Abstract
Rationale
Orai1 and the associated calcium release-activated calcium (CRAC) channel were discovered in the immune system. Existence also in endothelial cells has been suggested but the relevance to endothelial biology is mostly unknown.
Objective
The aim of this study was to investigate the relevance of Orai1 and CRAC channels to vascular endothelial growth factor (VEGF) and endothelial tube formation.
Methods and Results
In human umbilical vein endothelial cells Orai1 disruption by short-interfering RNA or dominant-negative mutant Orai1 inhibited calcium-release-activated (store-operated) calcium entry, VEGF-evoked calcium-entry, cell migration, and in vitro tube formation. Expression of exogenous wild-type Orai1 rescued the tube formation. VEGF receptor-2 and Orai1 partially co-localized. Orai1 disruption also inhibited calcium-entry and tube formation in endothelial progenitor cells from human blood. A known blocker of the immune cell CRAC channel (3-fluoropyridine-4-carboxylic acid (2′,5′-dimethoxybiphenyl-4-yl)amide) was a strong blocker of store-operated calcium entry in endothelial cells and inhibited calcium-entry evoked by VEGF in three types of human endothelial cell. The compound lacked effect on VEGF-evoked calcium-release, STIM1 clustering, and two types of endothelial TRP channel, TRPC6 and TRPV4. Without effect on cell viability the compound inhibited human endothelial cell migration and tube formation in vitro and suppressed angiogenesis in vivo in the chick chorioallantoic membrane. The compound showed 100-fold greater potency for endothelial compared with immune cell calcium entry.
Conclusions
The data suggest positive roles for Orai1 and CRAC channels in VEGF-evoked calcium entry and new opportunity for chemical modulation of angiogenesis.
Keywords: endothelium, angiogenesis, calcium channels, growth factors
Introduction
Angiogenesis is an endothelial migration and tube formation process underlying new vessel growth from pre-existing blood vessels1. It has roles in wide-ranging physiological events and pathologies that include diabetic retinopathy, peripheral vascular disease, endometriosis, tissue regeneration, atherosclerosis, obesity, rheumatoid arthritis, and cancer2-4. Strategies to enhance or suppress angiogenesis (depending on the clinical situation) have attracted much attention. In the case of efforts to stimulate angiogenesis for cardiovascular therapy, preclinical data have been encouraging whereas late stage clinical trials have been disappointing5, 6. Problems with the complexities of angiogenesis and the differing biology of old patients and young animal models have been suggested as explanations for the difficulties in translating to the clinic, suggesting that greater understanding and new intervention approaches are needed6. Strategies aimed at inhibiting angiogenesis to reduce tumour expansion have, however, seen success, although with limitations, for example, due to drug resistance, again because of the complexities of the angiogenesis3, 7. Perhaps surprisingly, angiogenesis inhibition may have advantage in cardiovascular disease settings because it could be a means to suppress obesity and metabolic diseases that drive atherosclerosis4.
Primary intercellular signaling molecules in angiogenesis are the vascular endothelial growth factors (VEGFs), which bind to VEGF receptors such as VEGFR21, 7. The receptors are tyrosine kinase receptors that link to down-stream signaling pathways such as monomeric G proteins and PI3 and MAP kinases but there is also evidence for associated intracellular Ca2+ signals originating from phospholipase Cγ and inositol 1,4,5-trisphosphate1, 8. These Ca2+ events show the classical Ca2+-release followed by Ca2+-entry characteristics8, 9. Jho et al9 suggested that TRPC1 channels contribute to the Ca2+-entry mechanism and other investigators have suggested roles of TRPC6 channels in angiogenesis10, 11. Synergism of VEGF and TRPC1 in zebrafish angiogenesis has been identified12. Nevertheless, the underlying Ca2+ channel mechanisms and their significance remain incompletely understood.
A Ca2+ channel type with emerging prominence is the Ca2+-release activated Ca2+ (CRAC) channel13-15. It is an extremely low conductance, highly calcium-selective channel that opens in response to any signal that depletes Ca2+ in intracellular stores14. A pore-forming subunit of the channels is Orai1, a member of the Orai family of tetraspanin-related membrane proteins13-15. The channel type was discovered in immune cells and individuals with disrupted Orai1 function, because of a R91W mutation, have severe combined immune deficiency13, 16. With the identification of Orai1, however, it has become increasingly apparent that the CRAC channel is widely distributed and thus not restricted to the immune system. Its general activation mechanism through STIM1 of depleted Ca2+ stores14 is common to many cell types, such that most mammalian cells exhibit store-operated Ca2+-entry that may be accounted for, at least partly, by Orai1. The general activation signal of store-depletion suggests that it is a nodal point in the signaling of multiple agonists, presenting an opportunity for overcoming problems of redundancy, compensation and drug resistance. Importantly, there is specific evidence that human umbilical vein endothelial cells (HUVECs) express Orai1, exhibit store-operated Ca2+-entry that depends on Orai1, and contain a small CRAC channel-like current17. HUVECs are commonly used as a basis for in vitro angiogenesis assays. The relevance of CRAC channels and Orai1 to the VEGF responses of these, or other, endothelial cells is unknown.
Here we investigated the relevance of Orai1 and CRAC channel-related Ca2+-entry to VEGF-evoked Ca2+-entry and endothelial cell behavior, using a variety of established angiogenesis assays1. In addition to molecular manipulation of Orai1, which may not only relate to the CRAC channel, we investigated a chemical blocker that has been found to have selectivity for CRAC channels in immune cells18, 19. HUVECs, endothelial cells cultured from human saphenous vein obtained at coronary artery bypass operations, and late outgrowth endothelial progenitor cells (EPCs) from healthy volunteers were used in addition to chicken embryo chorioallantoic membrane for in vivo angiogenesis. EPCs may be important in vasculogenesis, vascular repair, and tumour vessel formation3, 20, 21.
