been isolated from rice, which is homologous to one integral membrane component of the mammalian NADPH oxidase (Groom et al., 1996).
AOS production was also shown to be induced by physical stresses in plants and animals. Swelling of neutrophils induces anion superoxide production (Miyahara et al., 1993), and in soybean suspension cells, AOS production was activated by osmotic shock, physical pressure (Yahraus et al., 1995), and vigorous stirring of the suspension (Legendre et al., 1993a). The transduction pathway mediating this oxidative response activation has yet to be elucidated. Yahraus et al. (1995) showed that mechanically induced oxidative burst in soybean cells was prevented by Gd, an inhibitor of stretch-activated channels, therefore suggesting the involvement of these channels in oxidase activation. Ion, organic solute, and water fluxes caused by hypoosmotic stress may represent additional elements of the mechanical stress response. They are key elements of the osmoregulation process (Hallows and Knauf, 1994) in which oxidative burst may take part but the role of which has yet to be defined.
In this study we aimed to identify steps of the signaling pathway that are involved in the activation of the oxidative burst by osmotic stress and to bring information about the possible role of this response in osmoregulation, using suspension cells of tobacco. The oxidative burst induced by a hypoosmotic stress was characterized. Transduction events involved in oxidative burst activation were studied: Ca2+ requirement, opening of stretch-activated channels, and phosphorylation processes. Anion fluxes have been shown in parsley cells to play a key role in elicitor signaling, because the whole set of Phytophthora-induced defense responses, including oxidative burst, was inhibited by anion channel blockers (Jabs et al., 1997). Also, anion fluxes contribute to osmoregulation in guard cells, where they mediate the response to ABA by regulation of K+ fluxes (Schroeder, 1995; Ward et al., 1995). Involvement of anion channels in the activation of oxidative burst by osmotic stress was thus tested. A possible role of oxidative burst in osmotic stress response through modulation of ion fluxes, which constitute driving forces of osmoregulation in guard cells (Schroeder and Hedrich, 1989) or through cell wall modifications, was also evaluated.
MATERIALS AND METHODS
Tobacco (Nicotiana tabacum cv Xanthi) cells were cultured in B5 Gamborg's medium with 1 μm 2,4-D and 60 nm kinetin in constant light. Suspension cells were used after 4 d of subculturing with 60 to 100 mg fresh weight mL−1 cell density.
Inhibitors
Stock solutions of A9C and NPPB (100 mm) were prepared in DMSO and in water for DIDS. Stock solutions of GdCl3 and La(NO3)3 (500 and 250 mm) were prepared in water. Stock solutions of staurosporine, DMAP, and apigenin (2, 100, and 500 mm) were prepared in DMSO. Stock solutions of IDP chloride and glucosamine sulfate (20 mm and 1 m) were prepared in DMSO and water, respectively.
Osmotic Stress
Osmolarity was monitored using a freezing-point osmometer (Roebling, Berlin, Germany) on 100-μL aliquots.
Cells were washed and equilibrated for 3 h after repartition in aliquots in an ion-poor medium (160 mOsm), which is isoosmotic to the culture medium, containing 10 mm Mes-Tris, pH 5.2, 1 mm CaSO4, and 150 mm Suc. This medium allowed ion efflux measurements. Afterward, extracellular medium was replaced by the same volume of hypoosmotic medium (10 mm Mes-Tris, pH 5.2, 1 mm CaSO4, Suc-free, 15 mOsm), hyperosmotic medium (10 mm Mes-Tris, pH 5.2, 1 mm CaSO4, and 500 mm Suc, 640 mOsm), or fresh isoosmotic medium for control cells. The final osmotic strengths of extracellular mediums after transfer of cells were about 40 and 600 mOsm, respectively, for hypo- or hyperosmotic conditions. These variations from 15 to 40 or 640 to 600 mOsm were due to the presence of cells previously equilibrated in the isoosmotic medium. Hyperosmotic medium corresponds to a plasmolysis-inducing medium for tobacco cells. For extracellular pH measurements, 10 mm Mes-Tris was replaced by 1 mm Mes-Tris.
For the study of glucosamine effects, cells were equilibrated in their culture medium in the presence of 10 mm Mes-Tris, pH 5.2. One hour before the shock, 100 mm glucosamine solution, corresponding to a dilution in isoosmotic medium of the 1 m stock adjusted to pH 5.2 by NaOH, was added to the medium to get a 10 mm final concentration in glucosamine (an equal volume of extracellular medium was removed before addition), whereas Man was added in corresponding controls to reach the same osmolarity (180 mOsm) in both cases. Cells were then transferred either in isoosmotic medium composed of 150 mm Man, 10 mm Mes-Tris, and 1 mm CaSO4 for controls or in hypoosmotic medium containing 10 mm Mes-Tris and 1 mm CaSO4. The inhibitor (10 mm glucosamine) or the osmotic equivalent (30 mm Man) was also present after transfer. Glucosamine addition induced a very limited pH effect upon addition in the 10 mm buffered medium (lower than 0.1 pH unit) during pretreatment, but this effect was not detectable during the time of the experiments following transfer in a fresh medium containing 1 mm Mes-Tris (Fig. 6D, Iso-Gl).
Figure 6.
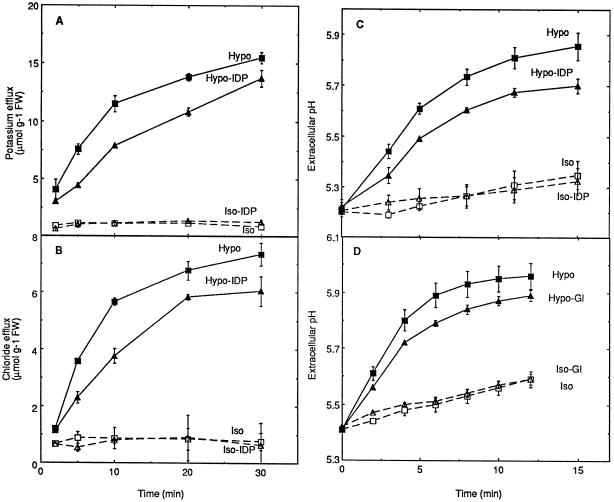
Effect of inhibitors of H2O2 production on the ion fluxes induced by hypoosmotic stress. Aliquots of cell suspension were treated by IDP (A–C, Hypo-IDP and Iso-IDP) or glucosamine (D, Hypo-Gl and Iso-Gl) as described in the legend of Figure 2 for each of the inhibitors, except the replacement of 10 mm by 1 mm Mes-Tris, pH 5.2, for the pH measurement experiments. Means ± se of at least two independent experiments are reported in each case. FW, Fresh weight.
