Abstract
Lamins are nuclear intermediate filament proteins. They provide mechanical stability, organize chromatin and regulate transcription, replication, nuclear assembly and nuclear positioning. Recent studies provide new insights into the role of lamins in development, differentiation and tissue response to mechanical, reactive oxygen species and thermal stresses. These studies also propose the existence of separate filament networks for A- and B-type lamins and identify new roles for the different networks. Furthermore, they show changes in lamin composition in different cell types, propose explanations for the more than 14 distinct human diseases caused by lamin A and lamin C mutations and propose a role for lamin B1 in these diseases.
Keywords: development, lamin, nuclear envelope, nuclear lamina, stress
See Glossary for abbreviations used in this article.
Glossary.
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and Rad3-related
- Chk1/2
checkpoint kinase 1/2
- DM0
Drosophila B-type lamin
- DNAPKcs
DNA-dependent protein kinase, catalytic subunit
- ERK
extracellular-signal-regulated kinase
- Fos
FBJ murine osteosarcoma viral oncogene homolog
- iPSC
induced pluripotent stem cell
- JNK
c-Jun N-terminal kinase
- LAP2(α/β)
lamina-associated polypeptide 2 (isoform alpha/beta)
- LEF1
lymphoid enhancer-binding factor
- LEM2
LAP2/emerin/MAN1 domain protein 2
- MAPK
mitogen-activated protein kinase
- miRNA
microRNA
- MyoD
myogenic differentiation 1
- NURD
nucleosome remodelling and histone deacetylation
- Oct1
POU class 2 homeobox 1
- PCNA
proliferating cell nuclear antigen
- PPARγ
peroxisome proliferator-activated receptor gamma
- pRb1
protein retinoblastoma 1
- PSA-NCAM
polysialylated neuronal cell adhesion molecule
- RBBP4/7
retinoblastoma binding protein 4/7
- Runx2
runt-related transcription factor 2
- siRNA
short interfering RNA
- Zmpste24
zinc metallopeptidase homologue of ste24
Lamins are evolutionarily conserved nuclear intermediate filament proteins. They are restricted to the animal kingdom and are the main constituents of the nuclear lamina, which is a meshwork of lamins at the nuclear periphery and their associated proteins. Similarly to most intermediate filament proteins, lamins have a conserved α-helical coiled-coil rod domain flanked by variable amino-terminal head and carboxy-terminal tail domains [1]. The tail domain of lamins contains an immunoglobulin-like fold motif and a nuclear localization signal. Except for lamin C, all lamins are translated as prelamins with a C-terminal CaaX motif, which undergoes farnesylation. In Xenopus oocytes, lamins form filaments of about 10 nm in diameter, which are arranged in a regular, parallel pattern [2,3]. The basic building-block for higher-order lamin assembly is the lamin dimer. The first step in this assembly involves head-to-tail polymerization of the lamin dimers [4]. These polymers associate laterally in an antiparallel fashion to form the protofilament, and then between three and four protofilaments form the lamin filament [5]. However, the structure of lamin in somatic cells in vivo still needs to be determined. There is an uneven distribution of the lamin subtypes during development and throughout human tissues [6,7,8]. All somatic cell types, including embryonic stem cells (ESCs), express lamin B1 and/or lamin B2 (B-type lamins), which are encoded by LMNB1 and LMNB2 genes, respectively. Lamin A and lamin C are expressed from the LMNA gene through alternative splicing (A-type lamins), and differ from each other in their C-terminal tail domain. They are developmentally regulated and are not essential for somatic cell survival. Lamin A, lamin B1 and lamin B2 originate from prelamins. Their C-terminal CaaX motif undergoes farnesylation, aaX cleavage and carboxymethylation. Only lamin A is further cleaved 15 amino acids away from its farnesylated cysteine by the protease Zmpste24 [9]. Recent studies used fluorescence microscopy techniques in mammalian cells to show that A-type and B-type lamins form separate networks in certain cell types [10,11]. However, in Xenopus oocytes, ectopic expression of lamin A induces its assembly on top of the endogenous lamin B3 [3]. In addition, a Förster resonance energy transfer (FRET) study suggested that although homeotypic interactions are favoured over heterotypic interactions, both forms were found in vivo [12]. In vitro studies showed the formation of heterotypic interactions between A-type and B-type lamins. These interactions were mediated by consensus motifs at either end of the α-helical rod domain [13,14]. Determining the exact composition of vertebrate lamin filaments in vivo is a major goal for future studies.
Lamins probably define the main nuclear architecture that provides structural support to the nucleus. Lamins are also required for most other nuclear functions including the organization of chromatin, assembly and disassembly of the nucleus and chromosome segregation during mitosis, DNA replication, RNA polymerase II (Pol II) transcription, cell signalling and apoptosis [15,16].
B-type lamins, development and organogenesis
B-type lamins are considered essential for cell survival. In support of this conclusion, downregulation of B-type lamin genes in human HeLa cells leads to apoptosis [17], and Caenorhabditis elegans embryos downregulated for lamin die at the 100–300-cell stage with many cellular phenotypes. Among these are cell-cycle aberrations, abnormal chromatin organization, mitotic defects, clustering of nuclear pore complexes, rapid changes in nuclear morphology and accelerated ageing [18,19,20]. In addition, the only known heritable disease associated with B-type lamins is autosomal-dominant leukodystrophy, which is caused by duplication in the LMNB1 gene [21], suggesting that other mutations in B-type lamins might be lethal [22]. Surprisingly, lamin B-null ESCs show no obvious difference when compared with wild-type ESCs, apart from a slight delay in entry to prometaphase, suggesting that B-type lamins might not be essential for mouse ESCs [23]. B-type lamins are also dispensable in some types of differentiated tissue, as hepatocytes and keratinocytes of mice deficient for B-type lamins demonstrate no apparent phenotypes [24]. Given that until these studies B-type lamins were always considered essential, it is probable that the observations in lamin B knockout mice reflect adaptive measures in the embryos.
B-type lamins have developmental roles in tissue differentiation and organ development. Drosophila cells, for example, mutated in the B-type lamin DM0 have aberrations in the compound eye, the exoskeleton, the male and female reproductive systems and the brain. Mouse embryos lacking both lamin B1 and lamin B2 show abnormal development in organs such as the lungs, the diaphragm and the brain [23,25]. The role of B-type lamins in the development of many organs raises the possibility that they evolved to facilitate the integration of different cell types into the highly elaborate tissue architecture found in animals. Such complex tissues include the brain, which requires B-type lamins for its proper organization. In Xenopus retinal ganglion cell axons, lamin B2 is expressed outside the nucleus. Interestingly, lamin B2-deficient axons have mitochondrial dysfunction and defects in axonal transport, suggesting that the non-nuclear lamin B in axons is required for normal axonal functions [26].
