Abstract
The mechanisms that lead to reticulin fibrosis of bone marrow (BM) in hairy cell leukemia (HCL) are not fully understood. We therefore investigated the involvement of TGF-β1, a potent fibrogenic cytokine, in this process. Immunoassays revealed that TGF-β1 is present at higher concentrations in BM, serum, and plasma of HCL patients in comparison with healthy donors (P < 0.001). RT-PCR and immunofluorescence studies showed that TGF-β1 is overexpressed at the mRNA and protein levels in peripheral blood, spleen, and BM mononuclear cells and that hairy cells (HCs) are the main source of TGF-β1. Active TGF-β1 correlated significantly with grades of BM fibrosis, infiltration with HCs, and serum procollagen type III aminoterminal propeptide (PIIINP). Ex vivo studies demonstrated that TGF-β1 significantly enhances the production and deposition of reticulin and collagen fibers by BM fibroblasts. In addition, BM plasma of HCL patients increased the synthesis of type I and type III procollagens, the main components of reticulin fibers, at the mRNA and protein levels. This fibrogenic activity of BM plasma was abolished by neutralizing anti–TGF-β1 antibodies. These results show, for the first time to our knowledge, that TGF-β1 is highly expressed in HCs and is directly involved in the pathogenesis of BM reticulin fibrosis in HCL.
Introduction
Hairy cell leukemia (HCL) is a chronic lymphoproliferative disorder characterized by the presence of hairy cells (HCs) in peripheral blood, bone marrow (BM), and spleen and is invariably associated with a unique type of BM fibrosis (1–3). Although a rare disease (2% of adult leukemia), HCL represents an excellent model for cancer biotherapy (4) and for understanding the deregulation of cytokines and growth factors in human neoplasia (5–7). The fibrotic process and the associated structural abnormality in BM of HCL patients are mainly due to accumulation of fine argyrophilic reticulin fibers, although collagen fibers can be observed in the advanced stages of the disease (8–11). The composition of reticulin fibers in HCL is not well defined. In normal and fibrotic BM, the distribution of reticulin fibers is identical to that of type III collagen and its precursor, type III procollagen (12, 13). Electron microscopic studies of human tissues revealed that reticulin fibers are individual collagen fibrils or small bundles of these fibrils embedded in the interfibrillar matrix of proteoglycans (14–16) and that they are composed mainly of type III collagen surrounding a core of type I collagen fibrils (17, 18). In addition to reticulin fibrosis, it has been recently demonstrated that the glycoprotein fibronectin, which is produced and assembled by HCs, contributes to the fibrotic process in BM of HCL patients (19). This process was also found to be particularly enhanced by bFGF, which is endogenously produced by the HCs (20). Since reticulin and fibronectin fibers were found to represent different structures in myelofibrotic BM (21), it appears that BM fibrosis in HCL is a complex process that involves accumulation and assembly of collagenous ECM components (reticulin) (22) and noncollagenous ECM components (fibronectin).
In BM, HCs are found in association with randomly dispersed fibroblastoid cells and are surrounded by reticulin fibers (9, 10, 23, 24). These fibroblastoid cells, which have been found in close association with collagen fibers, are the matrix-producing cells and are responsible for the synthesis of reticulin and collagen (9, 25–28). Other studies confirmed the increase in the collagen fibrils in the intercellular space around the HCs but did not find evidence that HCs give rise to these fibrils (10). These studies also showed that HCs are not argyrophilic (29), suggesting that they may not be the direct source of reticulin fibers. Since no massive increase in fibroblast numbers is observed in the BM of HCL patients (10), the increased production of reticulin and ECM proteins might be due to an accelerated differentiation of fibroblasts into matrix-producing cells rather than to an increased proliferation. This suggests that the fibroblastoid cells in HCL are exposed to mediators in the BM microenvironment, which mainly induce their differentiation and maturation without enhancing their proliferation. A possible candidate for such mediators is TGF-β, which is known as a potent fibrogenic cytokine (30) and exerts variable effects on fibroblasts in terms of proliferation and ECM synthesis (31). At low concentrations, TGF-β stimulates fibroblast proliferation, while at high concentrations it induces differentiation and collagen synthesis without increasing fibroblast numbers (32, 33). The fibrogenic property of TGF-β results not only from its induction of excessive production of ECM proteins but also from its inhibition of the synthesis of ECM-degrading enzymes (34, 35). In mammals, TGF-β is present in three isoforms, TGF-β1, -β2, and -β3 (36), and TGF-β1 is the most involved in fibrosis (30).
With regard to BM fibrosis, TGF-β has been implicated in the pathogenesis of idiopathic myelofibrosis and other myeloproliferative disorders (13, 37–39). In these diseases, megakaryocytes and monocytes have been recognized as sources of the fibrogenic cytokines (13, 37, 38). This situation may not be applicable to HCL, which is usually associated with monocytopenia and depletion of megakaryocytes (2, 40). Therefore, other cells such as the HCs might be the source of fibrogenic mediators. This suggestion would be in accordance with our previous observation that HCs produce high amounts of bFGF (5) and is underlined by the deregulated production of hematopoietic growth factors (6) and IFN-α (7) in HCL.
Therefore, we investigated the pattern of TGF-β1 expression in peripheral blood and BM of patients with HCL. We then studied the effect of TGF-β1 on deposition of reticulin and collagen fibers in vitro and its critical role in induction of BM reticulin fibrosis in HCL.
Methods
Patients.
Thirteen patients with HCL (ten male and three female), ten healthy donors (HDs), and five patients with B cell chronic lymphocytic leukemia (B-CLL) were investigated after informed consent was obtained. The ratio of male to female patients in our study, 4–5 to 1, closely matches the known predominance of the disease in men (2). Diagnosis of HCL was based on clinical presentation, the presence of HCs with typical morphology in the peripheral blood and BM, double immunofluorescence staining, and flow cytometric analysis using mAb’s against CD19 and CD11c and TRAP staining. Ten of the HCL patients were further analyzed for the relation between the degree of BM fibrosis and the concentrations of TGF-β1. BM reticulin fibrosis was evaluated using Gomori’s silver impregnation technique and the grading system proposed by Thiele et al. (41): 0, no increase in thickness and number of reticulin fibers; 1, borderline to minimal increase; 2, moderate increase; and 3, conspicuous increase. The degree of BM fibrosis was assessed by an investigator who was blinded to the results of TGF-β assays. The clinical and hematological data are shown in Table 1.
Table 1.
Clinical data of patients with HCL at the time of investigation
Serum and plasma collection.
Sera were prepared from nonheparinized peripheral blood by centrifugation. Peripheral blood and BM plasma (BMP) were separated from heparinized samples. To minimize platelet degradation and release of TGF-β from platelets, samples were kept on ice, centrifugation was performed at 4°C, and only the upper two-thirds of plasma was collected to avoid contamination with the platelets from the interface. All aliquots were immediately stored at –80°C until the time of the assays.
Cell isolation.