Methods
Cell preparation and culture
HUVECs were cultured in EGM-2 growth medium supplemented with EGM-2 bullet kit (Lonza) at 37 °C in a humidified atmosphere containing 5 % CO2. Provided in the Supplementary Information are the methods for culture of EPCs, endothelial cells from human saphenous vein, HEK 293 cells with and without stable expression of TRPC6, and CHO cells stably expressing TRPV4.
Short interfering (si) RNAs and cDNAs
Cells at 90 % confluence were transfected with 20 nmoles/L siRNA using Lipofectamine 2000 in OptiMEM as per the manufacturer’s instructions (Invitrogen). Sequences of siRNA probes are given in the Supplementary Information. Fresh EGM-2 growth medium was added after 4-6 h and the cells were analyzed 48 h after transfection. To validate effectiveness of siRNA probes, mRNA was isolated and quantified by real-time RT-PCR (see Supplementary Information). Orai1 siRNA 1 (Or1.si.1) effectiveness was also confirmed by western blotting (see Supplementary Information). When cells were transfected with human Orai1 (accession number BC013386) or its dominant negative (DN) mutant (R91W) in pcDNA6, 0.2 μg DNA was added. Orai1 cDNA was intended to saturate Or1.si.1 in rescue experiments. eYFP-STIM1 was in pDS (from J. Liou, Stanford, Calif.).
Intracellular Ca2+ measurement
Endothelial cells were incubated with fura-2AM for 1 h at 37 °C followed by a 0.5 h wash at room temperature (21± 2 °C). Fluo-4AM was used in place of fura-2AM for recordings of over-expressed TRPC6 and TRPV4 activity. Measurements were made at room temperature on a 96-well plate reader (FlexStation, Molecular Devices). The change () in intracellular calcium (Ca2+) concentration was indicated as the ratio of fura-2 emission intensities for 340 nm and 380 nm excitation (F ratio). Wells within columns of the 96-well plate were loaded alternately for test and control conditions. The recording solution contained (mmoles/L): 130 NaCl, 5 KCl, 8 D-glucose, 10 Hepes, 1.2 MgCl2, 1.5 CaCl2, titrated to pH 7.4 with NaOH. When Ca2+-free extracellular solution was used, CaCl2 was omitted; for Ca2+ addback, Ca2+ was 0.2 mmoles/L. S66 was added 15 min before Ca2+ measurements at room temperature.
Immunofluorescence
Cells were labeled with antibodies using standard immunofluorescence protocols (details are given in the Supplementary Information) and visualized using a deconvolution system (Applied Precision Instruments, Seattle, WA) based on the Olympus IX-70 inverted microscope with x60 objective (NA 1.4).
Whole-cell patch-clamp recordings
Methods are given in the Supplementary Information.
Cell migration and viability
Cell migration assays were performed using a modified Boyden chamber (8-μm pores; BD Biosciences, UK). HUVECs transfected with siRNA or pre-treated for 24 h with S66 were serum starved in EGM-2 for 4 h, resuspended at 5 × 104 cells/mL in EGM-2 containing 0.4 % foetal calf serum (FCS), and loaded in the upper chamber. The lower chamber contained 0.4 % FCS supplemented with the chemo-attractant 20 ng/mLVEGF (Sigma). After incubation for 4 h at 37 °C in a 5 % CO2 incubator, the inserts were fixed with 70 % ethanol for 2 min. Cells on duplicate membranes were scraped from the upper surface, and migrating cells underneath were stained with hematoxylin and eosin and evaluated by counting cells in 6 randomly chosen fields under light microscopy. Methods for measuring cell viability are given in the Supplementary Information.
In vitro tube formation
HUVECs were detached by trypsinization and, after neutralization of trypsin, were counted and resuspended in EGM-2 containing 1 % FCS and 2 ng/mL VEGF at 4 × 105 cells/mL. HUVECs were seeded at 2 × 104 cells per well in Matrigel pre-coated BD Biosciences 96-well plates (Catalog #354149). Tube formation was quantified 18-20 h later. Cells were treated with chemical agents for 24 h or transfected 48 h before seeding on the 96-well plate. Chemical agents were maintained throughout experiments. EPCs were plated on thin-layer Matrigel pre-coated 24-well plates (BD Biosciences, Catalog #354605) at 5 × 104 cells/well in 1% FCS EGM-2 with 2 ng/mL VEGF and tube formation was quantified 20 h later. Digital images of endothelial tubes were obtained by bright-field light microscopy. Tube formations were measured blind by an independent observer, giving: (i) total tube length per image; and (ii) number of complete loops (circles) per image.
In vivo angiogenesis
Chicken embryo chorioallantoic membrane angiogenesis assays were performed according to a published protocol22. Briefly, fertilized chicken eggs were cleaned with 70 % ethanol and incubated at 37 °C under 60 % humidity in an egg incubator. On day 3, albumen (2–3 mL) was aspirated at the acute pole using a sterile 25-G hypodermic needle in order to create an air sac directly over the chorioallantoic membrane. A square window (10 ×10 mm) was then cut in the shell and was sealed with tape. Eggs were returned to the incubator and, on day 8, the window was opened under sterile conditions and a sterilized gelatin sponge pre-infused with VEGF (100 ng/mL) and S66 (20 μmoles/L) was placed on the chorioallantoic membrane. On day 11, the vessels surrounding each sponge were counted.
Reagents
S66 (3-fluoropyridine-4-carboxylic acid (2′,5′-dimethoxybiphenyl-4-yl)amide) was a gift from GSK (GSK1349571A); its chemical identity was confirmed independently by LC-MS analysis (Leeds) and chemical synthesis. S66 is an abbreviation of “Synta 66” from patent WO 2005/00995418, 19. All other reagents were from Sigma unless specified in the Supplementary Information.
