SUMMARY
A large proportion of colorectal cancers (CRCs) display mutational inactivation of the TGF-beta pathway yet paradoxically, they are characterized by elevated TGF-beta production. Here, we unveil a prometastatic programme induced by TGF-beta in the microenvironment that associates with a high-risk of CRC relapse upon treatment. The activity of TGF-beta on stromal cells increases the efficiency of organ colonization by CRC cells whereas mice treated with a pharmacological inhibitor of TGFBR1 are resilient to metastasis formation. Secretion of IL11 by TGF-beta-stimulated cancer-associated fibroblasts (CAFs) triggers GP130/STAT3 signalling in tumour cells. This crosstalk confers a survival advantage to metastatic cells. The dependency on the TGF-beta stromal programme for metastasis initiation could be exploited to improve the diagnosis and treatment of CRC.
INTRODUCTION
Most CRCs originate from the intestinal epithelium as premalignant lesions called adenomas. Over time, a small fraction of adenomas are transformed to CRCs due to the accumulation of genetic alterations in a small set of driver genes including KRAS, TP53, SMAD4 or PIK3CA (Markowitz and Bertagnolli, 2009). Alterations in these and other cancer-causing genes have been associated with the different stages of the progression of the tumour (i.e. transition from normal intestinal mucosa to adenoma and further progression to CRC). In contrast, metastases either present at the time of diagnosis or developed as distant relapses after therapy are not strongly associated with alterations in any of these key genes (Walther et al., 2009). In addition, global genome sequencing of metastatic lesions and primary CRCs revealed hardly any metastasis-specific mutation (Jones et al., 2008). This drawback has hampered the development of metastasis specific therapies as well as the identification of CRC patients at risk of suffering metastatic disease.
Mutational inactivation of the TGF-beta signalling pathway is key during CRC progression. Alterations in TGF-beta pathway components are first detected in advanced adenomas and affect 40-50% of all CRCs (Markowitz et al., 1995; Markowitz and Bertagnolli, 2009). In mouse models, mutations in the tumour suppressor Apc combined with inactivation of TGF-beta signalling components in epithelial intestinal cells trigger the development of invasive adenocarcinomas (Munoz et al., 2006; Takaku et al., 1998). Restoration of a functional TGF-beta pathway in human CRC cells abrogates proliferation and tumourigenicity (Wang et al., 1995), implying that TGF-beta signalling exerts tumour suppressive effects. Hence, it has been proposed that TGF-beta imposes a selective pressure during CRC progression, which tumours avert by genetic inactivation of the TGF-beta receptors (TGFBR1 and TGFBR2) or of the SMAD intracellular mediators (SMAD4, SMAD2 and SMAD3) (Grady and Markowitz, 2002; Markowitz et al., 1995; Markowitz and Bertagnolli, 2009).
In addition to its tumour suppressor role in epithelial cells, TGF-beta signalling also acts as a negative regulator of tumour formation by conditioning mucosa-resident stromal cells. Mice with a deletion of Smad4 in T-cells develop gastrointestinal tumours (Hahn et al., 2011; Kim et al., 2006). Similarly, transgenic expression of a dominant negative TGFBR2 in T-cells accelerates azoxymethane-induced colon carcinogenesis (Becker et al., 2004). In both cases, T-cells lacking TGF-beta signals exacerbate the production of proinflammatory cytokines that spark off the transformation of the colonic epithelium (Becker et al., 2004) (Kim et al., 2006).
Whereas the above genetic and mutational data support a tumour suppressor role for TGF-beta signalling in intestinal carcinogenesis, high levels of TGFB1 in the serum of CRC patients associates with poor outcome in the clinical setting (Tsushima et al., 2001). The relevance of TGF-beta signalling for disease progression has been widely recognized in tumours where cancer cells retain a functional TGF-beta pathway, such as breast or prostate cancer (Massague, 2008). In these tumour cells, TGF-beta induces a variety of prometastatic programmes that range from induction of epithelial-to-mesenchymal transition to expression of genes that allow colonization of foreign organs (Massague, 2008). It is less clear, however, what CRC cells can gain from high TGF-beta levels once the pathway is fully inactivated by mutations and how this phenomenon links to an adverse outcome. To address this apparent paradox, we investigated whether TGF-beta may activate the tumour microenvironment to assist CRC cells in the metastatic process.
RESULTS
TGF-beta levels in CRC are a robust predictor of disease relapse
We first investigated whether differences in TGF-beta levels in primary tumours were associated with clinical disease progression in CRC. To this end, we interrogated a representative pooled cohort of 345 cases treated at three different hospitals for which transcriptomic profiles and clinical follow-up were publicly available. In this metacohort, overall TGF-beta levels were low in American Joint Cancer Committee (AJCC) Stage I patients compared to more advanced stages (Figure 1A, Table S1 and Table S2). The AJCC staging system has limited power to predict disease relapse as 10-20% of stage II and 30-50% of stage III CRC patients will develop recurrent cancer after therapeutic intervention. We found that for every increase in overall TGF-beta (TGFB) expression the risk of cancer recurrence augmented by 83% (Figure 1B). As a consequence, we observed large differences in the frequency of disease relapse after therapy in patients bearing tumours with high, medium or low TGFB (Figure 1C) or individual TGF-beta isoform levels (Figure S1A, B available online). During 10 years of follow-up, only patients with medium or high TGFB expression in the primary tumour (53 out of 220 patients) suffered cancer recurrence. Remarkably, all patients bearing TGFB-low tumours remained disease-free (Figure 1C - blue group). High TGFB levels were robustly associated with relapse in stage II and III patients whereas low TGFB characterized a small set of patients with no observed recurrences in both stages (Figure 1D; 17% and 11% of patients in these stages respectively). The rare relapses occurring in Stage I CRCs (2 out of 35 patients in this group) also expressed high TGFB levels (Figure 1D), albeit this comparison did not reach statistical significance probably due to the low incidence of recurrences in this stage. Cox proportional hazards multivariate analysis demonstrated that TGFB expression is an independent predictor of cancer recurrence that outperforms AJCC staging system in the identification of CRC patients that remained disease-free upon therapy (Figure 1E). The predictive power of TGFB levels also compared favourably to that of other genes that have been repeatedly associated with disease relapse in CRC (Table S3).
Figure 1. High TGF-beta expression predicts CRC relapse.
