Abstract
Neonatal diabetes mellitus (NDM) is a monogenic disorder caused by mutations in genes involved in regulation of insulin secretion from pancreatic β-cells. Mutations in the KCNJ11 and ABCC8 genes, encoding the adenosine triphosphate (ATP)-sensitive potassium (KATP) channel Kir6.2 and SUR1 subunits, respectively, are found in ∼50% of NDM patients. In the pancreatic β-cell, KATP channel activity couples glucose metabolism to insulin secretion via cellular excitability and mutations in either KCNJ11 or ABCC8 genes alter KATP channel activity, leading to faulty insulin secretion. Inactivation mutations decrease KATP channel activity and stimulate excessive insulin secretion, leading to hyperinsulinism of infancy. In direct contrast, activation mutations increase KATP channel activity, resulting in impaired insulin secretion, NDM, and in severe cases, developmental delay and epilepsy. Many NDM patients with KCNJ11 and ABCC8 mutations can be successfully treated with sulfonylureas (SUs) that inhibit the KATP channel, thus replacing the need for daily insulin injections. There is also strong evidence indicating that SU therapy ameliorates some of the neurological defects observed in patients with more severe forms of NDM. This review focuses on the molecular and cellular mechanisms of mutations in the KATP channel that underlie NDM. SU pharmacogenomics is also discussed with respect to evaluating whether patients with certain KATP channel activation mutations can be successfully switched to SU therapy.
Keywords: neonatal diabetes, KCNJ11, ABCC8, ATP-sensitive potassium channels
Introduction
Neonatal diabetes mellitus (NDM), either transient or permanent, is characterized by the occurrence of insulin-requiring diabetes in the first 6 months of life. The incidence of NDM is estimated to be 1 in ∼200,000 live births.1,2 The diabetes in 50%–60% of NDM is transient in nature, resolving within 18 months of birth and is thus termed TNDM.3 The remaining 40%–50% of NDM cases are permanent (PNDM) and require insulin treatment throughout life.3 In the most severe cases of NDM, the diabetes may be accompanied by marked developmental delay, muscle weakness, and epilepsy, termed DEND (developmental delay, epilepsy, and neonatal diabetes) syndrome.4 A form of NDM, between PNDM and DEND in severity, is known as intermediate DEND (iDEND), in which patients with PNDM show developmental delay or muscle weakness but not epilepsy.4
The evidence to date indicates that NDM is a monogenic disorder. Although mutations in multiple genes can cause NDM, such as INS (insulin gene) mutations5,6 and GCK (glucokinase gene) mutations,7,8 much attention has focused on the most common forms of NDM caused by heterozygous activation mutations in the KCNJ119–12 and ABCC813–15 genes that encode the two subunits Kir6.2 and SUR1, respectively, of the adenosine triphosphate (ATP)-sensitive potassium (KATP) channel that couples cellular metabolism to cellular excitability.16 KATP channels composed of Kir6.2 and SUR1 subunits are predominately expressed in endocrine tissues such as the pancreatic islet and nervous system. Therefore, the diabetic phenotype of NDM is believed to arise from KATP channel activation mutations in pancreatic β-cells,10,16 whereas neurological features associated with the more severe iDEND/DEND syndromes are likely the result of KATP channel activation mutations deleteriously affecting the nervous system.4,17
The physiological role of KATP channels in pancreatic β-cells
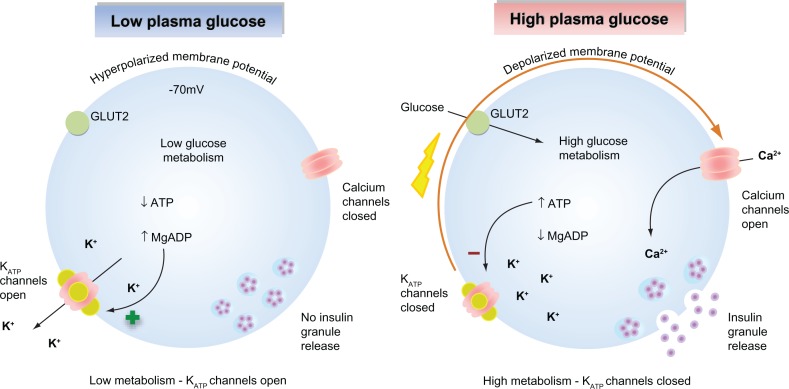
KATP channels sense changes in the cytosolic ATP/ADP ratio as a result of cellular metabolism and are a major regulator of the β-cell membrane potential. As glucose-stimulated insulin secretion is primarily controlled by the β-cell membrane potential, KATP channels serve to couple glucose metabolism to insulin secretion.16,18 When plasma glucose levels are low, the cytosolic ATP/ADP ratio is reduced, leading to a basal efflux of potassium ions from the cell via KATP channel activity that maintains the membrane potential of the β-cell at approximately −70 mV. This polarized membrane potential prevents calcium entry through voltage-gated calcium channels. As elevations in cytosolic calcium are the primary trigger for insulin granule exocytosis, insulin secretion is suppressed when plasma glucose levels are low (Figure 1A).19,20 When plasma glucose levels rise, glucose enters the β-cells via the glucose transporter 2. Subsequent glucose metabolism leads to an increase in the ratio of cytosolic ATP/ADP ratio, promoting KATP channel closure. The resultant decrease in potassium ion efflux depolarizes the β-cell membrane potential, leading to activation of voltage-gated calcium channels, calcium influx, and calcium-stimulated insulin granule exocytosis (Figure 1B).21 Graded increases in plasma glucose and subsequent metabolism lead to proportional decreases in KATP channel activity, resulting in an appropriate insulin secretory response that is tightly coupled to the plasma glucose concentration.
Figure 1.
Glucose-stimulated insulin secretion in pancreatic β-cells. (Left) When plasma glucose is low, the decreased ratio of ATP/Mg-ADP will increase KATP channel opening. Consequently, the cell membrane is hyperpolarized, preventing voltage-gated calcium channel opening, Ca2+ influx, and insulin secretion. (Right) When plasma glucose is high, glucose is transported into the cell via GLUT2. Glucose metabolism leads to an increased ratio of ATP/Mg-ADP, resulting in KATP channel closure, membrane depolarization, opening of voltage-gated calcium channels, Ca2+ influx, and insulin secretion.
As the electrical resistance of β-cell is high,22 only small changes in KATP channel activity are required to change β-cell excitability (and hence insulin secretion) via alterations in the β-cell membrane potential.23 Mutations within the KATP channel complex that change their intrinsic activity and/or ability to sense changes in either ATP or ADP will result in altered KATP channel activity that is correlated to the specific effects of the individual mutation on KATP channel activity.
