Abstract
Studies were conducted to identify a 64-kD thylakoid membrane protein of unknown function. The protein was extracted from chloroplast thylakoids under low ionic strength conditions and purified to homogeneity by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Four peptides generated from the proteolytic cleavage of the wheat 64-kD protein were sequenced and found to be identical to internal sequences of the chloroplast-coupling factor (CF1) α-subunit. Antibodies for the 64-kD protein also recognized the α-subunit of CF1. Both the 64-kD protein and the 61-kD CF1 α-subunit were present in the monocots barley (Hordeum vulgare), maize (Zea mays), oat (Avena sativa), and wheat (Triticum aestivum); but the dicots pea (Pisum sativum), soybean (Glycine max Merr.), and spinach (Spinacia oleracea) contained only a single polypeptide corresponding to the CF1 α-subunit. The 64-kD protein accumulated in response to high irradiance (1000 μmol photons m−2 s−1) and declined in response to low irradiance (80 μmol photons m−2 s−1) treatments. Thus, the 64-kD protein was identified as an irradiance-dependent isoform of the CF1 α-subunit found only in monocots. Analysis of purified CF1 complexes showed that the 64-kD protein represented up to 15% of the total CF1 α-subunit.
Light serves as both an energy source and as a regulatory signal for photosynthesis. The irradiance and spectral quality of light surrounding an individual leaf control the photosynthetic capacity of that leaf (Chow and Anderson, 1987a; Evans, 1987; Chow et al., 1990; Burkey and Wells, 1991). Light regulation is mediated through adjustments in the steady-state level of specific chloroplast proteins, including Rubisco (Chow and Anderson, 1987a; Prioul and Reyss, 1987), ATP synthetase (Chow and Anderson, 1987b; Chow and Hope, 1987; Davies et al., 1987; Burkey and Wells, 1991), and the light-harvesting chlorophyll-protein complexes (Leong and Anderson, 1984; De la Torre and Burkey, 1990a). Light also regulates photosynthetic electron transport capacity (Davies et al., 1986; Chow and Anderson, 1987a; Evans, 1987; De la Torre and Burkey, 1990b) through small adjustments in the PSII-to-PSI ratio (Wild et al., 1986; Chow and Anderson, 1987b; Chow and Hope, 1987; Evans, 1987; Lee and Whitmarsh, 1989; Chow et al., 1990; De la Torre and Burkey, 1990b) and somewhat larger changes in the concentration of the Cyt b6-f complexes (Wild et al., 1986; Chow and Anderson, 1987b; Chow and Hope, 1987; Evans, 1987; Lee and Whitmarsh, 1989; De la Torre and Burkey, 1990b) and plastocyanin (Burkey, 1993). Typically, the concentration of a given component varies by a factor of two or less over a wide range of light conditions.
During studies to examine the effects of growth irradiance on chloroplast biochemistry, a 64-kD thylakoid membrane protein of unknown function was discovered (Burkey, 1992). The steady-state level of this protein varied severalfold in response to a 10-fold difference in growth irradiance, a much larger effect than was observed for other thylakoid membrane components. In this report, the 64-kD protein was purified and identified as an isoform of the CF1 α-subunit.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
For purification of the 64-kD protein from barley (Hordeum vulgare cv Boone) and wheat (Triticum aestivum cv Pioneer 2548), plants were grown in trays of soil in controlled environment chambers with a PPFD of either 500 μmol photons m−2 s−1 (fluorescent and incandescent lamps) or 1000 μmol photons m−2 s−1 (microwave-powered fusion lamp, Fusion Lighting, Rockville, MD2). Plants were thinned weekly to reduce self-shading. Leaves from 2- to 4-week-old plants were used to prepare the thylakoids that served as a starting material for the isolation protocol.