A, Overall TGFB mRNA expression in CRC stages I-IV. Values are z-scores with standard deviation (SD) from the mean (**: p<0.01, two-tailed Student’s t test). B, Smooth function correlates overall TGFB mRNA expression with relative risk of recurrence. Stage IV patients were excluded from this analysis. Red dashed lines: 95% confidence interval (CI). Blue dashed lines: thresholds for patient selection into groups with low (L; blue: 44 patients), medium (M; gray; 86 patients) and high (H; red: 134 patients) TGFB expression. p-values and increase in HR per each increase in standard deviation of expression (+1 SD) are shown. C, Kaplan-Meier plots display recurrence-free survival (RFS) over time for groups defined in B. D, as in C, but grouped by AJCC stage. HR and p-values compare RFS over time for patients grouped according to TGFB levels (L vs M, L vs H and M vs H). E, Cox proportional hazards multivariate analysis of TGF-beta expression and AJCC staging in identifying patients that remained disease-free upon therapy. See also Figure S1
TGF-beta response signatures in tumour-associated stromal cells predict disease relapse in CRC
An increase in TGF-beta isoform levels was evident at the adenoma-CRC transition as shown by expression profiling of a small cohort of colon tumours (Figure S1C). Nuclear p-SMAD3, a marker for TGF-beta signalling, stained predominantly the stromal compartment in most CRCs (Figure S1D). In the majority of samples, epithelial CRC cells were markedly less stained compared to adjacent stromal cells or to the epithelial compartment of adenomas (Figure S1D-F). We characterized the stromal cell types stained by p-SMAD3 in CRCs but could not discriminate any obvious cell type-specificity. Rather, p-SMAD3 indiscriminately labelled all major types of stromal cells in CRCs including T-cells, macrophages, endothelial cells and fibroblasts (Figure S2A-H). We thus quantified the association of TGF-beta-activated stromal cell populations with disease progression. To this end, we used as surrogates the gene expression programmes induced by addition of TGF-beta (TGF-beta response signatures or TBRS) in cultures of normal-tissue-derived T-cells (T-), macrophages (Ma-), endothelial cells (End-) or fibroblasts (F-). To avoid biases, we used the full set of genes upregulated by TGF-beta signalling in these cell cultures (>2 fold, p<0.05) without additional filtering or refinement (Table S4). By Gene Set Enrichment Analysis (GSEA)(Subramanian et al., 2005), we determined that all stromal TBRSs were highly enriched in CRCs compared to adenomas (Figure 2A). Importantly, the expression levels of TGFB, F-TBRS, T-TBRS and Ma-TBRS showed robust direct correlations in the CRC patient cohort (Figure 2B) implying that they are to a large extent concurrently expressed in CRC. Significantly, these 3 signatures were excellent predictors of disease relapse in stage-I, -II and -III CRC patients and segregated a low-expression patient group with virtually no risk of developing recurrent cancer after therapy (Figure 2C, D, Blue group). This result paralleled that obtained with TGFB levels (Figure 1). In Stage IV patients that underwent potential curative therapy, high TGFB and stromal TBRS expression levels also correlated with higher risk of relapse (Figure S2I-L). However, a large proportion of these stage IV patients eventually relapsed likely due to lack of effective therapies to eliminate an overt metastatic disease. Consistent with their ability to predict disease progression, the stromal TBRS included several well-known prometastatic genes such as ANGPTL4 (Padua et al., 2008), PTHLH (Yin et al., 1999), HBEGF (Bos et al., 2009), CTGF (Kang et al., 2003), TNC (Oskarsson et al., 2011) or JAG1 (Sethi et al., 2011; Sonoshita et al., 2011), all of which encode for secreted or membrane-bound factors (Table S4).
Figure 2. Stromal TGF-beta gene signatures predict recurrence.
A, Gene set enrichment analyses (GSEA) of TGF-beta response signatures (TBRS) of Fibroblasts (F-TBRS), T-Cells (T-TBRS), Macrophages (Ma-TBRS) or Endothelial cells (End-TBRS) in carcinoma versus adenoma samples. ES: enrichment score, NES: normalized enrichment score, FDR: false discovery rate. B, Cross-correlations between TGFB, F-TBRS, T-TBRS, Ma-TBRS and End-TBRS in the patient cohort. Blue dots: patients. R: Correlation coefficients. Values are z-scores (*: p<0.05, ***: p<0.001). C, p-Values and increase in HR per each increase in expression (+1SD) in F-TBRS, T-TBRS, Ma-TBRS and End-TBRS, obtained via Cox proportional Hazards model. D, Kaplan-Meier plots display RFS of patients with low (blue), medium (gray) or high (red) F-TBRS, T-TBRS, Ma-TBRS or End-TBRS expression levels. E, FACS-purification of cell populations from primary CRC samples, enriched in the indicated cell types. F, Heat map shows relative expression levels of marker genes for epithelial (blue), leukocyte (red), endothelial (orange) cells and cancer-associated fibroblasts (green) in each cell population, Values are normalized intensities, log2. G, Relative expression of each TBRS in cell populations from (F). Whiskers represent minimum (Vmin) and maximum (Vmax) values. (log2; *: p<0.05, **: p<0.01, Student’s t test). H, Relative expression in each cell population of genes within the T-TBRS, Ma-TBRS, End-TBRS or F-TBRS associating positively with relapse. Whiskers: Vmin, Vmax (log2; *: p<0.05, **: p<0.01, Student’s t test). See also Figure S2.
To further analyse the cell-type specific expression of each stromal TBRS in vivo, we purified by FACS various cell populations from fresh CRC samples and assessed their gene expression profiles (Figure 2E, F). Relative levels of cell type-specific marker genes confirmed the purification of epithelial tumour cells (EPCAM+), leukocytes (CD45+), endothelial cells (CD31+) and fibroblasts (FAP+) (Figure 2F). A global comparative analysis revealed a trend towards high levels of all stromal TBRS in FAP+ CAFs. Conversely, epithelial tumour cells expressed lowest levels of each stromal TBRS (Figure 2G). Remarkably, these trends became very significant for those genes within the T-TBRS, Ma-TBRS or End-TBRS that associated positively with cancer relapse (HR>1, p<0.05; Figure 2H). Although the in vitro derived End-TBRS did not predict disease progression (Figure 2C, D), we observed elevated expression of recurrence-associated TGF-beta target genes in endothelial cells directly purified from tumours (Figure 2H). This observation may suggest that the response of in vitro cultured endothelial cells to TGF-beta signalling deviates significantly from that occurring in the tumour microenvironment. Altogether, these data highlight that the response of stromal cells to TGF-beta is an accurate predictor of disease relapse in CRC patients. Whereas high TGFB levels activate gene programmes in a wide range of tumour-associated stromal cell types, our in vivo data indicate that CAFs, and to a lower extent endothelial cells, are the main contributors to the association of stromal TGF-beta-driven programmes with poor outcome after therapy.