PBMCs and BM mononuclear cells (BMMCs) were isolated from heparinized blood samples and BM aspirates using Ficoll-Hypaque (Pharmacia Biotech AB, Uppsala, Sweden). HCs and normal B cells were purified from peripheral blood of four HCL patients and from four HDs by a magnetic cell-sorting technique (MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) with negative selection using a B cell isolation kit. Non-B cells (i.e., T cells, NK cells, monocytes, granulocytes, and erythroid cells) were magnetically labeled using a cocktail of biotin-conjugated antibodies against CD2, CD14, CD16, CD36, CD43, and CD235 and Anti-Biotin MicroBeads (Miltenyi Biotec GmbH). Depletion of magnetically labeled cells allowed the isolation of highly purified B cells. The purity of B cells and HCs was evaluated by FACS analysis using anti-CD19 and anti-CD11c antibodies. For isolation of BM fibroblasts (BMFs), BMMCs were suspended in α-MEM containing 20% FCS, and incubated in 25-cm2 tissue-culture flasks (Falcon; BD Biosciences Discovery Labware, Lincoln Park, New Jersey, USA) at 37°C in 5% CO2 in humidified air. After overnight incubation, nonadherent cells were removed and cultures were continued for 3–4 weeks with a weekly change of medium. At confluence, cells were trypsinized and subcultured in α-MEM (10% FCS). Cells from the third to fifth passages were used in the study. At these stages, cells were almost pure fibroblasts, as confirmed by morphology and staining with monoclonal mouse anti–human fibroblast antibodies (5B5; DAKO Diagnostics AG, Vienna, Austria). For isolation of BM stromal cells (BMSCs), BMMCs were incubated in α-MEM supplemented with 12% FCS, 12% horse serum, and hydrocortisone (10–6 M). Nonadherent cells were removed after 7 days of incubation, and adherent cells were fed weekly by replacement of the medium. After 4–6 weeks, adherent stromal cells reached confluence and consisted of fibroblasts, macrophages, and adipocytes as determined by their morphology and positive staining for 5B5, CD14, and oil red O, respectively.
Cell cultures and TGF-β1 immunoassays.
To measure the amounts of TGF-β1 produced by PBMCs and BMMCs, these cells were cultured at a density of 2 × 106 cells/ml in RPMI 1640 medium supplemented with 2% FCS. BMFs and BMSCs were cultured in α-MEM (2% FCS) (all reagents were GIBCO; Invitrogen Corp., Paisley, United Kingdom). The low concentration of FCS was used to maintain high cell viability and to prevent detachment of the fibroblasts and stromal cells throughout the incubation (1–3 days). After incubation, culture supernatants were collected and stored frozen at –80°C. TGF-β1 assays were carried out using the quantitative sandwich enzyme immunoassay (Quantikine; R&D Systems Inc., Minneapolis, Minnesota, USA), which detects the active form of TGF-β1. To detect the total amounts (active and latent forms) of TGF-β1, transient acidification was performed according to the manufacturer’s instructions. Since culture medium supplemented with 2% FCS contains detectable concentrations of TGF-β1, we measured the concentrations of TGF-β1 in cell-free cultures. The experiments were performed using the same batch of FCS that contained 12.2 ng/ml of TGF-β1, which was detectable only after activation procedures (i.e., medium supplemented with 2% FCS contained 0.24 ng/ml of TGF-β1). These concentrations were subtracted to calculate the quantity of TGF-β1 produced by the cells in cultures.
Detection of TGF-β1 and procollagens by immunofluorescence.
Indirect immunofluorescence was performed using mouse anti–human TGF-β1 antibodies, clone TB21, which react with active and latent TGF-β1 (BioSource International Inc., Camarillo, California, USA), and using mouse anti–human type I procollagen and rabbit anti–human type III procollagen mAb’s (Chemicon International Inc., Temecula, California, USA). Cytospin preparations of freshly isolated cells or fibroblasts cultured in tissue-culture chamber slides (Lab-Tek, Nunc Inc., Naperville, Illinois, USA) were fixed in cold methanol for 10 minutes and permeabilized with 0.05% NP40 in PBS for 10 minutes. Nonspecific binding was suppressed by incubation with 10% human AB serum (PAA Laboratories Gmbh, Linz, Austria) for 20 minutes. After washing with PBS, cells were incubated with the first antibodies overnight at 4°C and washed three times with PBS. Cells were then incubated with cyanine dye Cy3– or FITC-conjugated second antibodies for 45–60 minutes and washed extensively in PBS.
For localization of TGF-β1 in BM, double-immunofluorescence studies were performed on BM sections using anti–TGF-β1 antibodies (BioSource International Inc.) and anti-CD22 antibodies, clone SJ.10.1H11 (Immunotech-Coulter, Marseille, France), a marker for HCs (1, 2). Formalin-fixed and paraffin-embedded BM sections were deparaffinized and rehydrated through graded alcohol, and staining procedures were continued as above.
The specificity of the staining in all experiments was confirmed by negative controls, omitting the first antibodies from the staining process and using species- and isotype-matched unrelated antibodies (nonimmune mouse or rabbit IgG; BioSource International Inc.).
Detection of mature collagen and reticulin fibers in vitro.
BMFs were cultured in chamber slides in α-MEM supplemented with 10% FCS and allowed to reach confluence. Medium was changed and further supplemented with 10 μg/ml freshly prepared ascorbic acid (Sigma-Aldrich, St. Louis, Missouri, USA) to enhance collagen synthesis (42). Stationary cultures were continued for 4–6 weeks, and medium was changed weekly. Masson’s trichrome staining and Gomori’s silver impregnation staining were performed to detect collagen and reticulin fibers, respectively.
RIAs for serum procollagen.
Serum samples from ten HCL patients, with known degrees of BM fibrosis, and ten HDs were processed to measure the concentrations of procollagen type III aminoterminal propeptide (PIIINP), a noninvasive marker for ongoing BM fibrogenesis (43, 44). Specific PIIINP RIA kits (Orion Diagnostica, Espoo, Finland) were used according to the manufacturer’s instructions. The measurements were run in duplicate. Counting was performed using a gamma counter, and the final concentrations of PIIINP were determined by interpolation from the standard curve.
RT-PCR.
Total RNA was isolated by acid guanidinium thiocyanate-phenol-chloroform extraction techniques using RNAzol B (Tel-Test Inc., Friendswood, Texas, USA). cDNAs were synthesized from 1 μg of RNA. The synthesis efficiency in all samples was verified by 30 cycles of PCR reaction using β-actin–specific primers as previously described (7). For detection of TGF-β1 mRNA, the following primers were used for 35 cycles: upstream, 5′-CGGGCAGAGCTGCGTCTGCTGAGG-3′; downstream, 5′-GAGCTGAAGCAATAGTTGGTGTC-3′. For measurements of procollagen mRNA, two sets of specific primers were used for 35 cycles (45). The primers for type III (α1) procollagen were: upstream, 5′-GGTGAACGTGGCAGTCC-3′; downstream, 5′-GTTTCCATCTCTTCCAGGTT-3′ (fragment size 465 bp). The primers for type I (α1) procollagen were: upstream, 5′-TAAAGGGTCACCGTGGCTTC-3′; downstream, 5′-CGAACCACATTGGCATCATC-3′ (fragment size 355 bp). Primer pairs were driven from different exons to span introns so that the genomic DNA could be readily distinguished from cDNA based on size. The number of cycles was adjusted to the linear portion of the PCR amplification curve in preliminary experiments for detection of β-actin, TGF-β1, and procollagen mRNA expression. Amplified DNA was electrophoresed, stained with ethidium bromide, and photographed. The expression of the respective mRNA was corrected to β-actin mRNA in each sample.
Statistical analysis.
Grouped data are expressed as mean ± SEM. The results were compared for statistical significance using ANOVA, and P < 0.05 was considered statistically significant. Correlation between different parameters was calculated according to Pearson, using SPSS 10.0 software (SPSS Inc., Chicago, Illinois, USA).
Results
High levels of circulating TGF-β1 in HCL patients.