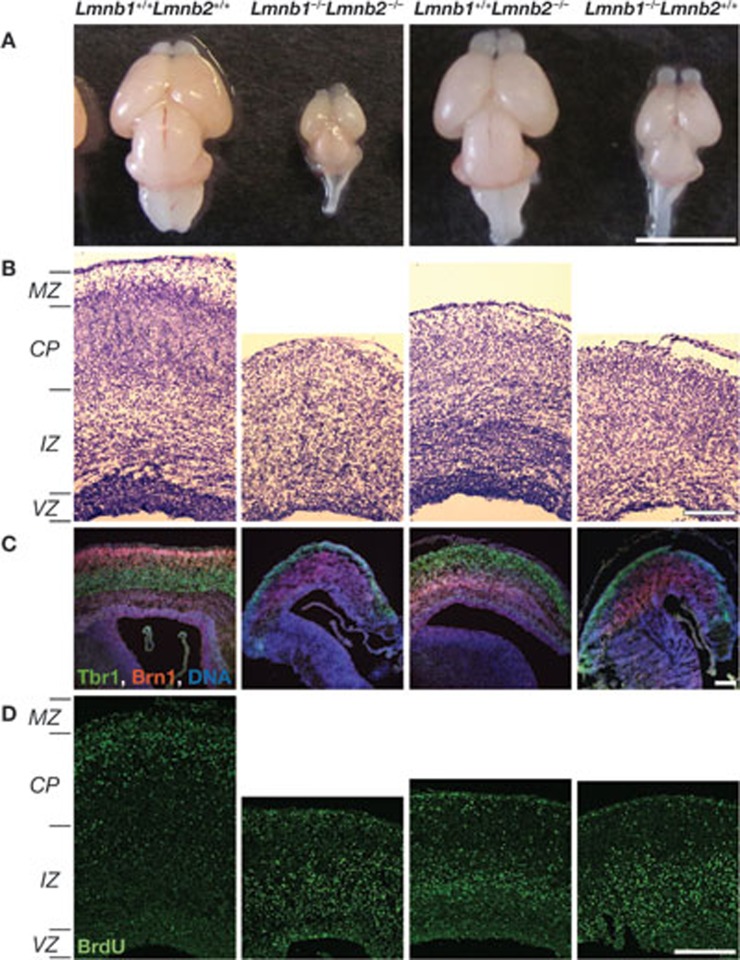
Lack of B-type lamins in mouse embryonic neurons causes abnormal nuclear morphology. These mutant mice also have severe neurodevelopmental abnormalities, including a reduced number of neurons and reduced cortex size (Fig 1A), as well as layer disorganization in the cerebral cortex (Fig 1; [23]). One explanation for the different outcomes of losing B-type lamins in different tissues could be a redundancy in the functions of B-type and A-type lamins. During neuronal differentiation, for example, there are alterations in lamin subtypes. Lamin A and lamin C are not expressed in PSA-NCAM-positive progenitor cells, whereas lamin B1 is expressed at high levels. The opposite is true for mature neurons in which lamin A and lamin C are highly expressed, whereas lamin B1 levels are reduced [27]. Another example of alterations in lamin subtypes is vascular cells and cells lining the surface of the brain, which express large amounts of lamin A. By contrast, little or no lamin A is observed in glial cells. A study shows that high levels of miR-9, a brain-specific miRNA, are responsible for the downregulation of lamin A—but not lamin C—in the brain [28].
Figure 1.
B-type lamins are required for brain development. The genotype of the brain and the brain sections is indicated above the panels. (A) Mice mutant for lamin B have smaller brains; brains are from embryonic day (E)18.5 mouse embryos. (B) Staining of E18.5 neocortex coronal sections of mouse embryos for nucleic acids. Neurons from lamin B-null mice have layer organization and migration defects. (C) Layer-specific labelling of the neocortex with Tbr1 (green) for early-born deep layer (V,VI) and Brn1 (red) for late-born outer neuronal layers (II–IV). DNA is labelled in blue. (D) BrdU (green) labelling of mid-to-late-born neurons from E18.5 mice embryos. The data for this figure have been used with permission from figure 4 in [23]. BrdU, bromodeoxyuridine; Brn1, POU domain, class 3, transcription factor 3; CP, cortical plate; IZ, intermediate zone; MZ, marginal zone; Tbr1, T-box, brain, 1; VZ, ventricular zone.
Lamin A and lamin C in development and organogenesis
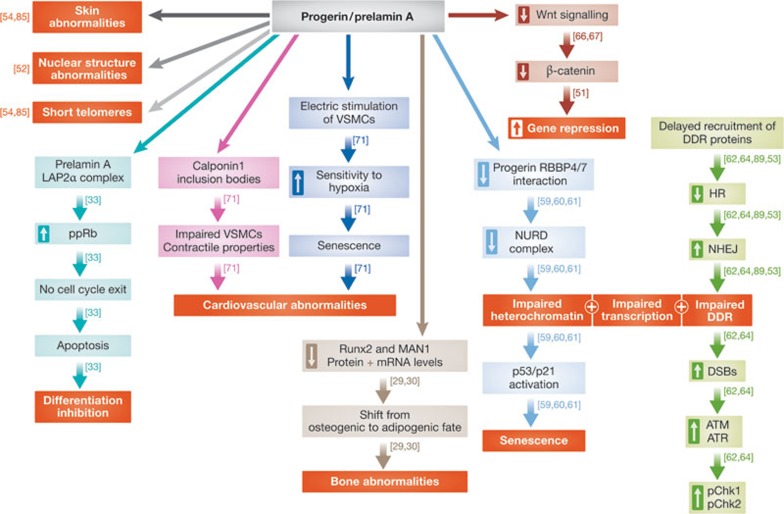
Lamin A and lamin C in osteoblastogenesis. Lamin A and lamin C are required for osteoblastogenesis and bone formation in vivo. They do so by helping to maintain the pool of mesenchymal stem cells (MSCs) by keeping their non-differentiation state. In support of this role, mice lacking lamin A and lamin C show characteristics of bone loss, due to a reduction in the number of stem cells, which leads to a shift from an osteogenic fate towards an adipogenic fate—phenotypes that are normally related to ageing. This shift is a result of changes in the expression and protein levels of Runx2- and MAN1-dependent pathways, which are transcription factors important for osteoblast differentiation (Fig 2, dark brown arrows; [29,30,31]).
