Abstract
Interleukin-1 (IL-1)-induced activation of the mTOR kinase pathway has major influences on Th17 cell survival, proliferation and effector function. Using biochemical and genetic approaches, the kinases IKKi and GSK3α were identified as the critical intermediate signaling components for IL-1-induced AKT activation, which in turn activated mTOR. Although insulin-induced AKT activation is known to phosphorylate and inactivate GSK3α and GSK3β, we found GSK3α, but not GSK3β formed a constitutive complex to phosphorylate and suppress AKT activation, showing that a reverse action from GSK to AKT can take place. Upon IL-1 stimulation, IKKi was activated to mediate GSK3α phosphorylation at S21, thereby inactivating GSK3α to promote IL-1-induced AKT-mTOR activation. Thus, IKKi has a critical role in Th17 cell maintenance and/or proliferation through the GSK-AKT-mTOR pathway, implicating the potential of IKKi as a therapeutic target.
Introduction
Invading pathogens are detected by the innate immune system through the recognition of pathogen-associated molecular patterns by various pattern recognition receptors including Toll-like receptors. The activation of pattern recognition receptors induces the production of inflammatory cytokines such as IL-1 and TNF, leading to inflammatory responses (Kawai and Akira, 2007). Activation of professional antigen- presenting cells through the pattern recognition receptors leads to the onset of adaptive immunity, triggering the differentiation and activation of CD4+ T helper (Th) lymphocytes, which is essential in regulating immune responses and autoimmune and inflammatory diseases (Janeway, Jr. and Medzhitov, 2002). Th17 cells have been defined as a distinct lineage of CD4+ Th cells that produce IL-17, IL-17F, IL-21 and IL-22 (Langrish et al., 2005; Bettelli et al., 2006; Harrington et al., 2005; Mangan et al., 2006; Park et al., 2005; Veldhoen et al., 2006). Th17 cells have attracted tremendous attention in immunology due to their important function in host defense against bacterial and fungal infection and potent pathogenic role in autoimmune and inflammatory diseases (Langrish et al., 2005; Park et al., 2005).
Several cytokines including TGF-β and IL-6 are required for Th17 cell differentiation upon T cell receptor (TCR) activation. Transcription factors including STAT3 together with lineage-specific factors RORα and RORγt to direct Th17 cell development and effector function through induction of a set of signature cytokines and cytokine receptors, including IL-23R and IL-1R (Korn et al., 2009; Wei et al., 2007; Ivanov et al., 2006; McGeachy et al., 2007). Although the detailed molecular mechanism is still unclear, mice lacking either IL-1 or IL-23 are resistant to disease induction in Th17 cell-dependent collagen induced arthritis (CIA), experimental autoimmune encephalomyelitis (EAE), and inflammatory bowel disease (IBD) (Ben-Sasson et al., 2009; Sutton et al., 2006; Murphy et al., 2003; Langrish et al., 2005; Chung et al., 2009).
Although the detailed molecular mechanism is still unclear, IL-1 has major influences on Th17 cell development and effector function, possibly through cell proliferation, and survival to maintain the differentiated state of Th17 cells (Korn et al., 2009; Gulen et al., 2010). IL-1 stimulation leads to strong and prolonged activation of the mammalian target of rapamycin (mTOR) pathway in Th17 cells, including phosphorylation of mTOR and downstream components 4E-BP1 and S6, which play a key role in controlling protein translation. 4E-BP1 is a repressor for protein translation through its interaction with translation initiation factor eIF4E. Phosphoylation of 4E-BP1 by mTOR disrupts its interaction with eIF4E, thereby promoting protein translation, especially on mRNAs with structured 5′ UTR including cMyc and Cyclin D1 (Hashemolhosseini et al., 1998; Nelsen et al., 2003). Although mTOR signaling is known to have a major impact on cell growth and proliferation, the mTOR inhibitor rapamycin abolishes IL-1-induced cell proliferation in Th17 cells, indicating the importance of IL-1-induced mTOR activation in IL-1-dependent Th17 cell maintenance and/or proliferation.
Here, we investigated the intermediate signaling events for IL-1-induced mTOR activation and how this pathway is modulated. We found that AKT was sequestered in a complex with IKKi and glycogen synthase kinase 3α (GSK3α) before IL-1 stimulation. GSK3α negatively regulates AKT activation by phosphorylating AKT at T312 in the substrate binding site, which inhibited IL-1-induced AKT activation and function. Importantly, although IKKi constitutively interacted with GSK3α, IL-1 stimulation induced the recruitment of IKKi to the TRAF6-TAK1 signaling complex. IKKi (also known as IKKε or I kappaB kinase ε) is then activated to mediate IL-1-induced GSK3α phosphorylation at S21 and consequent inactivation of GSK3α, resulting in AKT-mTOR activation. It is important to note that whereas PI3K-AKT are known to be upstream kinases for insulin-induced GSK3 phosphorylation and inactivation, this study provides an example that a reverse action from GSK to AKT can take place. We also demonstrate a distinct role of GSK3α versus GSK3β in IL-1 signaling, implicating the specific function of GSK3α in autoimmune inflammatory responses. IL-1-induced IKKi-GSK3α-mediated AKT-mTOR axis is probably not only critical for the modulation of Th17 cell proliferation and Th17 cell-dependent autoimmunity, but also important for coordinated regulation of immune responses and cell metabolism.
Results
PI3K and AKT are required for IL-1-induced mTOR-dependent Th17 cell proliferation
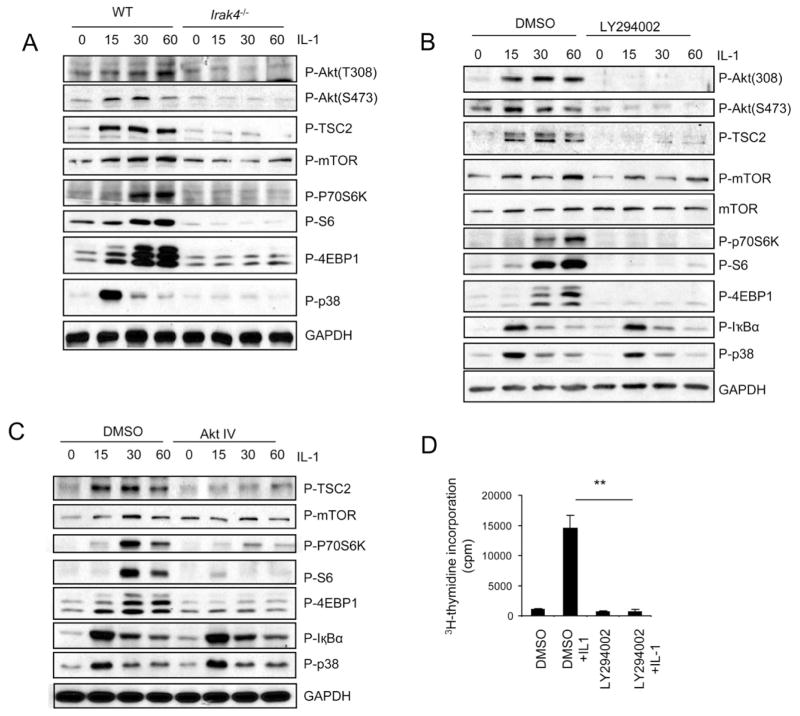
PI3K and AKT are upstream positive regulators of mTOR through the phosphorylation of TSC2 and consequent disruption of TSC1/TSC2 heterodimer (Manning et al., 2002; Inoki et al., 2002). IL-1 stimulation indeed induced AKT (T308 and S473) and TSC2 (T1462) phosphorylation in wild-type Th17 cells, which was abolished in IRAK4-deficient Th17 cells (Fig. 1A). To test the necessity of PI3K activity in IL-1 induced mTOR activation, Th17 cells were stimulated with IL-1 in the presence or absence of PI3K (LY294002) and AKT (AktIV) inhibitors. LY294002 and AktIV effectively inhibited IL-1-induced phosphorylation of mTOR and downstream components p70S6K, S6 and 4EBP1 (Fig. 1B–C), whereas phosphorylation of the kinases IκBα and p38 was not affected. Furthermore, IL-1-induced survival and/or proliferation of Th17 cells was also substantially reduced in the presence of LY294002 (Fig. 1D), indicating the requirement of PI3K-Akt axis in IL-1-mediated mTOR activation and Th17 cell maintenance and/or proliferation.
