Abstract
It was previously shown that a number of sulfhydryl [SH] group reagents (N-ethylmaleimide [NEM], iodoacetate, Ag+, HgCl2, etc.) can induce a marked, transitory stimulation of O2 uptake (QO2) in Egeria densa leaves, insensitive to CN− and salicylhydroxamic acid and inhibited by diphenylene iodonium and quinacrine. The phytotoxin fusicoccin (FC) also induces a marked increase in O2 consumption in E. densa leaves, apparently independent of the recognized stimulating action on the H+-ATPase. In this investigation we compared the FC-induced increase in O2 consumption with those induced by NEM and Ag+, and we tested for a possible interaction between FC and the two SH blockers in the activation of QO2. The results show (a) the different nature of the FC- and NEM- or Ag+-induced increases of QO2; (b) that FC counteracts the NEM- (and Ag+)-induced respiratory burst; and (c) that FC strongly reduces the damaging effects on plasma membrane permeability observed in E. densa leaves treated with the two SH reagents. Two alternative models of interpretation of the action of FC, in activating a CN−-sensitive respiratory pathway and in suppressing the SH blocker-induced respiratory burst, are proposed.
Previous investigations have shown that NEM and a number of other SH group reagents are able to induce a remarkable respiratory burst in Egeria densa Planchon leaves. This effect was not inhibited by CN−, SHAM, or propylgallate added separately or in combination, and was additive with the effect of the uncoupler carbonylcyanide-m-chlorophenylhydrazone, thus ruling out the participation of the mitochondrial (cytochromic and alternative) electron transport pathways. It was, however, completely suppressed by diphenylene iodonium and by quinacrine, two inhibitors of the plasmalemma NADPH oxidase activated by pathogens in granulocytes (Babior, 1992) and in plants (Auh and Murphy, 1995). This indicated an activating action of SH reagents on some redox systems that are inactive under normal conditions (Albergoni et al., 1996; Bellando et al., 1997).
To define experimental conditions able to influence the SH reagent-induced increase in O2 consumption, we studied the effects of the fungal toxin FC on the SH blocker-induced increase in QO2. This choice was based on previous evidence suggesting that FC might increase the reduction state of NADP and thiol components in the cell (Marrè et al., 1989a, 1994) and also the finding that FC, in addition to stimulating the H+-ATPase activity (Marrè et al., 1989b), induces a marked increase in O2 consumption in E. densa leaves, apparently independently of its stimulation on the proton pump. Leaves treated with the toxin under experimental conditions that are nonpermissive for proton extrusion had a respiration rate significantly higher than controls treated under conditions favorable for H+ extrusion (Beffagna et al., 1989; Marrè et al., 1993). Thus, we thought it would be interesting to compare this FC-induced increase in O2 consumption with the similar effect induced by NEM and to test for a possible interaction between FC and the SH blockers NEM and Ag+ in the activation of QO2.
In this paper we present data concerning E. densa leaves that demonstrate (a) the different nature of the FC- and the NEM- and Ag+-induced increases of QO2 and (b) the ability of FC to counteract the NEM- and Ag+-induced oxidative burst and associated effects on plasma membrane permeability.
MATERIALS AND METHODS
Egeria densa Planchon plants were grown in flowing tap water in a greenhouse. Young, fully expanded leaves were cut from branches about 2 to 4 cm from the apex, randomized, and then grouped into samples. The samples were preincubated for 1 h in 10 mL of 0.5 mm CaSO4, with continuous shaking at 20°C in the dark. The solution was renewed after 30 min. DCMU (5 μm) and, when applicable FC (0.1 mm) were added during the last 30 min of pretreatment.
QO2 Measurements
QO2 was measured on four leaves (about 30 mg fresh weight) in 2 mL of medium by using a Clark-type O2 electrode (unit KW2, control box CB1, Hansatech, Norfolk, UK), modified to allow quick (every 5–10 min) changes of the incubation solution. The electric current generated by the O2 consumption was applied to an mV recorder connected to a computerized unit differentiating continuous measurements. Data output were simultaneously converted to O2 consumption rate values expressed as nanomoles of O2 decrease per minute in the measurement chamber.
