Abstract
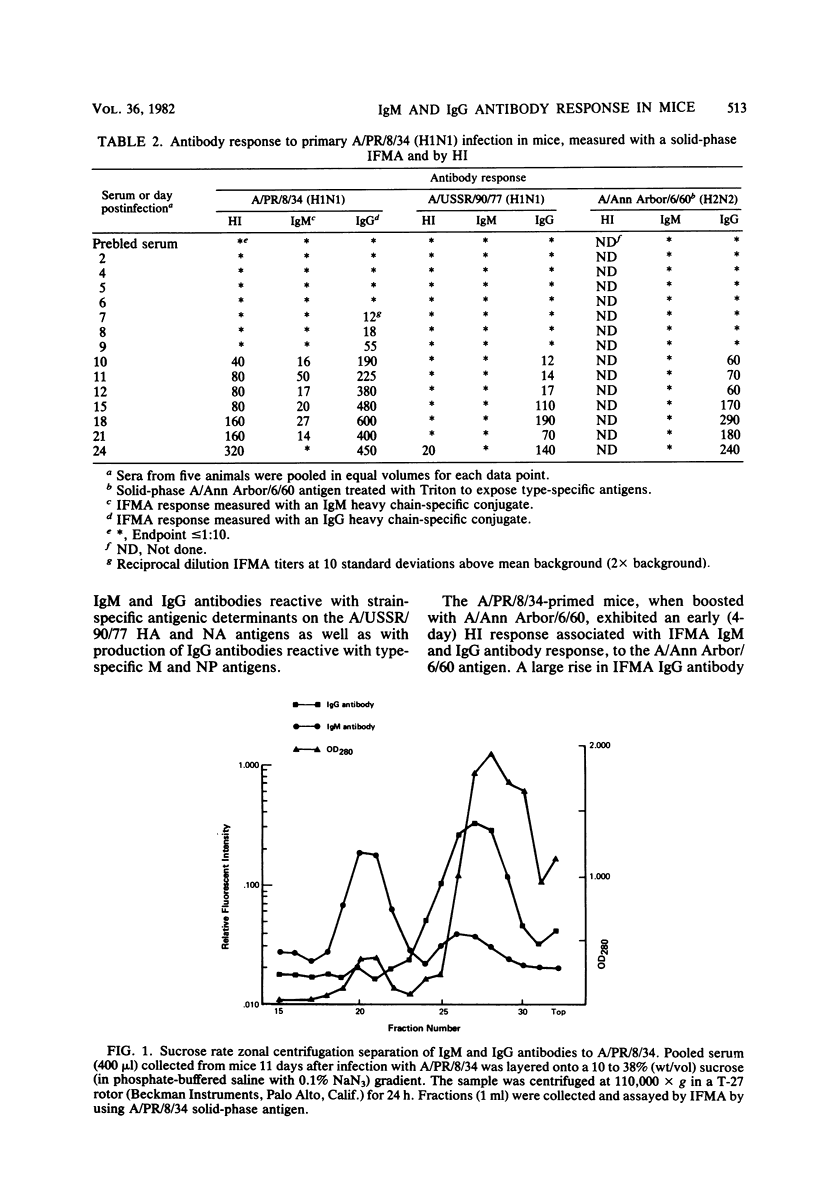
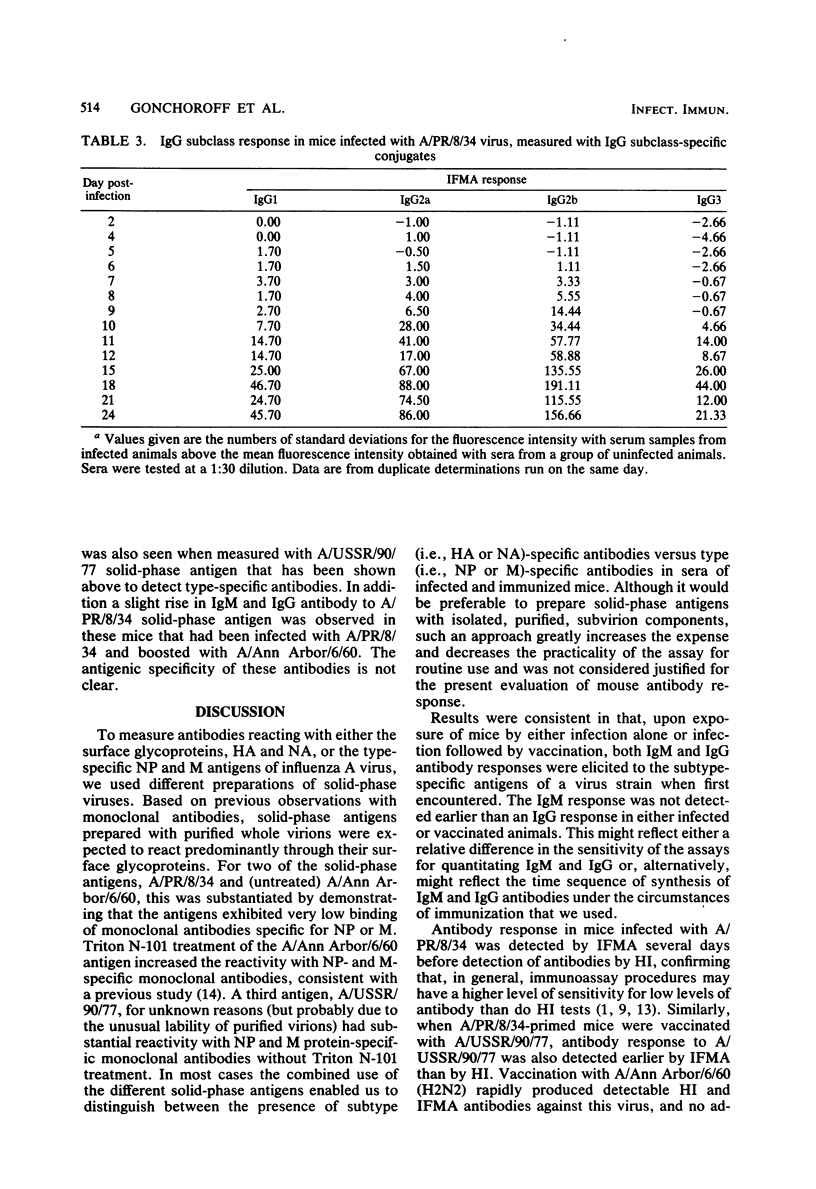
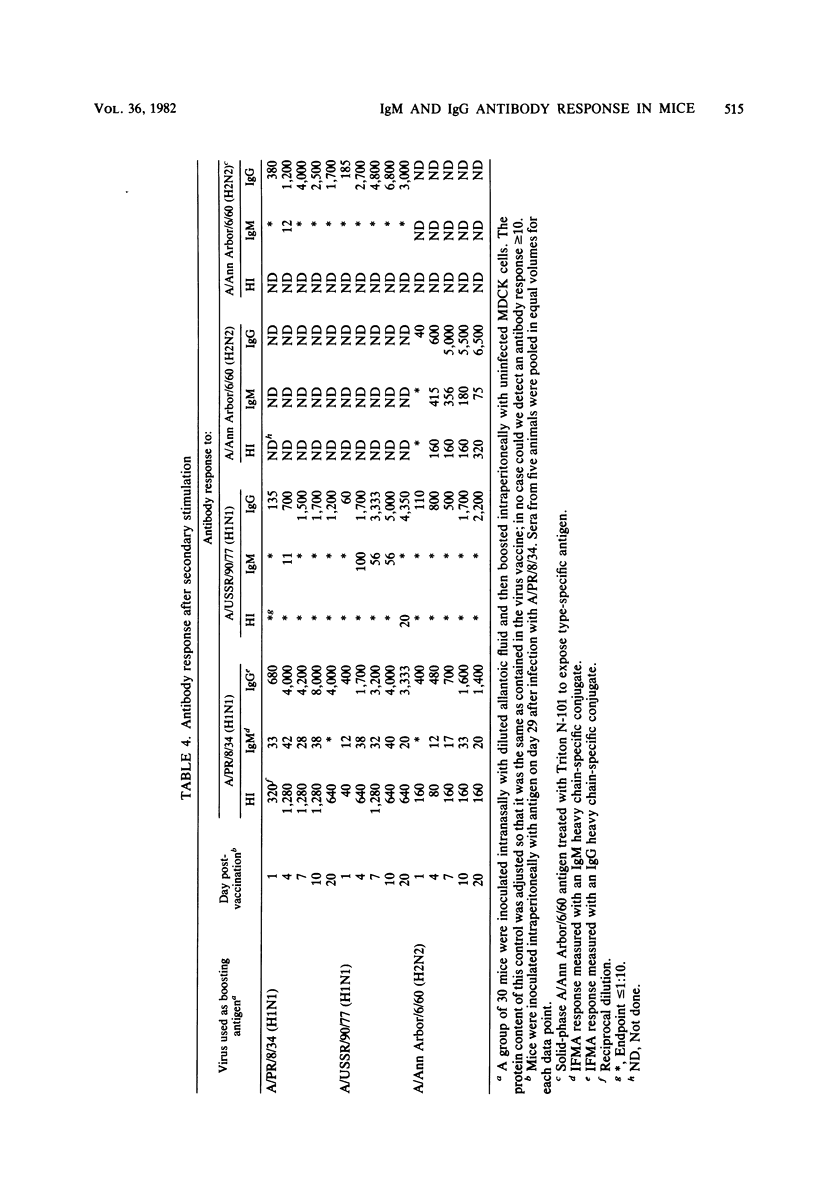
A mouse model of influenza infection was studied to help define parameters that may affect serodiagnosis of human infections by immunoassays. Antibodies to both type- and subtype-specific influenza A antigens were measured by a solid-phase immunofluorometric assay. Dilute mouse sera were added to purified influenza virus that had been covalently bound to polyaminostyrene microbeads, and the bound antibody was detected by fluorescein isothiocyanate-labeled isotype-specific antisera. Results were consistent in that upon exposure of mice by either infection alone or by vaccination after infection, both immunoglobulin M (IgM) and IgG antibodies reactive with newly encountered subtype specific viral antigens were measured. IgG antibody was usually detectable by the solid-phase immunofluorometric assay several days before it could be detected by a hemagglutination inhibition test. Increased levels of antibody of the IgG1, IgGa, IgG2b, and IgG3 subclasses were also measured during influenza infection. Surprisingly, response to type-specific viral antigens was of the IgG class in primary as well as in secondary exposure. The results suggest that for serodiagnosis of influenza infections by detection of specific IgM antibody, the assay should use subtype-specific antigens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishai F. R., Galli R. Enzyme-linked immunosorbent assay for detection of antibodies to influenza A and B and parainfluenza type 1 in sera of patients. J Clin Microbiol. 