Mechanical Stress
To generate a mechanical stress to cells, a cross-section rod was maintained in open vials containing aliquots of cells previously equilibrated for 2 h in culture medium containing 10 mm Mes-Tris, pH 5.2. The method that we used does not imply a physical pressure on the cells, which may damage the cells and induce oxidative burst. Introduction of the piston only allows increased contact of cells in the agitated culture. The contact surface with the cells was about 2-fold higher after introduction of the piston. It was verified that the cell viability was not modified after the application of mechanical stress.
Extracellular Alkalinization
Extracellular pH was monitored with a glass electrode in 6 mL of cell suspension aliquots previously equilibrated in isoosmotic medium containing 1 mm Mes-Tris, pH 5.2, in open vials on a shaker.
Oxidative Burst Assay
H2O2 concentration was measured using scopoletin fluorescence oxidative quenching (excitation wavelength 350 nm; emission 460 nm). To measure the H2O2 production rate, 500 mm stock solution of scopoletin in DMSO (240 μm final) and 2 mg mL−1 stock solution of peroxidase (10 μg mL−1 final) were added to 5-mL aliquots of a cell suspension previously equilibrated in buffered isoosmotic medium. Scopoletin was progressively oxidized, and the production of AOS was calculated from the fluorescence decrease using a calibration curve established in the presence of H2O2. Aliquots of medium were taken at various intervals and monitored by a fluorimeter (FluoroskanII, Labsystems, Helsinki, Finland) or a spectrofluorimeter SFM25 (Kontron Instruments, Montigny Le Bretonneux, France). To measure AOS accumulation, aliquots of extracellular medium were taken at various intervals and mixed with scopoletin and peroxidase to reach 1 μm and 8 μg mL−1 final concentrations, respectively.
Inhibition of soybean (Glycine max L.) peroxidase by IDP, excluding use of the peroxidase-dependent assay for H2O2 determination, has been reported (Dwyer et al., 1996). The effect of IDP on H2O2 determination using scopoletin was tested in tobacco cells. In usual conditions of assay (see above) the addition of IDP to obtain final concentrations of 5, 20, 50, or 100 μm did not modify the decrease of scopoletin fluorescence (154 ± 6 units) because of the addition of H2O2 (150 μm final). No inhibition of peroxidase by IDP could thus be detected in these assay conditions, allowing the use of scopoletin for H2O2 determination. It was verified that the other molecules, protein kinase inhibitors and anion channel blockers, did not modify the H2O2 determination assay in the same way.
Ion Concentration Measurements
Aliquots of cells (30 mL) were equilibrated in buffered isoosmotic medium for 3 h. After transfer to iso- or hypoosmotic medium, samples of the cell suspension were taken at various times and rapidly filtered. Aliquots were used for monitoring by specific K+ and Cl− electrodes (D821 and XS21, Radiometer, Copenhagen, Denmark) at a constant ionic strength in 0.1 m NaCl or 0.1 m K2SO4, respectively.
Cell Wall Protein Preparation
Cell wall proteins were extracted from untreated cells or from cells 5 min after transfer to iso- or hypoosmotic medium, 20 min after mechanical stress onset, or 5 min after treatment with 1 mm H2O2. To test the effect of IDP, 20 μm IDP was added for the last 15 min of equilibration and then during the mechanical or hypoosmotic stresses. H2O2 (2 mm) was also added for 5 min to the cell wall pellet from untreated cells.
Cells were filtered and plasmolyzed (15 min) at 4°C in 50 mm Mes-Tris, pH 6.0, 20% glycerol, 0.5 m Suc, 1 mm MgCl2, 10 mm ascorbic acid, 5 mm DTT, 0.6% PVP (w/v), and 0.5 μg mL−1 leupeptin. After the homogenate was ground in a pressure crusher, it was centrifuged at 10,000g for 10 min, resuspended in 10 mm Mes-Tris, pH 5.2, and centrifuged again (all centrifugations were held in the same conditions). The cell wall 10,000g pellet was frozen in liquid nitrogen, ground in a mortar, and homogenized resuspended with a Potter-Elvehjem homogenizer in 2% SDS, 80 mm Tris, and 1 m β-mercaptoethanol, pH 6.8. After centrifugation, proteins of the supernatant were precipitated in acetone overnight at −20°C. The resulting pellet was washed in 80% acetone and dried, and the proteins were resuspended in denaturating buffer without a reducing agent. They were stored at −20°C until use.
SDS-PAGE and Western-Blot Analysis
Cell wall proteins were analyzed by SDS-PAGE (10% polyacrylamide) and silver staining (Pharmacia-modified method from Blum et al., 1987). For the western blot, the SDS-PAGE gel was transferred using electrotransfer with a Mini Trans-blot Cell (Bio-Rad) in the presence of Tris-Gly buffer, 20% (v/v) ethanol, for 1 h at 100 V and 50 mA. The PVDF membrane was then saturated with 5% defatted milk in TBS containing 0.1% (v/v) Tween 20 (TBS-T) for 1 h at 37°C. The membrane was rinsed for 5 min three times in TBS-T. Hybridoma cell culture supernatant of monoclonal antibody MAC265 directed against p100 soybean extensin (Bradley et al., 1992) was a gift from Nick Brewin (John Innes Center, Norwich, UK) and was used at a final dilution of 1:10 in TBS-T for 3 h at room temperature. The membrane was then washed three times for 10 min in TBS-T before incubation with anti-rat IgG (1 mg mL−1) conjugated to alkaline phosphatase diluted 1:1000 in TBS-T for 1 h at room temperature. The membrane was washed for 5 min in TBS-T twice before alkaline phosphatase activity revelation by 5-bromo-4-chloro-3-indolyl phosphate staining in the dark at room temperature.