For the species comparison, plants were grown in pots of soil in a controlled environment chamber with a maximum PPFD of 600 μmol photons m−2 s−1 provided by fluorescent and incandescent lamps. Barley, oat (Avena sativa cv Brooks), and wheat were grown at 21°C with a 16-h photoperiod, and thylakoid membranes were isolated from primary leaves 14 DAP. Soybean (Glycine max Merr. cv Young) and maize (Zea mays cv Pioneer 3184) were grown at 25°C with a 16-h photoperiod, and thylakoid membranes were isolated from primary leaves at 14 DAP. Spinach (Spinacia oleracea cv Melody) and pea (Pisum sativum cv Progress 9) were grown at 21°C with a 10-h photoperiod, conditions that were selected to prevent flowering in spinach. Thylakoid membranes were isolated from the first true leaf of spinach or mature pea leaves at 22 DAP.
A study of manipulating growth irradiance was conducted with barley. Plants were grown in pots of soil in a controlled environment chamber at 21°C with a 16-h photoperiod. A maximum PPFD of 1000 μmol photons m−2 s−1 was provided by a microwave-powered fusion lamp (Fusion Lighting). A low-irradiance treatment of 80 μmol photons m−2 s−1 was established in a section of the chamber using a neutral-density shade cloth. Control plants were grown under either high or low irradiance until 10 DAP. Pots assigned to acclimation treatments were then transferred to the opposite light environment, and growth was continued for 7 d. Thylakoid membranes were isolated from primary leaves of control plants at 10 DAP and from all treatments at 17 DAP.
Thylakoid Membrane Isolation
Thylakoid membranes were isolated from leaf tissue as previously described (Burkey and Wells, 1991) using a grinding buffer that consisted of 0.4 m sorbitol, 10 mm NaCl, 5 mm MgCl2, and 50 mm Tricine-NaOH, pH 7.8. Thylakoid membranes used as starting material for the isolation of the 64-kD protein were further purified on Suc gradients (Burkey and Wells, 1991). The final membrane preparation was resuspended in grinding buffer, frozen with liquid nitrogen, and stored at −75°C prior to analysis of polypeptide composition or purification of the 64-kD protein.
Purification of the 64-kD Protein and Production of Antiserum
The 64-kD protein was extracted from isolated thylakoid membranes using the low-ionic-extraction procedure developed for the isolation of CF1 (Jagendorf, 1982). Purified barley or wheat thylakoid membranes were washed twice in cold 10 mm sodium pyrophosphate, pH 7.5, and collected by centrifugation at 15,000g for 5 min at 4°C. Washed membranes were resuspended in STT buffer (50 mm Suc and 2 mm Tricine-Tris, pH 8.0) at a final chlorophyll concentration of 0.2 mg mL−1, and stirred at room temperature in the dark for 15 min. The membranes were collected by ultracentrifugation at 100,000g for 30 min at 20°C. The supernatant containing the STT-extracted proteins was recovered and brought to 2 mm EDTA, 1 mm ATP, and 50 mm Tris-HCl, pH 7.5, by the addition of concentrated stock solutions. Solid (NH4)2SO4 was added to 50% saturation, and the solution was incubated at room temperature for 30 min to allow precipitate formation.
The precipitated proteins were collected by centrifugation at 10,000g for 10 min at 4°C and dissolved in 2 mm EDTA, 1 mm ATP, and 50 mm Tris-HCl, pH 7.5, followed by dialysis against the same buffer. The dialyzed preparation was clarified by centrifugation at 25,000g for 10 min. Sodium azide (3 mm final concentration) was added to the STT-extracted protein solution before storage at 4°C. For certain experiments, CF1 complexes were purified from the final STT-extracted protein preparation using Suc-gradient centrifugation, as described by Jagendorf (1982).
The 64-kD protein was purified from the STT-extracted protein preparation by two cycles of electrophoresis in preparative LiDS-PAGE gels. Gels were stained with Coomassie blue and destained. Following each cycle of electrophoresis, the 64-kD protein was recovered by electroelution in a buffer consisting of 0.1% (w/v) SDS, 25 mm Tris, and 192 mm Gly. The purified protein was precipitated with acetone and dissolved in 0.4% (w/v) SDS, 150 mm NaCl, and 10 mm NaH2PO4, pH 7.0. The concentration of the purified 64-kD protein was determined by densitometry of the Coomassie-stained gels using BSA as a standard. Rabbit antiserum was produced (Berkeley Antibody Co., Richmond, CA) using the purified barley 64-kD protein as the antigen.