TGF-beta signalling in the stroma promotes tumour initiation
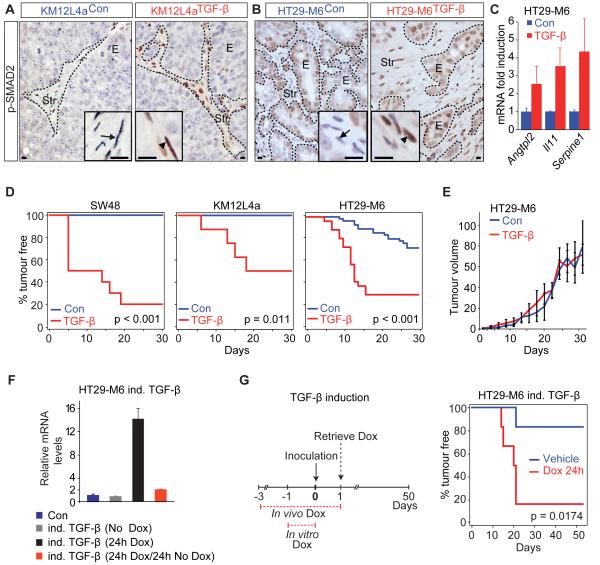
The above data suggest the possibility that elevated TGFB levels in CRC influence disease progression by acting on stromal cells. To functionally dissect this effect without the interference of TGF-beta signalling in epithelial cancer cells, we took advantage of the fact that virtually all late stage CRC–derived cell lines display mutational inactivation of the TGF-beta pathway. These CRC cell lines, however, did not induce robust stromal TGF-beta responses when injected into nude mice as shown by lack of p-SMAD2 accumulation in tumour-associated stromal cells (arrows in Figure 3A, B and data not shown). To enforce high TGF-beta signalling in xenografts, we engineered CRC cell lines to secrete active TGFB1. Subcutaneous tumours obtained from HT29-M6TGF-β, KM12L4aTGF-β cells and SW48TGF-β cells contained abundant p-SMAD2+ stromal cells (arrowheads in Figure 3A, B and not shown) and increased expression levels of stromal TBRS genes (Figure 3C and data not shown). Importantly, secretion of TGF-beta did not induce autocrine responses in these CRC cells (i.e. SMAD reporter activity, proliferation or EMT induction; Figure S3A-D), owing to homozygous mutations in TGFBR2 in KM12L4a and in SW48. HT29-M6 cells carry homozygous inactivation of the SMAD4 locus, which rendered this cell line unresponsive to TGF-beta (Figure S3A-D). Yet, this genetic alteration did not impede the nuclear accumulation of p-SMADs (Figure 3B) as previously reported for other SMAD4 mutant cell lines (Alarcon et al., 2009; Liu et al., 1997). Therefore, the xenografts derived from these cells combine a TGF-beta response in tumour stromal cells with lack of TGF-beta signalling in cancer cells, the scenario characteristic of advanced CRC. We inoculated CRC cells subcutaneously into nude mice in quantities that generated suboptimal engraftment in control conditions (see Methods). Elevated levels of TGF-beta dramatically increased the frequency of tumour formation and reduced the latency period in all cell lines (Figure 3D). Yet, after tumour initiation, the TGF-beta-activated microenvironment had no effect on xenograft growth rates (Figure 3E and data not shown). We further tested this early requirement by controlling the timing of TGF-beta production in HT29-M6 CRC cells via a doxycycline inducible promoter (Figure 3F, G). Secretion of TGF-beta during the first 24 hours following inoculation of tumour cells was sufficient to enhance tumour initiation (Figure 3G). Given the well-established role of TGF-beta in the polarization and suppression of the immune system in tumours (Flavell et al., 2010), we tested whether the enhancement of tumour initiation by TGF-beta signalling could be explained by modulation of the immune system. To this end, we injected CRC cells in severely immunocompromised mice of the NOD/SCID interleukin-2 receptor gamma chain null strain (NSG) (Shultz et al., 2007). In these NSG mice, high TGF-beta levels were also capable of enhancing tumour initiation, albeit fewer cancer cells were required to form tumours in this strain (Figure S3E).
Figure 3. TGF-beta activated stroma induces tumour initiation.
A, B, nuclear p-SMAD2 reactivity (arrowheads) in subcutaneous (subQ) tumours derived from TGF-beta secreting KM12L4a (A) or HT-29M6 (B) CRC cells and control cells. E: epithelial cells, Str: stromal cells. Scale bars = 10μm. C, Relative expression of some stromal TBRS genes in tumours from (B). Values are mean ±SEM (n = 3). D, Kaplan-Meier plots display tumour-free survival (TFS) for mice injected subQ with 3×104 TGF-beta secreting (red) or control cell lines (blue); SW48 (n = 8); KM12L4a (n = 8); HT29-M6 cells (n = 25 (red), n = 39 (blue). E, Growth over time for HT29-M6TGF-β (red; n = 13) and HT29-M6Con (blue; n = 16) derived tumours. Day 1: day of first detection. Values are mean ±SEM. F, Relative TGFB mRNA levels in HT29-M6 cells expressing active TGF-beta through a doxycycline (Dox) inducible promoter (HT29-M6 ind. TGF-beta) compared to control cells (Con, blue). Inducible TGF-beta cells were non-treated (gray), treated with Dox for 24h (black), or treated with Dox for 24h followed by 24h of Dox withdrawal prior to qRT-PCR (red). Values are mean ±SD (n = 3). G, Inducible TGF-beta cells were either untreated (blue) or pretreated with Dox in vitro 24h before subQ inoculation (3×104 cells; n = 6). Recipient mice received either Dox (red) or vehicle (blue) in drinking water ad libitum during 3 days prior to injection. Kaplan-Meier curves show TFS when TGF-beta secretion is induced in vivo for 24h (red) compared to control cells (blue). See also Figure S3
Stromal TGF-beta signalling is required for efficient metastasis initiation