Measurements were carried out in a medium containing: 0.5 mm CaSO4, 5 μm DCMU, and 10 mm Mes buffer, pH 6.0, with BTP. Other reagents were added at the concentrations specified in the legends to the figures. The data represent a group of at least five experiments for each experimental condition; the se did not exceed 10%.
Electrolyte Leakage Measurements
After pretreatment, samples of leaves (200 mg fresh weight) were incubated in 10 mL of a stirred solution, thermoregulated at 20°C, that contained 0.5 mm CaSO4, 5 μm DCMU, and 10 mm Mes buffer, pH 6.0, with BTP. Other reagents were added at the concentrations specified in the legends to the figures. The conductivity of the medium was measured continuously for the required time by means of a conductivity meter (model MO 101, Analytical Control, Milan, Italy) connected to a chart recorder. The data represent the change in medium conductivity (ΔμS) from the value (taken as zero) of conductivity at the end of the equilibration period. The data presented are those of a typical experiment out of at least five measurements for each set of experimental conditions. The se did not exceed 6%.
Electrophysiological Measurements
A single leaf held in a 5-mL plexiglass chamber was irrigated with a continuously flowing (approximately 15 mL min−1) aerated medium, thermoregulated at 20°C, that contained 0.5 mm CaSO4, 5 μm DCMU, and 10 mm Mes buffer, pH 6.0, with BTP. Other reagents were added at the concentrations specified in the legends to the figures.
Micropipettes (tip resistance 10–20 mΩ) filled with 1 m KCl were used as microsalt bridges of Ag/AgCl electrodes and inserted vertically into the tissue by means of a micromanipulator (Leitz, Wetzlar, Germany). Transmembrane electric potential difference was measured with a high-impedance electrometer amplifier (WPI K5–700, WP Instruments, New Haven, CT) connected to a chart recorder. The data presented are those of a typical experiment out of at least four experiments for each set of experimental conditions. The se did not exceed 6%.
Variations in Titratable H+ and K+ Concentrations in the Medium
Following pretreatment, the samples (120 mg fresh weight) were incubated in 7 mL of solution containing 0.5 mm Mes adjusted to pH 6.0 with BTP, 0.5 mm CaSO4, 0.25 mm K2SO4, and 5 μm DCMU with 0.1 mm FC and/or 0.2 mm NEM when applicable. After 45 min of incubation the leaves were removed from the medium, which was divided into two aliquots to determine titratable H+ extrusion (−ΔH+) and K+ net uptake (ΔK+) by the tissue.
H+ extrusion was determined by back titration of 4 mL of the medium from the final pH value to the initial value after removal of CO2, as described by Lado et al. (1981). K+ uptake was determined in 3 mL of the medium using an atomic absorption-flame spectrophotometer (model AA12175, Varian Techtron, Melbourne, Australia). Experiments were run in triplicate and repeated three times.