Gel Electrophoresis
Analytical or preparative PAGE was conducted under denaturing conditions according to the method of Laemmli (1970), with a gel concentration of 7% (w/v) acrylamide and with LiDS substituted for SDS. Thylakoid membranes were washed twice in 10 mm sodium pyrophosphate, pH 7.5, and resuspended in the same buffer prior to electrophoresis. Thylakoid membranes were solubilized in electrophoresis sample buffer at room temperature for 1 h. STT-extracted proteins from thylakoids were solubilized at 50°C for 15 min. Electrophoresis was conducted overnight at 8°C. Gels were either stained with Coomassie blue for photographic and densitometric analysis, or electroblotted to nitrocellulose for immunochemistry.
Chlorophyll and Protein Determinations
The chlorophyll content of thylakoid membrane preparations was determined by extraction of pigments with dimethylformamide followed by spectrophotometric assay (Moran, 1982). The protein content of STT extracts was determined by the method of Bradford (1976), with BSA as the standard.
Western Analysis
Polypeptides were transferred from polyacrylamide gels onto nitrocellulose membranes (Bio-Rad) using the buffer system of Towbin et al. (1979) without methanol. Electroblotting, nitrocellulose membrane treatments, and color development were conducted as described previously (Burkey, 1993). The primary antibody solution contained a 10,000-fold dilution of 64-kD protein antiserum or preimmune serum.
Determination of Protein Sequences
Purified wheat 64-kD protein was electroblotted from 7% (w/v) acrylamide gels onto PVDF membrane (Immobilon-P, Millipore) in the presence of 10 mm cyclohexylamino-propane sulfonic acid buffer, pH 11.0, 10% (v/v) methanol using a cooled tank system. The membrane was stained with Ponceau S to locate the protein.
The internal protein sequences were determined at the Emory University Microchemical Facility (Atlanta, GA). A portion of the PVDF membrane containing the 64-kD protein was digested using lysyl endoproteinase (EC 3.4.21.50, Wako BioProducts, Richmond, VA), as described previously (Fernandez et al., 1992). The peptides were isolated using microbore reverse-phase HPLC, collected manually, and stored at −20°C until used. Automated Edman degradation of the peptides was performed on Applied Biosystems model 470A/120A gas-phase, 477A/120A pulsed-liquid, and 491A/140S Procise sequencing systems, as described previously (Pohl, 1994). HPLC separation of the phenylthiohydantoin amino acids was performed on-line using solvent A3 (3.5% [v/v] aqueous tetrahydrofuran) containing the Premix buffer (19 mL L−1) and solvent B (acetonitrile) for the 470A and 477A systems or solvent B2 (9:1 [v/v] acetonitrile:2-propanol) for the 491A system.
RESULTS
Purification of the 64-kD Protein
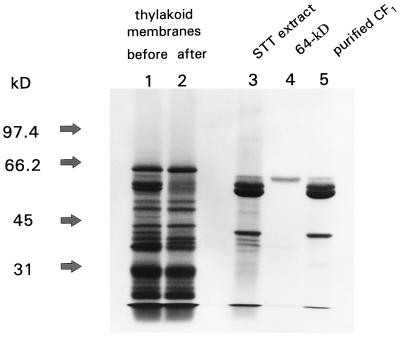
The polypeptide composition of the washed thylakoid membranes from barley before and after extraction with low-ionic-strength STT buffer is shown in Figure 1. Identical results were obtained with wheat (data not shown). The major polypeptides released from the membranes during STT extraction were the CF1 subunits. The 64-kD protein was the highest-molecular-mass component observed in the STT extract (Fig. 1, lane 3), but represented only a small percentage of the total protein at this stage of purification. The 64-kD protein and CF1 were also released from thylakoid membranes by extraction with 2 m NaBr (K.O. Burkey, unpublished data). After purification by preparative electrophoresis, the 64-kD protein consisted of a single polypeptide (Fig. 1, lane 4). Approximately 100 μg of purified 64-kD protein was obtained from thylakoid membranes equivalent to 50 mg of chlorophyll.
