Abstract
Objective
To compare the visual outcomes and adverse events of contact lens to primary intraocular lens (IOL) correction of monocular aphakia during infancy.
Methods
In a randomized, multicenter (12 sites) clinical trial, 114 infants with a unilateral congenital cataract were assigned to undergo cataract surgery between 1 to 6 months of age either with or without primary IOL implantation. Contact lenses were used to correct aphakia in patients who did not receive IOLs. Grating visual acuity was tested at 1 year of age by a masked traveling examiner
Main Outcome Measures
Grating visual acuity at 1 year of age.
Results
The median logMAR visual acuity was not significantly different between the treated eyes in the two groups (CL = 0.80, IOL = 0.97, p =.20). More patients in the IOL group underwent one or more additional intraocular surgeries than patients in the CL group (63% vs 12%; p <.0001). Most of these additional surgeries were performed to clear lens reproliferation and pupillary membranes from the visual axis.
Conclusions
There was no statistically significant difference in grating visual acuity at age 1 year between the IOL and CL groups; however, additional intraocular surgeries were performed more frequently in the IOL group.
Application to Clinical Practice
Until longer term follow-up data are available, caution should be exercised when performing IOL implantation in children 6 months of age or younger given the higher incidence of adverse events and the absence of an improved short-term visual outcome compared to contact lens use.
Since the 1970s, contact lenses have been the standard means of optically correcting unilateral aphakia in infancy.1–7 Their use during infancy, however, can be challenging due to problems with insertion and removal of lenses by parents, lens loss, difficulties with fitting the steep corneas of infants, and compliance problems. These factors among others probably contribute to the poor visual outcome of many children with unilateral aphakia. Intraocular lens (IOL) technology and microsurgical techniques have improved considerably in recent years. As a result, IOLs are being used increasingly for the optical correction of aphakia in infants following cataract surgery.8–10 However, the risks and benefits of IOL implantation during infancy have not been studied in the setting of a randomized clinical trial.
Intraocular lenses have the advantage of providing a partial optical correction at all times and more closely simulate the optics of the natural crystalline lens.8, 11 Such benefits, however, may be offset by complications that can be associated with IOL implantation and with the rapidly changing optical needs of a growing eye.12–15 Small case series have reported improved visual outcomes after IOL implantation during infancy.11, 16 However, these series have been retrospective and the number of patients included failed to provide the statistical power necessary to adequately assess the visual benefits of IOL implantation. Most series have also reported more postoperative complications than observed with leaving the eyes aphakic.15
The Infant Aphakia Treatment Study (IATS) is a multi-center, randomized, controlled clinical trial sponsored by the National Eye Institute. The objective of the study is to compare the use of immediate IOL implantation to the correction of aphakia with a contact lens after cataract surgery performed in infants with a unilateral congenital cataract between 1 and 6 months of age. This paper reports the clinical findings up to 12 months after surgery and the visual outcomes at 1 year of age by treatment group among the 114 patients enrolled in IATS.
METHODS
The study design, surgical technique, follow-up schedule, patching and optical correction regimens, evaluation methods, and patient characteristics at baseline have been reported in detail previously17 and are only summarized in this report. This study was approved by the institutional review boards of all the participating institutions and was in compliance with the Health Insurance Portability and Accountability Act. The off-label research use of the Acrysof SN60AT and MA60AC IOLs (Alcon Laboratories, Fort Worth, Texas) was covered by US Food and Drug Administration investigational device exemption # G020021.
Study Design
The main inclusion criteria were a visually significant congenital cataract (≥ 3 mm central opacity) in one eye and an age of 28 days to <210 days at the time of cataract surgery. Infants with a unilateral cataract due to persistent fetal vasculature (PFV) were allowed in the study as long as the PFV was not associated with visible stretching of the ciliary processes or involvement of the retina or optic nerve. The other main exclusion criteria were an acquired cataract, a corneal diameter <9 mm, a medical condition that might interfere with visual acuity testing, and prematurity (<36 gestational weeks). Patients were randomized to have either an IOL placed at the time of the initial surgery or to be left aphakic and corrected with a contact lens.
Surgical Technique
Infants randomized to the contact lens (CL) group underwent a lensectomy and anterior vitrectomy (Video 1: Cataract Extraction + IOL Implantation using IATS protocol). Infants randomized to the IOL group initially had the lens contents aspirated followed by the implantation of an AcrySof SN60AT IOL into the capsular bag (Video 2: Lensectomy using IATS protocol). In the event that both haptics could not be implanted into the capsular bag, an AcrySof MA60AC IOL was implanted into the ciliary sulcus. The IOL power was calculated based on the Holladay 1 formula targeting an 8 D undercorrection for infants 4–6 weeks of age and a 6 D undercorrection for infants older than 6 weeks. Following IOL placement, a posterior capsulectomy and an anterior vitrectomy were performed through the pars plana/plicata. When either a pre-existing opening was present or a rent developed intraoperatively in the posterior capsule and in some eyes with mild PFV, the posterior capsulectomy and anterior vitrectomy were performed through the anterior incision prior to IOL implantation.
Optical Correction
Within a week after cataract surgery, patients randomized to the CL group were fitted with a Silsoft (Bausch and Lomb, Rochester, NY) or a rigid gas permeable contact lens with a 2.0 D overcorrection to provide a near point focus. For patients randomized to the IOL group, spectacles were prescribed prior to the 1-month postoperative visit or at any later visit providing that one of the following conditions existed in the treated eye: hyperopia >1.0 D, myopia >3.0 D or astigmatism >1.5 D. The overall aim was to overcorrect the refractive error by 2.0 D to achieve a near point focus. The prescribed optical correction was to be worn at all times while the patient was awake.
Patching Regimen
Starting the second postoperative week, parents were instructed to have their child wear an adhesive occlusive patch over the unoperated eye for 1 hour/day per each month of age until age 8 months. Thereafter, patching was prescribed for all waking hours every other day or for one-half of the patient’s waking hours every day.
Follow-up: Clinical Examinations and Grating Visual Acuity Assessment
Follow-up examinations were performed by an IATS certified investigator at one day, one week, one month, and 3 months after cataract surgery. Subsequent follow-up examinations were obtained at 3 month (± 2 weeks) intervals. The investigator performed a standard clinical exam, checking the appropriateness of the optical correction and monitoring for adverse events. All of the patients underwent an examination-under-anesthesia 2–4 weeks prior to the grating visual acuity assessment. The patient’s optical correction was updated after the EUA prior to the grating acuity assessment.