KATP channels encoded by the KCNJ11 and ABCC8 genes are also expressed in other excitable tissues such as the nervous system. As KATP channels are involved in the control of neuronal excitability, mutations may also cause neuronal abnormalities, again dependent on the severity of the individual mutation.24–27
Molecular structure of pancreatic KATP channels
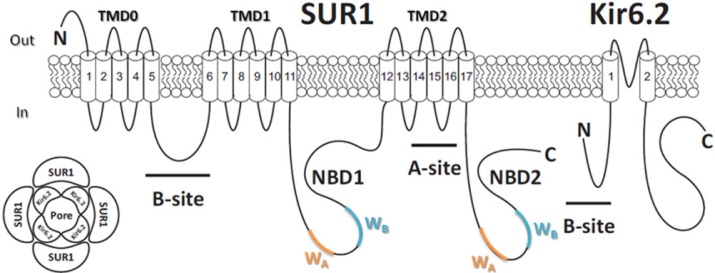
The KATP channel is a hetero-octameric membrane protein complex28,29 composed of four pore-forming inwardly rectifying potassium channel (Kir6.x) subunits and four regulatory sulfonylurea receptor (SURx) subunits (Figure 2A).30 There are two isoforms of the Kir6.x subunit, Kir6.1 and Kir6.2. Kir6.2 is more widely expressed than Kir6.1, which is predominately expressed in vascular smooth muscle.31,32 There are two isoforms of the SUR subunit (SUR1 and SUR2), and the subunit composition of KATP channel differs between tissue types.33 In pancreatic β-cells and neurons, KATP channels are assembled from Kir6.2 and SUR1 subunits.34 In cardiac tissue and skeletal muscle, KATP channels are composed of Kir6.2 and the SUR2A splice variant subunits,35 whereas in smooth muscle, KATP channels contain Kir6.1/Kir6.2 and SUR2A/SUR2B splice variant subunits.36,37
Figure 2.
Molecular make-up of the KATP channel complex. (Lower left) KATP channel is a hetero-octameric complex composed of four pore Kir6.2 subunits and four regulatory SUR1 subunits. (Right) Membrane topology of SUR1 and Kir6.2 subunits of the KATP channel. ATP binds to the Kir6.2 subunit, inhibiting KATP channels. Hydrolysis of MgATP within the SUR1 subunit nucleotide-binding domains (NBDs) leads to generation of stimulatory MgADP. The A and B sites for sulfonylurea drug binding on both subunits are labeled as indicated.
The Kir6.2 subunit contains ∼390 amino acid and is encoded by KCNJ11 gene, while ∼1,580 amino acid SUR1 subunits are encoded by ABCC8 gene. Both KCNJ11 and ABCC8 genes are located at the same chromosomal locus (11p15.1) and are only 4.5 kb apart.30 Each Kir6.2 subunit consists of two transmembrane (TM) helices connected by a pore-forming loop that confers potassium selectivity to the channel.38 The α-helix linking TM helix 1 (TM1) and intracellular N-terminus, termed as the “slide helix,” plays an important role in channel gating.39 Extensive interactions are found between the cytosolic N- and C-termini of adjacent Kir6.2 subunits that contribute to the formation of binding pocket for the inhibitory ATP molecule.40 Each SUR1 subunit consists of three TM domains (TMD) with a total of 17 TM segments.41 Each SUR1 subunit contains two nucleotide-binding domains (NBD1 and NBD2) that dimerize to form catalytic sites for the intrinsic Mg-ATPase activity of the channel complex, regulating channel activity through binding and hydrolysis of magnesium-bound ATP and the formation of stimulatory Mg-ADP.42,43 Each NBD contains two amino acid sequence nucleotide hydrolysis “Walker A” and “Walker B” motifs (Figure 2B).44,45 TMD0 and the cytosolic loop linking TMD0 and TMD1 of the SUR1 subunit are responsible for the interaction between Kir6.2 subunit.46 The Kir6.2 and SUR1 subunits each possess an endoplasmic reticulum retention motif that requires masking via subunit co-assembly to enable correct trafficking of the assembled hetero-octameric channel complex to the cell membrane.47
KATP channels are inhibited by ATP binding to the Kir6.2 subunits but are activated by the binding and hydrolysis of Mg-ATP in the NBD1/NBD2 dimers on SUR1 subunit, the resulting Mg-ADP generated antagonizes the inhibitory action of ATP on the Kir6.2 subunits. Therefore, the overall activity of the KATP channel complex, and hence the excitability of pancreatic β-cells, is primarily governed by the ratio of cytosolic ATP/ADP45,48 in the close vicinity of the KATP channel complex.
Mutations in either subunit that alter 1) the correct ATP/ADP-sensing machinery within the KATP channel complex, 2) subunit assembly, or 3) trafficking to the cell membrane may adversely affect the appropriate insulin secretion in response to plasma glucose. Inactivation mutations in the KATP channel complex decrease channel activity, causing over-secretion of insulin that is poorly coupled to plasma glucose levels. Indeed, mutations in SUR1 subunit that 1) reduce the stimulatory effect of Mg-ADP or 2) prevent correct trafficking of the channel complex to the cell membrane cause persistent hyperinsulinemia that presents as hypoglycemia in infancy (HI).49,50
Conversely, activation mutations in the KATP channel complex lead to increased channel opening, resulting in a suppression of insulin secretion and subsequent hyperglycemia. Consistent with the cellular regulation of KATP channel activity are the findings that mutations 1) in the Kir6.2 subunit that reduce sensitivity to inhibitory ATP and 2) in the SUR1 subunit that enhance the stimulatory effects of Mg-ADP may precipitate DEND,17 iDEND,4 PNDM,51 TNDM,52 MODY (maturity onset diabetes of the young),53 and type II diabetes (T2D).54,55
KATP channel inactivation mutations underlie HI
Inactivation mutations in both KATP channel subunits can cause HI, which is characterized by severe hypoglycemia.56 Mutant KATP channels with reduced or completely abolished channel activity lead to persistent depolarization of cell membrane, which results in continuous calcium influx and excessive insulin secretion that is uncoupled from the plasma glucose level, producing the hyperinsulinemic hypoglycemia phenotype.12,57,58 Compared to inactivation mutations in the KCNJ11 gene (Kir6.2 subunit), more inactivation mutations have been reported in the ABCC8 gene encoding the SUR1 subunit. Table 1 lists the reported inactivation mutations causing HI in both KCNJ11 and ABCC8 genes and their corresponding locations on each subunit.59,60 Inactivation mutations in the KATP channel complex can be divided into two functional classes: class I, a reduced number of functional KATP channels inserted into the cell membrane, and class II, mutant KATP channels that are correctly inserted but remain refractory to opening regardless of the cellular metabolic state of the cell.22 Class I mutations in either SUR1 or Kir6.2 subunits lead to reduced surface expression of KATP channels, which may result from a total loss of protein, defective channel assembly, or faulty trafficking to the cell membrane.61–63 Class II mutations impair the ability of Mg-ADP to stimulate channel activity,64–66 such that ATP inhibition becomes dominant and the KATP channel is permanently closed even at low glucose concentrations. The majority of class II mutations are located in the NBDs of SUR1 subunit, where the binding and hydrolysis of Mg-ATP occurs. In general, class I mutations produce a more severe phenotype, often requiring near-total or total pancreatectomy, whereas a number of class II mutations result in a milder phenotype as some residual response to stimulatory Mg-ADP may remain. However, there is no strict genotype–phenotype correlation as the same mutation in different patients can produce HI with differing degrees of severity. As HI class II mutations lead to cell membrane expression of dysfunctional KATP channels, less severe forms of HI can often be treated with KATP channel opener diazoxide.67
Table 1.
