Abstract
With the steady rise in the prevalence of obesity and its associated diseases, research aimed at understanding the mechanisms that regulate and control whole body energy homeostasis has gained new interest. Leptin and insulin, two anorectic hormones, have key roles in the regulation of body weight and energy homeostasis, as highlighted by the fact that several obese patients develop resistance to these hormones. Within the brain, the hypothalamic proopiomelanocortin and agouti-related protein neurons have been identified as major targets of leptin and insulin action. Many studies have attempted to discern the individual contributions of various components of the principal pathways that mediate the central effects of leptin and insulin. The aim of this review is to discuss the latest findings that might shed light on, and lead to a better understanding of, energy balance and glucose homeostasis. In addition, recently discovered targets and mechanisms that mediate hormonal action in the brain are highlighted.
Keywords: AgRP, FoxO1, insulin, leptin, POMC
See Glossary for abbreviations used in this article.
Glossary.
- AgRP
agouti-related protein
- AICAR
5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- AMPK
AMP-dependent kinase
- Arc
arcuate nucleus
- CART
cocaine-amphetamine-regulated transcript
- CNS
central nervous system
- FoxO1
Forkhead box protein O1
- HIF
hypoxia-inducible factor
- InsR
insulin receptor
- HGP
hepatic glucose production
- LepR-b
leptin receptor b
- mTOR
mammalian target of rapamycin
- NIR
neuron-specific insulin receptor
- NPY
neuropeptide Y
- PDK1
3-phosphoinositide-dependent protein kinase 1
- PI3K
phosphatidylinositol-3-kinase
- PIP2
phosphatidylinositol-4,5-biphospate
- PIP3
phosphatidylinositol-3,4,5-triphosphate
- POMC
proopiomelanocortin
- S6K
p70 S6 kinase
- shRNA
short hairpin RNA
- STAT3
signal transducer and activator of transcription 3
- SOCS3
suppressor of cytokine signalling 3
- WAT
white adipose tissue
Introduction
Although obesity has only recently become one of the more important health problems of developed societies, it has been studied for centuries. Obesity is no longer looked at as just a major health problem but is considered an economic predicament as well—placing an enormous financial burden on society for the care and treatment of patients. This metabolic state and its associated illnesses—for example, diabetes mellitus, dislipidaemia and hypertension—have increased healthcare costs beyond sustainable levels. It has been calculated that over the next 20 years, the healthcare costs attributable to obesity will rise to about 16% of the total of those costs in Western countries [1,2].
Early attempts by researchers to understand the biology and pathology of obesity has revealed several brain areas and factors involved in the regulation of food intake and body weight. These studies found that the CNS, mainly the hypothalamus, has a pivotal role in integrating signals from peripheral tissues—such as the pancreas, white adipose tissue and the gut—to regulate energy homeostasis (Fig 1). Secretions from these tissues act as peripheral signals that relay information regarding the energy status of the organism to the CNS. Two of the most well-known and studied peripheral signals are leptin and insulin, the expression levels of which correlate with total body fat mass [3]. In states of energy deficiency the blood levels of these two hormones are lower, whereas in states of energy surplus they are increased.
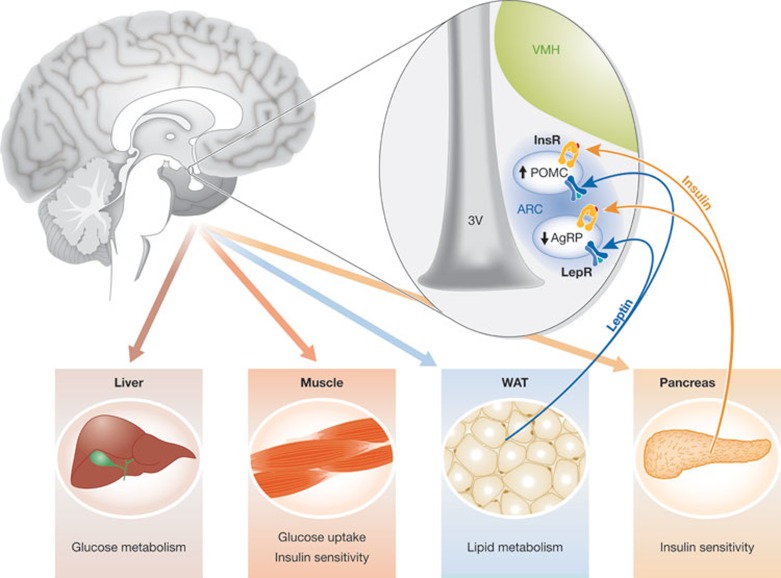
Figure 1.
Hypothalamic leptin and insulin signals regulate several peripheral functions. Leptin and insulin secreted by white adipose tissue (WAT) and the pancreas, respectively, inform the hypothalamus about the energy status of the organism. When LepR-b and InsR are activated by these hormones, they promote changes in hypothalamic neuropeptide expression, which cause alterations in peripheral functions to restore energy balance and glucose metabolism. Arc, arcuate nucleus; AgRP, agouti-related protein; InsR, insulin receptor; LepR-b, leptin receptor b; POMC, proopiomelanocortin; VMH, ventromedial hypothalamus.
