Abstract
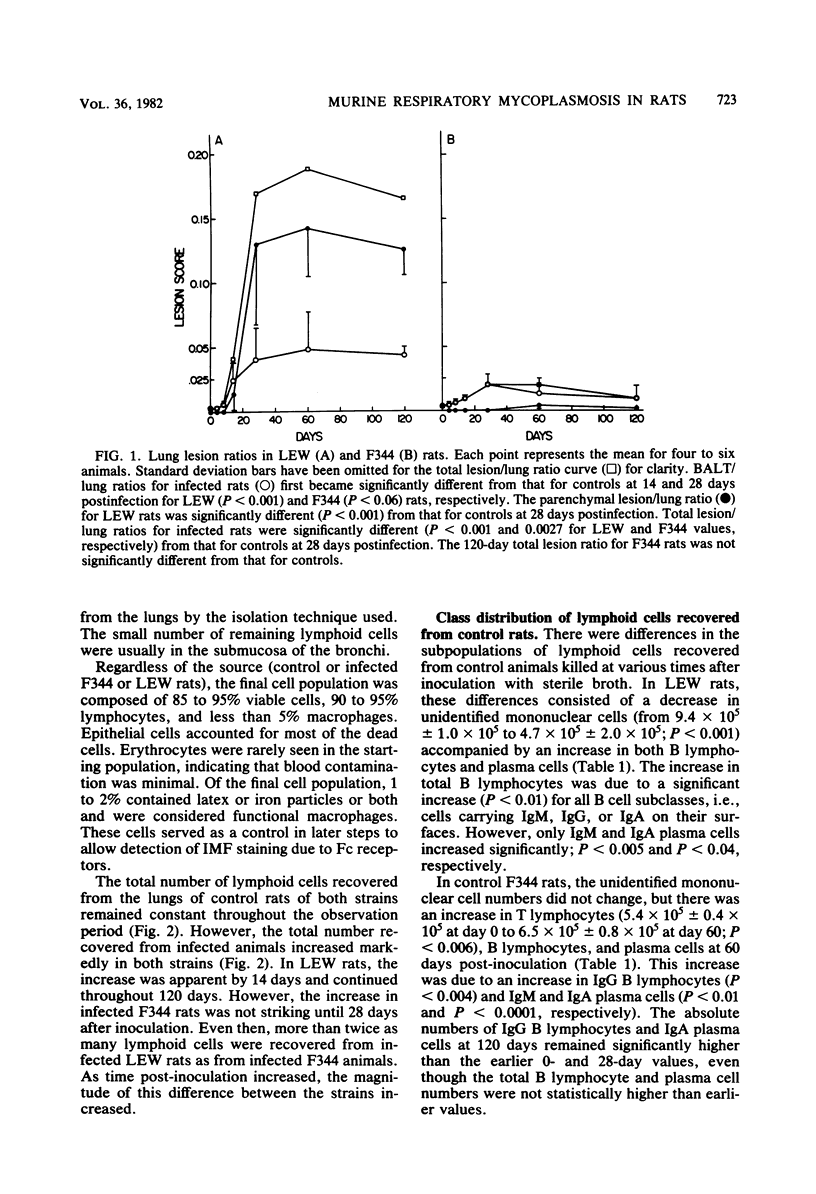
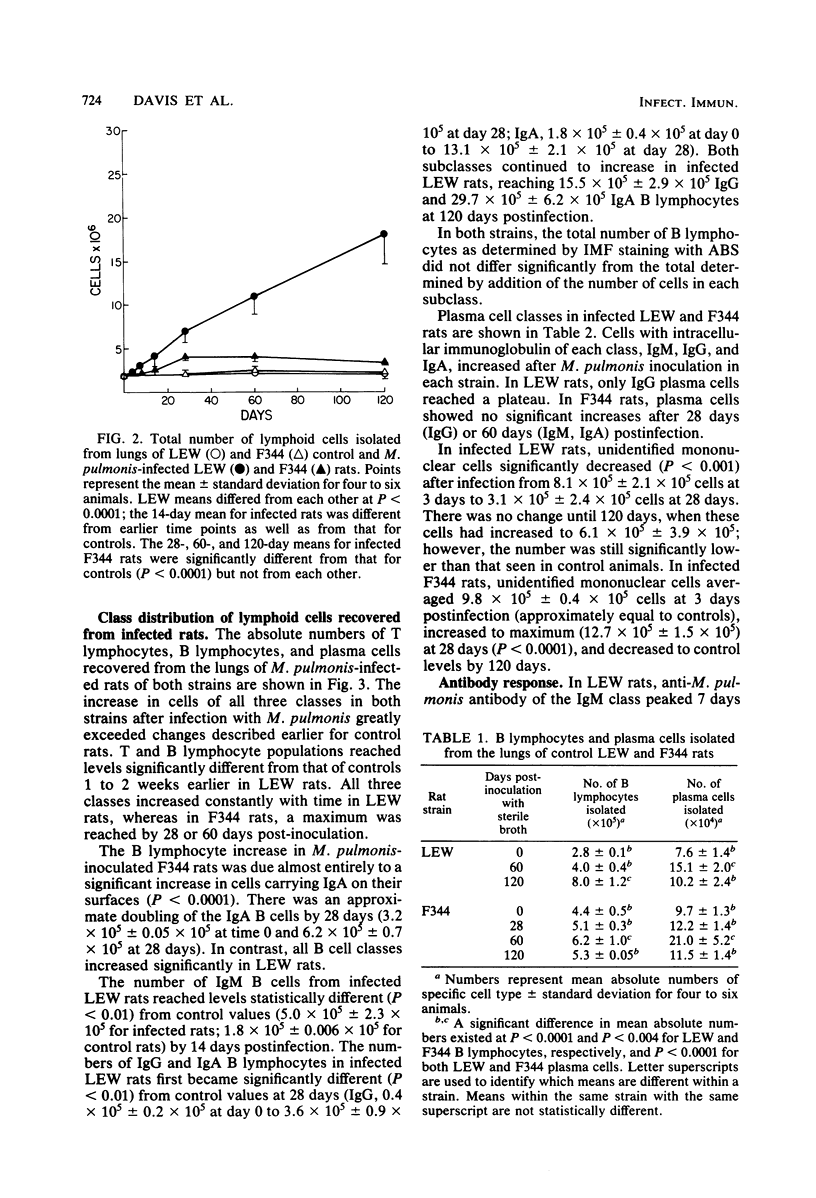
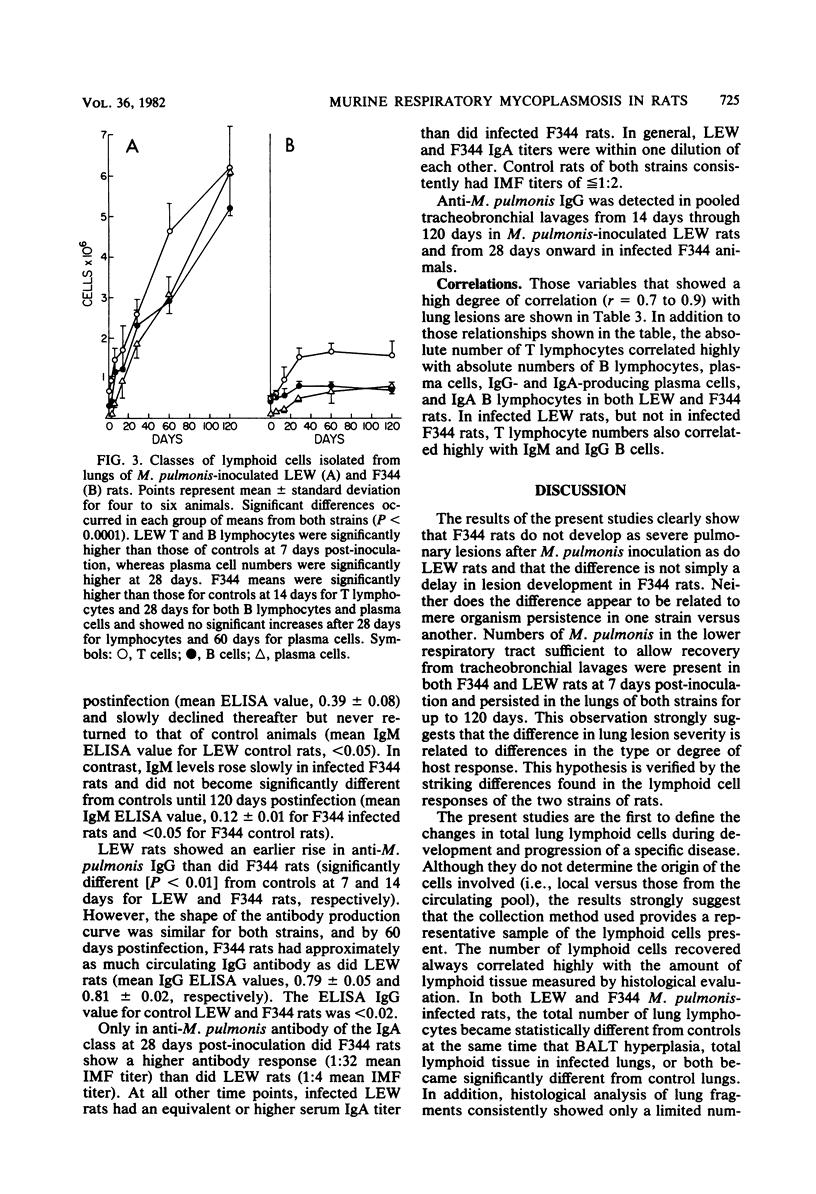
By comparison of two strains, LEW and F344, which are known to differ in susceptibility to Mycoplasma pulmonis respiratory disease, it was shown that differences in lesion severity and progression were associated with changes in lung lymphocyte populations. Lung lesions in LEW rats developed earlier after infection, became more severe, and were characterized by continued proliferation of all classes of lymphoid cells, T lymphocytes, B lymphocytes, and plasma cells, throughout the 120-day observation period. In contrast, lymphoid proliferation in F344 rats reached a plateau at 28 days and was restricted to an increase in T lymphocytes, immunoglobulin A (IgA)-bearing B lymphocytes, and IgA and IgG plasma cells. Although approximately 10 times as many IgG B cells and 4 times as many IgG plasma cells were found in infected LEW rats as compared with F344 rats, the specific anti-M. pulmonis IgG response in the two strains was roughly parallel. The same relationships held true, although to a lesser extent, for specific IgA antibody responses and cellular responses. Whereas lung lesions showed a tendency to resolve in F344 rats by 120 days, severe lesions persisted in LEW rats. The disparity between the cellular response and specific antibody response, the seemingly uncontrolled lymphocyte proliferation in LEW rats, and the mitogenic potential of M. pulmonis suggest that differences between LEW and F344 rats in lung lesion severity and progression are related to differences in the degree of nonspecific lymphocyte activation in the two strains, an imbalance in regulation of lymphocyte proliferation in LEW rats, or both.