Abstract
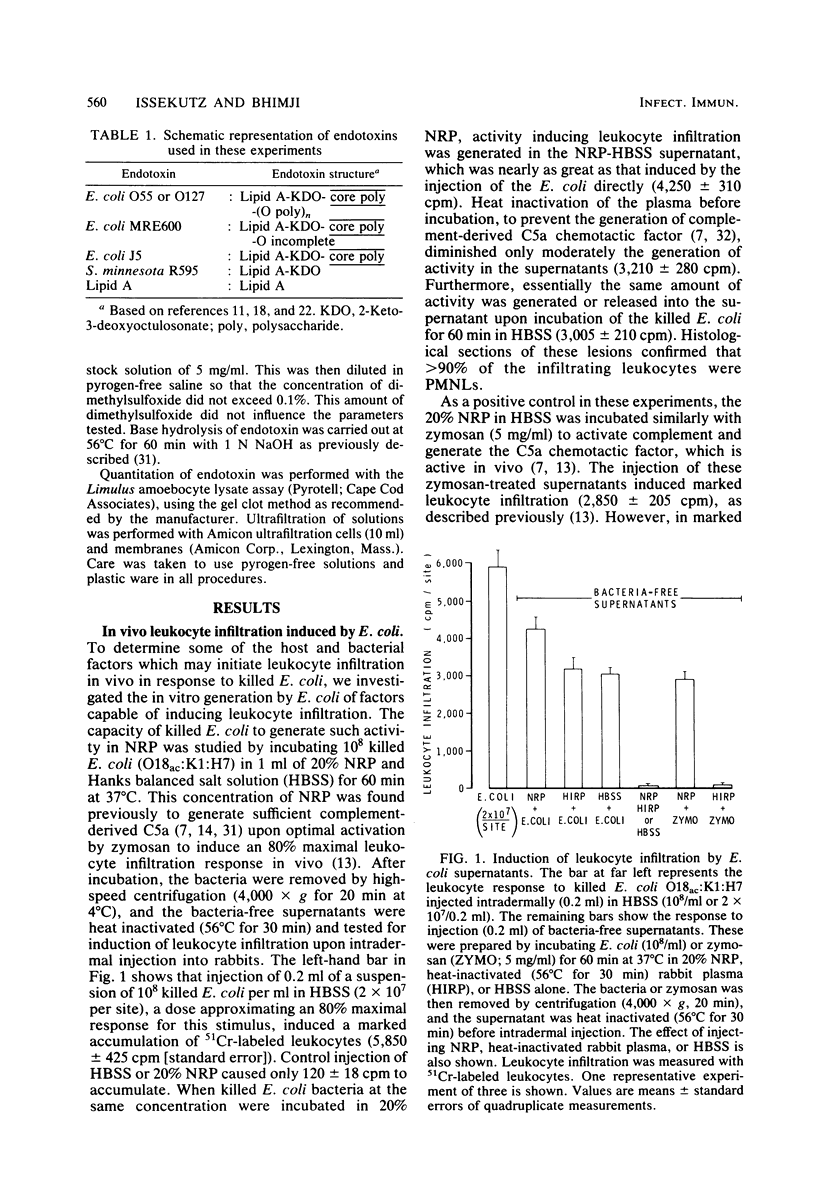
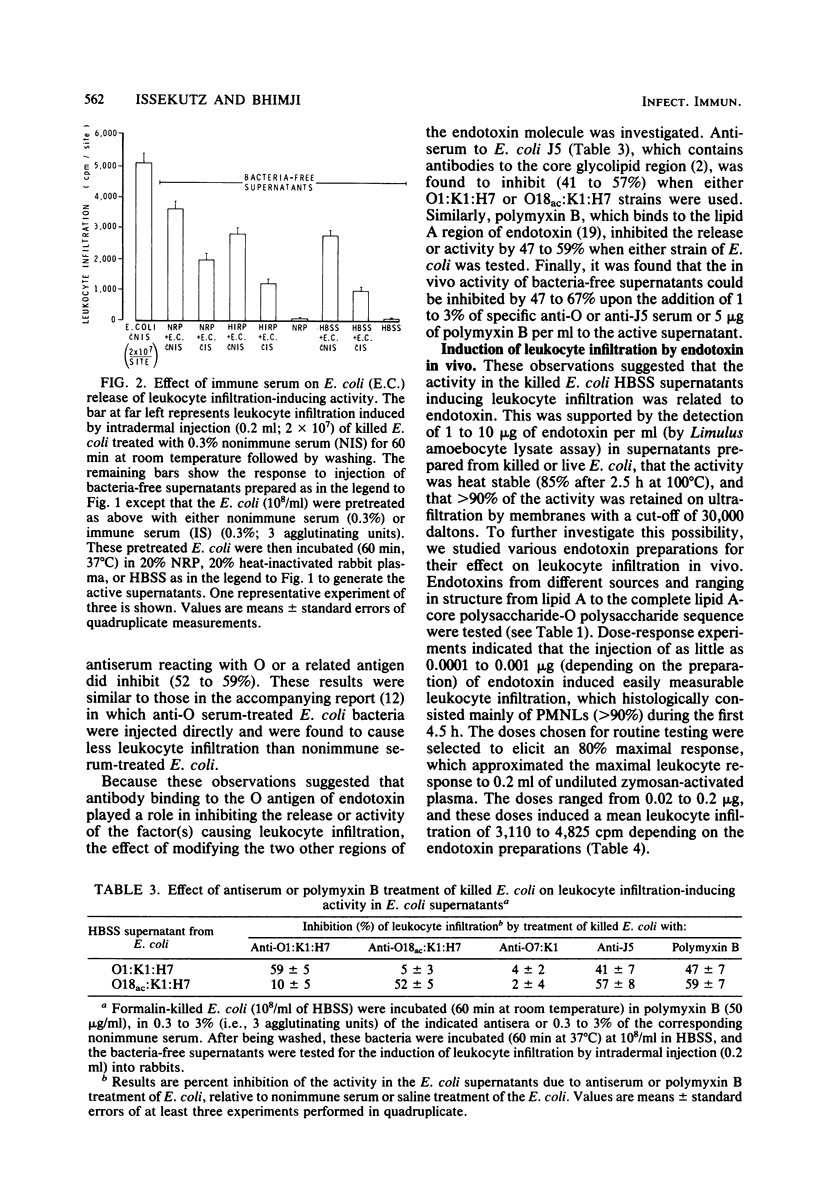
Escherichia coli organisms induce polymorphonuclear leukocyte (PMNL) infiltration during clinical infection and also in a rabbit dermal model of inflammation. We investigated the factors which may mediate this host response to E. coli. In vitro incubation of Formalin-killed E. coli in heat-inactivated rabbit plasma or balanced salt solution generated in the supernatant factors which induced in vivo PMNL infiltration upon intradermal injection into rabbits. However, these supernatants, in the presence or absence of plasma, did not induce PMNL migration in vitro. The in vivo activity was stable at 100°C and of high molecular weight (30,000). Antiserum to O antigen or to core glycolipid, but not to K or H antigen, as well as polymyxin B inhibited the release or activity of these E. coli-derived factors. The intradermal injection of 0.02 to 0.2 μg of four different endotoxin preparations or lipid A also induced marked PMNL infiltration in vivo. However, these preparations did not stimulate PMNL migration in vitro and failed to generate chemotactic activity in plasma except at very high concentrations (500 μg/ml). Anti-O serum inhibited PMNL infiltration induced by endotoxins with the corresponding O antigen and anti-core glycolipid serum inhibited all four endotoxins tested, whereas polymyxin B inhibited the activity of the endotoxins as well as that of lipid A. Base hydrolysis of endotoxin abolished PMNL infiltration. It is concluded that (i) endotoxin shed from E. coli (killed or live) may be one factor mediating the PMNL infiltration induced by this organism, (ii) endotoxin probably acts independent of in vivo complement activation, (iii) the activity is dependent on the lipid A moiety, and (iv) antibody binding to O or core glycolipid antigens can modify endotoxin so as to diminish its capacity to induce PMNL infiltration in vivo.