Data analysis
Averaged data are presented as mean±s.e.mean. Data were produced in pairs (test and control) and compared using t tests with statistical significance indicated by * (P<0.05) and no significant difference by NS (P>0.05). The number of independent experiments is indicated by n. For multi-well assays, the number of replicate wells is indicated by N.
Results
Orai1 role in store-operated and VEGF-evoked Ca2+ entry
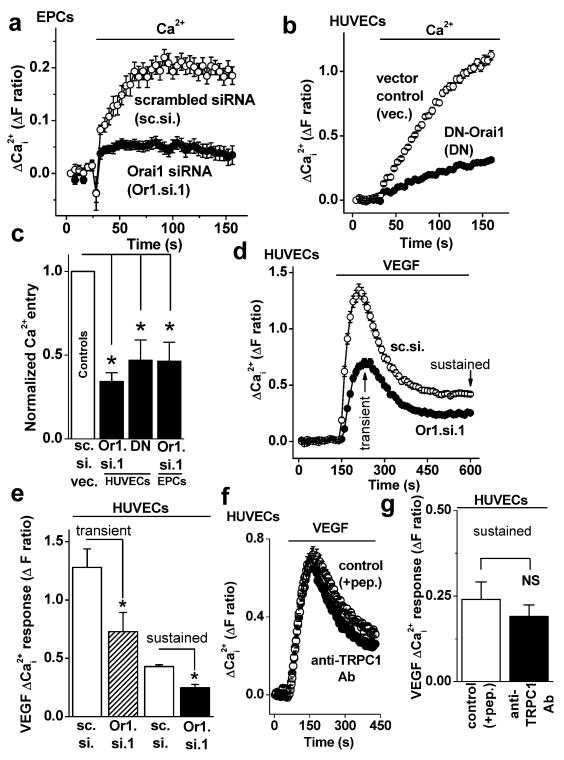
CRAC channel function was first detected as a component of store-operated Ca2+-entry, which was investigated using an intracellular Ca2+ indicator to detect Ca2+-entry when extracellular Ca2+ was added back to endothelial cells that had been store-depleted using the pharmacological agent thapsigargin (e.g. Fig 1a). The Ca2+ add-back signal was suppressed when Orai1 expression was knocked-down by short interfering (si) RNA, as shown by the example data for EPCs (Fig 1a, Supplementary Fig I and Fig II) and the mean data in Fig 1c. An alternative approach for disruption of Orai1 is expression of exogenous R91W mutant of Orai1, which acts as a dominant negative (DN) to inhibit endogenous Orai1 and CRAC channel function13, 23, 24. DN-Orai1 also inhibited the Ca2+-entry, as shown by comparative analysis in HUVECs (Fig 1b, c). The data confirm previous siRNA studies of HUVECs17 and extend the findings to DN-Orai1 and EPCs.
Figure 1. Orai1 role in Ca2+-entry.
(a) Example paired comparison of Ca2+-entry in EPCs transfected with Orai1 siRNA 1 (Or1.si.1) or scrambled (sc.si) siRNA (N=8 each). EPCs were store-depleted using 1 μmoles/L thapsigargin in Ca2+-free solution for 30 min prior to re-addition of extracellular Ca2+ (0.2 mmoles/L) to show Ca2+-entry. (b) As for (a) but a paired comparison of Ca2+-entry after transfection of HUVECs with dominant negative (DN) mutant (R91W) Orai1 or the DNA vector (vec.) only (N=8 each). (c) Summary data for experiments of the type shown in (a, b). Each test data set (black bars) was compared to its own control even though only one control bar (white) is shown (n/N=5/96, 4/48 and 4/72 for each paired experiment). Controls were sc. si. or vec. whereas tests were Or1.si.1 or DN Orai1. (d) Example HUVEC intracellular Ca2+ measurement in the presence of extracellular 1.5 mmoles/L Ca2+ showing responses to VEGF (100 ng/mL) after transfection with Or1.si.1 or sc.si (N=8). (e) Summary data for experiments of the type illustrated in (d) showing measurements for the transient and sustained effects of VEGF (n/N=3/24). (f) As for (d) but cells were pretreated with anti-TRPC1 antibody (Ab) or its peptide-preadsorbed control (+pep.) (N=16). (g) Mean data for independent experiments of the type shown in (f) (n/N=4/56).
It was hypothesized that the above Ca2+ signals were relevant to the action of VEGF because VEGF caused Ca2+ release from intracellular stores, as shown for example in HUVECs (Supplementary Fig III). In the continuous presence of extracellular Ca2+, VEGF evoked a transient followed by a sustained elevation of the intracellular Ca2+ concentration (Fig 1d), suggesting that there was Ca2+ entry after transient Ca2+ release. Orai1 siRNA reduced both the transient and sustained effects of VEGF by about 42 % (Fig 1d, e). Whole-cell patch-clamp recordings revealed that VEGF-evoked ionic current was below the detectable amplitude when using CRAC channel recording conditions in the presence of extracellular Ca2+ (Supplementary Figs IV-VIII), consistent with prior information on endothelial cells17. The data suggest that Orai1 contributes substantially to VEGF-evoked Ca2+ signals that are mediated by sub-pA ionic currents.
A previous report suggested that TRPC1 also confers VEGF-evoked Ca2+ entry9. We similarly observed suppression by anti-TRPC1 blocking antibody (Fig 1f) but the effect was relatively small and variable, not achieving statistical significance across all experiments (Fig 1g).
