A tumour is clonogenic in origin, dividing and accumulating accidental mutations over time to become a complex, heterogeneous mix of cells. By the time it is diagnosed, a typical tumour mass contains 30–80 somatic mutations [1]. This heterogeneity makes it difficult to eliminate all cancerous cells simultaneously by targeting mutated gene products [2], and we believe that even a single cancer cell left behind after surgery and/or chemotherapy might cause a recurrence of cancer [3,4,5]. We thought, therefore, that a simpler approach would be to target highly elevated levels of glycolysis common to most cancer types—the effect known as the ‘Warburg effect’. However, it turns out that targeting glycolysis and metabolic pathways in cancer might be just as complicated as targeting somatic mutations, if not more so.
In 1956, Otto Warburg characterized cancer cells as having higher glycolysis rates than normal healthy cells [6]. At the time, it was thought that cancer cells in the dense interior of the tumour survive the highly hypoxic conditions by generating energy through anaerobic respiration. However, advances in imaging technology have shown the existence of highly glycolytic cancer cells in oxygen-rich environments. In fact, in most cancer cells, glucose is used to synthesize lipids, amino acids and nucleotides for rapid cell division. In some cases, too much glucose is diverted from the tricarboxylic acid cycle, and the cycle must be supplied with glutamate from glutamine imported from an extracellular source, suggesting that complex metabolic changes take place in cancer cells. For example, Bishop and colleagues, have compared liver tumours induced by tissue-specific overexpression of either MYC or MET. MYC-induced mouse liver tumours significantly increase both glucose and glutamine catabolism, whereas MET-induced liver tumours use glucose to produce glutamine and import little glutamine [7]. MYC-induced liver tumours are associated with decreased levels of glutamine synthetase. Bishop's group also compared MYC-induced liver tumours with lung tumours induced by tissue-specific overexpression of MYC. They found that MYC-induced lung tumours have increased glutamine synthetase expression. Thus, cancer cells make complex changes on the basis of both their genetic background and their immediate environment. However, we do not yet know the exact molecular mechanisms behind these changes.
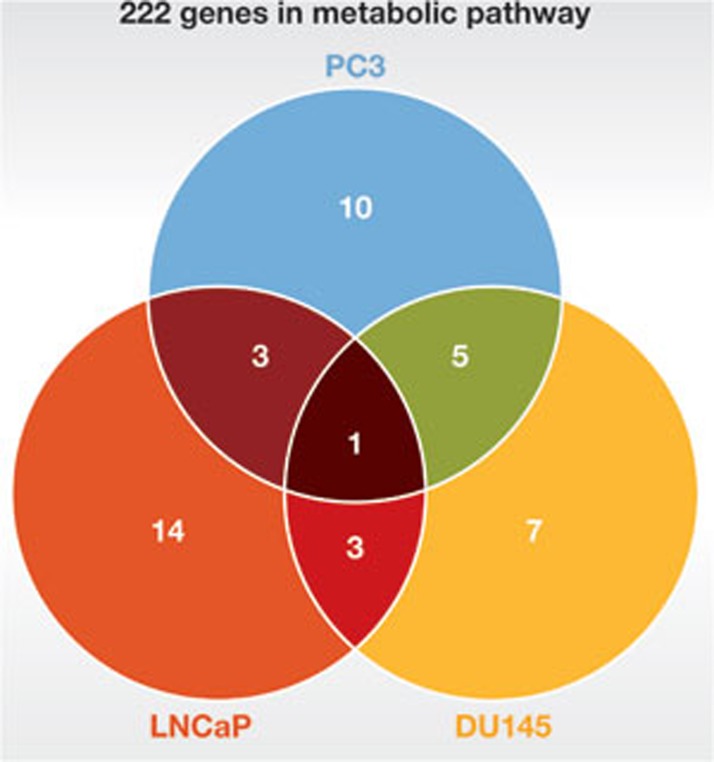
To gain a complete picture of cancer metabolomes in a particular type of cancer, Ros and colleagues took three prostate cancer cell lines and one non-malignant prostate epithelial cell line, and screened them with 222 siRNA knockdowns targeting metabolic enzymes, transporters and regulators located within the core glucose metabolism [8]. They then assayed for cell death. The three prostate cell lines varied from highly aggressive PC3 to intermediate yet androgen-independent DU145 and androgen-dependent LNCaP cancer cells. Ros and colleagues found 19 genes in metabolic pathways that are essential in PC3 cells, but only 4 of the 19 genes were among the 21 essential genes for LNCaP, whereas 6 of the 19 genes were among the 16 essential genes in DU145 (Fig 1). By extension, this suggests that if we had 19 drugs, each targeted to one of the 19 essential genes in PC3 cells, then, although all 19 drugs would induce apoptosis in PC3 cells, only 4 drugs might work in LNCaP cells and only 6 drugs might work for DU145, even though they are all derived from prostate cancer. In fact, there is a great deal of diversity among the cancer metabolomes of the three prostate cell lines studied. There are a total of 43 essential genes found across the three cell lines, but only one gene is essential to all three: PRKAB1, the β-1 non-catalytic subunit of AMP-activated kinase. As such, the importance of PRKAB1 was tested by depleting it from 20 cancer cell lines, but about half of the cells were unaffected by its absence. Thus, choosing effective metabolic inhibitors for a cancer patient might prove to be a difficult task.
