Abstract
1,3-Dimethylamylamine (1,3-DMAA) is a stimulant commercially sold in a variety of dietary supplements as a chemical species derived from geranium plants (Pelargonium graveolens). Whether 1,3-DMAA naturally occurs in geranium plants or other dietary ingredients, it has important regulatory and commercial ramifications. However, the analysis of 1,3-DMAA in geranium plants is not trivial due to low concentrations and a complex environmental matrix, requiring high selectivity and sensitivity. An extraction method combined with high performance liquid chromatography and tandem mass spectrometry is used to determine 1,3-DMAA and 1,4-dimethylamylamine (1,4-DMAA) concentrations in geranium plants with both external calibration and standard addition method. Samples from the Changzhou, Kunming, and Guiyang regions of China during both winter and summer were analyzed for 1,3-DMAA and 1,4-DMAA. The diastereomer ratios of the 1,3-DMAA stereoisomers of a racemic standard and the extracted plant were also quantified.
Keywords: DMAA, geranium, natural product analysis, HPLC, mass spectrometry
Introduction
There has been significant discussion of 1,3-dimethylamylamine (1,3-DMAA) in the literature concerning the presence of 1,3-DMAA in geranium plants (Pelargonium graveolens).1–6 1,3-DMAA, also known as 4-methyl-2-hexaneamine (MHA), 1,3-dimethylpentylamine, or 2-amino-4-methylhexane can be labeled as geranium extract in dietary supplements. Confirming the presence or absence of 1,3-DMAA as a natural product in geranium plants has important regulatory and commercial consequences for many dietary supplement companies.7
The chemical properties and concentrations of 1,3-DMAA and the associated matrix do not allow for simpler LC detection methods (UV-visible absorption or refractive index). Typically, GC-MS analysis requires derivatization to a higher molecular weight to increase boiling point and retention time. The geranium oil and plant matrix are sufficiently complex that most universal detectors, such as refractive index and flame ionization detectors, are likely to encounter significant matrix interferences. Thus, research and analytical effort for 1,3-DMAA analysis has focused on GC-MS1,3–5 and LC-MS/MS 1,2,4–6 analysis protocols for matrices, such as urine, geranium oil extracts and geranium plants.
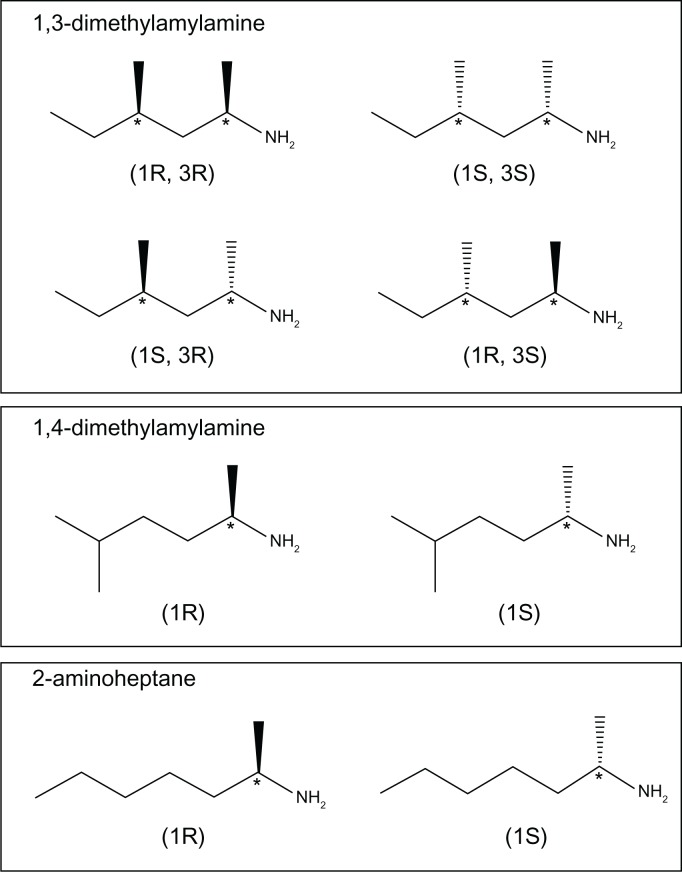
The World Anti-Doping Agency requires that compounds with chemical structure and biological activity similar to banned substances must be analyzed by anti-doping laboratories. 1,3-DMAA and 2-aminoheptane (a banned stimulant) have similar chemical structures and physiological stimulant effects (Fig. 1). The laboratory of Saudan1 developed a high performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for detection of 1,3-DMAA in urine samples. The method was calibrated over the range of 50 to 700 ng/mL with excellent intraday precision and accuracy of less than 6%. The results from the Saudan laboratory found that 1,3-DMAA could be detected in urine samples up to 105 hours after administration of a 40 mg dose.
Figure 1.
Chemical structures of the stereoisomers of 1,3-DMAA, 1,4-DMAA, and 2-aminoheptane with stereogenic carbons labeled (*) and their respective (R,S) configurations.
Subsequent research by Vorce et al2 used LC-MS/MS to confirm 1,3-DMAA as the cause of false positives in amphetamine screening kits used by the United States Department of Defense drug screening laboratories. 1,3-DMAA was suspected due to its inclusion in bodybuilding energy supplements available over the counter. Vorce et al reported that 1,3-DMAA would cause false positives at urine concentrations above 6.0 mg/L and confirmed the presence of 1,3-DMAA concentrations over the 6.0 mg/L limit in 92.3% of the false positive results for amphetamines.
The laboratory of Lisi3 conducted an analysis of five geranium oils which had origins in France, Egypt, and New Zealand. The geranium oils were analyzed using a derivatization and extraction procedure for 1,3-DMAA. None of these samples were reported to have 1,3-DMAA, but no limit of detection (LOD) was reported for the method. Supplements containing 1,3-DMAA were then administered and tested in a urine excretion study using a GC with a nitrogen-phosphorous detector. The results showed that 1,3-DMAA is excreted for at least 29 hours in agreement with a previous report.1
The research team of ElSohly et al4 used GC-MS, LC-MS/MS, and high resolution ultra-performance LC with quadrupole-time of flight-MS (UPLC-QTOF-MS) to analyze geranium oils and leaves from India as well as geranium leaves, stems, and freshly extracted oil from plants grown in Oxford, Mississippi. The GC-MS and LC-MS/MS-based methods used similar extraction procedures with a reported extraction efficiency of 35% (which is relatively low). However, the extraction was shown to have excellent accuracy (75%) and precision (less than 5%) on the control sample using GC-MS analysis. The limits of detection for the GC-MS, LC-MS/MS, and UPLC-QTOF-MS were 0.1 ppm, 2.5 ppb, and 10 ppb, respectively. The GC-MS analysis of the 0.1 ppm spikes of 1,3-DMAA in the geranium oil clearly showed the characteristic double peaks of the 1,3-DMAA diastereomer pairs. The authenticated geranium plant material showed a similar pattern to the spiked geranium oil, whereas the negative geranium oil and authenticated geranium oil did not. The GC-MS chromatograms of the authenticated geranium plant material suggested the presence of the 1,3-DMAA. However, the two more sensitive LC-MS/MS methods did not detect 1,3-DMAA in any of the samples analyzed. The LC-based methods do not exhibit the characteristic diastereomer double peak—possibly due to the chromatographic separation conditions.2,5,6
Zhang et al5 recently reported the analysis of eight different geranium oils, four from China and four from Egypt, and analysis of thirteen dietary supplements containing 1,3-DMAA. The goal of their paper was to determine whether the 1,3-DMAA in dietary supplements had synthetic or natural origins. The supplements were analyzed using GC-FID analysis with a chiral column. The 1,3-DMAA in the standards and supplements were derivatized by pentafluoropropionic anhydride (PFPA). The derivatized stereoisomer separation of 1,3-DMAA by GC-FID was excellent, showing all four stereoisomers present. The GC-FID analysis protocol did not have an LOD reported; however, the calibration curve range was 0.2 to 0.8 mg/mL of 1,3-DMAA. The dietary supplements were reported to contain the same stereoisomer ratios as the synthetic standards.