Figure 1. PI3K and AKT are required for IL-1-induced mTOR activation.
(A) Cell lysates from wild-type and Irak4−/− Th17 cells untreated or treated with IL-1 (10ng/ml) for different time points were analyzed by protein blot analysis using antibodies as indicated. (B–C) Wild-type Th17 cells were untreated or treated with IL-1 (10ng/ml) for different time points in the presence and absence of (B) 10μM PI3K inhibitor (LY294002) and 1μM Akt inhibitor (Akt IV) (C). Cell lysates were analyzed by protein blot analysis using antibodies as indicated. (D) Wild-type Th17 cells were rested for overnight, followed by incubation with 10ng/ml IL-1 in the presence and absence of 10μM PI3K inhibitor (LY294002). The treated cells were incubated one additional day with 3H for thymidine incorporation experiment. Error bars, s.d.; **, p<0.01 (two tailed t-test). Data are representative of three independent experiments.
IL-1-induced GSK3α phosphorylation is independent on PI3K-AKT kinase activity
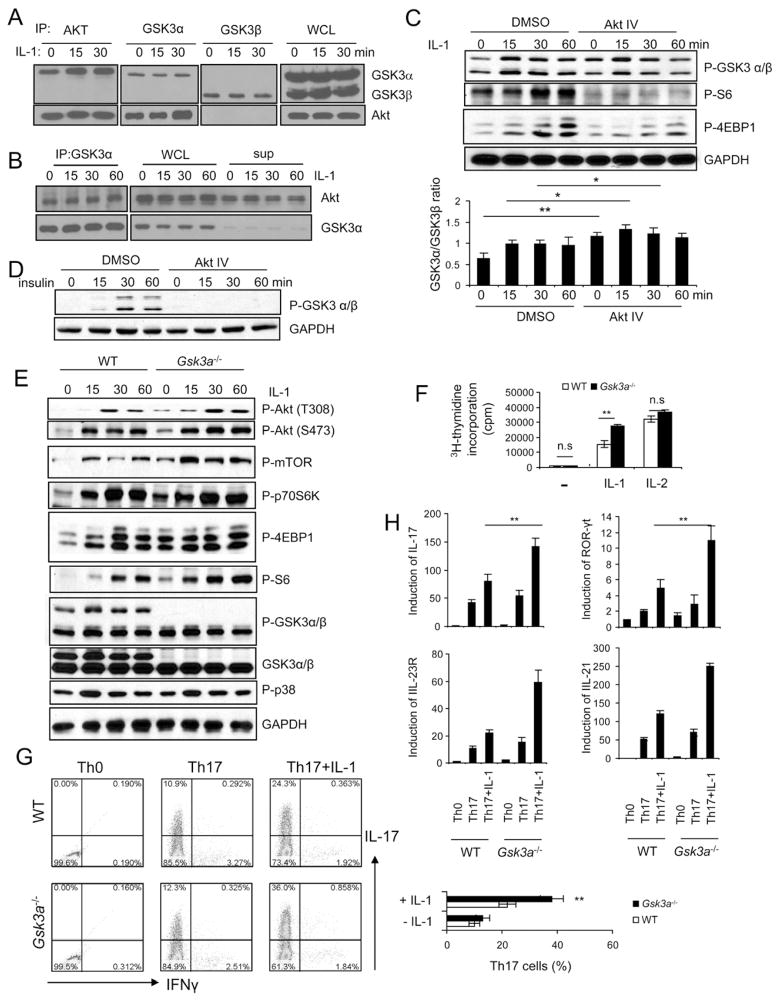
To identify intermediate signaling components that mediate IL-1-induced AKT activation, we searched for AKT interacting proteins. Co-immunoprecipitation of lysates from 293 cells transfected with IL-1R (293-IL-1R) showed that AKT interacted with GSK3α, but not GSK3β (Fig 2A). IL-1 stimulation further induced the interaction of AKT with GSK3α. GSK3α and GSK3β were not co-immunoprecipated, suggesting that they are probably not in the same complex (Fig. 2A). Furthermore, when GSK3α was depleted in the supernatant after two cycles of immunoprecipitation, there was still substantial amount of AKT in the supernatant, implicating GSK3α-AKT represents one of the AKT complexes in the cells (Fig. 2B). The activated AKT upon insulin stimulation are known to phosphorylate GSK3α (S21) and GSK3β (S9), inactivating GSK3 activity, which then removes its inhibitory effect on glycogen synthase (Cross et al., 1995). IL-1 stimulation also induced phosphorylation of GSK3α (S21) and GSK3β (S9) in wild-type Th17 cells, which was abolished in IRAK4-deficient cells (Suppl. Fig 1). Whereas PI3K and AKT inhibitors completely blocked insulin-induced GSK3α and β phosphorylation, both inhibitors partially attenuated IL-1-induced GSK3β but not GSK3α phosphorylation (Fig. 2C–D and Suppl. Fig. 2A). The inhibition of AktIV on IL-1-induced GSK3β phosphorylation is consistent with the previous finding that AKT mediates GSK3β phosphorylation to remove the inhibitory effect of GSK3β on mTOR activation (Cross et al., 1995). In contrast, these results implicate differential roles of GSK3α and GSK3β in IL-1 signaling.
Figure 2. IL-1R-mediated GSK3-α phosphorylation is independent on PI3K-AKT kinase activity.
(A) Lysates from 293-IL-1R cells were untreated or treated for 15 or 30 min with IL-1 (1 ng/ml) were immunoprecipitated with anti-AKT, anti-GSK3α and β, followed by protein blot analysis. WCL (whole cell lysates). (B) GSK3α was pulled down with two rounds of immunoprecipitation. Immunoprecipitated complex, whole cell lysate and the remaining supernatant were analyzed by western blot analysis using GSK3α and Akt1 antibody. (C) Wild-type Th17 cells were untreated or treated with IL-1 (10ng/ml) for different time points in the presence and absence of 1μM Akt inhibitor (Akt IV). Cell lysates were analyzed by western blot analysis using antibodies as indicated. Densitometry quantitation of pooled data is plotted below. (D) Wild-type bone-marrow macrophages were untreated or treated with insulin (10nM) for different time points in the presence and absence of 1μM Akt inhibitor (Akt IV), cell lysates were analyzed by western blot analysis using antibodies as indicated. (E) Cell lysates from wild-type and Gsk3a−/− Th17 cells untreated or treated with IL-1 (10ng/ml) for different time points were analyzed by western blot analysis using antibodies as indicated. (F) Wild-type and Gsk3a−/− Th17 cells were rested for overnight, followed by incubation with 10ng/ml IL-1 or IL-2 for three additional days and with 3H for one more day for thymidine incorporation experiment. (G) Naïve wild-type, Gsk3a−/−CD4+ T cells (CD4+CD44low) were polarized to Th17 cells in the presence and absence of IL-1β, followed by intracellular cytokine staining for IL-17 and IFN- γ (H) Real-time PCR analysis of relative expression of IL-17, IL-21, IL-23R, and ROR-γt in wild-type and Gsk3a−/− Th17 cells as compared to the Th0 cells. Error bars (F–H), s.d. **, p<0.01 (two tailed t-test). All data are representative of three independent experiments.