CRCs at stages I, II and III displaying low stromal TGF-beta signalling fail to form recurrences, which in the majority of CRC patients occur in the form of metastasis. To study whether stromal TGF-beta signalling may influence metastasis formation, we inoculated KM12L4aCon and KM12L4aTGF-β cells in the caecum of nude mice. Both cell lines gave rise to aggressive colorectal tumours, which killed mice by obstruction of the intestinal lumen. At death, KM12L4aCon and KM12L4aTGF-β had generated colon tumours of equivalent size (data not shown) with no significant differences in their degree of invasion, spread to local lymph nodes or major histological features (Figure 4A and Figure S4A-B). Yet, two thirds of mice bearing KM12L4aCon colon tumours remained metastasis-free, whereas 10 out 11 mice inoculated with KM12L4aTGF-β cells developed lung and/or liver metastasis (p<0.01, Figure 4B). These data imply that stromal TGF-beta signalling promotes metastasis formation. We further explored this phenomenon by using intra-splenic inoculation of CRC cells (Morikawa et al., 1988). Secretion of TGF-beta by KM12L4a cells increased the liver metastases burden in mice (Figure 4C). We also observed a large increase in the number of liver metastases generated by HT29-M6 secreting TGF-beta (Figure 4D). In addition, a significant fraction of mice inoculated with KM12L4aTGF-β or HT29-M6TGF-β developed lung metastases implying that TGF-beta signalling also facilitated secondary organ colonization (Figure 4E). The kinetics of liver metastasis revealed that control or TGF-beta secreting cells expanded with similar rates once tumour cells had taken root and resumed growth (from day 7 in Figure 4F). However, during the first few days following inoculation, most KM12L4aCon cells that reached the liver were progressively lost and by 7 days tumour cells were barely detectable (Figure 4F). A virtually complete loss of control metastatic cells was noticed during the first 24 hours upon inoculation of lower tumour cell numbers (Figure S4C). Secretion of TGF-beta significantly increased the amounts of KM12L4a cells detected at these early time points (Figure 4F and Figure S4C). To further test this early requirement, we used CRC cells that expressed TGF-beta from a doxycycline-inducible promoter. A short pulse of TGF-beta (24h) at the moment of intrasplenic inoculation was sufficient to increase metastasis burden by facilitating metastasis initiation without affecting subsequent tumour growth (Figure 4G, H and Figure S4D; p<0.05). Thus, high levels of TGF-beta specifically act to enhance the colonization capability of CRC cells at the initial phase of metastasis. Because KM12L4a and HT29-M6 cells harbour an inactivated TGF-beta pathway, enhanced metastasis initiation by TGF-beta secretion must be the consequence of changes in the tumour microenvironment. Indeed, metastasis derived by both TGF-beta secreting cell lines displayed enhanced desmoplastic reaction with abundant p-SMAD2 accumulation in stromal cells (Figure 4I, J, and Figure S4E-H and S5I-L) and elevated expression of stromal TBRS genes (Figure 4K and not shown).
Figure 4. TGF-beta activated stroma induces CRC metastasis.
A, Pie charts for submucosal or lymphatic vessel invasion in KM12L4aconand KM12L4aTGF-β-derived caecum tumours. B, Incidence of metastases in mice from (A) (**: p<0.01; Fisher’s exact test). C-D, Number of liver metastases after intrasplenic (IS) injection with (C) 105 KM12L4aCon or KM12L4aTGF-β cells; or (D) 2×106 HT29-M6Con or HT29-M6TGF-b cells *: p<0.05, two-tailed Student’s t test). Whiskers: Vmin, Vmax. Representative livers are shown from injections with 5×105 cells (C) or 2×106 cells (D). Arrows: metastatic nodules. E, Incidence of metastasis induced in (C), (D) and S4C. F, Normalized bioluminescence over time after injection of 5×105 KM12L4aCon or KM12L4aTGF-β cells. Intensities were normalized to day 0 and arbitrarily set to 100. Values are mean ±SEM (*: p<0.05). G-H, Inducible TGF-beta cells and recipient mice were treated as in Figure 3G. G, Bioluminescence over time after injection with 5×105 cells. Values, normalized as in (F), are mean ±SEM (*: p<0.05). H, Number of liver metastases at time of sacrifice. Whiskers: Vmin, Vmax (*: p<0.05, two-tailed Student’s t test). I-J, p-SMAD2 staining in liver metastasis generated from KM12L4aCon (I) or KM12L4aTGF-β cells (J). E: epithelial cells, Str: stromal cells. Scale bars = 10μm. K, Relative expression levels of some stromal TBRS genes in dissected metastatic nodules. Values are mean ±SEM (n = 3). See also Figure S4
Pharmacological inhibition of stromal TGF-beta signalling blocks metastasis initiation
We have recently described the purification of Colon Cancer Stem Cells (CoCSCs) from CRC biopsies via surface expression of the receptor tyrosine kinase EPHB2 (Merlos-Suarez et al., 2011). We isolated EPHB2-high CoCSCs from the primary tumour of a Stage IV CRC patient and cultured them in conditions similar to those used for expansion of normal colon stem cells (Jung et al., 2011; Sato et al., 2011). EPHB2-high tumour cells embedded in matrigel expanded as epithelial tumour organoids (Figure 5A), which retained high expression levels of colon stem cell marker genes such as LGR5 and ASCL2 (data not shown). Genomic analysis of the tumour organoids revealed that the two TGFBR2 alleles were inactivated by mutations in this patient (data not shown).
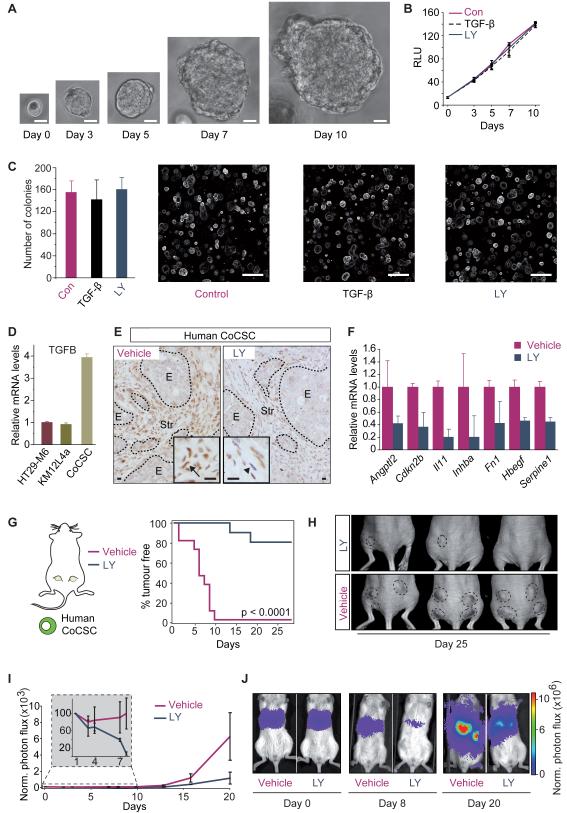
Figure 5. Inhibition of TGF-beta response in the stroma blocks tumour initiation.