From earlier publications reporting that pituitary adenomas cause obesity [4,5], up to studies that have used more innovative methods and new techniques, the hypothalamus has been identified as the main regulator of feeding. Efforts to understand the molecular basis of obesity focused on the hypothalamus and its modulation by peripheral signals. The first clues about the importance of the hypothalamus in the control of feeding came from studies showing that lesions in some of its areas cause alterations in food intake and body weight [6,7,8,9]. The hypothalamus, located in the mediobasal part of the brain, is organized into well-structured nuclei (ventromedial (VMH), paraventricular (PVH) and lateral (LHA)). Within each nucleus, neuronal populations are characterized by their expression of neuropeptides, which are involved in the control of food intake. Thus, it has been observed that lesions in the VMH are associated with hyperphagia and obesity [8], whereas lesions in the LHA cause hypophagia and a decrease in body weight [7]. Within the basal part of the hypothalamus, the Arc contains a special modification in the blood–brain barrier to allow for the entry of nutrients, hormones and other molecules from the blood. Its ‘privileged’ location allows it to be the first sensor of peripheral signals [10]. As a result of this, it has been postulated that the Arc has a fundamental role in sensing the global energy status of the organism [10]. Within the Arc are two neuronal populations each characterized by the expression of specific neuropeptides, which have potent effects on energy homeostasis (Fig 1; [11]). One of these populations consists of the POMC and CART neurons, which provide a strong anorexigenic effect—secretion of the POMC and CART neuropeptides from these neurons decreases food intake and body weight. By contrast, the second population of AgRP and NPY neurons has a potent orexigenic effect—release of AgRP and NPY increases food intake. Thus, in a situation of negative energy balance, for example fasting, the expression of AgRP is increased and POMC expression is decreased. However, during a state of energy surplus, AgRP levels are diminished and POMC levels are elevated. Both hormones are also important regulators of peripheral metabolism; in fact, a report shows the importance of intact AgRP signalling in adult mice to maintain normal lipid and glucose homeostasis in peripheral tissues, such as the liver, muscles and the pancreas (Fig 1; [12,13]).
In the following sections, this review discusses insights into the leptin and insulin signalling pathways in the POMC and AgRP neurons that mediate the effects of the hormones on glucose and energy metabolism. In addition, the impact of new players, such as mTOR and AMPK, and new mechanisms, such as autophagy in the POMC and AgRP neurons, and their effect on energy homeostasis is highlighted.
Leptin and insulin signalling pathways
Leptin, an adipokine, is secreted by the WAT in positive relation to the total amount of body fat. Insulin is secreted by the pancreatic β-cells dependent on the blood glucose level in the short term and on the level of adiposity in the long term. Both peripheral signals present a strong anorexigenic effect [3]. Central administration of these molecules, which mimics a state of energy surplus, inhibits food intake and decreases body weight [14]. Furthermore, their central actions have a potent impact by regulating glucose homeostasis and lipid metabolism. The actions of leptin and insulin are mediated by the LepR-b and InsR, respectively, which are expressed in several areas of the CNS including the hypothalamus. Interestingly, LepR-b and InsR expression is high in the AgRP and POMC neurons of the Arc [15,16,17,18]. Given the role of these neuronal populations and the demonstration that they express high levels of LepR-b and InsR, it was proposed some years ago that the leptin and insulin signalling pathways in the POMC and AgRP neurons of the Arc are highly significant in the regulation of food intake, body weight and glucose homeostasis.
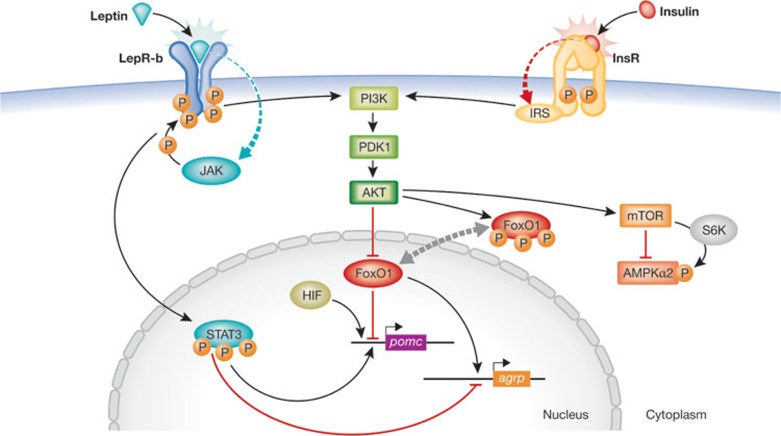
Leptin activates different signalling cascades. When this hormone binds to the extracellular domain of LepR-b, it recruits and activates the Janus kinase (JAK). JAK binds to and phosphorylates LepR-b [19], which activates STAT3. Once phosphorylated, STAT3 binds to pomc and agrp promoters, stimulating POMC expression and inhibiting AgRP [20,21]. The most studied targets of leptin action are the proteins involved in the PI3K signalling pathway. Leptin activates PI3K, which induces the synthesis of PIP3 from PIP2 [22,23,24]. Accumulation of PIP3 leads to PDK1 activation and thus activates protein kinase B (PKB, also known as AKT). Interestingly, the insulin signalling pathway, after insulin binds to the InsR and activates the insulin receptor substrate (IRS), converges with the leptin pathway at the same point, the activation of PI3K, to modulate body weight and glucose homeostasis [17,25]. AKT, one of the PDK1 downstream targets, has an important role in the regulation and activation of many proteins and transcription factors, among which are FoxO1 [26,27], AMPK [28,29] and mTOR [30]. FoxO1 acts as an inhibitor of POMC expression [31]. When leptin and insulin signalling leads to the phosphorylation of FoxO1, it is exported from the nucleus, thus allowing for the binding of STAT3 to the pomc promoter to stimulate its expression. In AgRP neurons, the nuclear export of FoxO1 abrogates agrp expression because STAT3 is able to bind to the agrp promoter and inhibit its expression (Fig 2; [32]).