Mechanism of VEGF-evoked Ca2+ entry
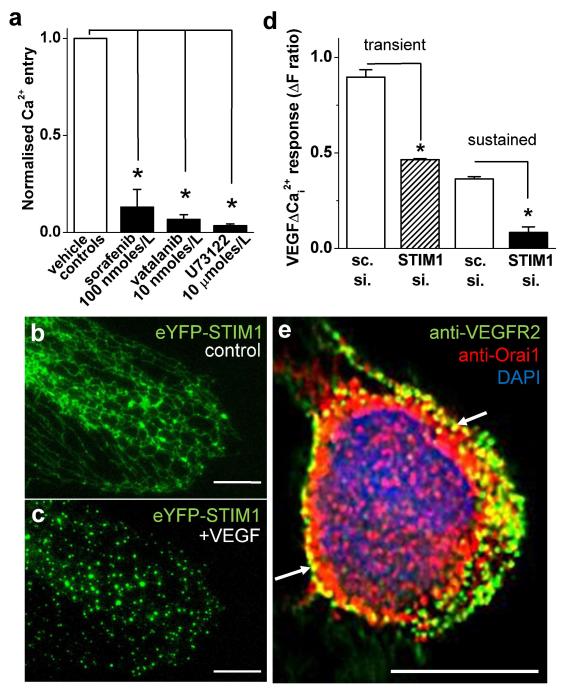
Previous research suggested that VEGFR2 is the mediator of VEGF-evoked Ca2+ signals9. Consistent with this mechanism the multikinase inhibitors sorafenib and vatalanib potently suppressed VEGF-evoked Ca2+ rises (Fig 2a, Supplementary Fig IX). Phospholipase Cγ has also been implicated9 and so a phospholipase C inhibitor U73122 was investigated. U73122 similarly inhibited VEGF-evoked Ca2+ rises (Fig 2a, Supplementary Fig IX). Inositol-1,4,5-trisphosphate generated by phospholipase C was, therefore, presumed to be the messenger evoking Ca2+-release. Studies of other cell types have suggested that the ensuing depletion of Ca2+ stores causes clustering of STIM1 in endoplasmic reticular membranes and that this STIM1 binds to and activates Orai1 channels in the plasma membrane14. To investigate this mechanism, HUVECs were transfected with eYFP-tagged STIM1 which showed the expected14 microtubule-like localization pattern (Fig 2b). VEGF caused marked clustering of eYFP-STIM1 (Fig 2c). Additional experiments were carried out where endogenous STIM1 was knocked-down by RNA interference (Fig 2d, Supplementary Fig I). STIM1 siRNA suppressed VEGF-evoked Ca2+ signals (Fig 2d).
Figure 2. Mechanisms of VEGF-activated Ca2+-entry.
All data were from HUVECs. (a) Mean 100 ng/mL VEGF-evoked Ca2+-entry in the presence of 100 nmoles/L sorafenib (n/N=3/40), 10 nmoles/L vatalanib (n/N=3/24) or 10 μmoles/L U73122 (n/N=3/24) normalised to their matched controls. (b, c) eYFP-STIM1 (green) before (b, control) and after exposure to 100 ng/mL VEGF (c). (d) Summary data for 100 ng/mL VEGF-evoked transient and sustained Ca2+ signals after transfection with STIM1.si or sc.si (n/N=3/64). (e) Example merged image of a cell labeled with anti-VEGFR2 antibody (green), anti-Orai1 antibody (red) and the nuclear counter stain, DAPI (blue). The image was captured at a focal plane midway through the nucleus and the cell had been exposed to VEGF. The white arrows point to areas of VEGFR2 and Orai1 colocalization. Scale bars of all images are 10 μm.
The data suggest that the pathway for VEGF to evoke Orai1-dependent Ca2+ entry is: VEGFR2; phospholipase Cγ; inositol-1,4,5-trisphosphate; inositol-1,4,5-trisphosphate receptor; Ca2+ release from intracellular stores; STIM1 activation and clustering; STIM1 binding to Orai1; Orai1 (CRAC) channel activation (summarized in Supplementary Fig X).
Subcellular colocalization of VEGFR2 with Orai1
Using specific mouse monoclonal anti-VEGFR2 and rabbit polyclonal anti-Orai1 antibodies (Supplementary Figs II, XI) we investigated the subcellular localizations of endogenous VEGFR2 and Orai1 in HUVECs (Fig 2e). Partial colocalization was observed in the vicinity of the plasma membrane, most obviously when observing in a focal plane above the adherent surface, where the nucleus was located (Fig 2e, Supplementary Fig XI-XIII). The image in Fig 2e was captured from a cell in the presence of VEGF but VEGF did not obviously change the degree of colocalization (Supplementary Fig XII). The data suggest that these proteins share a subcellular compartment, possibly to enhance signaling efficiency.
Orai1 role in cell migration and tube formation
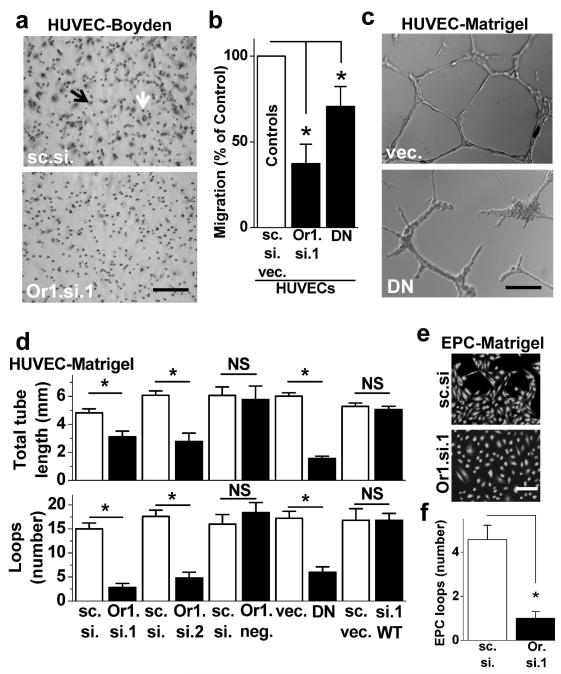
A key process regulated by VEGF is endothelial cell migration1 which can be quantified in modified Boyden chambers in which the cells migrate through 8 μm pores towards VEGF1 (Fig 3a, Supplementary Fig XIV). It was observed that Orai1 siRNA suppressed HUVEC migration, as shown by the example (Fig 3a) and mean data (Fig 3b). A similar but weaker effect of DN-Orai1 was observed (Fig 3b). The data suggest that Orai1 has a positive role in endothelial cell migration and may therefore play a role in angiogenesis.
