Abstract
A number of studies have suggested B7-H1, a B7 family member, inhibits T cell responses. Therefore, its expression on nonlymphoid tissues has been proposed to prevent T cell–mediated tissue destruction. To test this hypothesis, we generated transgenic mice that expressed B7-H1 on pancreatic islet β cells. Surprisingly, we observed accelerated rejection of transplanted allogeneic B7-H1–expressing islet β cells. Furthermore, transgenic B7-H1 expression broke immune tolerance, as some of the mice spontaneously developed T cell–dependent autoimmune diabetes. In addition, B7-H1 expression increased CD8+ T cell proliferation and promoted autoimmunity induction in a T cell adoptive transfer model of diabetes. Consistent with these findings, B7-H1.Ig fusion protein augmented naive T cell priming both in vitro and in vivo. Our results demonstrate that B7-H1 can provide positive costimulation for naive T cells to promote allograft rejection and autoimmune disease pathogenesis.
Introduction
B7-H1 (PD-L1) is a recently identified B7 family member whose expression is inducible in a variety of organs (1, 2). However, the role of B7-H1 in regulating T cell responses in vivo is not fully understood. Ligation of cognate receptor(s) on T cells by B7-H1 was first reported to stimulate predominantly IL-10 production (1, 3), which might negatively regulate cell-mediated immunity (4). An overwhelming number of studies support the role of B7-H1 as a negative regulator of T cell responses in vitro using immobilized mAb to CD3 and B7-H1.Ig fusion protein (5–9) or antibodies that blocked B7-H1 signaling in studies performed on human endothelial cells (10, 11), dendritic cells (12–14), liver nonparenchymal cells (15), and glioma cells (16). In vivo, the combination of B7-H1.Ig and cyclosporine A (CSA) or rapamycin promoted cardiac allograft survival and provided protection from chronic rejection (7). In addition, treatment with B7-H1.Ig plus mAb to CD154 induced long-term islet allograft survival (17). Still other studies have shown that B7-H1 expression on tumors prevented tumor rejection by inducing T cell apoptosis (2) or by promoting resistance to cytolysis (18). More recently, the inhibition of B7-H1–mediated signals has been shown to accelerate autoimmune diabetes (19). Together, these observations suggest an inhibitory role for B7-H1 in modulating T cell–mediated immune responses.
Given the demonstrations that B7-H1 can inhibit T cell responses both in vitro and in vivo, it has been hypothesized that B7-H1 expression on nonlymphoid tissues might help to prevent T cell–mediated tissue destruction (20). Therefore, to determine whether local nonlymphoid tissue expression of B7-H1 inhibits T cell responses and autoimmunity in vivo, we generated transgenic mice (rat insulin promoter [RIP].B7-H1) that expressed B7-H1 on pancreatic islet β cells. Unexpectedly, the transplantation of B7-H1–expressing islets resulted in accelerated allograft rejection in a B7-H1–dependent manner. Furthermore, a significant fraction of RIP.B7-H1 mice developed T cell–dependent spontaneous autoimmune diabetes. Finally, ectopic B7-H1 expression enhanced T cell priming and promoted autoimmunity induction in a T cell adoptive transfer model of diabetes. Thus, this study has revealed that tissue-specific expression of B7-H1 can costimulate T cell–mediated responses in vivo, resulting in accelerated allograft rejection, the breakdown of immune tolerance, and autoimmune disease pathogenesis.
Methods
Mice.
RIP–membrane ovalbumin (RIP.mOVA) and OT-I transgenic mice have been previously described (21) and were generous gifts from William Heath (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) and Susan Webb (The Scripps Research Institute, La Jolla, California, USA). C57BL/6 and 129 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). The mice were maintained under specific pathogen–free conditions. Animal care and use were in accordance with institutional and National Institutes of Health (NIH) guidelines.
Generation of the transgenic mice.
Full-length murine B7-H1 complementary deoxyribonucleic acid (cDNA) was synthesized by RT-PCR of splenic total RNA, and cloned into pCDNA3.1 (Invitrogen Corp., Carlsbad, California, USA) to produce the pCDNA3.1/B7-H1 construct. The construct was generated by insertion of the full-length murine B7-H1 cDNA into the ClaI site of RIP7 at position +180. A 10.6-kb fragment was excised by double digestion with NotI/SalI and was used for microinjection by the University of Chicago Cancer Research Center Transgenic Mice Facility. BamHI-digested tail DNA from mice was hybridized to a radiolabeled full-length murine B7-H1–specific probe. Two positive founders were generated directly on a C57BL/6 background with an approximate copy number of seven, as determined by phosphorimager analysis of a Southern blot. All of the mice used in this study were on the C57BL/6 background, except for the recipients of islet allografts, which were 129 (see below).
Immunofluorescence staining.
