Abstract
In mammals, transcriptional autorepression by Period (PER) and Cryptochrome (CRY) protein complexes is essential for the generation of circadian rhythms. We have identified CAVIN-3 as a new, cytoplasmic PER2-interacting protein influencing circadian clock properties. Thus, CAVIN-3 loss- and gain-of-function shortened and lengthened, respectively, the circadian period in fibroblasts and affected PER:CRY protein abundance and interaction. While depletion of protein kinase Cδ (PKCδ), a known partner of CAVIN-3, had little effect on circadian gene expression, CAVIN-3 required the PKCδ-binding site to exert its effect on period length. This suggests the involvement of yet uncharacterized protein kinases. Finally, CAVIN-3 activity in circadian gene expression was independent of caveolae.
Keywords: Cavin-3, Caveolin1, mammalian circadian gene expression, Period 2-interacting proteins, period length
INTRODUCTION
The currently held molecular model of the mammalian circadian oscillator is based on negative transcriptional feedback loops [1]. Its core consists of a positive limb, with the transcription factors BMAL1:CLOCK activating Period (Per) and Cryptochrome (Cry) genes, and a negative limb, with heterotypic PER:CRY complexes translocating to the nucleus to repress BMAL:CLOCK-mediated transcription. The timing of nuclear entry and residence time influences the pace of the clock and requires regulation by post-translational modifications, such as phosphorylations on PER2 [2, 3]. Given their role in transcription, PER:CRY complexes have been characterized predominantly in the nuclear compartment, and biochemical purifications of nuclear PER:CRY complexes have thus identified new nuclear interaction partners [4, 5].
We have identified a new and unexpected cytoplasmic PER2-interacting protein, CAVIN-3, in complexes purified from NIH3T3 fibroblasts. CAVIN-3 is a protein kinase C (PKC) δ substrate [6, 7] and a constituent of caveolae [7], specialized plasma membrane domains implicated in cellular signalling and protein trafficking. Using circadian reporter assays, we found that CAVIN-3 depletion and overexpression shortened and lengthened, respectively, the period of free-running oscillations. When CAVIN-3-depleted cells were synchronized by simulated body temperature rhythms, the phase of circadian gene expression was advanced. Moreover, the levels of CAVIN-3 inversely correlated with those of PER2 and CRY2, and CAVIN-3 might thus regulate both the abundance and the stability of PER:CRY complexes. The functions of CAVIN-3 in modulating circadian gene expression required its previously characterized PKC interaction domain [6, 7].
RESULTS AND DISCUSSION
CAVIN-3 physically interacts with the PER2 complex.
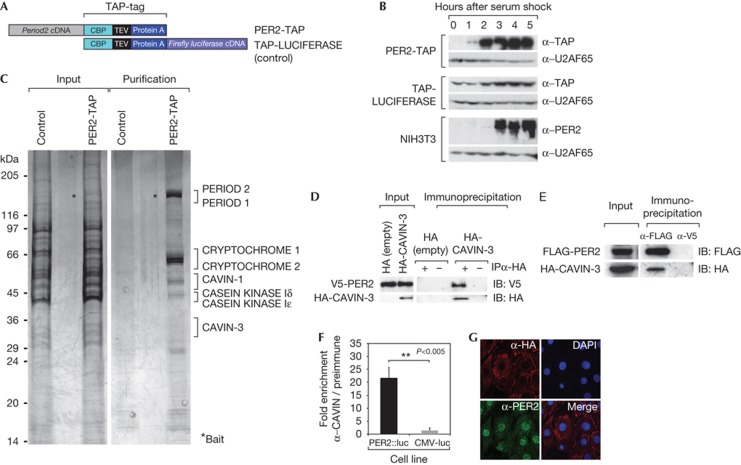
To purify new PER2-interacting proteins, we generated an NIH3T3 cell line stably expressing carboxy-terminally tagged murine PER2 protein (PER2-TAP) and a control cell line expressing tagged luciferase (TAP-LUC) (Fig 1A). As reported previously [8], Per2 overexpression compromised circadian clock function (supplementary Fig S1 online). However, treatment with 50% horse serum, which boosts the expression of Per2 and of immediate early genes [9], strongly induced both endogenous PER2 and transgenic PER2-TAP messenger RNA and protein with similar kinetics (Fig 1B; supplementary Fig S1A online). The stimulation of Per2-TAP transcription