Immunoassays revealed that active and latent forms of TGF-β1 are significantly increased in BMP, serum, and peripheral blood plasma (PBP) of HCL patients as compared with HDs and patients with B-CLL (Figure 1, A and B). The mean concentration of active TGF-β1 (± SEM) was 10.2 ± 2.41 ng/ml in HCL patients but only 0.6 ± 0.23 ng/ml in HDs, a 17-fold difference (P < 0.001). Total TGF-β1 in BMP of HCL patients amounted to 24.5 ± 4.30 ng/ml, fivefold higher than in HDs (4.9 ± 0.95 ng/ml; P < 0.001). Mean concentrations of TGF-β1 were also higher in PBP and serum of HCL patients as compared with HDs. In plasma, the mean level of active TGF-β1 was 62-fold higher in HCL patients than in HDs (4.6 ± 1.05 vs. 0.07 ± 0.02 ng/ml; P < 0.001), while total TGF-β1 was fourfold higher in HCL patients (17.2 ± 1.79 vs. 4.4 ± 1.01 ng/ml; P < 0.001). In serum, active TGF-β1 was 55-fold higher in HCL patients than in HDs (7.2 ± 1.54 vs. 0.13 ± 0.04 ng/ml; P < 0.001), while total TGF-β1 was threefold higher in HCL patients (27 ± 1.63 vs. 9.7 ± 2.76 ng/ml; P < 0.005). The amount of TGF-β1 was also measured in samples of five patients with B-CLL. The mean concentrations of active TGF-β1 in BMP, serum, and PBP of B-CLL patients were 0.59 ± 0.37, 0.11 ± 0.07, and 0.16 ± 0.8 ng/ml, respectively. These values were comparable to those for TGF-β1 in samples of HDs but significantly lower than in HCL patients. Total TGF-β1 in BMP, serum, and PBP was 7.05 ± 1.05, 12.32 ± 2.78, and 8.08 ± 2.12 ng/ml, respectively. These concentrations were higher than in HDs but significantly lower than in HCL patients. Since TGF-β might be released from the platelets during sample preparation, we studied the relation between TGF-β1 serum concentration and the number of platelets. No correlation between the two parameters was found, which suggests that the amounts of TGF-β1 detected in the samples reflect the concentrations of circulating TGF-β1 rather than the amount released from platelets.
Figure 1.
Levels of TGF-β1 in BMP, serum, and PBP. TGF-β1 was measured by immunoassays before and after acidification to detect active (A) and total (B) TGF-β1. The levels of active and total TGF-β1 were significantly higher in HCL patients (n = 13) than in HDs (n = 10) and B-CLL patients (n = 5). *P < 0.01; **P < 0.001. (C) High expression of TGF-β1 mRNA in PBMCs of HCL patients (lanes 7–12) in comparison with HDs (lanes 1–6). (D) Overexpression of TGF-β1 mRNA in BMMCs of three HCL patients and spleen (spl.) cells of two HCL patients compared with BMMCs of three HDs and PBMCs (lanes 9 and 10) and BMMCs (lanes 11 and 12) of B-CLL patients.
Overexpression of TGF-β1 mRNA in HCL.
To study the transcriptional regulation of TGF-β1 in HCL patients, PBMCs (from six HCL patients, six HDs, and two B-CLL patients), BMMCs (from three HCL patients, three HDs, and two B-CLL patients), and spleen cells obtained from postsplenectomy material (from two HCL patients) were isolated and immediately processed for RT-PCR analysis. As demonstrated in Figure 1, C and D, PBMCs from HCL patients expressed high levels of TGF-β1 mRNA as compared with HDs and B-CLL patients. The intensity of TGF-β1 mRNA signals was quantitated by scanning densitometry and corrected to β-actin mRNA signals. Comparison between TGF-β1 mRNA signals confirmed that TGF-β1 mRNA expression in HCL patients was significantly higher than in HDs and B-CLL patients (P < 0.001). TGF-β1 mRNA expression was also higher in BMMCs of HCL patients (>90% HCs) than in those of HDs and B-CLL patients (Figure 1D; P < 0.01). The expression of TGF-β1 mRNA in spleen cells of HCL patients (>95% HCs) was comparable to its levels in BM cells.
TGF-β1 production by PBMCs and HCs.
The overexpression of TGF-β1 at the transcriptional level in hematopoietic cells of HCL patients suggested that these cells may also produce high amounts of this cytokine and are the source of the circulating TGF-β1. To verify this suggestion, PBMCs of six HCL patients, six HDs, and five B-CLL patients were cultured for 48 hours, and the concentrations of TGF-β1 protein in supernatants was measured by ELISA. As illustrated in Figure 2A, PBMCs of HCL patients produced significantly higher amounts of TGF-β1 proteins (active and total) as compared with those of HDs (n = 6) (P < 0.01). While total TGF-β1 produced by PBMCs of HCL patients was fourfold higher, active TGF-β1 in HCL patients was 42-fold higher than in HDs (P < 0.001). Similarly, the amounts of TGF-β1 (active and total) produced by HCL cells were significantly higher than those produced by B-CLL cells. It is important to note that the amount of active TGF-β1 produced by B-CLL cells was similar to that produced by PBMCs of normal persons. To investigate the contribution of HCs to the production of TGF-β1, immunofluorescence staining for intracellular TGF-β1 was performed in purified HCs (>95% CD11c/CD19–positive cells) from peripheral blood of four HCL patients and compared with normal B cells (>95% CD19-positive cells) obtained from four HDs and PBMCs of four patients with B-CLL (<85% leukemic cells). A representative experiment is shown in Figure 2, where HCs (Figure 2B) show very intense cytoplasmic staining for TGF-β1 as compared with normal B cells (Figure 2C) and B-CLL cells (Figure 2D). Furthermore, the purified HCs and B cells were cultured for 48 hours, and TGF-β1 concentrations in culture supernatants were measured by ELISA. Significantly higher amounts of active and total TGF-β1 were produced by HCs in comparison with normal B cells (3.6 ± 0.7 vs. 0.06 ± 0.01 ng/ml and 16.1 ± 0.8 vs. 2.0 ± 0.1 ng/ml, respectively; P < 0.001). These data indicate that the HCs are more efficient in producing TGF-β1, particularly in its active form, than are normal B cells and B-CLL cells and that they represent a major source for TGF-β1 in HCL patients. We therefore focused our investigations on cells from HCL patients and HDs.
Figure 2.
TGF-β1 production by PBMCs and intracellular localization in normal B cells and HCs. (A) Cells were cultured for 48 hours and secreted TGF-β1 (active and total) was measured by ELISA. Significantly higher concentrations of active and total TGF-β1 were detected in supernatants of PBMCs of HCL patients as compared with HD and B-CLL cells. (B–E) Immunofluorescence staining using anti–TGF-β1 (TB21) antibody was performed on purified HCs, normal B cells, and B-CLL cells (>85% leukemic cells). A strong staining for TGF-β1 was found in HCs (B) in comparison with normal B cells (C) and B-CLL cells (D). (E) Nonimmune mouse IgG1 antibody was used as a negative control. A representative of four experiments is demonstrated. Original magnification, ×400.
Source of TGF-β1 in BM.