Zhang et al then used two LC-MS-based methods to analyze the geranium oils for 1,3-DMAA.5 The LOD of the linear ion trap method (HPLC-ESI-LIT) was 50 ppb and the LOD of the triple quadrupole instrument (HPLC-ESI-QQQ) was 10 ppb for derivatized 1,3-DMAA. The HPLC-ESI-LIT used a chiral-phase HPLC separation column. The HPLC-ESI-QQQ used a standard C18 separation phase. In both methods, 1,3-DMAA was not detected above the LOD, and both lacked the characteristic diastereomer double peak as expected (both possibly due to chromatographic separation choices).
Finally, the research team of Li et al6 developed an extraction and LC-MS/MS-based method for the analysis of 1,3-DMAA and 1,4-DMAA in geranium plants and oils (three distinct samples of each). The method validation was detailed and conducted according to United States Pharmacopeia guidelines. The traditional instrument LOD8 reported was 1 to 2 pg/g with a reported method quantification limit (LOQ) of 1 to 2 ng/g in the geranium sample. Li reported concentrations of 1,3-DMAA and 1,4-DMAA as present in three samples of geranium plants ranging from 13 to 365 ng/g and 3 to 35.3 ng/g, respectively. In the geranium oil, Li et al reported all three samples contained 1,3-DMAA ranging from 167 to 13,271 ng/g. In the sample containing 13,271 ng/g of 1,3-DMAA, 1,4-DMAA was detected at 220 ng/g. The other two geranium oil samples did not contain 1,4-DMAA above the LOD.
The research and sample analysis presented here used an adapted extraction and LC-MS-MS analysis6,9 to analyze both 1,3-DMAA and 1,4-DMAA in geranium plants. Linearity, method detection limit (MDL), accuracy, and precision studies were carried out followed by analysis of geranium plants from 3 distinct regions in China (Changzhou, Guiyang, and Kunming) during winter and summer months. An improved analysis protocol was developed that used standard addition analysis to re-analyze samples and confirm the reported concentrations of 1,3-DMAA and 1,4-DMAA. One of the Changzhou, China, samples was analyzed by another laboratory,6 and to the best of the authors’ knowledge, this represents the first inter-laboratory analysis and confirmation of 1,3-DMAA in an identical geranium sample. Additionally, the diastereomer ratio of 1,3-DMAA in geranium plants was measured and compared with synthetic standards and previously reported research.5
Experimental
Chemicals and reagents
All chemicals and reagents have a purity of 97% or greater. All standards and eluent were prepared in reagent-grade water with a resistivity of 18.2 MΩ · cm produced by a Barnstead e-pure four cartridge system. Glassware was cleaned with concentrated detergent and rinsed with reagent-grade water three times. 1,3-DMAA was purchased from 2A PharmaChem USA (purity confirmed by NMR) and 1,4-DMAA was purchased from Sigma-Aldrich. LC-MS grade acetonitrile and formic acid, HPLC grade ethanol and hexane, and ACS Certified Plus concentrated hydrochloric acid were purchased from Fisher Scientific.
Standard preparation
A combined stock solution was first prepared containing both standards (1,3-DMAA and 1,4-DMAA) with a concentration of 1000 mg/L each in ethanol. An intermediate standard solution is then diluted from the stock to prepare a standard with a concentration of 1000 μg/L in 0.5 N HCl for both 1,3-DMAA and 1,4-DMAA. Two external calibration curves were prepared for each analysis due to the unknown concentrations of 1,3-DMAA. The low range calibration was 1 to 20 μg/L, and the high range calibration was 3 to 100 μg/L. The standard addition spikes curves were prepared by analyzing sample spikes of 1,3-DMAA and 1,4-DMAA at 15.0 μg/L and 25.0 μg/L for each sample.
Sample preparation
Preliminary homogenization and extraction protocol
The preliminary extraction method was adapted from a standard analysis method.9 The method was scaled from 200 g to 50 g of geranium plant for analysis, and each subsequent step was appropriately scaled by a factor of four. The geranium plants were first cut into pieces having a mass ranging from 40 to 50 g and subsequently placed into a blender. A solution of 15 mL of 0.5 N HCl was added to extract the 1,3-DMAA and 1,4-DMAA analytes present in the plants. The mixture was homogenized at high speed for two minutes, filtered, and re-extracted with 7.5 mL of 0.5 N HCl. Both extracts were combined and diluted to a final volume of 25.00 mL. The solution was then sonicated, filtered, and analyzed by LC-MS/MS. A blank (no geranium plant) and spiked samples containing an additional 10.0 μg/L of the standard solution were also prepared by following the same procedure as those of the plant preparation. The spiked sample provides a percent recovery estimate for each sample matrix.
Optimized homogenization and extraction protocol
The preliminary analysis method was further modified6 to reduce matrix effects by adding a hexane partitioning step (hexane clean-up step). The geranium samples remained frozen at −20 °C prior to analysis and thawed for sample preparation. The wet geranium leaves and stems were cut into 1 to 2-cm pieces and subsequently ground with a high-speed grinder into finely chopped pieces. Then, 10 g of the chopped sample were weighed and placed into a standard food blender with 80 mL of 0.5 N HCl and homogenized at the highest blend setting for two minutes. The blended mixture was transferred into a 100-mL volumetric flask, and the blade and blender cup were rinsed with 15 mL of 0.5 N HCl and poured into the 100-mL volumetric flask. The blended geranium mixture was extracted by sonication for one hour at 50 °C. This solution was centrifuged at 3700 × g for ten minutes after cooling and filling to volume with 0.5 N HCl. Four mL of the supernatant and 2 mL of hexane were added to a 15-mL glass centrifuge tube with screw cap. This mixture was shaken by a vortex mixer for thirty seconds. The mixture was then centrifuged at 2000 × g for five minutes. The aqueous layer was filtered and analyzed by LC-MS/MS. For all sample analyses, a blank was analyzed with each sample to verify no carryover occurred from the previous analysis. For standard addition analysis, spiked samples were prepared by spiking standard prior to the blending process, such that the final added concentration was 15.0 and 25.0 μg/L in the volumetric flask.
This optimized method added and modified existing steps (grinding, sonication, and centrifuging) to the original extraction protocol to maximize the extraction efficiency of 1,3-DMAA and 1,4-DMAA from the plant matrix. The reduction of plant material extracted and increased volume of extractant resulted in a more practical extraction procedure and minimized sample handling errors. The sonication temperature was increased to 50 °C to increase the breakup and dissolution of the plant material in the acid extract and increase solvation of the analytes. The additional hexane extraction step minimized concentrations of the non-polar plant material in the 0.5 N HCl extraction solution. The non-polar plant material likely caused matrix effects during analysis by causing ion suppression in the ESI source. The combination of these steps provides an extract that contains a more representative concentration of 1,3-DMAA and 1,4-DMAA and a reduction of matrix effects. This means that the performance of the extraction method improves and this is demonstrated by the large improvement in percent recovery.