GSK3α negatively modulates IL-1-induced AKT-mTOR activity
An important question was then whether and how GSK3α contributes to IL-1-dependent effector function in Th17 cells. We first examined the impact of GSK3α deficiency on IL-1-mediated signaling in Th17 cells. While p38 phosphorylation was not affected, both constitutive and IL-1-induced AKT-mTOR activation (phosphorylation of AKT, mTOR, p70S6K and S6) were substantially enhanced in GSK3α-deficient Th17 cells compared to that in wild-type cells (Fig 2E and Suppl. Fig 2B). Consistent with the increased mTOR activity, GSK3α deficiency resulted in enhanced IL-1-mediated Th17 cell maintenance and/or proliferation (Fig. 2F). GSK3α did not have much impact on the response of Th17 cells to IL-2, indicating the specific function of this component in IL-1 pathway (Fig. 2F). WT and Gsk3a−/− naïve T cells were differentiated into Th17 cells with or without IL-1β stimulation. Similar numbers of IL-17-positive cells (10–12%) were detected in WT and Gsk3a−/− T cells under TGFβ+IL-6-mediated differentiation condition (in the absence of IL-1β stimulation) (Fig. 2G). Importantly, although IL-1β promoted Th17 cell differentiation in both WT and Gsk3a−/− T cells, addition of IL-1β resulted in a significantly higher IL-17-positive population in Gsk3a−/− T cells than that in WT T cells (Fig. 2G). Consistent with flow cytometry analysis, the expression of other Th17 cell-associated molecules (including IL-17, RORγt IL-21 and IL-23R) was also much higher in Gsk3a−/− T cells than that in WT cells in the presence of IL-1β stimulation (Fig. 2H). Taken together, these results suggest that GSK3α actually negatively regulates IL-1-induced AKT-mTOR activity and consequently suppresses IL-1-dependent Th17 cell differentiation and survival/proliferation. The fact that IL-1 induces specific interaction of GSK3α with PI3K and AKT (Fig. 2A) suggests that GSK3α might act upstream of PI3K and AKT and modulate their activity in response to IL-1 stimulation, which is consistent with the observation that GSK3α phosphorylation was not inhibited by PI3K and AKT inhibitors (Fig. 2C). Importantly, while IL-1 stimulation led to increased AKT and S6 phosphorylation in Gsk3a−/− MEFs, the insulin-induced AKT and S6 phosphorylation was comparable in wild-type and Gsk3a−/− MEFs (Suppl. Fig. 2C). These results support the notion that GSK3α has an inhibitory role in IL-1-, but not insulin-induced AKT activation.
GSK3 α phosphorylates Akt and inhibits its kinase activity
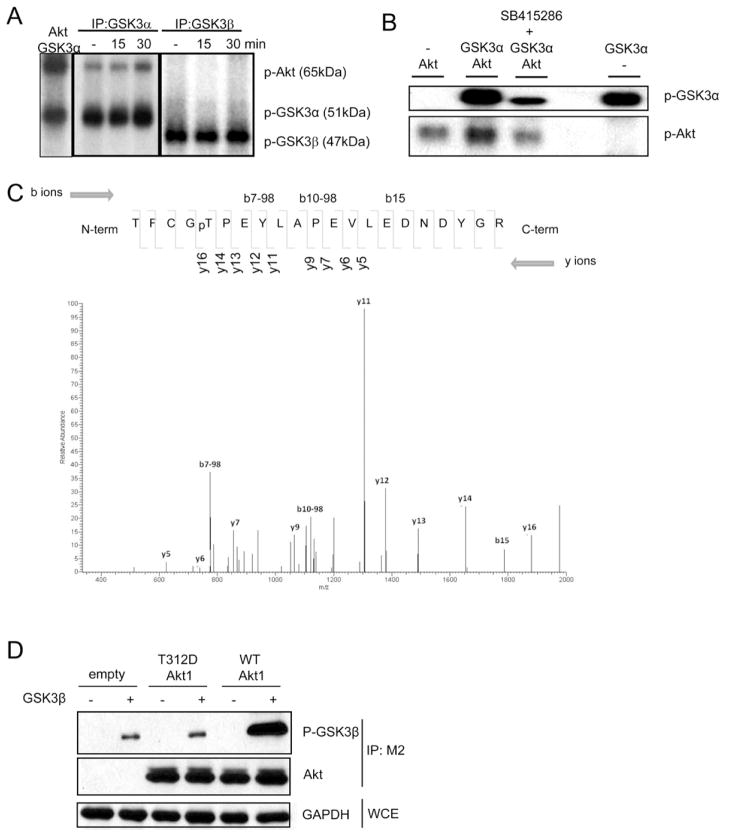
We then explored about how GSK3α inhibits IL-1-induced AKT-mTOR activation. Through co-immunoprecipitation of lysates from 293-IL-1R cells with anti-GSK3α or anti-GSK3β followed by in vitro kinase assay, it was noted that GSK3α, but not GSK3β, immunoprecipitates phosphorylated a protein at the size of AKT (Fig. 3A). These results are consistent with the observation that AKT was only co-immunoprecipiated with GSK3α but not with GSK3β (Fig. 2A). In vitro kinase assay with recombinant proteins showed that GSK3α indeed promoted AKT phosphorylation (Fig. 3B). Tandem MS revealed that GSK3α promoted AKT phosphorylation at T312 (Fig. 3C). Suppl. Fig. 3 showed the structure of active AKT kinase domain (green) bound to the substrate GSK3β peptide (Cyan). Phosphorylation of the activation loop at T308 (blue with phosphate group in red) is essential for AKT activation. T312 (yellow) of AKT is in the substrate binding site and the phosphorylation on its side chain hydroxyl group (red) will cause steric clash with the substrate, thereby inhibiting the AKT kinase activity. To assess the impact of phosphorylation of T312 on AKT activation and function, we mutated T312 to aspartic acid (D) to mimic phosphorylation and transfected empty vector, flag-tagged wild-type and AKT mutant (AKTT312D) into 293-IL-1R cells. The cell lysates from the transfected cells were immunoprecipitated with anti-flag (M2) to pull down wild-type and mutant AKT, followed in vitro kinase assay using recombinant GSK3β as a substrate. As shown in Figure 3D, AKTT312D displayed attenuated kinase activity compared to wild-type AKT, demonstrating that GSK3α-mediated phosphorylation of T312 is inhibitory for AKT activation and function.
Figure 3. GSK3α phosphorylates and inhibits Akt.
(A) Lysates from 293-IL-1R cells untreated or treated for 15 or 30 min with IL-1 (1 ng/ml) were immunoprecipitated with anti-GSK3α and anti-GSK3β, followed by in vitro kinase reaction in the presence (first lane) or absence of recombinant Akt. (B) Recombinant Akt and GSK3α proteins were incubated in kinase reaction in the presence and absence of GSK3 inhibitor (30μM SB415286). (C) Tandem mass spectrometry (ms2) of precursor ions in the Akt1 phosphopeptide (amino acids 308–328; sequence TFCGpTPEYLAPEVLEDNDYGR). (D) 293-IL-1R cells were transfected with flag-tagged wild-type Akt1, T312D mutant Akt1 or empty vectors. The cell lysates from the transfected cells were immunoprecipitated with anti-flag antibody (M2), followed in vitro kinase assay using recombinant GSK3β as a substrate. Data are representative of at least three separate experiments.
