Abstract
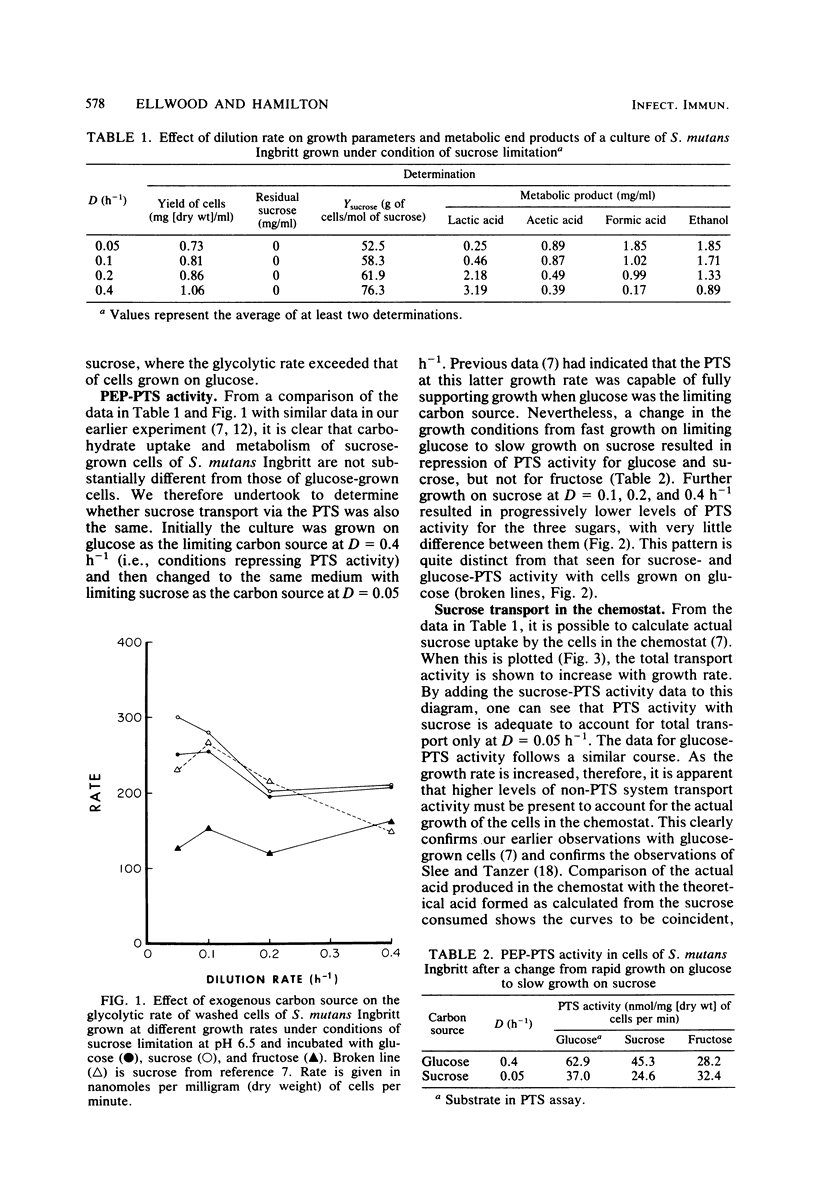
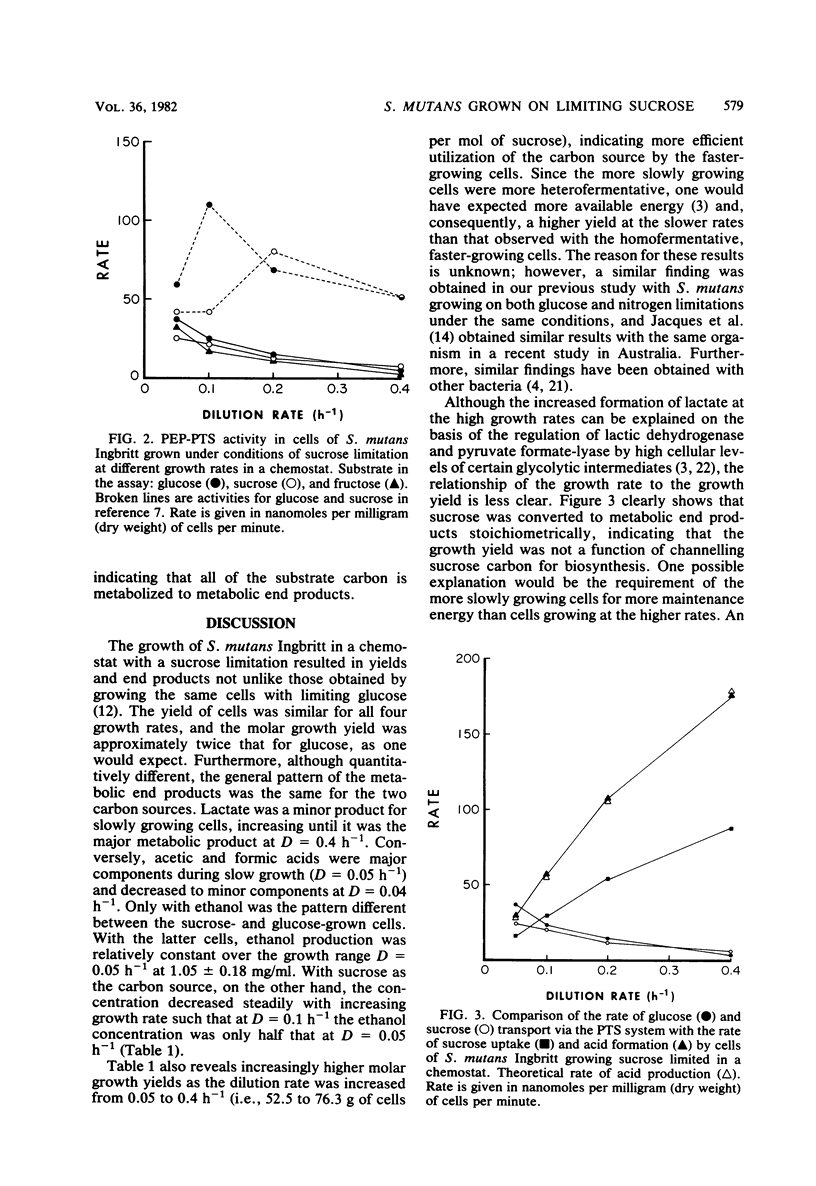
Growth of Streptococcus mutans Ingbritt on limiting sucrose in a chemostat at dilution rates of 0.05 to 0.4 h-1 (mean generation time, 14 to 1.7 h) resulted in a heterofermentative pattern of metabolic end products. During fast growth, lactic acid was the major end product, whereas at slower growth rates, acetic and formic acids, as well as ethanol, increased to be major end products. The patterns obtained were similar to those seen with the same organism growing on glucose. The glycolytic rate by washed cells was maximum at the lowest dilution rates and decreased as the cells were made to grow faster. Transport of sucrose, glucose, and fructose via the phosphoenolpyruvate phosphotransferase system (PTS) was repressed during growth on sucrose after growth on glucose. Uptake rates suggested that sucrose was transported in the PTS as the intact disaccharide. Comparison of the rate of sugar uptake in the chemostat with the rate of PTS activity in the cells at each growth rate indicated that the PTS was capable of supporting growth only at a dilution rate of 0.05 h-1. Growth on sucrose at faster growth rates required the activity of a second transport system, supporting our earlier observations with glucose that S. mutans contains at least two sugar transport systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J., Griffith C. J. Fermentation products and bacterial yields in glucose-limited and nitrogen-limited cultures of streptococci. Arch Oral Biol. 1974 Dec;19(12):1105–1109. doi: 10.1016/0003-9969(74)90238-6. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Nutritional requirements of Streptococcus mutans. Caries Res. 1970;4(4):305–320. doi: 10.1159/000259653. [DOI] [PubMed] [Google Scholar]