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Titus J. A., Segal D. M. Quantitation of Fc receptors and surface immunoglobulin is affected by cell isolation procedures using plasmagel and ficoll-hypaque. J Immunol Methods. 1978;22(3-4):263–272. doi: 10.1016/0022-1759(78)90034-0. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Feldman J. D. Thymus-dependent (T) lymphocytes in the rat. J Immunol. 1974 Jan;112(1):79–86. [PubMed] [Google Scholar]
- Blakeslee D., Baines M. G. Immunofluorescence using dichlorotriazinylaminofluorescein (DTAF). I. Preparation and fractionation of labelled IgG. J Immunol Methods. 1976;13(3-4):305–320. doi: 10.1016/0022-1759(76)90078-8. [DOI] [PubMed] [Google Scholar]
- Broderson J. R., Lindsey J. R., Crawford J. E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976 Oct;85(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- Butler J. E., Feldbush T. L., McGivern P. L., Stewart N. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry. 1978 Feb;15(2):131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Davis J. K. Protective effect of vaccination against Mycoplasma pulmonis respiratory disease in rats. Infect Immun. 1978 Jul;21(1):69–75. doi: 10.1128/iai.21.1.69-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Baker H. J. Immune response of pathogen-free mice inoculated intranasally with Mycoplasma pulmonis. J Immunol. 1974 Jan;112(1):124–136. [PubMed] [Google Scholar]
- Clagett J. A., Engel D. Polyclonal activation: a form of primitive immunity and its possible role in pathogenesis of inflammatory diseases. Dev Comp Immunol. 1978 Apr;2(2):235–241. doi: 10.1016/s0145-305x(78)80066-4. [DOI] [PubMed] [Google Scholar]
- Crum E. D., McGregor D. D. Functional properties of T and B cells isolated by affinity chromatography from rat thoracic duct lymph. Cell Immunol. 1976 May;23(2):211–222. doi: 10.1016/0008-8749(76)90187-8. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Altose M. D., Rowlands D. T., Jr Immunocompetent cells from the lower respiratory tract of normal human lungs. J Clin Invest. 1975 Oct;56(4):986–995. doi: 10.1172/JCI108179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. K., Shonnard J. W., Gill T. J., 3rd Genetic control of the immune response to poly(Glu52Lys33Tyr15) in neonatally thymectomized high and low responder rats. Am J Pathol. 1976 Jul;84(1):55–68. [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Maddox P. A., Thorp R. B., Cassell G. H. Immunofluorescent characterization of lymphocytes in lungs of rats infected with Mycoplasma pulmonis. Infect Immun. 1980 Jan;27(1):255–259. doi: 10.1128/iai.27.1.255-259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Immunoregulation in autoimmunity. J Allergy Clin Immunol. 1980 Jul;66(1):5–17. doi: 10.1016/0091-6749(80)90132-3. [DOI] [PubMed] [Google Scholar]
- Gasser D. L. Current status of rat immunogenetics. Adv Immunol. 1977;25:93–139. [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd, Cramer D. V., Kunz H. W. The major histocompatibility complex--comparison in the mouse, man, and the rat. A review. Am J Pathol. 1978 Mar;90(3):737–778. [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Gong H., Jr, Clements P. J., Eisenberg H. Pulmonary lymphocyte subpopulations. Variations in New Zealand black/white and C57BL/6 mice with age. Am Rev Respir Dis. 1979 Oct;120(4):821–827. doi: 10.1164/arrd.1979.120.4.821. [DOI] [PubMed] [Google Scholar]
- Gorenberg D. J., Daniele R. P. Characterization of immunocompetent cells recovered from the respiratory tract and tracheobronchial lymph node of normal guinea pigs. Am Rev Respir Dis. 1976 Dec;114(6):1099–1105. doi: 10.1164/arrd.1976.114.6.1099. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Kazmierowski J. A., Fauci A. S., Reynolds H. Y. Characterization of lymphocytes in bronchial lavage fluid from monkeys. J Immunol. 