IL-1 induces IKKi activation to mediate GSK3α phosphorylation
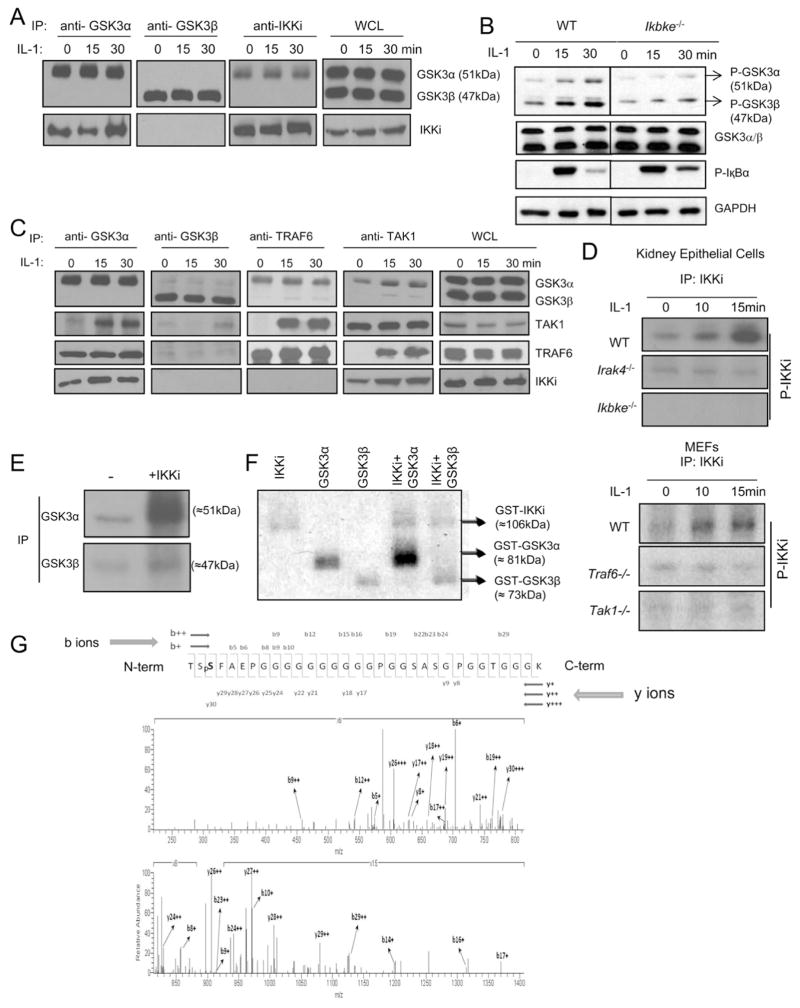
Because IL-1-induced GSK3α phosphorylation was not inhibited by PI3K and AKT inhibitors, a different kinase probably functions upstream of GSK3α and is responsible for the phosphorylation of GSK3α in response to IL-1 stimulation. Through co-immunoprecipitation of lysates from 293-IL-1R cells, we found that GSK3α, but not GSK3β, formed a complex with IKKi (Fig. 4A). Importantly, while IκBα phosphorylation was intact, IL-1-induced GSK3α and GSK3β phosphorylation was greatly reduced in IKKi-deficient Th17 cells compared to that in wild-type cells (Fig 4B), implicating that IKKi might be the upstream kinase of GSK3 in response to IL-1 stimulation.
Figure 4. IL-1 induces IKKi activation to mediate GSK3α phosphorylation.
(A) Lysates from 293/IL-1R cells were untreated or treated for 15 or 30 min with IL-1 (1 ng/ml) were immunoprecipitated with anti-GSK3α, anti-GSK3β and anti-IKKi, followed by protein blot analysis with anti-GSK3α/β, anti-IKKi. WCL (whole cell lysates) (B) Cell lysates from wild-type and Ikbke−/− Th17 cells untreated or treated with IL-1 (10ng/ml) for different time points were analyzed by protein blot analysis using antibodies as indicated. (C) Lysates from 293/IL-1R cells were untreated (0) or treated for 15 or 30 min with IL-1 (1 ng/ml) were immunoprecipitated with anti-GSK3α, anti-GSK3β, anti-TRAF6 and anti-TAK1 were analyzed by immunoblot with anti-GSK3α/β, anti-TRAF6, anti-TAK1 and anti-IKKi. (D) The lysates of kidney epithelial cells from wild-type, Irak4−/− and Ikbke−/− mice, or cell lysates from wild-type, Traf6−/− and Tak1−/− MEFs, were immunoprecipitated with anti-IKKi antibody, followed by in vitro kinase reaction. (E) The cell lysates from 293-IL-1R cells were immunoprecipitated with anti-GSK3α or anti-GSK3β antibody followed by in vitro kinase reaction in the absence and presence of recombinant IKKi protein. (F) Recombinant GSK3α and GSK3β proteins were incubated in kinase reaction in the presence and absence of recombinant IKKi protein. (G) Tandem mass spectrometry (ms2) of precursor ions in the GSK3α phosphopeptide (amino acids 19–50; sequence TSpSFAEPGGGGGGGGGGPGGSASGPGGTGGGK). All data are representative of at least three separate experiments.
Upon IL-1 stimulation, adaptor MyD88 is recruited to the IL-1 receptor, followed by the recruitment of serine and threonine kinases IRAKs (interleukin-1 receptor-associated kinases) and TRAF6. IRAK4 mediates the phosphorylation of IRAK1 and IRAK2, followed by the activation of TAK1 and IKK in a TRAF6-dependent manner, leading to NFκB activation (Yao et al., 2007). Interestingly, we found that the IL-1 stimulation induced the interaction of TRAF6/TAK1 with the IKKi/GSK3α complex in 293/IL-1R cells (Fig. 4C). Because IKKi is important in IL-1-induced GSK3 phsophorylation, we examined IL-1-mediated IKKi activation. We immunoprecipitated IKKi from cell lysates of MEFs and primary kidney epithelial cells untreated and treated with IL-1, followed by in vitro kinase assay. Increased IKKi phosphorylation was detected in lysates from both cell types 10–15 minutes after IL-1 stimulation (Fig. 4D), demonstrating IL-1-induced IKKi activation in cell culture model. IL-1-induced IKKi auto-phosphorylation was substantially reduced in IRAK4-deficient kidney epithelial cells and TRAF6- and TAK1-deficient MEFs. These results indicate that IL-1 stimulation specifically leads to IKKi activation in an IRAK4-TRAF6-TAK1-dependent manner. We then examined whether IKKi can directly phosphorylate GSK3 in vitro. Both GSK3α and GSK3β immunoprecipiated from 293-IL-1R cells was subjected to in vitro kinase assay with or without recombinant IKKi protein. Importantly, we observed that recombinant IKKi was able to effectively phosphorylate GSK3α but not GSK3β in vitro (Fig. 4E). Thus, IKKi only directly phosphorylates GSK3α, whereas the impact of IKKi on IL-1-induced GSK3β phosphorylation is likely to be indirect, probably through the activation of an intermediate kinase(s). In support of this, recombinant IKKi was also only able to phosphorylate recombinant GSK3α but not GSK3β in an in vitro kinase assay, indicating that IKKi is a direct upstream kinase of GSK3α (Fig. 4F).
IKKi-mediated inactivation of GSK3α is required for IL-1-induced AKT-mTOR activation
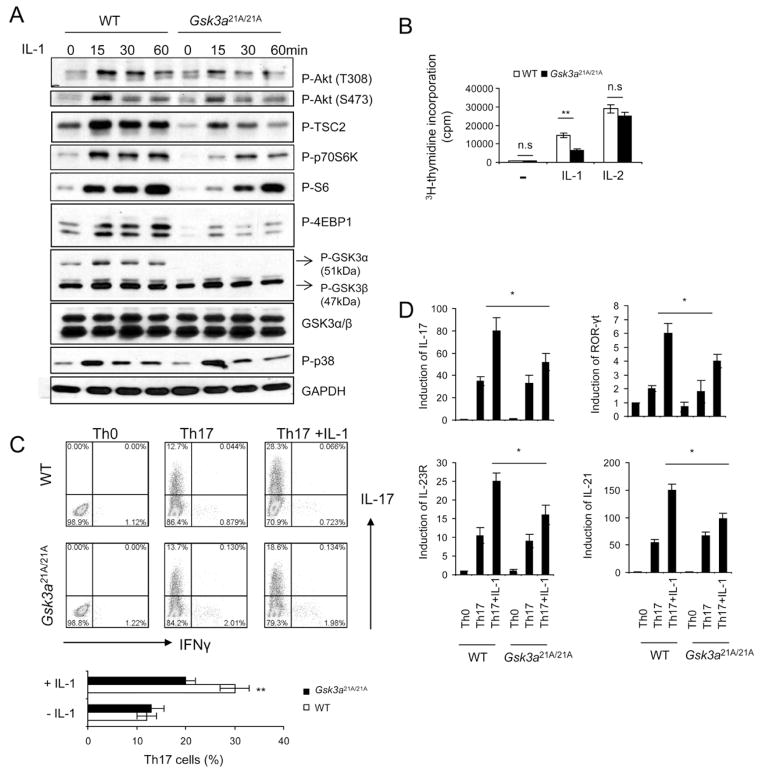
We next attempted to identify the IKKi-mediated phosphorylation sites of GSK3α by tandem mass spectrometry. A GSK3α peptide of amino acids 19–50 (TSSFAEPGGGGGGGGGGPGGSASGPGGTGGGK) was phosphorylated on Ser21 (Fig. 4G). Previous studies have shown that phosphorylation of Ser21 results in the N-terminal domain of GSK3α interacting with its phosphate binding pocket, preventing recognition of primed substrates (Frame et al., 2001). Therefore, it is possible that IKKi-mediated GSK3α phosphorylation at Ser21 also attenuates GSK3α kinase activity, thereby removing the inhibitory effect of GSK3α on IL-1-induced AKT-mTOR activity. To test this hypothesis, we employed GSK3α knockin mice, in which Ser21 was mutated to alanine. Whereas IL-1-induced p38 phosphorylation were intact, AKT (T308 and S473)-mTOR activation was substantially deceased in Gsk3a21A/21A Th17 cells as compared to that in wild-type cells (Fig 5A). Consistent with the decreased mTOR activity, IL-1-mediated cell survival and/or proliferation was reduced in Gsk3a21A/21A Th17 cells as compared to that in wild-type cells (Fig. 5B). WT and Gsk3a21A/21A naïve T cells were differentiated into Th17 cells with or without IL-1β stimulation. Similar numbers of IL-17-positive cells were detected in WT and Gsk3a21A/21A T cells under TGFβ+IL-6-mediated differentiation condition (in the absence of IL-1β stimulation) (Fig. 5C). Importantly, although IL-1β promoted Th17 cell differentiation in both WT and Gsk3a21A/21A T cells, addition of IL-1β resulted in a significantly reduced IL-17-positive population in Gsk3a21A/21A T cells than that in WT T cells (Fig. 5C). Furthermore, the expression of Th17 cell-associated molecules (including RORγt, IL-17, IL-21 and IL-23R) was also much lower in Gsk3a21A/21A T cells than that in WT cells in the presence of IL-1β stimulation (Fig. 5D). Thus, IKKi-mediated GSK3α phosphorylation at Ser21 is indeed required for the inactivation of GSK3α to remove GSK3α’s inhibitory effect on AKT, which is critical for IL-1-induced activation AKT-mTOR and Th17 cell maintenance and/or proliferation.