Figure 2.
Leptin and insulin signalling pathways in the Arc. Signal transducer and activator of transcription 3 (STAT3) is activated and phosphorylated when leptin binds to LepR-b. p-STAT3 binds to pomc and agrp promoters, stimulating pomc expression and inhibiting agrp. Leptin and insulin signalling pathways converge on PI3K. Activation of PI3K leads to phosphorylation and inactivation of FoxO1, a repressor of pomc expression. Phosporylation of FoxO1 provokes its nuclear export and allows STAT3 to bind to pomc and agrp promoters. It has been reported that inhibition of AMPK by leptin is mediated by mTOR [35]. AgRP, agouti-related protein; AKT, protein kinase B; AMPK, AMP-dependent kinase; FoxO1, Forkhead box protein O1; HIF, hypoxia-inducible factor; InsR, insulin receptor; IRS, insulin receptor substrate; JAK, Janus kinase; LepR-b, leptin receptor b; mTOR, mammalian target of rapamycin; PDK1, 3-phosphoinositide-dependent protein kinase 1; PI3K, phosphatidylinositol-3-kinase; POMC, proopiomelanocortin,; S6K,; STAT3, signal transducer and activator of transcription 3.
Leptin and insulin also regulate the AMPK signalling pathway in the hypothalamus [29]. Proteins involved in this pathway sense the global energy status and are activated by an energy deficiency [28,33]. Leptin and insulin, signals of energy surplus, inhibit the activation of AMPK and its downstream targets. The mTOR pathway is also important in the hypothalamic regulation of food intake, sensing nutrient availability and energy status [30,34]. In contrast to AMPK, mTOR expression is higher in situations of energy surplus; in fact it has been reported that intracerebroventricular (ICV) leptin administration increases mTOR expression [30]. mTOR is important as a mediator of leptin action in the hypothalamus: it has been demonstrated that an intact mTOR signalling pathway is necessary to maintain the anorexigenic effects of leptin [30]. A report also showed that mTOR mediates leptin-induced inhibition of the AMPK pathway [35]. Leptin-mediated activation of mTOR/S6K promotes the phosphorylation and inhibition of AMPK, and thereby regulates feeding behaviour (Fig 2; [35]). This new mechanism accentuates the importance of the mTOR pathway in mediating the effects of leptin. Furthermore, the role of mTOR in insulin signalling has been described. Insulin activates the mTOR pathway in the hypothalamus and mTOR/S6K, in turn, regulates the insulin-mediated suppression of HGP [36].
The single components of the insulin and leptin signalling pathways in the POMC and AgRP neurons, and their effects on glucose and energy homeostasis, are discussed in more detail later in this review.
Leptin in POMC and AgRP neurons
For several years, it has been known that leptin and insulin are important in the regulation of energy homeostasis [37]. It is expected that obese patients would present with lower serum leptin levels but, surprisingly, the opposite is true—high levels are expressed. This is believed to be the consequence of so-called leptin resistance, a state in which leptin signalling is impaired [38,39]. In mice, mutations of leptin (Lepob/ob) or LepR (Lepdb/db) are associated with increased body weight and impaired glucose homeostasis—for example, hyperglycaemia, hyperinsulinaemia, insulin resistance and impaired hepatic glucose production [40,41,42]. It has been reported that ICV leptin injections in these mice reduce body weight and food intake, and ameliorate glucose metabolism [14]. These findings, together with the evidence that the Arc neurons are important in the regulation of energy balance, focused attention on the POMC and AgRP neurons as the main targets of leptin action. It has been reported that leptin action depolarizes (activates) POMC [16] and hyperpolarizes (inhibits) AgRP neurons [43].
