Abstract
Glioblastoma is the most common primary brain tumor with a relatively poor prognosis. This article reviews the current standard therapy and discusses new developments in treatment of this disease. Surgical resection followed by radiation and chemotherapy has proven to be the most effective initial therapy. Recent advancement in molecular targeted therapies has led to the Food and Drug Administration (FDA) approval of bevacizumab in the setting of recurrent glioblastoma. The molecular pathways of glioblastoma growth are highlighted in this review. While numerous molecular targets are currently being intensely investigated, vascular endothelial growth factor (VEGF) receptor targeted therapy has been the only one to have shown clinical effect. The role of bevacizumab in this context provides a dynamic breakthrough in cancer therapy. Clinical trials have demonstrated significantly increased overall survival and six month progression free survival (PFS) in recurrent glioblastoma treated with bevacizumab alone or in combination with irinotecan. The use of this agent has also dramatically changed the imaging characteristics of glioblastoma. The anti-angiogenesis effects of bevacizumab have complicated the criterion for determining tumor growth. This may lead to redefinition of progressive disease based on non-invasive monitoring.
Keywords: glioblastoma, glioma, bevacizumab, vascular endothelial growth factor, avastin, angiogenesis, cancer
Introduction
Glioblastoma is the most common and aggressive primary brain tumor. The median life expectancy after diagnosis remains a mere 14 months. However, new advances have provided new optimism. The standard of care for treatment is resection followed by radiation with concurrent temozolomide.1 This regimen has had a significantly positive impact on progression free survival (PFS) and overall survival. Bevacizumab has recently received accelerated approval by the Food and Drug Administration (FDA) for the treatment of recurrent glioblastoma.
Chemotherapy has traditionally been targeted at inhibition of deoxyribonucleic acid (DNA) replication. This provided a non-specific mechanism by which to prevent cell growth. Our understanding of the pathways by which tumors are able to replicate and survive has expanded tremendously in recent years. This knowledge has shifted our experimental treatment strategy to specific molecular targeted therapies. Theoretically, individuals with aberrant signaling in one pathway are more likely to respond to agents that target those pathways then individuals with impairment in a different pathway. The focus has now shifted to intense research in clinical trials to find molecular targeted agents that can benefit patients with glioblastoma.2 Bevacizumab is the first such agent approved in the treatment of this disease.
Bevacizumab is a humanized monoclonal antibody against the vascular endothelial growth factor (VEGF). The receptor for this ligand is involved in the mediation of vascular proliferation. It is known that one of the cardinal histologic features of glioblastoma is vascular proliferation. This allows the tumor to have a continued supply of nutrients allowing continued growth. Bevacizumab neutralizes the VEGF signaling pathway and thus prevents glioblastoma from increasing its vascular supply. This in turn will hamper further tumor growth.
Standard therapy for glioblastoma
The current standard therapy for glioblastoma is surgical resection followed by radiation therapy with concurrent temozolomide therapy. This is followed by adjuvant temozolomide therapy for at least six cycles of a 5 day on, 23 day off schedule. Overall survival with this regimen is 14.6 months with a median PFS of 6.9 months. Two year survival rates are 25.6%.1 The results show that glioblastoma is still a fatal disease with a poor prognosis. The treatment for recurrent glioblastoma has been an area in desperate need of advancement. Traditional chemotherapies have long been evaluated. The most commonly used therapies include carmustine, carboplatin, irinotecan, BCNU wafers, and repeat surgical intervention. Bevacizumab was recently approved by the FDA for use in this setting becoming the standard of care.
Genetic variations in glioblastoma
While there are many mutations that are likely to lead to the development of glioblastoma there are three main pathways activated in the majority of glioblastoma tumors. They can be best thought of as the epidermal receptor tyrosine kinase (EGFR), retinoblastoma (RB), and p53 pathways.3 Numerous clinical trials have attempted to target the components of these pathways but none have shown any clinical efficacy.2 Given the lack of homogeneity in glioblastoma, the development of an effective targeted therapy has been challenging. Bevacizumab remains the only targeted agent which has significant response and clinical efficacy.