Figure 5. IKKi-mediated inactivation of GSK3α is required for IL-1-induced AKT-mTOR activation.
(A) Cell lysates from wild-type and GSK3α KI(S21A) Th17 cells untreated or treated with IL-1 (10ng/ml) for different time points were analyzed by western blot analysis using antibodies as indicated. (B) Wild-type and GSK3α KI Th17 cells were rested for overnight, followed by incubation with 10ng/ml IL-1 or IL-2 for three additional days. The treated cells were incubated one additional day with 3H for thymidine incorporation experiment. (C) Naïve wild-type, GSK3α KI CD4+ T cells were polarized to Th17 cells in the presence and absence of IL-1β, followed by intracellular cytokine staining for IL-17 and IFN-γ (D) Real-time PCR analysis of relative expression of IL-17, IL-21, IL-23R, and ROR-γt in wild-type and GSK3α KI Th17 cells as compared to the Th0 cells. All data are representative of at least three separate experiments. Error bars (B and D), s.d. **, p<0.01 (two tailed t-test).
IKKi deficiency leads to loss of IL-1-induced AKT-mTOR activation
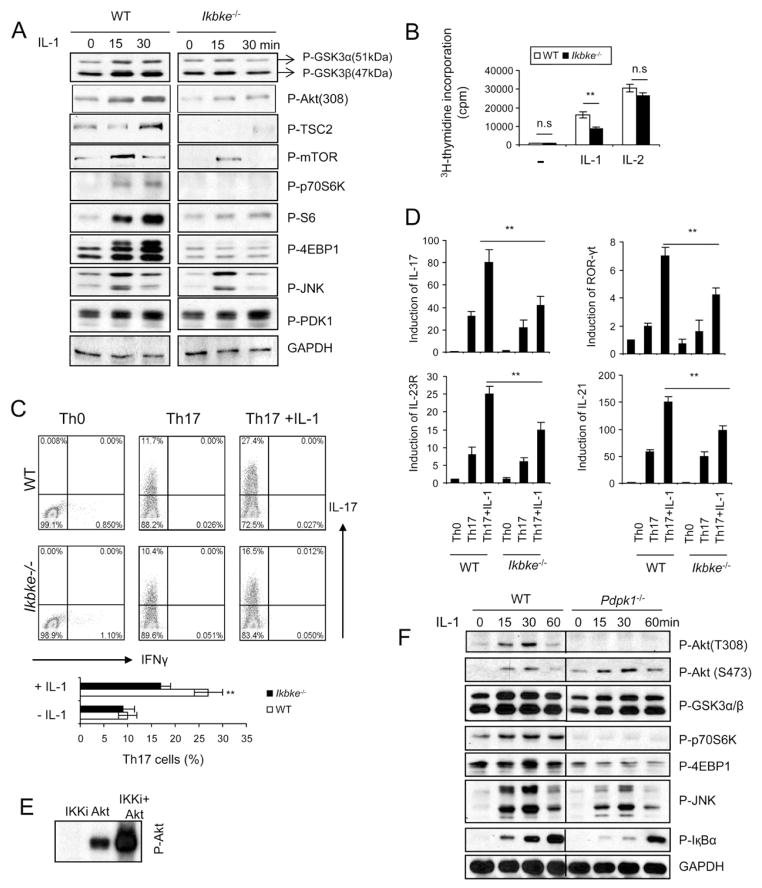
We examined the direct impact of IKKi deficiency on IL-1-mediated AKT-mTOR pathway and IL-1-dependent Th17 cell maintenance and/or proliferation. IL-1-induced phosphorylation of AKT (T308), TSC2, mTOR and downstream components p70S6K, S6 and 4EBP1 were markedly reduced in IKKi-deficient Th17 cells compared to that in wild-type cells (Fig 6A). Consistent with the decreased AKT-mTOR activation, IKKi deficiency substantially reduced IL-1-mediated impact on Th17 cell maintenance and/or proliferation compared to that in wild-type cells (Fig. 6B). However, IKKi deficiency did not have much impact on the response of Th17 cells to IL-2, indicating the specific function of IKKi in IL-1 pathway (Fig. 6B). Although IKKi deficiency did not have much impact on TGFβ+IL-6-mediated Th17 cell differentiation, addition of IL-1β resulted in a markedly reduced IL-17-positive population in Ikbke−/− T cells than that in WT T cells (Fig. 6C). Furthermore, the expression of Th17 cell-associated molecules (including RORγt, IL-17, IL-21 and IL-23R) was also much lower in Ikbke−/− T cells than that in WT cells in the presence of IL-1β stimulation (Fig. 6D). These results suggest that IKKi not only functions as an upstream kinase of GSK3α but also plays a critical role in IL-1-induced AKT-mTOR activation in Th17 cells and consequent Th17 cell maintenance and/or proliferation (Suppl. Fig. 5).
Figure 6. IKKi deficiency leads to loss of IL-1-induced AKT-mTOR activation.
(A) Cell lysates from wild-type and Ikbke−/− Th17 cells untreated or treated with IL-1 (10ng/ml) for different time points were analyzed by protein blots using antibodies as indicated. (B) Wild-type and Ikbke−/− Th17 cells were rested for overnight, followed by incubation with 10ng/ml IL-1 or IL-2 for three additional days. The treated cells were incubated for one additional day with 3H for thymidine incorporation experiment. (C) Naïve wild-type and Ikbke−/− CD4+ T cells (CD4+CD44low) were polarized to Th17 cells in the presence and absence of IL-1β, followed by intracellular cytokine staining for IL-17 and IFN-γ (D) Real-time PCR analysis of relative expression of IL-17, IL-21, IL-23R, and ROR-γt in wild-type and Ikbke−/− Th17 cells as compared to that in Th0 cells. Data (A–D) are representative of at least three separate experiments. Error bars (B and D), s.e.m **, p<0.01 (two tailed t-test). (E) Recombinant IKKi and Akt1 proteins were incubated in the in vitro kinase reaction. (F) Cell lysates from wild-type and Pdpk1−/− DLD1 cells untreated or treated with IL-1 (10ng/ml) for different time points were analyzed by protein blots using antibodies as indicated.