1978 Dec;8(6):648–656. doi: 10.1128/jcm.8.6.648-656.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner Y. I., Heath R. B., Collins J. V., Pattison J. R. Detection of antibodies of the IgM class in sera of patients recently infected with influenza viruses. J Clin Pathol. 1976 May;29(5):423–427. doi: 10.1136/jcp.29.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner Y. I., Heath R. B., Collins J. V., Pattison J. R. Serum IgM antibody and influenza A infection. J Clin Pathol. 1977 Aug;30(8):723–727. doi: 10.1136/jcp.30.8.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty H., Davis M. L., Kaye H. S. Immunoglobulin class of influenza antibodies investigated by radioimmunoassay (RIA). J Immunol. 1972 Oct;109(4):849–856. [PubMed] [Google Scholar]
- Ennis F. A., Wise T. G., McLaren C., Verbonitz M. W. Serological responses to whole and split A/New Jersey vaccines in humans and mice following priming infection with influenza A viruses. Dev Biol Stand. 1977 Jun 1;39:261–266. [PubMed] [Google Scholar]
- Hammond G. W., Smith S. J., Noble G. R. Sensitivity and specificity of enzyme immunoassay for serodiagnosis of influenza A virus infections. J Infect Dis. 1980 May;141(5):644–651. doi: 10.1093/infdis/141.5.644. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Cox N. J., Galphin J. C., Maassab H. F. Comparative studies of wild-type and cold-mutant (temperature-sensitive) influenza viruses: independent segregation of temperature-sensitivity of virus replication from temperature-sensitivity of virion transcriptase activity during recombination of mutant A/Ann Arbor/6/60 with wild-type H3N2 strains. J Gen Virol. 