Figure 3. Orai1 function in cell migration and tube formation.
(a) Example images of HUVEC migrations through 8 μm holes after transfection with control (sc.si) or Orai1 siRNA 1 (Or1.si.1). A typical cell is indicated by the black arrow and a typical hole by the white arrow. Scale bar = 20 μm. (b) Mean HUVEC migrations after Or1.si.1 or DN-Orai1 transfections and normalization to paired controls (n/N=4/8 for each experiment). (c) Example images of HUVEC tube formations after transfection with vector only (vec.) or DN-Orai1. Scale bar = 100 μm. (d) Two types of analysis of HUVEC tube formation after paired (control/test) transfections with the agents indicated (n/N=3/7, 3/3, 3/3, 3/7, 3/12, 3/7 for each experiments). Abbreviations: sc.si. (scrambled control siRNA); vec. (DNA empty vector); Or1.si.1 (Orai1 siRNA1); DN (R91W dominant negative Orai1); Or1.si.2 (Orai1 siRNA2); Or1.neg. (Orai1 negative control siRNA); WT (exogenous wild-type Orai1). (e) Example images of calcein-loaded EPCs after transfection with control or Orai1 siRNA. Scale bar = 100 μm. (f) Analysis of EPC loop formations in control and Orai1 siRNA groups (n/N=3/30).
To investigate the relevance to angiogenesis we first quantified HUVEC tube and loop formation on Matrigel as an in vitro assay of angiogenic potential1, 25. Examples of the structures formed by the cells are shown in Fig 3c. Disruption of Orai1 by DN-Orai1 inhibited the tube formation (Fig 3c). Therefore, extensive experiments were performed to test the hypothesis that Orai1 has a role in this phenomenon (Fig 3d). Experiments were carried out in test and control pairs where the white bars are mean control data and the black bars to the right are mean test data (Fig 3d). Two different Orai1 siRNAs (Or1.si.1 and Or1.si.2) independently inhibited tube length and loop formation, whereas an inactive siRNA (Or1.neg.) had no effect (Fig 3d). In cells subjected to Orai1 siRNA, normal tube length and formation could be rescued by expression of exogenous wild-type (WT) Orai1 clone (Fig 3d). EPCs were studied less extensively but loop structures formed by the cells were found to be largely prevented by Orai1 siRNA (Fig 3e, f).
The data suggest that Orai1 makes a significant contribution to angiogenic behaviour of endothelial cells.
Specific inhibition of Ca2+ entry by a CRAC channel blocker
The above experiments suggest a role of Orai1 but the results may not be explained by a role of CRAC channels because Orai1 could have multiple functions. Therefore, we sought a specific chemical blocker of CRAC channels as a further test of the role of the channels. The patent WO 2005/009954 describes the compound 3-fluoropyridine-4-carboxylic acid (2′,5′-dimethoxybiphenyl-4-yl)amide as an inhibitor of CRAC channels in immune cells. We refer to the compound as S66. It has previously been reported to specifically inhibit CRAC channel-related signals in immune cells with an IC50 of 1.4-3.0 μmoles/L18, 19.
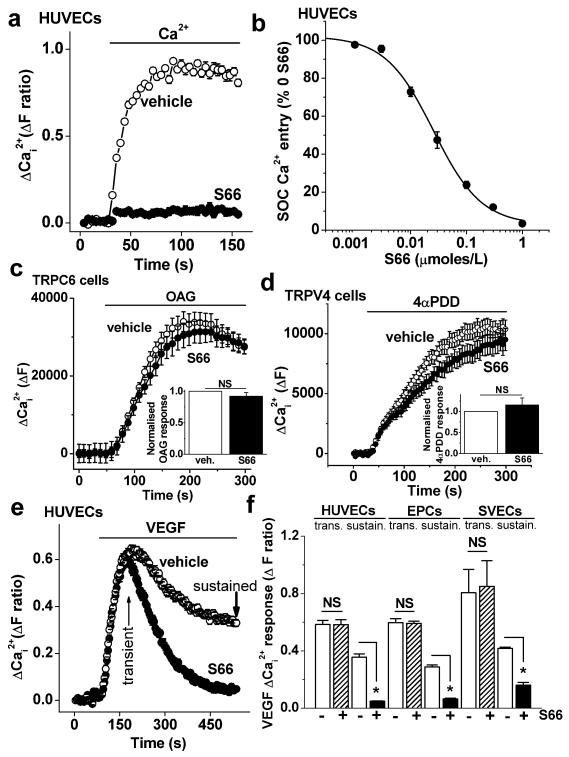
The Ca2+ add-back signal in store-depleted HUVECs was strongly suppressed by S66 (Fig 4a). The effect occurred with high potency, showing an IC50 of 25.5 nmoles/L (Fig 4b). Direct comparisons with immune cell Ca2+ entry under the same conditions confirmed that there was higher potency against HUVECs and EPCs (Supplementary Fig XV). Specificity of S66 was indicated by investigation of two other types of Ca2+ channel that are expressed and functional in endothelial cells, TRPC610, 11 and TRPV426, 27. Over-expressed TRPC6 was activated by the lipid 1-oleoyl-2-acetyl-sn-glycerol (Fig 4c) whereas over-expressed TRPV4 was activated by 4α-phorbol-didecanoate (Fig 4d). S66 (5 μmoles/L) had no significant effect on these channels (Fig 4c, d).