Mutations in KATP channel genes KCNJ11 and ABCC8 causing hyperinsulinism of infancy
| Genotype | Position in structure | Molecular mechanism | Phenotype |
|---|---|---|---|
| Kir6.2 subunit KCNJ11 | |||
| Y12Δ | N terminus | Immature Kir6.2 subunits | HI |
| R34H | Interface between Kir6.2 subunits | HI | |
| F55L | Interface with SUR1 subunits | HI | |
| K67N | Slide helix | HI | |
| W91R | Linker between TM1 and pore region | HI | |
| A101D | Linker between TM1 and pore region | HI | |
| S116P | Pore region | HI | |
| G134A | Linker between pore region and TM2 | HI | |
| R136L | Linker between pore region and TM2 | HI | |
| L147P | TM2 | HI | |
| A187V | ATP-binding site | HI | |
| P254L | ATP-binding site | HI | |
| H259R | ATP-binding site | Reduced trafficking of the channel | HI |
| P266L | C terminus | HI | |
| E282 K | C terminus | HI | |
| T294M | Gating | Reduced channel Po | HI |
| R301H | Gating | HI | |
| C344Δ | C terminus | Immature Kir6.2 subunits | HI |
| SUR1 subunit ABCC8 | |||
| G70E | Linker between TM1 and TM2 | HI | |
| R74Q/W | Linker between TM1 and TM2 | HI | |
| G111R | TM3 | HI | |
| A116P | TM3 | HI | |
| H125Q | Linker between TM3 and TM4 | HI | |
| V167L | TM5 | HI | |
| V187D | TM5 | HI | |
| N188S | TM5 | HI | |
| Q219Δ | Linker between TM5 and TM6 | Immature SUR1 subunits | HI |
| R248Δ | Linker between TM5 and TM6 | Immature SUR1 subunits | HI |
| N406D | Linker between TM7 and TM8 | HI | |
| N418R | Linker between TM7 and TM8 | HI | |
| L508P | Linker between TM9 and TM10 | HI | |
| F591L | NBD1 | HI | |
| R598Δ | NBD1 | Immature SUR1 subunits | HI |
| R620C | NBD1 | HI | |
| G716V | Walker A in NBD1 | HI | |
| C717Δ | Walker A in NBD1 | Immature SUR1 subunits | HI |
| R837Δ | NBD1 | Immature SUR1 subunits | HI |
| R842G | NBD1 | HI | |
| K890T | NBD1 | HI | |
| Q954Δ | NBD1 | Immature SUR1 subunits | HI |
| S957F | NBD1 | HI | |
| R999Δ | NBD1 | Immature SUR1 subunits | HI |
| T1139M | Linker between TM13 and TM14 | HI | |
| R1215Q/W | Linker between TM15 and TM16 | HI | |
| K1337N | NBD2 | HI | |
| W1339Δ | NBD2 | Immature SUR1 subunits | HI |
| G1343E | NBD2 | HI | |
| R1353P/H | NBD2 | HI | |
| V1361M | NBD2 | HI | |
| G1379R | Walker A in NBD2 | Reduced Mg–nucleotide binding | HI |
| G1382S | Walker A in NBD2 | Reduced Mg–nucleotide binding | HI |
| S1387F | NBD2 | HI | |
| F1388Δ | NBD2 | Immature SUR1 subunits | HI |
| R1394H | NBD2 | Impaired trafficking of SUR1 subunits | HI |
| G1401D | NBD2 | HI | |
| R1419H | NBD2 | HI | |
| R1421C | NBD2 | Reduced Mg–nucleotide binding | HI |
| R1437Q | NBD2 | HI | |
| A1458T | NBD2 | HI | |
| G1479R | NBD2 | Reduced Mg–nucleotide binding | HI |
| A1493T | NBD2 | HI | |
| R1494Q/W | NBD2 | HI | |
| E1507K | Walker B in NBD2 | Reduced Mg–nucleotide binding | HI |
| L1544P | NBD2 | Impaired trafficking of SUR1 subunits | HI |
| V1551D | NBD2 | Reduced Mg–nucleotide binding | HI |
| L1552V | NBD2 | Reduced Mg–nucleotide binding | HI |
| G1555S | C terminus | HI |
Abbreviations: HI, hyperinsulinism of infancy; SUR1, sulfonylurea receptor 1.
KATP channel activation mutations underlie NDM
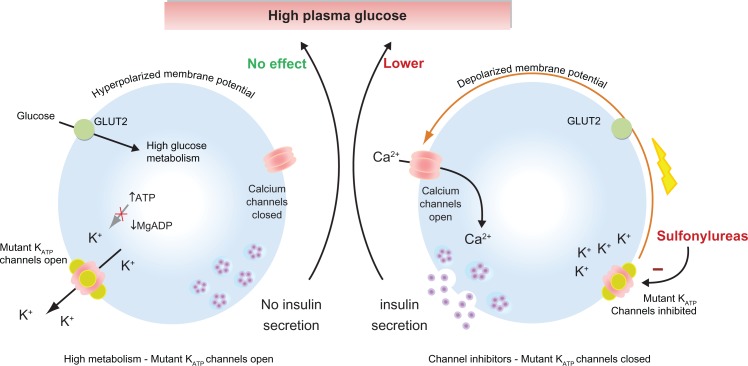
Monogenic activation mutations in the KCNJ11 and ABCC8 genes can be found in all forms of NDM (DEND,4,17 PNDM,51 TNDM,52 and MODY).53 Activation mutations result in a reduced coupling of channel activity to plasma glucose levels via glucose metabolism. In general, the more stimulatory the mutation, the greater the suppression of insulin secretion and the resulting level of hyperglycemia (Figure 3A).68–71 The underlying molecular mechanisms for the majority of activation mutations can be tested experimentally and correlated well with their specific locations within the KATP channel subunits as follows.