RESULTS
Induction of Oxidative Burst by Hypoosmotic and Mechanical Stimuli
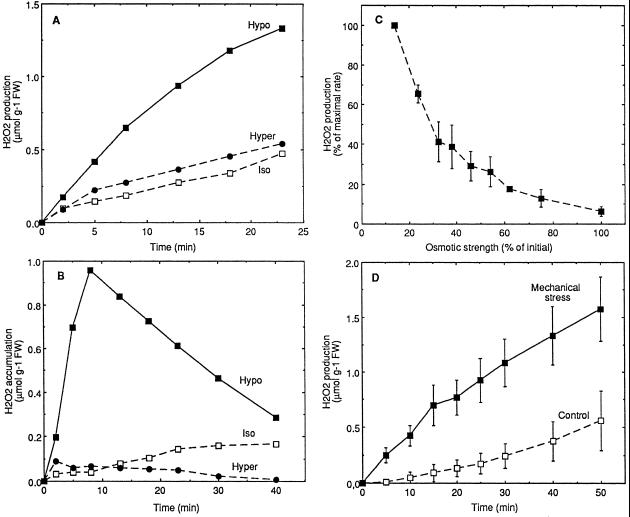
Production of H2O2 in suspension-cultured tobacco cells was assayed by following the scopoletin fluorescence decrease after osmotic or mechanical stresses. To study the effect of osmolarity, cells were transferred in media that were lypo-, iso-, or hyperosmotic relative to the cell culture medium. After the hypoosmotic shock, significant production of H2O2 was observed (Fig. 1A). AOS produced in hypoosmotic conditions accumulated in the extracellular medium, reaching a maximum after about 10 min (Fig. 1B). Afterward, H2O2 degradation was higher than the corresponding production, leading to the disappearance of H2O2 in the medium (Fig. 1B) In contrast, cells transferred to isoosmotic or hyperosmotic medium showed only weak H2O2 production (Fig. 1A) and no detectable accumulation (Fig. 1B). The oxidative burst could be stopped by return to isoosmotic conditions either by transfer back to isoosmotic medium or by addition of Suc (data not shown). The magnitude of the hypoosmotically induced oxidative burst was highly dependent on the osmotic strength of the transfer medium (Fig. 1C). The use of Man, sorbitol, PEG, or NaCl instead of Suc in the isoosmotic medium induced a similar oxidative burst for the similar osmotic strength decrease (data not shown). Increased AOS production was also observed when cells in their culture medium were subjected to a mechanical stress (Fig. 1D), in agreement with the possibility that the response to hypoosmotic stress may involve mechanical signaling. Increasing the intensity of the mechanical stress by use of syringe pistons differing in size, and thus differing in contact surface with cells, increased AOS production (data not shown).
Figure 1.
Oxidative burst induced by hypoosmotic (A–C) or mechanical (D) stress in suspension-cultured cells. H2O2 production (A) and accumulation in extracellular medium (B) by cells transferred at 0 time to hypoosmotic (Hypo, 40 mOsm), isoosmotic (Iso, 160 mOsm), or hyperosmotic (Hyper, 600 mOsm) medium buffered with 10 mm Mes-Tris, pH 5.2. One representative experiment of three independent experiments is illustrated in each case. C, Osmotic strength dependence of the oxidative burst triggered by transfer in hypoosmotic conditions. Aliquots of cells were transferred at 0 time in hypoosmotic media differing in osmotic strength (corresponding to differing Suc contents) and AOS production was followed. Maximal rate (100%) corresponds to H2O2 production rate for maximal osmotic shock. Means ± se of two independent experiments are reported. D, H2O2 production of cells subjected to a mechanical stress (see Methods). Means ± se of four independent experiments are reported. Aliquots of cell suspension were equilibrated for 3 h in isoosmotic (A–C) or in culture medium (D) buffered with 10 mm Mes-Tris, pH 5.2, before stress treatments. FW, Fresh weight.
Involvement of a Plasma Membrane NADPH Oxidase
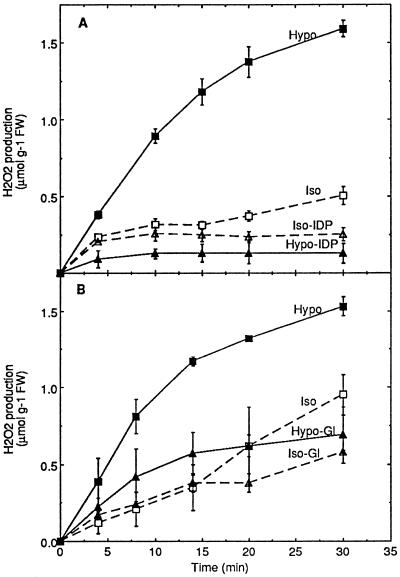
A possible homology between the AOS-generating system involved in the hypoosmotic response and the plasma membrane NADPH oxidase of phagocytic cells was tested for two properties: sensitivity to IDP and NADPH dependence. AOS production induced by hypoosmotic shock was completely prevented by 20 μm IDP (Fig. 2A). Complete inhibition was also found for the mechanically induced oxidative burst (data not shown). It was verified that IDP did not modify the H2O2 determination in the assay conditions used (see “Oxidative Burst Assay”). To test NADPH dependence, glucosamine was used to inhibit the pentose phosphate pathway, as previously shown on tobacco cells (Pugin et al., 1997). These authors demonstrated that in glucosamine-treated cells the NADPH-to-NADP+ ratio determined by 31P-NMR was largely decreased and that the elicitor-induced oxidative burst was completely prevented. The oxidative burst induced by hypoosmotic shock was clearly inhibited by 10 mm glucosamine (Fig. 2B). These data suggest that the AOS production system that was activated following hypoosmotic stress shares two properties with the mammalian NADPH oxidase: IDP sensitivity and NADPH dependence.