There were many attempts to investigate the exact mechanism by which these hormones act in the hypothalamus. Following the discovery and use of Cre–lox technology, the mechanisms of action of both hormones have become better understood, but are still unclear. The deletion or overexpression of LepR in POMC or AgRP neurons causes changes in body weight, food intake and glucose homeostasis [44,45,46]. Thus, the importance of POMC in leptin signalling has been intensely studied. It is well known that POMC neurons are essential in mediating leptin actions in the brain. Several studies have shown that deletion of LepR in these neurons promotes obesity, without changes in food intake or energy expenditure [44,45,46,47], and that overexpression of this receptor in POMC neurons of LepR-null mice partly rescues the obesity and hyperphagic phenotype characteristic of this mouse strain [46,48]. Despite this, there are controversies about the role of POMC in leptin signalling. Two reports have shown two reasons for the decrease in body weight seen in LepR-null mice that re-express LepR in POMC neurons. One report states that it is due to diminished food intake [46], whereas the other attributes it to increased energy expenditure [48]. In the latter, the authors postulated that this discordance could be due to LepR expression in subsets of POMC neurons. The mice that were used in the first study [46] overexpressed LepR in all POMC neurons, although in the study by Berglund et al [48] the reintroduction of LepR was restricted only to the subset of POMC neurons that originally express this receptor, and to the effects of leptin outside of the Arc that are important for the regulation of food intake. In both animal models, re-expression of LepR improved glucose levels and insulin sensitivity independently of body weight, indicating that leptin signalling in POMC neurons has a key role in regulating glucose homoeostasis [47,48]. The effects of leptin on AgRP neurons are not as clear. In these neurons, the deletion of LepR is associated with an increase in total body weight and adiposity [49]. AgRP and POMC are both involved in the maintenance of energy balance by leptin signalling. Mice with ablation of LepR in both the POMC and AgRP neurons present more severe obesity [49]. It has been postulated that these subsets of neurons work in a synergistic way to respond to leptin [49].
Insulin in POMC and AgRP neurons
Insulin, as with leptin, has been demonstrated to be an important regulator of several actions through the CNS [50,51,52,53]. It has been demonstrated that central insulin administration regulates feeding behaviour. Since the first evidence demonstrating that brain-specific deletion of InsR (NIR knockout mice) is associated with diet-sensitive obesity and increased food intake by female mice [51], many reports have tried to identify the neurons and mechanism by which this hormone affects the brain. In NIR knockout mice, obesity is related to high leptin levels [51], demonstrating that central leptin actions require intact insulin signalling in the CNS. Furthermore, central insulin is essential for the regulation of proper glucose homeostasis. As in the case of leptin, POMC and AgRP neurons are supposed to be crucial targets of insulin signalling. It has been shown that insulin acts on these subsets of neurons, as shown by the fact that its central administration provokes hyperpolarization of both POMC and AgRP neurons in the Arc [17,29,45,54]. Curiously, unlike leptin, the selective ablation of InsR in POMC or AgRP neurons has no effect on body weight or food intake [17]. However, transgenic deletions of InsR in POMC and AgRP neurons have an impact on glucose metabolism. Specifically, similarly to pharmacological blockade of insulin signalling, mice with an ablation of the InsR in AgRP—but not in POMC—neurons have impaired suppression of HGP [17], demonstrating the significance of insulin action on these neurons in the maintenance of glucose homeostasis. To investigate further the role of Arc neurons in mediating the effects of insulin, one study has analysed the effects of InsR re-expression in AgRP and POMC neurons in mice lacking InsR expression in the Arc [18]. In accordance with previous reports, these InsR knockout mice had potent suppression in HGP only when InsR is re-expressed in the AgRP neurons [18]. By contrast, re-expression in the POMC neurons increased HGP, and these mice also show increased food intake and locomotor activity compared with the InsR knockout mice [18]. Contrary to other reports, POMC neurons thus seem to have a role in energy homeostasis through insulin signalling. Taken together, these results demonstrate that AgRP and POMC neurons mediate insulin effects on glucose homeostasis. Other neuronal populations, for example, in hypothalamic nuclei—VMH was described as a regulator of food intake—are also probably involved in the effects of insulin on the regulation of body weight and food intake.
Mediators of leptin and insulin actions
When the Arc POMC and AgRP neurons were identified as major targets that mediate central leptin and insulin actions, several reports have tried to understand the exact mechanisms by which theses hormones modulate energy and glucose homeostasis. As stated above, leptin and insulin are involved in the activation of signalling pathways (Fig 2). Genetic modifications in these target genes have provided some ideas for how both hormones are able to trigger responses by the POMC and AgRP neurons. Since the finding that STAT3 deletion in the CNS induces an obese phenotype associated with lower mRNA levels of pomc, this gene has been largely studied as an important factor involved in leptin signalling [55,56]. Various work in which STAT3 was deleted or overexpressed in Arc neurons demonstrated a clear role of this gene in this mechanism [20,21,57]. In POMC neurons, it has been described that STAT3 activation causes mild obesity associated with increased food intake and lower pomc mRNA levels [21]. Furthermore, this animal model has an impaired leptin response. Peripheral leptin administration was unable to reproduce the expected effects on body weight and food intake seen in control mice—an observation that reinforces the importance of STAT3 signalling in POMC neurons mediating leptin effects [21,57]. In addition to this leptin resistance, which is probably mediated by increased expression of SOCS3—an inhibitor of leptin signalling—constitutive activation of STAT3 in POMC neurons leads to insulin resistance [21]. This study confirms the role of STAT3 as a mediator of not only leptin but also insulin signalling in POMC neurons. The role of STAT3 in AgRP neurons has also been examined [20,31,58]. Its activation by leptin has inhibitory effects on agrp mRNA levels. As expected, and because of the findings that STAT3 expression modifies agrp expression [31], constitutive activation or deletion of STAT3 in this neuronal population regulates energy balance [20,59]. STAT3 deletion shows a mild gain in mouse body weight, and unlike the effects of STAT3 in POMC neurons, this deregulation is due to effects on energy expenditure. Overexpression of STAT3 in AgRP neurons is associated with increased locomotor activity, with no effects on food intake [20], indicating that these factors could be responsible for the recovery of locomotor activity after restoration of LepR levels in the Arc of LepR knockout mice [20,60]. Furthermore, STAT3 has the effect of ameliorating glucose homeostasis in mice exposed to a high fat diet (HFD; [20]), indicating that this model is less sensitive to leptin. Altogether, these reports provide a clear implication of STAT3 in the mechanism(s) by which leptin and insulin elicit responses by the POMC and AgRP neurons. Overexpression of STAT3 in each separate neuron population results in different effects on energy balance and glucose homeostasis, indicating that synergy between the groups of neurons is necessary for a complete and appropriate response to leptin.