For immunofluorescence staining, cryostat sections 6 μm in thickness were incubated overnight with guinea pig anti-insulin (Dako Corp., Carpinteria, California, USA), then were incubated for 1 hour with biotinylated goat anti-guinea pig (Vector Laboratories Inc., Burlingame, California, USA) followed by a 1-hour incubation with streptavidin-FITC. The tissues were then stained overnight with rat mAb to mouse B7-H1–phycoerythrin (PE) (E-Biosciences, San Diego, California, USA).
Islet transplantation.
Donor pancreata were perfused in situ through the common bile duct with collagenase P (0.375 mg/ml; Roche Corp., Basel, Switzerland). Pancreata were harvested after perfusion and were incubated at 37°C for 10 minutes. Islets were released from the pancreata by gentle shaking. After being washed twice with Hank’s balanced salt solution (HBSS), islets were further purified on discontinuous Ficoll gradients. After centrifugation, the islets were harvested from the 1.096/1.069 gradient interface, washed twice in HBSS, and collected under the microscope. A total of 300 islets were transplanted under the renal capsule of each recipient 129 mouse rendered diabetic by a single intraperitoneal injection of streptozocin (STZ) (400 mg/kg; Pharmacia & Upjohn, Clayton, North Carolina, USA). Some mice were treated with 100 μg per mouse of hamster mAb to mouse B7-H1 (10B5; produced at the Mayo Clinic, Rochester, Minnesota, USA) on days 0 and 5. Allograft function was monitored by serial blood glucose measurements. Primary graft function was defined as a blood glucose concentration of less than 200 mg/dl on day 3 after transplantation, and graft rejection was defined as a rise in blood glucose concentration of more than 300 mg/dl after a period of primary graft function.
Measurement of blood glucose.
SureStep strips (Johnson and Johnson, Milpitas, California, USA) were used to measure glucose concentrations in blood obtained from a tail vein. Animals were considered diabetic after two consecutive measurements of blood glucose concentrations of more than 250 mg/dl.
Histological analysis of insulitis.
Pancreatic tissues were collected. Hematoxylin and eosin (H&E) staining was performed on sections 6 μm in thickness of 10% formalin-fixed tissue or tissue frozen at –70°C in optimal cutting temperature (OCT) compound. Sections were prepared on multiple levels, and 20–40 randomly chosen islets per mouse were evaluated for lymphocytic infiltration (insulitis) by a third-party pathologist. The degree of lymphocytic infiltration was assigned a score according to a previously described system as follows: 0, normal histology; 1, minimal cellular infiltrate into the islets, otherwise normal islet architecture; 2, extensive cellular infiltrate but preservation of islet architecture; 3, cellular infiltrate and loss of normal islet architecture (22).
Anti-CD3 treatment.
Diabetic mice were injected every other day for 3 days intraperitoneally with 15 μg/mouse of mAb to CD3 (145-2C11; a generous gift from Jeffrey A. Bluestone, UCSF Diabetes Center, San Francisco, California, USA).
Preparation of OT-I cells for adoptive transfer.
OT-I cells were prepared from lymph nodes and spleens of transgenic mice. The percentage of OT-I T cells was determined by iTAg MHC tetramer (H-2b-SIINFEKL-PE) staining following the manufacturer’s instructions (Beckman Coulter, Fullerton, California, USA). OT-I T cells (2 × 106) were injected intravenously (i.v.) into recipient mice. Cells were analyzed by flow cytometry on a FACScan (BD Biosciences, San Jose, California, USA).
Carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling of OT-I cells.
OT-I cells were resuspended in phosphate-buffered saline (PBS) at a concentration of 50 × 106 cells/ml. For fluorescence labeling, 50 × 106 cells/ml were incubated with 10 μM of CFSE (Molecular Probes Inc., Eugene, Oregon, USA) for 30 minutes at 37°C. At the time of transfer, as indicated, some mice were injected intraperitoneally with 100 μg hamster IgG or hamster mAb to mouse PD-1 (J43, hamster IgG; eBioscience, San Diego, California, USA). At 42 hours after adoptive transfer, tetramer-positive cells were analyzed in the pancreatic lymph node by flow cytometry on a FACScan or FACScalibur using FlowJo software (Tree Star, Ashland, Oregon, USA).
Generation of fusion proteins.
cDNA encoding the murine B7-H1 extracellular domain was isolated by RT-PCR of splenic total RNA, digested by NcoI/HindIII, and then fused to an IL-3 leader sequence in p30242 vector. The fusion fragment was subcloned into pX58 vector containing the IE-175 promoter and the Fc portion of human IgG1, which was transfected into BHK/VP16 cells to generate B7-H1.Ig. The B7-H1.Ig in culture supernatants was purified on a protein A column (Bio-Rad Laboratories, Hercules, California, USA) and dialyzed in lipopolysaccharide-free PBS. Control human IgG was obtained from Sigma-Aldrich (St. Louis, Missouri, USA). We repeated one of the published in vivo experiments (7) to establish that our B7-H1.Ig did not have different properties from the B7-H1.Ig fusion proteins generated by other investigators. Specifically, we transplanted BALB/c hearts into C57BL/6 CD28-deficient recipients, and each recipient was treated with either 100 μg control Ig or B7-H1.Ig for 14 consecutive days from the day of the transplantation (n = 3 per group). We found that mice in the control Ig group rejected their allografts on days 9, 10, and 16 after transplantation. In contrast, B7-H1.Ig–treated mice retained their graft for more than 30 days. This difference is highly significant even with this small number of mice (P < 0.02 by log rank test) and is similar to the published observation (7).