Figure 1.
Purification of the 64-kD protein. Protein fractions were analyzed in a 7% (w/v) acrylamide gel and stained with Coomassie blue. Lanes 1 and 2, Barley thylakoid membranes (20 μg of chlorophyll) before and after extraction with STT buffer; lane 3, STT-extracted proteins (40 μg protein); lane 4, purified 64-kD protein (approximately 2 μg of protein); and lane 5, CF1 complexes purified by Suc-gradient centrifugation (20 μg of protein). Molecular mass markers (in kD) are indicated at left.
The 64-kD protein exhibited the properties of an extrinsic membrane protein because it was released from thylakoid membranes by manipulation of ionic strength using the same conditions for the release of CF1. Further purification of the STT-extracted protein preparation revealed that the 64-kD protein was specifically associated with the CF1 complex. Some contaminating polypeptides were removed from CF1 during Suc-gradient centrifugation, but the 64-kD protein remained associated with the CF1 complex following purification (Fig. 1, lane 5).
Identification of the 64-kD Protein as an Isoform of the CF1 α-Subunit
The 64-kD protein from wheat was electroblotted onto PVDF membrane and subjected to several cycles of Edman degradation. No sequence signal could be obtained above the background signal, so it was concluded that the protein was N-terminally blocked. Since no attempt was made to chemically protect the N terminus during electrophoresis, a blocked N-terminal residue was not surprising. In the next step, a second portion of the PVDF membrane was treated with lysyl endopeptidase, and four peptides were purified and sequenced. Each peptide (Fig. 2) corresponded to an internal sequence of the wheat CF1 α-subunit gene (Howe et al., 1985). These results provided direct evidence that the 64-kD protein was an isoform of the CF1 α-subunit.
Figure 2.
Identification of peptides from the 64-kD protein as internal sequences of the α-subunit of CF1. The predicted amino acid sequence of the wheat CF1 α-subunit (Howe et al., 1985) is presented, with lysyl residues denoted by a box. Purified 64-kD protein from wheat was cleaved with a commercial lysyl endopeptidase, and four peptides from the digest were isolated and sequenced. The peptides corresponded to the four underlined sequences noted within the wheat CF1 α-subunit. Asterisks (*) indicate amino acid residues definitively identified; †, low signal level, putatively identified as Arg; −, residues not determined during sequencing due to low yield. Peptide 2 followed a methionyl residue, indicating a nonspecific cleavage by the commercial lysyl endopeptidase. Such cleavages have been noted previously in proteins cleaved by this enzyme (J. Pohl, personal communication).
Immunochemical Identification of CF1 α-Subunit Isoforms
The antiserum produced using purified 64-kD protein from barley detected two polypeptides in barley thylakoids (Fig. 3, lane 2), with no signal observed using preimmune serum (data not shown). The upper band on the western blot corresponded to the 64-kD protein, and is labeled α′ in Figure 3. The lower band, labeled α on the western blot, had a molecular mass of 61 kD and corresponded to the major Coomassie-stained band (Fig. 3, lane 1) of the CF1 α-subunit. Both α′ and α were observed on western blots when leaf tissue was extracted directly into hot (70°C) electrophoresis sample buffer (data not shown). The presence of two bands in whole-tissue extracts provided evidence that both isoforms existed in vivo and were not products of proteolysis during thylakoid isolation.
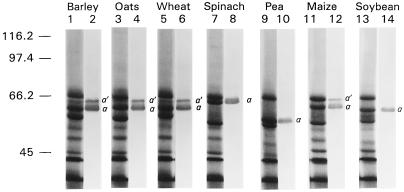
Figure 3.