The EGFR is an upstream receptor that is activated by the binding of epidermal growth factor to the extracellular domain. In glioblastoma there is often a ligand-independent mutation of the receptor, called EGFRvIII, which is constitutively active.4,13 This results in recruitment of PI3K to the cell membrane. PI3K in turn phosphorylates phosphatidylinositol (PI)-4,5-bisphosphate to PI-3-phosphate (PIP3). This active enzyme activates downstream molecules such as protein kinase B (AKT) and mTOR.14 This signaling cascade leads to cell proliferation and inhibition of apoptosis. Phosphate and tensin homology (PTEN) is a checkpoint in this system which inhibits PIP3 signaling. PTEN is involved in regulating cell migration and invasion by directly dephosphorylating focal adhesion kinase. It also has homology to the catalytic region of protein tyrosine phosphates which is important to the function of PIP3. The PTEN gene is located at 10q23.3. There is a mutation of this gene in 15%–40% of glioblastomas suggesting that deregulation of this pathway is common is glioblastoma.11,15,16 Ras proteins are also stimulated by EGFR signal transduction. These membrane associated GTPases require post-translational addition of a farnesyl group to the C-terminus. This is accomplished by farnesyl transferase.17 Once activated, Ras stimulates cellular proliferation, survival and angiogenesis.18
The p53 pathway has been shown to have mutation in 87% of glioblastomas.3 This pathway is strongly implicated in glioblastoma that has transformed from a lower grade tumor. TP53 controls cell response to DNA damage by stimulating apoptosis or senescence. Mutation or homozygous deletion of this gene which is encoded at chromosome 17p13.1 can lead to disruption of this regulatory control.19 MDM2 binds TP53 and inhibits its ability to activate transcription of promoter sequences.20 Amplification of MDM2 inhibits cells from entering apoptosis which can afford glioblastoma cells immortality. Upstream of MDM2 is the ARF gene product. The gene product binds to MDM2 and inhibits its ability to mediate p53 degradation and transactivational silencing.21,23 Homozygous deletion or promoter methylation of this gene product leading to loss of expression has been found frequently in glioblastoma. Promoter methylation is more common in secondary glioblastoma but loss of expression is not significantly different between primary and secondary glioblastoma.24 Disruption of MDM2, TP53, or ARF can lead to loss of normal cell function due to autoregulatory feedback between these gene products.
The RB protein is involved in the progression of the cell through the cell cycle. Mutations in this pathway have been found in 78% of glioblastomas.3 The cell is inhibited from progressing from the G1 phase to the S phase by the RB protein. One mechanism by which this is done is inhibition of E2F transcription factor which activates the gene involved the transition between phases.25 The RB protein is phosphylated by the CDK4/CCND2/CDK6 complexes which inhibits its activity. Amplification of these complexes are observed in 1%–18% of glioblastomas. The downstream effect is to increase cell replication. Upstream of these complexes is p16 and CDKN2B which act to inhibit these complexes. Homozygous deletions in these genes are seen frequently in primary glioblastoma. Alteration in expression in any of these genes, RB, or CDK4 complexes can lead to uncontrolled cell division.
Angiogenesis is another target of molecular therapies. Glomeruloid vascular proliferation is feature manifested in nearly all glioblastomas. This is typically mediated through the VEGF receptor. The majority of signal transduction is through the VEGF receptor 2 (VEGFR-2).26 The role of VEGF receptor 1 remains unclear. While VEGF has a stronger affinity for this receptor, the signal transduction is rather weak.27 The agonists for the VEGF receptor are a family of structurally related molecules termed VEGF-A, VEGF-B, VEGF-C, and VEGF-D. The major mediator of tumor angiogenesis is VEGF-A which is typically referred to simply as VEGF.26,28,29 Many of the pathways used in the EGFR pathways are common to the VEGF signaling cascade thus blocking downstream may prevent angiogenesis as well.30,31 As the tumor grows it characteristically encounters impaired oxygenation and nutrients with toxic metabolite build up. Hypoxia, hypoglycemia and acidosis stimulate VEGF and other angiogenesis factors. The secreted VEGF binds to VEGFR-2 receptors which are expressed on endothelial cells.32,33 This suggests VEGF has both autocrine and paracrine mechanisms of action. Endothelial cell activation can lead to increased vascular permeability, vasorelaxation, endothelial cell migration, proliferation and survival.34 This stimulates new blood vessel formation from surrounding and existing vessels affording glioblastoma with neovasculature. Integrin mediated signaling and matrix metalloporteinases are also involved in angiogenesis. These proteins allow blood vessels to penetrate into surrounding tissue.35,36 Targeting the angiogenesis process to prevent tumor growth may occur anywhere from the signaling pathway, to the invasion mechanism, to the receptor level via antibodies. It is the latter target by which bevacizumab elicits its effect on angiogenesis.