Figure 1.
Diversity in cancer metabolomes. Venn diagram of essential genes in three prostate cancer cell lines, among 222 metabolic enzymes, transporters and regulators located within the core glucose metabolism. Essential genes for non-malignant prostate epithelial cells are excluded. In each area, the number used indicates the number of essential genes shared by prostate cancer cell lines covering that area. Number 1 in the centre, for example, indicates that there is only one essential gene shared by all three cell lines.
When Ros and colleagues incubated cultured cancer cells in different media, they expected that there would be changes in metabolism in response to altering the availability of nutrients and/or oxygen [8]. The real surprise was that they observed so much diversity in the metabolic activites of cancer cells within the same cancer type cultured under identical conditions, suggesting that the genetic background of a cancer cell must have a substantial role in determining its metabolism.
Was the diversity of metabolomes found in these three prostate cell lines predictable from their genomes? DU145 cells contain one wild-type PTEN allele and a second variant allele (M134L); PC3 cells have sustained a homozygous deletion of PTEN; and LNCaP cells have a deletion of one allele and a mutation of the other PTEN allele. However, it seems unlikely that the diversity of the metabolomes, extending over 43 essential genes in three prostate cancer cell lines, can be explained by the status of PTEN alone. In choosing metabolic inhibitors for actual cancer therapy, having genome data is not enough. Careful analysis of the cancer metabolome should probably be performed for each cancer patient.
Given this metabolic flexibility, even if an efficacious target for a cancer patient can be found, might the cancer just elude a metabolic inhibitor-based therapy by altering its own metabolism? This has been shown in an established cell line by Robinson and colleagues [9]. Z138 mantel lymphoma cells incubated in glucose-depleted media supplemented with pyruvate and glutamine easily survived the adverse conditions by switching to aerobic respiration. In these cells, even the morphology of mitochondria was altered, such that the organelles showed well-developed cristae traversing their length—a characteristic high oxidative capacity phenotype. Moreover, the cells showed resistance to intrinsic cell death stimuli such as the BCL2 antagonist ABT-737 and ionizing radiation. The question is how many other cancer cell types can perform these tricks? If cancer cells can escape death from metabolic inhibitors by turning off the glycolytic switch and hiding among normal cells until the treatment is over, then the possibility exists that the cancer will come back after the cessation of treatment. We believe this is an important question that needs to be addressed.
There are drugs targeting 49 moieties in the glycolytic metabolism of tumours at various stages of development. Most of the targets are validated by siRNA-mediated knockdown in cancer cell lines. But validating a target in a cell line does not guarantee that its inhibitor will be effective at the clinical stage, as shown by Ros and colleagues and discussed above. Thus, we cannot know whether a metabolic inhibitor will work in a particular patient until we have analysed the cancer metabolome of that patient, determined the range of metabolic pathways that need to be targeted and have ensured that the glycolytic switch cannot simply be turned off in certain cells. Another concern is the safety of metabolic inhibitors in humans. As some healthy brain cells are highly glycolytic, drugs that target glycolytic pathways could adversely affect brain functions, yet drug safety studies concerning brain functions are difficult to do in animals.
Thus, in our opinion, metabolic inhibitors as cancer therapeutics to eliminate cancer cells face many difficult challenges. Even if only a few cancer cells survive treatment, they could cause recurrence of cancer and we should anticipate these obstacles and explore the ways to overcome them sooner rather than later.
However, in the case that some of these inhibitors have relatively few, mild side-effects, they might prove to be useful prophylactic agents, slowing or arresting cancer cell growth. By way of example, metformin works synergistically with doxorubicin [10] and 2-deoxyglucose works well with ABT-263 [2]. However, not all metabolic inhibitors act synergistically with other chemotherapeutics [2]. Thus by manipulating cancer metabolism, we sometimes make cancer cells more susceptible to chemotherapeutic agents but on other occasions, we make the cells more resistant to them. At the moment, we do not know a priori how a metabolic inhibitor will affect death-inducing pathways in cancer cells. Hence, careful studies must be conducted for each metabolic inhibitor before combining it with other agents.
Footnotes
The authors declare that they have no conflict of interest.
References
- Stratton MR (2011) Science 331: 1553–1558 [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Perkins G (2012) Cancer Res 72: 18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth J, Kahn M (1937) Am J Cancer 31: 276–282 [Google Scholar]
- Kelly PN et al. (2007) Science 317: 337. [DOI] [PubMed] [Google Scholar]
- Quintana E et al. (2008) Nature 456: 593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O (1956) Science 124: 269–270 [PubMed] [Google Scholar]
- Yuneva MO et al. (2012) Cell Metab 15: 157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros S et al. (2012) Cancer Discov 2: 328–343 [DOI] [PubMed] [Google Scholar]
- Robinson GL et al. (2012) Oncogene [Epub ahead of print] doi:; DOI: 10.1038/onc.2012.13 [DOI] [PubMed] [Google Scholar]
- Hirsch HA et al. (2009) Cancer Res 69: 7507–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]