An important question is how is AKT is activated upon IL-1 stimulation. IKKi can directly mediate AKT phosphorylation and activation in response to EGF stimulation (Xie et al., 2011; Guo et al., 2011). Because IKKi is required for IL-1-induced activation of AKT-mTOR signaling pathway, we examined a possible direct role of IKKi in IL-1-mediated AKT activation. Through co-immunoprecipitation, we found that IKKi forms a complex with AKT (data not shown). Furthermore, we observed that recombinant IKKi was able to effectively phosphorylate AKT in vitro (Fig. 6E), indicating the IKKi probably is able to directly phosphorylate AKT.
Because PI3K signaling is required for IL-1-induced for AKT-mTOR activation (Fig. 1B and 1D), we wondered whether PDK1 might also be involved in IL-1-induced AKT phosphorylation and activation. We found that IL-1-induced PDK1 phosphorylation was detected in both wild-type and IKKi-deficient Th17 cells, indicating that PDK1 activation is IKKi-independent (Fig. 6A). Although IKKi can directly phosphorylate AKT, it is unclear whether PDK1 is also required for IL-1-induced AKT activation. We found that IL-1-induced GSK3, JNK, IκBα phosphorylation were intact in PDK1-deficient colon cancer cells, whereas IL-1-induced phosphorylation of AKT T308 and downstream components of mTOR (p70S6K, S6, and 4EBP1) were abolished in the absence of PDK1 (Fig. 6F). PDK1 deficiency did not have much impact on IL-1-induced AKT phosphoryaltion at S473 (Fig. 6F), which might be directly phosphorylated by IKKi (Xie et al., 2011; Guo et al., 2011).
The impact of IKKi and GSK3α on Th17 cell effector function in induction of EAE
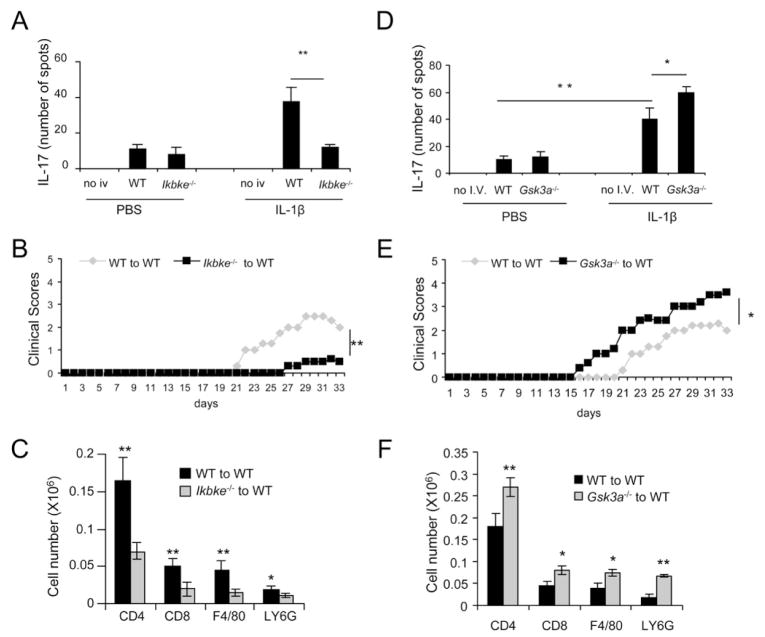
IL-1-mediated signaling in T cells is essential for in vivo Th17 cell differentiation and IL-17-dependent autoimmune diseases (Sutton et al., 2006). Here we showed that IKKi functions downstream of IRAK4 to mediate IL-1-induced AKT-mTOR activation and ex vivo Th17 cell survival and/or proliferation. To determine the importance of IKKi on IL-1-mediated impact on Th17 cells in vivo, we transferred MOG-specific Th17 cells from the WT and Ikbke−/− mice to sub-lethally irradiated Il1r−/− mice, followed by 7 days of injection of either PBS or IL-1β. Whereas IL-1β treatment induced IL-17 producing MOG specific T cells in Il1r−/− mice, IKKi deficiency reduced the impact of IL-1 on MOG-specific Th17 cells (Fig 7A). The functionality of MOG-specific Th17 cells from the WT and Ikbke−/− mice was also compared by adoptive transfer to WT recipient mice. Compared to MOG35–55-specific wild-type Th17 cells, MOG35–55-specific IKKi-deficient Th17 cells induced less severe EAE with a delayed onset of disease in recipient mice (Fig. 7B). Consistent with the clinical scores, infiltrating CD45+, CD4+, CD11b+ and Gr-1+ cells were reduced in the brains of mice in which Ikbke−/− MOG-specific Th17 cells were transferred compared to those received wild-type MOG-specific Th17 cells (Fig. 7C). Because our results showed that IKKi-mediated AKT-mTOR activation in Th17 cells requires the removal of the inhibitory effector of GSK3α on AKT, we also examined the effect of GSK3α deficiency on IL-1-induced Th17 cell maintenance/expansion in vivo and Th17 cell effector function in EAE model. We transferred MOG-specific Th17 cells from the WT and CD4CreGsk3afl/fl mice to sub-lethally irradiated Il1r−/− mice, followed by 7 days of injection of either PBS or IL-1β. GSK3α deficiency enhanced the impact of IL on MOG-specific Th17 cells (Fig 7D). The functionality of MOG-specific Th17 cell from the WT and Gsk3a−/− mice was also compared by adoptive transfer to WT recipient mice to induce EAE. Adoptive transfer of MOG35–55-specific Gsk3a−/− Th17 cells induced more severe EAE in recipient mice compared to WT Th17 cells (Fig. 7E–F). Thus, GSK3α deficiency enhanced Th17 effector function in vivo, leading to increased severity of EAE. Taken together, these data implicate that IL-1-induced IKKi-GSK3α-mediated AKT-mTOR axis is critical for the modulation of Th17 effector function in vivo, impacting on Th17 cell-dependent EAE pathogenesis.
Figure 7. IKKi/GSK3α axis is required for IL-1 mediated in vivo Th17 expansion/survival.
(A and D) WT, Gsk3a−/− and Ikbke−/− MOG specific Th17 cells were transferred to Il1r−/− mice. After 7 days of PBS or IL-1β injection, MOG specific IL-17 producing cells in the spleen were detected by ELISPOT. (B and E) Primed MOG35-55 specific wild-type, Gsk3a−/− and Ikbke−/− T cells (10 days) were re-stimulated with MOG35-55 in vitro in the presence of recombinant IL-23 for 4 days, and then transferred to naive wild-type mice. Graph represents the average clinical score after T-cell transfer. n=5. **, p<0.01 (ANOVA). (C and F) Immune cell infiltration in the brains of wild-type mice transferred with wild-type, Gsk3a−/− and Ikbke−/− Th17 cells was analyzed by flow cytometry (n = 5, 7 days after disease onset). Error bars (A, C, D and F), s.d. *, p<0.05; **, p<0.01 (two tailed t-test). All data are representative of three independent experiments.
Discussion
This study demonstrates a distinct role of GSK3α versus GSK3β in IL-1 signaling, which has a critical regulatory role in Th17 cell maintenance and/or proliferation. It is important to note that whereas PI3K-AKT are known to be upstream kinases for insulin-induced GSK3 phosphorylation and inactivation, we found here that GSK3α forms a constitutive complex to suppress AKT activation. This study showed that a reverse action from GSK3α to AKT can take place to impact on mTOR activation, whereas previous studies demonstrated how GSK3β can reversely act on mTOR activation by meditating the phosphorylation of TSC2 (Inoki et al., 2006; Wu and Pan, 2010). These pathways probably serve as important links between inflammatory pathways and cell metabolism. While we have reported the importance of IKKi in IL-17-mediated mRNA stabilization (Bulek et al., 2011), the study here showed that IKKi has a critical role in Th17 maintenance and/or proliferation through the GSK-AKT-mTOR pathway, which will have an important impact on the rationale and feasibility for IKKi to be a therapeutic target.