Figure 2.
Inhibition by IDP (A) and glucosamine (B) of the oxidative burst induced by hypoosmotic stress in tobacco cell suspensions. A, Aliquots of cell suspension were equilibrated for 3 h in isoosmotic medium before transfer at 0 time to hypoosmotic (Hypo), isoosmotic (Iso), hypoosmotic containing 20 μm IDP (Hypo-IDP), or isoosmotic containing 20 μm IDP (Iso-IDP) media. The inhibitor was also added during the last 15 min of the equilibration time at the concentration used after transfer. B, Aliquots of cell suspension were equilibrated for 2 h in buffered culture medium and for an additional 1 h in the same medium containing 10 mm glucosamine, before transfer in hypoosmotic medium containing glucosamine (Hypo-Gl) or isoosmotic medium containing glucosamine (Iso-Gl). Corresponding controls were treated similarly, except for the replacement of glucosamine by 30 mm Man during the last 1 h of equilibration and after transfer in hypoosmotic (Hypo) or isoosmotic (Iso) conditions. Means ± se of two independent experiments are reported in each case. FW, Fresh weight.
Transduction Events Involved in Oxidative Burst Induction
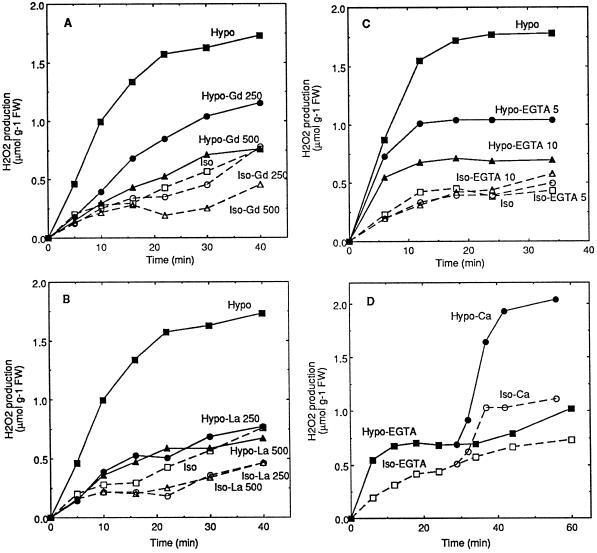
Activation of the oxidative burst by hypoosmotic and mechanical stresses suggested involvement of stretch forces at the plasma membrane level. Gd, reported as an inhibitor of stretch-activated channels (Yang and Sachs, 1989; Ding and Pickard, 1993), was thus tested. In tobacco cell suspensions, Gd was able to prevent the oxidative burst induced by hypoosmotic shock in a dose-dependent manner, with a nearly total inhibition efficiency for 500 μm Gd (Fig. 3A). Oxidative burst activation by mechanical stress was also inhibited by 500 μm Gd (data not shown). These results suggest that stretch-activated channels may be involved in oxidative burst activation.
Figure 3.
Inhibition of the hypoosmotically induced oxidative burst by the presence of Gd (A) or La (B) and by the lack of extracellular Ca2+ (C and D) in cell suspensions. A and B, Aliquots of cell suspension were equilibrated for 3 h in isoosmotic medium before transfer at time 0 to hypoosmotic (Hypo), isoosmotic (Iso), or hypoosmotic medium containing 250 or 500 μm La(NO3)3 or GdCl3 (Hypo-La and Hypo-Gd) or isoosmotic medium containing the same inhibitor concentrations (Iso-La and Iso-Gd). The inhibitor La(NO3)3 or GdCl3 was also added during the last 15 min of the equilibration time, at the concentration used after transfer. C and D, Aliquots of cell suspension were equilibrated for 3 h in isoosmotic medium deprived of Ca2+ before transfer at 0 time in hypoosmotic medium containing 5 or 10 mm EGTA (Hypo-EGTA) or without EGTA (Hypo) and in isoosmotic medium containing 5 or 10 mm EGTA (Iso-EGTA) or without EGTA (Iso). In D, 3 mm CaCl2 was added after about 30 min to the cells previously transferred in hypoosmotic (Hypo-Ca) or isoosmotic (Iso-Ca) media containing 10 mm EGTA. One representative experiment out of three independent experiments is illustrated in each part of the figure. FW, Fresh weight.
Two methods were used to investigate the role of Ca2+ in the induction of oxidative burst by hypoosmotic shock. The first one used La, which has been described as an inhibitor of Ca2+ channels (Ding and Pickard, 1993). The presence of La inhibited the oxidative burst with a maximal inhibition for 250 μm La (Fig. 3B). The second method was based on extracellular Ca2+ depletion by EGTA. Cells were equilibrated for 3 h in Ca2+-free medium and then transferred into a Ca2+-free hypoosmotic medium containing EGTA. The oxidative response was clearly reduced in a dose-dependent manner (Fig. 3C). The oxidative burst could be reactivated after 10 mm EGTA treatment for 30 min by addition of 3 mm CaCl2 (Fig. 3D). These data indicate the involvement of Ca2+ in the oxidative burst activation.