Figure 2.
A scheme depicting the different outcomes caused by the presence of progerin and prelamin in cells. The boxed hollow arrows show an increase and decrease in the mentioned phenotype. Each pathway is indicated by a different colour. The red boxes represent final outcomes. ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3-related; DDR, DNA damage response; DSB, double-strand break; HR, homologus recombination; LAP2α, lamina-associated polypeptide 2 isoform alpha; mRNA, microRNA; NHEJ, non-homologus end joining; NURD, nucleosome remodelling and histone deacetylation; pChk1/2, checkpoint kinase 1/2; ppRb, hyperphosphorylated retinoblastoma; RBBP4/7, retinoblastoma binding protein 4/7; Runx2, runt-related transcription factor 2; VSMC, vascular smooth muscle cell.
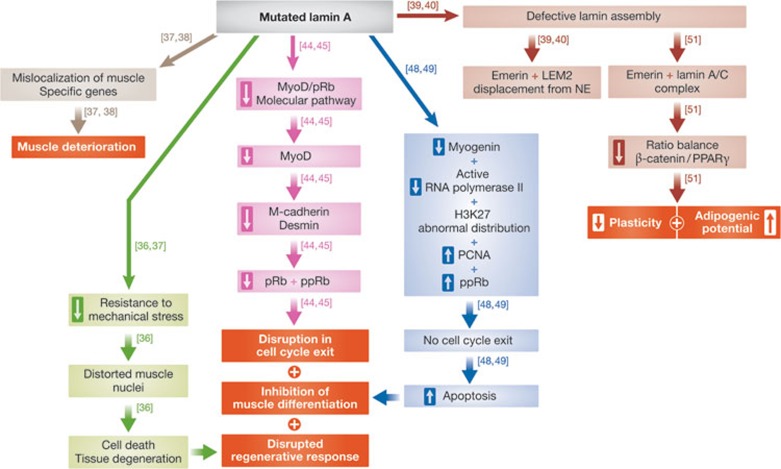
Lamin A, lamin C and muscle cells. Mutations in the LMNA gene cause several muscle degeneration diseases including Emery–Dreifuss muscular dystrophy (EDMD), limb–girdle muscular dystrophy, congenital muscular dystrophy, heart–hand syndrome and dilated cardiomyopathy [16]. These diseases target muscle cells and vary in the age of onset, severity of the disease and in the molecular mechanism leading to the disease. It is not clear why different lamin mutations affect different muscle cells and what causes the varying severity of these diseases. One probable explanation for the interfamilial variability of phenotypes observed in these types of disease is the contribution of modifier genes. Indeed, a chromosomal region linked to the variability in the age of onset of myopathic symptoms in striated muscle laminopathies has been identified [32]. Future studies will probably identify the specific modifier genes in this region. Mutations in genes encoding lamin-associated proteins such as emerin, LAP2 and LEM2 can also cause muscle diseases [33,34,35], suggesting that maintaining muscle integrity requires lamin-based protein complexes (Fig 3).
Figure 3.
A scheme depicting the different outcomes caused by the presence of mutated lamin in cells. The boxed hollow arrows show an increase and decrease in the mentioned phenotype. Each pathway is indicated by a different colour. The red boxes represent final outcomes. LEM2, LAP2/emerin/MAN1 domain protein 2; MyoD, myogenic differentiation 1; NE, nuclear envelope; PCNA, proliferating cell nuclear antigen; PPARγ, peroxisome proliferator-activated receptor gamma; pRB, protein retinoblastoma 1; ppRb, hyperphosphorylated retinoblastoma protein.
There are three non-mutually exclusive models that explain the muscle pathology caused by lamin mutations. Lamin A and lamin C are probably the main structural elements of the nucleus that protects it from mechanical stress [36]. The first model proposes that lamin A and lamin C mutations impair the ability of the lamin filaments to resist mechanical stress, leading to the distortion of muscle nuclei and abnormal mechanical signalling, which eventually leads to cell death and tissue degeneration [36]. Lamin A and lamin C filaments also serve as hubs for the formation of numerous protein complexes, and are required for developmental gene positioning. According to the second model, muscle-specific mutations in the LMNA gene cause mislocalization of muscle-specific genes, as well as abnormal formation of lamin A- and lamin C-containing muscle-specific protein complexes, which in turn lead to muscle deterioration. For example, an array of the muscle-specific promoter myo-3 is localized at the nuclear periphery in all C. elegans non-muscle cells. On differentiation of muscle cells, the myo-3 promoter migrates to the nuclear interior where it actively transcribes the gene [37]. Expression of the Y45C lamin EDMD mutation (Y59C in C. elegans lamin) inhibits the migration of the myo-3 promoter to the nuclear interior in most body muscle cells. In correlation, the expression of the promoter is significantly lower and the animal shows muscle-specific phenotypes. Interestingly, the effect of this EDMD-linked mutation is muscle-specific even in C. elegans, in which the position and expression of an array of the gut-specific promoter pha-4 is not affected by this EDMD lamin mutation [38]. An example of a potentially different mechanism leading to EDMD comes from analysis of the ΔLys 32 EDMD mutation (ΔLys 46 in C. elegans). C. elegans lamin containing the ΔLys 46 mutation have a defective lamin assembly in vitro, and displace emerin and LEM2 from the nuclear periphery in vivo [39]. C. elegans lamin is also implied in muscle integrity, as several lamin EDMD mutations or loss of emerin plus LEM2 disrupt muscle attachment to the cuticle [39,40]. The third model is based on the fact that the muscle phenotypes are post-natal. According to this model, lamins are required for regulation of cell-type-specific gene expression during adult stem cell differentiation [41]. The pRb1–MyoD molecular pathway is perturbed in satellite muscle cells taken from emerin-null mice or from knockout mice expressing the LMNA gene lacking in exons 8–11 [42,43]. This leads to a reduction in MyoD levels, as well as a reduction in its downstream targets desmin and M-cadherin. Reduced levels of both phosphorylated and non-phosphorylated pRb1 were also observed in these satellite cells. In these EDMD models a disruption in cell-cycle exit and differentiation is also evident (Fig 3, purple arrows; [44,45]). It would be important to test these signalling pathways in mice, which are completely null for lamin A expression.