1976 Mar;116(3):615–618. [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Montfort I., Pérez Tamayo R. Two antigenically different types of macrophages. Proc Soc Exp Biol Med. 1971 Oct;138(1):204–207. doi: 10.3181/00379727-138-35862. [DOI] [PubMed] [Google Scholar]
- Naot Y., Davidson S., Lindenbaum E. S. Mitogenicity and pathogenicity of Mycoplasma pulmonis in rats. I. Atypical interstitial pneumonia induced by mitogenic myeoplasmal membranes. J Infect Dis. 1981 Jan;143(1):55–62. doi: 10.1093/infdis/143.1.55. [DOI] [PubMed] [Google Scholar]
- Naot Y., Merchav S., Ben-David E., Ginsburg H. Mitogenic activity of Mycoplasma pulmonis. I. Stimulation of rat B and T lymphocytes. Immunology. 1979 Mar;36(3):399–406. [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Siman-Tov R., Ginsburg H. Mitogenic activity of Mycoplasma pulmonis. II. Studies on the biochemical nature of the mitogenic factor. Eur J Immunol. 1979 Feb;9(2):149–154. doi: 10.1002/eji.1830090211. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Hinrichs D. J. Suppressor cell influence in selected strains of inbred rats. I. Strain-dependent mitogen- and antigen-related low responsiveness. Cell Immunol. 1977 Mar 1;29(1):96–108. doi: 10.1016/0008-8749(77)90278-7. [DOI] [PubMed] [Google Scholar]
- Rudzik O., Clancy R. L., Perey D. Y., Bienenstock J., Singal D. P. The distribution of a rabbit thymic antigen and membrane immunoglobulins in lymphoid tissue, with special reference to mucosal lymphocytes. J Immunol. 1975 Jan;114(1 Pt 1):1–4. [PubMed] [Google Scholar]
- Stewart C. C., Lin H. S., Adles C. Proliferation and colony-forming ability of peritoneal exudate cells in liquid culture. J Exp Med. 1975 May 1;141(5):1114–1132. doi: 10.1084/jem.141.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem. 1972 Apr 25;247(8):2439–2446. [PubMed] [Google Scholar]


