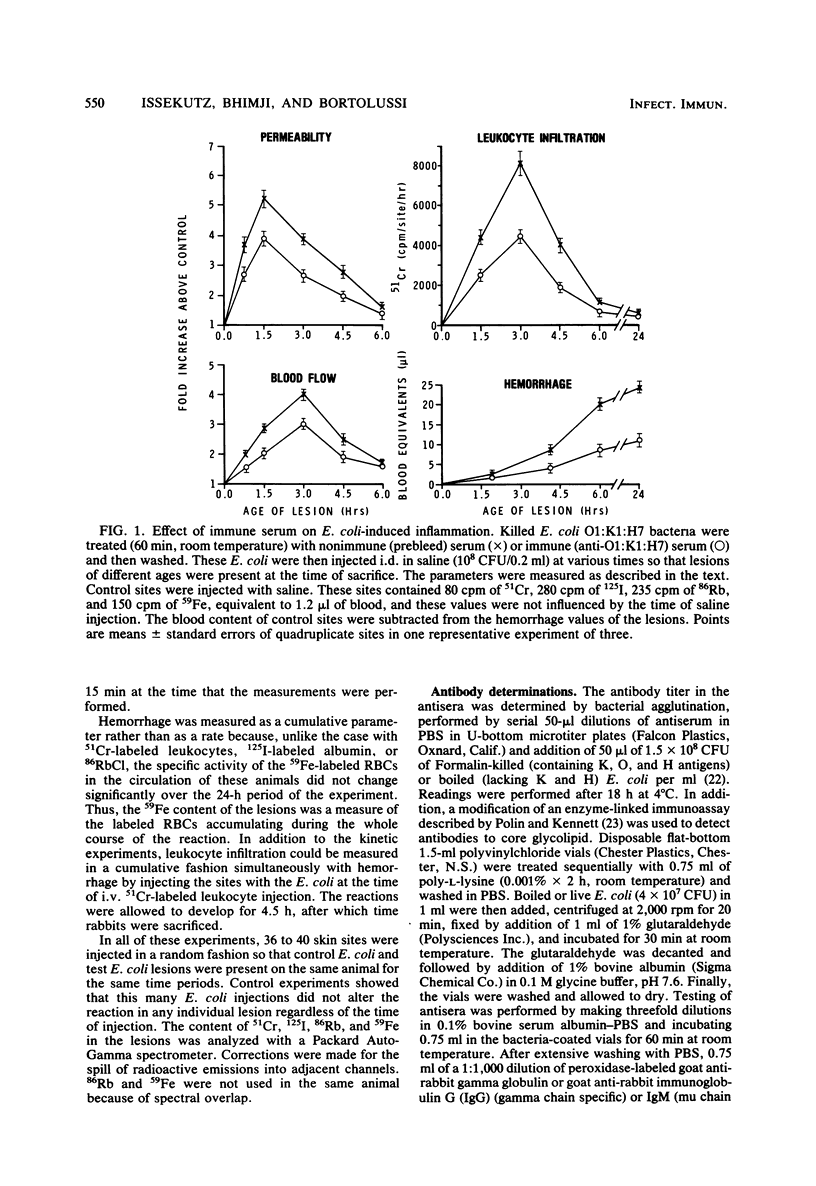
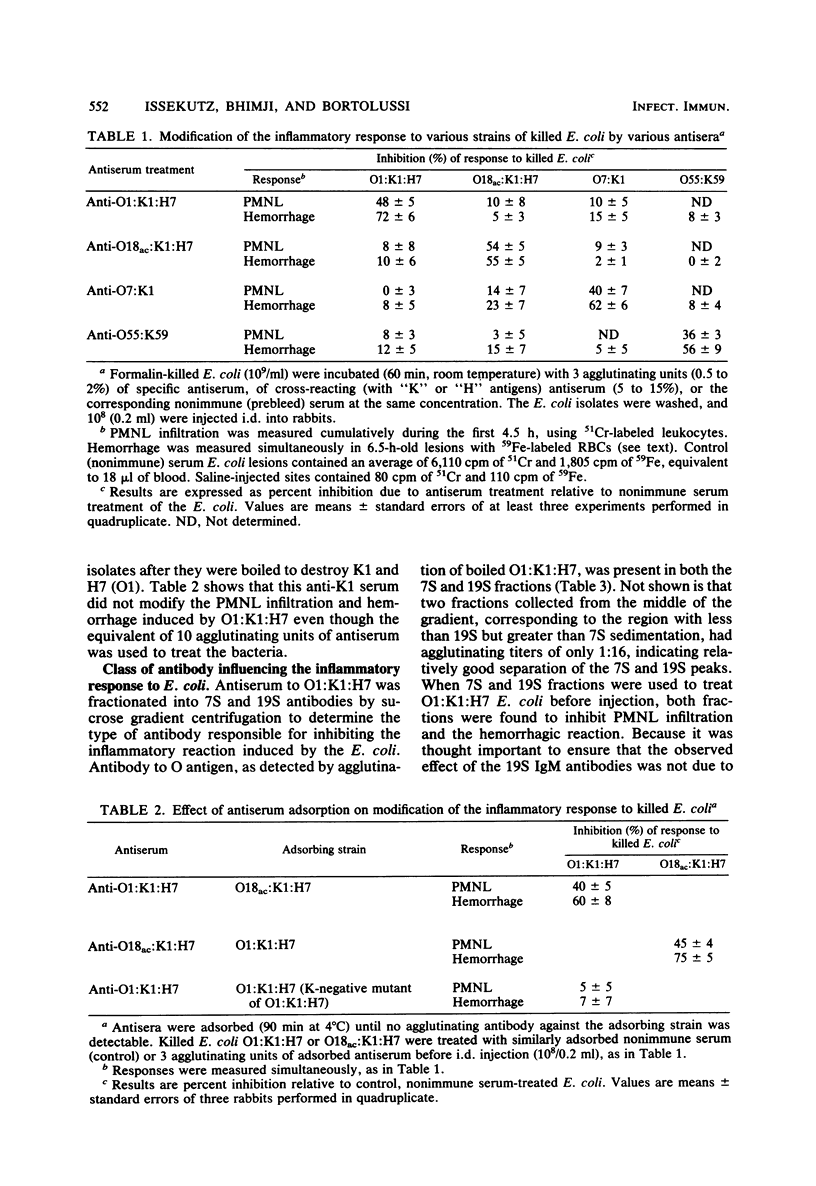
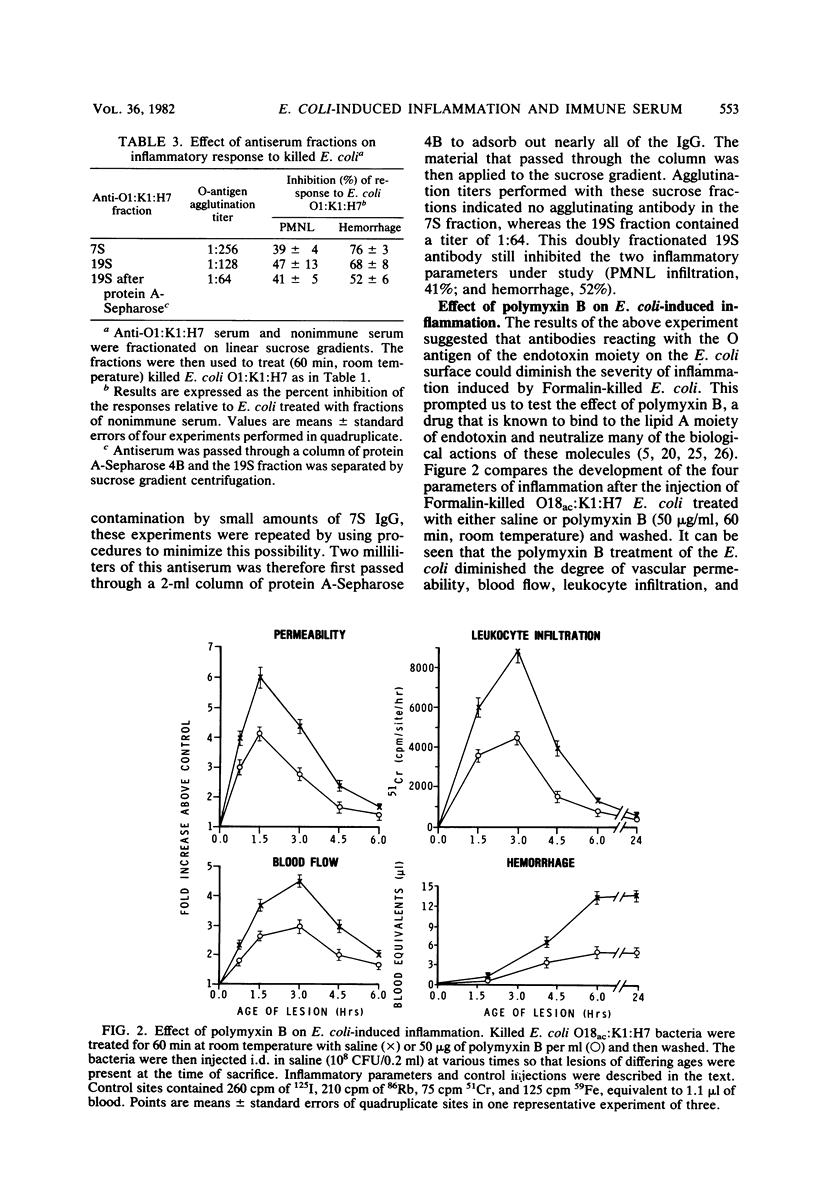
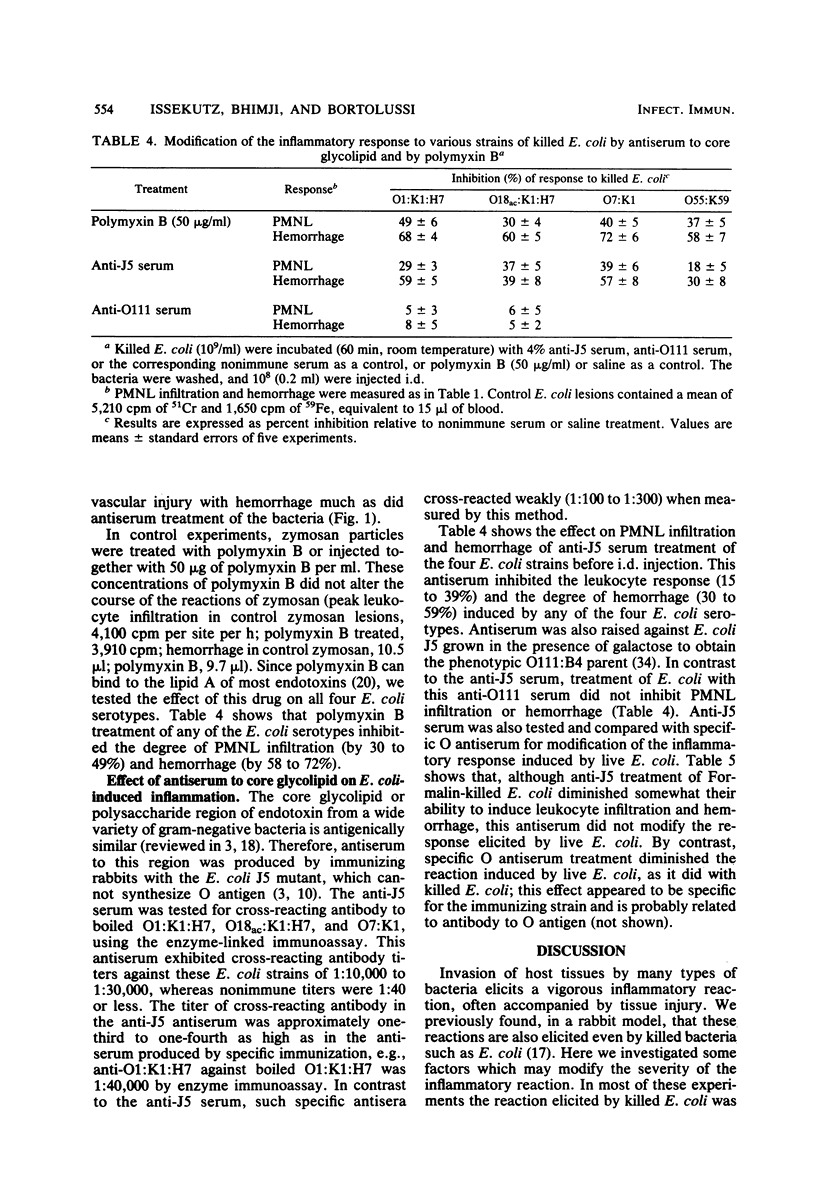
Abstract
Bacterial invasion of the tissues often stimulates a vigorous inflammatory reaction, which may limit the spread of microorganisms but may also be accompanied by serious vascular injury and tissue damage. We previously studied the inflammatory reaction induced by the injection of killed Escherichia coli into rabbit skin, a model suitable for the quantitation of various parameters of inflammation. Here we report the effect of immune serum treatment of the E. coli on their capacity to induce inflammation and vascular injury. Injection of killed E. coli treated with immune serum elicited a reaction which had a smaller increase in vascular permeability (protein exudation), measured with 125I-labeled albumin, less increase in blood flow, measured with 86RbCl, less leukocyte infiltration, measured with 51Cr-labeled leukocytes, and a lesser degree of hemorrhage, measured with 59Fe-labeled erythrocytes, than E. coli treated with nonimmune serum. Crossover experiments with four different E. coli serotypes and four different antisera indicated that antibody to specific O antigens or a related antigen, but not to K or H antigen, was important for modifying the