was likely owing to the CMV promoter, whose silencing and immediate early-like reactivation in fibroblasts by serum has been observed previously [10]. We thus concluded that the PER2-TAP cells were suitable to purify PER2-containing immediate early complexes. Whole-cell extracts [11] of serum-treated PER2-TAP and TAP-LUC cells were subjected to the TAP-tag purification protocol [12, 13[ and analysed by mass spectrometry (Fig 1C). In addition to known PER2 interaction partners (PER1, CRY1, CRY2 and CASEIN KINASE Iδ/ε), we identified ∼20 proteins with a MASCOT score of >20. CAVIN-3 attracted our particular attention, as this protein resides in the cytoplasm [7]. Moreover, we also identified CAVIN-1, one of its paralogs in the purified proteins. We first confirmed the PER2:CAVIN-3 interaction by co-immunoprecipitation experiments in NIH3T3 cells. Thus, haemagglutinin (HA)-tagged CAVIN-3 co-immunoprecipitated V5-tagged PER2 (Fig 1D) and, inversely, FLAG-tagged PER2 precipitated HA-CAVIN-3 (Fig 1E). We also examined the interaction of endogenous CAVIN-3 and PER2 proteins by a technique described by Maier et al [14] that uses cells from Per2::luciferase fusion knock-in mice [15]. We first confirmed CAVIN-3 expression in Per2::luc primary fibroblasts (supplementary Figs S2, S3 online) and measured luciferase activity co-precipitating with CAVIN-3 in extracts from these cells (Fig 1F). Anti-CAVIN-3 antibodies co-precipitated luciferase activity from Per2::luc extracts ≈20-fold more efficiently than from control extracts prepared from NIH3T3 cells expressing luciferase from a CMV promoter. We thus concluded that endogenous PER2 and CAVIN-3 proteins indeed interacted. This interaction probably occurred in the cytoplasm as CAVIN-3 is mostly cytoplasmic (Fig 1G; supplementary Fig S4 online) [16, 17, 18]. The timing of interaction was likely determined by rhythmic PER2 levels, given than neither CAVIN-3 nor other caveolar components showed rhythmic expression (supplementary Figs S5, S6 online).
Figure 1.
CAVIN-3 is a new PER2 interaction partner. (A) Schematic representation of TAP-tagged proteins used for the purification. (B) Immunoblot showing the serum induction of PER2-TAP and TAP-LUCIFERASE in the stable cell lines, and endogenous PER2 in NIH3T3 cells. U2AF65 served as a loading control. (C) Silver stain of purified protein complexes separated by 8–16% SDS–polyacrylamide gel electrophoresis. (D) Co-immunprecipitation of V5-tagged PER2 with HA-CAVIN-3. The pCI-HA empty vector was used as a negative control. A negative control IP was performed with beads devoid of antibodies. (E) Co-immunoprecipitation of HA-CAVIN-3 with FLAG-PER2. V5 antibody was used as an irrelevant mouse monoclonal antibody (negative control). (F) CAVIN-3 was immunoprecipitated from Per2::Luc cells with anti-CAVIN-3 serum. Co-immunoprecipitated PER2::Luciferase was assessed as luciferase activity. The fold enrichment represents the luciferase activity normalized to the signal from IPs with pre-immune serum. CMV-luc stable cells served as a control. (G) HA-CAVIN-3 localizes to the cytoplasm. HA-CAVIN-3-expressing synchronized NIH3T3 cells were stained with HA and PER2 antibodies. Nuclei were stained with DAPI. Merge: DAPI and α-HA straining. DAPI, 4,6-diamidino-2-phenylindole; HA, haemagglutinin; IB, immunoblotting; IP, immunoprecipitation; PER, Period.
CAVIN-3 influences circadian period length.