VEGF molecular targeted therapy
The main focus of research in the treatment of glioblastoma has aimed its sights on molecular targeted therapies. We know that there may be subgroups of glioblastoma patients that respond to treatment with different results. This may be due to the variable molecular genetics present in glioblastoma cells that allow them to procreate aberrantly. While there are likely to be many pathways that may be involved the signaling cascade that allows tumor growth it may be that subgroups of patients have tumors with a dominant pathway. Theoretically, blocking this pathway in this group of patients would prevent tumor growth while it would not necessarily do this in a subgroup with a different dominant pathway. Glioblastoma, regardless of the cellular pathways driving its growth, is known to have increased VEGF production. VEGF allows glioblastoma to stimulate vascular proliferation in the surrounding area. This allows increased blood flow, nutrient supply, and oxygen delivery. All of these factors contribute to the ability of glioblastoma to survive and growth. It is been shown that high levels of VEGF in glioblastomas are associated with a poor prognosis.37
When used with chemotherapy, bevacizumab has been associated with prolonged overall survival in metastatic colorectal38 and non-small-cell lung39 cancers. It has also been shown to have prolonged PFS when used with chemotherapy in metastatic breast40 and renal cancers.41 Just as it does in these cancers, bevacizumab works by the same mechanism of action in glioblastoma. The antibody binds the VEGF ligand and inhibits its ability to stimulate the signaling cascade which was previously described. Bevacizumab prevents secreted VEGFs from binding to their specific receptor thus disrupting activation of signaling cascades at the most upstream site. However, what is different in glioblastoma and renal cancer is that bevacizumab has benefits when used alone. This means that disruption of the VEGF pathway alone in glioblastoma is important for tumor control.42
Efficacy, safety and tolerability results
In the recurrent glioblastoma setting the use of bevacizumab alone or in combination with irinotecan has been shown to be a viable therapy.43,44 The BRAIN study was a multi-center phase II randomized non-comparative open label trial of irinotecan and bevacizumab versus bevacizumab alone.44 Historically, the six month PFS for relapsed or progressive glioblastoma is 9%–21%.45 The objective response rate is less than 10% and median overall survival is 30 weeks or less.46–48 The use of irinotecan as a single agent has a response rate of less than 15%.47–50 In the combination therapy there was a six month PFS of 50.3% and a median overall survival of 8.7 months. Median PFS has been reported as 5.6 months with an objective response rate of 37.8%. In the bevacizumab single agent arm six month PFS was 42.6% and median overall survival of 9.2 months. Median PFS has been reported as 4.2 months with an objective response rate of 28.2%.44 These results show at least a doubling of six month PFS compared to historical controls for irinotecan single agent therapy for recurrent glioblastoma. These results showed that bevacizumab was effective in combination with irinotecan or as a single agent compared to historical controls in the setting of recurrent glioblastoma. It was based on these early results that bevacizumab gained accelerated FDA approval for treatment of recurrent glioblastoma.
The main side effects reported with bevacizumab single agent therapy were fatigue, headache, hypertension, and proteinuria. Far less common but significant side effects included arterial (2.4%) or venous thromboembolism (3.6%), impaired wound healing (2.4%), and intracranial hemorrhage, gastrointestinal perforation (1.3%) and posterior reversible leukoencephalopathy.