In vitro proliferation and cytokine assays.
CD3+, CD4+, and CD8+ T cell populations (96–99% purity) were enriched by negative selection using a cocktail of mAb’s and magnetic beads (Miltenyi Biotec Inc., Auburn, California, USA). Anti-CD3, control IgG, and B7-H1.Ig were covalently attached to polyurethane-coated tosyl-activated Dynabeads (Dynal ASA, Oslo, Norway) according to the manufacturer’s instructions. For this, 3 μg of mAb to CD3 was added to 107 beads/ml in 0.1 M phosphate buffer, pH 7.4. Control IgG was added to the bead suspension in order to maintain a constant total Ig concentration of 5 μg/ml during binding. Similarly, anti–CD3/B7-H1.Ig beads were prepared with B7-H1.Ig representing 40% of the total bound protein (2 μg/107 beads). T cells (105) were cultured in 96-well flat-bottomed plates, and beads were added at a bead/cell ratio of 2:1. The cells were cultured for 96 hours, pulsed with 1 μCi of [3H]thymidine for 8 hours, and then harvested for liquid scintillation counting.
Results
The generation and characterization of transgenic mice expressing B7-H1 on pancreatic islet β cells.
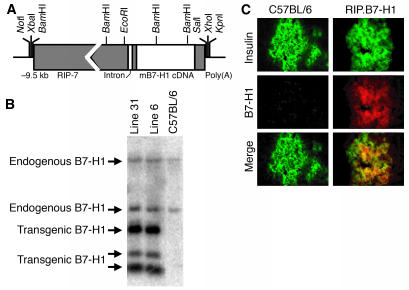
B7-H1 expression in peripheral tissues has been proposed to be a mechanism for inhibiting T cell–mediated immune responses and maintaining self-tolerance. We directly tested this hypothesis by generating transgenic mice (RIP.B7-H1) expressing B7-H1 on the pancreatic islet β cells under the control of the RIP (Figure 1, A and B). Tissue immunofluorescence staining was used to demonstrate that murine insulin and the B7-H1 transgene were expressed at the protein level. Both nontransgenic C57BL/6 and RIP.B7-H1 islets produced and expressed insulin (Figure 1C, top panels). However, the pancreatic islets in C57BL/6 mice did not express detectable levels of B7-H1, whereas in the transgenic mice the same cells that made insulin clearly expressed B7-H1 (Figure 1c, middle and bottom panels). B7-H1 protein expression on the transgenic islets was comparable by flow cytometry to levels found on splenic naive CD11b+ and CD11c+ cells (data not shown). It should be noted that transgenic expression of B7-H1 was detected only in the pancreatic islets and not in any of the other tissues examined (e.g., kidney and thymus; data not shown).
Figure 1.
Generation of RIP.B7-H1 transgenic mice. (A) A schematic of the RIP–murine B7-H1 hybrid gene. (B) Southern blot analysis of mouse genomic DNA digested with BamHI and hybridized with a full-length murine B7-H1 probe. (C) Immunofluorescence staining of insulin (green) and murine B7-H1 (red) in pancreata (original magnification, ×40) from C57BL/6 and RIP.B7-H1 mice.
To exclude the possibility that transgenic B7-H1 expression affected overall islet β cell function, we examined pancreatic islet cells from several young and old nondiabetic RIP.B7-H1 mice and found they had normal morphology based on histological tissue staining and electron microscopy. In addition, the pancreatic islets produced and secreted insulin comparably to those of C57BL/6 mice (data not shown). Finally, transgenic expression of B7-H1 did not affect the numbers, composition, or activation status (as determined by CD25, CD44 and CD69 expression levels) of lymphocytes in the thymus, spleen, and peripheral lymph nodes of RIP.B7-H1 compared with those of C57BL/6 mice (data not shown).
Accelerated rejection of B7-H1–expressing pancreatic islet β cells in an islet allograft setting.