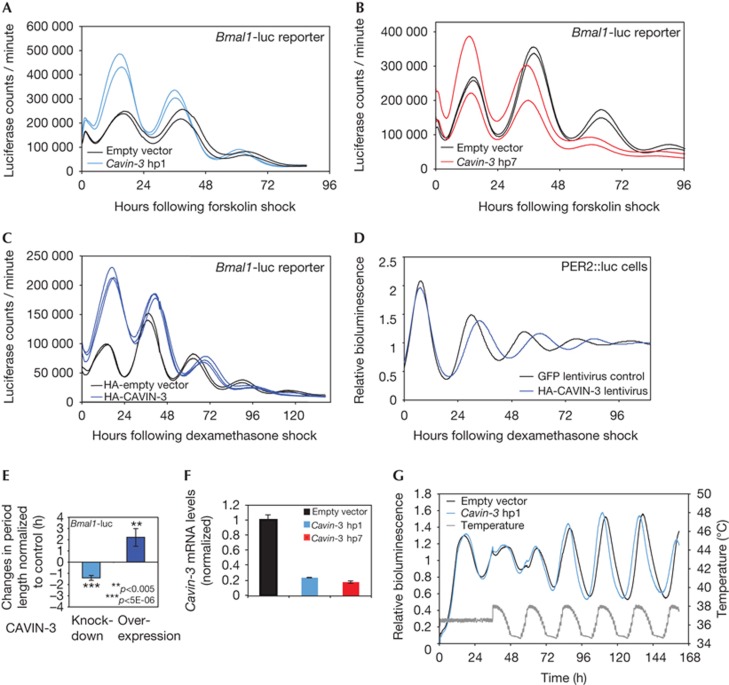
We next examined the role of CAVIN-3 in the circadian oscillator by loss- and gain-of-function experiments in cultured cells. To this end, we transfected NIH3T3 cells with luciferase-based circadian reporter plasmids and Cavin-3 short-hairpin RNAs (shRNAs) or a CAVIN-3 expression vector, and recorded circadian bioluminescence rhythms. Using a Bmal1-luciferase reporter [19], two different shRNAs downregulating endogenous Cavin-3 mRNA levels by ≈80% (Fig 2F) resulted in a period shortening of free-running oscillations by ≈1.5 h and in a slight phase advance (Fig 2A,B). Similar results were obtained by using Dbp-luciferase, another circadian reporter gene [4] (supplementary Fig S7 online). Data obtained with additional Cavin-3 shRNAs and an irrelevant shRNA are shown in supplementary Fig S8 online. In line with these RNA interference experiments, the overexpression of CAVIN-3 engendered a period lengthening by ≈2 h and a phase delay (Fig 2C,E). A period lengthening by ≈3 h was also observed in Per2::luc fibroblasts transduced with an HA-CAVIN-3-expressing lentiviral vector (Fig 2D; supplementary Fig S9 online). We also noticed that the strength of the period length phenotype depended to some extent on the chemical nature of the phase-resetting cues (supplementary Figs S10, S11 online).
Figure 2.
CAVIN-3 loss- and gain-of-function affects the circadian period length. (A) NIH3T3 cells were transiently co-transfected with the Bmal1-luciferase reporter and either an shRNA (hp1) targeting Cavin-3 mRNA (blue) or the empty plasmid (black). Individual oscillators were synchronized using forskolin. Data from duplicate transfections are shown. (B) Experiment as in A, but using another shRNA targeting Cavin-3 (hp7, red). (C) Bmal1-Luc bioluminescence recordings after co-transfection of an HA-CAVIN-3 expression vector (blue, in triplicates) or an empty vector control (black, duplicates). (D) Bioluminescence rhythms measured from Per2::luc primary cells transduced with HA-CAVIN-3 (blue) or GFP (black, control) expressing lentiviral vectors. The data were filtered with a low-pass filter (filfilt function in Matlab). Raw data were detrended using moving average transformation (window: 24 h). (E) Period length changes in CAVIN-3 loss- (hp1) and gain-of-function experiments normalized to values obtained with control cells. Mean±s.d., n?6. (F) shRNA-knockdown efficiencies on endogenous Cavin-3 mRNA analysed by quantitative PCR after FACS sorting of GFP-positive cells (encoded on shRNA vectors). (G) Bioluminescence recordings of cells expressing Bmal1-luciferase and Cavin-3 hp1 (blue) or the empty vector (black). Cells were synchronized by simulated body temperature rhythms (grey). The result of one representative experiment (out of three) is shown. Raw data were detrended using moving average transformation (window: 24 h). GFP, green fluorescent protein; HA, haemagglutinin; PER, Period; shRNA, short-hairpin RNA.
In vitro, cultured cells can be synchronized by simulated body temperature rhythms [20, 21, 22]. Under such conditions, CAVIN-3 depletion phase-advanced Bmal1-luciferase rhythms by ≈2 h (Fig 2G), as expected for a short-period phenotype. It is thus conceivable that the magnitude of CAVIN-3 expression in different tissues [6] contributes to different phase angles observed in various organs of intact animals [15].