Figure 3.
Sulfonylureas stimulate insulin secretion in neonatal diabetes caused by KATP channel mutations. (Left) Activation mutations in the KATP channel prevent channel closure in response to high plasma glucose. Consequently, the membrane potential remains hyperpolarized even, thereby preventing insulin secretion. (Right) Sulfonylureas bind directly to KATP channels causing channel inhibition that triggers membrane potential and insulin secretion resulting in a lowering of plasma glucose.
Activation mutations in the Kir6.2 subunit
Heterozygous activation mutations in Kir6.2 subunit have been identified in ∼50% of PNDM cases and also have been found in a large number of TNDM cases.3 To date, >40 activation mutations in Kir6.2 subunit have been reported at 30 distinct residues (Table 2).60 The locations of these mutations are clustered into three common regions in the Kir6.2 subunit. One cluster of mutations line the putative ATP-binding pocket (eg, R50, R201, and Y330) and reduce channel ATP inhibition by decreasing ATP-binding affin-ity.69,72–74 Another cluster of mutations reside in subunit regions involved in channel gating such as the slide helix (eg, V59), the cytosolic mouth of the channel (eg, I296), or gating loops (eg, C166) between ATP-binding site and the slide helix. These mutations decrease ATP inhibition by stabilizing the open conformation of the channel in both the absence and the presence of ATP, leading to increases in channel activity.75–77 The third cluster of mutations is located at the interface between the subunits such as the interface between adjacent Kir6.2 subunits (eg, F35 and E322) and the interface between Kir6.2 and SUR1 subunits (eg, Q52 and G53). These mutations likely alter channel activity by affecting the interactions between adjacent Kir6.2 and SUR1 subunits that are important for correct channel gating.78–82
Table 2.
Mutations in KATP channel genes KCNJ11 and ABCC8 causing diabetes in terms of DEND, PNDM, TNDM, MODY, and T2D
| Genotype | Position in structure | Molecular mechanism | Phenotype | Sensitivity to SU |
|---|---|---|---|---|
| Kir6.2 subunit KCNJ11 | ||||
| E23K | N terminus | T2D | Normal sensitivity | |
| R34C | Interface between Kir6.2 subunits | TNDM | ||
| F35L/V | Interface between Kir6.2 subunits | Increased channel Po | PNDM | Normal sensitivity |
| C42R | Interface between Kir6.2 subunits | Increased channel Po | PNDM/TNDM/MODY | |
| H46Y | Slide helix | Increased channel Po | PNDM | Normal sensitivity |
| H46 L | Slide helix | Increased channel Po | iDEND | Normal sensitivity |
| N48D | ATP-binding site | Decreased ATP-binding affinity | PNDM | |
| R50P/Q | ATP-binding site | Decreased ATP-binding affinity | PNDM | Normal sensitivity |
| Q52R | Interface with SUR1 subunits | Increased channel Po | DEND | Reduced sensitivity |
| G53R/S | Interface with SUR1 subunits | Decreased ATP-binding affinity | TNDM | Normal sensitivity |
| G53N/D | Interface with SUR1 subunits | Decreased ATP-binding affinity | PNDM | Normal sensitivity |
| V59G | Slide helix | Increased channel Po | DEND | Reduced sensitivity |
| V59M | Slide helix | Increased channel Po | iDEND | Normal sensitivity |
| F60Y | Slide helix | DEND | ||
| V64L | Slide helix | DEND | ||
| L164P | Gating | PNDM | Reduced sensitivity | |
| C166F/Y | Gating | DEND | Reduced sensitivity | |
| I167L | Gating | Increased channel Po | iDEND | Normal sensitivity |
| K170N/R/T | ATP-binding site | Decreased ATP-binding affinity | PNDM | Normal sensitivity |
| A174G | ATP-binding site | TNDM | ||
| R176C | ATP-binding site | PNDM | ||
| E179A | ATP-binding site | TNDM | ||
| I182V | ATP-binding site | Decreased ATP-binding affinity | TNDM | |
| K185E | ATP-binding site | Decreased ATP-binding affinity | DEND | |
| R201C | ATP-binding site | Decreased ATP-binding affinity | PNDM/DEND | Normal sensitivity |
| R201H/L | ATP-binding site | Decreased ATP-binding affinity | PNDM | Normal sensitivity |
| E227K/L | Gating | Increased channel Po | PNDM | Normal sensitivity |
| E229K | Gating | Increased channel Po | TNDM | |
| V252A | ATP-binding site | Decreased ATP-binding affinity | TNDM | |
| E292G | Gating | Increased channel Po | PNDM | |
| T293N | Gating | Increased channel Po | DEND | Reduced sensitivity |
| I296L | Pore | Increased channel Po | DEND | Reduced sensitivity |
| E322K | Interface between Kir6.2 subunits | Decreased ATP-binding affinity | PNDM | Normal sensitivity |
| Y330C/S | ATP-binding site | Decreased ATP-binding affinity | PNDM/DEND | Normal sensitivity |
| F333I | Interface with SUR1 subunits | Increased Mg-ATP hydrolysis by NBD2 in SUR1 subunits | PNDM | Normal sensitivity |
| G334D | ATP-binding site | Decreased ATP-binding affinity | DEND | Reduced sensitivity |
| I337V | ATP-binding site | T2D | ||
| R365H | C terminus | TNDM | ||
| SUR1 subunit ABCC8 | ||||
| P45L | TM1 | PNDM | Normal sensitivity | |
| N72S | Linker between TM1 and TM2 | PNDM | ||
| V86A/G | TM2 | PNDM | Normal sensitivity | |
| A90V | TM2 | PNDM | ||
| F132L/V | Linker between TM3 and TM4 | Reduced ATP inhibitory effect in Kir6.2 subunits | DEND | |
| L135P | TM4 | iDEND | ||
| R176C | TM5 | PNDM | ||
| P207S | Linker between TM5 and TM6 | Reduced ATP inhibitory effect in Kir6.2 subunits | PNDM | |
| E208K | Linker between TM5 and TM6 | Reduced ATP inhibitory effect in Kir6.2 subunits | PNDM | Normal sensitivity |
| D209E | Linker between TM5 and TM6 | Reduced ATP inhibitory effect in Kir6.2 subunits | PNDM/TNDM | Normal sensitivity |