Species screen of CF1 α-subunit isoforms. Thylakoids from seven species were subjected to LiDS-PAGE and immunoblot analysis. Odd-numbered lanes are gels from the indicated species that were stained for protein with Coomassie blue. Even-numbered lanes are immunoblots from the indicated species that were developed with barley 64-kD protein antisera. Lanes stained for protein contained thylakoids equivalent to 15 μg of chlorophyll. Lanes used for immunoblots contained thylakoids equivalent to 5 μg of chlorophyll. Molecular mass markers (in kD) are indicated at left.
Both the α′ and α isoforms of the CF1 subunit were present in barley, oats, wheat, and maize (Fig. 3). The molecular masses of the α′ and α isoforms were approximately 64 and 61 kD, respectively, in all monocots tested. In dicots, only the α isoform, corresponding to a major Coomassie-stained band, was present in thylakoids (Fig. 3). Large differences were observed in the apparent molecular mass for spinach (64 kD), pea (56 kD), and soybean (60 kD). The range of sizes (56–64 kD) for the CF1 α-subunit from different plants was similar to the range (57–62 kD) reported in a review by Merchant and Selman (1985). Typically, SDS-PAGE estimates of the CF1 α-subunit molecular mass are larger than molecular masses calculated from sequence data. For example, the α isoform of wheat migrated at 61 kD (Fig. 3), yet the molecular mass from sequence data is approximately 55 kD (Howe et al., 1985). This discrepancy may be related to unknown characteristics of the primary structure that affect migration in gels (see Discussion).
Effects of Growth Irradiance on Steady-State Levels of the 64-kD α′ Isoform of CF1
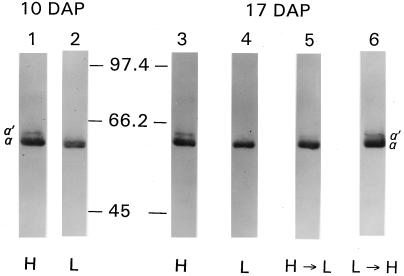
The 64-kD α′ isoform of CF1 was a distinct band on immunoblots of barley thylakoids from plants grown at high irradiance (Fig. 4, lanes 1 and 3), but was not detected in an equivalent amount of thylakoids from low-irradiance plants (Fig. 4, lanes 2 and 4). The extremely low level of the α′ isoform in low-irradiance plants required a larger sample size to detect on immunoblots (data not shown). The steady-state level of the α′ isoform exhibited a similar irradiance response in maize, oats, and wheat (data not shown).
Figure 4.
Effects of growth irradiance on the steady-state levels of the 64-kD protein. Barley was grown under high (1000 μmol photons m−2 s−1) or low (80 μmol photons m−2 s−1) irradiance. At 10 DAP, a portion of the plants were transferred to the opposite light environment and allowed to acclimate for 7 d until 17 DAP. Thylakoids from high (H) and low (L) irradiance control plants and from plants transferred from high to low (H→L) or low to high (L→H) irradiance were subjected to immunoblot analysis using 64-kD protein antisera. Each lane contained thylakoids equivalent to 2.5 μg of chlorophyll. Lane 1, H control thylakoids at 10 DAP; lane 2, L control thylakoids at 10 DAP; lane 3, H control thylakoids at 17 DAP; lane 4, L control thylakoids at 17 DAP; lane 5, H→L thylakoids at 17 DAP following a 7-d acclimation period; lane 6, L→H thylakoids at 17 DAP following a 7-d acclimation period. Molecular mass markers (in kD) are indicated between lanes 2 and 3.
The steady-state level of the α′ isoform was sensitive to changes in growth irradiance. The level of the α′ isoform declined in barley grown at a high irradiance after the plants were transferred to low irradiance for 7 d (Fig. 4, lane 5). The opposite response occurred in plants transferred from low to high irradiance, with the α′ isoform increasing during the 7-d acclimation period (Fig. 4, lane 6).