CAVIN-3 affects PER:CRY abundance and interactions.
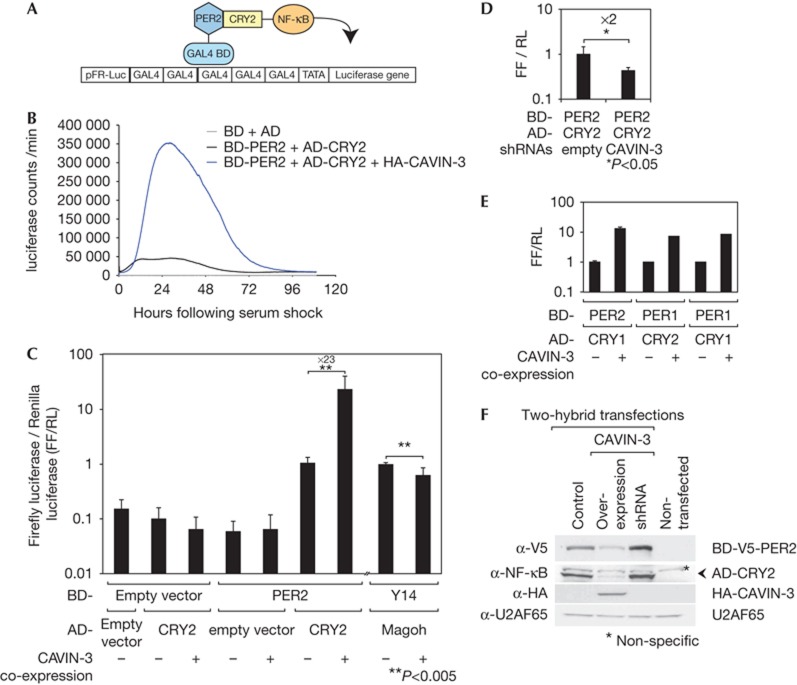
We first investigated the influence of CAVIN-3 on the interaction of PER and CRY proteins in NIH3T3 cells by using a two-hybrid system in which PER2 and CRY2 were fused to the GAL4 DNA-binding (BD) and NF-κB transactivation domains (AD), respectively. In this assay, the interaction of AD- and BD-tagged proteins is detected as bioluminescence produced from a co-transfected GAL4-firefly (FF) luciferase reporter plasmid (Fig 3A). Combining BD-PER2 and AD-CRY2 constructs gave readily detectable bioluminescence in the assay, as expected (Fig 3B). This signal was dramatically increased by co-expressing CAVIN-3 (Fig 3B,C). CAVIN-3 co-expression also increased the two-hybrid signals of other PER:CRY protein combinations (Fig 3E), but not those of the two unrelated interacting proteins, Y14 and MAGOH (Fig 3C; [23]). Moreover, CAVIN-3 depletion decreased PER2:CRY2 two-hybrid signals by twofold (Fig 3D). Interestingly, the downregulation of the paralog Cavin-2 decreased the BD-PER2:AD-CRY2 signal as well (supplementary Fig S12 online).
Figure 3.
CAVIN-3 influences PER and CRY expression levels and affects PER:CRY complex interactions. (A) Schematic representation of the mammalian two-hybrid system used to analyse PER:CRY interactions. (B) The PER2:CRY2 interaction measured after serum treatment (black). CAVIN-3 co-expression (blue) dramatically increased the PER2:CRY2 signal. Empty vectors are in grey. (C) Dual luciferase assay quantifying the effect of CAVIN-3 co-expression on the PER2:CRY2 interaction. Values represent FF luciferase signals normalized to RL luciferase (internal control) measured 40 h after synchronization. The Y14:Magoh interaction was used as a specificity control. Mean±s.d., n=10. (D) As in C, but quantifying the effect of Cavin-3 knockdown. Mean±s.d., n=5. (E) Effects of CAVIN-3 co-expression on PER2:CRY1, PER1:CRY2 and PER1:CRY1 interactions. Mean±s.d., n=5. (F) GAL4-V5-PER2 and NF-κB-CRY2 protein expression upon HA-CAVIN-3 overexpression and knockdown, analysed by immunoblotting using V5 and NF-κB antibodies, respectively. U2AF65 served as a loading control. CRY, Cryptochrome; FF, Firefly; HA, haemagglutinin; PER, Period; RL, Renilla.