Monocular grating acuity was assessed at 1 year ± 2 months of age by a traveling examiner using Teller Acuity Cards (Stereo Optical, Chicago, Illinois). Vision in the aphakic/pseudophakic eye was tested first. When nystagmus was present, monocular grating acuity was tested using a +10 D lens placed over the eye not being tested. Each site had a stage for presentation of the grating stimuli. The standard testing distance was 55 cm measured from the screen to the child’s eyes. Children with poor visual acuity were tested at nearer distances (e.g. 38, 19, 9.5 cm) and the Low Vision Card was used to determine the presence or absence of some pattern vision. Evaluations of LP or NLP visual acuities were performed following standard clinical protocols.
Adherence to Patching and Optical Correction
Adherence to patching and optical correction was assessed using 48-hour recall telephone interviews and 7-day diaries. The interviews were conducted every 3 months starting 3 months after surgery. Caregivers completed a 7-day patching diary two months following surgery and annually thereafter, one month after the child’s birthday. We calculated the proportion of waking time wearing optical correction at each assessment. Good adherence to patching was defined as reported patching at least 75% of prescribed time. Pseudophakic children who were not required to wear glasses because the refractive error was between +1.0 D and −3.0 D with less than 1.5 D of astigmatism were considered to be fully corrected without their spectacles on, and were therefore considered to be wearing optimal correction 100% of their waking hours.
The present analyses of adherence to patching and optical correction are limited to adherence assessments obtained prior to the visual acuity assessment. Data are available on 100 patients (51 CL, 49 IOL) two months after surgery, 110 patients (55 each from the CL and IOL groups) three months after surgery and 96 patients (48 from each group) six months after surgery. Data were available on fewer than half of the participants nine months after surgery (22 CL and 23 IOL) because the remainder of the interviews took place after the visual acuity assessment visit. The nine-month adherence data is therefore not presented because of potential biases and instability of the estimates.
Definitions for Adverse Events
Glaucoma was defined as IOP >21 mmHg with one or more of the following anatomical changes: 1) corneal enlargement; 2) asymmetrical progressive myopic shift coupled with enlargement of the corneal diameter and/or axial length; 3) increased optic nerve cupping defined as an increase of ≥ 0.2 in the cup-to-disc ratio, or 4) the use of a surgical procedure for IOP control. A patient was designated a Glaucoma suspect if there was either: 1) two consecutive IOP measurements above 21 mmHg on different dates after topical corticosteroids had been discontinued without any of the anatomical changes listed above; or 2) glaucoma medications were used to control IOP without any of the anatomical changes listed above. A pupillary membrane was defined as fibrous tissue extending across the pupil. Lens reproliferation into the visual axis was defined as lens material regrowth extending into the pupillary space and interfering significantly with vision. Children who had strabismus surgery were not classified as “orthotropic” even if they were later orthotropic on motility testing.
Statistical Considerations
The sample size was calculated to provide 80% power to detect a 0.2 logMAR difference in mean grating acuity between the two treatment groups using an independent groups t test with alpha=0.05 (two tailed) and standard deviation of visual acuity = 0.365 based on previously published data.16 The resulting sample size estimate was 57 patients per group and included an adjustment for a 5% lost to follow-up. As the visual acuity data accumulated, it became clear that the distribution of visual acuities for IATS patients was markedly skewed and that the independent groups t test would not be the appropriate statistical test. The sample size was evaluated in a simulation utilizing a re-sampling approach from a dataset provided by Dr. Eileen Birch consisting of 51 patients undergoing unilateral cataract surgery at less than 7 months of age treated with a contact lens. The outcome measure was optotype visual acuity at 5 years of age since IATS patients will be followed to age 5 to provide a more definitive measurement of visual acuity. The simulation incorporated the Wilcoxon rank-sum test and indicated that the power for detecting a 0.2 logMAR difference in visual acuity between the treatment groups was 0.74 with 57 patients per group and therefore the sample size was not changed.
The visual acuities at 1 year of age were compared between the treatment groups using the Wilcoxon rank-sum test. A nonparametric test was used because of the skewed distribution of the data and because of the assignment of visual acuity values for patients with vision below the level detectable with Teller acuity cards (see the IATS Protocol at www.sph.emory.edu/IATS for details). For other continuous factors the mean was compared between the treatment groups using the independent groups t test. The percent of patients experiencing adverse events or undergoing additional intraocular surgeries was compared between the treatment groups using Fisher’s exact test. All tests were conducted as two-sided. No adjustment was made for multiple testing. For the primary outcome, visual acuity, a p-value <0.05 was deemed statistically significant, whereas for other outcomes, the significance level was 0.01.