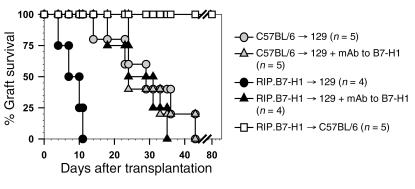
To study the regulatory role of B7-H1 in T cell–mediated immune responses in vivo, we chose a minor-mismatch transplantation model. We analyzed the function of B7-H1 expressed on islets by transplanting pancreatic islets from nondiabetic 10- to 15-week-old C57BL/6 and RIP.B7-H1 mice (C57BL/6 background) into STZ-induced diabetic 129 mice (all H-2b, mismatched for minor histocompatibility antigens). Surprisingly, B7-H1–expressing transplants promoted allograft rejection (median = 8.5 days) significantly faster than did control islets (median = 29 days; P < 0.01) (Figure 2). Two groups of recipients were transplanted with control islets or B7-H1–expressing islets and treated with mAb to B7-H1 (blocking antibody) to determine whether the accelerated rejection was B7-H1 dependent. As shown in Figure 2, we found that treatment with mAb to B7-H1 prevented the accelerated rejection of B7-H1–transgenic islets (median = 8.5 days for the RIP.B7-H1 group versus median = 27.5 days for the RIP.B7-H1 plus mAb to B7-H1 group; P < 0.01), whereas it did not affect the kinetics of rejection of control islets that did not express B7-H1. These data indicate that the accelerated rejection of B7-H1–expressing islets was B7-H1 dependent. To ensure that the accelerated rejection of B7-H1 transgenic islets was not due to reduced viability of the islet β cells, B7-H1 transgenic islets were also transplanted into STZ-induced diabetic syngeneic C57BL/6 mice. The transgenic islets survived indefinitely in syngeneic hosts; thereby demonstrating that RIP.B7-H1 islets can function normally long term in vivo (Figure 2). These results suggest that B7-H1 expression on the pancreatic islets can promote rather than inhibit T cell–mediated responses.
Figure 2.
Accelerated rejection of B7-H1–expressing pancreatic β cells in an islet allograft setting. Isolated islet β cells from C57BL/6 and RIP.B7-H1 (line 31) mice were transplanted into 129 recipient mice (C57BL/6 → 129 and RIP.B7-H1 → 129, respectively), and graft survival was determined. As indicated, some of the mice were treated with mAb to B7-H1. As an isograft control, islets from RIP.B7-H1 mice were transplanted into syngeneic C57BL/6 mice (RIP.B7-H1 → C57BL/6). The statistical significance between groups was determined using the Kaplan-Meier log-rank test method.
B7-H1 expression on islet β cells breaks immune tolerance and promotes autoimmunity.
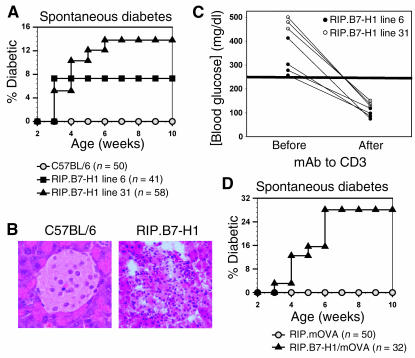
Mice in the C57BL/6 background are resistant to the development of autoimmune diabetes, even in many of those engineered to overexpress cytokines, chemokines, or T cell costimulatory molecules in the pancreatic islets (23–28). Unexpectedly, we found 7–14% of the transgenic mice expressing B7-H1 on islet β cells developed spontaneous diabetes within 3–6 weeks of age (Figure 3A). Histological examination of islets from these diabetic mice revealed grade 2 insulitis marked by lymphocytic infiltration (Figure 3B). To determine whether the diabetes was T cell dependent, low doses of mAb to CD3 were administered to diabetic mice, as this treatment has been shown to reverse T cell–mediated autoimmune diabetes in nonobese diabetic (NOD) mice by inducing tolerance (29, 30). After treatment, blood glucose levels in all diabetic mice returned to normal, suggesting that the spontaneous diabetes in RIP.B7-H1 mice was T cell dependent and autoimmune in nature (Figure 3C). These results demonstrate for the first time to our knowledge that B7-H1 expression on pancreatic islets can promote T cell–dependent autoimmunity.
Figure 3.
RIP.B7-H1 mice develop T cell–mediated spontaneous diabetes. (A) Incidence of spontaneous diabetes was evaluated in RIP.B7-H1 mice. (B) H&E staining (original magnification, ×40) of pancreata from C57BL/6 and diabetic RIP.B7-H1 mice. (C) Treatment of diabetic RIP.B7-H1 mice with mAb to CD3. As indicated in the graph, blood glucose levels were detected immediately before treatment onset and 4 days after treatment termination. (D) Incidence of spontaneous diabetes was evaluated in RIP.B7-H1/mOVA mice.
Ectopic pancreatic islet β-cell B7-H1 expression promotes CD8+ T cell priming and autoimmune disease induction.
