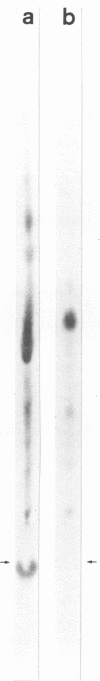
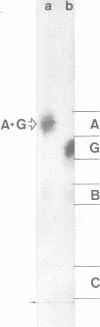
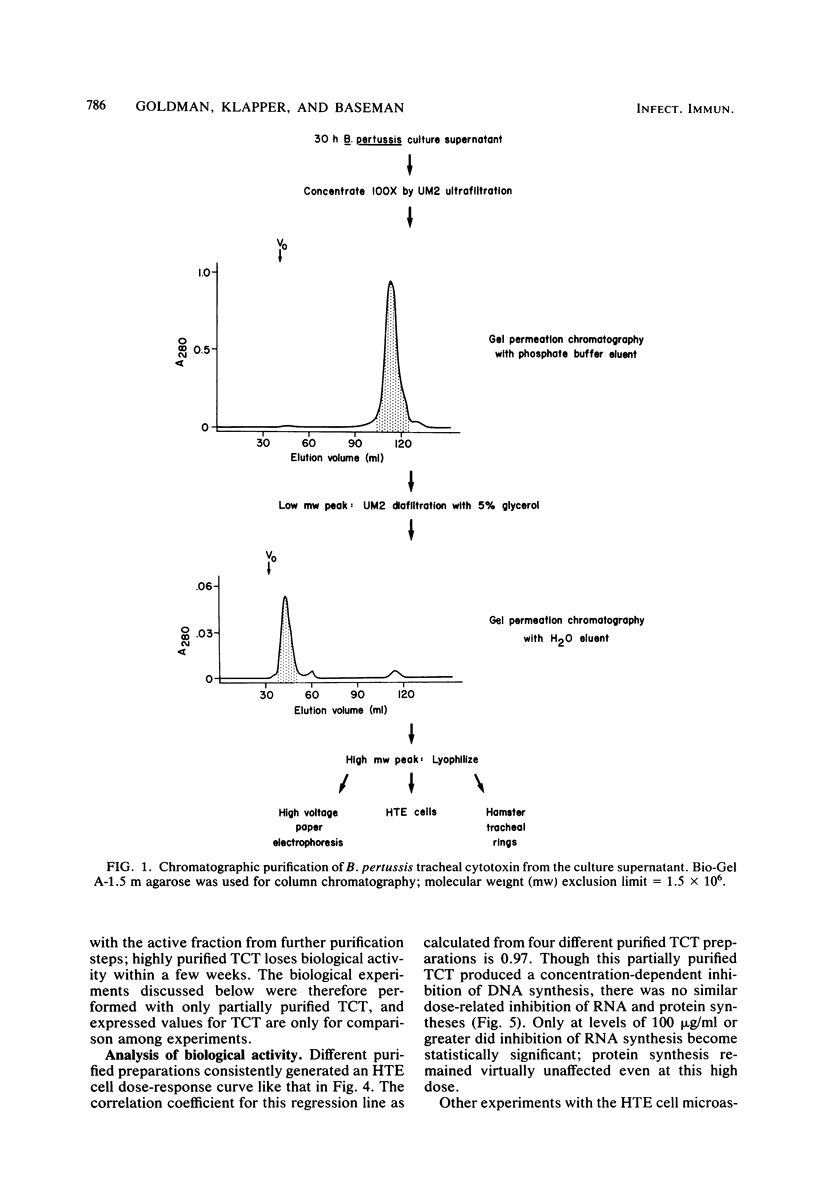
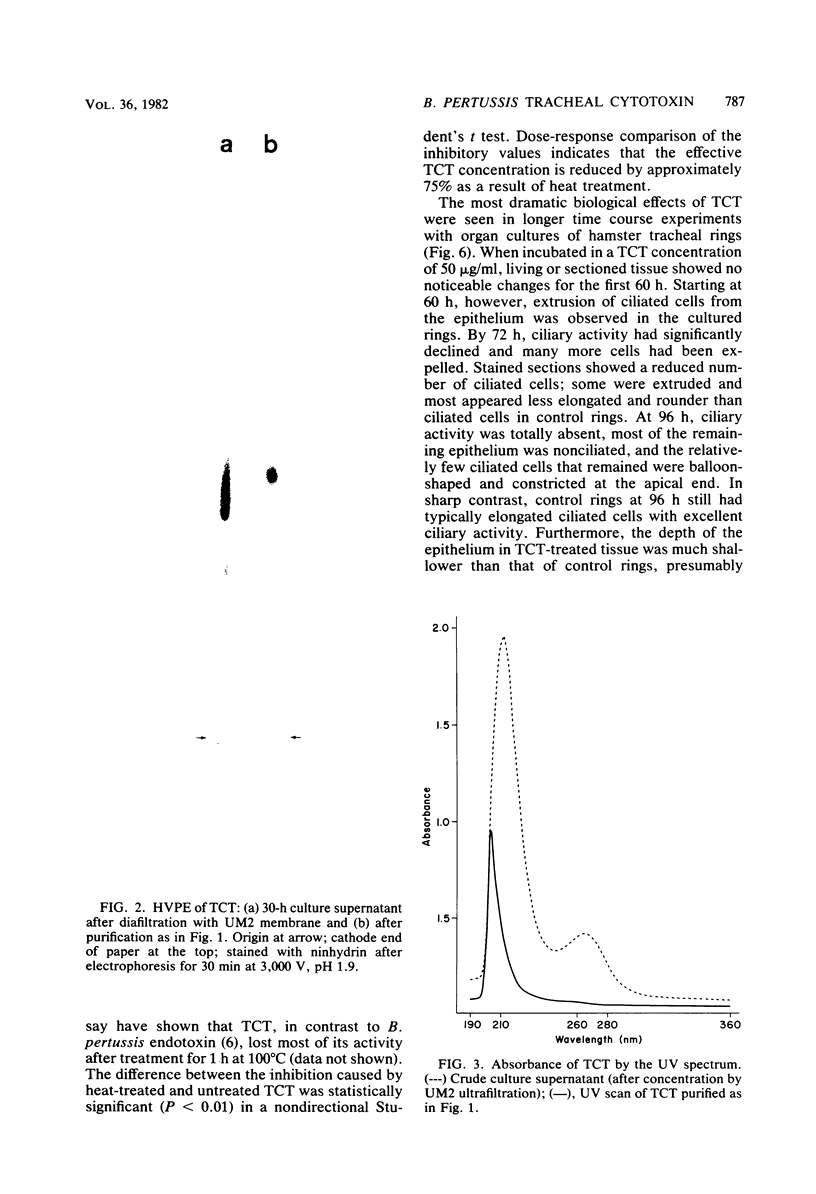
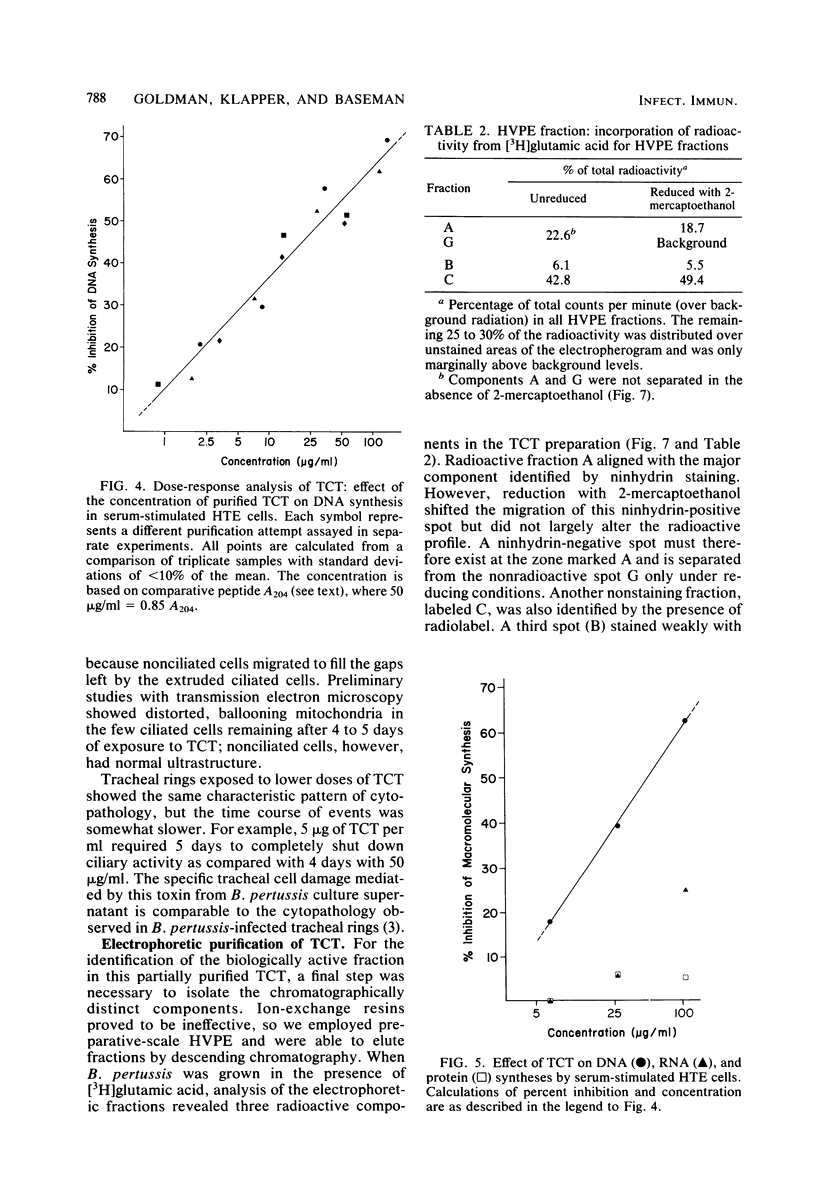
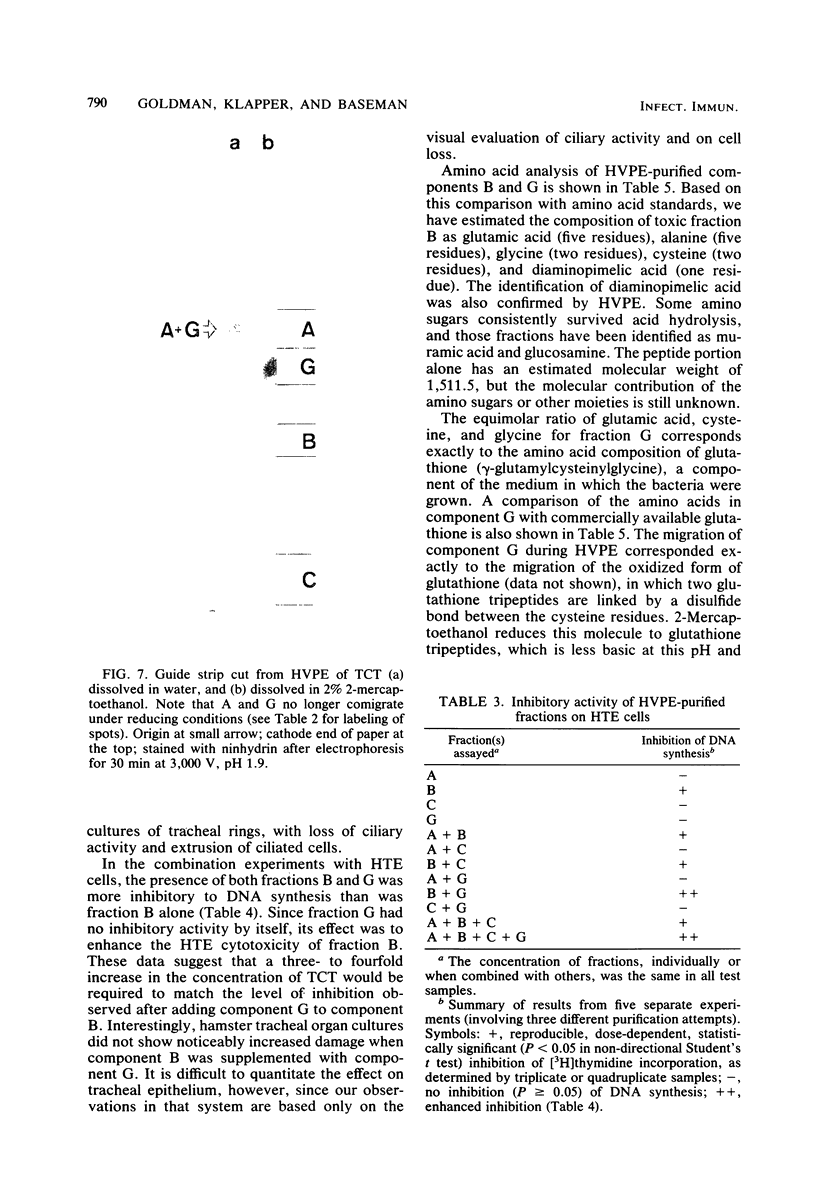
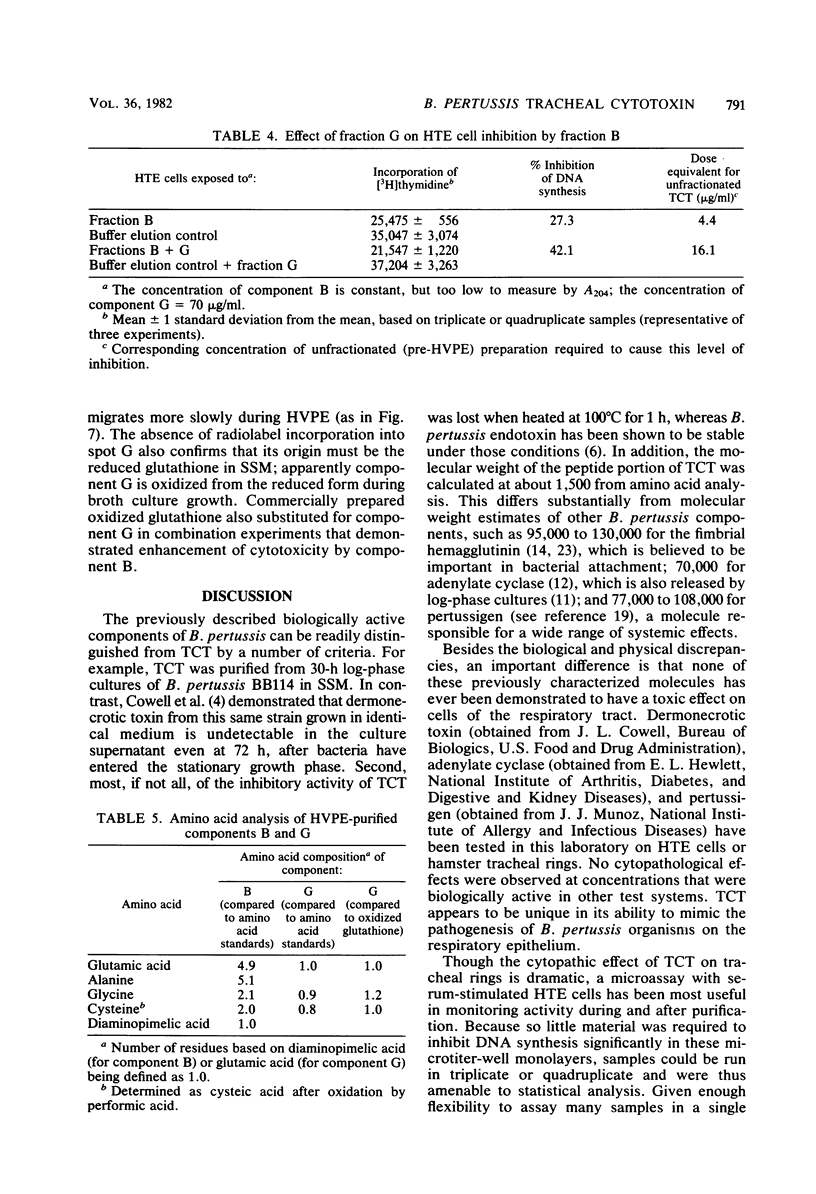
Abstract
Cultured hamster trachea epithelial cells were selected as an in vitro model system to study Bordetella pertussis in the respiratory tract. DNA synthesis by serum-stimulated tracheal cells, in contrast to other cell types tested, was inhibited by the supernatant from log-phase B. pertussis broth cultures. A sensitive microassay with these tracheal cells permitted the development of a chromatographic purification scheme based on aggregation of the biological activity under salt-free conditions. The active fraction from this first stage of purification caused a dose-dependent inhibition of DNA synthesis without a similar effect on RNA or protein synthesis. Organ cultures