inflammatory response. Treatment of four different E. coli serotypes with antiserum to “core” glycolipid, produced by immunization with the E. coli J5 mutant, inhibited the inflammatory response to all four E. coli serotypes. Finally, treatment of killed E. coli with polymyxin B also inhibited their inflammation-inducing potential. These results suggest that it may be possible to diminish the magnitude of local vascular and tissue injury associated with E. coli infections by the use of antisera or polymyxin B, which bind to endotoxin on the E. coli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L. Neutrophil dysfunction associated with states of chronic and recurrent infection. Pediatr Clin North Am. 1980 May;27(2):377–401. doi: 10.1016/s0031-3955(16)33857-3. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Krishnan C., Armstrong D., Tovichayathamrong P. Prognosis for survival in neonatal meningitis: clinical and pathologic review of 52 cases. Can Med Assoc J. 1978 Jan 21;118(2):165–168. [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Ziegler E. J., Douglas H., McCutchan J. A. Antibody to cell wall glycolipid of Gram-negative bacteria: induction of immunity to bacteremia and endotoxemia. J Infect Dis. 1977 Aug;136 (Suppl):S167–S173. doi: 10.1093/infdis/136.supplement.s167. [DOI] [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Bell B. M. Endotoxin-induced intravascular coagulation: prevention with polymyxin B sulfate. J Lab Clin Med. 1971 May;77(5):802–810. [PubMed] [Google Scholar]