| Q211K | Linker between TM5 and TM6 | Reduced ATP inhibitory effect in Kir6.2 subunits | PNDM | Normal sensitivity |
| D212I/N | Linker between TM5 and TM6 | Reduced ATP inhibitory effect in Kir6.2 subunits | TNDM | |
| L213R | Linker between TM5 and TM6 | Reduced ATP inhibitory effect in Kir6.2 subunits | DEND | Normal sensitivity |
| L225P | Linker between TM5 and TM6 | PNDM | Normal sensitivity | |
| T229I | Linker between TM5 and TM6 | TNDM | Normal sensitivity | |
| Y263D | Linker between TM5 and TM6 | PNDM | Normal sensitivity | |
| A269D | Linker between TM5 and TM6 | PNDM | ||
| R306H | TM6 | TNDM | ||
| V324M | TM6 | TNDM | ||
| Y356C | TM7 | T2D | ||
| E382K | Linker between TM7 and TM8 | PNDM | ||
| C435R | TM8 | TNDM | ||
| L438F | TM8 | PNDM | ||
| L451P | TM9 | TNDM | ||
| L582V | TM11 | TNDM | ||
| R826W | NBD1 | Increased channel activation by Mg-nucleotide | TNDM | |
| H1024Y | TM12 | TNDM | Normal sensitivity | |
| R1183Q/W | Linker between TM15 and TM16 | TNDM | ||
| A1185E | Linker between TM15 and TM16 | PNDM | ||
| M1290V | TM17 | PNDM | ||
| R1314H | NBD2 | TNDM | Normal sensitivity | |
| E1327K | NBD2 | PNDM | ||
| S1369A | NBD2 | T2D | ||
| R1380C/H/L | Walker A in NBD2 | Increased ATPase activity in NBD2 | TNDM | Normal sensitivity |
| G1401R | NBD2 | PNDM | Normal sensitivity | |
| I1425V | NBD2 | Increased channel activation by Mg-nucleotide | PNDM | Normal sensitivity |
| V1524L/M | NBD2 | PNDM | Normal sensitivity |
Abbreviations: DEND, developmental delay, epilepsy, and neonatal diabetes; PNDM, permanent neonatal diabetes mellitus; TNDM, transient neonatal diabetes mellitus; MODY, maturity onset diabetes of the young; T2D, type II diabetes; iDEND, intermediate developmental delay, epilepsy, and neonatal diabetes; Po, open probability; SUR1, sulfonylurea receptor 1.
To directly study the ability of ATP to inhibit the KATP channel via the Kir6.2 subunit, Mg-free experimental conditions can be used to eliminate the channel stimulatory effect of Mg-ATP on the SUR1 subunit of the channel.83 In Mg-free conditions, homomeric channels containing Kir6.2 activation mutations are less sensitive to ATP inhibition compared to wild-type channels. There are two major molecular mechanisms by which Kir6.2 activation mutations elicit a reduction in ATP sensitivity. 1) An increase in the maximal open probability (Po) of the channel in the absence of ATP.17,84 In the ATP-unbound state of channels (ATP absent), mutations in the region involved in channel gating (eg, V59G)4 exhibit a higher maximal channel Po compared to wild-type channels. (In the absence of Mg2+, IC50 was 7.0 ± 1.1 μmol/L and 7.4 ± 1.5 mmol/L for wild-type channels and homomeric V59G channels, respectively; P < 0.001. Channel Po was 0.53 ± 0.02 and 0.83 ± 0.01 for wild-type channels and homomeric V59G channels, respectively; P < 0.001.)4 2) A decrease in ATP-binding affinity.51,85,86 Homomeric channels containing mutations in the ATP-binding region (eg, R201C)4 display altered ATP inhibition, yet their maximal Po in the absence of ATP is not significantly different compared to wild-type channels. (In the absence of Mg2+, IC50 was 7.0 ± 1.1 and 106 ± 12 μmol/L for wild-type channels and homomeric R201C channels, respectively; P < 0.001. Channel Po was 0.53 ± 0.02 and 0.6 ± 0.03 for wild-type channels and homomeric R201C channels, respectively; P is not significant.)4
Activation mutations in the SUR1 subunit
There are more than 30 individual activation mutations in SUR1 subunit that have been reported to cause NDM (Table 2).60 Many of these mutations are dispersed throughout the SUR1 subunit sequence, although a large number of mutations reside in two specific regions of the SUR1 subunit. One cluster of mutations is concentrated in TMD0 and the cytosolic loop linking TMD0 and TMD1.14,87–91 As this region is known to interact with adjacent Kir6.2 subunit, mutations in this region are believed to reduce ATP inhibition via the Kir6.2 subunit.92,93 The second cluster of mutations resides in the NBD2 of the SUR1 subunit, where stimulatory Mg–nucleotide diphosphates such as Mg-ADP bind.94–96 Therefore, NBD2 mutations are thought to either increase direct Mg-ADP stimulation or enhance MgATPase activity in NBD2, leading to increased Mg-ADP stimulation. (For example, R1380 L, Vmax of ATPase activity was 60.8 ± 1.8 and 104.3 ± 9.9 nmol/min/mg for wild-type NBD2 and R1380L NBD2, respectively; P < 0.01. Km of ATPase activity was 0.41 ± 0.04 and 0.55 ± 0.09 mmol/L for wild-type NBD2 and R1380L NBD2, respectively. P is not significant.)94
Genotype–phenotype correlation in NDM caused by mutations in KATP channels
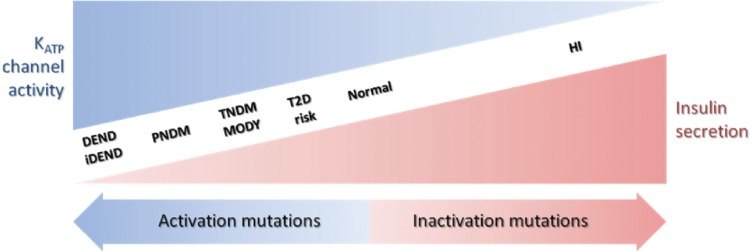
There is a wide spectrum of NDM severity associated with different degrees of insulin secretion deficiency and neuronal defects caused by activation mutations in KATP channels (Figure 4). The severity of these clinical phenotypes increases in the order of T2D < MODY/TNDM < PNDM < iDEND/DEND.22 In general, the greater the activation of KATP channels, the more severe the phenotype; however, several factors need to be considered when attempting to predict the clinical severity caused by a specific mutation.
Figure 4.
Relationship between insulin secretion and KATP channel activity in a spectrum of clinical presentations of hypo- and hyperglycemia. The clinical severity of the disease is correlated with the extent of KATP channel activity caused by the mutations.