RESULTS
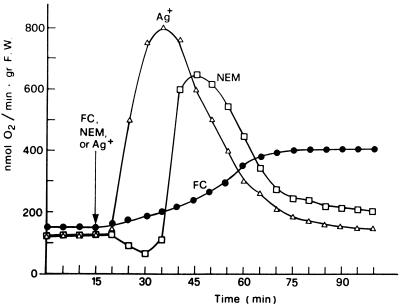
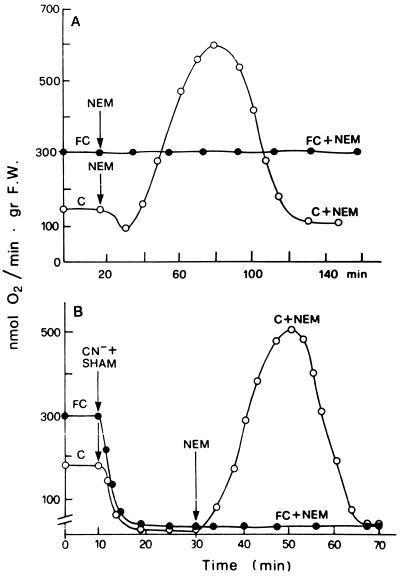
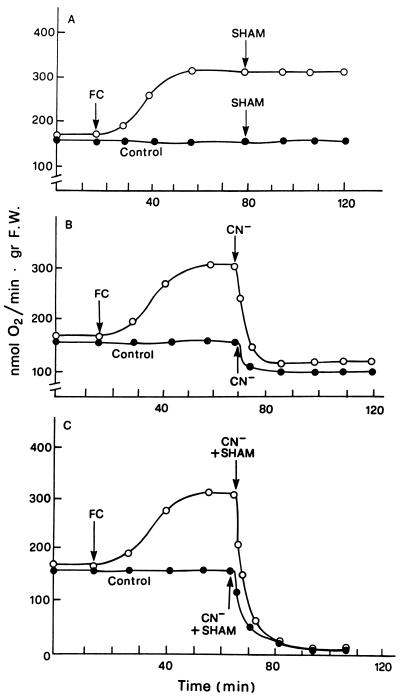
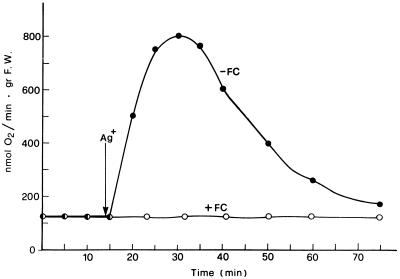
Figure 1 shows the changes induced by FC, or alternatively by NEM or Ag+, on the rate of O2 consumption by E. densa leaves. The substitution of the control solution, with one containing 0.2 mm NEM or 2 μm Ag+, caused the typical respiratory changes previously described for E. densa and Potamogeton crispus (Albergoni et al., 1996; Bellando et al., 1997). With NEM an initial phase in which a slight QO2 decrease occurred was followed by the onset of a marked but transitory stimulation of O2 consumption, whereas with Ag+, no lag phase was usually observed. The NEM- or the Ag+-induced respiratory burst was essentially unchanged, even in conditions of drastic inhibition of the basal QO2 induced by combined treatment with CN− and SHAM (Bellando et al., 1997; Fig. 3B, control curve). The effect of FC on respiration had a completely different pattern. As shown in Figure 1, a stimulation of QO2 was already measurable 15 min after the toxin was added and reached its maximum in about 50 min, remaining constant for more than 2 h. This FC-induced increase in respiration was not influenced by SHAM (Fig. 2A) but was completely suppressed by CN− alone (Fig. 2B) or in combination with SHAM (Fig. 2C). Even more interesting was the finding that FC completely suppressed the NEM-induced stimulation of QO2 both in the absence and in the presence of CN− and SHAM (Fig. 3). A similar suppression of the respiratory burst was observed when FC was added 5–8 min after the addition of NEM (data not shown). Figure 4 shows that FC was equally able to inhibit the increase in QO2 induced in E. densa leaves by Ag+.
Figure 1.
Changes in the rate of QO2 induced in E. densa leaves by the presence of 10−4 m FC and of 0.2 mm NEM, or 2 μm Ag+, in the incubation medium. Basal composition of the medium was 0.5 mm CaSO4, 5 μm DCMU, and 10 mm Mes-BTP buffer, pH 6.0, and Ag+ was added as the SO42− salt. gr F.W., Grams fresh weight.
Figure 3.
Counteracting effect of FC on the oxidative burst induced by 0.2 mm NEM in E. densa leaves in the absence (A) and in the simultaneous presence (B) of 2 mm NaCN and 1 mm SHAM. Other experimental conditions were as described for Figure 1. gr F.W., Grams fresh weight.
Figure 2.
Effects of 2 mm NaCN and 1 mm SHAM, fed separately or in combination, on the basal and FC-induced increase in O2 consumption. Other experimental conditions were as described for Figure 1. gr F.W., Grams fresh weight.
Figure 4.
Counteracting effect of FC on the respiratory burst induced by 2 μm Ag+ in E. densa leaves. Other experimental conditions were as described for Figure 1. gr F.W., Grams fresh weight.