LMNA mutations can not only cause myofibre damage and degeneration of post-mitotic myofibres, but also directly compromise satellite cell performance, thus exacerbating myofibre damage and degeneration. They do so by compromising its regenerative response [46]. The specific contribution of each proposed mechanism to the different LMNA disease phenotypes remains to be determined. Lamin A and lamin C have an additional role in transcriptional activation during the early stages of muscle differentiation. During the initial stages of myoblast differentiation, prelamin A is found in euchromatic complexes with its binding partner LAP2α. The protein levels and cellular localization of prelamin A and LAP2α regulate the expression of caveolin 3, thus triggering early differentiation events. When the formation of this complex is compromised it probably contributes to the muscle phenotypes observed in these diseases [47].
A role for prelamin A and LAP2α is also suggested in the triggering of early differentiation events in the mouse myoblast cell line C2C12 (Fig 2, turquoise arrows). C2C12 cells expressing the EDMD lamin mutations R545C or R453W have an abnormally structured nuclear lamina. In addition, myogenic differentiation is inhibited at an early step due to a lack of myogenin, the level of active RNA Pol II is reduced and the distribution of the transcription repression chromatin marker H3K27 is abnormal. In the myoblast line C2C12, the persistent pool of hyperphosphorylated retinoblastoma protein (ppRb) enables elevated PCNA expression, which causes cells to continue to cycle and become hypersensitive to apoptosis induction (Fig 3, dark blue arrows; [48,49]). A similar persistence of ppRb is found in mice lacking emerin expression, which leads to an inhibition in the onset of myoblast differentiation. The muscle phenotypes of the emerin-null mice are less severe than those in mice with LMNA mutations. A possible explanation for this difference could be an upregulation of lamin A in the emerin-null mice, partly compensating for the loss of emerin, and thus leading to the amelioration of the muscle phenotypes. However, in the case of mice lacking LMNA, emerin cannot adequately compensate for the loss of lamin A and lamin C due to its dependence on A-type lamins for its proper localization to the nuclear envelope [50]. Emerin, lamin A and lamin C also influence cellular differentiation and nuclear remodelling by modulating the expression level and distribution of β-catenin, a central regulatory molecule of the Wnt signalling pathway (Fig 3, dark red arrows). Absence of emerin, a key β-catenin mediator, would limit plasticity and adipogenic potential of the cells by impairing the balance between β-catenin and PPARγ signalling [51].
Lamin, Hutchinson–Gilford progeria syndrome and ageing. Hutchinson–Gilford progeria syndrome (HGPS) is a premature ageing disease. Most HGPS patients carry a de novo heterozygous mutation in exon 11 of the LMNA gene, which causes accumulation of a truncated form of permanently farnesylated lamin A, termed ‘progerin’. HGPS patients have bone and skin abnormalities, impaired growth and cardiovascular problems that ultimately lead to death at about 13 years of age. HGPS cells have many phenotypes including distorted nuclear structure, loss of peripheral heterochromatin, impaired DNA damage response (DDR), shortened telomeres and early senescence. These abnormalities are also observed in cells lacking the lamin A-processing protease Zmpste24 suggesting that permanent farnesylation is a key player in the toxic effects of progerin [52,53,54]. It is worth noting that progerin is also detected in cells from normal ageing individuals, where it causes similar phenotypes, and thus could potentially contribute to the normal ageing process [55,56,57]. Despite these observations, the extent to which lamin A and lamin C contribute to cell senescence and ageing in the general population is unknown [58].
In HGPS fibroblasts, there is a decreased interaction of progerin with RBBP4/7 (Fig 2, light blue arrows). In turn this leads to depletion of the NURD complex, which affects heterochromatin formation, transcription regulation and the DDR. Dysfunctional chromatin and altered telomeres induce the activation of the p53/p21-dependent senescence programme (Fig 2, light blue arrows; [59,60,61]). Embryonic fibroblasts of mice deficient for Zmpste24 delay recruiting DDR proteins, which compromises DNA repair leading to defective homologous recombination and enhanced non-homologous end joining (Fig 2, green arrows). miR-29, a tumour suppressor miRNA, probably regulates the DDR in a p53-dependent fashion. Human cells with a depletion of lamin A and lamin C also show an increase in DNA double-strand breaks, which leads to activation of the cell-cycle checkpoint proteins ATM and ATR, and to high levels of Chk1 and Chk2 phosphorylation (Fig 2, green arrows; [62,63,64]).
One of the models for HGPS suggests that premature exhaustion of stem cells is the reason for the inhibition of tissue regeneration. This is especially true for tissues with an elevated degree of cell turnover. These tissues are likely to rapidly deplete their pool of stem cells [65]. In support of this model, expression of progerin in skin cells resulted in impaired function and depletion of epidermal stem cells, as well as downregulation of both the Notch and Wnt signalling pathways [66]. Downregulation of the Wnt signalling pathway and accumulation of prelamin A were also observed in the hair follicle stem cells of Zmpste24-deficient mice, leading to their reduced proliferation capacity [67]. Mice carrying the progeric mutation LMNAΔ9 show compromised extracellular matrix synthesis, which is caused by reduced function of LEF1 due to inhibition of the Wnt signalling pathway [68]. Progerin functions downstream from the Notch signalling pathway, as demonstrated by the deregulation of several downstream effectors from the Notch signalling pathway and the disruption of differentiation pathways in human MSCs expressing progerin. Indeed, MSCs give rise to many of the affected tissues in HGPS [69].
Autopsy- and biopsy-based research of HGPS patients is limited, due to the rarity of the disease and their death at an early age. Our understanding of the pathophysiology of this disease stems from patient-derived fibroblasts and progerin expression in cell lines. Animal models fail to mimic all of the symptoms seen in the human disease. The use of pluripotent HGPS cells (HGPS-iPSCs) can provide one way to overcome this problem. This unique model system for studying human premature ageing is based on the fact that HGPS-iPSCs are indistinguishable from normal iPSCs and human ESCs in many aspects. When the HGPS-iPSCs are differentiated to a mesodermal lineage they show a characteristic HGPS phenotype [70]. The diminished DNAPKcs/Ku80 protein expression in HGPS-iPSCs is also found in normal ageing cells, and thus can serve as a marker for both a normal and premature ageing process [70]. When HGPS-iPSCs are differentiated into vascular smooth muscle cells (VSMCs), the contractile properties of these cells are changed, probably due to an accumulation of cytoplasmic calponin 1 inclusion bodies (Fig 2, pink arrows). When subjected to repeated pulses of electrical stimulation, these HGPS-VSMCs also show increased senescence (Fig 2, blue arrows) and are hypersensitive to hypoxia [71].