DISCUSSION
In four representative monocots, the α-subunit of CF1 consisted of two isoforms with apparent molecular masses of 61 and 64 kD. The 61-kD isoform was the major polypeptide in thylakoids routinely identified as the CF1 α-subunit (Merchant and Selman, 1985). The 64-kD protein referred to as α′ in this study was identified as a previously unrecognized isoform of the CF1 α-subunit (Figs. 1 and 2). Based on densitometry of Coomassie-stained gels of purified CF1 (e.g. Fig. 1, lane 5), the α′ isoform represented 14 ± 1% (mean ± se, n = 7) of the total CF1 α-subunit under high-irradiance growth conditions. The three dicots examined in this study did not contain α′ (Fig. 3), suggesting that it is found exclusively in monocots.
Possible mechanisms for the formation of the α′ isoform of CF1 are an alternative translation start site that generates a larger polypeptide or a posttranslational modification event. No sequence evidence exists for an alternative translation start site in the wheat chloroplast gene encoding the α-subunit of CF1 that could explain the 3-kD difference in molecular mass between the α′ and α isoforms (Howe et al., 1985). Formation of α′ by a posttranslational modification mechanism is a possible explanation because evidence exists for protein glycosylation within CF1 (Maione and Jagendorf, 1984). If protein modification is responsible for the conversion of α into α′, then such a mechanism might also explain the discrepancy between SDS-PAGE estimates of α-subunit molecular mass and calculated molecular masses from sequence data.
In conclusion, we have identified a novel isoform of the CF1 α-subunit referred to as α′, which accumulates in CF1 complexes at a level proportional to growth irradiance. The light-regulated α′ isoform was found in monocots but not in dicots. Future studies should focus on the molecular basis for the formation of α′ and the functional role of α′ within the CF1 complex.
ACKNOWLEDGMENTS
The authors thank Sharyn R. Caudell and Gary A. Little for their excellent technical assistance, and Barbara L. Leach and Tom Beggs for assistance in the preparation of text and figures. The authors also thank Jan Pohl and Frantisek Hubalek for determination of the internal peptide sequences by the Emory University Microchemical Facility.
Abbreviations:
- CF1
chloroplast coupling factor
- DAP
days after planting
- LiDS
lithium dodecylsulfate
Footnotes
This work is based on cooperative investigations of the U.S. Department of Agriculture, Agricultural Research Service, and the North Carolina Agricultural Research Service, Raleigh, NC 27695–7643; and the State University of West Georgia, Carrollton, GA 30118. Support for the Emory University Microchemical Facility was provided by the Shared Instrumentation Grant (NIH RR02878). Partial support for this work was provided by the State University of West Georgia Faculty Research Grants Program through the Learning Resources Committee.
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture, the North Carolina Agricultural Research Service, or the State University of West Georgia, and does not imply its approval to the exclusion of other products that mayalso be suitable.