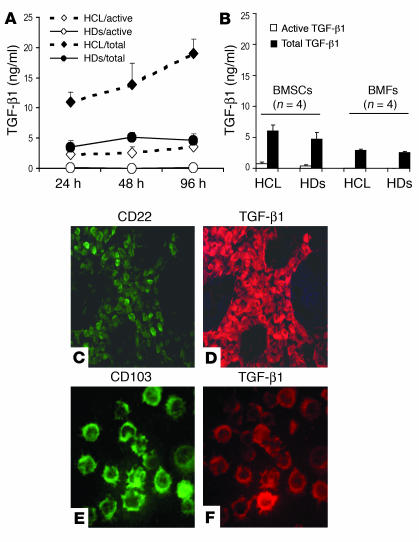
To identify the source of the increased TGF-β1 proteins in BM of patients with HCL, a set of experiments was performed using different cellular components of BM, including BMMCs, purified BMFs, and BMSCs. BM cells from four patients with 50–90% HC infiltration and from four HDs were cultured for 24, 48, and 96 hours. Supernatants were collected and assayed for TGF-β1 production. As shown in Figure 3A, at each time point, BMMCs from HCL patients produced significantly higher amounts of active and total TGF-β1 than BMMCs of HDs (P < 0.001). There was also a continuous increase in the production of TGF-β1 throughout the incubation, while BMMCs of HDs reached a maximum level after 2 days with relative decrease thereafter. Since BMSCs and BMFs can also produce TGF-β, these cells were isolated from four HCL patients and four HDs and cultured for 48 hours, and supernatants were assayed to measure the secreted TGF-β1. As shown in Figure 3B, BMSCs and BMFs from HCL patients produced amounts of active and total TGF-β that were comparable to those produced by HD cells. In addition, the amounts of TGF-β1 produced by BMFs and BMSCs were much lower than those produced by the BMMCs. These experiments demonstrated that the elevated levels of TGF-β1 in the BM of HCL patients could be due to an increased production by the hematopoietic cells rather than by BMSCs or BMFs. To localize the TGF-β1–producing cells in BM of HCL patients, BM sections and aspirates were subjected to double immunofluorescence staining using anti–TGF-β1 antibody in combination with anti-CD22, a marker for HCs (1, 2). As demonstrated in Figure 3, C and D, a very intense staining for TGF-β1 was found in the majority of HCs (CD22-positive). TGF-β1 immunoreactivity was also observed in the intercellular space. Furthermore, cytospin preparations of BMMCs (>90% HCs) were double-stained with anti–TGF-β1 and with anti–B-ly7 antibodies (Immunotech/Coulter), which recognize CD103, a specific HC marker (1, 2). As illustrated in Figure 3, E and F, TGF-β1 was also found to be highly expressed specifically in the HCs (CD103-positive). Collectively, these results confirm that the HCs represent a major source for TGF-β1 within the hematopoietic cells and are thus responsible for the elevated levels of TGF-β1 in BM and peripheral blood of HCL patients.
Figure 3.
Secreted and intracellular TGF-β1 in BM cells. (A) BMMCs of HCL patients produced significantly higher amounts of TGF-β1 throughout the incubation than BMMCs of HDs. (B) The amounts of TGF-β1 secreted by BMSCs and BMFs of HCL patients were comparable to the amounts secreted by HD cells. (C and D) Double immunofluorescence staining of BM sections of an HCL patient (representative of four patients tested) shows the colocalization of CD22 (an HC marker) (C) and TGF-β1 (D). (E and F) Cytospin preparation of BMMCs (>90% HCs) double-stained with antibody against CD103 (B-ly7, a specific HC marker) and against TGF-β1 (E and F), confirming the presence of TGF-β1 in the HCs. Original magnification, ×100 (C and D) and ×400 (E and F).
Correlation among TGF-β1, BM fibrosis, and serum PIIINP.
To study the relation between TGF-β1 and the process of BM fibrosis, the concentrations of this cytokine in BMP of ten HCL patients were compared with the degree of BM fibrosis (41). A significant positive correlation was found between the TGF-β1 levels (active and total) in BMP and the degree of BM fibrosis (Figure 4, A and B). There was also a significant correlation between the levels of active and total TGF-β1 and the percentage of HCs in BM (Figure 4, C and D). Since serum concentration of PIIINP is considered a noninvasive marker for the ongoing accumulation of interstitial collagen in BM and correlates with the degree of BM reticulin fibrosis (43, 44, 46), we studied the relation among serum PIIINP, the degree of BM fibrosis (41), and TGF-β1 concentrations (active and total) in ten HCL patients and ten HDs. Serum PIIINP was significantly higher in HCL patients than in HDs (mean 6.22 ± 3.63 vs. 3 ± 0.8 μg/ml; P < 0.04). As illustrated in Figure 4E, there was a significant correlation between serum PIIINP and the degree of BM fibrosis (r = 0.836). There was a significant correlation between serum PIIINP and active TGF-β1 in BMP (Figure 4F), serum and PBP (r = 0.755 and 0.674, respectively) (not shown). In addition, serum PIIINP correlated significantly with the percentage of HCs in the BM (r = 0.763; not shown). These results clearly demonstrate the close relation among TGF-β1, extent of BM fibrosis, and infiltration of the BM with leukemic cells. They also suggest that the concentrations of circulating active TGF-β1 may reflect the degree of BM fibrosis and infiltration with the leukemic cells as well as the ongoing processes of BM fibrogenesis.
Figure 4.
Correlation among TGF-β1 concentrations, grades of BM fibrosis, and the percentage of HCs in the BM. The concentrations of active and latent TGF-β1 in BMP of HCL patients (n = 10) correlate significantly with grades of BM fibrosis (A and B) and with percentage of HCs in BM (C and D). Serum levels of PIIINP significantly correlate with grades of BM fibrosis (E) and with active TGF-β1 in BMP (F). The gray symbols represent the mean value of TGF-β1 in BM (A–D) and PIIINP in serum (E and F) of ten HDs.
TGF-β1 induces reticulin synthesis and deposition in vitro.
To get a closer insight into the role of BMFs and TGF-β1 in the induction of BM reticulin fibrosis in HCL, in vitro experiments were performed using BMFs from HCL patients and HDs. Stationary fibroblast cultures were established, and the production of collagens and reticulin was evaluated by Masson’s trichome and Gomori’s silver impregnation stainings under basal conditions and upon stimulation with TGF-β1. As illustrated in Figure 5 (which shows a representative of five experiments), early passages (the third through the fifth) of BMFs from HDs (Figure 5, A and F) produced spontaneously lower amounts of collagen and reticulin fibers than BMFs of HCL patients (Figure 5, B and G). These fibers had smaller diameters and were running in parallel to the fibroblasts of HDs, while in HCL patients the fibers were thicker and running in different directions. Addition of TGF-β1 (2.5–5 ng/ml) further enhanced the deposition of both collagen and reticulin fibers (Figure 5, C and H). However, the effect of TGF-β1 on reticulin was more dramatic and resulted in an obvious increase in the number and thickness of these fibers and led to the formation of a tight network of reticulin. Addition of neutralizing mouse anti–human TGF-β1 mAb (TB21) significantly decreased the number and thickness of collagen and reticulin fibers (Figure 5, D and I), while equivalent amounts of isotype-matched control antibody (mouse IgG1) had no effect (Figure 5, E and J). It is important to note that later passages of BMFs of HCL patients and HDs (not shown) exhibited a similar pattern of response to TGF-β1 and TGF-β1–neutralizing antibody in terms of reticulin and collagen synthesis. This situation could be explained by the normal, reactive and non-neoplastic nature of BMFs in patients with myelofibrotic disorders (47–49). These results point to an activated state of BMFs of HCL patients and show that TGF-β1 plays a substantial role in the synthesis and deposition of collagen and reticulin fibers by these fibroblasts.
Figure 5.
In vitro production of collagen and reticulin fibers by BMFs and the effect of TGF-β1. Confluent stationary fibroblast cultures (4–6 weeks) were performed, mature collagen fibers were visualized by Masson’s trichrome staining (A–E), and reticulin fibers were stained by Gomori’s silver impregnation technique (F–J). Under basal conditions, BMFs of HCL patients produced more collagen and reticulin fibers (B and G) than did fibroblasts of HDs (A and F). TGF-β1 (5 ng/ml) increased the number and thickness of collagen and reticulin fibers (C and H), while anti–TGF-β1 antibody significantly decreased fiber deposition (D and I). Control antibody had no effect (E and J). Original magnification, ×600.
Biologically active TGF-β1 in BMP of HCL patients.