Figure 4. Inhibition of Ca2+-entry by S66.
(a) Example paired comparison of Ca2+-entry in HUVECs treated with 5 μmoles/L S66 (N=24) and store-depleted with 1 μmoles/L thapsigargin in Ca2+-free solution for 30 min prior to re-addition of extracellular Ca2+ (0.2 mmoles/L). (b) As for (a) but mean concentration-response data (n/N=3/24 for each point) with a fitted Hill equation (IC50 25.5 nmoles/L, slope 1.01). The data are percentages of the signal amplitude in the absence of S66 (% 0 S66). (c) The main graph is example data showing the effect of 5 μmoles/L S66 on TRPC6 channel activity evoked by 100 μmoles/L 1-oleoyl-2-acetyl-sn-glycerol, OAG (N=6). Inset are the mean data showing no significant effect (n/N=4/22). (d) As for (c) but showing TRPV4 activity evoked by 1 μmoles/L 4α-phorbol-didecanoate, 4α-PDD (N=9), with inset mean data showing no significant effect (n/N=4/32). (e) Example Ca2+ responses to VEGF (100 ng/mL) in the presence of 5 μmoles/L S66 or vehicle (N=24), showing the transient and sustained components of the response. (f) Mean transient and sustained responses to VEGF for the type of experiment shown in (e) (n/N=3/56), and for EPCs (n/N=4/64) and saphenous vein endothelial cells (SVECs, n/N=3/14).
The data suggest that S66 is an inhibitor of endothelial store-operated Ca2+-entry and lacks effect on TRPC6 and TRPV4.
Inhibition of VEGF-evoked Ca2+ entry by the CRAC channel blocker
S66 was tested against VEGF-evoked Ca2+ signals in the presence of extracellular Ca2+ (Fig 4e, f). It caused strong inhibition of sustained Ca2+-entry while lacking effect on the initial transient response (Fig 4e, f). These data contrast with the Orai1 siRNA data of Fig 1 (d, e) because there was specificity for the sustained Ca2+ response, suggesting that S66 did not affect VEGF receptors or Ca2+-release. S66 also did not affect STIM1 clustering (Supplementary Fig XVI). To investigate the relevance of the action of S66 to other types of endothelial cell, VEGF responses were investigated in EPCs and cultures of saphenous vein endothelial cells (SVECs) from patients undergoing coronary artery bypass graft surgery. As observed in HUVECs, S66 was a strong inhibitor of the sustained VEGF response but not the transient response (Fig 4f). The residual S66-resistant Ca2+ entry in HUVECs might be accounted for by TRPC1 (Fig 1f, g). In saphenous vein endothelial cells the S66-resistant component was larger (Fig 4f), as was the TRPC1 contribution (Supplementary Fig XVII).
The data are consistent with CRAC channels mediating a substantial fraction of the sustained Ca2+-entry evoked by VEGF and with the amplitude of this contribution varying across endothelial cell types.
Inhibition of tube formation and angiogenesis by the CRAC channel blocker
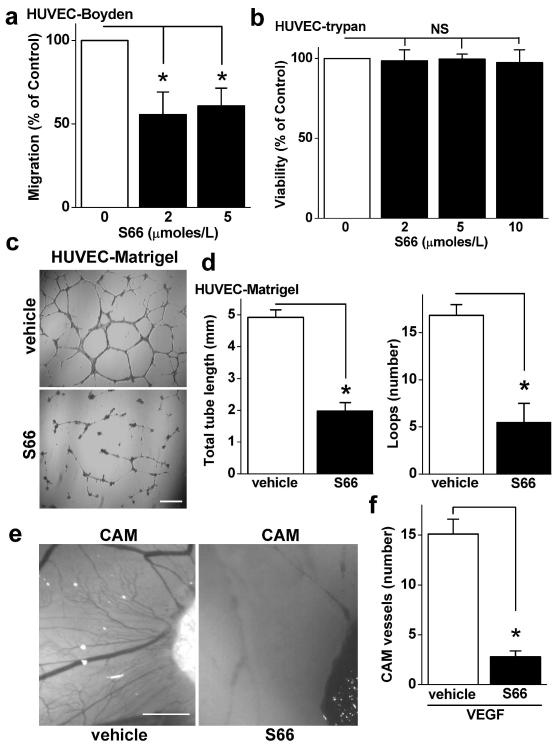
S66 was also investigated against endothelial cell migration. The cell migration was inhibited by about 50 % with maximum effect at 2 μmoles/L S66 (Fig 5a), suggesting that the S66-sensitive mechanism had a positive modulator role. S66 lacked effect on endothelial cell viability (Fig 5b). To investigate the impact on angiogenesis we performed in vitro and in vivo assays. In in vitro assays S66 restricted tube length and reduced the number of loops, conferring 60-70 % inhibition (Fig 5c, d). For in vivo analysis we used the established angiogenesis assay of the chick chorioallantoic membrane, where new blood vessels form around VEGF-infused sponges1, 22. S66 had striking inhibitory effects on the growth of these vessels (Fig 5e, f). The data suggest that S66 was an inhibitor of VEGF-induced endothelial cell migration and angiogenesis without effect on cell viability.
Figure 5. Inhibition of cell migration and angiogenesis by S66.
(a) Mean HUVEC migration in the presence of 2 or 5 μmoles/L S66 or vehicle (n/N=3/6). (b) Mean normalized data for HUVEC viability in the presence of 2, 5 or 10 μmoles/L S66 for 24 h (n/N=3/10). (c) Example images of HUVEC tubes after treatment with vehicle or 5 μmoles/L S66. Scale bar = 100 μm. (d) As for (c) but mean data for analysis of tube length (left) and loops (right) (n/N=3/15). (e) Example images of chorioallantoic membrane blood vessels around a VEGF-infused gelatin sponge (which is seen on the right of each image) with or without 20 μmoles/L S66 in the sponge. Scale bar = 50 μm. (f) For the type of experiment shown in (e), the mean number of blood vessels around the sponge for vehicle and S66 (n=15 and 10).
