Abstract
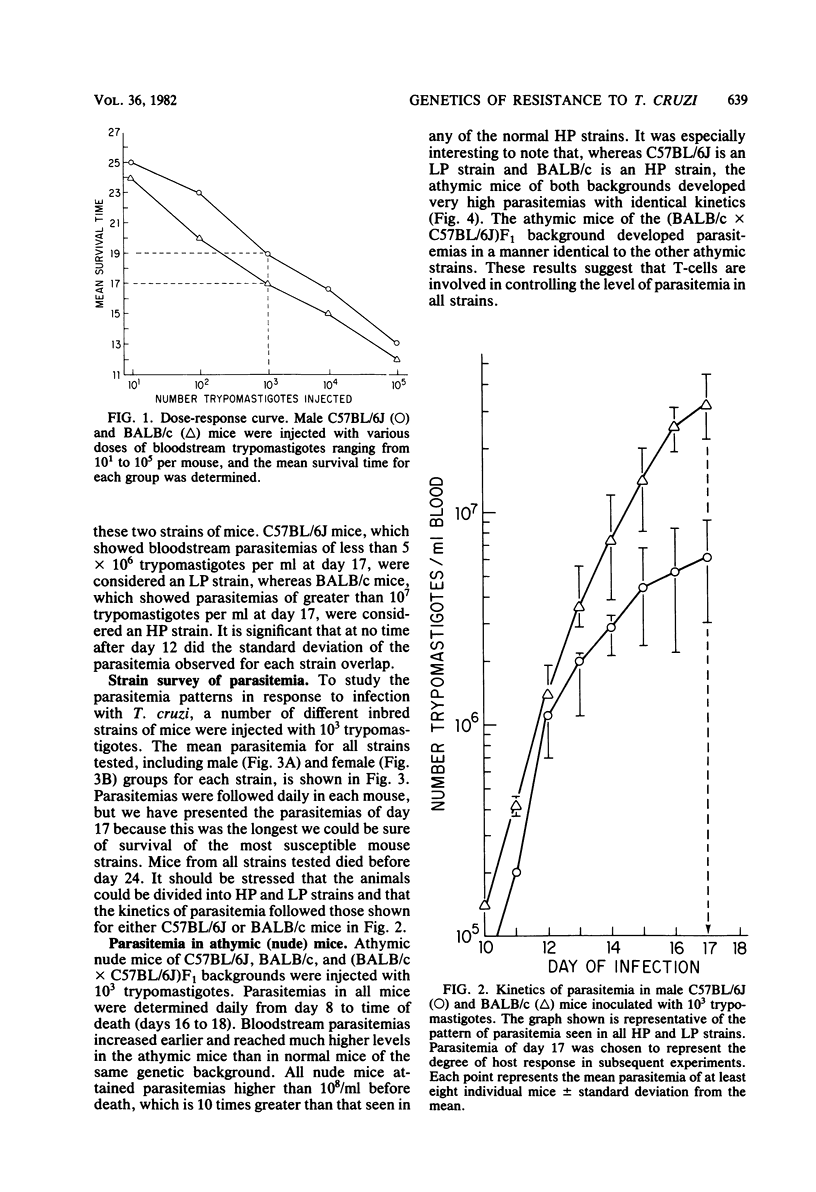
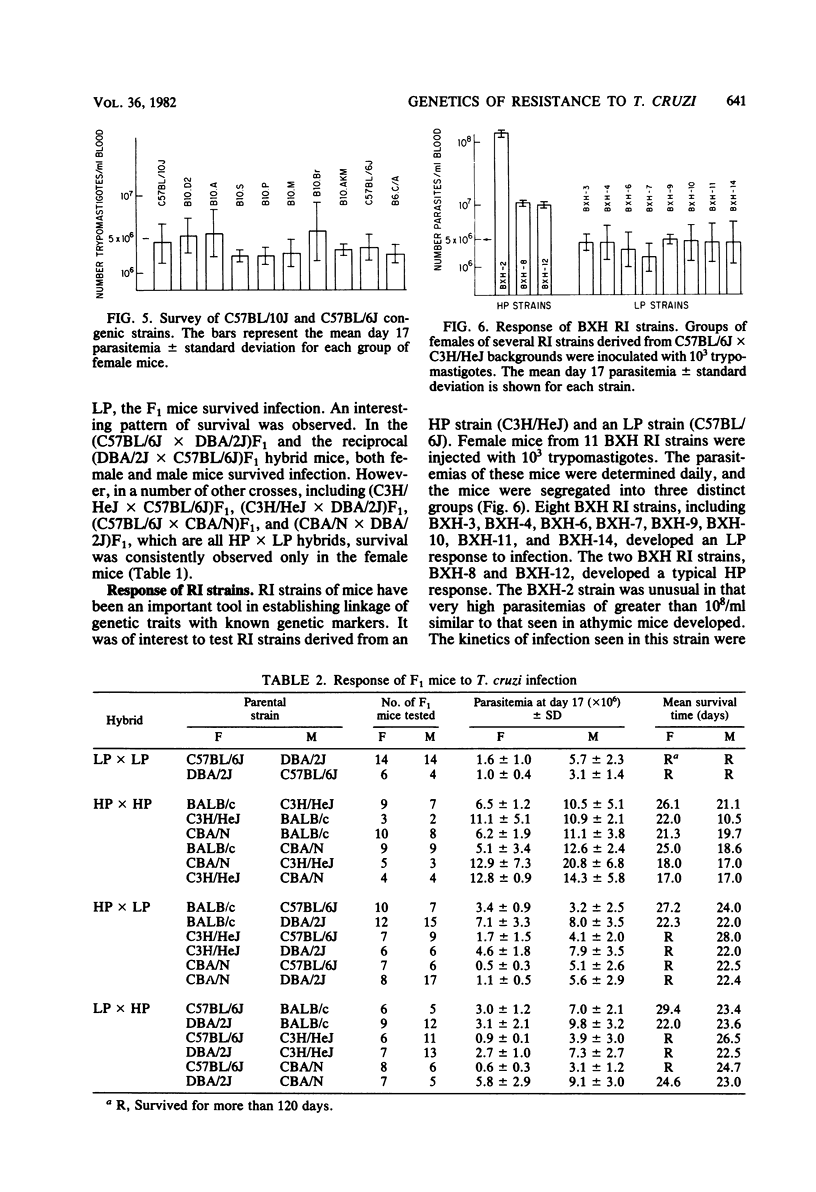
Inbred strains of mice can be divided into two groups based on the level of parasitemia which develops after injection with 10(3) trypomastigotes of Trypanosoma cruzi (Peru). Strains which developed parasitemias of greater than 10(7) trypomastigotes per ml by day 17, including C3H/HeJ, BALB/c, and CBA/N mice, were termed high parasitemia strains. Low parasitemia strains, including C57BL/6J and DBA/2J mice, developed parasitemias of less than 5 x 10(6) trypomastigotes per ml by day 17 of infection. Congenic mice from C57BL/10J, C57BL/6J, and BALB/c backgrounds which differed at the H-2 region were injected with 10(3) trypomastigotes to determine the effect of the H-2 locus on response to infection. The H-2 locus had no effect on the level of parasitemia attained during infection. However, one strain, B10.S (H-2s), was unusual in that most of the mice survived infection. The results of infection of F1 hybrid progeny with T. cruzi (Peru) suggest that the low parasitemia response in inherited in a dominant manner and that survival may be influenced by several other genes. The response to T. cruzi infection in inbred mice, as measured by parasitemia and survival time, was influenced by several genes. One or more genes, located outside the H-2 region, were involved in regulating the level of parasitemia reached during infection. Another H-2-linked gene(s) was involved in survival of the infection and appeared to be unique to the H-2s haplotype.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. W. Recombinant-inbred strains. An aid to finding identity, linkage, and function of histocompatibility and other genes. Transplantation. 1971 Mar;11(3):325–327. doi: 10.1097/00007890-197103000-00013. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Zeledon R. Comparison of infectivity of strains of Trypanosoma cruzi (Chagas, 1909). J Parasitol. 1970 Aug;56(4):663–670. [PubMed] [Google Scholar]