There are more than 400 mutations described in the LMNA locus. The effect of these mutations on tissue development and maintenance is not restricted to bones, muscle cells or ageing. In mouse 3T3-L1 cells expressing the familial partial lipodystrophy mutations R482Q or R482W, for example, lipid accumulation is inhibited and the ability of these cells to differentiate to adipocytes is hampered [72]. One therapeutic approach that could be used to treat laminopathies is the use of helper-dependent adenoviral vectors (HDAdVs). These vectors allow the insertion of long homologous DNA regions that facilitate targeted integration through homologous recombination and maintain genetic and epigenetic cell profiles. Genetic correction of HGPS-iPSCs using this method eliminated the expression of progerin, restored the expression of wild-type lamin A, corrected the nuclear architecture and established a normal cell senescence programme [71]. Other potential therapeutic approaches towards treatment of HGPS include reactivating the Wnt signalling pathway, which is downregulated in HGPS, and inhibiting the JNK and ERK pathways in cardiomyopathy [66,73].
Lamins and stress
Lamins and mechanical strain. Lamins have a key role in protecting the nucleus from mechanical insults. This includes regulation of cell and nuclear shape and stiffness, and induction of mechanical-strain-induced transcription regulation. However, the importance of lamins in determining these properties varies between tissues [36]. Although mouse ESCs lacking all lamins had no apparent phenotype, LMNA-null mouse embryo fibroblasts showed nuclear deformation, reduced nuclear stiffness and defective mechanotransduction [23]. The viability of fibroblasts lacking lamin A and lamin C was significantly reduced under mechanical strain and the transcriptional response to mechanical stimuli was impaired, whilst the viability under unstrained conditions was similar to wild-type fibroblasts [74]. Mice that are heterozygous for LMNA—reduced lamin A and lamin C expression—show less resistance to mechanical stress and impaired induction of the mechanotransduction response [75]. Slightly different results were obtained in HGPS cells. In the presence of progerin, nuclei showed higher stiffness in late passages, but also reduced viability under mechanical strain [76].
Lamins and heat shock. When exposed to high temperatures, cells activate a heat shock response that includes post-translational modifications of specific proteins, changes in protein localization and function, as well as an increase in the translation of a group of proteins termed heat shock proteins (HSPs). Most HSPs have a role in the proper folding or unfolding of other proteins, specifically during stressful conditions [77]. The exact nature of the heat shock response varies not only between organisms, but also between tissues in an organism and depends on the severity of the heat shock [78]. When subjected to severe hyperthermia, human U-1 melanoma and HeLa CCL2 cells show an increase in lamin B expression, starting early after heat exposure and gradually increasing [79]. By contrast, HeLa S3 cells show no significant response during or shortly after heat shock. In these cells, lamin B1 protein levels decrease after 20 h of recovery [80]. Interestingly, there is no correlation between the lamin B1 expression levels and cell survival, suggesting that lamin B1 has a role in the recovery phase of the heat shock response [79,81]. Changes in protein levels after heat shock were also reported for emerin. Following a mild heat shock and a 12 h recovery period, emerin levels increased briefly and then decreased significantly [80]. By contrast, both the localization and expression levels of lamin A and lamin C, or the LAP2β protein, did not change [80].
αB-crystallin is a heat shock protein that is expressed in multiple tissues. Heat shock of the undifferentiated mouse C2C12 myoblast cells increases the expression levels of αB-crystallin and the protein translocates into the nucleus. In the nucleus, αB-crystallin and Hsp25, but not Hsp70, co-localize with nucleoplasmic lamin A, lamin C and the splicing factor SC-35. Speckles containing lamin A and lamin C help to organize splicing factors and RNA Pol II transcription. The interaction of lamin and αB-crystallin/Hsp25 might stabilize the internal architecture of the speckles. This interaction is developmentally specific, as it is detected in myoblasts but not in myotube nuclei [82]. It is worth noting that αB-crystallin also interacts with cytoplasmic intermediate filaments including desmin [83], and mutations in αB-crystallin can cause desmin-related myopathy [84]. Thus, small HSPs might have a general role in regulating intermediate-filament-related structures.
In summary, heat shock can cause multiple changes in the levels and composition of nuclear lamina proteins, as well as changes in protein complexes containing nuclear lamina proteins and HSPs. The roles of these heat-shock-induced changes in nuclear lamina organization and activity, and the cellular response to heat shock, require further investigation.
Lamins and DNA repair. Insults to DNA molecules, either due to metabolism or external factors, are common and must be addressed promptly to enable normal cell function and to prevent senescence or malignant transformations. Lamin A and lamin C have key roles in promoting both DNA repair and preventing DNA damage formation. These include helping to maintain the integrity of telomeres, stabilizing 53BP1, promoting non-homologous end joining (NHEJ) and preventing senescence. Specifically, loss of LMNA leads to mislocalization and shortening of telomeres, as well as alterations to the chromatin structure of the telomeres [85]. In turn, abnormal telomeres cause genomic instability, including an increase in telomeric signal-free ends and DNA double-strand breaks [86]. Loss of LMNA also prevents dysfunctional telomere maintenance by NHEJ through increased 53BP1 degradation [85,87]. Low levels of lamin A and lamin C probably contribute to tumour formation and progression, as they might lead to an abnormal DDR [88].
As mentioned above, the roles of lamin A and lamin C in genome stability are also observed in patients and in cell lines derived from patients. For example, cells expressing progerin have increased DNA damage and defective DNA repair [87,89,90]. Specifically, the recruitment of 53BP1 and RAD51 to DNA damage sites is impaired [87]. As progerin affects telomere organization, these phenotypes might be mediated by telomere-localized DNA damage signalling. In support of this hypothesis, overexpression of the catalytic subunit of telomerase can rescue these phenotypes [91].
Lamins and oxidative stress. Reactive oxygen species (ROS) are natural by-products of metabolism and are important in cell signalling and homeostasis. However, under stress, their amount might increase to potentially harmful levels that can jeopardize the cell. Several antioxidant enzymes, including glutathione transferase, catalase and glutathione peroxidase, are probably associated with the nuclear periphery, thus potentially preventing damaging agents from entering the nuclear interior [92]. Lamin B1 acts to sequester Oct1, and lack of lamin B1 leads to activation of several Oct1 target genes and to elevated ROS levels. Lamin B1 deficiency also makes cells more sensitive to extrinsic oxidative stress, a phenotype that can be rescued by Oct1 siRNA in mouse embryonic fibroblasts [93].