The PI3K–PKD1–FoxO1 signalling pathway acts to integrate leptin and insulin signals. The primary effector of this pathway, PI3K, has been shown to be a mediator of both hormones (Fig 2). PI3K is required for the actions of leptin and insulin to activate or inhibit POMC and AgRP neurons [17,23,29,43]. It has been demonstrated that the PI3Kp110β subunit has a fundamental role in regulating body weight, food intake and glucose homeostasis through leptin and insulin signals [61]. Once more attention was focused on the importance of PI3K in mediating the effects of both hormones, it became obvious that the mechanisms by which it provokes changes in energy homeostasis were not clearly identified. Studies reported PDK1 as the main PI3K target to affect energy homeostasis. It was demonstrated that PDK1 is involved in the ability of POMC and AgRP neurons to sense leptin and insulin inputs [22,62,63]. In POMC neurons, the deletion of PDK1 promotes a gain in body weight associated with increased food intake [22,62]. As expected, mice with this ablation have lower pomc expression [22,62], which is responsible for the changes in food intake seen in these animals. Furthermore, PDK1 deletion in POMC neurons affects glucose homeostasis. Administering leptin to these animals cannot reproduce the body weight loss provoked in control mice, suggesting that leptin action in POMC neurons is dependent on PDK1 [62]. It has also been reported that PDK1 is involved in insulin action. The lack of PDK1 in POMC neurons leads to increased insulin sensitivity in adult mice, and higher blood glucose levels in young and obese mice [22]. The deletion of PDK1 in this neuronal population has no effects on energy expenditure. By contrast, in AgRP neurons, PDK1 was shown to regulate energy balance by provoking changes in food intake and energy expenditure [63]. As anticipated, and contrary to its effect in POMC neurons, the deletion of PDK1 in the AgRP population promoted body weight loss as a consequence of diminished food intake and enhanced energy expenditure—specifically, increased locomotor activity [63]. In addition, these mice were more sensitive to leptin. Chronic peripheral administration of leptin induced greater reductions in body weight and food intake [63].
PDK1 activates AKT, which then regulates many kinases and transcription factors. One of the most studied is FoxO1, which has been involved in hypothalamic neuropeptide expression and food intake. As stated above, when FoxO1 is phosphorylated and inactivated by leptin in POMC neurons, it acts to promote pomc transcription and, conversely, inhibits neuropeptide expression in AgRP neurons (Fig 2). As expected, previous data from the two PDK1 knockout models described above are in agreement with the FoxO1 effects on feeding. PDK1 deletion in POMC neurons impairs FoxO1 phosphorylation and thus, this transcription factor remains in the nucleus to inhibit pomc expression. In AgRP neurons, PDK1 ablation leads to nuclear accumulation of FoxO1 and decreased food intake [63]. These data indicate that FoxO1 could be the last effector regulating leptin signalling. In fact, in the PDK1 deletion studies, inhibition of FoxO1 rescues the phenotype. Its inactivation in PDK1ΔPOMC mice abolished the change in body weight, pomc expression and glucose homeostasis [22,62]. In AgRP neurons, FoxO1 inactivation, which leads to its exclusion from the nucleus, rescues the phenotype seen in the AgRP–PDK1 knockout mice [63]. These studies emphasize the importance of FoxO1 as a regulator of leptin and insulin actions.
Several studies have described the specific action of FoxO1 in the regulation of food intake, energy expenditure and glucose homeostasis. Leptin and insulin require FoxO1 signalling in the Arc to provoke their anorexigenic effects that lead to increased leptin and insulin plasma levels. Given these clues, the role of FoxO1 in POMC and AgRP neurons was investigated [26,31,64,65]. In POMC neurons, the deletion of FoxO1 leads to body weight loss associated with decreased food intake [64]. This change in feeding behaviour is not associated with changes in pomc expression, but the authors linked it with increased levels of α-melanocyte stimulating hormone, a proteolytic product of POMC, and they found that FoxO1 actions on energy balance are mediated by carboxypeptidase E, a prohormone processing convertase [64]. No changes in energy expenditure were found in this model. Furthermore, FoxO1 expression in POMC neurons has an important role in leptin and insulin action [64,65]. The deletion of this transcription factor in POMC neurons increased leptin sensitivity [64]. A report investigated the role of FoxO1 in AgRP neurons [26]. FoxO1 signalling is required in AgRP neurons to regulate energy homeostasis. The specific ablation of FoxO1 in this neuronal population was associated with decreased food intake and locomotor activity [26]. Interestingly, body weight was not affected. In addition, it has been shown that the lack of FoxO1 in AgRP neurons is involved in glucose homeostasis. This animal model presents altered HGP due to enhanced insulin sensitivity of the AgRP neurons [26]. In this work, G-protein-coupled receptor 17 (Gpr17) is identified as the downstream target from which FoxO1 affects energy homeostasis in the AgRP neurons [26].