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bortolussi R., Krishnan C., Armstrong D., Tovichayathamrong P. Prognosis for survival in neonatal meningitis: clinical and pathologic review of 52 cases. Can Med Assoc J. 1978 Jan 21;118(2):165–168. [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Ziegler E. J., Douglas H., McCutchan J. A. Antibody to cell wall glycolipid of Gram-negative bacteria: induction of immunity to bacteremia and endotoxemia. J Infect Dis. 1977 Aug;136 (Suppl):S167–S173. doi: 10.1093/infdis/136.supplement.s167. [DOI] [PubMed] [Google Scholar]
- Corrigan J. J., Jr, Bell B. M. Endotoxin-induced intravascular coagulation: prevention with polymyxin B sulfate. J Lab Clin Med. 1971 May;77(5):802–810. [PubMed] [Google Scholar]
- Cutler J. E., Munoz J. J. A simple in vitro method for studies on chemotaxis. Proc Soc Exp Biol Med. 1974 Nov;147(2):471–474. doi: 10.3181/00379727-147-38367. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Sun F. F. Generation of unique mono-hydroxy-eicosatetraenoic acids from arachidonic acid by human neutrophils. J Exp Med. 1979 Aug 1;150(2):406–411. doi: 10.1084/jem.150.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath E. C., Mayer R. M., Edstrom R. D., Beaudreau C. A. Structure and biosynthesis of the cell wall lipopolysaccharide of Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):315–333. doi: 10.1111/j.1749-6632.1966.tb52374.x. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980 Mar;42(3):310–317. [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of zymosan-activated plasma. Clin Exp Immunol. 1980 Sep;41(3):505–511. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Movat H. Z. Kinetics of acute inflammation induced by E coli in rabbits. Quantitation of blood flow, enhanced vascular permeability, hemorrhage, and leukocyte accumulation. Am J Pathol. 1980 Feb;98(2):485–498. [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Niemetz J., Morrison D. C. Lipid A as the biologically active moiety in bacterial endotoxin (LPS)-initiated generation of procoagulant activity by peripheral blood leukocytes. Blood. 1977 Jun;49(6):947–956. [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Stepney R. J., Higgs G. A., Eakins K. E. Chemokinetic activity of arachidonic and lipoxygenase products on leuocyctes of different species. Prostaglandins. 1980 Aug;20(2):411–418. doi: 10.1016/s0090-6980(80)80058-x. [DOI] [PubMed] [Google Scholar]
- Polin R. A., Kennett R. Use of monoclonal antibodies in an enzyme-linked inhibition assay for rapid detection of streptococcal antigen. J Pediatr. 1980 Oct;97(4):540–544. doi: 10.1016/s0022-3476(80)80005-9. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Hill R. B., Jr Neutralization of the Shwartzman reactions by polymyxins B. J Immunol. 1967 Sep;99(3):564–569. [PubMed] [Google Scholar]
- Sahu S., Lynn W. S. Lipid chemotaxins isolated from culture filtrates of Escherichia coli and from oxidized lipids. Inflammation. 1977 Mar;2(1):47–54. doi: 10.1007/BF00920874. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Showell H. V., Corcoran B. A., Ward P. A., Smith E., Becker E. L. The isolation and partial characterization of neutrophil chemotactic factors from Escherichia coli. J Immunol. 1975 Jun;114(6):1831–1837. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Cohen S., Bigazzi P. E., Kurasuji T., Amsden A. Inflammatory mediators in culture filtrates of Escherichia coli. Am J Pathol. 1975 Nov;81(2):389–400. [PMC free article] [PubMed] [Google Scholar]