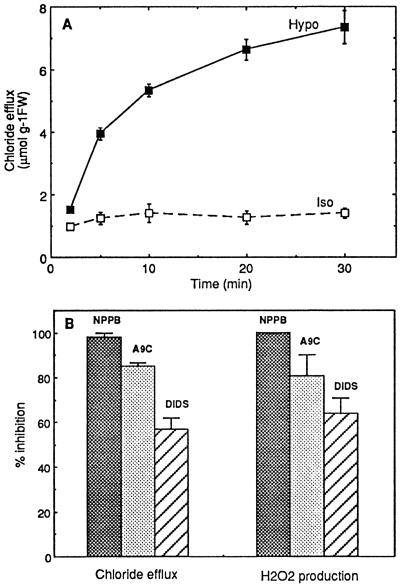
Hypoosmotic stress induced rapid Cl− efflux (Fig. 4A). The possibility that anion efflux involved in osmoregulation may play a role in the induction of the oxidative burst was investigated. Three structurally unrelated inhibitors of anion channels, NPPB, A9C, and DIDS, shown to be active on guard cells (Marten et al., 1992; Ward et al., 1995) and tobacco cell suspensions (S. Zimmerman, J.M. Frachisse, S. Thomine, H. Barbier-Brygoo, and J. Guern, unpublished data), were tested on hypoosmotically stressed cells. These molecules were able to inhibit the Cl− effluxes induced by hypoosmotic shock with varying efficiencies for a same inhibitor concentration (Fig. 4B). The oxidative response was clearly susceptible to the three anion channel blockers, with about 100, 80, and 60% inhibition for NPPB, A9C, and DIDS, respectively. These products did not interfere with the H2O2 determination in the assay conditions used (data not shown). These results suggest that Cl− efflux could be a part of the signaling pathway for hypoosmotically induced oxidative burst.
Figure 4.
Cl− efflux induced by hypoosmotic stress (A) and inhibition of hypoosmotically induced responses by anion channel blockers NPPB, A9C, and DIDS (B). A, Aliquots of cell suspension were equilibrated for 3 h in isoosmotic medium before transfer at 0 time to hypoosmotic (Hypo) or isoosmotic (Iso) medium. Means ± se of two independent experiments are reported. B, Aliquots of cell suspension were equilibrated for 3 h in isoosmotic medium before transfer at time 0 to hypoosmotic medium containing 100 μm NPPB, 100 μm A9C, or 100 μm DIDS. Inhibitions by each molecule of the Cl− efflux (left part) and H2O2 production (right part) initial rates, in comparison with control cells deprived of inhibitor, are reported. Each inhibitor was also added during the last 15 min of the equilibration time at the concentration used after transfer. Means ± se of at least two independent experiments are reported in each case. FW, Fresh weight.
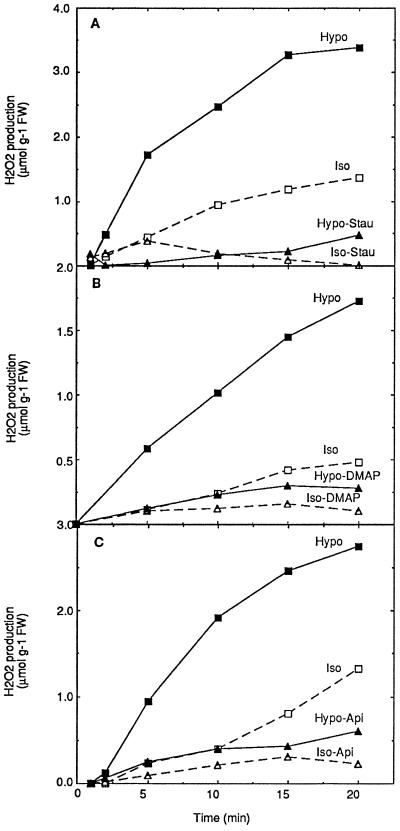
Involvement of protein phosphorylation in the hypoosmotically induced oxidative response was tested with various protein kinase inhibitors, among which three structurally unrelated protein kinase inhibitors, staurosporine, DMAP, and apigenin, were effective. All three inhibitors were able to inhibit completely the oxidative burst, at 0.5 μm for staurosporine and 500 μm for the other two molecules (Fig. 5). Although these inhibitors were reported to block specifically protein kinase C for staurosporine, cyclin-dependent kinases for DMAP, and MAP kinases for apigenin, it is most probable that these compounds may have broader specificities at these concentrations.
Figure 5.
Involvement of protein phosphorylation in the activation of oxidative burst by hypoosmotic stress. Aliquots of cell suspension were equilibrated for 3 h in isoosmotic medium before transfer at 0 time to hypoosmotic (Hypo), isoosmotic (Iso), or hypoosmotic medium containing 0.5 μm staurosporine (A, Hypo-Stau), 500 μm DMAP (B, Hypo-DMAP), 500 μm apigenin (C, Hypo-Api), or isoosmotic medium containing the same inhibitor concentrations (A, Iso-Stau; B, Iso-DMAP; and C, Iso-Api). One representative experiment of three independent experiments is illustrated in each case. FW, Fresh weight.
Effect of the Oxidative Burst on Ion Fluxes
Hypoosmotic stress induced K+ and Cl− effluxes, as well as extracellular alkalinization (Fig. 6, Hypo and Iso curves). IDP and glucosamine were shown to inhibit the oxidative response to hypoosmotic stress (Fig. 2). The possibility that AOS production may play a role in the activation of ionic responses to the hypoosmotic signal was evaluated. First, oxidative burst inhibition by IDP resulted in a slight decrease of K+ efflux (Fig. 6A), Cl− efflux (Fig. 6B), and extracellular alkalinization (Fig. 6C) induced by hypoosmotic shock. Second, extracellular alkalinization was partially inhibited in the presence of glucosamine (Fig. 6D). Although the effect seems weak (20–30% inhibition), it was highly reproducible.
