Abstract
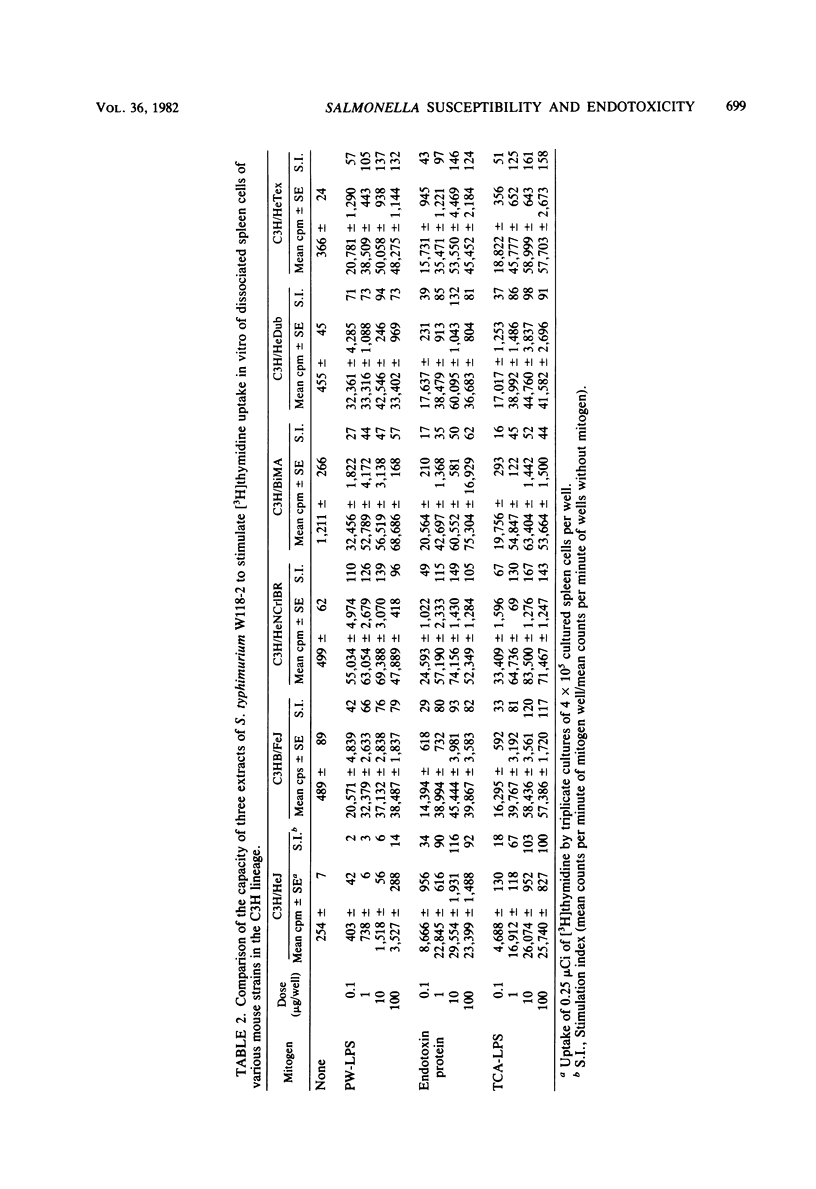
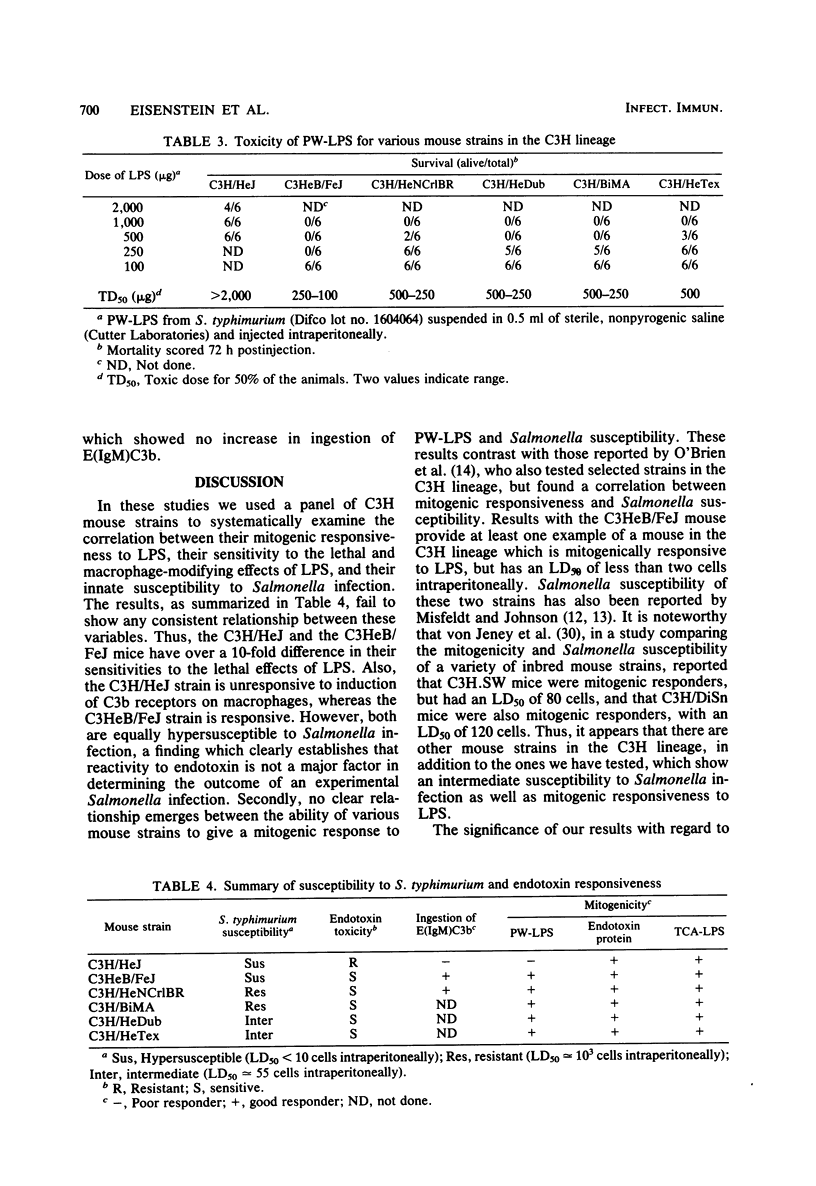
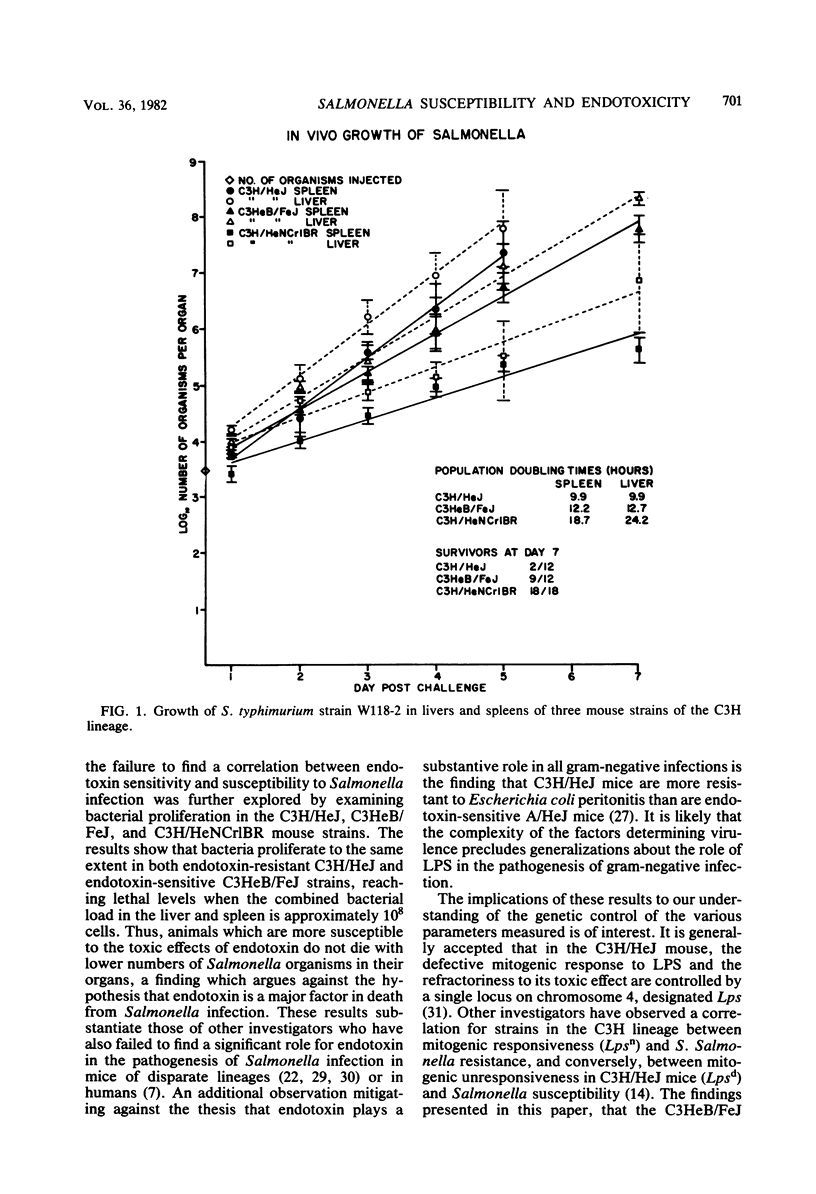
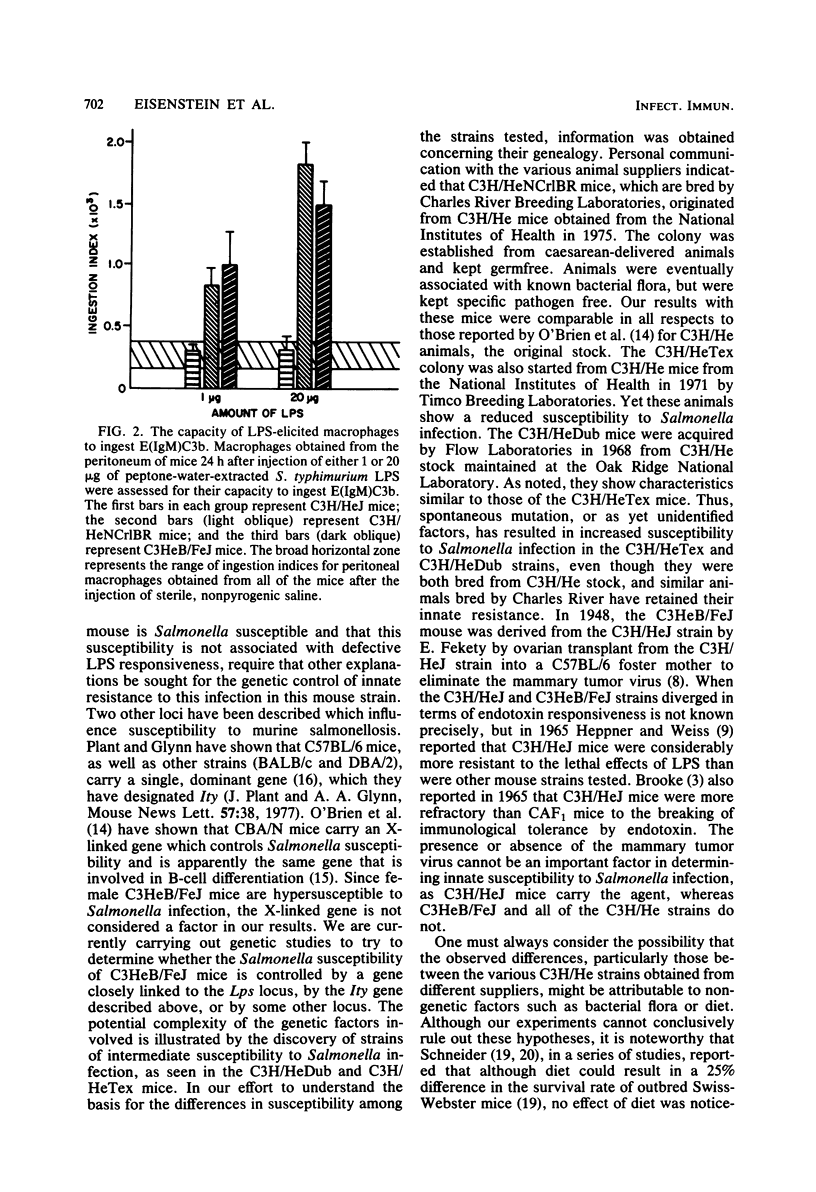
Studies of various mouse strains in the C3H lineage have shown that there is no correlation between innate susceptibility to Salmonella infection and sensitivity to the toxic or mitogenic effects of lipopolysaccharide (LPS). C3H/HeNCrlBR mice were Salmonella resistant, but sensitive to the toxic and mitogenic effects of LPS, whereas C3HeB/FeJ mice were Salmonella susceptible as the C3H/HeJ mice, yet were mitogenically responsive to LPS and sensitive to its lethal effects. Furthermore, other mouse strains (C3H/HeTex and C3H/HeDub) displayed intermediate susceptibility to Salmonella infection and were responders to the mitogenic and toxic effects of LPS. These results are interpreted to mean that endotoxemia cannot be a major factor in the pathogenesis of Salmonella infection and provide evidence for the involvement of multiple factors in the control of innate resistance