Abstract
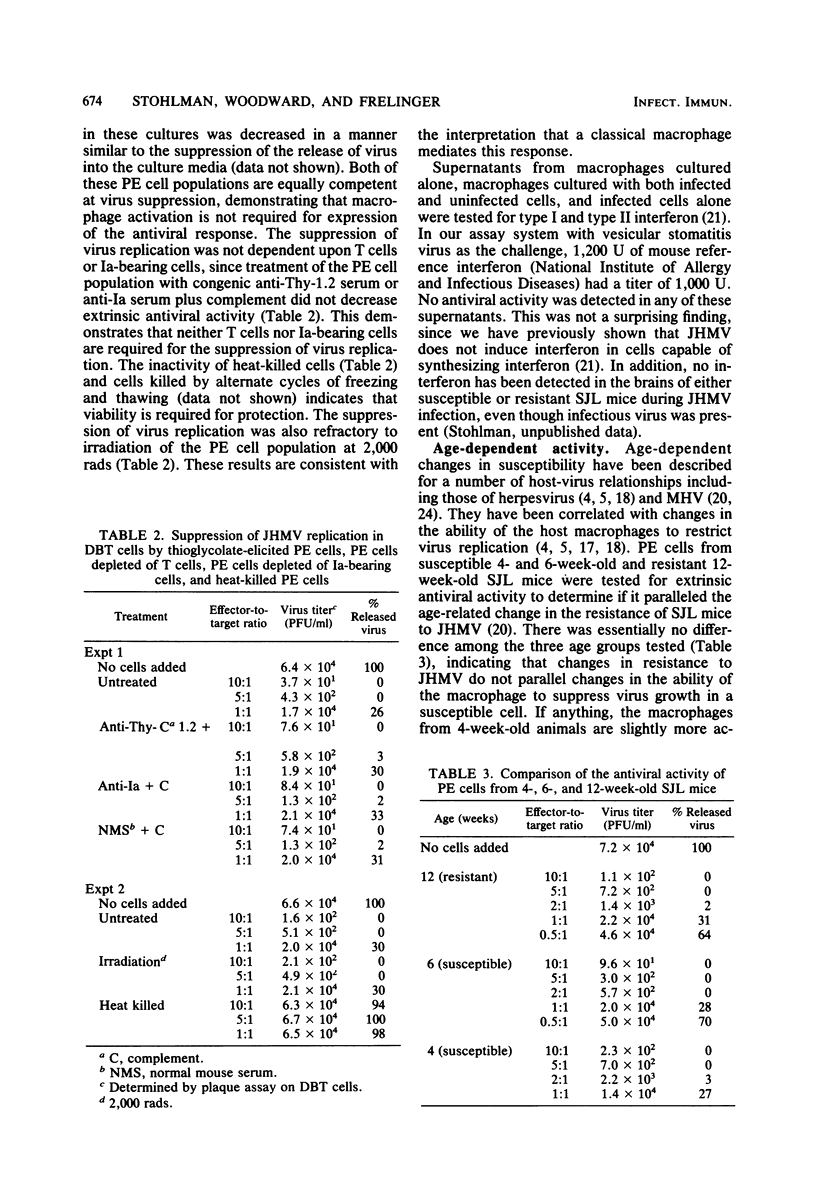
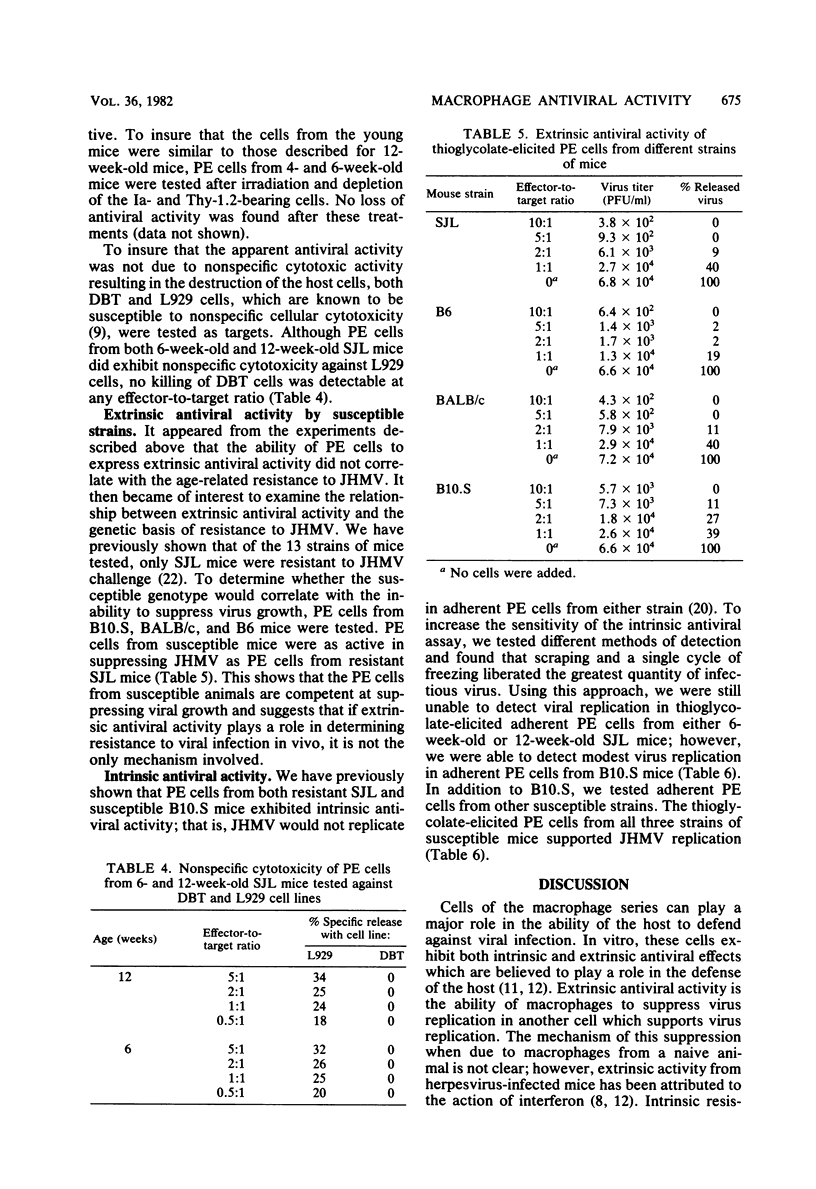
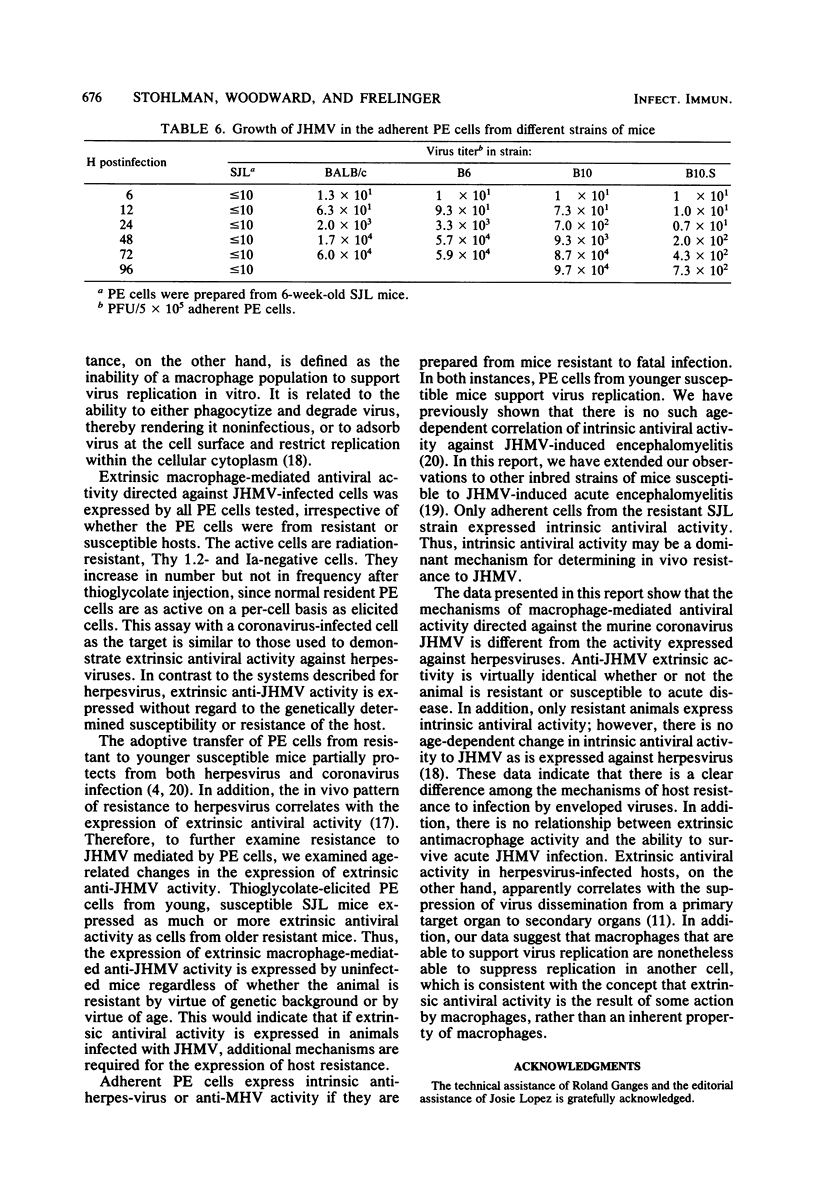
Peritoneal exudate cells from strains of mice both resistant and susceptible to challenge with mouse hepatitis virus strain JHM were examined for extrinsic and intrinsic antiviral activity. Thioglycolate-elicited and resident peritoneal cells from uninfected mice were able to suppress viral growth in a permissive cell. The active cell in both populations is an adherent, radiation-resistant, Thy-1.2 antigen- and Ia antigen-negative cell. The suppression of virus replication was not related to nonspecific cellular cytotoxicity directed against the permissive host cell, and no interferon was detected. The expression of extrinsic antiviral activity was not related to the ability of the host to resist mouse hepatitis virus infection by virtue of either age or genetic background. The expression of intrinsic antiviral activity, on the other hand, correlated with the ability of the host to resist virus challenge, indicating a characteristic distinction between these two in vitro mechanisms of macrophage-mediated antiviral activity with regard to host resistance to viral infection. Further, the ability of a macrophage to support viral replication itself was independent of the ability of the macrophage to suppress virus growth in another cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon R. M., Griffin D. E., McCormick U., Weiner L. P. Mouse hepatitis virus-induced recurrent demyelination. A preliminary report. Arch Neurol. 1975 Jan;32(1):32–35. doi: 10.1001/archneur.1975.00490430054008. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Zisman B., Allison A. C. Macrophages and age-dependent resistance to Herpes simplex virus in mice. J Immunol. 1970 May;104(5):1160–1165. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Stohlman S. A. RNA of mouse hepatitis virus. J Virol. 1978 May;26(2):236–242. doi: 10.1128/jvi.26.2.236-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Leblond E., Oth D., Dupuy J. M. Genetic study of mouse sensitivity to MHV3 infection: influence of the H-2 complex. J Immunol. 1979 Apr;122(4):1359–1362. [PubMed] [Google Scholar]
- Martinez D., Lynch R. J., Meeker J. B., Field A. K. Macrophage dependence of polyriboinosinic acid-polyribocytidylic acid-induced resistance to herpes simplex virus infection in mice. Infect Immun. 1980 Apr;28(1):147–153. doi: 10.1128/iai.28.1.147-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Tucker R. W., Sanford K. K., Leonard E. J. Interaction of BCG-activated macrophages with neoplastic and nonneoplastic cell lines in vitro : quantitation of the cytotoxic reaction by release of tritiated thymidine from prelabeled target cells. J Natl Cancer Inst. 1975 May;54(5):1177–1184. doi: 10.1093/jnci/54.5.1177. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Gould J. The role of macrophages in mice infected with murine cytomegalovirus. J Gen Virol. 1978 Oct;41(1):143–153. doi: 10.1099/0022-1317-41-1-143. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Morse S. S., McGeorge M. G. Macrophage extrinsic antiviral activity during herpes simplex virus infection. J Gen Virol. 1980 Feb;46(2):291–300. doi: 10.1099/0022-1317-46-2-291. [DOI] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978 Oct 13;44(1):63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhuber J. E., Frelinger J. A., Dugan E., Coutinho A., Shreffler D. C. Effects of anti-Ia serum on mitogenic responses. I. Inhibition of the proliferative response to B cell mitogen, LPS, by specific anti-Ia sera. J Immunol. 