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. S., Kuhn R. E., Rowland E. C. Suppression of humoral responses during Trypanosoma cruzi infections in mice. Infect Immun. 1978 Oct;22(1):155–160. doi: 10.1128/iai.22.1.155-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso A. P., Jarvinen J. A. Experimental Chagas' disease in complement-deficient mice and guinea pigs. Infect Immun. 1980 May;28(2):434–440. doi: 10.1128/iai.28.2.434-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Hedrick S. M., Watson J. D. Genetic control of the immune response to collagen. I. Quantitative determination of response levels by multiple I-region genes. J Immunogenet. 1980 Jun;7(3):271–283. doi: 10.1111/j.1744-313x.1980.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Hoff R. A method for counting and concentrating living Trypanosoma cruzi in blood lysed with ammonium chloride. J Parasitol. 1974 Jun;60(3):527–528. [PubMed] [Google Scholar]
- Khoury E. L., Ritacco V., Cossio P. M., Laguens R. P., Szarfman A., Diez C., Arana R. M. Circulating antibodies to peripheral nerve in American trypanosomiasis (Chagas' disease). Clin Exp Immunol. 1979 Apr;36(1):8–15. [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Pienkowski M. M. Thymus-dependent control of host defense mechanisms against Trypanosoma cruzi infection. Infect Immun. 1979 Apr;24(1):117–120. doi: 10.1128/iai.24.1.117-120.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress Y., Tanowitz H., Bloom B., Wittner M. Trypanosoma cruzi: infection of normal and activated mouse macrophages. Exp Parasitol. 1977 Apr;41(2):385–396. doi: 10.1016/0014-4894(77)90110-2. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976 Mar;116(3):755–760. [PubMed] [Google Scholar]
- Lanar D. E. Growth and differentiation of Trypanosoma cruzi cultivated with a Triatoma infestans embryo cell line. J Protozool. 1979 Aug;26(3):457–462. doi: 10.1111/j.1550-7408.1979.tb04653.x. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Gordon S., Cohn Z. Trypanosoma cruzi: modification of macrophage function during infection. J Exp Med. 1977 Jul 1;146(1):157–171. doi: 10.1084/jem.146.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Trischmann T., Tanowitz H., Wittner M., Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol. 1978 Aug;45(2):160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M. Thymus and lymphohemopoietic cells: their role in T cell maturation in selection of T cells' H-2-restriction-specificity and in H-2 linked Ir gene control. Immunol Rev. 1978;42:224–270. doi: 10.1111/j.1600-065x.1978.tb00264.x. [DOI] [PubMed] [Google Scholar]