Recently bevacizumab has been evaluated in the upfront setting for newly diagnosed glioblastoma in a phase II study. This study has entered its expansion phase and may soon allow us to interpret the synergistic effect of bevacizumab with radiation and temozolomide in the upfront setting. Early results presented at the Society of Neuro-Oncology meeting in 2009 showed a median PFS of 14.1 months compared to historical controls of 6.9 months and institutional controls of 7.9 months. Survival data showed a median overall survival at 20.3 months in the treatment arm versus 14.6 months of the historical control and 21.1 months in the institutional control.51 Bevacizumab and temozolomide during and after radiation therapy was well-tolerated. The trial observed improved PFS but not overall survival compared to an internal control cohort. Additional studies are warranted to determine if bevacizumab administered upfront is superior to bevacizumab at recurrence.
Imaging
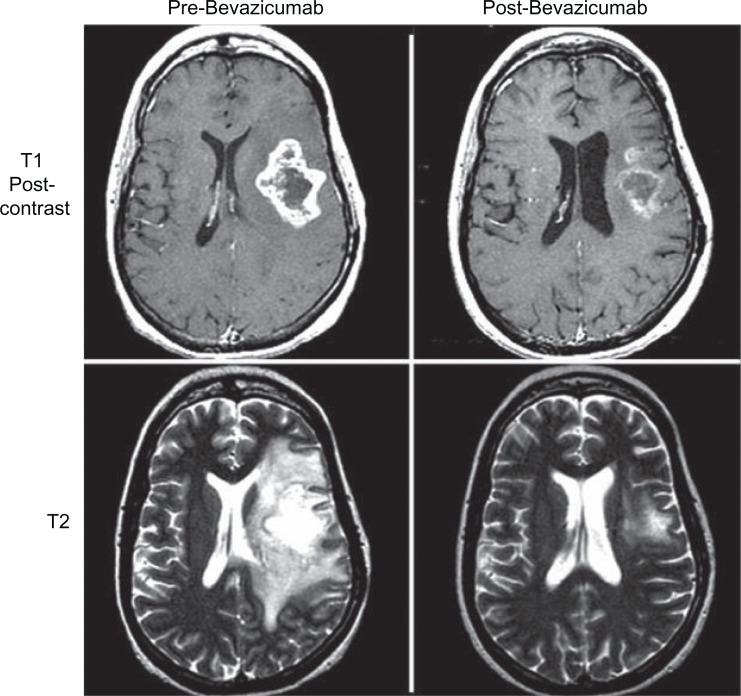
The anti-angiogenesis effect of bevacizumab has significant impact on the imaging characteristics of glioblastoma on magnetic resonance imaging (MRI). MRI is the main imaging modality for providing anatomic data on tumor size. The standard surrogate for tumor burden has been the change in contrast enhancing tumor size as described in the MacDonald criteria.52 Glioblastoma has extensive abnormal vasculature at the blood brain barrier which allows contrast material to leak out of blood vessels. This results in an increased signal on T1 weighted images (enhancement)53 (see Figure 1). Unfortunately, there are other causes of contrast enhancing change in the setting of glioblastoma; therefore, contrast enhancement is not specific for tumor growth. Bevacizumab causes a reduction in the intensity of contrast enhancement.54 These effects can be seen as early as two weeks after initiation of therapy.54,55 Given the lack of specificity of contrast indicating tumor burden, the changes may be due to, or independent of, effect on tumor burden. Even in patients with stable tumor size, edema and enhancement may be reduced significantly. It has been well documented that steroid requirements are reduced following bevacizumab therapy.44,56 This is thought to be due to reduced edema which can persist even with tumor progression.55 The clinical benefit of diminished edema and reduced steroid use has provided rationale for clinicians to continue bevacizumab therapy even with tumor progression. Preclinical data of bevacizumab treated rats having fewer symptoms despite larger tumor size then control rats, suggests that edema control has clinical benefit despite tumor progression.57 These anti-permeability effects, which are mediated through the VEGF pathway, have been seen in brain metastases from non-primary brain tumors as well.58,60 A concerning side effect seen in the pre-clinical setting of anti-angiogenesis therapy has been stimulated migration of glioblastoma cells.61,62 These findings have also been observed in trials of anti-angiogenesis agents in the clinical setting though not directly targeting VEGF.63 There have been trials showing that the use of bevacizumab in combination with other therapies to treat recurrent glioblastoma showed patterns of progression suggestive of a diffuse phenotype,64,65 however, analysis of the bevacizumab only arm in the BRAIN study showed no evidence of an increase in the diffuse phenotype.66 This may a significant effect on non-contrast MRI findings in this class of agents.