Increased two-hybrid signals upon CAVIN-3 co-transfection could be indicative of a tighter BD-PER2:AD-CRY2 interaction. However, changes in two-hybrid partner protein abundance could also affect the bioluminescence readout. We thus inserted a V5 epitope into the BD-PER2 construct (supplementary Fig S13 online) and measured AD-CRY2 and BD-V5-PER2 protein levels using anti-NF-κB and anti-V5 antibodies, respectively. Surprisingly, we found that CAVIN-3 co-expression decreased AD-CRY2 and BD-V5-PER2 levels, while the Cavin-3 knockdown had an opposite effect (Fig 3F; supplementary Fig S14 online). Importantly, endogenous PER2 protein was also downregulated ≈2-fold on HA-CAVIN-3 expression (supplementary Fig S15 online). It was possible that the lower PER and CRY levels were responsible for the CAVIN-3-mediated increase in two-hybrid signals, because excessive BD-PER and AD-CRY levels might have competed with cofactors important for productive two-hybrid activity. However, titrating the amount of transfected BD-PER2- and AD-CRY2-expressing plasmids to fivefold lower and twofold higher levels did not reveal a general sensitivity of the two-hybrid assay to BD-PER2 and AD-CRY2 quantities (supplementary Fig S16 online). Thus, CAVIN-3 increased the two-hybrid signal over the entire titration range. Hence, we concluded that CAVIN-3 had two effects regarding PER and CRY proteins. First, it regulated the abundance of PER2 and possibly other negative limb components, such as CRY2. Second, it increased the strength of interaction within PER2:CRY2 complexes. Conceivably, both effects might be mechanistically linked, in that a stronger PER2:CRY2 interaction might engender a reduced metabolic stability of these proteins.
CAVIN-3 acts independently of PKCδ and caveolae.
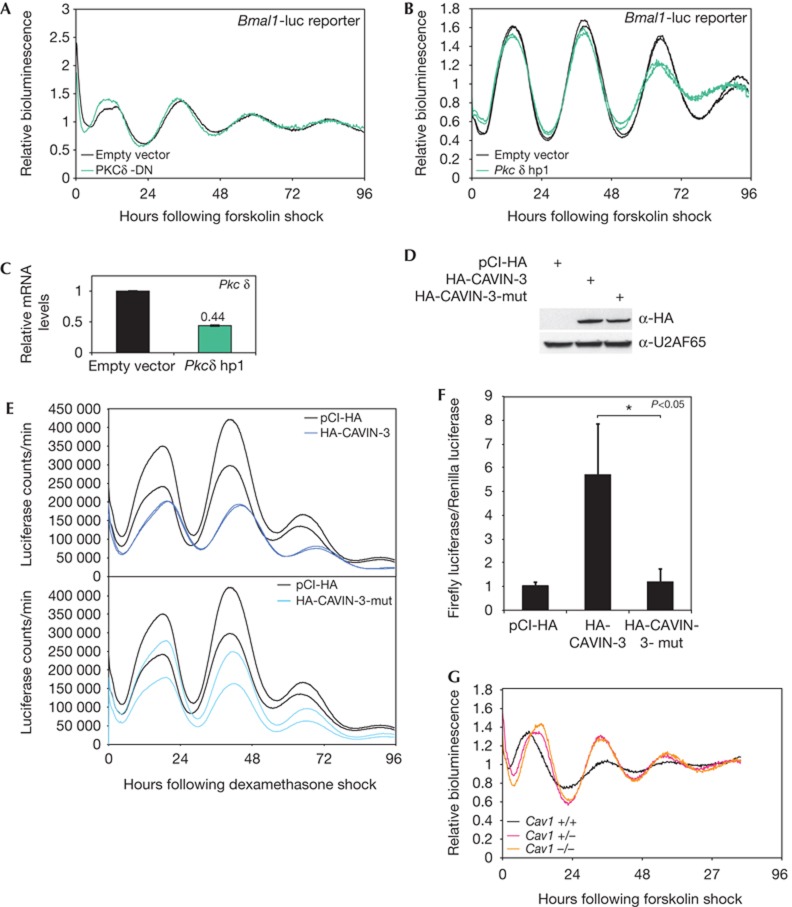
Various kinases regulate PER2 phosphorylation and stability, and thereby control circadian period length [3, 24]. CAVIN-3 interacts with PKCδ [6], but neither the co-transfection of a dominant-negative PKCδ variant (PKCδ-DN) [25] nor that of a Pkcδ-directed shRNA led to changes in Bmal1-luciferase rhythms (Fig 4A,B). The efficiency of Pkcδ knockdown was assessed against a background of non-transfected cells (Fig 4C). Although transfection efficiencies were typically <50%, our observations suggested that PKCδ did not act as a critical regulator of circadian clock function, and that the effects of CAVIN-3 were likely not mediated by this kinase. Conceivably, however, the PKC-binding domain within CAVIN-3, which is itself subject to phosphorylation [6, 7], could serve as a binding platform for several other kinases. To uncouple CAVIN-3 from the activity of such kinases, we created a mutant version of the protein (CAVIN-3-mut), in which the putative phosphorylation sites within the PKC-binding region were mutated to alanines (supplementary Fig S17 online). While mutant and wild-type CAVIN-3 accumulated to comparable levels in transfected cells (Fig 4D), CAVIN-3-mut failed to provoke the period lengthening (Fig 4E) and the increase in BD-PER2:AD-CRY2 two-hybrid signal (Fig 4F). We thus concluded that the role of CAVIN-3 in regulating the circadian clock required its known kinase interaction site. It is hence likely that a protein kinase other than PKCδ is involved in the underlying mechanism.