RESULTS
Study Population
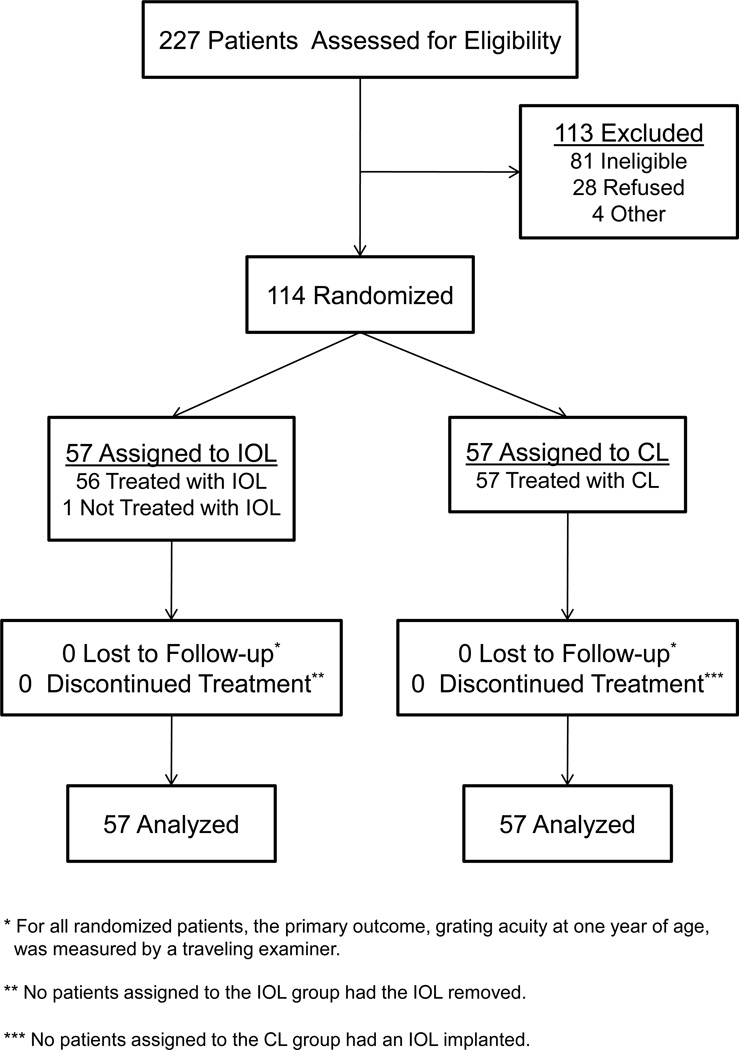
There were 114 patients enrolled in the study with 57 randomized to each treatment group (Figure 1). Two patients with PFV were enrolled in the study despite having an exclusion criterion (e.g. PFV with stretching of the ciliary processes). In the first case, the patient was randomized into the study because stretching of the ciliary processes was not visible preoperatively even after pupillary dilation. This patient was randomized to the IOL group. However, the investigator decided intraoperatively that an IOL could not be safely implanted in this eye; the patient was left aphakic and treated with a contact lens. In the second case, the investigator noted stretched ciliary processes preoperatively, but did not think the stretching severe enough to warrant exclusion from the study. This patient was randomized to the IOL group and had a lens implanted in the ciliary sulcus. A post-hoc review of this surgical video by the IATS steering committee determined that the stretching of the ciliary processes in this eye did indeed meet the PFV exclusion criterion. Both patients were analyzed in the IOL group following the intent-to-treat principle. Fifty two (93%) of the patients in the IOL group had the IOL implanted in the capsular bag, while 4 (7%) had the IOL implanted in the sulcus.
Figure 1.
Flow diagram illustrating the progress of patients throughout the IATS.
The median (25th, 75th percentiles) age at the time of surgery was 1.8 (1.1, 3.1) months for the CL group (n=57) and 1.8 (1.2, 3.2) for the IOL group (n=57). The 114 patients distributed into age categories as follows: 4 to 6 weeks, 50 patients (CL group, 25, IOL group 25) (44%); 7 weeks to 3 months, 32 patients (CL group, 17, IOL group 15) (28%); 3.1 to 5 months, 19 patients (CL group, 9, IOL group 10) (17%); and 5.1 to 6.9 months, 13 patients (CL group, 6, IOL group 7) (11%). There were 60 girls (53%) and 97 whites (85%). The baseline clinical characteristics of the patients in total and as individual treatment groups have been previously published.17 None of the patients was lost to follow-up during the first 12 months after surgery and all patients had their vision measured by a traveling tester at 1 year of age (Figure 1). The percent of completed postoperative follow-up visits were: 1 day (100%), 1 week (97%), 1 month (99%), 3 months (100%), 6 months (98%), 9 months (97%), and 12 months (92%).
Visual Acuity
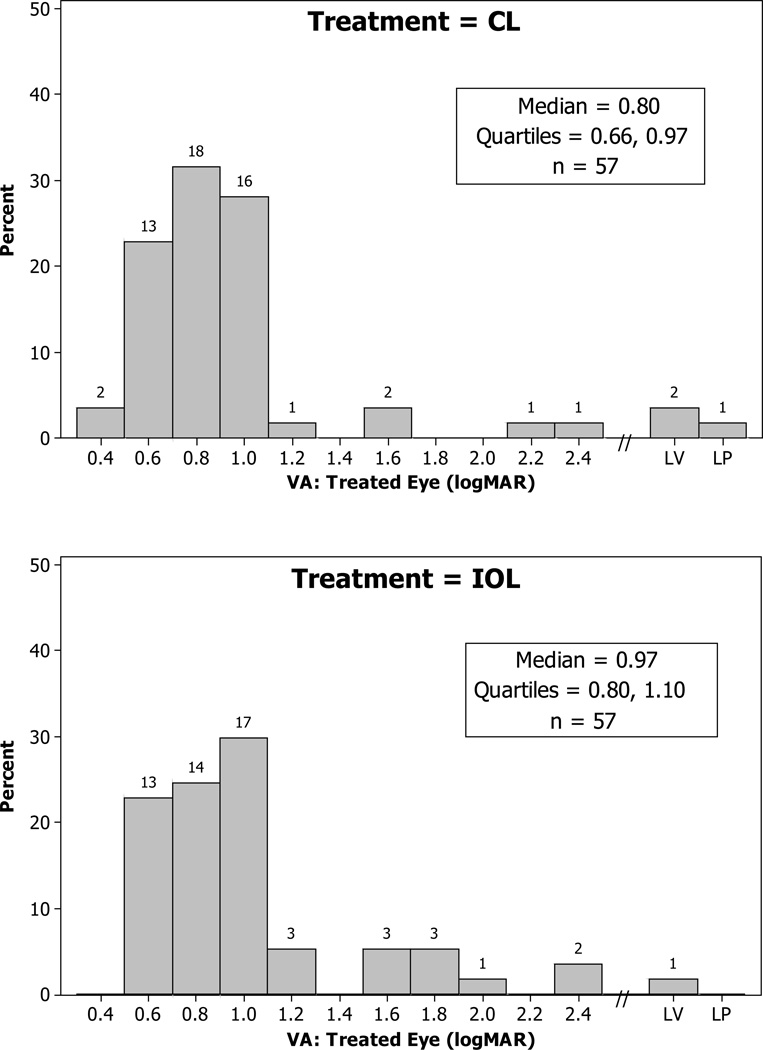
The visual acuity assessments were conducted by two traveling examiners, one of whom performed the majority of the assessments (74%). The percent of patients tested by this examiner was not significantly different in the two treatment groups (CL, 72%; IOL, 75%; p =.83). Of the 114 patients, 111 (97%) were examined within 30 days of their age 1 year birthday. Two patients were examined outside the 12 ± 2 month window (ages 17.6 and 18.6 months). The median logMAR visual acuity was not significantly different between the treated eyes in the two groups (CL = 0.80, IOL = 0.97, p =.19) (Figure 2). The difference between the medians of the two groups was 0.17 logMAR which was slightly larger than the interval between each of the Teller Acuity Cards (0.5 octaves or 0.15 logMAR) and slightly smaller than the difference the study was designed to detect (0.20 logMAR). Very poor vision was present in the treated eye in 3 patients in the CL group (2 with pattern vision only detectable with the low vision card and 1 with light perception) and 1 treated eye in the IOL group (pattern vision only detectable with the low vision card). The median logMAR visual acuity was 0.66 in the untreated eyes of both treatment groups (Figure 3).