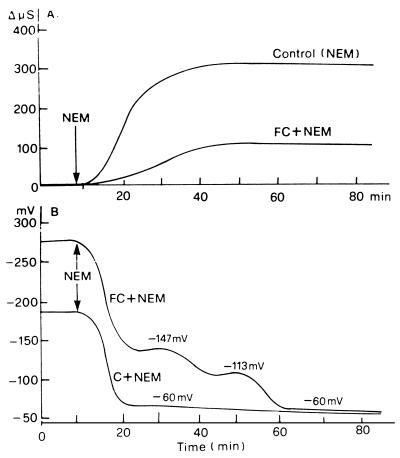
In a further exploration of this clear-cut antagonism between FC action and the ability of NEM to induce the respiratory burst, we investigated how FC influenced the effects of NEM on the membrane electrical potential and on electrolyte leakage: two responses to NEM or other SH blockers usually associated with that of QO2 (Bellando et al., 1997; for the toxic effect of SH blockers on plasma membrane functionality, see Delrot et al., 1980; Lichtner and Spanswick, 1981; Loos and Lüttge, 1984). Figure 5A shows that FC efficiently, but only partially, inhibited the NEM-induced increase in electrolyte leakage, in contrast to its ability to completely suppress the NEM-induced respiratory activation. The effect of FC on the membrane potential of the NEM-treated cells is more complex, and the interpretation is difficult because of the interference of the strong hyperpolarizing action of FC. Figure 5B highlights two phases in the phenomenon. In an earlier phase (lasting approximately 25 min after the addition of NEM to the FC-pretreated leaves), the presence of FC does not prevent the NEM-induced depolarization; however, the cells treated with FC and NEM remain hyperpolarized by about 40 mV above those treated with NEM alone. In a second phase, the FC-induced hyperpolarization disappears as well; therefore, after 90 min the same transmembrane electric potential difference values are reached by all samples, independently of the presence of FC. A possible interpretation could be that NEM acts on more than one target and that its initial action on membrane potential is not suppressed by FC, whereas in the second phase, it attacks other systems, in particular the H+-ATPase, the activation of which is responsible for the hyperpolarizing action of FC (Marrè et al., 1989b; for the inhibition by NEM of the H+-ATPase, see Brooker and Slayman, 1982).
Figure 5.
Counteracting effect of FC on the increase in medium conductivity and on membrane depolarization induced by 0.2 mm NEM in E. densa leaves. Basal medium consisted of 0.5 mm CaSO4, 5 μm DCMU, and 10 mm Mes buffer, pH 6.0, with BTP.
The contrast between the total inhibition of the respiratory response to NEM and the partial inhibition of the electrolyte response is similar to the behavior previously observed in treatments with the two oxidase inhibitors diphenylene iodonium and quinacrine, which only partially inhibit NEM-induced electrolyte leakage and depolarization, while completely suppressing the respiratory burst (Bellando et al., 1997). In both cases, the simplest interpretation is that of a relative independence of the effects of the SH blocker on both the general organization of the membrane and on the enzyme system responsible for the respiratory burst (Bellando et al., 1997). The results reported in Table I that show that FC only partially counteracted the basification of the medium, and the marked release of K+ induced by NEM in E. densa leaves are in agreement with this interpretation.
Table I.
Effects of NEM and FC on titratable proton extrusion (−ΔH+) and on K+ net uptake (ΔK+) in E. densa leaves
| Treatment | −ΔH+ | ΔK+ |
|---|---|---|
| μmol H+ g−1 fresh wt 45 min−1 | μmol K+ g−1 fresh wt 45 min−1 | |
| Control | 2.03 ± 0.08 | 1.55 ± 0.1 |
| FC | 6.25 ± 0.28 | 7.25 ± 0.19 |
| NEM | −3.5 ± 0.13 | −47.8 ± 2.4 |
| NEM + FC | −1.97 ± 0.09 | −28 ± 1.57 |
Basal composition of the medium (control) consisted of the following: 0.5 mm Mes adjusted to pH 6.0 with BTP, 0.5 mm CaSO4, 0.25 mm K2SO4, and 5 μm DCMU, with 0.1 mm FC and/or 0.2 mm NEM when present.