Discussion
The study suggests that a CRAC channel protein (Orai1) is important for store-operated and VEGF-evoked Ca2+ entry in endothelial cells, with Orai1 disruption leading to reduced endothelial cell migration and tube formation (see the summary diagram of Supplementary Fig X). Because Orai1 may have CRAC channel independent roles we sought to further investigate the hypothesis that CRAC channels are involved by seeking a specific chemical blocker of the channels. S66 was found to be a strong, potent and specific inhibitor of store-operated Ca2+-entry. Importantly, it replicated the effects of Orai1 disruption on sustained endothelial cell Ca2+-entry and migration and tube formation, and inhibited VEGF-evoked angiogenesis in vivo. Suppression of the transient VEGF Ca2+-response by Orai1 siRNA but not S66 may be explained by partial store-depletion resulting from the long-term disruption of CRAC channels or a CRAC channel-independent role of Orai1.
CRAC channels and Orai1 have mostly been associated with immune cell function13, 28. Therefore, inhibitors of CRAC channels and Orai1 are predicted to be immune-suppressive. Our S66 data suggest that it may be possible to minimize such effects because of the higher potency in endothelial cells. Nevertheless, inhibitor effects on immune cell function could be advantageous in conditions such as obesity or cancer where, for example, low-grade long-term inflammation and late-stage aggressive immune responses have deleterious effects29, 30. Although the effect of S66 seems to open new therapeutic potential it is a limitation that we do not yet know its mechanism of action. Our data suggest that it does not affect Ca2+-release (Fig 4e) or STIM1 clustering (Supplementary Fig XVI), putting its site of action downstream and thus perhaps at Orai1 or another CRAC channel component.
Disease in Orai1-deficient patients is severe but apparently limited to immunodeficiency, congenital myopathy and ectodermal dysplasia16, 31. These patients have intact vasculatures and so Orai1 is not obligatory for vasculogenesis in humans. Orai1-deficient mice are either runted and display immune deficiency or die just before or after birth for reasons that are not yet clear32, 33. Such observations from patients and mutant mice are consistent with our data showing suppression but not abolition of endothelial migration and tube formation following Orai1 disruption. That is, we hypothesize that Orai1’s role in endothelial function is as a positive modulator rather than obligatory factor. It is, therefore, conceivable that there are compensatory mechanisms for Orai1-deficiency, perhaps involving other Orai proteins or other types of Ca2+ channel protein that have roles in VEGF responses and angiogenesis. Indeed, our data are consistent with a contribution of TRPC1 and there is good evidence for roles of TRPC610, 11 which may be particularly relevant to slowly-developing VEGF effects outside the time-frame of our Ca2+i measurements (see Supplementary Fig X). Integration of Orai1 and TRPC1 is indicated to occur, for example, through STIM134 (Supplementary Fig X).
In summary, positive roles of Orai1 in endothelial cell VEGF Ca2+i-responses, migration and tube formation are indicated. The effects appear to result substantially from Orai1’s role in CRAC channels because of the additional finding that a specific CRAC channel blocker had mostly similar effects to Orai1 disruption. Unexpected high potency of the blocker at endothelial compared with immune cells suggested, nevertheless, a distinct characteristic of the endothelial CRAC channel and the opportunity for endothelial specificity. In addition to the mechanistic insight provided by this study there is the suggestion that the process could be an attractive target for therapeutic modulation of angiogenesis in inflammatory disease situations that include cancer and metabolic syndrome associated with obesity.
Novelty and Significance.
What Is Known?
Orai1 is a membrane protein component of a T cell Ca2+ entry mechanism that is defective in severe combined immune deficiency
Depletion of intracellular Ca2+ stores is an activation signal for CRAC channels in T cells
Orai1 is expressed in human umbilical vein endothelial cells, which contain CRAC channel-like signals
What New Information Does This Article Contribute?
Endothelial Orai1 stimulates Ca2+ entry, cell migration and tube formation evoked by vascular endothelial growth factor (VEGF)
A specific chemical blocker of CRAC channels inhibits VEGF-evoked Ca2+-entry, endothelial cell migration and endothelial tube formation in vitro, and angiogenesis in vivo
Endothelial Ca2+-entry is more sensitive to the CRAC channel blocker than immune cell Ca2+-entry
This study was conducted because of paucity of information about the significance of Orai1 and CRAC channels in endothelial cell biology and to determine if a pharmacological approach exists that could enhance mechanistic insight and facilitate clinical exploitation in the cardiovascular system. It was confirmed that disruption of Orai1 suppressed Ca2+-entry evoked by pharmacological store-depletion in human umbilical vein endothelial cells and the finding was extended to endothelial progenitor cells derived from blood of healthy volunteers, which are thought to be involved in endothelial repair and pathological angiogenesis. Furthermore, Ca2+-entry evoked by the physiological factor, VEGF, was also shown to depend on Orai1. Because VEGF has pivotal roles in evoking endothelial cell migration and angiogenesis we investigated if Orai1 is relevant to these key endothelial cell properties. By using an established in vitro angiogenesis assay we were able to carry out a thorough molecular investigation of the role of Orai1, including rescue of function by exogenous Orai1 clone after knock-down of native expression. The data show conclusively that Orai1 is important in tube formation. Reduced capacity for cell migration was implicated as a contributing factor because this parameter also depended on Orai1 and is central to angiogenesis. Because Orai1 may have functions in addition to CRAC channel formation we sought a specific CRAC channel blocking substance for further studies. We found that a substance with previously described specificity but modest potency at immune cell CRAC channels has 100-fold higher potency at endothelial Ca2+ entry. The substance blocked VEGF-evoked Ca2+-entry but not Ca2+-release, and endothelial cell migration and tube formation were suppressed without effect on cell viability. Importantly, the substance also inhibited VEGF-evoked angiogenesis in vivo. The study has shown us that Orai1 and CRAC channels are relevant to major aspects of endothelial biology: VEGF signaling, endothelial cell migration, and angiogenesis. Identification of a specific and potent chemical modulator increases the likelihood that there will be opportunity for therapeutic benefit.