It is well known that insulin receptor signaling results in the activation of PI3K pathway and subsequent phosphorylation GSK3α (S21) and GSK3β (S9) by AKT, leading to inactivation of GSK3 activity and removal of its inhibitory effect on glycogen synthase (Cross et al., 1995; Cross et al., 1994; Cross et al., 1997). Therefore, it has been generally accepted that GSK3 is a downstream substrate of AKT. Our results unexpectedly showed that both PI3K and AKT inhibitors failed to inhibit IL-1-induced GSK3α phosphorylation, whereas they partially inhibited GSK3β phosphorylation. GSK3α and GSK3β, encoded by different genes (Woodgett, 1990), seem to be functionally redundant in some signaling pathways (Doble et al., 2007), although most of the previous studies have focused on GSK3β. An important finding in this study is the identification of the constitutive interaction of GSK3α (but not GSK3β) with AKT, which allows GSK3α to specifically phosphorylate AKT at T312 to suppress AKT activation and function. While GSK3α and GSK3β share nearly identical sequences in their kinase domains, a glycine-rich extension at the N-terminus of GSK3α accounts for the molecular weight differences between GSK3α (51kD) and GSK3β(47kD) (Woodgett, 1990). The last 76 amino acids within the C-terminus region of GSK3α and GSK3β exhibit only 36% homology (Woodgett, 1990). It is likely that these structural differences between GSK3α and GSK3β contribute to their differential interactions with signaling complexes, including the specific interaction of GSK3α, but not GSK3β, with AKT. It is important to note that when GSK3α was depleted in the supernatant after two cycles of immunoprecipitation, there was still substantial amount of AKT in the supernatant, implicating additional AKT complexes in the cells. It is possible that GSK3α-AKT might be a specific complex preserved to regulate inflammatory response.
Tandem MS showed that GSK3α phosphorylates AKT at T312, which is in the substrate binding site according to the structure of AKT kinase domain. The phosphorylation on the side chain hydroxyl group of T312 will cause steric clash with the substrate, thereby inhibiting the AKT kinase activity. Interestingly, while GSK3α deficiency enhanced IL-1-induced phosphorylation of the activation loop at T308, GSK3α kinase-active knockin blocked IL-1-induced phosphorylation at T308. While the phosphorylation of T308 is essential for AKT activation, the activation loop does not overlap with the substrate binding site. Therefore, it is not clear how the phosphorylation at T312 affects the phosphorylation of the activation loop at T308. A possibility is that the interaction of GSK3α with AKT and/or phosphorylation at T312 might interfere with the access of AKT to the kinase (e.g. PDK1 and/or IKKi, discussed below) for the phosphorylation of the activation loop of AKT.
Consistent with the fact that PI3K and AKT inhibitors failed to inhibit IL-1-induced GSK3α phosphorylation (S21), IKKi deficiency impaired IL-1-induced GSK3α phosphorylation (S21), implicating the possibility of IKKi as the upstream kinase for GSK3α. In support of this, in vitro kinase assays clearly indicate that IKKi indeed can directly phosphorylate GSK3α. Interestingly, tandem MS showed that IKKi phosphorylates GSK3α at S21, suggesting that IKKi is responsible for IL-1-induced GSK3α phosphorylation at S21. GSK3α S21 is a known inhibitory phosphorylation site for GSK3α kinase activity, which mainly blocks the binding of GSK3α to its “primed substrates” (Frame et al., 2001). Most of the best described GSK3 substrates require pre-phosphorylation at a residue 4 or 5 amino acids (or further) C-terminal to GSK3 target residue, a phenomenon referred to as “priming” (Fiol et al., 1987). Although it is unclear whether AKT is a “primed” substrate, GSK3α S21 to A21 attenuated IL-1-induced AKT phosphorylation, presumably due to prolonged kinase activity of GSK3α S21A and consequent inhibitory phosphorylation of AKT at T312. It is interesting to note that AKT contains several glutamic acid residues (2, 7 and 10 amino acids) and aspartic acid residues (11 and 13 amino acids) C-terminal to T312, which might serve as “priming” residues for the phosphorylation of T312 by GSK3α. Previous studies have shown that phosphorylation of S21 of GSK3α results in N-terminal domain of GSK3 interacting with its phosphate binding pocket, preventing recognition of primed substrates. We speculate that IKKi-mediated GSK3α phosphorylation at S21 might inhibit the recognition of acidic amino acids C-terminal to T312, thereby blocking AKT T312 phosphorylation by GSK3α. Future studies are required to test this hypothesis.
IKKi can directly mediate AKT phosphorylation and activation in response to EGF stimulation (Xie et al., 2011; Guo et al., 2011). In addition to IKKi’s role in removal of the inhibition of GSK3α on AKT, an important question is whether IKKi is the direct kinase for AKT phosphorylation and activation in response to IL-1 stimulation. We found that recombinant IKKi was able to effectively phosphorylate AKT in vitro, [Au: No figure callouts in the Discussion, pls.] indicating the IKKi probably is able to directly phosphorylate AKT. However, since PI3K signaling is required for IL-1-induced AKT-mTOR activation, it is possible that PDK1 might also be involved in IL-1-induced AKT phosphorylation and activation. It is well known that PI3K phosphorylates phosphatidylinositol-4, 5-bisphosphate (PIP2) to generate phosphatidylinositol-3, 4, 5-trisphosphate (PIP3). While AKT and PDK1 bind to PIP3 at the plasma membrane, PDK1 phosphorylates the activation loop of AKT at T308 (Alessi et al., 1997). Interestingly, our results showed IL-1-induced phosphorylation of AKT T308 and downstream components of mTOR (p70S6K, S6, and 4EBP1) were abolished in the absence of PDK1. These results suggest that whereas IKKi is necessary for the phosphorylation of GSK3α at S21 to remove the inhibitory effect of GSK3α on AKT (T312), PDK1 might be the direct kinase responsible for AKT phosphorylation at T308. It is important to point out that PDK1 deficiency did not have much impact on IL-1-induced AKT phosphoryaltion at S473. Therefore, after the removal of the inhibitory effect of GSK3α on AKT (T312), IKKi might directly phosphorylate AKT at S473 (Guo et al., 2011; Xie et al., 2011).
It is intriguing to note that PI3K and AKT inhibitors did partially inhibit IL-1-induced GSK3β phosphorylation at S9, which demonstrates the importance of PI3K-AKT in GSK3β phosphorylation. It is plausible that the activated AKT through the action of IKKi-GSK3α in turn mediates the phosphorylation and inactivation of GSK3β (S9). Consistently, GSK3β was not detected in the IKKi complex and IKKi can only weakly phosphorylate GSK3β in vitro. As a matter of fact, previous studies have shown that since GSK3β inhibits mTOR activation through phosphorylation of TSC2 (Inoki et al., 2006), AKT-mediated GSK3β phosphorylation at S9 should alleviate the inhibitory effect of GSK3β on mTOR, contributing to mTOR activation. It is important to note that although IKKi is unable to phosphorylate GSK3β in vitro, IKKi deficiency abolished IL-1-induced GSK3β phopshorylation in Th17 cells. It remains unclear whether there is another kinase that mediates IL-1-induced GSK3β phosphorylation, since AKT inhibitor only partially blocked IL-1-induced GSK3β phosphorylation. Future studies are required for identify the candidate kinase (s) that participates in IL-1-induced GSK3β phosphorylation.
Although it is well-established that AKT phosphorylates and inactivates GSK3α and β (e.g. in insulin signaling), we showed GSK3α can reversely phosphorylate AKT and suppress AKT activation in resting cells and IL-1 stimulation activates IKKi to phosphorylate GSK3α, resulting in inactivation of GSK3α to promote AKT-mTOR activation. This study has been mainly carried out in Th17 cells, demonstrating the critical role of the IKKi-GSK3α-AKT-mTOR axis in IL-1-dependent Th17 cell maintenance and/or proliferation and function. The kinase mTOR has emerged as an important regulator of the differentiation of helper T cells (Delgoffe et al., 2009; Delgoffe et al., 2011). The mTOR complex promotes phosphorylation of the translational regulators S6K1 and 4E-BP1 and is believed to have a central role in regulating cellular growth and proliferation by modulating metabolism. In addition to the impact of mTOR-mediated 4E-BP1 on the translation of cell cycle regulators cMyc and Cyclin D1, recent studies indicate that mTOR activation is required HIF1α induction, a transcription factor driving the glycolytic pathway (Shi et al., 2011; Dang et al., 2011). While aerobic glycolysis has been associated with lymphocyte proliferation, HIF1α-dependent glycolytic pathway plays a critical role in Th17 cell differentiation. We found that IL-1 stimulation also strongly induced HIF1α expression in differentiated Th17 cells, which is specially blocked by rapamycin (data not shown). Future studies are required to elucidate how IL-1-induced mTOR activation leads to Th17 cell proliferation and effector function.