Effect of the Oxidative Burst on Cell Wall Protein Cross-Linking
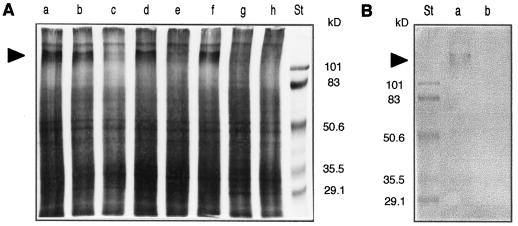
Insolubilization of cell wall proteins in response to hypoosmotic and mechanical stresses was evaluated. Analysis by SDS-PAGE and silver staining of SDS-extracted cell wall proteins (Fig. 7A) revealed the disappearance of a protein of approximately 115 kD in hypoosmotically (lane c) or mechanically (lane e) stressed cells compared with untreated cells (lane a) or isoosmotically transferred cells (lane b). The protein was not insolubilized after hypoosmotic shock or mechanical stress when the oxidative burst was inhibited by 20 μm IDP (lanes d and f, respectively). AOS involvement in the 115-kD protein insolubilization was also confirmed by addition of H2O2 to cells (lane g) or to cell walls (lane h). These results indicate that the oxidative burst activated by hypoosmotic shock or by mechanical stress induces the cross-linking of a 115-kD protein. The MAC265 antibody directed against a 100-kD extensin in soybean (Bradley et al., 1992) was used to identify the 115-kD protein. Analysis by western blot (Fig. 7B) revealed that a large band at approximately 115 kD, like that in silver staining, was recognized by the MAC265 antibody in control cells (lane a) and that the protein disappeared in extracts from hypoosmotically stressed cells (lane b).
Figure 7.
Cross-linking of cell wall proteins by oxidative burst induced by hypoosmotic or mechanical stress in tobacco cells. A, SDS-PAGE and silver staining of cell wall proteins extracted from untreated cells (lane a), 5 min after cell transfer in isoosmotic medium (lane b), hypoosmotic medium (lane c), hypoosmotic medium containing 20 μm IDP (lane d), after 20 min of mechanical stress in culture medium (lane e) or culture medium containing 20 μm IDP (lane f), after 5 min of in vivo treatment of cells by 1 mm H2O2 (lane g) or 5 min of in vitro treatment of proteins by 2 mm H2O2 (lane h). B, Western blot of cell wall proteins corresponding to cells transferred in hypoosmotic medium containing 20 μm IDP (a) or hypoosmotic medium without IDP (b). St, Molecular mass standards (in kilodaltons). Arrowheads in A and B indicate the 115-kD bands.
DISCUSSION
The present study documents the induction of an oxidative burst in suspension-cultured tobacco cells transferred to hypoosmotic medium. Specificity of the rapid production and extracellular accumulation of AOS was shown by comparison with transfer experiments in iso- or hyperosmotic conditions (Fig. 1, A and B). These results extend a previous demonstration of oxidative burst induction by dilution with water of soybean cell suspension (Yahraus et al., 1995). A similar production of AOS was measured when cells were subjected to a mechanical stress, independently of any change in the medium composition (Fig. 1D). This result suggests that physical perturbations at the plasma membrane level may take part in the transduction pathway originating from the hypoosmotic signal. In agreement with this idea, Gd, known as a stretch-activated channel inhibitor, was efficient to prevent the activation of the hypoosmotically induced oxidative burst (Fig. 3A). However, it cannot be excluded that Gd may prevent oxidative burst by inhibition of Ca2+ channels as a lanthanide-type element. It would be interesting to further assess the parallel between transduction steps that correspond to hypoosmotic shock and mechanical stimuli, to approach the eventual complexity of the osmotic signal.
Several transduction events located upstream of the oxidase activated by hypoosmotic stress could be identified, even if the sequence of the steps was not yet determined within the cascade. The induction of the oxidative burst was dependent on Ca2+ channels (Fig. 3B) and external Ca2+ (Fig. 3, C and D). The role of Ca2+ in the activation of oxidative burst in plants was previously demonstrated in response to several elicitors (for review, see Low and Merida, 1996) and, therefore, seems common in these two different kinds of stimuli. Ca2+ was evidenced as a response to hypoosmotic shock in Lamprothamium succinctum cells (Okasaki and Tazawa, 1986), and intracellular Ca2+ increase in BY2 tobacco cells following hypoosmotic shock has recently been reported (Takahashi et al., 1997a). Ca2+ influx was also evidenced as a response to mechanical stress in plants (Braam and Davis, 1990; Ding and Pickard, 1993). Ca2+ may transduce the hypoosmotic signal through Ca2+-dependent protein kinases. This hypothesis has been tested in algae (Yuasa and Muto, 1992, 1996) and in BY2 tobacco cells (Takahashi et al., 1997b). Activations of protein kinases were evidenced in the hypoosmotic response, but these kinases do not require Ca2+ for their activities and the postulated Ca2+-dependent protein kinases have not yet been identified. Phosphorylations are also key elements in the responses to mechanical stress (Piotrowski et al., 1996). In the present study staurosporine, DMAP, and apigenin, three structurally unrelated inhibitors of protein kinases, prevented oxidative burst activation following hypoosmotic shock (Fig. 5), and the nature of the involved protein kinases is currently being investigated. Inhibition of Cl− fluxes by different anion channel blockers, NPPB, A9C, and DIDS, is concomitant with corresponding oxidative burst prevention (Fig. 4). It suggests a role of Cl− effluxes as a step in hypoosmotic signal transduction in tobacco cells, leading to oxidase activation. Similarly, in parsley cell suspensions, various anion channel blockers were shown to inhibit with the same efficiency elicitor-induced Cl− and K+ effluxes, H2O2 production, and phytoalexin accumulation, thus placing anion channels as the most upstream elements so far identified within the elicitor-signaling cascade (Jabs et al., 1997). The mechanisms by which anion fluxes are required to initiate and/or maintain the oxidative burst are not yet understood.
The AOS-producing system activated by hypoosmotic shock was shown to be IDP sensitive (Fig. 2A) and NADPH dependent (Fig. 2B), thus sharing these properties with NADPH oxidase from neutrophils. The AOS-generating oxidase activated by elicitors in plants is also IDP sensitive and was recently shown to be NADPH dependent by the original approach, allowing NADPH depletion, as established by Pugin et al. (1997). Therefore, it seems that this system can be activated by different kinds of signals, such as elicitor and hypoosmotic shock. However, involved signal transduction pathways are likely to differ from one signal to another (Chandra et al., 1996b).