Figure 4.
CAVIN-3 requires its PKC-binding site, but Pkcδ and Caveolin1 seem not to be involved in setting the circadian period length. (A) Bmal1-luciferase reporter rhythms in NIH3T3 cells expressing a dominant-negative PKCδ (PKCδ-DN, green) or the empty vector (black). Raw data were detrended using moving average transformation. (B) Bmal1-luciferase rhythms in cells co-transfected with a vector encoding a Pkcδ-specific shRNA (green) or an empty shRNA vector (black). Raw data were detrended using moving average transformation. (C) Pkcδ-knockdown efficiency measured by quantitative PCR. (D) Expression levels of HA-CAVIN-3 and HA-CAVIN-3-mut assessed by anti-HA immunoblotting. (E) Bmal1-luciferase reporter rhythms in dexamethasone-shocked NIH3T3 cells co-transfected with plasmids encoding HA-CAVIN-3 (dark blue), HA-CAVIN-3-mut (light blue) or the empty plasmid (black). Data from duplicate transfections are shown. (F) Dual luciferase assay quantifying the effect of HA-CAVIN-3 and HA-CAVIN-3-mut co-expression on the PER2:CRY2 interaction. The assay was performed as in Fig 3. Mean±s.d., n=3. (G) Bioluminescence recorded in Bmal1-luciferase lentivector-transduced primary fibroblasts prepared from wt (black), heterozygous (pink) and homozygous (orange) Caveolin1-knockout animals. Raw data were detrended using moving average transformation. CRY, Cryptochrome; HA, haemagglutinin; PER, Period; PKC, protein kinase C; PKCδ-DN, dominant-negative PKCδ variant; shRNA, short-hairpin RNA.
Finally, we also examined a potential role of caveolae in the circadian clock using Caveolin1-knockout animals that lack caveolae [26]. As shown in supplementary Fig S18 online, Caveolin1 loss-of-function had little if any effect on the circadian period length of locomotor activity. Likewise, the loss of Caveolin1 did not affect the free-running period length of Bmal1-luciferase rhythms in cultured NIH3T3 cells, although it did delay the first peak by several hours (Fig 4G). As perturbations in CAVIN-3 expression levels elicited more severe phenotypic changes than the lack of Caveolin1, we consider likely that CAVIN-3 did not act through caveolar structures in influencing circadian clock function.
In summary, we have identified CAVIN-3 as a new cytoplasmic PER2-interacting protein that influenced period length and phase of circadian gene expression. Conceivably, CAVIN-3 and PER2 engage in transient interactions in the cytosol before or concomitant with the assembly and nuclear import of PER:CRY complexes. Whereas further experiments will be required to assess the precise molecular mechanisms through which CAVIN-3 affects clock function, our data indicate that CAVIN-3 might regulate PER and CRY protein abundance in addition to their interaction. Furthermore, the phosphorylation of CAVIN-3 by a yet unidentified kinase appears to be required for this action. Future experiments will thus include the search of this kinase and its effect on PER2 and/or CRY proteins.