Figure 2.
Histograms of logMAR visual acuity of treated eyes at 1 year of age for the treatment groups. Visual acuity was assessed using Teller acuity cards. The numbers above the bars indicate the number of patients in the acuity category (LV = Low Vision Card; LP = Light Perception).
Figure 3.
Histograms of logMAR visual acuity of untreated eyes at 1 year of age for the treatment groups. Visual acuity was assessed using Teller acuity cards. The numbers above the bars indicate the number of patients in the acuity category (LV = Low Vision Card; LP = Light Perception).
Intraoperative Complications
There was a trend for a greater occurrence of intraoperative complications in the IOL group than the CL group. Sixteen (28%) IOL patients experienced one or more complications compared to 6 (11%) CL patients (p =.031) (Table 1). The difference was primarily due to a higher incidence of iris prolapse in the IOL group. Iris prolapse occurred during surgery in 12 (21%) eyes in the IOL group compared to only 2 (4%) eyes in the CL group (p =.008). The frequency of other intraoperative complications was not significantly different between the two treatment groups.
Table 1.
Intraoperative Complications with Initial Cataract Surgery by Treatment Group
| Complication | Treatment | |
|---|---|---|
| CL (57 Patients) |
IOL (57 Patients) |
|
| Iris prolapse | 2 (4%) | 12 (21%) |
| Hyphema | 3 (5%) | 2 (4%) |
| Iris damage | 1 (2%) | 3 (5%) |
| Retained cortex | 1 (2%) | 1 (2%) |
| Cornea cloudy | 1 (2%) | 1 (2%) |
| Iris sphincterotomy | 0 (0%) | 1 (2%) |
| Lens fragment in vitreous | 0 (0%) | 1 (2%) |
| Rupture posterior capsule | 0 (0%) | 1 (2%) |
| At least 1 complication* | 6 (11%) | 16 (28%) |
Comparison of treatment groups: p-value = .031
Adverse Events
Forty-four (77%) patients in the IOL group had one or more of the adverse events compared to 14 (25%) patients in the CL group (p < .0001) (Table 2). In the IOL group, the most common complications were lens reproliferation into the visual axis, pupillary membranes, and corectopia. Lens reproliferation into the visual axis developed in 24 (42%) eyes in the IOL group and in 1 (2%) eye in the CL group (p <.0001). Pupillary membranes developed in 17 (30%) eyes in the IOL group but in none of the eyes in the CL group (p < 0.0001). Eleven (19%) eyes in the IOL group developed corectopia compared to 1 (2%) eye in the CL group (p =.004). None of the eyes in the IOL group developed IOL capture, decentration or dislocation into the vitreous. In the CL group, 3 (5%) eyes developed contact lens associated complications (1 eye each with presumed bacterial keratitis (the eye was not cultured), corneal abrasion, and corneal opacity due to tight contact lens) (Table 2). Other vision threatening complications that occurred in the CL group included 1 (2%) eye with Hemophilus influenza endophthalmitis and 2 (4%) eyes with retinal detachments; one after undergoing a pars plana membranectomy and the second after being treated for endophthalmitis. Both of these eyes have poor vision and one eye has developed phthisis bulbi.
Table 2.
Postoperative Adverse Events by Treatment Group
| Adverse Event | Treatment | |
|---|---|---|
| CL (57 Patients) |
IOL (57 Patients) |
|
| Lens Reproliferation into Visual Axis | 1 (2%) | 24 (42%) |
| Pupillary Membrane | 0 (0%) | 17 (30%) |
| Corectopia | 1 (2%) | 11 (19%) |
| Glaucoma | 3 (5%) | 7 (12%) |
| Glaucoma Suspect | 2 (4%) | 2 (4%) |
| Vitreous Hemorrhage | 2 (4%) | 4 (7%) |
| Retinal Hemorrhage | 2 (4%) | 2 (4%) |
| Hyphema | 1 (2%) | 3 (5%) |
| Retained Cortex | 2 (4%) | 3 (5%) |
| Retinal Detachment | 2 (4%) | 0 (0%) |
| Endophthalmitis | 1 (2%) | 0 (0%) |
| Phthisis Bulbi | 1 (2%) | 0 (0%) |
| Contact Lens Associated Bacterial Keratitis | 1 (2%) | 0 (0%) |
| Corneal Abrasion | 1 (2%) | 0 (0%) |
| Corneal Opacity Due to Tight Contact Lens | 1 (2%) | 0 (0%) |
| Corneal Edema >30 days | 0 (0%) | 1 (2%) |
| Capsular Phimosis | 1 (2%) | 0 (0%) |
| Wound Leak / Dehiscence | 0 (0%) | 1 (2%) |
| At Least 1 Adverse Event* | 14 (25%) | 44 (77%) |
Comparison of treatment groups: p-value < .0001
Glaucoma developed in 3 (5%) eyes in the CL group and 7 (12%) eyes in the IOL group (p =.32). Two (4%) eyes in the CL group and 2 (4%) eyes in the IOL group were glaucoma suspects.
Additional Surgeries
Patients in the IOL group underwent additional intraocular surgeries more often than patients in the CL group. In the IOL group, 36 (63%) patients underwent one or more additional intraocular surgeries compared to only 7 (12%) patients in the CL group (p < .0001) (Table 3). In the IOL group 10 (18%) eyes underwent 2 or more additional intraocular surgeries compared to only 2 (4%) eyes in the CL group. The most commonly performed procedure was an operation to clear the visual axis (Table 4). One of the eyes in the IOL group underwent an IOL exchange due to a large myopic shift. None of the patients randomized to the CL group had a secondary IOL implanted.