Summarizing, the individual contributions of important factors in the leptin and insulin signal transduction pathways have been described. STAT3 and FoxO1—downstream transcription factors from both pathways—are shown to have key roles in energy balance and glucose homeostasis and can act independently within either of the POMC or AgRP neurons in the Arc. In addition, all of these factors modify the overall effects of leptin and insulin on food intake, energy expenditure and glucose metabolism, indicating that there must be a co-operative, that is, synergistic, interaction between pathways for the AgRP and POMC neurons to regulate energy homeostasis.
mTOR is also a downstream target of the PI3K pathway, and its activation is dependent on nutrient availability [30,34]. In addition to its role in sensing glucose or leucine levels, it has been shown that this pathway mediates the anorexigenic effects of leptin, as noted above [30,66,67]. Pharmacological studies have demonstrated that mTOR inhibition by rapamycin blunts the effects of leptin on food intake [30]. Furthermore, these results were corroborated by other reports using transgenic models. Mice with a selective lack of S6K do not respond to leptin treatment, showing that this pathway is involved in leptin resistance [66]. The activation of the mTOR pathway in POMC neurons attenuates body weight loss and decreases food intake after leptin treatment [68]. These data support the idea that the mTOR pathway is an important mediator of the onset of leptin resistance.
HIF, which has been linked to the mTOR pathway [69,70] is regulated by oxygen availability. Under hypoxic conditions HIF is stabilized and under normoxia it is degraded [71,72]. It has been demonstrated that this transcription factor is involved in the control of glucose metabolism [73]. The deletion of HIF in POMC neurons curtails the changes on pomc expression caused by glucose injection, demonstrating that HIF mediates glucose action on POMC neurons [73]. This role of HIF was independent of leptin signalling. AMPK and mTOR are nutrient sensors. The activation of HIF counteracts food intake provoked by AICAR—an AMPK activator. In the same way, the orexigenic effects of rapamycin are blocked by HIF activation. Furthermore, decreased food intake caused by the activation of mTOR in the mediobasal hypothalamus is reduced in mice lacking HIF in their POMC neurons. This report shows a potent action of HIF in glucose sensing, which in POMC neurons involves AMPK and mTOR signalling [73]. However, HIF is not only involved in glucose sensing. In the same report, the authors propose that HIF acts as a therapeutic target to oppose obesity. The animal model used in this study is more sensitive than normal mice to a high fat diet; however, the constitutive activation of HIF protected these mice against diet-induced obesity [73].
Role of autophagy in glucose homeostasis
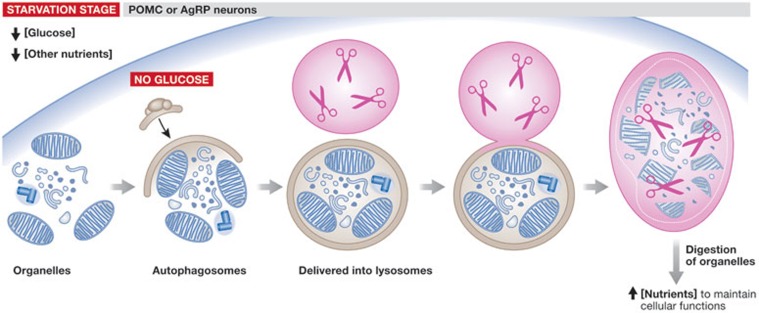
Macroautophagy—referred to as autophagy—is a new mechanism involved in the maintenance of cellular homeostasis. Autophagy is a process whereby cytoplasmic organelles are sequestered and delivered into lysosomes for degradation to provide the cell with nutrients under starvation conditions (Fig 3; [74]). Although the role of autophagy has been tested in other tissues [75,76], this mechanism had not been studied in the hypothalamus. In the past year, some reports have implicated autophagy in the regulation of food intake [74,77,78,79,80]. Mice that lack the autophagy-related gene 7 (Atg7) in POMC or AgRP neurons show altered energy homeostasis. In AgRP neurons, inhibition of autophagy promotes body weight loss associated only with increases in locomotor activity. In addition, these mice have a reduced rebound effect on food intake after fasting, suggesting that they might develop enhanced leptin sensitivity [74]. The deletion of Atg7 in POMC neurons leads to obesity, and changes in food intake and energy expenditure [77], supporting the idea that autophagy regulates energy homeostasis. In agreement with the findings in AgRP neurons, the inhibition of autophagy in POMC neurons provokes potent effects on glucose homeostasis [77,78,80] and causes structural alterations—such as diminished neuronal projections—in these neurons. These mice show leptin resistance and this is related to the inability of leptin to activate and phosphorylate STAT3 [78,80]. Furthermore, the lack of Atg7 in POMC neurons increased blood glucose levels despite elevated insulin concentrations, indicating that these mice develop insulin resistance [77,80]. These data clearly demonstrate an implication of autophagy in glucose homeostasis. Beyond these animal models in which autophagy is abolished in specific neuronal populations, another study shows the effect of impaired autophagy in the mediobasal hypothalamus (MBH; [79]). In this report, the authors used a strategy of shRNA stereotaxic delivery to abrogate Atg7 expression in the MBH. Surprisingly, these mice showed the same phenotype as the Atg7ΔPOMC mice: the appearance of obesity was due to elevated food intake and impaired energy expenditure [79]. The reason why autophagy seems to have more important effects on POMC neurons than on AgRP cells remains unclear. However, the fact that the lack of Atg7 in POMC neurons provokes a loss of neuronal projections from these cells to the PVH [80], an important hypothalamic nucleus involved in the regulation of food intake, might provide a clue as to why POMC autophagic effects prevail over those of AgRP. Further efforts are needed however to investigate the role of autophagy in promoting structural changes in the AgRP neurons. In spite of this, the mechanism by which autophagy affects energy balance is still unknown (Sidebar A). Noteworthy, it has been proposed that the mTOR pathway can act to mediate autophagy-dependent changes in energy homeostasis [74,77].