METHODS
Animal housing. Animal studies were in accordance with regulations of the State of Geneva veterinary office, including standard animal housing conditions (12-h-light–12-h-dark), with free access to food/water. Caveolin1-knockout mice [26] and the recording of wheel-running activities [21] have been described previously.
Construction of stable cell lines and protein purification. Per2 and luciferase complementary DNAs were cloned into pCMV-C-term-TAP, linearized plasmids were transfected in NIH3T3 cells and clonal stable cell lines were selected on neomycin. TAP-tag purifications were performed as described [13]. After separation by SDS–polyacrylamide gel electrophoresis (SDS–PAGE), silver-stained protein bands were excised, reduced, alkylated and trypsin-digested as described [27]. Liquid chromatography–tandem mass spectrometry and the interpretation of peptide data sets using Mascot (Matrix Science, UK) were as detailed in Supplementary Material.
Lentivirus production and transduction. Bmal1-luciferase and HA-CAVIN-3 lentivector particles were produced as described [28]. Lentivirally transduced cells were selected on Blasticidin (Invitrogen).
Co-immunoprecipitation and immunoblotting. Co-immunoprecipitations were performed from RIPA extracts of serum-synchronized cells with the indicated antibodies and protein G magnetic beads (Invitrogen), using standard protocols. For the examination of endogenous PER2:CAVIN-3 interaction, RIPA extracts from serum-synchronized Per2::Luc and CMV-luc cells were incubated with pre-blocked protein G agarose beads (Roche) and pre-immune or anti-CAVIN-3 serum. Luciferase activity was determined in a plate reader using a luciferase assay system (Promega). SDS–PAGE and immunoblot analysis were performed according to standard protocols.
RNA analysis by quantitative real-time reverse transcriptase PCR. RNAs were extracted from cultured cells using Trizol (Invitrogen) and from mouse liver as in ]ref. 21]. cDNA synthesis (SuperScript II, Invitrogen) and SYBR-green PCR-amplification (Roche) were performed according to the suppliers’ indications. Mean levels calculated from triplicate PCR assays were normalized to the control mRNAs Cyclophilin A or Rps9. Primers are listed in supplementary Table S1 online.
Immunostaining. Immunostaining was performed on paraformaldehyde-fixed cells using the indicated primary antibodies and Alexa594- or Alexa488-conjugated secondary antibodies (Molecular Probes). Nuclei were stained with 4,6-diamidino-2-phenylindole. Microscopic images were taken on a Leica SP2 confocal microscope.
Two-hybrid assay. Cells were transfected with the plasmids specified in the text and the figure legends. At 24 h after transfection, cells were synchronized by serum shock, and luciferase activity was recorded in real-time as described [19]. For dual luciferase assays, cells were co-transfected with pRL-control encoding Renilla luciferase. Samples were synchronized 24 h after transfection and harvested 40 h later. Dual luciferase measurements were carried out using the Dual Glo Luciferase Assay System (Promega), and FF luciferase signals were normalized to Renilla luciferase signals after background subtraction.
Antibody production. CAVIN-3 protein was bacterially expressed as an amino-terminal glutathione S-transferase and 6xHis fusion, and purified under denaturing conditions on Ni-NTA beads (Qiagen). After dialysis against PBS, polyclonal antisera were generated in rabbits (Charles River)
Further Methods can be found in supplementary information online.
Supplementary Material
Acknowledgments
We thank J. Ripperger and R. Loewith for antibodies; E. Izaurralde, S. Thore, S. Brown and M. Shong for plasmids; Olivier Schaad and Guillaume Rey for help in the mathematical detrending of bioluminescence tracings; N. Roggli for the artwork; and members of the Schibler lab for comments on the manuscript. Particular thanks go to A. Liani, Y.-A. Poget and G. Severi, who designed and constructed the bioluminescence and temperature recording systems. Research in the laboratory of U.S. was supported by the Swiss National Science Foundation (individual grants SNF 31-113565 and 31-128656/1, and NCCR Frontiers in Genetics), the European Research Council (ERC- 2009-AdG 20090506), the State of Geneva, the Louis Jeantet Foundation of Medicine and the 6th European Framework Project EUCLOCK. D.G. received FEBS and HFSP long-term fellowships; D.G.’s laboratory is supported by SNF Professorship grant PP00P3_128399.