Table 3.
Number of Additional Intraocular Surgeries by Treatment Group
| Number of Additional Intraocular Surgeries |
Treatment | |
|---|---|---|
| CL (57 Patients) |
IOL (57 Patients) |
|
| 0 | 50 (87%) | 21 (37%) |
| 1 | 5 (9%) | 26 (46%) |
| 2 | 1 (2%) | 8 (14%) |
| 3 | 1 (2%) | 1 (2%) |
| 4 | 0 (0%) | 1 (2%) |
Comparison of treatment groups: p-value < .0001
Table 4.
Additional Intraocular Surgeries by Treatment Group
| Surgical Procedure | Treatment | |
|---|---|---|
| CL (57 Patients) |
IOL (57 Patients) |
|
| Clearing Visual Axis Opacities | 6 (11%) | 34 (60%) |
| Glaucoma Surgery | 1 (2%) | 4 (7%) |
| Repair Retinal Detachment | 2 (4%) | 0 (0%) |
| Repair Wound Dehiscence | 0 (0%) | 1 (2%) |
| IOL Exchange | 0 (0%) | 1 (2%) |
| Iridectomy / Iridotomy | 1 (2%) | 1 (2%) |
| Scleral Patch Graft | 0 (0%) | 1 (2%) |
| Lysis of Vitreous Wick | 0 (0%) | 1 (2%) |
| Laser Treatment of Lattice Degeneration | 1 (2%) | 0 (0%) |
| At Least 1 Surgical Procedure* | 7 (12%) | 36 (63%) |
Comparison of treatment groups: p-value < .0001
In addition to the intraocular surgeries described above, non-mandated EUAs were performed on 5 (9%) patients in the CL group and 8 (14%) patients in the IOL group generally to assess the intraocular pressure in the treated eyes. Strabismus surgery was performed on 10 (18%) patients in the CL group and 6 (11%) patients in the IOL group. Finally, a nasolacrimal duct procedure was performed on 1 patient (2%) in the CL group.
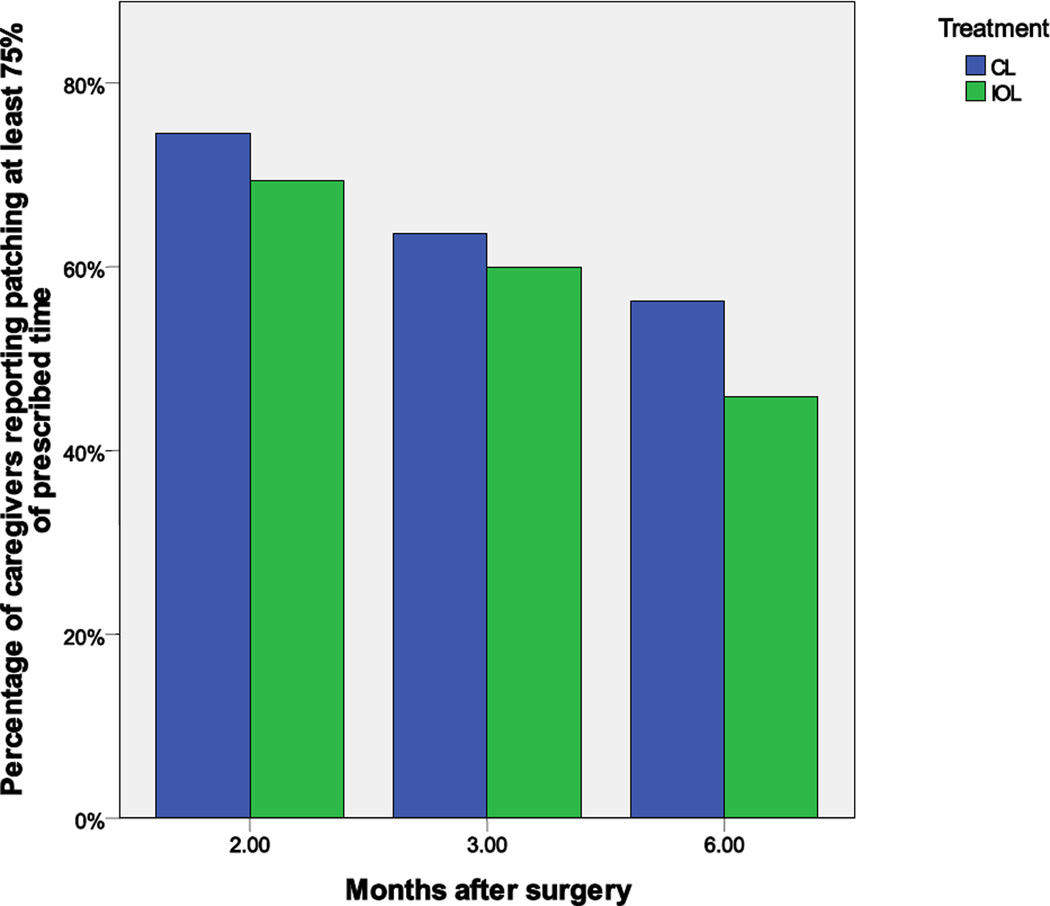
Adherence with Patching and Optical Correction
Caregivers reported a wide range of compliance with patching regimens. The proportion of caregivers reporting “good” (i.e., at least 75% of prescribed) adherence to patching decreased over time and was higher at all time points among aphakic than pseudophakic groups (Figure 4). These differences did not meet the criterion for statistical significance for secondary outcomes.
Caregivers of aphakic children reported that their children wore a contact lens on average more than eighty percent of waking hours (86%) (84% at 2 months, 85% at 3 months, and 89% at 6 months). Three-quarters of the caregivers reported that their children wore a contact lens at least 88% of waking hours prior to the visual acuity assessment.
Caregivers of pseudophakic children reported spectacle use on average slightly less than one-half of their waking hours (49%) (55% at 2 months, 53% at 3 months, and 62% at 6 months). At each time point, the percentage of waking hours of spectacle wear ranged from 0% to more than 97%. Three months after surgery, 4 pseudophakic children did not require spectacles; there were 11 such children at six months.