HPLC-MS/MS instrumentation
The LC-MS/MS system consists of an Agilent 1100 HPLC system equipped with an autosampler, coupled to a triple quadrupole mass spectrophotometer (Waters Quattro Ultima) operated in ESI+ mode. The injection volume was 100 μL with separation performed on a Phenomenex Kinetex C18 phase column (4.6 × 150 mm, 2.6 μm) with a column temperature set at 25 °C and flow rate at 0.4 mL/min. The HPLC eluent ratio was 82:18 of mobile phase A (1% of formic acid in reagent water) to mobile phase B (acetonitrile). The column effluent was split at a ratio of 1:1 prior to introduction to the mass spectrometer.
The mass spectrometer operating conditions were as follows: the capillary voltage was 3.0 kV, the cone voltage was 20 V, the source temperature was set at 120 °C with a flow of 108 L/hr, and the desolvation temperature was 350 °C with a flow of 635 L/hr. The dwell time was 0.5 second and the interscan delay was 0.1 second. The collision voltage was set to 8 eV with a collision gas (argon) pressure at 7 psi. The detection of the analytes was done using the MRM function with a pair of mass transitions of 116/99.7 m/z and 116/57 m/z to produce a single chromatogram for both 1,3-DMAA and 1,4-DMAA.
All chromatogram integrations were performed with Waters MassLynx MS software. Each chromatogram was prefiltered with a peak-to-peak noise amplitude of 2000. Chromatograms were submitted to a Savitzky Golay10 smoothing method within the MassLynx software. The Savitzky Golay method takes an average of the intensities of the data points weighted by a quadratic curve.
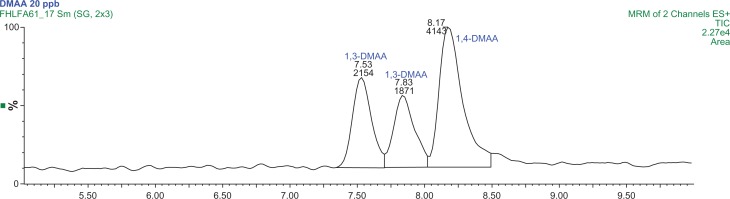
The LC-MS/MS total analysis time was 10 minutes. Figure 2 presents a typical standard chromatogram of a 20 μg/L standard of 1,3-DMAA and 1,4-DMAA. Additional standards are presented in the supplementary materials (Figs. S1–S3). It is important to mention that the compound 1,3-DMAA has two chiral centers that result in four stereoisomers (Fig. 1). These stereoisomers include two diastereomers that have different physical properties and can be separated. Therefore, 1,3-DMAA is detected as two peaks in the chromatogram. All values referenced to 1,3-DMAA_total or 1,3-DMAA are calculations based on the summation of both peak areas.2,6,9 The compound 1,4-DMAA exists as two enantiomers which cannot be separated. Therefore, only one peak was detected for 1,4-DMAA.
Figure 2.
Typical MRM Chromatogram at 20 μg/L each for 1,3 and 1,4-DMAA analytes.
Note: The retention times for the 1,3-DMAA diastereomers are 7.53 and 7.83 minutes, and 1,4-DMAA retention time is 8.17 minutes.
Results and Discussion
Detection limits, accuracy, precision, and linearity studies
Before sample analysis was conducted, detection limit,11–13 accuracy,14 precision,14 and linearity8 studies were conducted to evaluate and ensure acceptable instrument performance. The typical practice for United States Environmental Protection Agency (USEPA) MDL studies in the laboratory is to construct a 5-point calibration curve and analyze a check standard halfway between the two lowest calibration points. The USEPA MDL reported here represents the lowest concentration distinguishable from noise and determined on the variation of the analytical signal of a check standard expected to be within a factor of 2 to 5 of the detection limit. At these analytical conditions, the MDL study provides a worst-case estimate of the analyzer performance. The accuracy of the analysis is estimated using the mean percent recovery of the check standard analysis.14 The precision is estimated as the percent relative standard deviation (% RSD).14
Another estimate for the detection limit is the propagation of uncertainty MDL (Unc. MDL).13 The Unc. MDL is determined using the standard deviations of the slope (m), y-intercept (b), and signal (y) as determined by the LINEST function in Microsoft Excel. These standard deviations are then used to propagate and determine the error on “x” in the linear regression line.13 The propagated error represents the lowest concentration of analytical significance.
Detailed MDL, accuracy, and precision studies of 1,3-DMAA and 1,4-DMAA are presented in Tables 1 and 2, respectively, for all sample analysis conducted (Analysis Sets 1 to 3). The reported values for Analysis set 1 were based on the preliminary extraction protocol. Analysis Sets 2 and 3 were conducted using a hexane clean-up step as well as standard addition analysis. Typically, an MDL, accuracy and precision study was conducted with two different check standard concentrations prior to each set of sample analysis. For Analysis Sets 1 and 2, the MDLs at 3.0 μg/L were based on the calibration curves from 1 to 20 μg/L (low range calibration). The MDLs at 8.0 μg/L were based on the 3 to 100 μg/L calibration curves (high range calibration). In Analysis Set 3, the calibration curve for the 2.0 μg/L check standard was 1 to 100 μg/L, and the calibration curve for the 3.0 μg/L check standard was 2 to 100 μg/L. The R2 values for all studies with both DMAA species were greater than 0.99.
Table 1.
Detection limits, accuracy, and precision studies for 1,3-DMAA for all sample analysis.
| Analysis Set | Check standard (μg/L) | USEPA MDL (μg/L) | Unc. MDL (μg/L) | Mean % recovery | % RSD | MDL factor | Equation of linear regression | r2 |
|---|---|---|---|---|---|---|---|---|
| Analysis Set 1 | 3.0 | 1.1 | 0.4 | 126 | 9 | 2.8 | y = 195.81x − 19.049 | 0.999 |
| 8.0 | 1.8 | 3.4 | 73 | 10 | 4.5 | y = 148.84x + 420.8 | 0.999 | |
| Analysis Set 2 | 3.0 | 2.3 | 0.5 | 71 | 35 | 1.3 | y = 121.94x + 72.43 | 0.998 |
| 3.0 | 1.8 | 0.8 | 95 | 20 | 1.7 | y = 90.587x − 43.177 | 0.994 | |
| 8.0 | 2.5 | 1.5 | 62 | 16 | 3.2 | y = 114.66x + 199.46 | 0.999 | |
| 8.0 | 3.2 | 1.4 | 60 | 21 | 2.5 | y = 78.45x + 131.18 | 0.999 | |
| Analysis Set 3 | 2.0 | 1.4 | 2.6 | 103 | 21 | 1.5 | y = 111.83x + 60.259 | 0.996 |
| 3.0 | 0.6 | 1.4 | 63 | 10 | 4.9 | y = 135.07x + 190.33 | 0.999 |
Table 2.