The AOS-generating system, as a component of a highly regulated system of membrane transporters, and the produced AOS, through modification of cell wall structure, could play a role in cell volume and turgor regulation. Ion, organic solute, and water fluxes drive osmoregulation, and it was shown here that oxidative burst contributes to a small but significant part of Cl− and K+ effluxes and extracellular alkalinization induced by hypoosmotic stress (Fig. 6). Because oxidative burst induction depends on anion effluxes, it suggests a feedback regulation of oxidative burst on anion effluxes. Strong reduction of oligogalacturonide-induced extracellular alkalinization by oxidative burst inhibition was also previously observed (Rouet-Mayer et al., 1997). The way oxidative burst participates in the regulation of these fluxes is still in question. Oxidative burst could cause membrane lipid peroxidation, which would modify plasma membrane permeability. Activated oxygen species could interact with ion channel activity and oxidase-dependent electron transfer could drive a depolarization, both effects leading to channel activation. The regulation of K+ channels by H2O2 (Vega-Saenz de Miera and Rudy, 1992; Duprat et al., 1995) or by a membrane oxidoreductase activity (Fehlau et al., 1989) has been reported in animals. Electrophysiological studies should give new insights into the possible role of the oxidative burst in osmoregulation.
Oxidative burst could also induce reinforcement of the cell wall. It was shown that a 115-kD protein was cross-linked within 5 min upon hypoosmotic stress, and this cross-linking was also observed upon addition of exogenous H2O2 (Fig. 7). This protein was recognized by an antibody directed against soybean extensin. Extensin, a Hyp-rich protein, is one of the major cell wall proteins. Its cross-linking could contribute to cell wall reinforcement, along with other cross-linked cell wall components, leading to cell volume increase limitation after hypoosmotic stress. Cross-linking may also contribute to turgor pressure increase and, thus, contribute to the induction of osmosensing. High-osmolarity-adapted cell suspension displayed diminished cell volume, as well as cell wall-insoluble protein increase, and it was also shown that Hyp-rich proteins are responsible for the cell wall tensile strength (Iraki et al., 1989). Cell volume and turgor regulations are also involved in cellular elongation and growth (Hohl and Schopfer, 1995). At the plant level, oxidative burst could limit growth by inducing protein peroxidase-dependent cross-linking and lignification. Inhibition of peroxidase secretion was observed in the case of gibberellin-stimulated growth (Fry, 1979), and reticulation of Hyp-rich proteins increased with diminished growth (Taiz, 1984). H2O2 inhibited elongation of coleoptiles (Schopfer, 1996) and was associated with lignification (Olson and Varner, 1993). Bradley et al. (1992) showed a correlation between the end of growth and protein cross-linking in bean hypocotyls (Bradley et al., 1992), suggesting that it could be one of the molecular bases for the measured modification of cell wall elasticity and plasticity. Cross-linking not only limits growth but also contributes to the plant morphology (Carpita and Gibeaut, 1993). Oxidative burst induction by mechanical stress suggested that it could be involved in thigmomorphogenesis (Legendre et al., 1993a). From these results, a general role of oxidative burst in plant development is suggested.
ACKNOWLEDGMENTS
We wish to warmly thank Jean Guern, who initiated and showed constant interest for this work. We also thank Nick Brewin for the gift of monoclonal antibody.
Abbreviations:
- AOS
active oxygen species
- A9C
anthracene-9-carboxylic acid
- DIDS
4,4′-diisothiocyanostilbene-2,2′-disulfonic acid
- DMAP
6-dimethylaminopurine
- IDP
iodonium diphenyl
- NPPB
5-nitro-2-(3-phenyl propylamino)-benzoic acid
LITERATURE CITED
- Baker CJ, Orlandi EW, Mock NM. Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 1993;102:1341–1344. doi: 10.1104/pp.102.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- Bottin A, Véronési C, Pontier D, Esquerré-Tugayé MT, Blein JP, Rusterucci C, Ricci P. Differential responses of tobacco cells to elicitors from two Phytophtora species. Plant Physiol Biochem. 1994;32:373–378. [Google Scholar]
- Braam J, Davis RW. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Chandra S, Heinstein PF, Low PS. Activation of phospholipase A by plant defense elicitors. Plant Physiol. 1996a;110:979–986. doi: 10.1104/pp.110.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Low PS. Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc Natl Acad Sci USA. 1995;92:4120–4123. doi: 10.1073/pnas.92.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Martin GB, Low PS. The Pto kinase mediates a signaling pathway leading to the oxidative burst in tomato. Proc Natl Acad Sci USA. 1996b;93:13393–13397. doi: 10.1073/pnas.93.23.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Desikam R, Hancock JT, Coffey MJ, Neill SJ. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 1996;382:213–217. doi: 10.1016/0014-5793(96)00177-9. [DOI] [PubMed] [Google Scholar]
- Devlin WS, Gustine DL. Involvement of the oxidative burst in phytoalexin accumulation and the hypersensitive reaction. Plant Physiol. 1992;100:1189–1195. doi: 10.1104/pp.100.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JP, Pickard BG. Mechanosensory calcium-selective cation channels in epidermal cells. Plant J. 1993;3:83–110. doi: 10.1111/j.1365-313x.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci USA. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer SC, Legendre L, Low PS, Leto TL. Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim Biophys Acta. 1996;1289:231–237. doi: 10.1016/0304-4165(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Fehlau R, Grygorczyk R, Fuhrmann GF, Schwarz W. Modulation of the Ca2+- or Pb2+-activated K+-selective channels in human red cells. II. Parallelisms to modulation of the activity of a membrane-bound oxidoreductase. Biochim Biophys Acta. 1989;978:37–42. doi: 10.1016/0005-2736(89)90495-1. [DOI] [PubMed] [Google Scholar]
- Fry SC. Phenolic components of the primary cell wall and their possible role in the hormonal regulation of growth. Planta. 1979;146:343–351. doi: 10.1007/BF00387807. [DOI] [PubMed] [Google Scholar]
- Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JDG. rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- Hallows KR, Knauf PA. Principles of cell volume regulation. In: Strange K, editor. Cellular and Molecular Physiology of Cell Volume Regulation. Boca Raton, FL: CRC Press; 1994. pp. 3–29. [Google Scholar]
- Henderson LM, Chappell JB. NADPH oxidase of neutrophils. Biochim Biophys Acta. 1996;1273:87–107. doi: 10.1016/0005-2728(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Hohl M, Schopfer P. Rheological analysis of viscoelastic cell wall changes in maize coleoptiles as affected by auxin and osmotic stress. Physiol Plant. 1995;94:499–505. [Google Scholar]
- Iraki NM, Bressan RA, Hasegawa PM, Carpita NC. Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol. 1989;91:39–47. doi: 10.1104/pp.91.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Heinstein PF, Low PS. Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem. 1992;267:20140–20147. [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993a;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Yueh YG, Crain R, Haddock N, Heinstein PF, Low PS. Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. J Biol Chem. 1993b;268:24559–24563. [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense: function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Marten I, Zeilinger C, Redhead C, Landry DW, AI-Awqati Q, Hedrich R. Identification and modulation of a voltage-dependent anion channel in the plasma membrane of guard cells by high-affinity ligands. EMBO J. 1992;11:3569–3575. doi: 10.1002/j.1460-2075.1992.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu Y, Sanchez FJ, Droillard M-J, Lapous D, Laurière C, Guern J. Involvement of protein phosphorylation in the early steps of transduction of the oligogalacturonide signal in tobacco cells. Plant Physiol Biochem. 1996;34:399–408. [Google Scholar]
- Mehdy MC, Sharma YK, Sathasivan K, Bays NW. The role of activated oxygen species in plant disease resistance. Physiol Plant. 1996;98:365–374. [Google Scholar]
- Miyahara M, Watanabe Y, Edashige K, Yagyu KI. Swelling-induced O2-generation in guinea-pig neutrophils. Biochim Biophys Acta. 1993;1177:61–70. doi: 10.1016/0167-4889(93)90158-l. [DOI] [PubMed] [Google Scholar]
- Nurnberger T, Nennstiel D, Hahlbrock K, Scheel D. Covalent cross-linking of the Phytophtora megasperma oligopeptide elicitor to its receptor in parsley membranes. Proc Natl Acad Sci USA. 1995;92:2338–2342. doi: 10.1073/pnas.92.6.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasaki Y, Tazawa M. Involvement of calcium ion in turgor regulation upon hypotonic treatment in Lamprothamnium succinctum. Plant Cell Environ. 1986;9:185–190. [Google Scholar]
- Olson PD, Varner JE. Hydrogen peroxide and lignification. Plant J. 1993;4:887–892. [Google Scholar]
- Otte O, Barz W. The elicitor-induced oxidative burst in cultured chickpea cells drives the rapid insolubilization of two cell wall structural proteins. Planta. 1996;200:238–246. [Google Scholar]
- Piotrowski M, Liss H, Weiler EW. Touch-induced protein phosphorylation in mechanosensitive tendrils of Bryonia dioica Jacq. J Plant Physiol. 1996;147:539–546. [Google Scholar]
- Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells. Involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;11:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet-Mayer MA, Mathieu Y, Cazalé AC, Guern J, Lauriè C. Extracellular alkalinization and oxidative burst induced by fungal pectin lyase in tobacco cells are not due to the perception of oligogalacturonide fragments. Plant Physiol Biochem. 1997;35:321–330. [Google Scholar]
- Schopfer P. Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta. 1996;199:43–49. [Google Scholar]
- Schroeder J. Anion channels as central mechanisms for signal transduction in guard cells and putative functions in roots for plant-soil interactions. Plant Mol Biol. 1995;28:353–361. doi: 10.1007/BF00020385. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989;14:187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Schwacke R, Hager A. Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein-kinase activity. Planta. 1992;187:136–141. doi: 10.1007/BF00201635. [DOI] [PubMed] [Google Scholar]
- Taiz L. Plant cell expansion: regulation of cell wall mechanical properties. Annu Rev Plant Physiol. 1984;35:585–657. [Google Scholar]
- Takahashi K, Isobe M, Knight MR, Trewavas AJ, Muto S. Hypoosmotic shock induces increases in cytosolic Ca2+ in tobacco suspension-culture cells. Plant Physiol. 1997a;113:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Isobe M, Muto S. An increase in cytosolic calcium ion concentration precedes hypoosmotic shock-induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett. 1997b;401:202–206. doi: 10.1016/s0014-5793(96)01472-x. [DOI] [PubMed] [Google Scholar]
- Tavernier E, Wendehenne D, Blein JP, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction, in tobacco cells. Plant Physiol. 1995;109:1025–1031. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA. 1995;92:4158–4163. doi: 10.1073/pnas.92.10.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E, Rudy B. Modulation of K+ channels by hydrogen peroxide. Biochem Biophys Res Commun. 1992;186:1681–1687. doi: 10.1016/s0006-291x(05)81602-x. [DOI] [PubMed] [Google Scholar]
- Viard MP, Martin F, Pugin A, Ricci P, Blein JP. Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Pei Z-M, Schroeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Binet MN, Blein JP, Ricci P, Pugin A. Evidence for specific high affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 1995;374:203–207. doi: 10.1016/0014-5793(95)01108-q. [DOI] [PubMed] [Google Scholar]
- Yahraus T, Chandra S, Legendre L, Low PS. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995;109:1259–1266. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Yu LM. Elicitins from Phytophotora and basic resistance in tobacco. Proc Natl Acad Sci USA. 1995;92:4088–4094. doi: 10.1073/pnas.92.10.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Muto S. Change of protein phosphorylation of Dunaliella tertiolecta cells in osmotic shock. In: Murata M, editor. Research in Photosynthesis, Vol 4. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 263–266. [Google Scholar]
- Yuasa T, Muto S. Plant Cell Physiol. 1996;37:35–42. [Google Scholar]