DISCUSSION AND CONCLUSION
The respiratory activation by the SH blockers NEM and Ag+, and by FC, involve two clearly distinct oxidative pathways: a nonmitochondrial, CN−- and SHAM-insensitive one and one completely blocked by CN−. The finding that FC completely blocks the respiratory effect of NEM even in the presence of CN− and SHAM (in spite of the total inhibition of the effects of FC on respiration under these conditions) indicates that the stimulation of CN−-sensitive respiration by the toxin is not a necessary step in the repression of NEM capability to induce the oxidative burst. A possible interpretation of the action of FC, both in activating a CN−-sensitive respiratory pathway and in suppressing the NEM- and Ag+-induced respiratory burst, is that the toxin could primarily interact with a receptor located in the plasma membrane. Recently, this FC receptor has been identified with a protein belonging to the 14-3-3 group (Korthout and De Boer, 1994; Marra et al., 1994; Oecking et al., 1994), several members of which have been shown to be involved in the regulation of a number of enzyme activities (Aitken et al., 1992; Moorhead et al., 1996; De Boer, 1997). The FC-receptor complex might then be responsible for the activation of CN−-sensitive respiration and also, independently, for the repression of the effects of SH blockers on QO2 and electrolyte leakage.
The hypothesis that the primary interaction of FC with its receptor might give rise to a multiplicity of effects (De Boer, 1997), beyond the well-established one on the H+-ATPase (De Michelis et al., 1996), has already been proposed to interpret the FC effect on respiration (Marrè et al., 1993). The ability of FC to efficiently counteract the effects of SH blockers demonstrated here is in agreement with previous evidence suggesting that FC increases the reduction state of NADP and thiol compounds (Rasi-Caldogno et al., 1978; Trockner and Marrè, 1988; Marrè et al., 1989a, 1994), a view also supported by some recent results from this laboratory showing a statistically significant increase (20%) of NADPH in E. densa leaves treated with FC in the absence of K+ (P. Vergani, personal communication). Because the respiratory burst induced by NEM or by other SH blockers depends on the reaction between these reagents and the SH groups of cell components, the suppression of the respiratory effect of SH blockers by FC might be due to its capability to increase the reducing power of the pyridine coenzyme-SH system in the cell, thus protecting SH groups from attack by the SH blockers. In this respect it is worth pointing out that a well-conserved Cys is present in a definite region of the 14-3-3 proteins so far studied (Oecking et al., 1994), thus suggesting the hypothesis of an involvement of this protein family in both the effects of FC and of SH reagents on respiration.
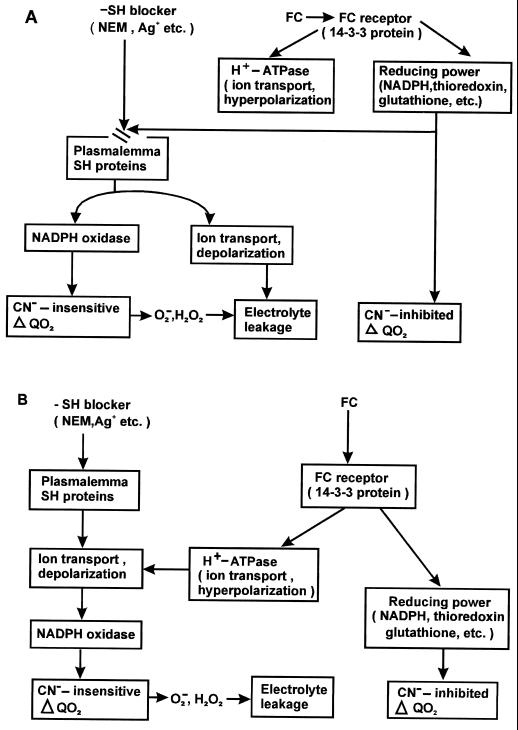
An interesting aspect of the results reported here is that they converge with recent reports (De Boer, 1997) that propose that the model in which FC specifically influences the plasma membrane H+-ATPase should be corrected to a new one. In this model FC would primarily bind to a 14-3-3 protein, modifying it so as to give rise to a series of responses, including activation of the H+ pump, inhibition of nitrate reductase (Moorhead et al., 1996), stimulation of respiration, and the protection of SH groups from SH blockers. This interpretation is depicted in Figure 6A, where the interaction between FC and the SH blockers would occur at the level of some SH group-containing cell components.