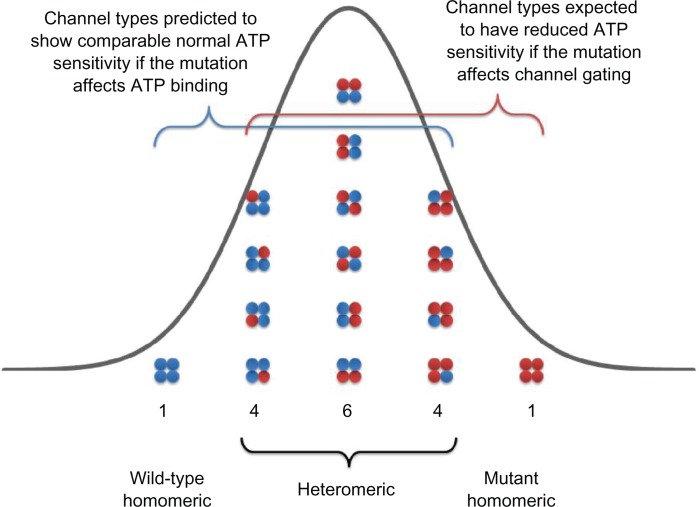
Heterozygosity is an important factor affecting the clinical phenotype of a mutation. NDM patients with activation mutations in either Kir6.2 or SUR1 subunits are heterozygous77 for the mutation; thus, both wild-type and mutant subunits are expressed in the same cell. The assembly of Kir6.2 subunits can be used to explain the nature of heterozygosity in NDM patients. In a heterozygous NDM patient carrying one copy of normal (wild type) and one copy of mutant KCNJ11 gene,28 there will be a mixed population of channels, each of which carries from 0 to 4 mutant Kir6.2 subunits (Figure 5).4,22 Two factors determine the inhibitory ATP sensitivity of any individual channel in this population. One is the number of mutant subunits that an individual channel contains and the other is the contribution of mutant subunits to overall channel ATP sensitivity. This contribution is also linked to the molecular mechanism of each activation mutation in Kir6.2 subunit.
Figure 5.
Schematic of the KATP channel Kir6.2 subunit compositions expected when wild-type (blue) and mutant (red) Kir6.2 are co-expressed in the heterozygous state. If the co-assembly wild-type (blue) and mutant (red) Kir6.2 subunits is independent and random and follows a binomial distribution, as a single KATP channel is made of 4 Kir6.2 subunits, there will be 1/16 channel with 0 mutant subunit, 4/16 channel with 1 mutant subunit, 6/16 channel with 2 mutant subunits, 4/16 channel with 3 mutant subunits, and 1/16 channel with all 4 mutant subunits.
If the mutation impairs ATP-binding affinity alone, there will be only a small reduction in ATP sensitivity in heterozygous population compared to wild-type population. (For example, R201H of Kir6.2 subunit. In the absence of Mg2+, IC50 were 7.0 ± 1.1, 12.5 ± 1.1, and 298 ± 25 μmol/L for wild type, heterozygous, and homomeric R201H channels, respectively; P < 0.05 and P < 0.001 vs wild-type, respectively.)4 This is because binding of a single ATP molecule to 1 of 4 ATP-binding sites is sufficient to inhibit the KATP channel.97 The ATP sensitivity of the channel will only be substantially impaired when all four subunits contain the mutation; otherwise, the mutant Kir6.2 subunit’s effects will be largely compensated for by the presence of the other wild-type subunits. This can be explained by using a simple statistical probability model. If the co-assembly of wild-type and mutant Kir6.2 subunits is independent and random and follows a binomial distribution, as a single channel is made of 4 Kir6.2 subunits, then only 1 out of 16 channels in the heterozygous population will contain all 4 mutant Kir6.2 subunits that display a significant decrease in ATP sensitivity (Figure 5).4 The other 15 channels (4/16 with 3 mutant subunits; 6/16 with 2 mutant subunits; 4/16 with 1 mutant subunit, and 1/16 with 0 mutant subunits) will have comparable ATP sensitivity to the channel containing all wild-type subunits, so the resulting ATP sensitivity of heterozygous population is very close, but not identical, to that of a pure wild-type channel population. However, this small shift of ATP sensitivity in the heterozygous channel population leads to NDM for the following reasons. Under physiological conditions, intracellular concentration of ATP is in the range of 1–5 mM, such that KATP channels exhibit very low activity. Additionally, the β-cell membrane possesses a high electrical resistance such that only a small reduction in ATP sensitivity to the channel results in a small increase in KATP channel activity that holds the β-cell membrane potential in a more polarized state and suppresses insulin secretion.23 Therefore, even a very modest reduction in heterozygous KATP channel ATP sensitivity can lead to significantly impaired insulin secretion resulting in NDM.
In direct contrast, if the mutation in question increases intrinsic KATP channel Po (in the absence of ATP), there will be a significant reduction in ATP sensitivity in heterozygous population compared to wild-type population, as the presence of one single mutant subunit will increase the intrinsic Po of KATP channels. (For example, Q52R of Kir6.2 subunit. Channel Po were 0.53 ± 0.02, 0.70 ± 0.03, and 0.83 ± 0.01 for wild type, heterozygous, and homomeric Q52R channels, respectively. P < 0.001 and P < 0.001, vs wild-type, respectively.)4 This can be explained by using the same statistical model described in detail earlier. Fifteen out of 16 channels will contain at least one mutant subunit in a heterozygous channel population (Figure 5) and exhibit a marked decrease in ATP sensitivity. Thus, the ATP sensitivity of heterozygous population is significantly reduced compared to that of wild-type population and is associated with a more severe DEND syndrome phenotype.17 This provides a rational explanation as to why mutations that increase the intrinsic Po produce a more severe clinical phenotype, such as DEND, whereas mutations that decrease ATP-binding affinity lead to a milder clinical phenotype such as PNDM.
The specific location of mutations within either subunit also correlates well with the severity of the clinical phenotype. In general, mutations in Kir6.2 subunit are typically associated with PNDM, iDEND, and DEND, whereas mutations in SUR1 subunit are more frequently associated with TNDM. This may be accounted for by the overriding ability of ATP to inhibit channel activity within wild-type Kir6.2 subunits even when there is an enhanced stimulatory effect of Mg-ADP via the effects of a SUR1 subunit activation mutation causing TNDM.74 Furthermore, although some activation KATP channel mutations lead to transient diabetes, these patients are at increased risk of developing T2D later in life. Interestingly, the common genetic variants E23K in KCNJ11 and S1369A in ABCC8 form a haplotype and are associated with an increased risk to T2D.98–100 The precise molecular mechanisms that underlie this increased risk likely result from even subtler alterations of ATP/ADP sensitivity101 than those described for the monogenic mutations that cause overt forms of NDM.