- DODGE P. R., SWARTZ M. N. BACTERIAL MENINGITIS--A REVIEW OF SELECTED ASPECTS. II. SPECIAL NEUROLOGIC PROBLEMS, POSTMENINGITIC COMPLICATIONS AND CLINICOPATHOLOGICAL CORRELATIONS. N Engl J Med. 1965 May 13;272:1003–CONCL. doi: 10.1056/NEJM196505132721906. [DOI] [PubMed] [Google Scholar]
- Faulkner R. S., Gough D. A. Rubella 1974 and its aftermath, congenital rubella syndrome. Can Med Assoc J. 1976 Jan 24;114(2):115–119. [PMC free article] [PubMed] [Google Scholar]
- Heath E. C., Mayer R. M., Edstrom R. D., Beaudreau C. A. Structure and biosynthesis of the cell wall lipopolysaccharide of Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):315–333. doi: 10.1111/j.1749-6632.1966.tb52374.x. [DOI] [PubMed] [Google Scholar]
- Herman P. G., Lyonnet D., Fingerhut R., Tuttle R. N. Regional blood flow to the lymph node during the immune response. Lymphology. 1976 Sep;9(3):101–104. [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S. Role for endotoxin in the leukocyte infiltration accompanying Escherichia coli inflammation. Infect Immun. 