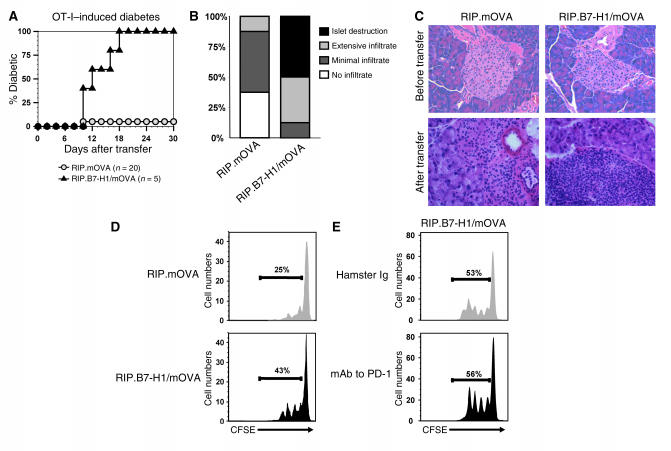
Antigen-specific autoreactive T cells are difficult to trace in RIP.B7-H1 mice because their T cells have a broad T cell receptor (TCR) repertoire and very low frequencies of each clone. To study an antigen-specific T cell–mediated autoimmune disease, we crossed the RIP.B7-H1 mice to RIP.mOVA mice to generate double-transgenic mice (RIP.B7-H1/mOVA mice) that express the well-defined antigen mOVA in addition to B7-H1 on islet β cells. These mice also developed spontaneous T cell–dependent autoimmune diabetes with similar kinetics but at an even higher frequency (∼30%; Figure 3D) compared with RIP.B7-H1 mice (7–14%, Figure 3A). We reasoned the transfer of OT-I T cells (OVA-specific CD8+ T cells) into mice with OVA-expressing islets would allow us to examine the role of local B7-H1 expression in regulating antigen-specific CD8+ T cell responses. We subsequently found that the transfer of 4 × 106 OT-I T cells (OVA-specific CD8+ T cells) into RIP.mOVA mice induced diabetes in all (six of six) mice within 14 days (data not shown), whereas the transfer of 2 × 106 OT-I T cells promoted diabetes in only one of twenty of the mice in our facility (Figure 4A). In contrast, the transfer of 2 × 106 OT-I T cells into 10-week-old RIP.B7-H1/mOVA mice (those that did not develop spontaneous diabetes) resulted in overt diabetes in all double-transgenic mice (five of five) within 10–18 days after transfer (Figure 4A). Histological examination of the pancreas at the time of diabetes initiation for RIP.B7-H1/mOVA mice or day 22 after transfer for RIP.mOVA mice revealed the presence of infiltrating T cells in both groups of mice, but β-cell destruction occurred solely in RIP.B7-H1/mOVA mice (Figure 4, B and C). The data suggest that local expression of B7-H1 in nonlymphoid tissues can promote autoimmune diseases.
Figure 4.
B7-H1 expression in the pancreatic islets promotes CD8+ T cell priming and autoimmune diabetes induction. (A) OT-I T cells (2 × 106) were adoptively transferred into RIP.mOVA and RIP.B7-H1/mOVA mice. Incidence of diabetes was evaluated at daily intervals. (B) Pancreata were stained with H&E, and individual islets from three mice per group were assigned scores for insulitis at the time of diabetes onset (RIP.B7-H1/mOVA mice) or day 22 after transfer for those mice that did not develop diabetes. (C) H&E staining of pancreata from RIP.mOVA and RIP.B7-H1/mOVA mice before and after transfer of 2 × 106 OT-I T cells. Original magnifications: top panels, ×20; bottom panels, ×40. (D) CFSE-labeled OT-I T cells (2 × 106) were adoptively transferred into RIP.mOVA and RIP.B7-H1/mOVA mice. Then, 42 hours later, the pancreatic draining lymph node cells were analyzed by flow cytometry. The numbers indicate the percentage of dividing OT-I T cells. (E) CFSE-labeled OT-I T cells (2 × 106) were adoptively transferred into RIP.B7-H1/mOVA mice. At the time of transfer, the mice were treated with 100 μg hamster IgG (top panel) or mAb to mouse PD-1 (bottom panel). Then, 42 hours later, the pancreatic draining lymph node cells were analyzed by flow cytometry. The numbers indicate the percentage of dividing OT-I T cells. Each of the experiments was repeated at least three times.
To determine whether transgenic expression of B7-H1 regulated T cell priming, we transferred 2 × 106 CFSE-labeled naive OT-I T cells into 10-week-old RIP.mOVA and RIP.B7-H1/mOVA mice. Then, 42 hours later, cells were recovered from the pancreatic draining lymph node and examined for proliferation based on CFSE dilution. This analysis revealed a significantly greater number of dividing T cells in RIP.B7-H1/mOVA recipients (Figure 4D). It should be noted that there were absolutely no dividing CFSE-labeled cells in the nondraining lymph nodes. The increased percentages of proliferating T cells in RIP.B7-H1/mOVA could be inhibited to levels observed in RIP.mOVA mice by administration of a mAb to B7-H1 at the time of OT-I transfer (data not shown). These results suggest that B7-H1 promotes CD8+ T cell proliferation and diabetes induction in vivo, although we cannot rule out the possibility that inflammatory cytokines produced at the site of islet destruction are contributing to enhancing T cell proliferation.