Ocular Alignment
Approximately two-thirds of the patients in both treatment groups were orthotropic at the 1 month examination. At the 3, 6 and 9 months examinations, however, an increasing number of the patients in the CL group were no longer orthotropic whereas in the IOL group the prevalence of orthotropia remained relatively constant. At the 12 month examination, there was a trend for more of the patients in the IOL group (58%) to be orthotropic compared to patients in the CL group (38%) (p =.051).
Pre-Visual Acuity Assessment EUA
At the time of the pre-visual acuity assessment EUA, the lenses, corneas, irides, optic discs and retinas of the fellow eyes were all normal with the exception of the retina of one patient in the IOL group who developed high myopia in his fellow eye and who, following enrollment, was diagnosed with Stickler syndrome. One aphakic eye with PFV was noted to have peripapillary traction without macular involvement and another aphakic eye developed a posterior staphyloma with tilting of the optic disc. The aphakic eye treated for endophthalmitis had pigmentary changes in the macula as well as gliosis of the optic disc.
DISCUSSION
At one year of age there was no significant difference between the median visual acuity in the treated eyes of children with a unilateral congenital cataract who were optically corrected either with a contact lens or with an IOL after undergoing cataract surgery during the first six months of life. There was, however, a five-fold increase in additional intraocular surgeries in the IOL group, most of which were performed to remove visual axis opacities and a five-fold increase in iris prolapse during cataract surgery in the IOL group. There was a trend for higher incidence glaucoma in the IOL group and higher incidence of strabismus in the CL, but these differences were not statistically significant.
The median logMAR grating acuity in the treated eyes of the IOL group was 0.97 compared to 0.80 in the CL group. This difference was not statistically significant (p =.19). Although, the IOL group was somewhat less adherent to the prescribed patching regimen than the CL group, preliminary analysis suggests that patching compliance differences between the treatment groups was unlikely to have had a major effect on the primary outcome because these differences were relatively small in the first few months after surgery and because of the large amount of variation in patching within both groups. The median logMAR acuity of the fellow eyes in both groups was 0.66.
Our results differ from those that have been reported previously in some small case series. Autrata and coworkers11 reported better logMAR acuity in eyes treated with IOLs (0.43) versus contact lenses (0.58) following unilateral cataract surgery during the first six months of life. However, their sample size was much smaller (n=41) and the study was non-randomized. In addition, visual acuity was not assessed in a uniform manner by a masked examiner. In our previous small non-randomized pilot study of children with unilateral congenital cataracts who underwent cataract surgery when <7 months of age, we found that the mean logMAR visual acuity was better in eyes treated with IOLs (0.70) compared to eyes treated with contact lenses (0.87).16 In that pilot study, however, the patients were older at the time of grating visual acuity testing (mean age: CL group, 18 months; IOL group, 15 months) and the testing itself was incomplete (13 of 26 eligible CL patients and 12 of 13 eligible IOL patients). In contrast, all 114 patients in the IATS had their grating visual acuity uniformly assessed by a masked traveling examiner at age 1 year. In our pilot study we also found better mean logMAR visual acuities in the fellow eyes (IOL group, 0.44; CL group, 0.37) compared to the fellow eyes in our clinical trial. This discrepancy may reflect the younger age of the patients in our clinical study at the time their grating visual acuity was tested. Mayer et al18 have reported a mean monocular visual acuity of 12 month old phakic children to be 6.42 ± 0.29 cy/deg (0.67 logMAR). These numbers are equivalent to the median logMAR visual acuity we report in the untreated eyes of both treatment groups (0.66 logMAR). Birch and coworkers19 have shown that the grating visual acuity of children steadily improves between the ages of 12 months and 4 years. In their study, the mean logMAR visual acuity of pseudophakic eyes improved from 0.76 at age 12 months to 0.45 at age 4 years and the logMAR visual acuity of aphakic eyes improved from 0.63 at age 1 year to 0.44 at age 4 years. On this basis, we anticipate that the visual acuity of both the treated and fellow eyes in our study will improve over time. To this end we plan to retest the visual acuity of these children when they reach 4.5 years of age using the Amblyopia Treatment Study (ATS)-HOTV test. It is certainly possible that visual acuities will be better in one or the other treatment group at this age either owing to differing degrees of adherence to patching, spectacle and contact lens wear or to late postoperative vision-threatening complications.
In the IOL group there was a 2 1/2-fold increase in the rate of intraoperative complications, of which the most common was iris prolapse. This finding likely reflects the larger wound size and the greater intraocular manipulation required to implant an IOL. Infantile eyes are more prone to this complication than adult eyes because of their lack of scleral rigidity. This complication was likely mitigated by the use of implanted foldable acrylic lenses inserted through a 3 mm wound. Other intraoperative complications were evenly distributed between the two treatment groups.
The need for additional intraocular surgeries was five times higher in the IOL group. In the IOL group, 63% patients underwent one or more additional intraocular operations. Most were performed to remove visual axis opacities. Visual axis opacities likely occurred more often in the IOL group because the IOL prevented the anterior and posterior leaflets of the capsular bag from fusing together thereby allowing reproliferating lens material to migrate into the visual axis.20 In contrast, in aphakic eyes the capsular leaflets typically fuse together preventing the reproliferating lens material from migrating into the visual axis. In addition, the IOL may act as scaffolding facilitating the spread of lens epithelial cells across the visual axis. Other studies evaluating IOL implantation during infancy have also reported high rates of visual axis opacities requiring additional surgeries. In our pilot study, 6 of 12 (50%) eyes undergoing IOL implantation during infancy underwent an additional surgery to remove visual axis opacities.16 Plager and coworkers21 reported additional surgeries to remove visual axis opacities in 12 of 15 (80%) eyes undergoing IOL implantation at 6 months of age or younger. In their study, additional surgeries were performed on average 4.5 months following cataract surgery and included two patients who required multiple operations. Lundvall and Zetterstrom10 reported that 70% of children who underwent IOL implantation during infancy required additional operations to remove visual axis opacities.