of hamster tracheal rings, when exposed to this partially purified fraction, developed epithelial cytopathology comparable to that seen during B. pertussis infection. Ciliary activity showed and eventually ceased as ciliated cells were extruded from the ring, leaving an intact but mostly nonciliated epithelium. Further purification of this biological activity was achieved with preparative-scale high-voltage paper electrophoresis. Based on ninhydrin staining and the radioactive profile of material purified from radiolabeled B. pertussis cultures, four fractions were eluted from the paper by descending chromatography. Only component B caused a dose-dependent inhibition of cultured tracheal cell DNA synthesis and epithelial cytopathology in tracheal rings. Combination experiments also demonstrated enhanced inhibition by component B in the presence of component G (oxidized glutathione), a copurifying molecule from the growth medium. Amino acid analysis (five residues), glycine (two residues), cysteine (two residues), and diaminopimelic acid (one residue), as well as muramic acid and glucosamine.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannai S., Tsukeda H. The export of glutathione from human diploid cells in culture. J Biol Chem. 1979 May 10;254(9):3444–3450. [PubMed] [Google Scholar]
- Collier A. M., Peterson L. P., Baseman J. B. Pathogenesis of infection with Bordetella pertussis in hamster tracheal organ culture. J Infect Dis. 1977 Aug;136 (Suppl):S196–S203. doi: 10.1093/infdis/136.supplement.s196. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Hewlett E. L., Manclark C. R. Intracellular localization of the dermonecrotic toxin of Bordetella pertussis. Infect Immun. 1979 Sep;25(3):896–901. doi: 10.1128/iai.25.3.896-901.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Baseman J. B. Glycoprotein secretion by cultured hamster trachea epithelial cells: a model system for in vitro studies of mucus synthesis. In Vitro. 