Figure 1.
T1 post-contrast imaging shows significant response to the enhancing tumor burden following bevazicumab therapy. The T2 imaging highlights the impact of edema with bevazicumab therapy with a significant decrease in the T2 hyperintensity.
The assessment of the effect of bevacizumab based on Macdonald criteria becomes problematic, as it is unclear if the observed response is a change in contrast enhancement or a change in tumor burden. Tumor progression is typically noted due to an increase in contrast enhancement. Given impact of bevacizumab on vascular permeability and thus contrast enhancement, tumor progression following bevacizumab therapy manifests as T2/FLAIR signal changes not T1 post contrast enhancement.65 It has been shown that compared to the ease of identifying an enhancing tumor, a non-enhancing tumor is less easy to define.67 Increased non-enhancing tumor is not addressed when using Macdonald criteria for tumor progression. These changes can be difficult to differentiate from gliosis as a treatment reaction from surgery or radiation. These observations have lead to new recommendation for tumor assessment in neurooncology.42 Numerous MR techniques such as MR spectroscopy,68 MR perfusion,69 MR diffusion70 are being evaluated to help differentiate treatment related changes from tumor response. Metabolic imaging techniques with PET or SPECT have also been studied.71 Thus far, these techniques have not proved clinically effective. At UCLA, we are investigating neutral amino acid imaging (FDOPA-PET) as a new biomarker for tumor burden which is not dependent on vascular permeability to provide a signal. The use of pretreatment apparent diffusion coefficient (ADC) histograms may also have a predictive role for bevacizumab responsive tumor.72
Conclusions
The treatment of glioblastoma continues to evolve. Further advances in molecular targeted therapies will hopefully allow individualized treatment based on specific tumor markers. Bevacizumab is the first FDA approved targeted therapy based on its efficacy in the treatment of recurrent glioblastoma. The front line therapy remains resection and subsequent temozolomide with radiation therapy followed by adjuvant temozolomide treatments. Bevacizumab is quickly becoming the first choice agent for use in the recurrent setting. Its use is associated with higher response rates, longer PFS and maintenance of quality of life. Bevacizumab as a targeted therapy represents the first step in a paradigm shift in the treatment of glioblastoma and cancer in general. Previous therapies have been aimed at non-specifically inhibiting cell division or growth. Molecular targeted therapy allows us to target specific pathways that are abnormally functioning. This practice requires an intimate knowledge of the aberrant molecular pathways involved in glioblastoma formation and growth. EGFR, RB, p53 signal pathways and angiogenesis are all area of future targets.
The effect on imaging is likely to be more profound than the actual effect on tumor burden. This leaves us with relatively impaired ability to evaluate further tumor growth with the traditional McDonald criteria. New methods for assessing non-contrast enhancing tumor are currently being evaluated. This may lead to new criteria by which the oncologist determines tumor progression. Bevacizumab has put us on the precipice of this change. Further research also needs to be done on predicting bevacizumab response based on imaging characteristics and molecular profiles.