Cataract surgery during infancy has been reported to be a risk factor for the development of glaucoma.22–24 In our study, glaucoma developed in 3 eyes in the CL group and 7 eyes in the IOL group. In addition, 2 eyes in each group were categorized as glaucoma suspects. The IATS protocol required that cataract surgery be deferred until children were at least 4 weeks of age because two patients in our pilot study developed glaucoma after undergoing cataract surgery between the ages of 2 to 4 weeks.16 Vishwanath and coworkers23 have reported an increased incidence of glaucoma in children undergoing cataract surgery during the first month of life. Asrani and coworkers25 reported a lower incidence of glaucoma in children following cataract surgery who underwent primary IOL implantation compared to children who were left aphakic . However the children in their study were older at the time of cataract surgery. Trivedi and coworkers26 reported a similar incidence of glaucoma in infants following cataract surgery with or without IOL implantation. However, they noted that glaucoma developed at an earlier age in eyes that underwent IOL implantation versus eyes that were left aphakic. A major difference between our study and other studies evaluating the incidence of glaucoma following IOL implantation is that age at surgery was controlled in our study eliminating this as a variable that could potentially confound disease severity and outcome. Given the long latency of glaucoma following cataract surgery in children,27 our planned follow-up to 5 years of age should allow us to better assess both the incidence and time course for the development of glaucoma in these eyes.
In the present study, a higher percentage of children in the IOL group were orthotropic at one year of age (58% vs 38%, p =.051). This was a suggestive trend, but did not meet the criterion for statistical significance for secondary outcomes. France and France28 have also reported a high incidence of strabismus in children with a unilateral congenital cataract treated with contact lenses. Ben Ezra29 reported a lower incidence of strabismus in older children treated with an IOL versus a contact lens. The higher incidence of orthotropia in the IOL group is intriguing particularly since we observed no differences in acuity measurements between the two groups. It is conceivable that the constancy of the optical correction in the IOL group succeeded in providing sufficient binocular input to the visual cortex yielding simultaneous vision adequate to maintain alignment of the two eyes. Again a longer follow-up will allow us to determine if this difference persists.
This study was undertaken to assess whether the risks of implanting IOLs in very young children were reasonable given the potential for improved visual outcome that might occur with IOL implantation over time. Our results demonstrate no visual acuity benefit at age 1 year for primary IOL implantation and an increased incidence of complications requiring additional surgical interventions in the IOL group. These findings are not entirely unexpected. We knew from previous studies that visual axis opacities requiring removal commonly develop in the first 3–6 months after IOL implantation in infants so we assumed that they would likely occur in this study. It was reassuring to find that despite the increased number of additional intraocular surgeries in the IOL group to remove visual axis opacities, the median visual outcome in their study eyes was no different than the CL group at 1 year of age. Assessing the risks and benefits of IOL implantation at 1 year of life may lead to premature conclusions. We anticipate that the real benefit of IOL implantation may occur later, especially if children in the CL group become less compliant with contact lens use as they become older. If this is true, it is possible that the children in the IOL group will have an increasing visual advantage with their pseudophakic correction alone as they become older and approach emmetropia. We plan to retest the visual acuity of these children when they are age 4 ½ years using the ATS-HOTV acuity test.
Another caveat with our study is that it provided contact lenses, spectacles and patches for participants at no charge. In addition, regular monitoring of their adherence to these treatments may have improved compliance. As a result, our outcomes may reflect efficacy (benefit under ideal conditions) rather than effectiveness (benefit under usual conditions).30
In conclusion, IOL and contact lens correction following unilateral cataract surgery during infancy resulted in similar grating visual acuity outcomes at age 1 year in our cohort of children. Infants treated with IOLs had more intraoperative complications and required more intraocular surgeries postoperatively to clear visual axis opacities. Thus there appears to be no short-term visual benefit and some increased risk to implanting IOLs in infants. However, since there remains a possibility that IOLs may be found to be beneficial after a longer follow-up, we feel it would be premature to recommend that IOLs not be implanted in infants. In addition, the theoretical long term benefit of having the IOL in the capsular bag versus implanted in the ciliary sulcus as a secondary procedure cannot be quantified at this point. We suggest that practitioners continue to exercise caution when considering implanting IOLs in infants. The ultimate role for IOL implantation during infancy may be further clarified after a longer follow-up of these children.
Supplementary Material
Acknowledgments
Lucy Yang, M.D. assisted with the editing of the manuscript and Olga Villanueva, M.D. translated the informed consent and HIPPA waiver into Spanish.
Supported by National Institutes of Health Grants U10 EY13272 and U10 EY013287 and in part by NIH Departmental Core Grant EY06360 and Research to Prevent Blindness, Inc, New York, New York
Footnotes
Proprietary interests: none
Videos
Video 1: Cataract Extraction + IOL Implantation using the IATS protocol
Video 2: Lensectomy using the IATS protocol
References
- 1.Frey T, Friendly D, Wyatt D. Re-evaluation of monocular cataracts in children. Am J Ophthalmol. 1973 Sep;76(3):381–388. doi: 10.1016/0002-9394(73)90495-9. [DOI] [PubMed] [Google Scholar]
- 2.Beller R, Hoyt CS, Marg E, Odom JV. Good visual function after neonatal surgery for congenital monocular cataracts. Am J Ophthalmol. 1981 May;91(5):559–565. doi: 10.1016/0002-9394(81)90053-2. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz B, Worle J. Visual results in congenital cataract with the use of contact lenses. Graefes Arch Clin Exp Ophthalmol. 1991;229(2):123–132. doi: 10.1007/BF00170543. [DOI] [PubMed] [Google Scholar]