1980 Apr;16(4):320–329. doi: 10.1007/BF02618338. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Baseman J. B. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro. 1980 Apr;16(4):313–319. doi: 10.1007/BF02618337. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt L. B. The pathology and immunology of Bordetella pertussis infection. J Med Microbiol. 1972 Nov;5(4):407–424. doi: 10.1099/00222615-5-4-407. [DOI] [PubMed] [Google Scholar]
- Irons L. I., MacLennan A. P. Isolation of the lymphocytosis promoting factor-haemagglutinin of Bordetella pertussis by affinity chromatography. Biochim Biophys Acta. 1979 Sep 29;580(1):175–185. doi: 10.1016/0005-2795(79)90208-3. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Lane B. P., Gordon R. Regeneration of rat tracheal epithelium after mechanical injury. I. The relationship between mitotic activity and cellular differentiation. Proc Soc Exp Biol Med. 1974 Apr;145(4):1139–1144. doi: 10.3181/00379727-145-37968. [DOI] [PubMed] [Google Scholar]
- Muse K. E., Collier A. M., Baseman J. B. Scanning electron microscopic study of hamster tracheal organ cultures infected with Bordetella pertussis. J Infect Dis. 1977 Dec;136(6):768–777. doi: 10.1093/infdis/136.6.768. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAM E., MOORE S., BIGWOOD E. J. Chromatographic determination of cystine as cysteic acid. Biochem J. 1954 May;57(1):33–37. doi: 10.1042/bj0570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Grau E. M., Meister A. Conversion of glutathione to glutathione disulfide by cell membrane-bound oxidase activity. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2715–2719. doi: 10.1073/pnas.76.6.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. M. DNA synthesis by cultured lymphocytes: a modified method for measuring 3H-thymidine incorporation. Cell Immunol. 1973 Dec;9(3):435–444. doi: 10.1016/0008-8749(73)90058-0. [DOI] [PubMed] [Google Scholar]