A, In vitro expansion of a CoCSC. Scale bars = 10μm. B, In vitro growth of human CoCSCs upon addition of TGF-beta or LY2157299 (LY). Values are mean relative absorbance (RLU) over time ±SD (n = 3). C, Number of organoids from human CoCSCs treated with TGF-beta or LY2157299. Values are mean ±SD (n = 3). Representative images are shown. Scale bars = 200μm. D, qRT-PCR for TGFB in the indicated CRC cells. Values are mean ±SEM. E, p-SMAD2 staining in subQ tumours derived from CoCSCs injected in NSG mice treated with LY2157299 for 3 days (LY; right panel, arrowhead) or untreated (left panel; arrow). E: epithelial cells, Str: stromal cells. Scale bars = 10μm. F, Relative expression of some stromal TBRS genes in tumours from (E). Values are mean ±SEM (n = 2) G, Kaplan–Meier curves show TFS for mice injected subQ with 106 CoCSCs. Mice received LY2157299 (blue) or vehicle (purple) from 3 days prior to inoculation until sacrifice (n = 12). H, Representative images from (G). I, Bioluminescence over time after IS inoculation of 5×105 CoCSCs cells in NSG mice treated as in (G). Values, normalized as in Figure 4F, are mean ±SEM. J, Representative images from (I).
Indeed, treatment with TGFBR1-specific inhibitor LY2157299 (Bueno et al., 2008) or addition of active TGF-beta did not modify in vitro growth rates, morphology or organoid forming activity of this CoCSC-derived culture (Figure 5A-C). Primary CoCSCs expressed higher TGFB levels than CRC cells lines (Figure 5D). When injected in immunodeficient mice, they generated tumours with abundant p-SMAD2+ stromal cells (Figure 5E, left panel), implying that this primary CoCSCs elicited a TGF-beta response in the tumour microenvironment. Feeding mice bearing macroscopic tumours from CoCSCs-derived cultures with LY2157299 blocked TGF-beta signalling in the tumour stroma as shown by reduced stromal p-SMAD2 positivity (Figure 5E, right panel) and downregulated levels of stromal TBRS genes (Figure 5F). Importantly, treatment with LY2157299 conferred resistance to the formation of subcutaneous tumours by primary CoCSC-derived cells (Figure 5G, H). Remarkably, this TGF-beta inhibitor regime also reduced formation of liver metastasis by CoCSCs inoculated via the spleen (Figure 5I, J). Kinetics of metastatic colonization showed that LY2157299 reduced the number of cells that engrafted the liver immediately after inoculation (Figure 5I, – inset), the opposite behaviour of that induced by secretion of high TGF-beta levels in CRC cell lines. Of note, these experiments were performed in NSG mice, which rules out that LY2157299 blocked metastasis through changes in the function of the immune system. Altogether, the clinical and functional data described so far indicate that a TGF-beta programme activated in the tumour microenvironment confers the competence to overcome the initial phase of metastasis to CRC cells.
Metastasis initiation by TGF-beta-stimulated stromal cells depends on GP130/STAT3 signalling in tumour epithelial cells
We next sought to understand the mechanisms behind the potent effect of stromal TGF-beta programme on the capacity of CRC cells to initiate metastasis. We discovered that subcutaneous tumours and metastases generated in the context of a TGF-beta activated microenvironment displayed prominent accumulation of p-STAT3 in CRC cells compared with those derived from control cells (Figure 6A, Figure S5A-H). STAT3 signalling depended on GP130 as shown by strong reduction of epithelial p-STAT3 levels upon GP130 shRNA-mediated knockdown in CRC cells (Figure 6A and Figure S5I-J). These results suggest that TGF-beta induces the expression of GP130-binding cytokines in the tumour microenvironment, which in turn switches on STAT3 signalling in tumour epithelial cells. To study the relevance of this signalling cycle for tumourigenesis, we inoculated subcutaneously into nude mice HT29-M6 cells that secreted active TGFB1 and at the same time were knockdown for GP130 (HT29-M6shGP130/TGF-β). These experiments revealed that GP130/STAT3 signalling in cancer cells participated in the enhancement of tumour initiation mediated by stromal TGF-beta signalling (Figure 6B), but it was not important for the growth of tumours once established (Figure 6C). HT29-M6shCon or HT29-M6shGP130 cells grew with identical kinetics in vitro (Figure S5K) and, in the absence of a source of TGF-beta, formed tumours in immunodeficient mice with equivalent latency and frequency (Figure S5L).
Figure 6. Metastasis initiation driven by stromal TGF-beta signalling requires GP130/STAT3 in tumour cells.
A, p-STAT3 staining in subQ tumours derived from control (HT29-M6shCon, left panel) cells, HT29-M6 cells that secrete TGF-beta (HT29-M6shCon/TGF-β; middle panel) or HT29-M6 shGP130 cells that also secrete TGF-beta (HT29-M6shGP130/TGF-β; right panel). Arrows point to epithelial tumour cell nuclei. E: epithelial cells, Str: stromal cells. Scale bars = 50μm. B, Kaplan–Meier curves for mice injected with 3×104 HT29-M6shGP130/TGF-β (blue) or HT29-M6shCon/TGF-β cells (red; n = 16). C, Growth kinetics of HT29-M6shGP130/TGF-β (blue) and HT29-M6shCon/TGF-β (red) subQ xenografts (n = 5). Day 1: day of first detection. Values are mean ±SEM. D, Bioluminescence over time after IS inoculation of 2×106 HT29-M6shCon/TGF-β (red) or HT29-M6shGP130/TGF-β (blue) cells. Values, normalized as in Figure 4F, are mean ±SEM (n = 4; *: p<0.05). E, Bioluminescence from (D) 24h after IS injection. Whiskers: Vmin, Vmax (**: p<0.01, two-tailed Student’s t test). F, In vivo measurement of caspase3/7 activity in CRC cells 6h post IS injection. Values are mean ratios between caspase activity and total cellular bioluminescence ±SEM (n = 3). See also Figure S5
Importantly, these GP130-knockdown HT29-M6 cells secreting TGF-beta displayed decreased liver take rates during the first hours following intrasplenic inoculation (Figure 6D, E) compared to control cells. After the initial phase, the number of tumour cells detected in the liver progressively decreased and, after two weeks, both HT29-M6shCon/TGF-β and HT29-M6shGP130/TGF-β cells reinitiated expansion with similar kinetics (Figure 6D). The initial clearance of tumour cells is concurrent with apoptosis as shown by measurement of in vivo caspase activity few hours after inoculation (Figure 6F). In the absence of GP130/STAT3 signalling, apoptosis levels were increased, suggesting that this pathway promotes survival in the context of liver colonization.
