Abstract
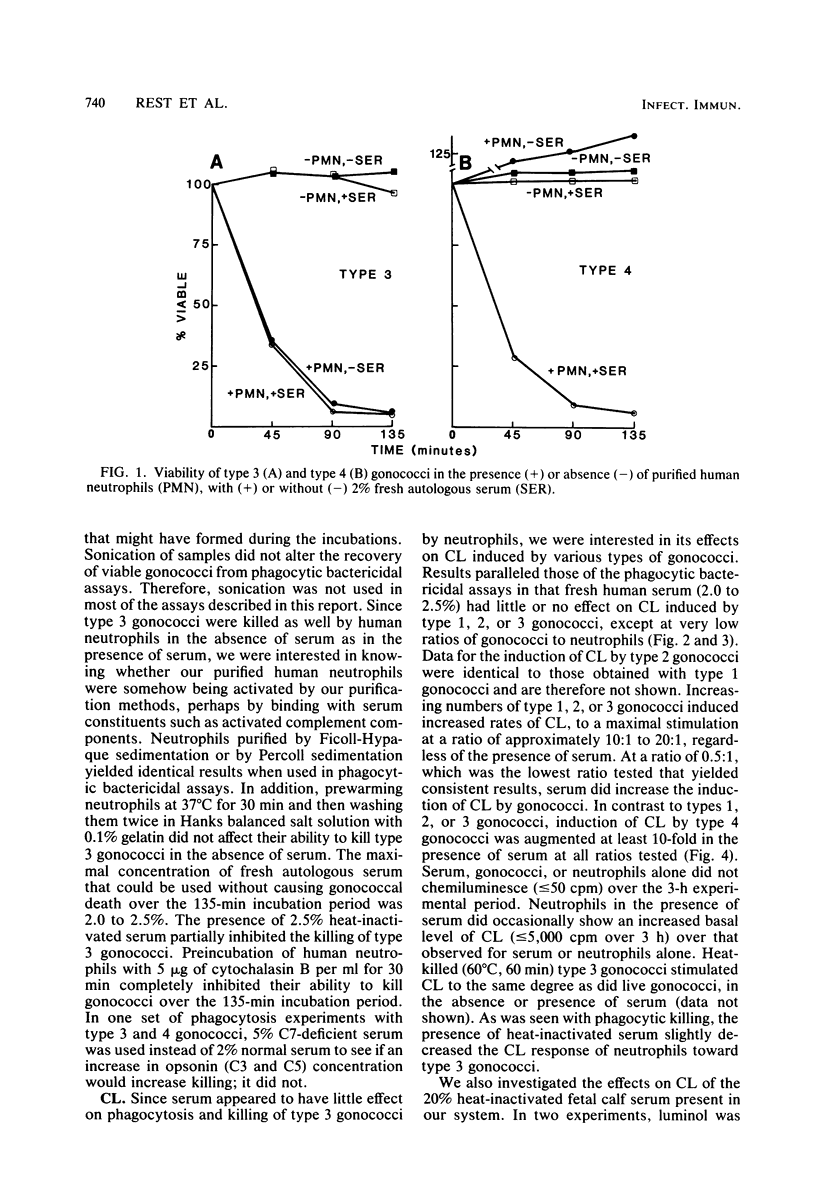
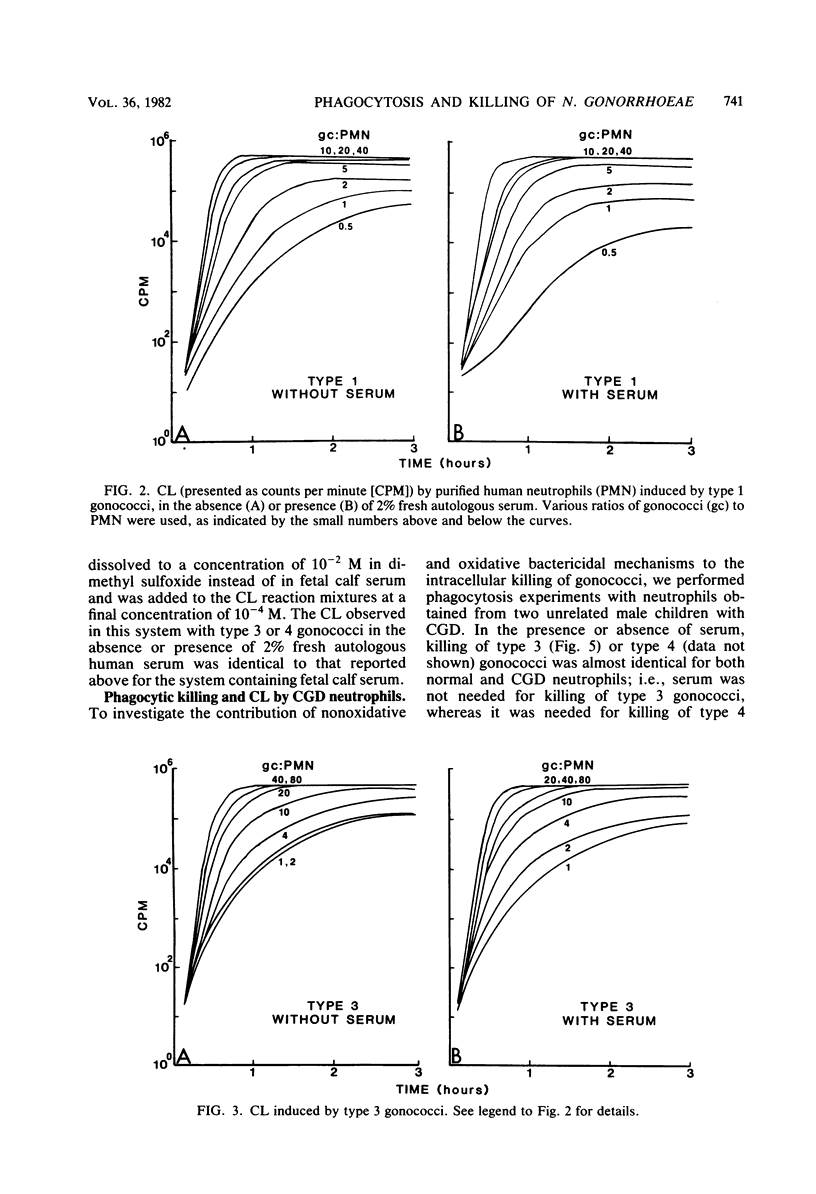
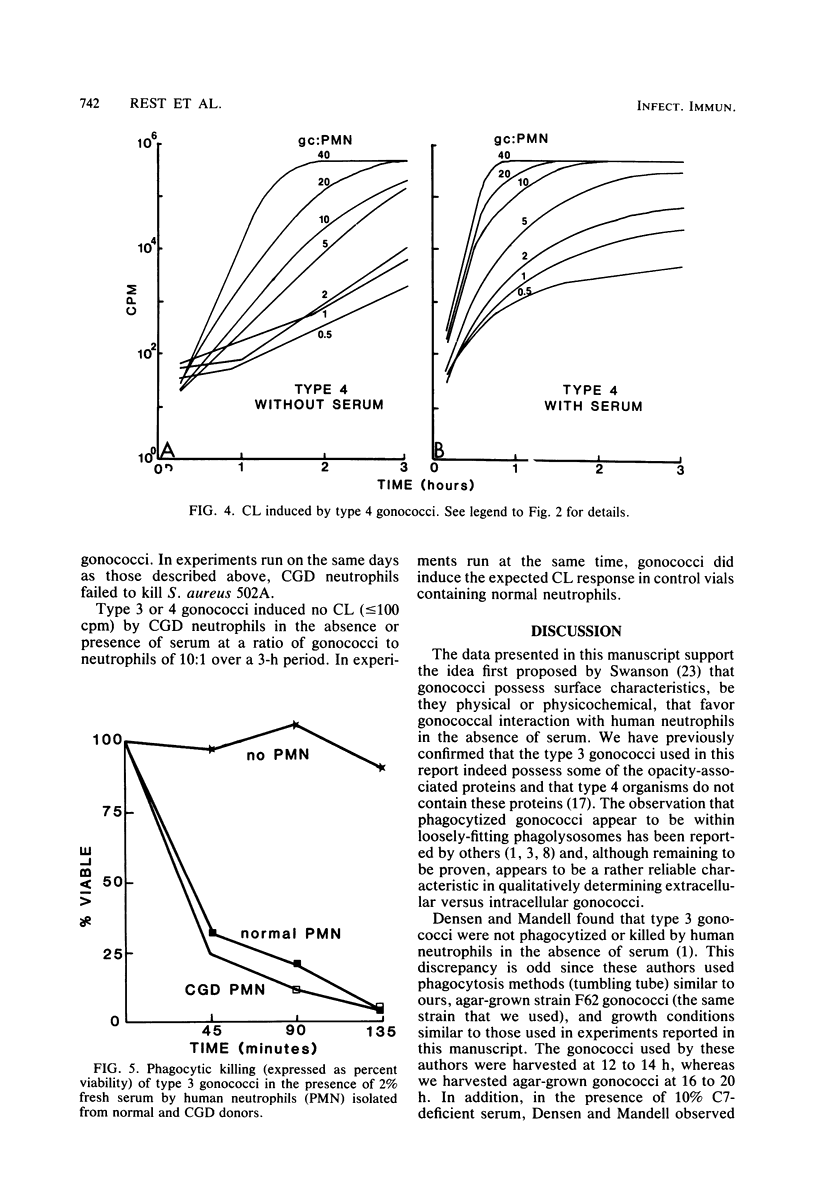
Serum-sensitive strains of Neisseria gonorrhoeae were incubated with suspensions of normal or chronic granulomatous disease human neutrophils in the absence or presence of fresh or heat-inactivated human serum; phagocytosis, gonococcal viability, and chemiluminescence were measured. Nonpiliated opaque or transparent gonococci (colony types 3 and 4, respectively) were used for phagocytic bactericidal assays. In the presence of 2.0% fresh human serum, normal neutrophils killed >90% of types 3 and 4 gonococci by 135 min. Serum alone at this concentration was not bactericidal. In the absence of serum, type 4 gonococci were not killed, whereas type 3 gonococci were killed to the same degree as in the presence of serum. Interestingly, heat-inactivated normal serum slightly inhibited phagocytic killing of type 3 gonococci. Results almost identical to those above were obtained when 5% fresh human serum deficient in complement component 7 was substituted for 2% normal autologous serum. This indicated that the later components of complement were not involved in the observed results. To investigate the mechanisms responsible for the intracellular killing of the gonococci, we used neutrophils from patients with chronic granulomatous disease. These neutrophils are deficient in an activable NADPH oxidase and do not produce bactericidal oxygen products upon phagocytic stimulation. Neutrophils from two unrelated boys with chronic granulomatous disease killed type 3 and 4 gonococci to the same degree as did normal neutrophils. As with normal neutrophils, serum was needed for killing type 4 organisms. As expected, neutrophils from these patients showed absolutely no increased chemiluminescence in the presence of type 3 or 4 gonococci, with or without serum. The effects of serum on gonococcus-induced chemiluminescence by normal neutrophils was also investigated. For these studies, in addition to type 3 and 4 gonococci, we also used