1975 Dec;115(6):1672–1676. [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Defense mechanisms against infectious bovine rhinotracheitis virus: inhibition of virus infection by murine macrophages. Infect Immun. 1975 Mar;11(3):505–511. doi: 10.1128/iai.11.3.505-511.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabach A. J., Martinez D., Field A. K., Tytell A. A. Resistance of C58 mice to primary systemic herpes simplex virus infection: macrophage dependence and T-cell independence. Infect Immun. 1979 Nov;26(2):615–620. doi: 10.1128/iai.26.2.615-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman S. A., Frelinger J. A., Weiner L. P. Resistance to fatal central nervous system disease by mouse hepatitis virus, strain JHM. II. Adherent cell-mediated protection. J Immunol. 1980 Apr;124(4):1733–1739. [PubMed] [Google Scholar]
- Stohlman S. A., Sakaguchi A. Y., Hiti A. Interferon production and activity in mouse neuroblastoma cells. Arch Virol. 1978;57(1):91–96. doi: 10.1007/BF01315640. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Weiner L. P. Chronic central nervous system demyelination in mice after JHM virus infection. Neurology. 1981 Jan;31(1):38–44. doi: 10.1212/wnl.31.1.38. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Weiner L. P. Stability of neurotropic mouse hepatitis virus (JHM strain) during chronic infection of neuroblastoma cells. Arch Virol. 1978;57(1):53–61. doi: 10.1007/BF01315637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Yamada A., Fujiwara K. Factors involved in the age-dependent resistance of mice infected with low-virulence mouse hepatitis virus. Arch Virol. 1979;62(4):333–340. doi: 10.1007/BF01318107. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C. Correlation of persistent mouse hepatitis virus (MHV-3) infection with its effect on mouse macrophage cultures. Arch Virol. 1976;50(4):279–285. doi: 10.1007/BF01317953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]