Figure 3.
Autophagy in POMC and AgRP neurons. During starvation, when glucose levels are low, certain cytoplasmic organelles are sequestered in autophagosomes. In turn, these organelles are delivered into lysosomes, where they are broken into necessary nutrients to maintain cellular functions. AgRP, agouti-related protein; POMC, proopiomelanocortin.
Sidebar A | In need of answers.
Is there canonical signalling of leptin and insulin?
What is the precise signalling modality between hypothalamic and extrahypothalamic areas in mediating the actions of leptin and insulin on integrative physiology?
What is leptin and insulin resistance in relation to neurons?
What is the exact mechanism by which hypothalamic autophagy affects energy balance?
In conclusion, the individual contributions of each significant component of the JAK, STAT3 and PI3K pathways in the regulation of energy balance and glucose homeostasis have been described. Furthermore, studies of STAT3 and FoxO1, the last effectors of these pathways, have been shown to have vital roles in leptin and insulin actions. The POMC and AgRP neurons of the Arc of the hypothalamus have been identified as key central targets of leptin and insulin action. New targets, such as mTOR, have been detected to be potent mediators of leptin and insulin signalling, and new mechanisms, such as autophagy, have been discovered to be involved in energy and glucose homeostasis. It was also emphasized that specific subpopulations of neurons might have differential roles in feeding compared with glucose homeostasis regulation [56]. Despite all these, more efforts are needed to understand the individual contribution of each factor discussed here and the global mechanisms by which they operate, with the ultimate goal of obtaining a molecular and systemic understanding of the onset of leptin and insulin resistance (see Sidebar A). This would, thereby, advance the development of future therapeutic treatments to combat obesity and its associated metabolic syndrome.

Luis Varela

Tamas L Horvath
Acknowledgments
L.V. was supported by a Fulbright Fellowship from the Spanish Ministry of Education.
Footnotes
The authors declare that they have no conflict of interest.
References
- Wang Y et al. (2008) Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 16: 2323–2330 [DOI] [PubMed] [Google Scholar]
- Wang YC et al. (2011) Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378: 815–825 [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ (2000) Adiposity signals and the control of energy homeostasis. Nutrition 16: 894–902 [DOI] [PubMed] [Google Scholar]
- Mohr B (1840) Hypertrophie der hipófisis cerebral und druck adurch bedingter auf die hirngrundflache, complementos besondere auf die sehnerven, das quiasma derselben und den linkseitigen hirnschinkel. Wschr des Heilk 6: 565–571 [Google Scholar]
- Frohlich A (1901) Ein fall von tumor der hypophysis cerebri ohne akromegalie wien klin. Rundschau 15: 883–906 [Google Scholar]
- Brobeck JR, Tepperman J, Long CN (1943) Experimental hypothalamic hyperphagia in the albino rat. Yale J Biol Med 15: 831–853 [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR (1951) Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med 77: 323–324 [DOI] [PubMed] [Google Scholar]
- Stellar E (1954) The physiology of motivation. Psychol Rev 61: 5–22 [DOI] [PubMed] [Google Scholar]
- Aschner B (1912) Uber die funktion der hypophyse. Pflüg Arch ges Physiol 146: 1–146 (s [Google Scholar]
- Cone RD et al. (2001) The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25 (Suppl 5): S63–S67 [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL (2009) Feeding signals and brain circuitry. Eur Neurosci J 30: 1688–1696 [DOI] [PubMed] [Google Scholar]
- Joly-Amado A et al. (2012) Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J [Epub ahead of print] doi:; DOI: 10.1038/emboj.2012.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela L, Horvath TL (2012) AgRP neurons: a switch between peripheral carbohydrate and lipid utilization. EMBO J [Epub ahead of print] doi:; DOI: 10.1038/emboj.2012.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM et al. (2006) Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav 89: 687–691 [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA (1997) Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138: 4489–4492 [DOI] [PubMed] [Google Scholar]
- Cowley MA et al. (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484 [DOI] [PubMed] [Google Scholar]
- Konner AC et al. (2007) Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449 [DOI] [PubMed] [Google Scholar]
- Lin HV et al. (2010) Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59: 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C et al. (1996) Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97 [DOI] [PubMed] [Google Scholar]
- Mesaros A et al. (2008) Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab 7: 236–248 [DOI] [PubMed] [Google Scholar]
- Ernst MB et al. (2009) Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. Neurosci J 29: 11582–11593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF et al. (2008) PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab 7: 291–301 [DOI] [PubMed] [Google Scholar]
- Hill JW et al. (2008) Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 118: 1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L et al. (2006) Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116: 1886–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD et al. (2003) Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231 [DOI] [PubMed] [Google Scholar]
- Ren H et al. (2012) FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell 149: 1314–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS et al. (2006) Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci 9: 901–966 [DOI] [PubMed] [Google Scholar]
- Minokoshi Y et al. (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574 [DOI] [PubMed] [Google Scholar]
- Claret M et al. (2007) AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117: 2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D et al. (2006) Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930 [DOI] [PubMed] [Google Scholar]
- Kitamura T et al. (2006) Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12: 534–540 [DOI] [PubMed] [Google Scholar]
- Morrison CD et al. (2005) Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289: E1051–E1057 [DOI] [PubMed] [Google Scholar]