- 4.Amos CF, Lambert SR, Ward MA. Rigid gas permeable contact lens correction of aphakia following congenital cataract removal during infancy. J Pediatr Ophthalmol Strabismus. 1992 Jul-Aug;29(4):243–245. doi: 10.3928/0191-3913-19920701-13. [DOI] [PubMed] [Google Scholar]
- 5.Neumann D, Weissman BA, Isenberg SJ, Rosenbaum AL, Bateman JB. The effectiveness of daily wear contact lenses for the correction of infantile aphakia. Arch Ophthalmol. 1993 Jul;111(7):927–930. doi: 10.1001/archopht.1993.01090070045017. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd IC, Dowler JG, Kriss A, et al. Modulation of amblyopia therapy following early surgery for unilateral congenital cataracts. Br J Ophthalmol. 1995 Sep;79(9):802–806. doi: 10.1136/bjo.79.9.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaya LG, Speedwell L, Taylor D. Contact lenses for infant aphakia. Br J Ophthalmol. 1990 Mar;74(3):150–154. doi: 10.1136/bjo.74.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert SR, Lynn M, Drews-Botsch C, et al. Intraocular lens implantation during infancy: perceptions of parents and the American Association for Pediatric Ophthalmology and Strabismus members. J AAPOS. 2003 Dec;7(6):400–405. doi: 10.1016/j.jaapos.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Fan DS, Rao SK, Yu CB, Wong CY, Lam DS. Changes in refraction and ocular dimensions after cataract surgery and primary intraocular lens implantation in infants. J Cataract Refract Surg. 2006 Jul;32(7):1104–1108. doi: 10.1016/j.jcrs.2006.01.097. [DOI] [PubMed] [Google Scholar]
- 10.Lundvall A, Zetterstrom C. Primary intraocular lens implantation in infants: complications and visual results. J Cataract Refract Surg. 2006 Oct;32(10):1672–1677. doi: 10.1016/j.jcrs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Autrata R, Rehurek J, Vodickova K. Visual results after primary intraocular lens implantation or contact lens correction for aphakia in the first year of age. Ophthalmologica. 2005 Mar-Apr;219(2):72–79. doi: 10.1159/000083264. [DOI] [PubMed] [Google Scholar]
- 12.Ashworth JL, Maino AP, Biswas S, Lloyd IC. Refractive outcomes after primary intraocular lens implantation in infants. Br J Ophthalmol. 2007 May;91(5):596–599. doi: 10.1136/bjo.2006.108571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BenEzra D. Cataract surgery and intraocular lens implantation in children, and intraocular lens implantation in children. Am J Ophthalmol. 1996 Feb;121(2):224–226. doi: 10.1016/s0002-9394(14)70595-1. [DOI] [PubMed] [Google Scholar]
- 14.Dahan E, Drusedau MU. Choice of lens and dioptric power in pediatric pseudophakia. J Cataract Refract Surg. 1997;23(Suppl 1):618–623. doi: 10.1016/s0886-3350(97)80043-0. [DOI] [PubMed] [Google Scholar]
- 15.Lambert SR, Buckley EG, Plager DA, Medow NB, Wilson ME. Unilateral intraocular lens implantation during the first six months of life. J AAPOS. 1999 Dec;3(6):344–349. doi: 10.1016/s1091-8531(99)70043-1. [DOI] [PubMed] [Google Scholar]
- 16.Lambert SR, Lynn M, Drews-Botsch C, et al. A comparison of grating visual acuity, strabismus, and reoperation outcomes among children with aphakia and pseudophakia after unilateral cataract surgery during the first six months of life. J AAPOS. 2001 Apr;5(2):70–75. doi: 10.1067/mpa.2001.111015. [DOI] [PubMed] [Google Scholar]
- 17.Group TIATS. The Infant Aphakia Treatment Study: Design and Clinical Measures at Enrollment. Arch Ophthalmol. 2010 doi: 10.1001/archophthalmol.2009.350. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer DL, Beiser AS, Warner AF, Pratt EM, Raye KN, Lang JM. Monocular acuity norms for the Teller Acuity Cards between ages one month and four years. Invest Ophthalmol Vis Sci. 1995 Mar;36(3):671–685. [PubMed] [Google Scholar]
- 19.Birch EE, Cheng C, Stager DR, Jr, Felius J. Visual acuity development after the implantation of unilateral intraocular lenses in infants and young children. J AAPOS. 2005 Dec;9(6):527–532. doi: 10.1016/j.jaapos.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi RH, Wilson ME, Jr, Bartholomew LR, Lal G, Peterseim MM. Opacification of the visual axis after cataract surgery and single acrylic intraocular lens implantation in the first year of life. J AAPOS. 2004 Apr;8(2):156–164. doi: 10.1016/j.jaapos.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Plager DA, Yang S, Neely D, Sprunger D, Sondhi N. Complications in the first year following cataract surgery with and without IOL in infants and older children. J AAPOS. 2002 Feb;6(1):9–14. doi: 10.1067/mpa.2002.121169. [DOI] [PubMed] [Google Scholar]
- 22.Rabiah PK. Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol. 2004 Jan;137(1):30–37. doi: 10.1016/s0002-9394(03)00871-7. [DOI] [PubMed] [Google Scholar]
- 23.Vishwanath M, Cheong-Leen R, Taylor D, Russell-Eggitt I, Rahi J. Is early surgery for congenital cataract a risk factor for glaucoma? Br J Ophthalmol. 2004 Jul;88(7):905–910. doi: 10.1136/bjo.2003.040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhli-Hattenbach C, Luchtenberg M, Kohnen T, Hattenbach LO. Risk factors for complications after congenital cataract surgery without intraocular lens implantation in the first 18 months of life. Am J Ophthalmol. 2008 Jul;146(1):1–7. doi: 10.1016/j.ajo.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Asrani S, Freedman S, Hasselblad V, et al. Does primary intraocular lens implantation prevent "phakic" glaucoma in children? J AAPOS. 2000 Feb;4(1):33–39. doi: 10.1016/s1091-8531(00)90009-0. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi RH, Wilson ME, Jr, Golub RL. Incidence and risk factors for glaucoma after pediatric cataract surgery with and without intraocular lens implantation. J AAPOS. 2006 Apr;10(2):117–123. doi: 10.1016/j.jaapos.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Egbert JE, Christiansen SP, Wright MM, Young TL, Summers CG. The natural history of glaucoma and ocular hypertension after pediatric cataract surgery. J AAPOS. 2006 Feb;10(1):54–57. doi: 10.1016/j.jaapos.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.France TD, Frank JW. The association of strabismus and aphakia in children. J Pediatr Ophthalmol Strabismus. 1984 Nov-Dec;21(6):223–226. doi: 10.3928/0191-3913-19841101-06. [DOI] [PubMed] [Google Scholar]
- 29.BenEzra D, Cohen E, Rose L. Traumatic cataract in children: correction of aphakia by contact lens or intraocular lens. Am J Ophthalmol. 1997 Jun;123(6):773–782. doi: 10.1016/s0002-9394(14)71126-2. [DOI] [PubMed] [Google Scholar]
- 30.Wilson ME, Trivedi RH. Multicenter randomized controlled clinical trial in pediatric cataract surgery: efficacy and effectiveness. Am J Ophthalmol. 2007 Oct;144(4):616–617. doi: 10.1016/j.ajo.2007.06.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.