Lamin B1 was also shown to be involved in DNA damage signalling [94]. Ataxia telangiectasia is an autosomal-recessive genetic disorder, resulting from a mutation in the ATM gene, a kinase activated by DNA double-strand breaks. In ataxia telangiectasia cells, lamin B1 levels are elevated and the nuclear envelope becomes lobulated (Fig 4). This phenotype is also seen in cells from aged individuals or in cells expressing progerin and is generally associated with senescence. The role of lamin B1 was confirmed by overexpression in primary fibroblasts, where it induces senescence [95]. The high levels of lamin B1 probably result, at least in part, from the high ROS levels present in ataxia telangiectasia cells. Indeed, treating ataxia telangiectasia cells with the antioxidant N-acetyl-l-cysteine reduces the levels of lamin B1, whereas treating lymphoblasts and primary fibroblasts with pro-oxidants increases the amount of lamin B1. Interestingly, in these ataxia telangiectasia cells, the levels of lamin B1 are regulated by the p38 MAPK, in an ATM-independent manner [95].
Figure 4.
Impact on nuclear shape by oxidative stress or by an ataxia telangiectasia cell mutation. Upper panel: wild-type fibroblast not treated (NT) or treated with H2O2 stained with lamin B1 antibodies. Lower panel: wild-type and ataxia telangiectasia primary cells stained with lamin B1 antibodies. Arrows indicate examples of alterations in nuclear morphology. The data have been taken with permission from figures 2 and 5 in [95].
Cells lacking lamin A also have elevated ROS levels. Lamin A has an additional role in the DDR by serving as a sensor of oxidative damage in the nucleus. The cysteine residues in the C-terminal tail domain of lamin A are damaged by oxidative stress, and under low oxidative stress conditions it could act as a sink to protect less abundant proteins, as well as lamin-binding proteins, from oxidative damage. However, oxidized lamin A also promotes cellular senescence, presumably by changing its interactions with key pathways such as the Rb–LAP2α pathway or the ERK–fos pathway [96].
Conclusions and future perspectives
Nuclear lamins are essential components of the nuclear architecture and are important in development and differentiation. Lamins are also required for the response to internal and external stress conditions. Studies in mammalian cells, mouse models and C. elegans provide important new insights into the mechanisms by which the lamin-based response to signals that drive development and differentiation are governed, and into lamin-mediated protection of cells from different stress conditions.
Many interesting properties of lamins remain to be determined (Sidebar A). These include understanding the assemblies of B-type and A-type lamins in vivo both in the cell periphery and in the nucleoplasm, as well as elucidating the roles of lamins in metabolism, the DDR, Pol II transcription and regulating euchromatin–heterochromatin transition during differentiation and ageing.
Sidebar A | In need of answers.
What is the composition of lamin complexes during development, in different cell types and under stress conditions?
How do lamins regulate chromatin modifications and promote gene silencing?
Why do mutations in lamin A and lamin C residues cause more then 14 heritable diseases with variable pathologies?
How do the A-type and B-type lamin networks interact with each other?

Noam Zuela

Daniel Z Bar

Yosef Gruenbaum
Acknowledgments
We acknowledge funding from the Morasha Legacy 1798/10, The Muscular Dystrophy Association (MDA), Israel Ministry of Health (2965), the Israeli Science Foundation, the Binational Israel–USA Science Foundation (2007215) and the COST NANONET (BM1002).
Footnotes
The authors declare that they have no conflict of interest.
References
- Stuurman N, Heins S, Aebi U (1998) Nuclear lamins: their structure, assembly, and interactions. J Struct Biol 122: 42–66 [DOI] [PubMed] [Google Scholar]
- Aebi U, Cohn J, Buhle L, Gerace L (1986) The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323: 560–564 [DOI] [PubMed] [Google Scholar]
- Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R (2008) Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci 121: 215–225 [DOI] [PubMed] [Google Scholar]
- Heitlinger E, Peter M, Haner M, Lustig A, Aebi U, Nigg EA (1991) Expression of chicken lamin B2 in Escherichia coli: characterization of its structure, assembly, and molecular interactions. J Cell Biol 113: 485–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Harush K, Wiesel N, Frenkiel-Krispin D, Moeller D, Soreq E, Aebi U, Herrmann H, Gruenbaum Y, Medalia O (2009) The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol 386: 1392–1402 [DOI] [PubMed] [Google Scholar]
- Broers JL, Machiels BM, Kuijpers HJ, Smedts F, van den Kieboom R, Raymond Y, Ramaekers FC (1997) A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol 107: 505–517 [DOI] [PubMed] [Google Scholar]
- Rober RA, Gieseler RK, Peters JH, Weber K, Osborn M (1990) Induction of nuclear lamins A/C in macrophages in in vitro cultures of rat bone marrow precursor cells and human blood monocytes, and in macrophages elicited in vivo by thioglycollate stimulation. Exp Cell Res 190: 185–194 [DOI] [PubMed] [Google Scholar]
- Stewart C, Burke B (1987) Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell 51: 383–392 [DOI] [PubMed] [Google Scholar]
- Weber K, Plessmann U, Traub P (1989) Maturation of nuclear lamin A involves a specific carboxy-terminal trimming, which removes the polyisoprenylation site from the precursor; implications for the structure of the nuclear lamina. FEBS Lett 257: 411–414 [DOI] [PubMed] [Google Scholar]
- Shimi T et al. (2008) The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 22: 3409–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimi T et al. (2011) The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 25: 2579–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre E, Tramier M, Coppey-Moisan M, Gaillard C, Courvalin JC, Buendia B (2006) The truncated prelamin A in Hutchinson–Gilford progeria syndrome alters segregation of A-type and B-type lamin homopolymers. Hum Mol Genet 15: 1113–1122 [DOI] [PubMed] [Google Scholar]
- Kapinos LE, Schumacher J, Mücke N, Machaidze G, Burkhard P, Aebi U, Strelkov SV, Herrmann H (2010) Characterization of the head-to-tail overlap complexes formed by human lamin A, B1 and B2 ‘half-minilamin’ dimers. J Mol Biol 396: 719–731 [DOI] [PubMed] [Google Scholar]
- Schirmer EC, Guan T, Gerace L (2001) Involvement of the lamin rod domain in heterotypic lamin interactions important for nuclear organization. J Cell Biol 153: 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar DZ, Barkan R, Meshorer E, Gruenbaum Y (2009) Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med 13: 1059–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer TA, Misteli T (2011) The lamin protein family. Genome Biol 12: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K (2001) Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci 114: 4557–4565 [DOI] [PubMed] [Google Scholar]