transparent colony types of lightly (type 1) and heavily (type 2) piliated organisms. Chemiluminescence induced by type 1, 2, or 3 gonococci (i.e., gonococci possessing either pili or opacity-associated proteins, but not both) was augmented only slightly by serum and then only at low ratios of gonococci to neutrophils. On the other hand, chemiluminescence induced by type 4 gonococci (i.e., gonococci possessing neither pili nor opacity-associated proteins) was substantially increased in the presence of serum. Stimulation of chemiluminescence by type 1, 2, 3, or 4 gonococci was dose dependent in the absence or presence of serum. Heat-killed type 3 gonococci induced chemiluminescence to the same degree as did viable organisms. Since the gonococci used in this research was strongly catalase positive, as are gonococci in general, and since it was killed by chronic granulomatous disease neutrophils, the results indicate that gonococci can be effectively killed within neutrophils, i.e., within phagolysosomes, by nonoxidative bactericidal mechanisms. Whereas type 3 gonococci were phagocytized and killed by neutrophils equally well with or without serum, serum was obligatory for phagocytic killing of type 4 gonococci, i.e., gonococci lacking opacity-associated proteins. In addition, either pili or opacity-associated proteins were apparently necessary for maximal stimulation of neutrophil chemiluminescence. The submaximal stimulation of chemiluminescence by gonococci lacking both pili and opacity-associated proteins, i.e., type 4 gonococci was augmented by low concentrations of nonimmune serum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D. L., Roberts R. B. The interaction in vitro between human polymorphonuclear leukocytes and Neisseria gonorrhoeae cultivated in the chick embryo. J Exp Med. 1975 Jan 1;141(1):155–171. doi: 10.1084/jem.141.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger A. G., Schiller N. L., Roberts R. B. Gonococci-human polymorphonuclear leukocyte interactions: metabolic studies associated with attachment and ingestion. Infect Immun. 