Detection limits, accuracy, and precision studies for 1,4-DMAA for all sample analyses.
| Analysis Set | Check standard (μg/L) | USEPA MDL (μg/L) | Unc. MDL (μg/L) | Mean % recovery | % RSD | MDL factor | Equation of linear regression | r2 |
|---|---|---|---|---|---|---|---|---|
| Analysis Set 1 | 3.0 | 1.4 | 0.7 | 127 | 12 | 2.1 | y = 201.78x − 78.268 | 0.996 |
| 8.0 | 2.7 | 4.6 | 60 | 18 | 2.9 | y = 147.57x + 552.07 | 0.998 | |
| Analysis Set 2 | 3.0 | 2.0 | 0.6 | 73 | 30 | 1.5 | y = 130.39x + 31.787 | 0.996 |
| 3.0 | 0.9 | 0.6 | 93 | 10 | 3.4 | y = 85.06x − 38.131 | 0.997 | |
| 8.0 | 2.4 | 2.9 | 48 | 20 | 3.3 | y = 109.18x + 340.17 | 0.995 | |
| 8.0 | 2.1 | 0.9 | 81 | 10 | 3.9 | y = 79.501x − 7.9734 | 0.999 | |
| Analysis Set 3 | 2.0 | 0.8 | 2.6 | 98 | 13 | 2.4 | y = 95.314x + 76.382 | 0.996 |
| 3.0 | 0.8 | 1.6 | 76 | 11 | 3.7 | y = 121.63x + 118.7 | 0.999 |
The MDL values11,12 for 1,3-DMAA range from 0.6 to 3.2 μg/L and for 1,4-DMAA, range from 0.8 to 2.7 μg/L. Accuracy14 for 1,3-DMAA ranges between 60% and 126% and for 1,4-DMAA, ranges between 48% and 127%. The precision (estimated as % RSD)14 for 1,3-DMAA is in the range of 9% to 35%, and for 1,4-DMAA, precision ranges between 10% and 30%. With the exception of one mean percent recovery analysis in Analysis Set 2, the reported mean percent recoveries and % RSD are within the guidelines set by the USEPA14 for check standard analysis. The USEPA reports that mean percent recovery can range from 50% to 150%, and the % RSD can be up to 30% when samples are analyzed within a factor of 2 to 5 of the MDL.14 As the MDL factor decreases, the % RSD of the check standard analysis increases, and below an MDL factor of 2, the % RSD can dramatically increase beyond 30%.15
Ideally, MDL, accuracy and precision studies should provide estimates that are similar to each other.15–17 Further confidence of these MDL values is gained when the USEPA MDLs are compared to the Unc. MDL. Both sets of detection limit values are within 2 μg/L of each other in absolute terms and within a factor of 5 in all cases. This similarity indicates the MDL values for the calibration and analysis protocols are realistic estimates for both 1,3-DMAA and 1,4-DMAA.
A linearity study was conducted to estimate the upper limit of linearity for the LC-MS/MS analysis.8 A calibration curve was prepared and analyzed over the range of 1 to 250 μg/L for 1,3-DMAA and 1,4-DMAA, with both species being linear over the entire range as evidenced by the excellent R2 values (>0.99). The linearity study resulted in a linear regression equation for 1,3-DMAA of y = 149.08x + 380.91 and for 1,4-DMAA of y = 148.05x + 473.94.
DMAA concentrations in the plant material
The reported concentration of the DMAA species in the geranium herb was determined using the calculated concentration from the calibration curve, final extraction volume, and mass of geranium (Equation 1, below). The MDL, accuracy, and precision studies (Tables 1 and 2) were conducted with prepared standards in solution (no extraction). However, the MDLs in the analyzed plant would vary with the amount of plant mass used and the final extracted volume. For Analysis Set 1, the amount of plant material used was 50 g extracted into 25.00 mL. This resulted in MDLs that ranged from 0.6 to 1.4 ng DMAA/g geranium. In Analysis Sets 2 and 3, 10 g of plant material were extracted into 100.00 mL, which resulted in MDLs ranging from 6 to 32 ng DMAA/g geranium. While the MDLs increased for the second extraction method, the percent recovery of DMAA analysis also increased for all samples. The increase in percent recovery is likely due to the hexane clean-up step as well as a more practical increase in the extraction solvent volume. If the mass of plant material were doubled, the MDLs of the optimized extraction protocol would likely increase by a factor of two.
| (1) |
Authenticated Pelargonium graveolens samples
The Pelargonium graveolens (geranium) samples were collected and authenticated as all belonging to the genus and species Pelargonium graveolens by Xu YouKai of the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences. Samples were collected from three regions in China: Changzhou, Guiyang, and Kunming, during three different harvest seasons. The Chinese Academy received the geranium herbs as potted plants originally grown in the field. Multiple plants (ranging from two to ten in number) were collected from each location. The plants from each location were combined prior to shipment to The University of Memphis. Therefore, concentrations of 1,3-DMAA and 1,4-DMAA in individual plants and variations thereof are not reported here. The samples were sent by express airmail from Dr. Yi Jin of Yunnan University directly to the University of Memphis where the samples were immediately stored at −20 °C. Analysis Sets 1 and 2 consisted of a Changzhou sample collected on June 9, 2011 (Changzhou S11-1 and Changzhou S11–2), a Kunming, China, sample collected March 20, 2012 (Kunming 1 and 2); a Guiyang, China, sample collected March 16, 2012 (Guiyang 1 and 2); and an additional Changzhou, China, sample collected on March 10, 2012 (Changzhou 1). Analysis Set 3 consisted of a Changzhou sample collected on May 18, 2012 (Changzhou 3), a Guiyang sample collected May 20, 2012 (Guiyang 3), and a Kunming sample collected May 23, 2012 (Kunming 3). The Changzhou S11 sample was received from Intertek Labs (Detroit, MI, USA) and frozen upon arrival. The Changzhou S11 sample is an identical sample previously analyzed and reported by Li,6 providing an inter-laboratory analysis of a sample. The numbers for each region identifier signify the various Analysis Sets.
Sample Analysis set 1: preliminary extraction protocol
The concentrations of 1,3-DMAA and 1,4-DMAA in the three winter geranium samples and Changzhou S11 sample are presented in Table 3. The Changzhou S11-1 analysis was conducted in duplicate and the winter samples were analyzed in singlet. A spike sample was analyzed to determine the percent recovery for that particular plant sample. There is no reported spike analysis for Changzhou 1 due to a sample loss during analysis. No additional sample was available. The percent recovery of the spike was calculated using equation 2:13
| (2) |
Table 3.
Analysis Set 1: preliminary extraction protocol results of geranium samples from Changzhou, Kunming, and Guiyang.
|
1,3-DMAA
|
1,4-DMAA
|
|||||
|---|---|---|---|---|---|---|
| Sample (ng/g) | Spike level (μg/L) | Percent recovery (%) | Sample (ng/g) | Spike level (μg/L) | Percent recovery (%) | |
| Changzhou S11-1 | 94.7 ± 15.1 | 10.0 | 19 | 13.5 ± 1.8 | 10.0 | 65 |
| Kunming 1 | <0.5* | 10.0 | 44 | <0.7* | 10.0 | 32 |
| GuiYang 1 | <0.5* | 10.0 | 36 | <0.7* | 10.0 | 23 |
| Changzhou 1 | 213 | N/A | N/A | 52.0 | N/A | N/A |
Note:
The results are less than the MDL values.
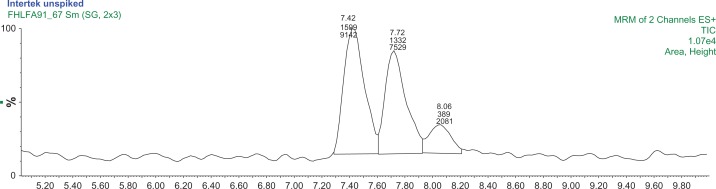
Of the four samples in Analysis Set 1, only the Changzhou S11-1 and Changzhou 1 sample contained 1,3-DMAA and 1,4-DMAA above the MDLs of the method (Table 3). Figures 3 and 4 present an MRM chromatogram of Changzhou S11-1 and Changzhou 1 samples, respectively. Additional sample and spike chromatograms are presented in the supplementary materials (Figs. S4–S8). The average concentration of 1,3-DMAA in the Changzhou S11-1 sample was 94.7 ± 15.1 ng/g geranium, with a percent recovery of 19% on the 10 μg/L spike. The average concentration of 1,4-DMAA in Changzhou S11-1 was 13.5 ± 1.8 μg/L with a 65% recovery on a 10 μg/L spike. The concentrations of 1,3-DMAA and 1,4-DMAA in Changzhou 1 samples were 213 and 52 ng/g respectively. The reported 1,3-DMAA concentrations for Changzhou S11-1 and Changzhou 1 samples were outside the calibration range but within the linearity of the analyzer. A 1:1 dilution of both samples was analyzed and resulted in calculated concentrations within 9% of the original concentration reported in Table 3.