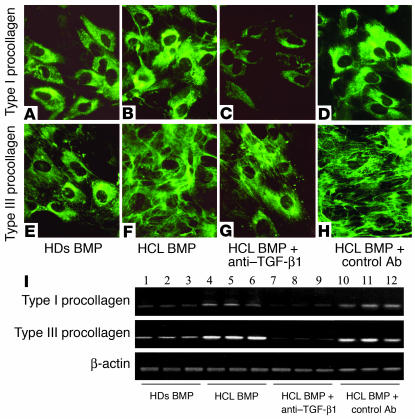
To test whether TGF-β1 in BM is present in a biologically active form that contributes to the synthesis of collagen and reticulin fibers, three separate sets of experiments on stationary BMF cultures were performed. BMFs of three patients with HCL were cultured and supplemented with 10% of BMP obtained from three HDs and three HCL patients. After 48 hours, immunofluorescence staining was performed using antibodies against type I (α1) and type III (α1) procollagens, which are the precursors of type I and type III collagens and are the major components of reticulin fibers (12, 13, 17, 18). A representative experiment is shown in Figure 6, A–H. BMP of HCL patients was more potent in enhancing the synthesis of both types of procollagen (Figure 6, B and F) as compared with BMP of HDs (Figure 6, A and E). This effect was more prominent on type III procollagen, which was also induced to form a dense extracellular network (Figure 6F). The enhancing effect of BMP of HCL patients was abolished by anti–TGF-β1 mAb (Figure 6, C and G). An equal amount of control antibody had no effect (Figure 6, D and H). In another set of experiments, BMFs were cultured and treated as above, and cells were processed for RT-PCR analysis. As demonstrated in Figure 6I, BMP of HCL patients significantly increased the expression of type I and type III procollagen mRNA (lanes 4–6) as compared with BMP of HDs (lanes 1–3). The effect of BMP on type III procollagen mRNA was more pronounced than the effect on type I procollagen mRNA. The enhancing effect of BMP of HCL patients was also abolished by anti–TGF-β1 antibody (lanes 7–9) but not by control antibody (lanes 10–12). There was also a relative decrease in the expression of type I and type III procollagens at the mRNA and protein levels upon addition of anti–TGF-β1 antibody, but not upon addition of control antibody, to fibroblasts treated with BMP of HDs (not shown). These data demonstrate that the fibrogenic activity in the BMP of HCL patients is due to the high content of biologically active TGF-β1. In addition, the enhancing effect of BMP is more prominent on type III procollagen (the main component of reticulin) at the mRNA and protein levels.
Figure 6.
Induction of type I and type III procollagen synthesis by BMP of HCL patients. (A–H) BMFs of three HCL patients were cultured and supplemented with 10% BMP of HDs or HCL patients alone or in the presence of neutralizing anti–TGF-β1 antibodies. A representative of three experiments is demonstrated. Type I and type III procollagen was detected mainly intracellularly in the presence of HD BMP (A and E) and was significantly increased in response to HCL BMP (B and F). HCL BMP dramatically increased extracellular deposition of type III procollagen. The effect of BMP was abolished by anti–TGF-β1 antibody (C and G). Control antibody had no effect (D and H). Original magnification, ×400. (I) The effect of BMP on mRNA expression of type 1 (α1) and type III (α1) procollagen in BMFs of three patients with HCL was also investigated, and a representative experiment is shown. BMP of HCL patients (n = 3) had a stronger enhancing effect on the mRNA expression of both types of procollagen (lanes 4–6) than BMP of three HDs (lanes 1–3). The effect of HCL BMP was also abolished by anti–TGF-β1 antibody (lanes 7–9), while the control antibody had no effect (lanes 10–12).
In vitro interaction between HCs and BMFs.
Since HCs are found in close association with fibroblastoid cells in the BM (9, 10, 23, 24), we studied the interaction between the two cell types in vitro. To mimic the in vivo situation, we performed autologous cocultures using purified HCs and BMFs and studied the expression of TGF-β1 and type III procollagen (a major component of reticulin fiber and ECM of BM). As demonstrated in Figure 7A, HCs were closely associated with and adhered to BMFs. While HCs showed a very intense intracellular staining for TGF-β1, the fibroblasts were strongly positive for intracellular type III procollagen, which was also deposited extracellularly, forming fibrillar structures. TGF-β1 was also found to be deposited in the extracellular space on the matrix produced by the fibroblasts. Addition of neutralizing anti–TGF-β1 antibody significantly decreased the intracellular contents of type III procollagen in BMFs and inhibited the deposition of the fibrillar matrix (Figure 7B). Consequently, minimal or no TGF-β1 was detected extracellularly. Equivalent amounts of control antibody did not show this effect (Figure 7C). Cocultures were also performed using normal B cells and BMFs. Results showed that expression of TGF-β1 was weak in normal B cells and that type III procollagen was present intracellularly in the fibroblasts, and no deposition of TGF-β1 or fibrillar matrix was observed extracellularly (Figure 7D). These experiments suggest that HCs produce high amounts of TGF-β1, which activates the fibroblasts in their proximity to produce type III procollagen and other matrix proteins. They also illustrate that the secreted TGF-β1 can be stored in the extracellular space bound to the matrix proteins produced by the fibroblasts.
Figure 7.
Interaction between HCs and BMFs in vitro. Purified HCs or B cells were cultured on top of BMFs in the absence or presence of anti–TGF-β1 antibody. After 24 hours of incubation, immunofluorescence was performed, and a representative of four experiments is demonstrated. (A) An intense immunoreactivity for TGF-β1 (red fluorescence) was found intracellularly in the HCs (large arrows) and on the matrix produced by the fibroblasts (small arrows). Green fluorescence demonstrates intracellular type III procollagen in the fibroblasts and its extracellular deposition. (B and C) Anti–TGF-β1 antibody significantly inhibited the synthesis of type III procollagen, formation of fibrillar matrix, and deposition of TGF-β1 on this matrix (B), while control antibody had no effect (C). (D) Coculture of normal B cells and BMFs showed a weak TGF-β1 immunoreactivity in B cells (arrow) and absence of extracellular deposition of TGF-β1 and fibrillar matrix. Original magnification, ×400.
Discussion
BM fibrosis in HCL is caused by the formation of a fine network of reticulin fibers (2, 3, 8–10). The mechanisms and mediators responsible for induction and progression of this unique type of fibrosis are not completely defined. In this study we sought to identify the pattern of TGF-β1 expression in HCL patients and to explore the involvement of this cytokine in the pathogenesis of reticulin fibrosis of the BM in HCL patients. The results reported here demonstrate that BMP, serum, and PBP of HCL patients contain significantly high amounts of active and latent TGF-β1. They also show that TGF-β1 is highly expressed at the mRNA and protein levels in peripheral blood, spleen, and BMMCs of HCL patients and that the HCs represent the major source of TGF-β1. In addition, we were able to illustrate that TGF-β1 is directly involved in enhancing the synthesis of reticulin fibers by BMFs of HCL patients.
Detection of high levels of circulating TGF-β1 in HCL patients is an important finding and is in agreement with the recent data of Rameshwar et al., who demonstrated that systemic TGF-β1 is elevated in patients with idiopathic and secondary myelofibrosis (50). TGF-β is usually produced as a large latent complex, and the active form has to be released to produce biological effects (51). The high concentrations of circulating active and latent TGF-β1 and increased production by HCs could be related to the malignant phenotype of HCL, as has been reported for B-CLL (52, 53). They could be also attributed to the activated state of the HCs (1), or to the perturbed regulation of cytokine production in HCL, as we have previously demonstrated (5–7). Although the molecular mechanisms leading to activation of TGF-β1 in HCL are not completely understood, it is possible that HCs produce proteases that activate latent TGF-β in a manner similar to that of some neoplastic cells (54). In addition, direct contact between HCs and BMSCs may also induce activation of TGF-β1 through plasmin, as has been reported in cocultures of endothelial cells, pericytes, and smooth muscle cells (55). Another important factor that may contribute to the overproduction and activation of TGF-β in the BM is bFGF, which is also present at high concentrations in BM of HCL patients (5). bFGF was shown to increase the release of TGF-β1 (56), to induce its activation (57), and to enhance its fibrogenic activity (58). On the other hand, TGF-β1 induces bFGF mRNA expression (56) and release of matrix-bound bFGF (59). It is therefore possible that mutual interaction between both cytokines within the BM microenvironment of HCL patients leads to the increased production and activation of TGF-β1 and also enhances its fibrogenic activity, as has been suggested in other myelofibrotic disorders (60, 61).