While there is good evidence for a clear genotype–phenotype relationship with several activation mutations in KATP channels, the association between phenotype and genotype is not absolute, as there is often a different severity of clinical phenotype among patients carrying the same mutation. This strongly implies that there are other factors (such as underlying polygenic diabetes risk, diet, or environment) that influence the development of clinical phenotype besides the presence of a single KCNJ11 or ABCC8 mutation in NDM patients.102,103
Pharmacotherapy for NDM patients carrying KCNJ11 and ABCC8 mutations
Before the discovery that mutations in a number of genes underlie NDM, daily insulin therapy was the only effective treatment for patients. As mentioned earlier, NDM can be the result of mutations in multiple genes (eg, KCNJ11, ABCC8, GCK, INS, FOXP3, EIF2AK3, and ZAC/HYMAI).104 Since 2004, many NDM patients with either KCNJ11 or ABCC8 mutations have been successfully treated with a pharmacological approach, removing the requirement for insulin injections.105,106 The SU drugs, a class of KATP channel inhibitor, have been widely used as a treatment of T2D for over 50 years. SUs bind directly to the KATP channel complex, leading to channel closure and subsequent stimulation of insulin secretion (Figure 3B). Recent studies now demonstrate that glycemic control in NDM patients with KATP channel activation mutations can be managed with SU therapy alone.107,108 Therefore, SUs should be considered as an attractive alternative therapy to treat NDM patients carrying mutations in KCNJ11 and ABCC8 genes. As mutations in a number of genes can cause NDM and the causal mutation in each patient may differ greatly, a pharmacogenomic approach to treatment may be possible to “tailor” SU therapy based on specific NDM genotype.
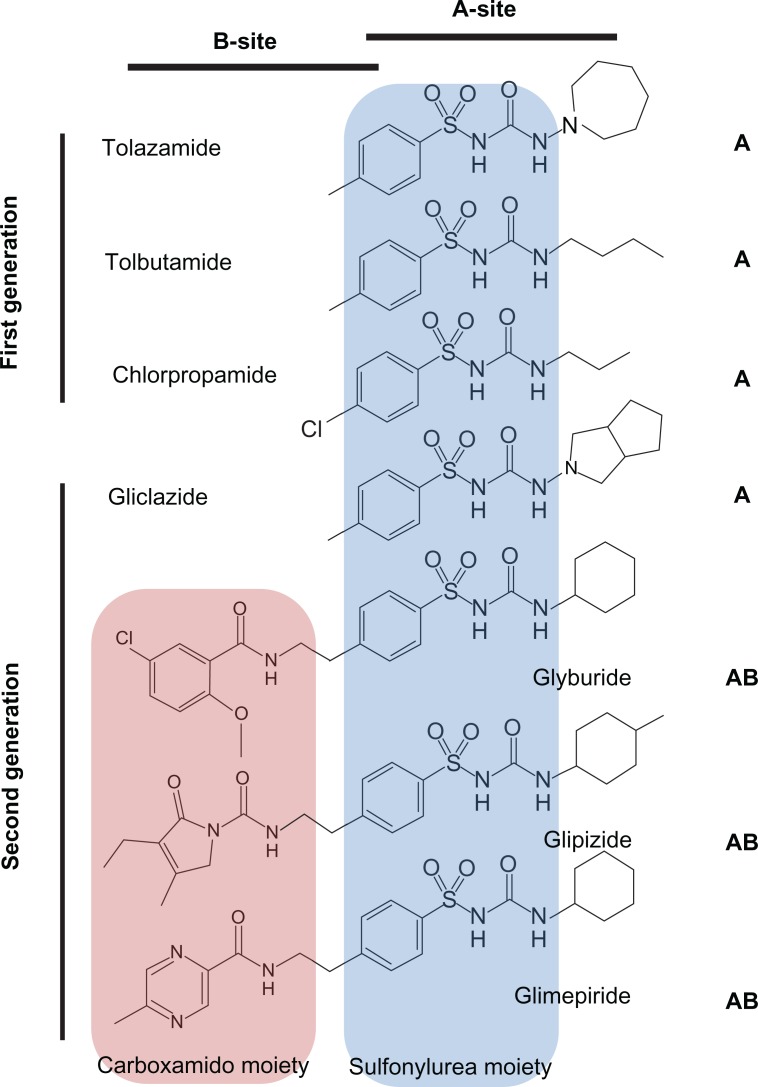
SUs can be classified according to their historical discovery with first generation SUs, including tolazamide, tolbutamide, and chlorpropamide, and second generation SUs, including glyburide, glipizide, and glimepiride (Figure 6). Compared to the first generation SUs, the second generation SUs are now more commonly used in the treatment of NDM as they are more potent and tend to have a longer duration of action.
Figure 6.
Structures and binding-site classification of clinically used sulfonylurea drugs.
There are two SU-binding sites within the KATP channel complex109 that have been identified to date. The “A-site” is located in the intracellular loops connecting TM segments 14–16 on SUR1 subunit. The “B-site” is composed of the intracellular loop between TM segments 5–6 in the SUR1 subunit and the N-terminus in Kir6.2 subunit (Figure 2B). Therefore, SUR1 subunit possesses a bipartite pocket with distinct A- and B-binding sites. Furthermore, SUs can be classified as A-site or AB-site drugs based on where they bind to the channel (Figure 6). The A site within SUR1 subunit binds the SU moiety, whereas the B site binds the non-SU carboxamido moiety of the molecule.109 Most of the first generation SUs (eg, tolbutamide and chlorpropamide) are A-site drugs, whereas the majority of the second generation SUs (eg, glyburide, glipizide, and glimepiride) are AB-site drugs, which also accounts for the higher potency of the second generation SUs. An exception to this is gliclazide, which is an A-site SU with potency comparable to the AB-site SUs.
A key issue in the optimization of SU therapy in NDM is whether mutant KATP channels can be inhibited by SUs in a similar concentration range to wild-type channels. For NDM patients with SUR1 subunit mutations, there are no reports of mutations in the ABCC8 altering SU inhibition. This may be because NDM patients with mutant SUR1 subunits often exhibit a milder clinical NDM phenotype. Therefore, SU therapy should be effective for most NDM patients with SUR1 subunit mutations.
In contrast, several studies suggest that NDM patients with Kir6.2 subunit mutations respond differently to SU therapy,110–112 which is likely related to the underlying molecular mechanisms of mutations that alter KATP channel function. Mutations that reduce binding affinity of inhibitory ATP causing TNDM or PNDM show adequate efficacy of SUs.113 However, mutations that enhance intrinsic channel Po causing DEND or iDEND have a reduced inhibitory efficacy of SUs.114 In general, the greater the ability of a specific mutation to increase the intrinsic channel Po, the higher the SU dosage required to achieve the same level of channel inhibition seen in with mutations causing PNDM and TNDM.115,116 As SUs are unable to sufficiently inhibit KATP channels with mutations that cause a greatly enhanced intrinsic channel Po, DEND patients with activation mutations in Kir6.2 subunit often require a combination of SU and insulin therapy rather than SU therapy alone. SU dosage for NDM patients can be quite high (up to 2.5 mg/kg/day of glyburide) compared with the dosage for patients with T2D (∼0.2 mg/kg/day).