Interleukin-11, a TGF-beta target gene in CAFs, enhances metastasis initiation
Interleukin-11 (IL11), a GP130-binding cytokine, was among the genes highly upregulated by TGF-beta in colon fibroblast cultures (F-TBRS, Table S4), although microarray measurements of IL11 mRNA levels did not associate with cancer recurrence in the CRC patient cohort (Table S4). HT29-M6 CRC cells responded to recombinant IL11 by activating the GP130/STAT-3 pathway (Figure 7A). Genes upregulated by IL11 in this cell line constituted an IL11-Response Signature (IL11RS, Table S4). We found that IL11RS expression correlates tightly with both TGFB and FTBRS levels in CRC samples (Figure 7B) and predicts disease relapse (Figure 7C, D). Purification of stromal populations from primary human CRC samples showed that CAFs were the only source of IL11 in tumours (Figure 7E). Experimental metastasis generated from TGF-beta secreting KM12L4a cells showed a marked upregulation of IL11 (Figure 7F). Importantly, IL11 mRNA was reduced to control levels upon LY2157299 treatment of mice bearing CRC stem cell-derived tumours (Figure 7F).
Figure 7. Stromal TGF-beta-induced IL11 increases metastasis initiation by CRC cells.
A, Western blot of p-STAT3 and total STAT3 in CRC epithelial cells upon addition of rhIL11. B, Cross-correlation analysis between expression of IL11 response signature (IL11RS) and TGFB or F-TBRS in the CRC patient cohort. Blue dots: patients. R are indicated. (***: p<0.001). C, Smooth function correlates IL11RS expression with relative risk of recurrence, stage IV patients excluded. Red dashed lines: 95% CI. Blue dashed lines: thresholds for selection into groups with low (L; blue, 42 patients), medium (M; gray, 88 patients) and high (H; red, 134 patients) IL11RS expression levels. HR (+1SD) and p-values are indicated. D, Kaplan-Meier plots with RFS for groups defined in (C). E, IL11 mRNA levels in the indicated tumour cell populations from six patients. qRT-PCR values are mean ±SD. F, Relative expression levels of IL11 mRNA in liver metastasis from IS injection with KM12L4aCon (blue) or KM12L4aTGF-β (red) (n = 3); and from subQ tumours generated by CoCSCs treated with either vehicle (Con, purple) or LY2157299 (dark blue) (n = 2). Values are mean ±SEM. G, Pie charts evaluate submucosal or lymphatic vessel invasion in KM12L4acon (n = 7) and KM12L4aIL11 (n = 8)-derived caecum tumours. Table shows incidence of metastasis in mice (**: p<0.01, Fisher’s exact test). H-L, Mice inoculated IS with 2×106 HT29-M6Con cells (blue; n = 7) or HT29-M6IL11 cells (gray; n = 4). H, Quantification and representative pictures of liver metastases (arrows) at time of sacrifice (*: p<0.05, two-tailed Student’s t test). Whiskers: Vmin, Vmax. I, Bioluminescence over time after IS inoculation. Values, normalized as in Figure 4F, are mean ±SEM (**: p<0.01). J, Representative images from (I). K, Bioluminescence 24h after injection in mice from (I). Whiskers: Vmin, Vmax (**: p<0.01, two-tailed Student’s t test). L, In vivo caspase3/7 activity 6 hours post IS injection of HT29-M6con or HT29-M6IL11 cells. Values are mean ±SEM. See also Figure S6
We tested the contribution of IL11 to metastasis by engineering CRC cells to autonomously produce this cytokine. Upon implantation in the caecum of mice, we observed no significant differences in the size (not shown), degree of invasion (Figure 7G) or histological appearance of primary tumours developed by control or study groups (Figure S6A-C). Yet, KM12L4aIL11 cells effectively colonized liver, lungs, distant lymph nodes and brain whereas control cells displayed limited metastatic capacity (Figure 7G, Table). In the same set of experiments, KM12L4a cells expressing IL6, a cytokine closely related to IL11, displayed a marginal increase in metastatic capacity (Figure S6D-E). Intrasplenic inoculation of HT29-M6IL11 tumour cells confirmed that IL11 enhanced the liver metastatic potential of CRC cells (Figure 7H-K). The initial kinetics of metastasis upon intrasplenic inoculation demonstrated that IL11-expressing cells were more proficient to colonize livers than control cells (Figure 7I, J), an effect that was evident as early as 24h after inoculation (Figure 7K). IL11 rescued apoptosis of tumour cells during the first hours of liver colonization (Figure 7L). This behaviour paralleled that induced by stromal TGF-beta through GP130 signalling in CRC cells shown in Figure 6D-F.
DISCUSSION
Metastasis involves the regeneration of a full-blown tumour from few disseminated cancer cells. This process is intrinsically inefficient mainly due to the inability of isolated tumour cells to colonize host tissues and reinitiate tumour growth in a different environment (Luzzi et al., 1998; Valastyan and Weinberg, 2011). The most accepted view is that competences to overcome this initial bottleneck result from Darwinian selection of appropriate genetic alterations in CRC cells. It is not clear, however, how functions required for colonizing a foreign organ could be selected in the primary tumour where the specific constraints imposed by a different tissue environment are not present (Luzzi et al., 1998; Valastyan and Weinberg, 2011). Additionally, metastatic traits could be acquired after cancer cells have reached the metastatic site, yet this event would necessarily require that tumour cells gain extravasation capacity and survive in the host environment. Our data argue that key functions required to overcome the initial phase of metastasis are provided by the TGF-beta-activated microenvironment. Without the activity of this stromal program, fully aggressive CRC cells fail to colonize the host organ. We speculate that those tumour cells capable of initiating metastasis with high efficiency would possess the capacity to raise such TGF-beta response in the environment. During metastatic colonization, tumour cells may instruct the stroma of the host organ by either secreting TGF-beta or recruiting TGF-beta-producing cells such macrophages, CAFs or platelets. An alternative hypothesis is that a TGF-beta-driven premetastatic niche could be specified by the primary tumour in foreign tissues through secretion of systemic cytokines, including TGF-beta itself.
Among the benefits that CRCs obtain via crosstalk with the TGF-beta-subverted microenvironment is GP130/STAT3 signalling, which suppresses apoptotic stimuli encountered during the colonization of the metastatic site. Fitting these observations, it has been shown that p-STAT3 accumulation in primary CRC samples associates to advanced disease and poor outcome (Kusaba et al., 2006; Morikawa et al., 2011). Indeed, the GP130-binding cytokine IL11, which is secreted by TGF-beta stimulated CAFs, confers robust metastatic capacity to CRC cells. On a side note, IL11 is also a megakaryopoietic cytokine that stimulates platelet production (Musashi et al., 1991; Teramura et al., 1992) and recombinant IL11 is an efficient supportive therapy in those patients with malignancies that develop thrombocytopenia as a side effect of chemotherapeutic treatment (Bhatia et al., 2007). Therefore, the prometastatic effect of IL11 described here calls for a reassessment of the use of this cytokine in an adjuvant setting. On the other hand, correlations between elevated platelet counts, referred to as thrombocytosis, and poor prognosis have been reported for many solid cancers including gastrointestinal tumours (Ikeda et al., 2002; Nouso et al., 2008). Platelets protect circulating tumour cells from immune system, as well as assist them during extravasation (Gay and Felding-Habermann, 2011). In addition, platelets are a rich source of TGF-beta (Labelle et al., 2011). It is thus possible that tumor-derived TGF-beta may promote IL11 production from stromal cells to increase platelet activation, which may further enhance stromal TGF-beta response.