1979 Aug;44(2):443–456. doi: 10.1099/0022-1317-44-2-443. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Phillips D. J., Webster R. G., Galland G. G., Reimer C. B. Effect of test system on the ability of monoclonal antibodies to detect antigenic drift in influenza A(H1N1) virus haemagglutinins. J Gen Virol. 1981 Jun;54(Pt 2):253–261. doi: 10.1099/0022-1317-54-2-253. [DOI] [PubMed] [Google Scholar]
- Masihi K. N., Lange W. Enzyme-linked immunosorbent assay for the detection of influenza type-specific antibodies. J Immunol Methods. 1980;36(2):173–179. doi: 10.1016/0022-1759(80)90041-1. [DOI] [PubMed] [Google Scholar]
- McLaren C., Potter C. W. Immunity to influenza in ferrets. VII. Effect of previous infection with heterotypic and heterologous influenza viruses on the response of ferrets to inactivated influenza virus vaccines. J Hyg (Lond) 1974 Feb;72(1):91–100. doi: 10.1017/s0022172400023251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Verbonitz M. W., Daniel S., Grubbs G. E., Ennis F. A. Effect of priming infection on serologic response to whole and subunit influenza virus vaccines in animals. J Infect Dis. 1977 Dec;136 (Suppl):S706–S711. doi: 10.1093/infdis/136.supplement_3.s706. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Phelan M. A., Nelson D. L., Yarchoan R., Tierney E. L., Alling D. W., Chanock R. M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981 Mar;13(3):554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Tierney E. L., Barbour B. A., Yolken R. H., Alling D. W., Holley H. P., Jr, Mayner R. E., Chanock R. M. Use of the enzyme-linked immunosorbent assay to detect serum antibody responses of volunteers who received attenuated influenza A virus vaccines. Infect Immun. 