Figure 3.
A MRM chromatogram of Changzhou S11-1 sample.
Notes: The first two peaks are 1,3-DMAA diastereomer pairs with retention times of 7.51 minutes and 7.81 minutes. The 1,4-DMAA peak retention time is 8.15 minutes. The chromatogram is produced using two mass transitions 116/99.7 m/z and 116/57 m/z.
Figure 4.
A MRM chromatogram of the Changzhou 1 sample.
Notes: The first two peaks are 1,3-DMAA diastereomer pairs with retention times of 7.51 minutes and 7.81 minutes. The 1,4-DMAA peak retention time is 8.15 minutes. The mass transitions used are 116/99.7 m/z and 116/57 m/z.
While the percent recovery of the DMAA species is not ideal, the relative concentrations should be considered for the spike. For Changzhou S11-1 sample, the concentrations of 1,3-DMAA in volumetric flask after extraction averaged 190 μg/L. The % RSD error of analysis from the MDL study was of 9% to 10% for Analysis Set 1 and translates to ∼18 μg/L error. This is more than twice the 10 μg/L spike and thus a likely contributor to the low percent recovery (high error). When 1,4-DMAA was examined, the 10 μg/L spike addition was outside the error of analysis (2.7 μg/L) and gave a more reasonable 65% recovery. Additionally, the low percent recoveries across all samples indicated the presence of a matrix effect. Previous reports6 have suggested that extraction protocols are likely to be extracting lipids from the cell membranes and contributing to ion suppression in the ESI source.
Analysis Set 2: optimized extraction protocol analysis of Changzhou S11 and winter geranium samples
The matrix effect identified in Analysis Set 1 was minimized by the addition of a hexane clean-up step. Additionally, the optimized method was more efficient as it used less plant sample mass per extraction. This efficiency provided an opportunity to re-analyze Changzhou S11, Kunming, and Guiyang winter samples. Each sample was extracted and analyzed with two different spike concentrations (15.0 μg/L and 25.0 μg/L) for both 1,3-DMAA and 1,4-DMAA and in duplicate. The spiked samples were analyzed concurrently with the unspiked ones, and the percent recovery was subsequently calculated.13 Detailed analysis results are presented in Table 4.
Table 4.
Analysis set 2: optimized extraction protocol results of geranium samples from Changzhou S11, Kunming, and Guiyang.
|
1,3-DMAA
|
1,4-DMAA
|
|||||
|---|---|---|---|---|---|---|
| Sample (ng/g) | Spike level (μg/L) | Percent recovery (%) | Sample (ng/g) | Spike level (μg/L) | Percent recovery (%) | |
| Changzhou S11–2 | 254 ± 17 | 15.0 | 54 ± 5 | 39.8** | 15.0 | 76 ± 2 |
| 25.0 | 55 ± 8 | 25.0 | 65 ± 1 | |||
| Kunming 2 | <20 ± 4* | 15.0 | 83 ± 11 | <14 ± 8 | 15.0 | 78 ± 10 |
| 25.0 | 67 ± 1 | 25.0 | 63 ± 5 | |||
| Guiyang 2 | <20 ± 4* | 15.0 | 107 ± 23 | <14 ± 8 | 15.0 | 82 ± 16 |
| 25.0 | 81 ± 2 | 25.0 | 78 ± 6 | |||
Notes:
The results are less than the MDL values;
one duplicate was less than MDL for the sample (23.9 ng/g).
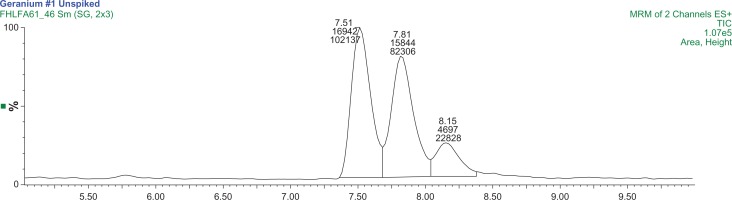
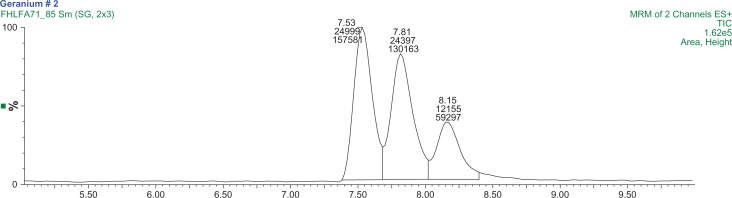
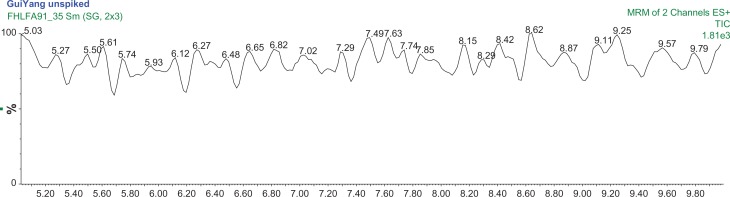
Changzhou S11 concentrations were expected to be high and thus analyzed on the high range calibration of 3 to 100 μg/L of both DMAA species. The concentrations of 1,3-DMAA and 1,4-DMAA were 254 ng/g and 39.8 ng/g, respectively, and an optimized extraction chromatogram of Changzhou S11–2 is presented in Figure 5. The percent recovery13 for 1,3-DMAA was approximately 55% for both spike levels. Both Kunming and Guiyang (Fig. 6) samples were analyzed using the low range calibration curves (1 to 20 μg/L of each DMAA species). The concentrations of 1,3-DMAA and 1,4-DMAA are reported in Table 4. All are less than the MDL of the analysis. The percent recovery for all remaining samples ranged from 63% to 107%, indicating that the matrix effect previously identified was substantially mitigated by the optimized extraction protocol. The chromatograms for samples and 15.0 μg/L spikes of Analysis Set 2 are also presented in the supplementary materials (Figs. S9–S14).
Figure 5.
A MRM chromatogram of the optimized extraction protocol for Changzhou S11–2 showing the presence of 1,3-DMAA diastereomers (peaks 1 and 2) and 1,4-DMAA (peak 3).
Note: The mass transitions used are 116/99.7 m/z and 116/57 m/z.
Figure 6.
A typical MRM chromatogram of the Guiyang 2 sample demonstrating the absence of 1,3-DMAA and 1,4-DMAA in the geranium plant.
Note: The mass transitions used are 116/99.7 m/z and 116/57 m/z.
A comparison of the two extraction protocols using Changzhou S11 geranium sample demonstrates that the preliminary extraction protocol underestimated the concentrations of both DMAA species as indicated by the percent recovery results. However, it is clear that Changzhou S11 geranium samples contain 1,3-DMAA species and the concentrations are well above the MDL of both analysis. In contrast, Kunming and Guiyang samples did not contain 1,3-DMAA or 1,4-DMAA species at significant concentrations above the MDL of analysis (20 ng/g).