- Andrews ZB et al. (2008) UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature 454: 846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela L et al. (2012) Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. Pathol J 227: 209–222 [DOI] [PubMed] [Google Scholar]
- Dagon Y et al. (2012) p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab 16: 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H et al. (2008) Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest 118: 2959–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Bruning JC (2010) CNS leptin and insulin action in the control of energy homeostasis. Ann NY Acad Sci 1212: 97–113 [DOI] [PubMed] [Google Scholar]
- Considine RV et al. (1995) Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest 95: 2986–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M et al. (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA et al. (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543 [DOI] [PubMed] [Google Scholar]
- Zhang Y et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432 [DOI] [PubMed] [Google Scholar]
- Chen H et al. (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495 [DOI] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D (2004) Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7: 493–494 [DOI] [PubMed] [Google Scholar]
- Balthasar N et al. (2004) Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42: 983–991 [DOI] [PubMed] [Google Scholar]
- Hill JW et al. (2010) Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11: 286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L et al. (2009) Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9: 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamber KM et al. (2012) Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity. PLoS ONE 7: e30485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED et al. (2012) Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest 122: 1000–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wall E et al. (2008) Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149 1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S et al. (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382 [DOI] [PubMed] [Google Scholar]
- Bruning JC et al. (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125 [DOI] [PubMed] [Google Scholar]
- Obici S et al. (2002) Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572 [DOI] [PubMed] [Google Scholar]
- Inoue H et al. (2006) Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 3: 267–275 [DOI] [PubMed] [Google Scholar]
- Williams KW et al. (2010) Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. Neurosci J 30: 2472–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q et al. (2004) Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 101: 4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG Jr (2004) LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53: 3067–3073 [DOI] [PubMed] [Google Scholar]
- Xu AW et al. (2007) Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 148: 72–80 [DOI] [PubMed] [Google Scholar]
- Kaelin CB et al. (2006) Signal transducer and activator of transcription (stat) binding sites but not stat3 are required for fasting-induced transcription of agouti-related protein messenger ribonucleic acid. Mol Endocrinol 20: 2591–2602 [DOI] [PubMed] [Google Scholar]
- Gong L et al. (2008) Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide Y neurons for normal energy homeostasis. Endocrinology 149: 3346–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R et al. (2005) The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab 1: 63–72 [DOI] [PubMed] [Google Scholar]
- Al-Qassab H et al. (2009) Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab 10: 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar K et al. (2010) PDK-1/FoxO1 pathway in POMC neurons regulates Pomc expression and food intake. Am J Physiol Endocrinol Metab 298: E787–E798 [DOI] [PubMed] [Google Scholar]
- Cao Y et al. (2011) PDK1–Foxo1 in agouti-related peptide neurons regulates energy homeostasis by modulating food intake and energy expenditure. PLoS ONE 6: e18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L et al. (2009) The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med 15: 1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L et al. (2012) InsR/FoxO1 signaling curtails hypothalamic POMC neuron number. PLoS ONE 7: e31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D et al. (2008) The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. Neurosci J 28: 7202–7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouet C, Ono H, Schwartz GJ (2008) Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab 8: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H et al. (2009) Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 9: 362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK et al. (2004) mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10: 594–601 [DOI] [PubMed] [Google Scholar]
- Hudson CC et al. (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22: 7004–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poellinger L, Johnson RS (2004) HIF-1 and hypoxic response: the plot thickens. Curr Opin Genet Dev 14: 81–85 [DOI] [PubMed] [Google Scholar]
- Gordan JD, Simon MC (2007) Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev 17: 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H et al. (2011) Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol 9: e1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S et al. (2011) Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab 14: 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R et al. (2009) Autophagy regulates lipid metabolism. Nature 458: 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R et al. (2009) Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 119: 3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S et al. (2012) Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep 13: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan W et al. (2012) Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology 153: 1817–1826 [DOI] [PubMed] [Google Scholar]
- Meng Q, Cai D (2011) Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF–kappaB pathway. J Biol Chem 286: 32324–32332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe B et al. (2012) Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab 15: 247–255 [DOI] [PMC free article] [PubMed] [Google Scholar]