- Liu J, Rolef-Ben Shahar T, Riemer D, Spann P, Treinin M, Weber K, Fire A, Gruenbaum Y (2000) Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell 11: 3937–3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit A, Liu J, Fridkin A, Wilson KL, Gruenbaum Y (2005) A lamin-dependent pathway that regulates nuclear organization, cell cycle progression and germ cell development. Novartis Found Symp 264: 231–240 [PubMed] [Google Scholar]
- Cohen M, Tzur YB, Neufeld E, Feinstein N, Delannoy MR, Wilson KL, Gruenbaum Y (2002) Transmission electron microscope studies of the nuclear envelope in Caenorhabditis elegans embryos. J Struct Biol 140: 232–240 [DOI] [PubMed] [Google Scholar]
- Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptacek LJ, Fu YH (2006) Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet 38: 1114–1123 [DOI] [PubMed] [Google Scholar]
- Coffinier C et al. (2011) Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell 22: 4683–4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y (2011) Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 334: 1706–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, de Jong PJ, Fong LG, Young SG (2011) An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet 20: 3537–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osouda S, Nakamura Y, de Saint Phalle B, McConnell M, Horigome T, Sugiyama S, Fisher PA, Furukawa K (2005) Null mutants of Drosophila B-type lamin Dm0 show aberrant tissue differentiation rather than obvious nuclear shape distortion or specific defects during cell proliferation. Dev Biol 284: 219–232 [DOI] [PubMed] [Google Scholar]
- Yoon BC, Jung H, Dwivedy A, O'Hare CM, Zivraj KH, Holt CE (2012) Local translation of extranuclear lamin B promotes axon maintenance. Cell 148: 752–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori Y, Tamura Y, Kataoka Y, Cui Y, Seo S, Kanazawa T, Kurokawa K, Yamada H (2007) Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur J Neurosci 25: 1653–1662 [DOI] [PubMed] [Google Scholar]
- Jung HJ et al. (2012) Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc Natl Acad Sci USA 109: E423–E431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Yeo LS, Vidal C, McCorquodale T, Herrmann M, Fatkin D, Duque G (2011) Decreased bone formation and osteopenia in lamin A/C-deficient mice. PLoS ONE 25: e19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter R, Rivas D, Geneau G, Drissi H, Duque G (2009) Effect of lamin A/C knockdown on osteoblast differentiation and function. J Bone Miner Res 24: 283–293 [DOI] [PubMed] [Google Scholar]
- Duque G, Troen BR (2008) Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J Am Geriatr Soc 56: 935–941 [DOI] [PubMed] [Google Scholar]
- Bertrand AT, Chikhaoui K, Yaou RB, Bonne G (2011) Clinical and genetic heterogeneity in laminopathies. Biochem Soc Trans 39: 1687–1692 [DOI] [PubMed] [Google Scholar]
- Naetar N et al. (2008) Loss of nucleoplasmic LAP2alpha-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol 10: 1341–1348 [DOI] [PubMed] [Google Scholar]
- Morris GE (2001) The role of the nuclear envelope in Emery–Dreifuss muscular dystrophy. Trends Mol Med 7: 572–577 [DOI] [PubMed] [Google Scholar]
- Huber MD, Guan T, Gerace L (2009) Overlapping functions of nuclear envelope proteins NET25 (Lem2) and emerin in regulation of extracellular signal-regulated kinase signaling in myoblast differentiation. Mol Cell Biol 29: 5718–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerger M, Ho CY, Lammerding J (2011) Nuclear mechanics in disease. Annu Rev Biomed Eng 13: 397–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister P, Towbin BD, Pike BL, Ponti A, Gasser SM (2010) The spatial dynamics of tissue-specific promoters during C. elegans development. Genes Dev 24: 766–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout A, Pike BL, Towbin BD, Bank EM, Gonzalez-Sandoval A, Stadler MB, Meister P, Gruenbaum Y, Gasser S (2011) An EDMD mutation in C. elegans lamin blocks muscle-specific gene relocation and compromises muscle Integrity. Curr Biol 21: 1603–1614 [DOI] [PubMed] [Google Scholar]
- Bank EM, Ben-Harush K, Wiesel-Motiuk N, Barkan R, Feinstein N, Lotan O, Medalia O, Gruenbaum Y (2011) A laminopathic mutation disrupting lamin filament assembly causes disease-like phenotypes in Caenorhabditis elegans. Mol Biol Cell 22: 2716–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank EM, Ben-Harush K, Feinstein N, Medalia O, Gruenbaum Y (2011) Structural and physiological phenotypes of disease-linked lamin mutations in C. elegans. J Struct Biol 177: 106–112 [DOI] [PubMed] [Google Scholar]
- Gotzmann J, Foisner R (2006) A-type lamin complexes and regenerative potential: a step towards understanding laminopathic diseases? Histochem Cell Biol 125: 33–41 [DOI] [PubMed] [Google Scholar]
- Sullivan T, Escalente-Alcalde D, Bhatt H, Anver M, Naryan B, Nagashima K, Stewart CL, Burke B (1999) Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 147: 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn D, Schramm S, Schnölzer M, Heilmann CJ, de Koster CG, Schütz W, Benavente R, Alsheimer M (2012) A truncated lamin A in the Lmna−/− mouse line: implications for the understanding of laminopathies. Nucleus 3: 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner D, Vlcek S, Foeger N, Gajewski A, Makolm C, Gotzmann J, Hutchison CJ, Foisner R (2006) Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma–E2F pathway. J Cell Biol 173: 83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boban M, Braun J, Foisner R (2010) Lamins: ‘structure goes cycling’. Biochem Soc Trans 38: 301–306 [DOI] [PubMed] [Google Scholar]
- Gnocchi VF, Ellis JA, Zammit PS (2008) Does satellite cell dysfunction contribute to disease progression in Emery-Dreifuss muscular dystrophy? Biochem Soc Trans 36: 1344–1349 [DOI] [PubMed] [Google Scholar]
- Capanni C et al. (2008) Prelamin A is involved in early steps of muscle differentiation. Exp Cell Res 314: 3628–3637 [DOI] [PubMed] [Google Scholar]
- Kandert S, Wehnert M, Müller CR, Buendia B, Dabauvalle MC (2009) Impaired nuclear functions lead to increased senescence and inefficient differentiation in human myoblasts with a dominant p.R545C mutation in the LMNA gene. Eur J Cell Biol 88: 593–608 [DOI] [PubMed] [Google Scholar]
- Favreau C, Higuet D, Courvalin JC, Buendia B (2004) Expression of a mutant lamin A that causes Emery-Dreifuss muscular dystrophy inhibits in vitro differentiation of C2C12 myoblasts. Mol Biol Cell 24: 1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcon G et al. (2006) Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet 15: 637–651 [DOI] [PubMed] [Google Scholar]