to Salmonella infection in mice of the C3H lineage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. S. Conversion of immunological paralysis to immunity by endotoxin. Nature. 1965 May 8;206(984):635–636. doi: 10.1038/206635a0. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. The effect of the intestinal flora on the growth rate of mice, and on their susceptibility to experimental infections. J Exp Med. 1960 Mar 1;111:407–417. doi: 10.1084/jem.111.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Glode L. M., Rosenstreich D. L. Genetic control of B cell activation by bacterial lipopolysaccharide is mediated by multiple distinct genes or alleles. J Immunol. 1976 Dec;117(6):2061–2066. [PubMed] [Google Scholar]
- Greisman S. E., Hornick R. B., Wagner H. N., Jr, Woodward W. E., Woodward T. E. The role of endotoxin during typhoid fever and tularemia in man. IV. The integrity of the endotoxin tolerance mechanisms during infection. J Clin Invest. 1969 Apr;48(4):613–629. doi: 10.1172/JCI106020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner G., Weiss D. W. High Susceptibility of Strain A Mice to Endotoxin and Endotoxin-Red Blood Cell Mixtures. J Bacteriol. 1965 Sep;90(3):696–703. doi: 10.1128/jb.90.3.696-703.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Role of endotoxin contamination in ribiosomal vaccines prepared from Salmonella typhimurium. Infect Immun. 1977 Jul;17(1):98–104. doi: 10.1128/iai.17.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Variability of protection in inbred mice induced by a ribosomal vaccine prepared from Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):652–659. doi: 10.1128/iai.14.3.652-659.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol. 1979 Aug;123(2):720–724. [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The susceptibility of mice to bacterial endotoxins. J Exp Med. 1961 Mar 1;113:559–570. doi: 10.1084/jem.113.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senterfitt V. C., Shands J. W., Jr Salmonellosis in mice infected with Mycobacterium tuberculosis BCG. I. Role of endotoxin in infection. J Bacteriol. 1968 Aug;96(2):287–292. doi: 10.1128/jb.96.2.287-292.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic analysis of lymphocyte activation by lipopolysaccharide Endotoxin. Infect Immun. 1976 Jun;13(6):1579–1584. doi: 10.1128/iai.13.6.1579-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic factors in leucocyte responses to endotoxin: further studies in mice. J Immunol. 1969 Jul;103(1):32–38. [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Vas S. I., Roy R. S., Robson H. G. Endotoxin sensitivity of inbred mouse strains. Can J Microbiol. 1973 Jul;19(7):767–769. doi: 10.1139/m73-125. [DOI] [PubMed] [Google Scholar]
- Watson J., Kelly K., Largen M., Taylor B. A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978 Feb;120(2):422–424. [PubMed] [Google Scholar]
- von Jeney N., Günther E., Jann K. Mitogenic stimulation of murine spleen cells: relation to susceptibility to Salmonella infection. Infect Immun. 1977 Jan;15(1):26–33. doi: 10.1128/iai.15.1.26-33.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