LITERATURE CITED
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burkey KO. Novel light-regulated chloroplast membrane protein. Plant Physiol. 1992;98:1211–1213. doi: 10.1104/pp.98.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey KO. Effect of growth irradiance on plastocyanin levels in barley. Photosynth Res. 1993;36:103–110. doi: 10.1007/BF00016275. [DOI] [PubMed] [Google Scholar]
- Burkey KO, Wells R. Response of soybean photosynthesis and chloroplast membrane function to canopy development and mutual shading. Plant Physiol. 1991;97:245–252. doi: 10.1104/pp.97.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow WS, Anderson JM. Photosynthetic responses of Pisum sativum to an increase in irradiance during growth. I. Photosynthetic activities. Aust J Plant Physiol. 1987a;14:1–8. [Google Scholar]
- Chow WS, Anderson JM. Photosynthetic responses of Pisum sativum to an increase in irradiance during growth. II. Thylakoid membrane components. Aust J Plant Physiol. 1987b;14:9–19. [Google Scholar]
- Chow WS, Hope AB. The stoichiometries of supramolecular complexes in thylakoid membranes of spinach chloroplasts. Aust J Plant Physiol. 1987;14:21–28. [Google Scholar]
- Chow WS, Melis A, Anderson JM. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA. 1990;87:7502–7506. doi: 10.1073/pnas.87.19.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies EC, Chow WS, LeFay JM, Jordan BR. Acclimation of tomato leaves to changes in light intensity: effects on the function of the thylakoid membrane. J Exp Bot. 1986;37:211–220. [Google Scholar]
- Davies EC, Jordan BR, Partis MD, Chow WS. Immunochemical investigation of thylakoid coupling factor protein during photosynthetic acclimation to irradiance. J Exp Bot. 1987;38:1517–1527. [Google Scholar]
- De la Torre WR, Burkey KO. Acclimation of barley to changes in light intensity: chlorophyll organization. Photosynth Res. 1990a;24:117–125. doi: 10.1007/BF00032592. [DOI] [PubMed] [Google Scholar]
- De la Torre WR, Burkey KO. Acclimation of barley to changes in light intensity: photosynthetic electron transport activity and components. Photosynth Res. 1990b;24:127–136. doi: 10.1007/BF00032593. [DOI] [PubMed] [Google Scholar]
- Evans JR. The relationship between electron transport components and photosynthetic capacity in pea leaves grown at different irradiances. Aust J Plant Physiol. 1987;14:157–170. [Google Scholar]
- Fernandez J, DeMott M, Atherton D, Mische SM. Internal protein sequence analysis: enzymatic digestion for less than 10 μg of protein bound to polyvinylidene difluoride or nitrocellulose membranes. Anal Biochem. 1992;201:255–264. doi: 10.1016/0003-2697(92)90336-6. [DOI] [PubMed] [Google Scholar]
- Howe CJ, Fearnley IM, Walker JE, Dyer TA, Gray JC. Nucleotide sequences of the genes for the alpha, beta and epsilon subunits of wheat chloroplast ATP synthetase. Plant Mol Biol. 1985;4:333–345. doi: 10.1007/BF02418255. [DOI] [PubMed] [Google Scholar]
- Jagendorf AT (1982) Isolation of chloroplast coupling factor (CF1) and of its subunits. In M Edelman, RB Hallick, N-H Chua, eds, Methods in Chloroplast Molecular Biology. Elsevier Biomedical Press, Amsterdam, The Netherlands, pp 881–898
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W-J, Whitmarsh J. Photosynthetic apparatus of pea thylakoid membranes: response to growth irradiance. Plant Physiol. 1989;89:932–940. doi: 10.1104/pp.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong T-Y, Anderson JM. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth Res. 1984;5:105–115. doi: 10.1007/BF00028524. [DOI] [PubMed] [Google Scholar]
- Maione TE, Jagendorf AT. Partial deglycosylation of chloroplast coupling factor 1 (CF1) prevents the reconstitution of photophosphorylation. Proc Natl Acad Sci USA. 1984;81:3733–3736. doi: 10.1073/pnas.81.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S, Selman BR. Photosynthetic ATPases: purification, properties, subunit isolation and function. Photosynth Res. 1985;6:3–31. doi: 10.1007/BF00029044. [DOI] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982;68:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl J (1994) Sequence analysis of peptide resins from boc/benzyl solid-phase synthesis. In BM Dunn, MW Pennington, eds, Methods in Molecular Biology, Vol 36: Peptide Analysis Protocols. Humana Press, Totowa, NJ, pp 107–129 [DOI] [PubMed]
- Prioul J-L, Reyss A. Acclimation of ribulose bisphosphate carboxylase and mRNAs to changing irradiance in adult tobacco leaves. Plant Physiol. 1987;84:1238–1243. doi: 10.1104/pp.84.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild A, Hopfner M, Ruhle W, Richter M. Changes in stoichiometry of photosystem II components as an adaptive response to high-light and low-light conditions during growth. Z Naturforsch. 1986;41c:597–603. [Google Scholar]