If blockade of PD-1 could reverse the costimulatory effect of ectopic B7-H1, we would conclude that the augmentation of T cell proliferation induced by B7-H1 was PD-1 dependent. To test this hypothesis, we adoptively transferred 2 × 106 CFSE-labeled OT-I T cells into RIP.B7-H1/mOVA mice and treated one group with hamster Ig and the other with hamster mAb to mouse PD-1 (blocking mAb) (Figure 4E). Administration of mAb to PD-1 did not reduce the enhanced T cell proliferation observed in RIP.B7-H1/mOVA mice. Although it was possible that B7-H1–mediated proliferation was PD-1 independent, this experiment does not rule out that the increased T cell proliferation was PD-1 dependent, as either mAb to PD-1 or ectopic B7-H1 could have, for instance, desensitized the PD-1 receptor.
B7-H1.Ig costimulates T cell responses both in vitro and in vivo.
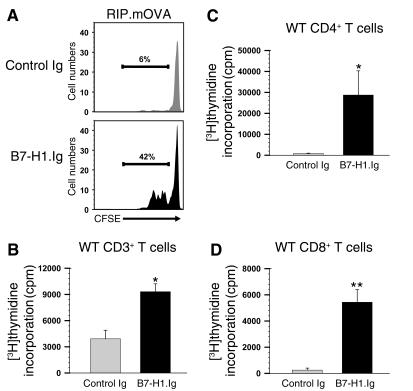
We generated a B7-H1.Ig fusion protein to further substantiate the role of B7-H1 as a costimulator of T cell immune responses. The specificity of B7-H1.Ig was confirmed by its ability to bind to a mAb to B7-H1 a well as to 293 cells expressing PD-1 (data not shown). To determine whether B7-H1.Ig could also enhance T cell proliferative responses in vivo, CFSE-labeled OT-I T cells were adoptively transferred into RIP.mOVA mice. At the time of transfer, the recipients were injected intraperitoneally with B7-H1.Ig or control Ig. Forty-two hours later, cells were recovered from the pancreatic draining lymph node and examined for proliferation based on CFSE dilution. A greater percentage of OT-I T cells isolated from B7-H1.Ig–treated mice (43%) had undergone cell division than had those from control mice (6%) (Figure 5A), whereas no dividing CFSE-labeled cells were observed in the nondraining lymph nodes. This is consistent with our data shown in Figure 4D, in which transgenic expression of B7-H1 in the pancreatic islets also increased T cell proliferation (42% versus 25%). It appears that in some settings B7-H1–mediated signals might be able to promote early T cell priming in vivo.
Figure 5.
B7-H1.Ig provides T cell costimulation both in vitro and in vivo. (A) CFSE-labeled OT-I T cells (2 × 106) were adoptively transferred into RIP.mOVA mice treated with 20 μg control Ig or B7-H1.Ig intraperitoneally at the time of transfer. Then, 42 hours later, the pancreatic draining lymph node cells were analyzed by flow cytometry. The numbers indicate the percentage of dividing OT-I T cells. (B–D) Purified CD3+ T cells (B), CD4+ T cells (C), and CD8+ T cells (D) from C57BL/6 mice were stimulated with bead-bound anti-CD3/control Ig or anti-CD3/B7-H1.Ig for 96 hours. The statistical significance between groups was determined using Student’s t-test: *P < 0.05; **P < 0.01.
To determine the role of B7-H1.Ig in regulating T cell responses in vitro, we stimulated purified T cells with bead-bound anti-CD3/B7-H1.Ig or anti-CD3/control Ig. We found that B7-H1.Ig costimulated CD3+, CD4+, and CD8+ T cell proliferation (Figure 5, B–D). It should be noted that in each of the bead-bound assays, anti-CD3/CD86.Ig was used as positive controls and resulted in slightly greater T cell proliferation than anti-CD3/B7-H1.Ig (data not shown). Together, these results demonstrate that B7-H1.Ig can costimulate T cell immune responses both in vitro and in vivo.
Discussion
Many recent studies have suggested that B7-H1 could inhibit T cell–mediated immune responses (5, 6, 31, 32). However, we have now presented the unexpected finding that B7-H1.Ig can augment naive T cell proliferation both in vitro and in vivo. Furthermore, ectopic expression of B7-H1 accelerated the rejection of allogeneic islet cells, and it promoted diabetes pathogenesis by breaking tolerance. Our results were especially striking because RIP.B7-H1 mice were generated on the C57BL/6 background that usually confers genetic resistance to autoimmune diabetes pathogenesis. Many cytokines, chemokines, or costimulatory molecules previously implicated in promoting autoimmunity have been expressed on C57BL/6 islet β cells, but have failed, on their own, to induce autoimmune diabetes. However, the combination of some of these molecules was shown to be pathogenic (23–28). For example, less than 1.5% of RIP.CD80 mice developed diabetes, but coexpression of CD80 and TNF in the pancreatic islets dramatically increased the rate of autoimmune diabetes (24).