1982 May;36(2):558–566. doi: 10.1128/iai.36.2.558-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980 Mar;42(3):310–317. [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of rabbit C5ades arg: probable role of polymorphonuclear leucocyte lysosomes. Clin Exp Immunol. 1980 Sep;41(3):512–520. [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of zymosan-activated plasma. Clin Exp Immunol. 1980 Sep;41(3):505–511. [PMC free article] [PubMed] [Google Scholar]
- Ito J. I., Jr, Wunderlich A. C., Lyons J., Davis C. E., Guiney D. G., Braude A. I. Role of magnesium in the enzyme-linked immunosorbent assay for lipopolysaccharides of rough Escherichia coli strain J5 and Neisseria gonorrhoeae. J Infect Dis. 1980 Oct;142(4):532–537. doi: 10.1093/infdis/142.4.532. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Burrowes C. E., Movat H. Z. The quantitation of hemorrhage in the skin. Measurement of hemorrhage in the microcirculation in inflammatory lesions and related phenomena. Proc Soc Exp Biol Med. 1980 Jan;163(1):126–131. doi: 10.3181/00379727-163-40733. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Movat H. Z. Kinetics of acute inflammation induced by E coli in rabbits. Quantitation of blood flow, enhanced vascular permeability, hemorrhage, and leukocyte accumulation. Am J Pathol. 1980 Feb;98(2):485–498. [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Mize S. G. A controlled study of intrathecal antibiotic therapy in gram-negative enteric meningitis of infancy. Report of the neonatal meningitis cooperative study group. J Pediatr. 1976 Jul;89(1):66–72. doi: 10.1016/s0022-3476(76)80929-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin R. A., Kennett R. Use of monoclonal antibodies in an enzyme-linked inhibition assay for rapid detection of streptococcal antigen. J Pediatr. 1980 Oct;97(4):540–544. doi: 10.1016/s0022-3476(80)80005-9. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Hill R. B., Jr Neutralization of the Shwartzman reactions by polymyxins B. J Immunol. 1967 Sep;99(3):564–569. [PubMed] [Google Scholar]
- Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967 Apr;93(4):1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Stevens P., Chu C. L., Young L. S. K-1 antigen content and the presence of an additional sialic acid-containing antigen among bacteremic K-1 Escherichia coli: correlation with susceptibility to opsonophagocytosis. Infect Immun. 1980 Sep;29(3):1055–1061. doi: 10.1128/iai.29.3.1055-1061.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Huang S. N., Welch W. D., Young L. S. Restricted complement activation by Escherichia coli with the K-1 capsular serotype: a possible role in pathogenicity. J Immunol. 1978 Dec;121(6):2174–2180. [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]