To understand the relevance of circulating TGF-β1 to the fibrogenic processes in BM, we studied the correlation between the cytokine and the grade of reticulin fibrosis as well as the serum levels of PIIINP, a noninvasive marker for the process of fibrogenesis (21, 44, 46). The results demonstrated a significant correlation between TGF-β1 concentrations, particularly its active form, in BM, serum, and plasma, and the histological grade of BM reticulin fibrosis, as well as the serum levels of PIIINP. Moreover, the concentrations of active TGF-β1 significantly correlated with the extent of BM infiltration with the leukemic cells. These results point to a strong link between active TGF-β1 concentrations, the ongoing processes of BM fibrosis, and the extent of BM infiltration with HCs. Therefore, the levels of circulating active TGF-β1 may have a significant clinical relevance and represent a potential noninvasive marker for BM fibrosis and infiltration with HCs. This suggestion however, needs to be substantiated by studies on a larger number of patients.
To get insight into the role of TGF-β1 in the activation of BMFs and induction of BM fibrosis in HCL, we isolated BMFs and assessed their ability to synthesize collagen and reticulin fibers under basal conditions and after TGF-β1 stimulation. Early passages of BMFs of HCL patients were more efficient in producing collagen and reticulin than were fibroblasts from HDs. Exposure of BMFs to TGF-β1 further enhanced the synthesis and deposition of both collagen and reticulin fibers and led to the formation of a tight reticulin network. This suggests that fibroblasts of HCL patients exhibit an activated phenotype due to exposure to fibrogenic activities in the BM, that this phenotype is retained in vitro, and that TGF-β1 is a component of this activity. In support of this suggestion is that exposure of BMFs to BMP obtained from HCL also enhanced the mRNA expression and protein synthesis of type I and type III procollagens (the main components of reticulin fibers). This effect was completely abolished by anti–TGF-β1 antibody. Therefore, these data confirm that TGF-β1 is present in BM of HCL patients in a biologically active form, which contributes substantially to the activation of BMFs and induction of reticulin fibrosis.
The in vitro data presented in this work are closely related to the situation in vivo in BM of HCL patients. Several studies have shown a close association among HCs, the fibrous network, and fibroblastoid cells in the BM (9, 10, 23, 24), suggesting that HCs may induce activation of the fibroblastoid cells to produce the fibrous network. Here, we demonstrate, in coculture experiments, that HCs adhere to and are in close association with BMFs and also contain high amounts of TGF-β1. The cytokine was also found to be deposited on the fibrillar matrix actively produced by the fibroblasts. This in vitro observation appeared similar to the immunoreactivity of TGF-β1 in BM sections of HCL patients, where TGF-β1 was detected in the HCs and also in the extracellular space. It is also in agreement with the reported distribution of TGF-β1 in BM of patients with hematological malignancies and myelofibrosis (62). Therefore, it is conceivable that HCs produce high amounts of TGF-β1 in BM, and that the TGF-β1 is stored near BMFs, activates them, and ultimately leads to excessive deposition of ECM proteins and fibrosis.
Since no massive increase in fibroblast number is generally observed in BM of HCL patients, it appears that the high activity of TGF-β1 in the BM substantially enhances the fibrogenic properties of the fibroblasts without increasing their proliferation rate. In support of this view is that the concentrations of TGF-β1 detected in the BM in this study are similar to those that inhibit proliferation but effectively enhance ECM production by BMFs in vitro (32). Similarly, exposure of fibroblasts to vitamin A and retinoic acid also leads to a decrease in growth rate of the fibroblasts but enhances synthesis and accumulation of collagen (63). In addition, it is known that the fibroblasts are more efficient in the synthesis of ECM proteins during the stationary stage of growth than during active proliferation (42, 64–66).
Although other cytokines, such as bFGF, PDGF, EGF, and calmodulin are also involved in myelofibrosis through their mitogenic effect on the fibroblasts (38, 60, 67), TGF-β seems to play an important role in the pathogenesis of BM fibrosis in HCL particularly through its enhancing effect on the synthesis and deposition of ECM proteins (32, 34–36). Moreover, because of its inhibitory effect on the mitogenic response of the fibroblasts (68), TGF-β1 may limit the fibrotic process in HCL to the characteristic fine reticulin fibrosis (mainly type III collagen) and slow down its progression into advanced collagen fibrosis (mainly type I collagen), which is found in other myelofibrotic disorders (12, 16, 69, 70). This notion is substantiated by our findings that TGF-β1 exhibited a stronger effect on the synthesis and deposition of type III procollagen and reticulin than on type I procollagen and mature collagen fibers.
In conclusion, the studies presented here shed light on the critical role of TGF-β1 in the induction of reticulin fibrosis in HCL and as a potential indicator for disease progression. Furthermore, they suggest that targeting of TGF-β1 represents a possible therapeutic approach to the prevention of BM fibrosis in HCL. These results will also raise questions about the possible involvement of this cytokine, as a potent inhibitor of hematopoiesis and an immunoregulatory agent (71), in the pathogenesis of pancytopenia and recurrent infection in HCL (2).
Acknowledgments
This study was partly supported by grants to M. Shehata from the Commission of Oncology (University of Vienna) and from the Medizinisch-Wissenschaftlicher Fonds des Buergermeisters der Bundeshauptstadt Wien (project no. 1480 and no. 1808).