Besides IL11, the TGF-beta response signatures include some previously described prometastatic factors in other tumour types such as ANGPTL4 (Padua et al., 2008), PTHLH (Yin et al., 1999), HBEGF (Bos et al., 2009), CTGF (Kang et al., 2003), TNC (Oskarsson et al., 2011) and JAG1 (Sethi et al., 2011; Sonoshita et al., 2011). For instance, ANGPTL4 mRNA levels are induced by TGF-beta in fibroblasts (Table S4). This secreted factor has been previously shown to mediate intravasation of breast cancer cells into lungs (Padua et al., 2008). Consistent with this observation, our assays show enhancement of lung metastatic capacity by CRC cells upon activation of stromal TGF-beta programme. JAG1 participates in breast cancer metastasis to the bone (Sethi et al., 2011) and activation of Notch signalling in CRC cells by endothelial cell-expressed JAG1 promotes transendothelial migration during liver and lung metastasis (Sonoshita et al., 2011). Indeed, we found that JAG1 is a TGF-beta response gene in endothelial cells (Table S4). Therefore, besides survival during the colonization phase of metastasis, the programme activated by TGF-beta in the microenvironment likely influences additional functions required to complete the metastasic process. Importantly, in contrast to CRC, the expression of ANGPTL4, PTHLH, CTGF or JAG1 is induced autonomously in breast cancer cells activated by TGF-beta (Kang et al., 2003; Padua et al., 2008; Yin et al., 1999). IL11 itself is a TGF-beta target gene in breast cancer cells, with an important role during bone metastasis formation (Kang et al., 2005; Kang et al., 2003). It thus appears that in the context of a lack of response to TGF-beta, CRC cells instead achieve similar abilities by engaging the microenvironment in a TGF-beta-dependent manner. It would be interesting to analyse whether this may be a general response in other cancer types that bear inactivating mutations in TGF-beta pathway components, such as pancreatic cancer.
The invasive adenocarcinomas developed in mouse models bearing compound mutations in Smad4 and Apc course with a prominent accumulation of reactive stroma (Kitamura et al., 2009; Takaku et al., 1998). Whereas it is not clear whether this effect depends on increased levels of TGF-beta signalling in the microenvironment, Tgfbr2 deletion in an Apc mutant background raises production of TGFB1 in tumours (Munoz et al., 2006). It is thus plausible that CRCs evolve towards a favourable scenario for metastasis by combining an increase of TGF-beta signalling in stromal cells with the acquisition of inactivating mutations in TGF-beta pathway components in the cancer cells. The majority of CRCs display moderate to high TGF-beta expression levels, which may help explain the high rates of CRC metastasis. Importantly, we discovered a subgroup of tumours (10-20% of all CRCs), displaying invasion and/or local dissemination (i.e. AJCC Stage II and III) yet low TGF-beta production that did not relapse after surgical intervention. Therefore, besides AJCC staging, our findings call for the assessment of TGF-beta pathway activation in stromal cells as a central criterion for patient stratification. Several targeted therapies against TGF-beta signalling including LY2157299 are currently being evaluated for treatment of different cancer types, (Tan et al., 2009; Yingling et al., 2004). Whereas their efficacy is not yet known, our observations predict that pharmacological inhibition of TGF-beta signalling may prevent CRC relapse and metastasis when treating patients at early time point of the process.
EXPERIMENTAL PROCEDURES
Profile datasets
We defined overall TGF-beta levels (TGFB) as the average expression of TGFB1, TGFB2 and TGFB3 mRNAs in a given sample (see supplemental Information). Datasets corresponding to human colon adenomas and carcinomas have been previously described (Sabates-Bellver et al., 2007; Van der Flier et al., 2007). To correlate TGFB and TBRS expression with clinical disease progression, we pooled two publicly available sets of Affymetrix transcriptomic profiles (GSE17537 and GSE14333) corresponding to primary CRCs for which clinical follow-up was available. GSE17537 (Smith et al., 2010) is composed of 55 colon cancer patients treated at Vanderbilt University Medical Center (Vanderbilt, USA). GSE14333 (Jorissen et al., 2009) contains a pool of 290 CRC patients treated at two different hospitals; Peter MacCallum Cancer Center (Australia) and H. Lee Moffitt Cancer Center (USA). The representation of tumour samples at different AJCC stages in these cohorts follows the natural distribution of CRC patients receiving standard treatment in the aforementioned hospitals. Descriptive statistics (Table S1) as well as univariate analysis of clinical progression parameters (Table S2) in this meta-cohort are included as supplementary information. The TGF-beta response signatures used in this study are detailed in Table S4.
Orthotopic mouse studies
All experiments with mouse models were approved by the animal care and use committee of the Barcelona Science Park (CEEA-PCB) and the Catalan Government. Cells were injected subcutaneously in 5 to 6 weeks old Swiss nude or NSG mice, (Jackson Labs), which were followed for the periods described. Tumour appearance was assessed by palpation. Five to six weeks old Balb/c nude or NSG mice (Jackson Labs) were used to perform metastasis experiments by intra-splenic injection (Warren et al., 1995) or intra-caecum injection (Cespedes et al., 2007).
Clinical material
Biological samples were obtained from individuals treated at the Hospital del Mar (Barcelona, Spain) or from Hospital Clinic (Barcelona, Spain) under informed consent and approval of the Bank Tumour Committees of each hospital according to Spanish Ethical regulations. The study followed the guidelines of the Declaration of Helsinki and patient’s identity of pathological specimens remained anonymous in the context of this study. Experiments were approved by the ethics committee of IRB/Hospital Clinic (project ERC-208488/CRCprogramme).
Supplementary Material
HIGHLIGHTS.
TGF--beta pathway mutant cells require a stromal TGF--beta program for metastasis
CRC patients with low levels of stromal TGF--beta program do not relapse
Pharmacological blockade of TGF--beta stromal signaling prevents metastasis initiation
A TGF--beta/IL11/GP130 signaling cycle confers metastatic organ colonization capacity
SIGNIFICANCE.