Analysis set 3: optimized extraction protocol of summer geranium samples
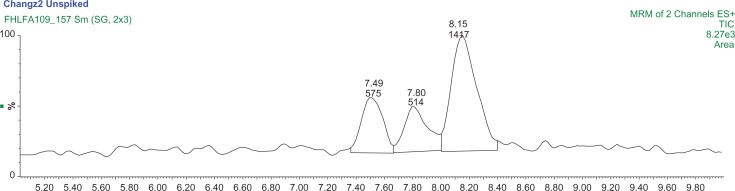
An additional round of samples was collected from a summer harvest of geranium plants and analyzed using the same protocols from Analysis Set 2 (with two spike levels, in duplicate). The Changzhou 3 sample (Fig. 7) contained 1,3-DMAA and 1,4-DMAA concentrations of 68.8 ± 36.5 ng/g and 118 ± 45 ng/g, respectively (Table 5). Both Kunming 3 and Guiyang 3 had concentrations of 1,3-DMAA and 1,4-DMAA below the MDL (less than 10 ng/g). These results are consistent with the previous winter sample analysis. Both DMAA species were detected and quantified in the Changzhou samples, but no DMAA species were detected above the MDL in Kunming and Guiyang samples (See Supplementary materials Figs. S15–S20). The percent recovery for all samples was excellent and ranged between 64% and 86%.
Figure 7.
A MRM chromatogram of Changzhou 3 sample showing the presence of 1,3-DMAA at a lower concentration than 1,4-DMAA.
Note: Mass transitions are 116/99.7 m/z and 116/57 m/z.
Table 5.
Analysis set 3: optimized extraction protocol results of geranium summer samples from Kunming, Guiyang, and Changzhou.
|
1,3-DMAA
|
1,4-DMAA
|
|||||
|---|---|---|---|---|---|---|
| Sample (ng/g) | Spike level (μg/L) | Percent recovery (%) | Sample (ng/g) | Spike level (μg/L) | Percent recovery (%) | |
| Kunming 3 | <10 ± 6* | 15.0 | 68 ± 3 | <8.2 ± 0.3* | 15.0 | 64 ± 2 |
| 25.0 | 74 ± 6 | 25.0 | 75 ± 9 | |||
| Guiyang 3 | <10 ± 6* | 15.0 | 75 ± 4 | <8.1 ± 0.2* | 15.0 | 78 ± 1 |
| 25.0 | 81 ± 8 | 25.0 | 84 ± 6 | |||
| Changzhou 3 | 68.8 ± 36.5 | 15.0 | 76 ± 13 | 118 ± 45 | 15.0 | 86 ± 4 |
| 25.0 | 79 ± 13 | 25.0 | 77 ± 7 | |||
Note:
The results are less than the MDL values.
Winter versus summer sample analysis
Previous research has shown that concentrations of chemical species in natural products can be highly variable.18,19 A seasonal comparison is possible between the winter harvest (March 2012) and the summer harvest (May 2012) for Kunming, Guiyang and Chang-zhou samples. Neither the winter nor summer harvest samples of Kunming and Guiyang samples contained 1,3-DMAA or 1,4-DMAA species above the MDLs of the analysis. However, Changzhou sample resulted in similar concentrations of 1,3-DMAA and 1,4-DMAA in the June 2011 and March 2012 samples. From March 2012 to May 2012, 1,3-DMAA resulted in a factor of 3 decrease in concentration while 1,4-DMAA about doubled in concentration. These results indicate a potential seasonal effect of 1,3-DMAA and 1,4-DMAA concentrations in agreement with previously reported research discussing environmental effects on chemical composition.18,19 It is also possible the concentrations of 1,3-DMAA in Changzhou winter samples were higher due to an apparent underestimation of 1,3-DMAA concentrations by the preliminary extraction protocol as evidenced by Changzhou S11 analysis.
Standard addition analysis of 1,3-DMAA and 1,4-DMAA
A standard addition analysis protocol was developed for sample analysis. Standard addition analysis compensates for matrix effects found in geranium plants caused by chemical species other than DMAA affecting analytical signal (either positive or negative).13 In the standard addition method, known quantities of the 1,3-DMAA and 1,4-DMAA standards are added to the sample extract. This is termed “spiking” the sample. The added standard is affected by matrix effects just as the analyte in the sample. The unknown concentration can then be derived from a plot of signal versus spike concentration as long as the analyte has been previously established to have a linear signal response. Thus, the standard addition method resolves matrix interferences present in the complex geranium sample composition.13
The standard addition protocol was applied to both Analysis Sets 2 and 3 (Changzhou S11, Kunming, and Guiyang winter samples and Changzhou, Kunming, and Guiyang summer samples). For this study, a three-point standard addition plot was constructed using the unspiked sample, a 15.0 μg/L spike each of 1,3-DMAA and 1,4-DMAA, and a 25.0 μg/L spike each of 1,3-DMAA and 1,4-DMAA. The signal was plotted against the spike concentration (0, 15, and 25 μg/L), and a linear regression analysis was performed. The slope (m) and y-intercept (b) of the calibration curve were used to calculate the concentration of analyte (x) in the sample.13 The equation for determining the x-intercept is x = −b/m, and in standard addition, the negative of the x-intercept is the concentration present in the unspiked sample.
The standard addition analysis results showed some matrix effects were still present in the optimized procedure and the external calibration analysis likely underestimated DMAA concentrations. However, the standard addition analysis agreed overall with the external calibration results. Samples reported to contain 1,3-DMAA by external calibration also contained 1,3-DMAA by standard addition. Concentrations of 1,3-DMAA species were quantified in both Changzhou S11–2 and Changzhou 3 samples at 496 ± 46 ng/g and 97 ± 20 ng/g, respectively. The concentrations of 1,4-DMAA in Changzhou S11–2 and Changzhou 3 samples were 68 ± 7 ng/g and 162 ± 48 ng/g, respectively. All concentrations were well above the MDL of the analysis and clearly demonstrated 1,3-DMAA and 1,4-DMAA were present in geranium herbs from the Changzhou region.
The standard addition results for winter and summer samples of Kunming and Guiyang agreed with the external calibration results. Concentrations of 1,3-DMAA and 1,4-DMAA were all less than the MDL previously reported, or so close to the MDL that the confidence of analysis was extremely low. One of the Kunming 3 duplicates resulted in a 1,3-DMAA concentration of 21 ng/g, while the other duplicate was below the MDL of 14 ng/g. Similarly, one of the Kunming 2 duplicates resulted in a 1,4-DMAA concentration of 10 ng/g, whereas the other duplicate had concentrations less than the 20 ng/g MDL of that particular analysis.
Measurement of the diastereomer ratios of 1,3-DMAA in the Changzhou geranium samples
Zhang et al5 measured the diastereomer ratios (reported as first peak/second peak) of synthetic standards and dietary supplements containing 1,3-DMAA using GC-FID analysis. The reported results showed the diastereomer ratio of a Sigma-Aldrich standard of 1,3-DMAA was 1.22 ± 0.06 and the ChromaDex standard ratio was 1.42 ± 0.09. The dietary supplements had identical ratios to those of the standards suggesting that both standards and supplements were of synthetic origin.