- Tilgner K, Wojciechowicz K, Jahoda C, Hutchison C, Markiewicz E (2009) Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of beta-catenin. J Cell Sci 122: 401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RD et al. (2004) Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. Proc Natl Acad Sci USA 101: 8963–8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T (2005) Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nat Med 11: 440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS (2011) Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest 121: 2833–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, Djabali K (2007) The mutant form of lamin A that causes Hutchinson–Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE 2: e1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimen P et al. (2009) A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci USA 106: 20788–20793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bökenkamp R, Raz V, Venema A, DeRuiter MC, van Munsteren C, Olive M, Nabel EG, Gittenberger-de Groot AC (2011) Differential temporal and spatial progerin expression during closure of the ductus arteriosus in neonates. PLoS ONE 6: e23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ (2006) Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev 86: 967–1008 [DOI] [PubMed] [Google Scholar]
- Meshorer E, Gruenbaum Y (2009) NURD keeps chromatin young. Nat Cell Biol 11: 1176–1177 [DOI] [PubMed] [Google Scholar]
- Moiseeva O, Bourdeau V, Vernier M, Dabauvalle MC, Ferbeyre G (2011) Retinoblastoma-independent regulation of cell proliferation and senescence by the p53–p21 axis in lamin A/C-depleted cells. Aging Cell 10: 789–797 [DOI] [PubMed] [Google Scholar]
- Pegoraro G, Kubben N, Wickert U, Göhler H, Hoffmann K, TM (2009) Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol 11: 1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B et al. (2005) Genomic instability in laminopathy-based premature aging. Nat Med 11: 780–785 [DOI] [PubMed] [Google Scholar]
- Osorio FG, Ugalde AP, Mariño G, Puente XS, Freije JM, López-Otín C (2011) Cell autonomous and systemic factors in progeria development. Biochem Soc Trans 39: 1710–1714 [DOI] [PubMed] [Google Scholar]
- Musich PR, Zou Y (2009) Genomic instability and DNA damage responses in progeria arising from defective maturation of prelamin A. Aging (Albany NY) 1: 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzmann J, Foisner R (2005) A-type lamin complexes and regenerative potential: a step towards understanding laminopathic diseases? Histochem Cell Biol 125: 33–41 [DOI] [PubMed] [Google Scholar]
- Rosengardten Y, McKenna T, Grochová D, Eriksson M (2011) Stem cell depletion in Hutchinson–Gilford progeria syndrome. Aging Cell 10: 1011–1020 [DOI] [PubMed] [Google Scholar]
- Espada J et al. (2008) Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J Cell Biol 181: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L et al. (2010) Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev Cell 19: 413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RC (2006) Hutchinson–Gilford progeria syndrome: review of the phenotype. Am J Med Genet A 140: 2603–2624 [DOI] [PubMed] [Google Scholar]
- Liu GH et al. (2011) Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 472: 221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J et al. (2011) A human iPSC model of Hutchinson–Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8: 31–35 [DOI] [PubMed] [Google Scholar]
- Boguslavsky RL, Stewart CL, Worman HJ (2006) Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 15: 653–663 [DOI] [PubMed] [Google Scholar]
- Wu W, Muchir A, Shan J, Bonne G HJW (2011) Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene. Circulation 123: 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RS (2004) Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 113: 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupesi M, Yoshioka J, Gannon J, Kudinova A, Stewart CL, Lammerding J (2010) Attenuated hypertrophic response to pressure overload in a lamin A/C haploinsufficiency mouse. J Mol Cell Cardiol 48: 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J (2008) Increased mechanosensitivity and nuclear stiffness in Hutchinson–Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell 7: 383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger MJ (1994) How the cell copes with stress and the function of heat shock proteins. Pediatr Res 36: 1–6 [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Calderwood SK (2005) Regulation of heat shock gene transcription in neuronal cells. Int J Hyperthermia 21: 433–444 [DOI] [PubMed] [Google Scholar]
- Dynlacht JR, Story MD, Zhu WG, Danner J (1999) Lamin B is a prompt heat shock protein. J Cell Physiol 178: 28–34 [DOI] [PubMed] [Google Scholar]
- Haddad N, Paulin-Levasseur M (2008) Effects of heat shock on the distribution and expression levels of nuclear proteins in HeLa S3 cells. J Cell Biochem 105: 1485–1500 [DOI] [PubMed] [Google Scholar]
- Malhas AN, Vaux DJ (2011) The nuclear envelope and its involvement in cellular stress responses. Biochem Soc Trans 39: 1795–1798 [DOI] [PubMed] [Google Scholar]
- Adhikari AS, Sridhar Rao K, Rangaraj N, Parnaik VK, Mohan Rao C (2004) Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell Res 299: 393–403 [DOI] [PubMed] [Google Scholar]
- Houck SA, Landsbury A, Clark JI, Quinlan RA (2011) Multiple sites in αB-crystallin modulate its interactions with desmin filaments assembled in vitro. PLoS ONE 6: e25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicart P et al. (1998) A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20: 92–95 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez I et al. (2009) Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J 28: 2414–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller SP (2006) Dysfunction of lamin A triggers a DNA damage response and cellular senescence. DNA Repair (Amst) 5: 286–289 [DOI] [PubMed] [Google Scholar]
- Liu B et al. (2005) Genomic instability in laminopathy-based premature aging. Nat Med 11: 780–785 [DOI] [PubMed] [Google Scholar]
- Prokocimer M, Margalit A, Gruenbaum Y (2006) The nuclear lamina and its proposed roles in tumorigenesis: projection on the hematologic malignancies and future targeted therapy. J Struct Biol 155: 351–360 [DOI] [PubMed] [Google Scholar]
- Hutchison CJ (2011) The role of DNA damage in laminopathy progeroid syndromes. Biochem Soc Trans 39: 1715–1718 [DOI] [PubMed] [Google Scholar]
- Liu B, Zhou Z (2008) Lamin A/C, laminopathies and premature ageing. Histol Histopathol 23: 747–763 [DOI] [PubMed] [Google Scholar]
- Benson EK, Lee SW, Aaronson SA (2010) Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci 123: 2605–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrini R et al. (2010) Nuclear shield: a multi-enzyme task-force for nucleus protection. PLoS ONE 5: e14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhas AN, Lee CF, Vaux DJ (2009) Lamin B1 controls oxidative stress responses via Oct-1. J Cell Biol 184: 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CJ (2012) B-type lamins and their elusive roles in metazoan cell proliferation and senescence. EMBO J 31: 1058–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P (2012) Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J 31: 1080–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, Wenhert M, von Zglinicki T, Hutchison CJ (2011) Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell 10: 1067–1079 [DOI] [PubMed] [Google Scholar]