Author contributions: This study was designed by D.G., K.S. and U.S. D.G. purified the PER2 protein complexes, T.Kö. identified PER2-associated proteins by mass spectrometry, and K.S. confirmed the interaction of PER2 with CAVINs and performed all loss- and gain-of-function experiments. T.A. cloned CRY1 in the pCMV-AD vector and T.Ku. provided Caveolin1-knockout mice and advice on caveolae biology. K.S., D.G. and U.S. wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72: 517–549 [DOI] [PubMed] [Google Scholar]
- Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C (2011) The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci USA 108: 16451–16456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanselow K, Kramer A (2007) Role of phosphorylation in the mammalian circadian clock. Cold Spring Harb Symp Quant Biol 72: 167–176 [DOI] [PubMed] [Google Scholar]
- Brown SA, Ripperger J, Kadener S, Fleury-Olela F, Vilbois F, Rosbash M, Schibler U (2005) PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science 308: 693–696 [DOI] [PubMed] [Google Scholar]
- Duong HA, Robles MS, Knutti D, Weitz CJ (2011) A molecular mechanism for circadian clock negative feedback. Science 332: 1436–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Hirai S, Tamai Y, Fujise-Matsuoka A, Nishimura Y, Ohno S (1997) A protein kinase Cdelta-binding protein SRBC whose expression is induced by serum starvation. J Biol Chem 272: 7381–7389 [DOI] [PubMed] [Google Scholar]
- McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, Luby-Phelps K, Anderson RG (2009) SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J 28: 1001–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Yagita K, Okamura H (2005) Role of cyclic mPer2 expression in the mammalian cellular clock. Mol Cell Biol 25: 1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929–937 [DOI] [PubMed] [Google Scholar]
- Brightwell G, Poirier V, Cole E, Ivins S, Brown KW (1997) Serum-dependent and cell cycle-dependent expression from a cytomegalovirus-based mammalian expression vector. Gene 194: 115–123 [DOI] [PubMed] [Google Scholar]
- Lavery DJ, Schibler U (1993) Circadian transcription of the cholesterol 7 alpha hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev 7: 1871–1884 [DOI] [PubMed] [Google Scholar]
- Forler D, Kocher T, Rode M, Gentzel M, Izaurralde E, Wilm M (2003) An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nat Biotechnol 21: 89–92 [DOI] [PubMed] [Google Scholar]
- Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B (2001) The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24: 218–229 [DOI] [PubMed] [Google Scholar]
- Maier B, Wendt S, Vanselow JT, Wallach T, Reischl S, Oehmke S, Schlosser A, Kramer A (2009) A large-scale functional RNAi screen reveals a role for CK2 in the mammalian circadian clock. Genes Dev 23: 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH et al. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205 [DOI] [PubMed] [Google Scholar]
- Yagita K, Yamaguchi S, Tamanini F, van Der Horst GT, Hoeijmakers JH, Yasui A, Loros JJ, Dunlap JC, Okamura H (2000) Dimerization and nuclear entry of mPER proteins in mammalian cells. Genes Dev 14: 1353–1363 [PMC free article] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, Yasuda M, Hoeijmakers JH, van der Horst GT, Okamura H (2002) Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J 21: 1301–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U (2004) Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119: 693–705 [DOI] [PubMed] [Google Scholar]
- Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U (2002) Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol 12: 1574–1583 [DOI] [PubMed] [Google Scholar]
- Reinke H, Saini C, Fleury-Olela F, Dibner C, Benjamin IJ, Schibler U (2008) Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev 22: 331–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini C, Morf J, Stratmann M, Gos P, Schibler U (2012) Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev 26: 567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg S, Gatfield D, Izaurralde E, Conti E (2003) A novel mode of RBD-protein recognition in the Y14-Mago complex. Nat Struct Biol 10: 433–439 [DOI] [PubMed] [Google Scholar]
- Virshup DM, Eide EJ, Forger DB, Gallego M, Harnish EV (2007) Reversible protein phosphorylation regulates circadian rhythms. Cold Spring Harb Symp Quant Biol 72: 413–420 [DOI] [PubMed] [Google Scholar]
- Soh JW, Weinstein IB (2003) Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem 278: 34709–34716 [DOI] [PubMed] [Google Scholar]
- Drab M et al. (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858 [DOI] [PubMed] [Google Scholar]
- Saini C, Suter DM, Liani A, Gos P, Schibler U (2011) The Mammalian Circadian Timing System: synchronization of peripheral clocks. Cold Spring Harb Symp Quant Biol 76: 39–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.