EXPERIMENTAL PROCEDURES
Mice
Wild type mice were purchased from Taconic Laboratories. Il1r−/− mice were purchased from Jackson Laboratories. Gsk3a−/− mice, Gsk3afl/fl mice and Gsk3a21A/21A mice were generated as described (MacAulay et al., 2007; Doble et al., 2007; McManus et al., 2005). Mice were housed in animal facility (SPF condition) at the Cleveland Clinic Foundation in compliance with the guidelines set by Institutional Animal Care and Use Committee.
T Cell Differentiation
Naive CD4+CD44lo T cells from wild type C57BL/6, CD4CreGsk3afl/fl and Gsk3a21A/21A mice were sorted by flow cytometry and activated with plate-bound 3 mg/ml anti-CD3 and 3 mg/ml anti- CD28 and in the presence of 5 ng/ml TGF-β (Peprotech), 10 ng/ml IL-6 (Peprotech), 5 mg/ml anti-IL-4 (11B11), 5 mg/ml anti-IFN-γ (XMG 1.2), 10 ng/ml IL-1β (R&D system), or combination of these stimuli. For intracellular staining, cells were stimulated with PMA and ionomycin in the presence of Golgi-stop for 4 hr, after which IL-17- and IFN-g-producing cells were analyzed with intracellular staining.
Proliferation
Th17 cells were cultured (20× 103 cells/well) at a final volume of 200 ml/well in 96 well plates. In some wells, rapamaycin (50 nM) (Sigma) was added. After incubation for 96 hr, wells were pulsed with [methyl-3H]thymidine (1.0 μCi/well, specific activity 6.7 Ci/mM; New England Nuclear) and harvested 16 hr later by aspiration onto glass fiber filters. For proliferation assay, levels of incorporated radioactivity were determined by scintillation spectrometry.
Quantitative Real-Time RT-PCR
Expression of the genes encoding IL-17A, IL-23R, IL-21 and ROR-γt were quantified with the SYBER Green PCR Master Mix kit (Applied Biosystems) with primer pairs selected for amplification of each individual cytokine (Gulen et al., 2010). Relative gene expression was determined as the ratio of cytokine to b-actin gene expression levels for each sample.
EAE Induction
Mice were immunized with MOG(35–55) plus complete Freund’s adjuvant in conditions that induce active EAE. Th17 cells from lymph nodes of the immunized mice were re-stimulated with MOG (35–55) (20 mg/ml) and IL-23 (20 ng/ml) as described before (Gulen et al., 2010). After 4 days of culture, cells were collected and injected into recipient WT mice that had been sublethally irradiated (600 rads) at 4 h before injection. While 2 × 107 total WT cells were transferred to each mouse, the numbers of Gsk3a−/− and Ikbke−/− cells transferred were normalized against WT based on the ELISPOT analysis of the MOG specific Th17 cell numbers after the cell culture (Supple. Fig. 4). For the detection of IL-1β effect on the MOG specific Th17 cells, cells were injected by I.V. into irradiated (600 rads) Il1r−/− mice. The mice were intraperitoneally injected daily either with PBS or 400ng IL-1β (R&D) for 7 days. After 7 days, ELISPOT analysis was performed for the MOG specific IL-17 producing cells from spleens of Il1r−/− mice using mouse IL-17 ELISPOT kit (R&D) according to manufacturer instructions.
Transfection, Coimmunoprecipitations, and Immunoblots
Procedures for transfection, coimmunoprecipitations, and immunoblots with 293 cells were previously described (Yao et al., 2007). Antibodies used in this study include primary anti-Akt, GSK3α/β (Santa Cruz), GSK3α, GSK3β, p-GSK3α/β, p-Akt, p-4EBP1, 4EBP1, p-70S6K, p-IκBα, p-JNK, p-mTOR, mTOR, p-S6, p-TSC2, TSC2, PI3K, IKKi (Cell Signaling), and GAPDH (Ambion).
In vitro Kinase Assay
Recombinant proteins (GST-GSK3α (CellScinces), GST-GSK3β (R&D) and GST-IKKi/IKKε (Active Motif)) or immunoprecipitated beads were subjected to in vitro kinase reaction as previously described(Bulek et al., 2011).
Trypsin digestion, LC/ESI-MS/MS and MS data analysis
The detail methods for Trypsin digestion, LC/ESI-MS/MS have been previously described (Pang et al., 2002; Gao et al., 2004). MSMS data were searched against human database by Sequest through Sorcerer (Sage-N Research, Milpitas, CA). The searched dataset was processed by TPP(Trans-Proteomics Pipeline) and filtered with Peptide Prophet (Keller et al., 2002) as well as Sorcerer Sequest scores (Xcorr>2.0, DelCN>0.095, Rsp<5) (Pang et al., 2002; Gao et al., 2004). Additionally, the phospho-peptides that passed the filtering criteria were manually inspected to confirm the assignments generated by the data searching algorithm.
Statistics
ANOVA was used for EAE clinical score studies. The Student’s t test was used to assess all other statistical values. p values were determined and error bars represent standard error of the mean (SEM).
Supplementary Material
Acknowledgments
This work was supported by grants from NIH (2PO1 HL 029582-26A1; 2PO1CA062220-16A1). The Pdpk1−/− DLD1 and HCT116 cells were provided by Dr.Bert Vogelstein of Johns Hopkins Kimmel Cancer Center, Baltimore, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, Lauer M, Hascall V, Misra S, Chance MR, Aronica M, Hamilton T, Li X. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. 2011;12:844–852. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Vandenheede JR, McDowell HE, Hundal HS, Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303(Pt 1):21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Watt PW, Shaw M, van der Kaay J, Downes CP, Holder JC, Cohen P. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997;406:211–215. doi: 10.1016/s0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987;262:14042–14048. [PubMed] [Google Scholar]
- Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Gao J, Ann GL, Storm SM, Hefta SA, Opiteck GJ, Lin JH, Moulin F, Dambach DM. Identification of in vitro protein biomarkers of idiosyncratic liver toxicity. Toxicol In Vitro. 2004;18:533–541. doi: 10.1016/j.tiv.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Bak-Jenson K, McGeachy MJ, Do JS, Xiao H, Delgoffe GM, Min B, Powell JD, Tuohy VK, Cua DJ, Li X. The Receptor SIGIRR Suppresses Th17 Cell Proliferation via Inhibition of the Interleukin-1 Receptor Pathway and mTOR Kinase Activation. Immunity. 2010 doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JP, Coppola D, Cheng JQ. IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J Biol Chem. 2011;286:37389–37398. doi: 10.1074/jbc.M111.287433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivieres S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem. 1998;273:14424–14429. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, Nagy A, Woodgett JR. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007;6:329–337. doi: 10.1016/j.cmet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen CJ, Rickheim DG, Tucker MM, Hansen LK, Albrecht JH. Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J Biol Chem. 2003;278:3656–3663. doi: 10.1074/jbc.M209374200. [DOI] [PubMed] [Google Scholar]
- Pang JX, Ginanni N, Dongre AR, Hefta SA, Opitek GJ. Biomarker discovery in urine by proteomics. J Proteome Res. 2002;1:161–169. doi: 10.1021/pr015518w. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Zhang D, Zhao B, Lu MK, You M, Condorelli G, Wang CY, Guan KL. IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Acad Sci U S A. 2011;108:6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kim TW, Qin J, Jiang Z, Qian Y, Xiao H, Lu Y, Qian W, Gulen MF, Sizemore N, DiDonato J, Sato S, Akira S, Su B, Li X. Interleukin-1 (IL-1)-induced TAK1-dependent Versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J Biol Chem. 2007;282:6075–6089. doi: 10.1074/jbc.M609039200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.