Supplementary Material
Acknowledgments
Sources of Funding
The work was supported by the British Heart Foundation, Wellcome Trust, and Medical Research Council. LAW was supported by a BBSRC-AstraZeneca PhD Studentship, MSA by a scholarship from the Egyptian Ministry of Higher Education, and BH by a Scholarship from the University of Leeds and the China Scholarship Council.
Non-standard abbreviations and acronyms
- CRAC
Calcium-release-activated calcium
- VEGF
vascular endothelial growth factor
- HUVEC
human umbilical vein endothelial cell
- EPC
endothelial progenitor cell
- STIM1
stromal interaction molecule-1
- VEGFR2
VEGF receptor-2
- TRPC
Transient Receptor Potential Canonical
- TRPV
Transient Receptor Potential Vanilloid
- sc.si.
scrambled control siRNA
- vec.
empty DNA vector
- Or1.si.1
Orai1 siRNA1
- DN
R91W dominant negative Orai1
- Or1.si.2
Orai1 siRNA2
- Or1.neg.
Orai1 negative control siRNA
- STIM1.si
STIM1 siRNA
- WT
exogenous wild-type Orai1
Footnotes
Disclosures
None.
Subject Codes
[152] Ion channels/membrane transport
[129] Angiogenesis
[147] Growth factors/cytokines
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. Therapeutic angiogenesis: Another passing phase? Circ Res. 2006;98:1115–1116. doi: 10.1161/01.RES.0000223485.43020.9e. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. Vegf receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 8.Faehling M, Kroll J, Fohr KJ, Fellbrich G, Mayr U, Trischler G, Waltenberger J. Essential role of calcium in vascular endothelial growth factor a-induced signaling: Mechanism of the antiangiogenic effect of carboxyamidotriazole. FASEB J. 2002;16:1805–1807. doi: 10.1096/fj.01-0938fje. [DOI] [PubMed] [Google Scholar]
- 9.Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Angiopoietin-1 opposes vegf-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2+ influx. Circ Res. 2005;96:1282–1290. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- 10.Hamdollah Zadeh MA, Glass CA, Magnussen A, Hancox JC, Bates DO. VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation. 2008;15:605–614. doi: 10.1080/10739680802220323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge R, Tai Y, Sun Y, Zhou K, Yang S, Cheng T, Zou Q, Shen F, Wang Y. Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett. 2009;283:43–51. doi: 10.1016/j.canlet.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Yu PC, Gu SY, Bu JW, Du JL. TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ Res. 2010;106:1221–1232. doi: 10.1161/CIRCRESAHA.109.207670. [DOI] [PubMed] [Google Scholar]
- 13.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in orai1 causes immune deficiency by abrogating crac channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 14.Cahalan MD. Stimulating store-operated Ca2+ entry. Nat Cell Biol. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG. STIM1 gates the store-operated calcium channel orai1 in vitro. Nat Struct Mol Biol. 2010;17:112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feske S. Orai1 and stim1 deficiency in human and mice: Roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. STIM1 and orai1 mediate crac currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng SW, di Capite J, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283:31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 19.Di Sabatino A, Rovedatti L, Kaur R, Spencer JP, Brown JT, Morisset VD, Biancheri P, Leakey NA, Wilde JI, Scott L, Corazza GR, Lee K, Sengupta N, Knowles CH, Gunthorpe MJ, McLean PG, MacDonald TT, Kruidenier L. Targeting gut T cell Ca2+ release-activated Ca2+ channels inhibits t cell cytokine production and T-box transcription factor t-bet in inflammatory bowel disease. J Immunol. 2009;183:3454–3462. doi: 10.4049/jimmunol.0802887. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125–136. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- 21.Pearson JD. Endothelial progenitor cells - hype or hope? J Thromb Haemost. 2009;7:255–262. doi: 10.1111/j.1538-7836.2008.03214.x. [DOI] [PubMed] [Google Scholar]
- 22.Ribatti D, Nico B, Vacca A, Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc. 2006;1:85–91. doi: 10.1038/nprot.2006.13. [DOI] [PubMed] [Google Scholar]
- 23.Bergmeier W, Oh-Hora M, McCarl CA, Roden RC, Bray PF, Feske S. R93W mutation in orai1 causes impaired calcium influx in platelets. Blood. 2009;113:675–678. doi: 10.1182/blood-2008-08-174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derler I, Fahrner M, Carugo O, Muik M, Bergsmann J, Schindl R, Frischauf I, Eshaghi S, Romanin C. Increased hydrophobicity at the n terminus/membrane interface impairs gating of the severe combined immunodeficiency-related orai1 mutant. J Biol Chem. 2009;284:15903–15915. doi: 10.1074/jbc.M808312200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: State of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 26.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Cao J, Luo J, Nilius B, Huang Y, Ambudkar IS, Yao X. Depletion of intracellular Ca2+ stores stimulates the translocation of TRPV4-C1 heteromeric channels to the plasma membrane. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.212084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32:235–245. doi: 10.1016/j.tibs.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S. Orai1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 2009;124:1311–1318 e1317. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking orai1. Mol Cell Biol. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of transient receptor potential canonical (TRPC) channels tunes their store-operated mode. J Biol Chem. 2010;285:38666–38673. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.