About 40-50% of all colorectal cancer patients will present with metastasis either at the time of diagnosis or as recurrent disease upon intended curative therapy. In the absence of genetic alterations that explicate these processes, it remains a major challenge to predict which patients will develop metastatic disease or to design targeted therapies. Here we show that metastasis depends on a gene programme expressed by the tumour microenvironment upon TGF-beta stimulation. Low stromal TGF-beta signalling is a robust predictor of disease-free survival after therapy, which improves on the current AJCC staging system. Furthermore, colonization of foreign organs requires TGF-beta signalling in stromal cells and therefore may be a target for therapeutic intervention at early time points of the metastatic process.
ACKNOWLEDGMENTS
We thank IRB Functional Genomics Core Facility for technical assistance in microarray hybridization experiments, Jonathan Cheng (Fox Chase Cancer Center) for the anti-FAP antibody, Mark Taketo (Kyoto University) for mouse tumour samples, Elisabeth Calderón and Azucena Salas (IDIBAPS, Barcelona) for support with tumour samples, Xavier Hernando for assistance in the animal facility, Jacob Sabates and Giancarlo Marra (IMZR, Zurich) for sharing the Ad-CRC Affymetrix patient dataset, Jaume Comas and Ricard Álvarez, (UB, Barcelona) for expert technical assistance with the FACS, Iris Joval for assistance in mounting figures and all members of the Batlle Lab for support and discussions. A.C. and D.V.F.T hold a Juan de la Cierva postdoctoral fellowship, E.E. an FPI PhD fellowship (both from Spanish Ministry of Science and Innovation). JM is an Investigator of the Howard Hughes Medical Institute. This work has been supported by grants from Instituto de Salud Carlos III FEDER (RD09/0076/00036) and the “Xarxa de Bancs de tumors sponsored by Pla Director d’Oncologia de Catalunya (XBTC), to E.B. from the European Research Council (Starting grant - 208488) and Consolider programmes (MICINN), to E.S. and A.C. by the Spanish Ministry of Science and Innovation, to JM by NIH grant CA34610 and to RM by grants PS09/00965 (MICINN) and NanoCoMets (CIBERBBN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Davenport V, Cairo MS. The role of interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with solid tumors, lymphoma, acute myeloid leukemia and bone marrow failure syndromes. Leuk Lymphoma. 2007;48:9–15. doi: 10.1080/10428190600909115. [DOI] [PubMed] [Google Scholar]
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, Troconiz IF. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Cespedes MV, Espina C, Garcia-Cabezas MA, Trias M, Boluda A, Gomez del Pulgar MT, Sancho FJ, Nistal M, Lacal JC, Mangues R. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol. 2007;170:1077–1085. doi: 10.2353/ajpath.2007.060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer. Annu Rev Genomics Hum Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. [DOI] [PubMed] [Google Scholar]
- Hahn JN, Falck VG, Jirik FR. Smad4 deficiency in T cells leads to the Th17-associated development of premalignant gastroduodenal lesions in mice. J Clin Invest. 2011;121:4030–4042. doi: 10.1172/JCI45114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, Tatsuta M, Satomi T. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9:287–291. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, Traulsen A, Nowak MA, Siegel C, Velculescu VE, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen RN, Gibbs P, Christie M, Prakash S, Lipton L, Desai J, Kerr D, Aaltonen LA, Arango D, Kruhoffer M, et al. Metastasis-Associated Gene Expression Changes Predict Poor Outcomes in Patients with Dukes Stage B and C Colorectal Cancer. Clin Cancer Res. 2009;15:7642–7651. doi: 10.1158/1078-0432.CCR-09-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massague J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Biyajima K, Aoki M, Oshima M, Taketo MM. Matrix metalloproteinase 7 is required for tumor formation, but dispensable for invasion and fibrosis in SMAD4-deficient intestinal adenocarcinomas. Lab Invest. 2009;89:98–105. doi: 10.1038/labinvest.2008.107. [DOI] [PubMed] [Google Scholar]
- Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, Nagayasu T, Sekine I. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006;15:1445–1451. [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Pouponnot C, Massague J. Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes. Genes Dev. 1997;11:3157–3167. doi: 10.1101/gad.11.23.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, Sevillano M, Hernando-Momblona X, da Silva-Diz V, Munoz P, et al. The Intestinal Stem Cell Signature Identifies Colorectal Cancer Stem Cells and Predicts Disease Relapse. Cell Stem Cell. 2011 doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–6871. [PubMed] [Google Scholar]
- Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS, Ogino S. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452–1462. doi: 10.1158/1078-0432.CCR-10-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz NM, Upton M, Rojas A, Washington MK, Lin L, Chytil A, Sozmen EG, Madison BB, Pozzi A, Moon RT, et al. Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res. 2006;66:9837–9844. doi: 10.1158/0008-5472.CAN-06-0890. [DOI] [PubMed] [Google Scholar]
- Musashi M, Yang YC, Paul SR, Clark SC, Sudo T, Ogawa M. Direct and synergistic effects of interleukin 11 on murine hemopoiesis in culture. Proc Natl Acad Sci U S A. 1991;88:765–769. doi: 10.1073/pnas.88.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouso K, Ito Y, Kuwaki K, Kobayashi Y, Nakamura S, Ohashi Y, Yamamoto K. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br J Cancer. 2008;98:1161–1165. doi: 10.1038/sj.bjc.6604282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA, Bujnicki JM, Menigatti M, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Deane NG, Wu F, Merchant NB, Zhang B, Jiang A, Lu P, Johnson JC, Schmidt C, Bailey CE, et al. Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology. 2010;138:958–968. doi: 10.1053/j.gastro.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hashida H, Takabayashi A, Sasaki M, Robine S, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19:125–137. doi: 10.1016/j.ccr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- Tan AR, Alexe G, Reiss M. Transforming growth factor-beta signaling: emerging stem cell target in metastatic breast cancer? Breast Cancer Res Treat. 2009;115:453–495. doi: 10.1007/s10549-008-0184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramura M, Kobayashi S, Hoshino S, Oshimi K, Mizoguchi H. Interleukin-11 enhances human megakaryocytopoiesis in vitro. Blood. 1992;79:327–331. [PubMed] [Google Scholar]
- Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, Imai Y, Nagashima R, Misawa H, Takeda H, et al. Circulating transforming growth factor beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258–1262. [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9:489–499. doi: 10.1038/nrc2645. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun L, Myeroff L, Wang X, Gentry LE, Yang J, Liang J, Zborowska E, Markowitz S, Willson JK, et al. Demonstration that mutation of the type II transforming growth factor beta receptor inactivates its tumor suppressor activity in replication error-positive colon carcinoma cells. J Biol Chem. 1995;270:22044–22049. doi: 10.1074/jbc.270.37.22044. [DOI] [PubMed] [Google Scholar]
- Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.