To rule out the possibility that the B7-H1–expressing islets were more susceptible to cytotoxicity or apoptosis, we compared the islets from RIP.B7-H1 mice and C57BL/6 mice using several experimental approaches (data not shown). The morphology of the transgenic islets appeared to be normal based on histological and ultrastructural appearance (light microscopy and electron microscopy). Morphometric analysis demonstrated that the sizes of the transgenic islets were similar to those of C57BL/6 and RIP.mOVA islets. In addition, TUNEL-positive cells were not increased in transgenic islets. We also cultured “hand-picked” islets from transgenic and control mice, collected the supernatants, and stained the islets with Annexin V and propidium iodide, and found there was no difference in the numbers and percentages of apoptotic cells over time. Similar results were obtained in islets cultured in the presence of plate-bound PD-1.Ig, suggesting that the transgenic islets were not being rejected as a result of reverse signaling through B7-H1. In particular, because pancreatic IL-10 has been shown to accelerate diabetes (33), we also examined whether cultured RIP.B7-H1 islets produced IL-10 either spontaneously or after B7-H1 engagement by immobilized PD-1.Ig. A sensitive ELISA did not reveal detectable IL-10 in the supernatants of these islets. Finally, RIP.B7-H1 islets were accepted in syngeneic recipients, thereby indicating that these islets performed normally long term in vivo (Figure 2). Therefore, the tissue destruction presented in this study was consistent with T cell–mediated damage in a B7-H1–dependent manner.
It was unclear at this point whether the in vivo B7-H1–mediated effects were PD 1 dependent. Two reported features of PD-1 might be consistent with the possibility that PD-1 is able to play a costimulatory role in this setting. First, PD-1 might be able to transduce both positive and negative signals, as its cytoplasmic tail contains an immunoreceptor tyrosine-based switch motif (34), which has recently been described as being capable of providing diametrically opposed signals in CD150 subfamily members (35, 36). Second, a recent study has suggested that to deliver inhibitory signals, PD-1 needs to be in close proximity with the TCR (8). Ectopic B7-H1 on pancreatic islets might pull PD-1 away from TCRs that are interacting with APCs or target cells or might induce desensitization of the PD-1 signal transduction machinery, therefore preventing PD-1 from inhibiting T cell responses. The levels of cell surface B7-H1 expression could contribute to this effect, as we have found that the transgenic islets expressed B7-H1 at physiological levels similar to those observed on naive CD11b+ and CD11c+ splenocytes. Administration of blocking mAb to PD-1 did not reverse the B7-H1–mediated augmentation of T cell responses in our model (Figure 4E). It is conceivable that either the mAb to PD-1 or ectopic B7-H1 could prevent the inhibitory activity of PD-1 by precluding the spatial redistribution or cross-linking requirements necessary for its negative effects, or by desensitizing PD-1. More intriguingly, the result of our experiment using mAb to PD-1 could be interpreted to suggest that the effects of ectopic B7-H1 were PD-1 independent. A recent study has provided some evidence for an independent, non–PD-1 costimulatory receptor that can bind to B7-H1, using comparative molecular modeling and site-directed mutagenesis of the B7-H1 molecule (37). In such a case, one might envision that the outcome of an immune response might depend on the expression kinetics of the putative costimulatory receptor and PD-1, or on a balance of signaling between the two receptors, as postulated for CD28 and CTLA-4 (38). We attempted to directly address this issue by generating OT-I T cells deficient in PD-1. Unfortunately, due to defects in thymic selection, less than 1% of the T cells in the periphery expressed the transgenic TCR Vα2+Vβ7+ (our unpublished observations), precluding the assessment of whether antigen-specific PD-1–deficient T cells would fail to have enhanced proliferative responses in our vivo systems.
To ensure that the augmented T cell responses observed in B7-H1 transgenic animals were not specific to the ectopic expression of B7-H1, we utilized an alternative in vivo experimental approach in which RIP.mOVA mice were treated with B7-H1.Ig. In this model, the administration of B7-H1.Ig resulted in increased antigen-specific T cell responses. This was in contrast with published observations that B7-H1.Ig administration attenuated T cell responses. For instance, B7-H1.Ig in combination with a mAb to CD154 promoted islet allograft survival, whereas B7-H1.Ig alone had no therapeutic effects (17). Similarly, B7-H1.Ig treatment enhanced cardiac allograft survival and protected against chronic rejection (7). In contrast, a more recent study showed that the administration of B7-H1.Ig accelerated the lethality of graft-versus-host disease (39), a result similar to our previous observation that B7-H1.Ig promoted antibody production and CD4+ T cell responses to keyhole limpet hemocyanin (3). In another study, the administration of a mAb to B7-H1 promoted the development of autoimmune diabetes in NOD mice (19). In contrast, the same mAb to B7-H1 inhibited the development of chronic intestinal inflammation (40). Thus, it appears that the type of B7-H1 response might depend on the nature and stage of disease pathogenesis.