Figure 6.
Alternative models of the interaction between FC and SH blockers. The interaction occurs at the level of the SH systems redox state in A, and at the level of ion transport and membrane polarization in B.
The present data are also open to a different interpretation, which is depicted in Figure 6B. Recent reports suggest that the induction of the oxidative burst by pathogenic elicitors might be caused by changes in ion fluxes at the membrane level (Jabs et al., 1997; for the role of Ca2+ transport in oxidative burst, see Atkinson et al., 1990; Schawacke and Hager, 1992; Levine et al., 1996). If the view is accepted that in our system as well the effects of NEM or Ag+ on ion transport and membrane potential mediate the activation of NADPH oxidase, then the interaction between FC and SH blockers might be interpreted as occurring at the ion transport level. Here the stimulation of K+ and Cl− uptake and of H+ extrusion and the hyperpolarization of the membrane induced by the toxin counteract the release of K+ and Cl−, the increase in H+ influx, and the depolarization induced by the SH blockers (Table I; Fig. 5; Bellando et al., 1997).
The choice between the two interpretations presented cannot be made on the basis of available data, and a more detailed description of the ion fluxes (in particular of Ca2+) and of the electrophysiological parameters of the phenomenon are required.
ACKNOWLEDGMENTS
We are grateful to Prof. Erasmo Marré for useful advice and to Prof. Ida de Michelis for critical reading of the manuscript. Thanks are also due to Prof. Robert Jennings for revising the English manuscript.
Abbreviations:
- BTP
1,3-bis Tris(hydroxymethyl)methylamino-propane
- FC
fusicoccin
- NEM
N-ethylmaleimide
- QO2
O2 uptake
- SH
sulfhydryl
- SHAM
salicylhydroxamic acid
Footnotes
This work was supported by the Ministero Italiano dell' Università e della Ricerca Scientifica e Tecnologica (40%).
LITERATURE CITED
- Aitken A, Collinge DB, Van Heusden BPH, Isobe T, Roseboom PH, Rosenfeld G, Soll J. 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins. Trends Biochem Sci. 1992;17:498–501. doi: 10.1016/0968-0004(92)90339-b. [DOI] [PubMed] [Google Scholar]
- Albergoni F, Rocco P, Bellando M, Sacco S, Marrè MT. The induction of oxidative burst in Elodea densa by sulfhydryl reagents is independent of the inhibiting action of these reagents on photosynthesis. Rend Fis Acc Lincei. 1996;9:171–177. [Google Scholar]
- Atkinson MM, Keppler LD, Orlandi EW, Baker CJ, Mischke CF. Involvement of plasma membrane calcium influx in bacterial induction of the K+/H+ and hypersensitive responses in tobacco. Plant Physiol. 1990;92:215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auh CK, Murphy TM. Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. The respiratory burst oxidase. Adv Enzymol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- Beffagna N, Romani G, Marrè E (1989) ATP level and proton pump activity in Elodea leaves. In J Dainty, MI De Michelis, E Marrè , F Rasi-Caldogno, eds, Plant Membrane Transport: The Current Position. Proceedings of the Eighth International Workshop on Plant Membrane Transport, Venice, Italy, June 25–30, 1989
- Bellando M, Sacco S, Albergoni F, Rocco P, Marrè MT. Transient stimulation of oxygen uptake induced by sulfhydryl reagents in Egeria densa and Potamogeton crispus leaves. Bot Acta. 1997;110:388–394. [Google Scholar]
- Brooker RJ, Slayman CW. Inhibition of the plasma membrane H+-ATPase of Neurospora crassa by N-ethylmaleimide. Protection by nucleotides. J Biol Chem. 1982;257:12051–12057. [PubMed] [Google Scholar]
- De Boer B. Fusicoccin—a key to multiple 14-3-3 locks? Trends Plant Sci. 1997;2:60–66. [Google Scholar]