1980 Aug;29(2):342–347. doi: 10.1128/iai.29.2.342-347.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. J., Kendal A. P., Webster R. G., Feorino P. M., Reimer C. B. Detection of monoclonal influenza antibodies synthesized in culture by hybridoma cells with a solid-phase indirect immunofluorometric assay. J Virol Methods. 1980;1(5):275–283. doi: 10.1016/0166-0934(80)90024-5. [DOI] [PubMed] [Google Scholar]
- Phillips D. J., Reimer C. B., Wells T. W., Black C. M. Quantitative characterization of specificity and potency of conjugated antibody with solid-phase, antigen bead standards. J Immunol Methods. 1980;34(4):315–327. doi: 10.1016/0022-1759(80)90104-0. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., Rees R. C., McLaren C. Antibody response of hamsters to A2-Hong Kong virus vaccine after priming by heterotypic virus infection. Infect Immun. 1973 Aug;8(2):137–144. doi: 10.1128/iai.8.2.137-144.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. M., Liew F. Y. T cells primed by influenza virion internal components can cooperate in the antibody response to haemagglutinin. Nature. 1979 Jul 12;280(5718):147–148. doi: 10.1038/280147a0. [DOI] [PubMed] [Google Scholar]
- SCHULMAN J. L., KILBOURNE E. D. INDUCTION OF PARTIAL SPECIFIC HETEROTYPIC IMMUNITY IN MICE BY A SINGLE INFECTION WITH INFLUENZA A VIRUS. J Bacteriol. 1965 Jan;89:170–174. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