- Dalland E., Hofstad T. Growth of Bacteroides fragilis in continuous culture and in batch cultures at controlled pH. Appl Microbiol. 1974 Nov;28(5):856–860. doi: 10.1128/am.28.5.856-860.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Hunter J. R., Longyear V. M. Growth of Streptococcus mutans in a chemostat. Arch Oral Biol. 1974 Aug;19(8):659–664. doi: 10.1016/0003-9969(74)90134-4. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRENBY T. H. The effects of some carbohydrates on experimental dental caries in the rat. Arch Oral Biol. 1963 Jan-Feb;8:27–30. doi: 10.1016/0003-9969(63)90088-8. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON B. E., QUENSEL C. E., LANKE L. S., LUNDQVIST C., GRAHNEN H., BONOW B. E., KRASSE B. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954 Sep;11(3-4):232–264. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Phipps P. J., Ellwood D. C. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect Immun. 1979 Dec;26(3):861–869. doi: 10.1128/iai.26.3.861-869.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Campbell L. K., Knox K. W., Evans J. D., Wicken A. J. Effect of carbohydrate source and growth conditions on the production of lipoteichoic acid by Streptococcus mutans Ingbritt. Infect Immun. 1979 Dec;26(3):1079–1087. doi: 10.1128/iai.26.3.1079-1087.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem J. 1972 Aug;128(5):1339–1344. doi: 10.1042/bj1281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Rowan J., Straffon L. H., Loos P. J. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975 Jun;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E. Sucrose, the arch criminal of dental caries. Odontol Revy. 1967;18(4):373–386. [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Effect of growth conditions on sucrose phosphotransferase activity of Streptococcus mutans. Infect Immun. 1980 Mar;27(3):922–927. doi: 10.1128/iai.27.3.922-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in five serotypes of Streptococcus mutans. Infect Immun. 1979 Nov;26(2):783–786. doi: 10.1128/iai.26.2.783-786.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