1980 Jun;28(3):991–1000. doi: 10.1128/iai.28.3.991-1000.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- McMillan A., McNeillage G., Young H. Antibodies to Neisseria gonorrhoeae: a study of the urethral exudates of 232 men. J Infect Dis. 1979 Jul;140(1):89–95. doi: 10.1093/infdis/140.1.89. [DOI] [PubMed] [Google Scholar]
- Norrod P., Morse S. A. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1287–1294. doi: 10.1016/0006-291x(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Hughes M., Miler J. J., Syrett C., Turner W. H., Harris J. R., MacLennan I. P. Studies on the mechanism of pathogenicity of Neisseria gonorrhoeae. J Med Microbiol. 1977 Aug;10(3):347–365. doi: 10.1099/00222615-10-3-347. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect Immun. 1979 Aug;25(2):574–579. doi: 10.1128/iai.25.2.574-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Pretzer E. Degradation of gonococcal outer membrane proteins by human neutrophil lysosomal proteases. Infect Immun. 1981 Oct;34(1):62–68. doi: 10.1128/iai.34.1.62-68.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Friedman G. L., Roberts R. B. The role of natural IgG and complement in the phagocytosis of type 4 Neisseria gonorrhoeae by human polymorphonuclear leukocytes. J Infect Dis. 1979 Nov;140(5):698–707. doi: 10.1093/infdis/140.5.698. [DOI] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Zeligs B. Studies on gonococcus infection. VI. Electron microscopic study on in vitro phagocytosis of gonococci by human leukocytes. Infect Immun. 1974 Sep;10(3):645–656. doi: 10.1128/iai.10.3.645-656.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C. Inhibition of adherence of Neisseria gonorrhoeae by human genital secretions. J Clin Invest. 1977 Jan;59(1):117–124. doi: 10.1172/JCI108608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale D. R., Goldner M., Penn C. W., Ward J., Smith H. The intracellular survival and growth of gonococci in human phagocytes. J Gen Microbiol. 1979 Aug;113(2):383–393. doi: 10.1099/00221287-113-2-383. [DOI] [PubMed] [Google Scholar]