Footnotes
Nonstandard abbreviations used: B cell chronic lymphocytic leukemia (B-CLL); bone marrow (BM); BM fibroblast (BMF); BM mononuclear cell (BMMC); BM plasma (BMP); BM stromal cell (BMSC); hairy cell (HC); HC leukemia (HCL); healthy donor (HD); peripheral blood plasma (PBP); procollagen type III aminoterminal propeptide (PIIINP).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Pettitt AR, Zuzel M, Cawley JC. Hairy-cell leukaemia: biology and management. Br. J. Haematol. 1999;106:2–8. doi: 10.1046/j.1365-2141.1999.01500.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodman GR, Bethel KJ, Saven A. Hairy cell leukemia: an update. Curr. Opin. Hematol. 2003;10:258–266. doi: 10.1097/00062752-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Golomb HM, Catovsky D, Golde DW. Hairy cell leukemia: a clinical review based on 71 cases. Ann. Intern. Med. 1978;89:677–683. doi: 10.7326/0003-4819-89-5-677. [DOI] [PubMed] [Google Scholar]
- 4.Vedantham S, Gamliel H, Golomb HM. Mechanism of interferon action in hairy cell leukemia: a model of effective cancer biotherapy. Cancer Res. 1992;52:1056–1066. [PubMed] [Google Scholar]
- 5.Gruber G, Schwarzmeier JD, Shehata M, Hilgarth M, Berger R. Basic fibroblast growth factor is expressed by CD19/CD11c-positive cells in hairy cell leukemia. Blood. 1999;94:1077–1085. [PubMed] [Google Scholar]
- 6.Schwarzmeier JD, et al. Inadequate production of hematopoietic growth factors in hairy cell leukemia: up-regulation of interleukin 6 by recombinant IFN-alpha in vitro. Cancer Res. 1996;56:4679–4685. [PubMed] [Google Scholar]
- 7.Shehata M, et al. Reconstitution of endogenous interferon a by recombinant interferon in hairy cell leukemia. Cancer Res. 2000;60:5420–5426. [PubMed] [Google Scholar]
- 8.Podzimek K, et al. The value of bone marrow biopsy in the prognosis of hairy cell leukemia (HCL) Neoplasma. 1994;41:325–330. [PubMed] [Google Scholar]
- 9.Vykoupil KF, Thiele J, Georgii A. Hairy cell leukemia. Bone marrow findings in 24 patients. Virchows Arch. A Pathol. Anat. Histol. 1976;370:273–289. doi: 10.1007/BF00445773. [DOI] [PubMed] [Google Scholar]
- 10.Katayama I, Schneider GB. Further ultrastructural characterization of hairy cells of leukemic reticuloendotheliosis. Am. J. Pathol. 1977;86:163–182. [PMC free article] [PubMed] [Google Scholar]
- 11.Laughlin M, et al. Effect of alpha-interferon therapy on bone marrow fibrosis in hairy cell leukemia. Blood. 1988;72:936–939. [PubMed] [Google Scholar]
- 12.Lisse I, Hasselbalch H, Junker P. Bone marrow stroma in idiopathic myelofibrosis and other haematological diseases. An immunohistochemical study. APMIS. 1991;99:171–178. doi: 10.1111/j.1699-0463.1991.tb05135.x. [DOI] [PubMed] [Google Scholar]
- 13.Reilly JT. Pathogenesis of idiopathic myelofibrosis: role of growth factors. J. Clin. Pathol. 1992;45:461–464. doi: 10.1136/jcp.45.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 2002;65:109–126. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- 15.Montes GS, et al. Histochemical and morphological characterization of reticular fibers. Histochemistry. 1980;65:131–141. doi: 10.1007/BF00493161. [DOI] [PubMed] [Google Scholar]
- 16.Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol. Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- 17.Fleischmajer R, et al. Immunochemical analysis of human kidney reticulin. Am. J. Pathol. 1992;140:1225–1235. [PMC free article] [PubMed] [Google Scholar]
- 18.Fakoya FA. Reticulin fibres in the tunica albuginea and peritubular tissue of seminiferous tubules of adult male Wistar rats. Acta Histochem. 2002;104:279–283. doi: 10.1078/0065-1281-00646. [DOI] [PubMed] [Google Scholar]
- 19.Burthem J, Cawley JC. The bone marrow fibrosis of hairy-cell leukemia is caused by the synthesis and assembly of a fibronectin matrix by the hairy cells. Blood. 1994;83:497–504. [PubMed] [Google Scholar]
- 20.Aziz KA, et al. The role of autocrine FGF-2 in the distinctive bone marrow fibrosis of hairy-cell leukemia (HCL) Blood. 2003;102:1051–1056. doi: 10.1182/blood-2002-12-3737. [DOI] [PubMed] [Google Scholar]
- 21.Reilly JT, Nash JR, Mackie MJ, McVerry BA. Immuno-enzymatic detection of fibronectin in normal and pathological haematopoietic tissue. Br. J. Haematol. 1985;59:497–504. doi: 10.1111/j.1365-2141.1985.tb07336.x. [DOI] [PubMed] [Google Scholar]
- 22.Charron D, Robert L, Couty MC, Binet JL. Biochemical and histological analysis of bone marrow collagen in myelofibrosis. Br. J. Haematol. 1979;41:151–161. doi: 10.1111/j.1365-2141.1979.tb05843.x. [DOI] [PubMed] [Google Scholar]
- 23.Hounieu H, et al. Hairy cell leukemia. Diagnosis of bone marrow involvement in paraffin-embedded sections with monoclonal antibody DBA.44. Am. J. Clin. Pathol. 1992;98:26–33. doi: 10.1093/ajcp/98.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Bartl R, et al. Bone marrow histology in hairy cell leukemia. Identification of subtypes and their prognostic significance. Am. J. Clin. Pathol. 1983;79:531–545. doi: 10.1093/ajcp/79.5.531. [DOI] [PubMed] [Google Scholar]
- 25.Bentley SA, Alabaster O, Foidart JM. Collagen heterogeneity in normal human bone marrow. Br. J. Haematol. 1981;48:287–291. [PubMed] [Google Scholar]
- 26.Bentley SA. Bone marrow connective tissue and the haemopoietic microenvironment. Br. J. Haematol. 1982;50:1–6. doi: 10.1111/j.1365-2141.1982.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 27.Apaja-Sarkkinen M, Autio-Harmainen H, Alavaikko M, Risteli J, Risteli L. Immunohistochemical study of basement membrane proteins and type III procollagen in myelofibrosis. Br. J. Haematol. 1986;63:571–580. doi: 10.1111/j.1365-2141.1986.tb07535.x. [DOI] [PubMed] [Google Scholar]
- 28.Brown DC, Gatter KC. The bone marrow trephine biopsy: a review of normal histology. Histopathology. 1993;22:411–422. doi: 10.1111/j.1365-2559.1993.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 29.Catovsky D, Pettit JE, Galton DA, Spiers AS, Harrison CV. Leukaemic reticuloendotheliosis (‘Hairy’ cell leukaemia): a distinct clinico-pathological entity. Br. J. Haematol. 1974;26:9–27. doi: 10.1111/j.1365-2141.1974.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 30.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 31.Sudarshan C, Yaswen L, Kulkarni A, Raghow R. Phenotypic consequences of transforming growth factor beta1 gene ablation in murine embryonic fibroblasts: autocrine control of cell proliferation and extracellular matrix biosynthesis. J. Cell. Physiol. 1998;176:67–75. doi: 10.1002/(SICI)1097-4652(199807)176:1<67::AID-JCP8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Kimura A, Katoh O, Hyodo H, Kuramoto A. Transforming growth factor-beta regulates growth as well as collagen and fibronectin synthesis of human marrow fibroblasts. Br. J. Haematol. 1989;72:486–491. doi: 10.1111/j.1365-2141.1989.tb04310.x. [DOI] [PubMed] [Google Scholar]
- 33.Shi DL, Savona C, Chambaz EM, Feige JJ. Stimulation of fibronectin production by TGF-beta 1 is independent of effects on cell proliferation: the example of bovine adrenocortical cells. J. Cell. Physiol. 1990;145:60–68. doi: 10.1002/jcp.1041450110. [DOI] [PubMed] [Google Scholar]
- 34.Rizzino A. Transforming growth factor-beta: multiple effects on cell differentiation and extracellular matrices. Dev. Biol. 1988;130:411–422. doi: 10.1016/0012-1606(88)90337-5. [DOI] [PubMed] [Google Scholar]
- 35.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J. Invest. Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 36.Massague J. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 37.Martyre MC. TGF-beta and megakaryocytes in the pathogenesis of myelofibrosis in myeloproliferative disorders. Leuk. Lymphoma. 1995;20:39–44. doi: 10.3109/10428199509054751. [DOI] [PubMed] [Google Scholar]
- 38.Tefferi A. Myelofibrosis with myeloid metaplasia. N. Engl. J. Med. 2000;342:1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 39.Chagraoui H, et al. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100:3495–3503. doi: 10.1182/blood-2002-04-1133. [DOI] [PubMed] [Google Scholar]