Footnotes
Disclosures
The authors report no conflicts of interest in this work.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Mischel PS, Cloughesy TF. Targeted molecular therapy of GBM. Brain Pathol. 2003;13:52–61. doi: 10.1111/j.1750-3639.2003.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekstrand AJ, Sugawa N, James CD, Collins VP. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc Natl Acad Sci U S A. 1992;89:4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 6.Hayashi Y, Ueki K, Waha A, Wiestler OD, Louis DN, von Deimling A. Association of EGFR gene amplification and CDKN2 (p16/MTS1) gene deletion in glioblastoma multiforme. Brain Pathol. 1997;7:871–875. doi: 10.1111/j.1750-3639.1997.tb00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuan CT, Wikstrand CJ, Bigner DD. EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer. 2001;8:83–96. doi: 10.1677/erc.0.0080083. [DOI] [PubMed] [Google Scholar]
- 9.Nagane M, Lin H, Cavenee WK, Huang HJ. Aberrant receptor signaling in human malignant gliomas: mechanisms and therapeutic implications. Cancer Lett. 2001;162(Suppl):S17–S21. doi: 10.1016/s0304-3835(00)00648-0. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JS, Tachibana I, Passe SM, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6:217–223. doi: 10.1111/j.1750-3639.1996.tb00848.x. discussion 23–24. [DOI] [PubMed] [Google Scholar]
- 13.Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD. The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol. 1998;4:148–158. doi: 10.3109/13550289809114515. [DOI] [PubMed] [Google Scholar]
- 14.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, James CD, Frederick L, Alderete BE, Jenkins RB. PTEN/MMAC1 mutations and EGFR amplification in glioblastomas. Cancer Res. 1997;57:5254–5257. [PubMed] [Google Scholar]
- 16.Schmidt EE, Ichimura K, Goike HM, Moshref A, Liu L, Collins VP. Mutational profile of the PTEN gene in primary human astrocytic tumors and cultivated xenografts. J Neuropathol Exp Neurol. 1999;58:1170–1183. doi: 10.1097/00005072-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH. Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res. 2001;7:1438–1445. [PubMed] [Google Scholar]
- 18.Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 19.Bogler O, Huang HJ, Kleihues P, Cavenee WK. The p53 gene and its role in human brain tumors. Glia. 1995;15:308–327. doi: 10.1002/glia.440150311. [DOI] [PubMed] [Google Scholar]
- 20.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 21.Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci U S A. 1998;95:8292–8297. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pomerantz J, Schreiber-Agus N, Liegeois NJ, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 23.Mercer WE, Shields MT, Amin M, et al. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990;87:6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohgaki H, Kleihues P. Genetic Pathways to Primary and Secondary Glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 26.Kerbel RS. Tumor Angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 29.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 30.Brat DJ, Van Meir EG. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: a new world of angiogenesis research. Am J Pathol. 2001;158:789–796. doi: 10.1016/S0002-9440(10)64025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 32.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 33.Jakeman LB, Winer J, Bennett GL, Altar CA, Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992;89:244–53. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieder C, Wiedenmann N, Andratschke N, Molls M. Current status of angiogenesis inhibitors combined with radiation therapy. Cancer Treat Rev. 2006;32:348–364. doi: 10.1016/j.ctrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Bello L, Lucini V, Carrabba G, et al. Simultaneous inhibition of glioma angiogenesis, cell proliferation, and invasion by a naturally occurring fragment of human metalloproteinase-2. Cancer Res. 2001;61:8730–8736. [PubMed] [Google Scholar]
- 36.Zagzag D, Capo V. Angiogenesis in the central nervous system: a role for vascular endothelial growth factor/vascular permeability factor and tenascin-C. Common molecular effectors in cerebral neoplastic and non-neoplastic “angiogenic diseases”. Histol Histopathol. 2002;17:301–321. doi: 10.14670/HH-17.301. [DOI] [PubMed] [Google Scholar]
- 37.Carlson MR, Pope WB, Horvath S, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res. 2007;13:2592–2598. doi: 10.1158/1078-0432.CCR-06-2772. [DOI] [PubMed] [Google Scholar]