In this report, both pairs of diastereomers were detected in the Changzhou region samples as well as the synthetic calibration standards. By inspection of the chromatograms (Figs. 3, 4, 5, and 7), both standards and geranium samples present similar diastereomer ratios. Quantitatively, the average ratio of 1,3-DMAA diastereomers (first peak/second peak) in typical 20, 50 and 100 μg/L calibration standards is 1.14 ± 0.08. The diastereomer ratio of Changzhou S11-1 sample was 1.10 ± 0.01, Changzhou 1 was 1.02, Changzhou S11–2 was 1.25 ± 0.03, and Changzhou 3 was 1.16 ± 0.10. The results of the geranium plant diastereomer ratios are similar to the ratios of the synthetic standards presented here, as well as the standards and supplements analyzed by Zhang et al. This indicates that supplements containing both 1,3-DMAA diastereomer pairs couldbenaturallyproducedandextractedfromgeranium plants.
Conclusion
In conclusion, geranium plants (Pelargonium graveolens) from three different regions of China (Kunming, Guiyang, and Changzhou) and three different harvests (June 2011, March 2012, and May 2012) were analyzed for 1,3-DMAA and 1,4-DMAA. An extraction and HPLC-MS/MS analysis method was used to determine concentrations of 1,3-DMAA and 1,4-DMAA with both external calibration and standard addition analysis. The extraction and external calibration analysis likely suffered from matrix effects and thus underestimated concentrations of 1,3-DMAA and 1,4-DMAA in geranium plants. The matrix effects were largely solved by the standard addition analysis, as expected. This demonstrates that future analysis should use standard addition to minimize matrix effects and increase confidence of analysis with little additional labor. All extraction and calibration protocols reported 1,3-DMAA and 1,4-DMAA concentrations in geranium plants from the Changzhou region of China above the reported MDLs. The reported concentrations of 1,3-DMAA ranged from 68 to 496 ng/g and 1,4-DMAA ranged from 13 to 162 ng/g. Similarly, 1,3-DMAA and 1,4-DMAA were not detected above the MDL in samples from Guiyang and Kunming regions. To the best of the authors’ knowledge, this is the first reported inter-laboratory analysis confirming the presence of 1,3-DMAA in a geranium plant (specifically Changzhou S11 sample). Finally, the diastereomer ratios of the 1,3-DMAA in geranium plants from Changzhou are similar to those of the synthetic standards. This indicates that 1,3-DMAA could be a natural product extract, fulfilling a requirement of the Dietary Supplement Health and Education Act.20
The results reported here provide evidence that 1,3-DMAA naturally occurs in geranium plants in agreement with Li et al,6 but clearly in disagreement with other previously reported articles by well-respected chemists and organizations.4,5 However, this may not be a case of right or wrong. In analytical chemistry, the critical review of data is important for explaining differences in reported results. These differences can also provide insight into why analysis of seemingly identical plant species can result in very different outcomes. Khan has published an extensive review showing that it is not uncommon for plants of different locations to exhibit variations in their chemical compositions.18 For example, studies show that fluctuating geographical dynamics such as water stress and nutrient availability in the soil are associated with the variations in cyanide concentration in the cassava plant.19
The published research to date includes a substantial amount of geranium plant and oil analysis.1–6 However, until now, none of the samples analyzed have been identical or reported as from the same region. Thus, regional environmental variations18,19 could explain the presence of 1,3-DMAA in the Changzhou S11, Changzhou March 2012, and Changzhou May 2012 samples and the absence of 1,3-DMAA concentrations in Kunming and Guiyang geranium samples reported here; the Indian and Mississippi samples reported by ElSohly et al,4 the France, Egypt, and New Zealand samples reported by Lisi et al,3 and the China and Egypt samples reported by Zhang et al.5 A possible solution to this discrepancy would be a multiple laboratory and blind analysis of identical samples expected to have 1,3-DMAA (such as Changzhou region samples) as well as samples that are not expected to contain 1,3-DMAA. Using this approach, a satisfactory answer for the national regulatory agencies as well as the commercial interests could be provided.
Acknowledgments
The authors would like to acknowledge and thank Dr. Yi Jin of Yunnan University for overseeing the geranium sample collection and shipment to The University of Memphis.
Footnotes
Video Abstract Available from http://la-press.com/t.php?i=10445
Author Contributions
Conceived and designed the experiments: PSS, HLF, PLR. Analysed the data: HLF, PLR, PSS. Wrote the first draft of the manuscript: HLF, PLR, PSS. Contributed to the writing of the manuscript: HLF, PLR, PSS. Agree with manuscript results and conclusions: HLF, PLR, PSS. Jointly developed the structure and arguments for the paper: HLF, PLR, PSS. Made critical revisions and approved final version: HLF, PLR, PSS. All authors reviewed and approved of the final manuscript.
Funding
The authors would like to acknowledge and thank USP Labs, LLC for funding portions of this work. Authors confirm that USP Labs, LLC had no influence over the content of this paper and contributing research.
Competing Interests
PSS has received consulting fees from CirQuest Labs for consulting work on analysis for pharmaceutical and implantable devices. All other authors disclose no competing interests.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Supplemental Materials
Figure S1 is an example of a typical, original (bottom) and smoothed (top) chromatogram for a sample blank. Figures S2 and S3 are examples of 1,3 and 1,4-DMAA standard chromatograms. Two concentrations are shown: 3 (Fig. S2) and 20 (Fig. S3) μg/L each DMAA. 1,3-DMAA elutes as the first two peaks, followed by 1,4-DMAA as the third peak. Figures S4 to S20 are chromatograms for each geranium herb sample. Unspiked and spiked chromatograms are shown for each sample where possible. Each chromatogram is labeled by the corresponding table number found in the paper. As with the standards, 1,3-DMAA elutes as the first two peaks followed by 1,4-DMAA as the third peak. Figures S4 to S8 are typical chromatograms from Analysis set 1 and were used to determine the concentration of Guiyang 1, Kunming 1, and Changzhou S11–2 in Table 3. Figures S9 to S14 are typical chromatograms from Analysis set 2 and were used to determine the concentration of Guiyang 2, Kunming 2, and Changzhou S11–2 in Table 4. Figures S15 to S20 are typical chromatograms from Analysis set 3 and were used to determine the concentration of Guiyang 3, Kunming 3, and Changzhou 3 in Table 5.
An example of a blank chromatogram.
Notes: The blank sample is prepared in the same way as an unspiked sample but there is no addition of geranium herb to the blender. The original chromatogram is presented on the bottom and the smoothed chromatogram is presented on the top.
Typical MRM chromatogram of 3 μg/L 1,3-DMAA and 1,4-DMAA.
Typical MRM chromatogram of 20 μg/L 1,3-DMAA and 1,4-DMAA.
Analysis set 1—Changzhou S11-1, unspiked.
Analysis set 1—Changzhou S11-1, spike 10 μg/L.
Analysis set 1—Guiyang 1, unspiked.
Analysis set 1—Guiyang 1, spike 10 μg/L.
Analysis set 1—Changzhou 1, unspiked.
Analysis set 2—Guiyang 2 unspiked.
Analysis set 2—Guiyang 2, spike 15 μg/L.
Analysis set 2—Kunming 2, unspiked.
Analysis set 2—Kunming 2, spike 15 μg/L.
Analysis set 2—Changzhou S11–2 unspiked.
Analysis set 2—Changzhou S11–2, spike 15 μg/L.
Analysis set 3—Guiyang 3, unspiked.
Analysis set 3—Guiyang 3, spiked 15 μg/L.
Analysis set 3—Kunming 3, unspiked.
Analysis set 3—Kunming 3, spiked 15 μg/L.
Analysis set 3—Changzhou 3, unspiked.
Analysis set 3—Changzhou 3, spiked 15 μg/L.