- 40.Dalal BI, Freier L, Johnston JB, Merry CC, Israels LG. Peripheral blood and bone marrow changes following 2’-deoxycoformycin therapy in hairy cell leukemia. Results of 200 weeks’ follow-up. Cancer. 1989;63:14–22. doi: 10.1002/1097-0142(19890101)63:1<14::aid-cncr2820630103>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 41.Thiele J, Moedder B, Kremer B, Zankovich R, Fischer R. Chronic myeloproliferative diseases with an elevated platelet count (in excess of 1,000,000/microliter): a clinicopathological study on 46 patients with special emphasis on primary (essential) thrombocythemia. Hematol. Pathol. 1987;1:227–237. [PubMed] [Google Scholar]
- 42.Booth BA, Polak KL, Uitto J. Collagen biosynthesis by human skin fibroblasts. I. Optimization of the culture conditions for synthesis of type I and type III procollagens. Biochim. Biophys. Acta. 1980;607:145–160. doi: 10.1016/0005-2787(80)90228-2. [DOI] [PubMed] [Google Scholar]
- 43.Reilly JT, et al. Bone marrow and serum connective tissue polypeptides in idiopathic myelofibrosis. Clin. Lab. Haematol. 1995;17:35–39. doi: 10.1111/j.1365-2257.1995.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 44.Taube T, Franssila K, Risteli L, Risteli J, Elomaa I. Monitoring of multiple myeloma and bone marrow fibrosis with aminoterminal propeptide of type III collagen (PIIINP) Br. J. Haematol. 1992;82:32–37. doi: 10.1111/j.1365-2141.1992.tb04590.x. [DOI] [PubMed] [Google Scholar]
- 45.Chan D, Cole WG. Low basal transcription of genes for tissue-specific collagens by fibroblasts and lymphoblastoid cells. Application to the characterization of a glycine 997 to serine substitution in alpha 1(II) collagen chains of a patient with spondyloepiphyseal dysplasia. J. Biol. Chem. 1991;266:12487–12494. [PubMed] [Google Scholar]
- 46.Hasselbalch H, Junker P, Lisse I, Bentsen KD. Serum procollagen III peptide in chronic myeloproliferative disorders. Scand. J. Haematol. 1985;35:550–557. doi: 10.1111/j.1600-0609.1985.tb02827.x. [DOI] [PubMed] [Google Scholar]
- 47.Castro-Malaspina H, et al. Characteristics of bone marrow fibroblast colony-forming cells (CFU-F) and their progeny in patients with myeloproliferative disorders. Blood. 1982;59:1046–1054. [PubMed] [Google Scholar]
- 48.Wang JC, Lang HD, Lichter S, Weinstein M, Benn P. Cytogenetic studies of bone marrow fibroblasts cultured from patients with myelofibrosis and myeloid metaplasia. Br. J. Haematol. 1992;80:184–188. doi: 10.1111/j.1365-2141.1992.tb08898.x. [DOI] [PubMed] [Google Scholar]
- 49.Reilly JT. Cytogenetic and molecular genetic aspects of idiopathic myelofibrosis. Acta Haematol. 2002;108:113–119. doi: 10.1159/000064708. [DOI] [PubMed] [Google Scholar]
- 50.Rameshwar P, Chang VT, Thacker UF, Gascon P. Systemic transforming growth factor-beta in patients with bone marrow fibrosis: pathophysiological implications. Am. J. Hematol. 1998;59:133–142. doi: 10.1002/(sici)1096-8652(199810)59:2<133::aid-ajh6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 51.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J. Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 52.Lotz M, Ranheim E, Kipps TJ. Transforming growth factor beta as endogenous growth inhibitor of chronic lymphocytic leukemia B cells. J. Exp. Med. 1994;179:999–1004. doi: 10.1084/jem.179.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kremer JP, Reisbach G, Nerl C, Dormer P. B-cell chronic lymphocytic leukaemia cells express and release transforming growth factor-beta. Br. J. Haematol. 1992;80:480–487. doi: 10.1111/j.1365-2141.1992.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 54.Horimoto M, et al. Identification of a transforming growth factor beta-1 activator derived from a human gastric cancer cell line. Br. J. Cancer. 1995;72:676–682. doi: 10.1038/bjc.1995.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J. Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips AO, Topley N, Morrisey K, Williams JD, Steadman R. Basic fibroblast growth factor stimulates the release of preformed transforming growth factor beta 1 from human proximal tubular cells in the absence of de novo gene transcription or mRNA translation. Lab. Invest. 1997;76:591–600. [PubMed] [Google Scholar]
- 57.Flaumenhaft R, Abe M, Mignatti P, Rifkin DB. Basic fibroblast growth factor-induced activation of latent transforming growth factor beta in endothelial cells: regulation of plasminogen activator activity. J. Cell Biol. 1992;118:901–909. doi: 10.1083/jcb.118.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shinozaki M, et al. Induction of subcutaneous tissue fibrosis in newborn mice by transforming growth factor beta: simultaneous application with basic fibroblast growth factor causes persistent fibrosis. Biochem. Biophys. Res. Commun. 1997;240:292–297. [PubMed] [Google Scholar]
- 59.Falcone DJ, McCaffrey TA, Haimovitz-Friedman A, Garcia M. Transforming growth factor-beta 1 stimulates macrophage urokinase expression and release of matrix-bound basic fibroblast growth factor. J. Cell. Physiol. 1993;155:595–605. doi: 10.1002/jcp.1041550317. [DOI] [PubMed] [Google Scholar]
- 60.Bousse-Kerdiles MC, Martyre MC. Involvement of the fibrogenic cytokines, TGF-beta and bFGF, in the pathogenesis of idiopathic myelofibrosis. Pathol. Biol. (Paris). 2001;49:153–157. doi: 10.1016/s0369-8114(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 61.Bousse-Kerdiles MC, Martyre MC. Dual implication of fibrogenic cytokines in the pathogenesis of fibrosis and myeloproliferation in myeloid metaplasia with myelofibrosis. Ann. Hematol. 1999;78:437–444. doi: 10.1007/s002770050595. [DOI] [PubMed] [Google Scholar]
- 62.Johnston JB, et al. Deposition of transforming growth factor-beta in the marrow in myelofibrosis, and the intracellular localization and secretion of TGF-beta by leukemic cells. Am. J. Clin. Pathol. 1995;103:574–582. doi: 10.1093/ajcp/103.5.574. [DOI] [PubMed] [Google Scholar]
- 63.Demetriou AA, Levenson SM, Rettura G, Seifter E. Vitamin A and retinoic acid: induced fibroblast differentiation in vitro. Surgery. 1985;98:931–934. [PubMed] [Google Scholar]
- 64.Rodemann HP, et al. The underlying cellular mechanism of fibrosis. Kidney Int. Suppl. 1996;54:S32–S36. [PubMed] [Google Scholar]
- 65.Bayreuther K, Francz PI, Gogol J, Kontermann K. Terminal differentiation, aging, apoptosis, and spontaneous transformation in fibroblast stem cell systems in vivo and in vitro. Ann. N. Y. Acad. Sci. 1992;663:167–179. doi: 10.1111/j.1749-6632.1992.tb38660.x. [DOI] [PubMed] [Google Scholar]
- 66.Goldberg B, Green H. An analysis of collagen secretion by established mouse fibroblast lines. J. Cell Biol. 1964;22:227–258. doi: 10.1083/jcb.22.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reilly JT. Cytogenetic and molecular genetic aspects of idiopathic myelofibrosis. Acta Haematol. 2002;108:113–119. doi: 10.1159/000064708. [DOI] [PubMed] [Google Scholar]
- 68.Fontenay M, Bryckaert M, Tobelem G. Transforming growth factor-beta 1 inhibitory effect of platelet-derived growth factor-induced signal transduction on human bone marrow fibroblasts: possible involvement of protein phosphatases. J. Cell. Physiol. 1992;152:507–519. doi: 10.1002/jcp.1041520310. [DOI] [PubMed] [Google Scholar]
- 69.Hasselbalch H. Idiopathic myelofibrosis: a review. Eur. J. Haematol. 1990;45:65–72. doi: 10.1111/j.1600-0609.1990.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 70.Thiele J, et al. Bone marrow features and clinical findings in chronic myeloid leukemia: a comparative, multicenter, immunohistological and morphometric study on 614 patients. Leuk. Lymphoma. 2000;36:295–308. doi: 10.3109/10428190009148850. [DOI] [PubMed] [Google Scholar]
- 71.Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood. 2000;96:2022–2036. [PubMed] [Google Scholar]