- 38.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus iri-notecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 39.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or meta-static non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 41.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen PY, Macdonald D vdBM, Reardon DA, et al. Proposal for updated response criteria in malignant gliomas [abstract] Neurooncology. 2009;11:622–623. [Google Scholar]
- 43.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 45.Ballman KV, Buckner JC, Brown PD, et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 47.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 48.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8:189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cloughesy TF, Filka E, Kuhn J, et al. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer. 2003;97:2381–2386. doi: 10.1002/cncr.11306. [DOI] [PubMed] [Google Scholar]
- 50.Raymond E, Fabbro M, Boige V, et al. Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapy-naive patients with glioblastoma. Ann Oncol. 2003;14:603–614. doi: 10.1093/annonc/mdg159. [DOI] [PubMed] [Google Scholar]
- 51.Lai A, et al. Updated results of phase II trial of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly-diagnosed glioblastoma. Society of Neuro-Onocology Meeting; 2009 Oct; [DOI] [PubMed] [Google Scholar]
- 52.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 53.Rees JH, Smirniotopoulos JG, Jones RV, Wong K. Glioblastoma multiforme: radiologic-pathologic correlation. Radiographics. 1996;16:1413–1438. doi: 10.1148/radiographics.16.6.8946545. quiz 62–63. [DOI] [PubMed] [Google Scholar]
- 54.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 55.Ananthnarayan S, Bahng J, Roring J, et al. Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol. 2008;88:339–347. doi: 10.1007/s11060-008-9573-x. [DOI] [PubMed] [Google Scholar]
- 56.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 57.Jahnke K, Muldoon LL, Varallyay CG, Lewin SJ, Kraemer DF, Neuwelt EA. Bevacizumab and carboplatin increase survival and asymptomatic tumor volume in a glioma model. Neuro Oncol. 2009;11:142–150. doi: 10.1215/15228517-2008-077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Labidi SI, Bachelot T, Ray-Coquard I, et al. Bevacizumab and paclitaxel for breast cancer patients with central nervous system metastases: a case series. Clin Breast Cancer. 2009;9:118–121. doi: 10.3816/CBC.2009.n.021. [DOI] [PubMed] [Google Scholar]
- 59.Mathews MS, Linskey ME, Hasso AN, Fruehauf JP. The effect of bevacizumab (Avastin) on neuroimaging of brain metastases. Surg Neurol. 2008;70:649–653. doi: 10.1016/j.surneu.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 60.Karimi S, Lis E, Gilani S, D’Ambrosio N, Holodny A. Nonenhancing Brain Metastases. J Neuroimaging. 2009 doi: 10.1111/j.1552-6569.2009.00389.x. [DOI] [PubMed] [Google Scholar]
- 61.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 62.Lamszus K, Brockmann MA, Eckerich C, et al. Inhibition of glioblastoma angiogenesis and invasion by combined treatments directed against vascular endothelial growth factor receptor-2, epidermal growth factor receptor, and vascular endothelial-cadherin. Clin Cancer Res. 2005;11:4934–4940. doi: 10.1158/1078-0432.CCR-04-2270. [DOI] [PubMed] [Google Scholar]
- 63.Tuettenberg J, Grobholz R, Seiz M, et al. Recurrence pattern in glioblastoma multiforme patients treated with anti-angiogenic chemotherapy. J Cancer Res Clin Oncol. 2009;135:1239–1244. doi: 10.1007/s00432-009-0565-9. [DOI] [PubMed] [Google Scholar]
- 64.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 66.Pope WB, Das Q, Hambleton A, et al. Patterns of progression in patients with glioblastoma at first or second relapse treated wtih bevacizumab alone or in combination with irinotecan in the BRAIN study. Neuro Oncol. 2009;11:563–699. [Google Scholar]
- 67.Hayward RM, Patronas N, Baker EH, Vezina G, Albert PS, Warren KE. Inter-observer variability in the measurement of diffuse intrinsic pontine gliomas. J Neurooncol. 2008;90:57–61. doi: 10.1007/s11060-008-9631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peca C, Pacelli R, Elefante A, et al. Early clinical and neuroradiological worsening after radiotherapy and concomitant temozolomide in patients with glioblastoma: tumour progression or radionecrosis? Clin Neurol Neurosurg. 2009;111:331–334. doi: 10.1016/j.clineuro.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223:11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 70.Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25:201–209. [PMC free article] [PubMed] [Google Scholar]
- 71.Siepmann DB, Siegel A, Lewis PJ. Tl-201 SPECT and F-18 FDG PET for assessment of glioma recurrence versus radiation necrosis. Clin Nucl Med. 2005;30:199–200. doi: 10.1097/00003072-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252:182–189. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]