Supplementary data
A video abstract by the authors of this paper is available.
References
- 1.Perrenoud L, Saugy M, Saudan C. Detection in urine of 4-methyl-2-hexaneamine, a doping agent. J Chromatogr B. 2009;877(1):3767–70. doi: 10.1016/j.jchromb.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Vorce SP, Holler JM, Cawrse BM, Magluilo J. Dimethylamylamine: A drug causing positive immunoassay results for amphetamines. J Anal Toxicol. 2011;35(3):183–7. doi: 10.1093/anatox/35.3.183. [DOI] [PubMed] [Google Scholar]
- 3.Lisi A, Hasick N, Kazlauskas R, Goebel C. Studies of methylhexaneamine in supplements and geranium oil. Drug Test Anal. 2011;3(11–2):873–6. doi: 10.1002/dta.392. [DOI] [PubMed] [Google Scholar]
- 4.ElSohly MA, Gul WG, ElSohly KM, Murphy TP, et al. Pelargonium oil and methyl hexaneamine (MHA): Analytical approaches supporting the absence of MHA in authenticated pelargonium graveolens plant material and oil. J Anal Toxicol. 2012;36(7):457–71. doi: 10.1093/jat/bks055. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Woods RM, Breitbach ZS, Armstrong DW. 1,3-Dimethylamylamine (DMAA) in supplements and geranium products: natural or synthetic? Drug Test Anal. 2012 Jul 12; doi: 10.1002/dta.1368. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 6.Li JS, Chen M, Li ZC. Indentification and quantification of dimethylamylamine in geranium by liquid chromatography tandem mass spectrometry. Anal Chem Insights. 2012;7:47–58. doi: 10.4137/ACI.S9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Food and Drug Administration. FDA challenges marketing of DMAA products for lack of safety evidence. FDA News Release. Apr 27, 2012. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm302133.htm. Accessed Aug 10, 2012.
- 8.Skoog DA, Holler FJ, Crouch SR. Principles of Instrumental Analysis. 6th ed. Belmont, CA: Thomson Higher Education; 2007. [Google Scholar]
- 9.Li J. Standard Analytical Method AACL-SAM 11044: 1,3- and 1,4-Dimethylpentylamines Geranium Plant by LC/MS/MS. Champaign, IL: Intertek-AAC Labs; 2011. [Google Scholar]
- 10.Savitzky M, Golay JE. Smoothing and differentiation of data by simplified least squares procedures. Anal Chem. 1964;36(8):1627–39. [Google Scholar]
- 11.Glaser JA, Foerst DL, McKee GD, Quave SA, Budde WL. Trace Analysis for Wastewaters. Environ Sci Tech. 1981;15(12):1426–35. [Google Scholar]
- 12.United States Environmental Protection Agency. Code of Federal Regulations. Title 40—Protection of Environment. 40 CFR Appendix B to Part 136-Definition and Procedure for the Determination of the Method Detection Limit-Revision 1.11. Cincinnati, OH: US Environmental Protection Agency; 1996. pp. 303–6. [Google Scholar]
- 13.Harris DC. Quantitative Chemical Analysis. 8th ed. New York, NY: WH Freeman and Company; 2010. [Google Scholar]
- 14.United States Environmental Protection Agency. DBP/ICR Analytical Methods Manual. Cincinnati, OH: US Environmental Protection Agency, Office of Water; 1996. EPA 814-B-96-002. [Google Scholar]
- 15.Ranaivo PL, Henson CM, Simone PS, Emmert GL. Analysis of haloacetic acids in drinking water using post-column reaction-ion chromatography with on-line internal standardization. Anal Methods. 2011;3:2873–80. [Google Scholar]
- 16.Brown MA, Emmert GL. On-line purge and trap as chromatography for monitoring of THMs in drinking water distribution systems. Anal Chim Acta. 2007;592(2):154–61. doi: 10.1016/j.aca.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Simone PS, Jr, Ranaivo PL, Geme G, Brown MA, Emmert GL. On-line monitoring of nine haloacetic acid species at the μg L−1 level using post-column reaction-ion chromatography with nicotinamide fluorescence. Anal Chim Acta. 2009;654(2):133–40. doi: 10.1016/j.aca.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Khan IA. Issues related to botanicals. Life Sci. 2006;78(18):2033–8. doi: 10.1016/j.lfs.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Burns AE, Gleadow RM, Zacarias AM, Cuambe CE, Miller RE, Cavagnaro TR. Variations in the chemical compositin of cassava (Manihot esculenta Crantz) leaves and roots as affected by genotypic and environmental variation. J Agric Food Chem. 2012;60(19):4946–56. doi: 10.1021/jf2047288. [DOI] [PubMed] [Google Scholar]
- 20.National Institutes of Health. Dietary Supplement Health and Education Act of 1994. Bethesda, MD: National Institutes of Health, Office of Dietary Supplements; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 is an example of a typical, original (bottom) and smoothed (top) chromatogram for a sample blank. Figures S2 and S3 are examples of 1,3 and 1,4-DMAA standard chromatograms. Two concentrations are shown: 3 (Fig. S2) and 20 (Fig. S3) μg/L each DMAA. 1,3-DMAA elutes as the first two peaks, followed by 1,4-DMAA as the third peak. Figures S4 to S20 are chromatograms for each geranium herb sample. Unspiked and spiked chromatograms are shown for each sample where possible. Each chromatogram is labeled by the corresponding table number found in the paper. As with the standards, 1,3-DMAA elutes as the first two peaks followed by 1,4-DMAA as the third peak. Figures S4 to S8 are typical chromatograms from Analysis set 1 and were used to determine the concentration of Guiyang 1, Kunming 1, and Changzhou S11–2 in Table 3. Figures S9 to S14 are typical chromatograms from Analysis set 2 and were used to determine the concentration of Guiyang 2, Kunming 2, and Changzhou S11–2 in Table 4. Figures S15 to S20 are typical chromatograms from Analysis set 3 and were used to determine the concentration of Guiyang 3, Kunming 3, and Changzhou 3 in Table 5.
An example of a blank chromatogram.
Notes: The blank sample is prepared in the same way as an unspiked sample but there is no addition of geranium herb to the blender. The original chromatogram is presented on the bottom and the smoothed chromatogram is presented on the top.
Typical MRM chromatogram of 3 μg/L 1,3-DMAA and 1,4-DMAA.
Typical MRM chromatogram of 20 μg/L 1,3-DMAA and 1,4-DMAA.
Analysis set 1—Changzhou S11-1, unspiked.
Analysis set 1—Changzhou S11-1, spike 10 μg/L.
Analysis set 1—Guiyang 1, unspiked.
Analysis set 1—Guiyang 1, spike 10 μg/L.
Analysis set 1—Changzhou 1, unspiked.
Analysis set 2—Guiyang 2 unspiked.
Analysis set 2—Guiyang 2, spike 15 μg/L.
Analysis set 2—Kunming 2, unspiked.
Analysis set 2—Kunming 2, spike 15 μg/L.
Analysis set 2—Changzhou S11–2 unspiked.
Analysis set 2—Changzhou S11–2, spike 15 μg/L.
Analysis set 3—Guiyang 3, unspiked.
Analysis set 3—Guiyang 3, spiked 15 μg/L.
Analysis set 3—Kunming 3, unspiked.
Analysis set 3—Kunming 3, spiked 15 μg/L.
Analysis set 3—Changzhou 3, unspiked.
Analysis set 3—Changzhou 3, spiked 15 μg/L.
A video abstract by the authors of this paper is available.