One possibility for the discrepancies of our data with previously published results could be attributed to potential differences in the B7-H1.Ig fusion proteins used in each of the studies. We obtained and tested some of the B7-H1.Ig proteins generated in other laboratories, and we found that they exhibited comparable costimulatory activities in our experimental model systems (data not shown). Conversely, we have utilized our B7-H1.Ig protein and reproduced the attenuation of immune responses previously reported in CD28-deficient mice transplanted with allogeneic hearts when treated with B7-H1.Ig (7). Together, these validations suggest that the diversity in the modulation of immune responses by B7-H1.Ig was not due to intrinsic differences in the reagents but was due to the complexities of immune interactions in vivo.
In summary, our findings support the notion that B7-H1 upregulation in some peripheral tissues can promote T cell priming and T cell–dependent tissue destruction rather than facilitate T cell tolerance. The apparent complicated dual effect of B7-H1 warrants further investigation to better predict the nature of responses before clinical trials are undertaken. It is possible that B7-H1 can deliver either positive or negative signals depending on the nature of the disease, the type of immune cells involved, the activation status of the T cells, and the genetic background of the mice. Blocking B7-H1 in specific settings might provide a novel approach for preventing allograft rejection and treating T cell–mediated autoimmune diseases while enhancing immune response for tumor rejection.
Acknowledgments
This research was supported by grants from the NIH (R01-HD37104, DK58897, P01-CA09296-01). Y. Sun is the recipient of an NIH training fellowship (T32 HL07237). We would like to give special thanks to Youjin Lee for technical assistance; Gorden Bowie for photographic assistance; Jerrold Turner for microscopy assistance; Louis Philipson and Yong Wang for helping us perform assays to examine islet functionality; and Anthony Montag for his insightful pathological observations.
Footnotes
Maria-Luisa Alegre and Yang-Xin Fu contributed equally to this work.
Nonstandard abbreviations used: cyclosporine A (CSA); membrane ovalbumin (mOVA); phycoerythrin (PE); rat insulin promoter (RIP); streptozocin (STZ); T cell receptor (TCR).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 3.Tamura H, et al. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809–1816. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 4.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter L, et al. PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur. J. Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Ozkaynak E, et al. Programmed death-1 targeting can promote allograft survival. J. Immunol. 2002;169:6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 8.Bennett F, et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 9.Gao W, Demirci G, Li XC. Negative T cell costimulation and islet tolerance. Diabetes Metab. Res. Rev. 2003;19:179–185. doi: 10.1002/dmrr.345. [DOI] [PubMed] [Google Scholar]
- 10.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 11.Rodig N, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 12.Selenko-Gebauer N, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J. Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 13.Brown JA, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 14.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 15.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wintterle S, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 17.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76:994–999. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- 18.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 21.Kurts C, et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herold KC, Montag AG, Buckingham F. Induction of tolerance to autoimmune diabetes with islet antigens. J. Exp. Med. 1992;176:1107–1114. doi: 10.1084/jem.176.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Transgenic tumor necrosis factor (TNF)-α production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-α and TNF-β transgenic mice. J. Immunol. 1993;150:4136–4150. [PubMed] [Google Scholar]
- 24.Guerder S, Picarella DE, Linsley PS, Flavell RA. Costimulator B7-1 confers antigen-presenting-cell function to parenchymal tissue and in conjunction with tumor necrosis factor alpha leads to autoimmunity in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5138–5142. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuff CA, Sacca R, Ruddle NH. Differential induction of adhesion molecule and chemokine expression by LTα3 and LTαβ in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J. Immunol. 1999;162:5965–5972. [PubMed] [Google Scholar]
- 26.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen SC, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J. Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 28.Luther SA, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 29.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc. Natl. Acad. Sci. U. S. A. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Q, et al. CD28/B7 regulation of anti-CD3-mediated immunosuppression in vivo. J. Immunol. 2003;170:1510–1516. doi: 10.4049/jimmunol.170.3.1510. [DOI] [PubMed] [Google Scholar]
- 31.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 32.Greenwald RJ, Latchman YE, Sharpe AH. Negative co-receptors on lymphocytes. Curr. Opin. Immunol. 2002;14:391–396. doi: 10.1016/s0952-7915(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 33.Balasa B, et al. Islet-specific expression of IL-10 promotes diabetes in nonobese diabetic mice independent of Fas, perforin, TNF receptor-1, and TNF receptor-2 molecules. J. Immunol. 2000;165:2841–2849. doi: 10.4049/jimmunol.165.5.2841. [DOI] [PubMed] [Google Scholar]
- 34.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidorenko SP, Clark EA. The dual-function CD150 receptor subfamily: the viral attraction. Nat. Immunol. 2003;4:19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- 36.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, et al. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J. Exp. Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 39.Blazar BR, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-γ-dependent mechanism. J. Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 40.Kanai T, et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J. Immunol. 2003;171:4156–4163. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]