- Delrot S, Despeghel JP, Bonnemain JL. Phloem loading in Vicia faba leaves: effects of N-ethylmaleimide and parachloromercuribenzenesulfonic acid on H+ extrusion, K+ and sucrose uptake. Planta. 1980;149:144–148. doi: 10.1007/BF00380875. [DOI] [PubMed] [Google Scholar]
- De Michelis MI, Rasi-Caldogno F, Pugliarello MC, Olivari C. Fusicoccin binding to its plasma membrane receptor and the activation of the plasma membrane H+-ATPase. Plant Physiol. 1996;110:957–964. doi: 10.1104/pp.110.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado P, Cerana R, Bonetti A, Marrè MT, Marrè E. Effects of calmodulin inhibitors in plants. I. Synergism with fusicoccin in the stimulation of growth and H+ secretion and in the hyperpolarization of the transmembrane electric potential. Plant Sci Lett. 1981;23:253–262. [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Lichtner FT, Spanswick RM. Electrogenic sucrose transport in developing soybean cotyledons. Plant Physiol. 1981;67:869–874. doi: 10.1104/pp.67.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos S, Lüttge U. Effects of HgCl2 on membranes of Lemna gibba in the energized and non-energized state. Physiol Veg. 1984;22:171–179. [Google Scholar]
- Korthout HAAJ, de Boer AH. A fusicoccin binding protein belongs to the family of 14-3-3 brain protein homologs. Plant Cell. 1994;6:1681–1692. doi: 10.1105/tpc.6.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M, Fullone M, Fogliano V, Pen J, Mattei M, Masi S, Aducci P. The 30-kilodalton protein present in purified fusicoccin receptor preparations is a 14-3-3-like protein. Plant Physiol. 1994;106:1497–1501. doi: 10.1104/pp.106.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrè E, Bellando M, Albergoni F, Sacco S. Stimulation of oxygen uptake by fusicoccin in conditions of inhibited H+ extrusion. Rend Fis Acc Lincei. 1993;9:173–178. [Google Scholar]
- Marrè E, Marrè MT, Moroni A, Venegoni A, Albergoni F. Metabolism-mediated control of ATP-driven H+ extrusion: some evidence and a working hypothesis. In: Chiatante D, Gallon J, Smith C, Zocchi G, editors. Biochemical Mechanisms Involved in Growth Regulation, Phytochemical Society of Europe. Milan, Italy: Oxford University Press; 1994. pp. 245–258. [Google Scholar]
- Marrè MT, Albergoni FG, Moroni A, Marrè E. Light-induced activation of electrogenic H+ extrusion and K+ uptake in Elodea densa depends on photosynthesis and is mediated by the plasma membrane H+-ATPase. J Exp Bot. 1989a;40:343–352. [Google Scholar]
- Marrè MT, Albergoni FG, Moroni A, Pugliarello MC. On the involvement of the ATP-driven H+ pump in H+ extrusion in Elodea densa leaves. Plant Sci. 1989b;62:21–28. [Google Scholar]
- Moorhead G, Douglas P, Morrice N, Scabel M, Aitken A, MacKintosh C (1996) Phosphorylated nitrate reductase from spinach leaves is inhibited by 14-3-3 proteins and activated by fusicoccin. Curr Biol 6: 1104–1113 [DOI] [PubMed]
- Oecking C, Eckerskorn C, Weiler EW. The fusicoccin receptor of plants is a member of the 14-3-3 superfamily of eukaryotic regulatory proteins. FEBS Lett. 1994;352:163–166. doi: 10.1016/0014-5793(94)00949-x. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F, Lado P, Colombo R, Cerana R, Pugliarello MC (1978) Changes in Cellular Metabolism Associated with the Activation of H+/K+ Exchange by Auxin or FC in Pea Internode Segments. Proceeding of the Inaugural Meeting of the Federation of European Societies of Plant Physiology, Edinburgh, Scotland, pp 438–439
- Schawacke R, Hager A. Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein-kinase activity. Planta. 1992;187:136–141. doi: 10.1007/BF00201635. [DOI] [PubMed] [Google Scholar]
- Trockner V, Marrè E. Plasmalemma redox changes and H+ extrusion. II. Respiratory and metabolic changes associated with fusicoccin-induced and with ferricyanide-induced H+ extrusion. Plant Physiol. 1988;87:30–35. doi: 10.1104/pp.87.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]