SUs are extensively metabolized in the liver, primarily by the cytochrome P450 2C9 enzyme encoded by the CYP2C9 gene. To date, several pharmacogenomic studies have focused on the influence of common gene variants in the CYP2C9 gene on SU pharmacokinetics.117–119 As the activity of cytochrome P450 2C9 variants correlates well with serum levels of SUs, patients carrying CYP2C9 variants that reduce cytochrome P450 2C9 enzymatic activity possess elevated serum levels of SUs.120 Therefore, screening for these common CYP2C9 variants may provide additional information as to whether a NDM patient carrying a KCNJ11 or ABCC8 gene mutation may respond better to SU therapy.
Although insulin therapy may control glucose homeostasis in NDM patients with mutant KATP channels, it does not restore the normal KATP channel activity in nonpancreatic tissues such as the brain and skeletal muscle. On the other hand, SUs can inhibit KATP channels in many tissues such as the central nervous system (CNS), ameliorating the neurological dysfunction observed in iDEND/DEND.121–123
One potential concern is that the dosage for SUs needed to adequately control glucose homeostasis may not be enough to resolve neurological symptoms, as SUs have to cross the blood–brain barrier to exert inhibitory effect on CNS KATP channels. However, several studies showed that improvements in mental and motor function were found in patients carrying mutant KATP channels with DEND syndrome treated with SUs.121–123 These observations suggest that SUs are able to cross the blood–brain barrier at concentrations sufficient to inhibit KATP channels in the CNS.
Several recent studies now show that early diagnosis and treatment of DEND patients carrying KCNJ11 or ABCC8 gene mutations with SU therapy could reduce or even prevent the neurological dysfunction in addition to dramatically improving glycemic control.121–123 Most DEND patients who have successfully transferred to SU therapy were children at the time of transfer. Therefore, if the causal mutation is on either KCNJ11 or ABCC8 genes, then an early switch to SU therapy may minimize the extent of neurological problems. This also emphasizes the importance of early screening for mutations in KCNJ11 and ABCC8 genes in those NDM patients with neurological features.
Traditionally, PNDM patients with mutations in other genes such as GCK gene encoding glucokinase are treated with insulin therapy. A recent study reported that a PNDM patient with the T168A mutation in glucokinase exhibited modest responsiveness to SU therapy.124 Furthermore, MODY patients with mutations in HNF1α (hepatocyte nuclear factor) are very sensitive to SU therapy and many of them have been successfully transferred to SU therapy from insulin therapy.125,126 Therefore, the use of SU therapy should also be considered in NDM patients with mutations in genes other than KCNJ11 and ABCC8. These findings also highlight the central role that KATP channels play in regulating insulin secretion.
SUs exhibit differential potencies on KATP channels with different subunit compositions that are often expressed in a variety of tissues.127–130 The majority of first generation SUs and gliclazide (A site) are more selective for KATP channels containing the SUR1 subunit as found in the pancreas and CNS (IC50 was 50 nmol/L for gliclazide on KATP channels containing SUR1 subunits).131 Thus, KATP channels containing either SUR2A or SUR2B subunits (heart/skeletal/smooth muscle) would not be inhibited by these SUs at the same concentration (IC50 was 0.8 mmol/L for gliclazide on KATP channels containing SUR2A subunits).131 As second generation SUs (AB site) are nonselective, they will inhibit all KATP channels with similar potency (IC50 s were 3, 5.4, and 7.3 nmol/L for glimepiride on KATP channels containing SUR1, SUR2A, and SUR2B subunits, respectively).132
Recent studies implicate a role for skeletal muscle (Kir6.2 and SUR2A) KATP channels in peripheral insulin sensitivity.133,134 In NDM patients with Kir6.2 activation mutations, overactive KATP channels in skeletal muscle (Kir6.2 and SUR2A) may reduce insulin sensitivity in addition to decreasing insulin secretion, further contributing to the development of NDM.135 Inhibition of skeletal muscle KATP channels with Kir6.2 activation mutations with SUs may increase peripheral insulin sensitivity.136 This notion is supported by studies showing that better glycemic control is achieved with AB-site SUs, compared with A-site SUs.116,123,128,137 This is because skeletal muscle and β-cell are inhibited by AB-site SUs as both insulin secretion and insulin sensitivity are achieved. Therefore, AB-site SUs may be the better choice for NDM patients with Kir6.2 activation mutations.
In clinical practice, the two major treatments for NDM patients are insulin therapy and oral SUs and treatment for individual patient varies depending on the genetic cause of NDM.138 For 50% of PNDM patients and 10% of TNDM patients carrying mutant KATP channels, SU therapy is an attractive alternative to insulin therapy. However, for other PNDM patients carrying mutations in PTF1A, EIF2AK3, FOXP3 and 80% of TNDM patients carrying mutations in chromosome 6q24 (eg, ZAC/HYMAI), SU responsiveness is minimal and the patients’ only option is insulin therapy.3 Therefore, it is important to diagnose the underlying genetic cause of NDM to fully optimize treatment.139 Genetic testing is not only important for the correct diagnosis but may now also be used in the optimization of treatment in a large number of PNDM and TNDM patients with KATP channel mutations.140
Conclusion
KATP channels play a central physiological role in pancreatic β-cells, where they act as key regulators of insulin secretion in response to changes in plasma glucose. Inactivation or activation mutations in KATP channels lead to altered KATP channel activity, producing a phenotype of either HI or NDM. With respect to KATP channel mutations in NDM, the severity of the clinical phenotype correlates well with the magnitude of KATP channel activation.
To date, it is estimated that ∼90% of NDM patients carrying KATP channel activation mutations can discontinue daily insulin injections and show improved glycemic control when they are switched to a high-dose SU therapy. Besides improving the quality of life for NDM patients, switching from insulin injection to SU therapy can also reduce neurological symptoms associated with patients with more severe forms of NDM (iDEND/DEND).
Furthermore, genetic information, coupled with clinical factors, may help to improve the treatment of NDM by aiding in the appropriate selection of therapeutic strategies (insulin injection, or SU therapy, or a combination of both) and a more accurate adjustment of SU dosage. Future research will likely lead to improved glycemic control by the development of a rational pharmacogenomic approach to “tailor” SU therapy based on an NDM patient’s individual genotype.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wiedemann B, Schober E, Waldhoer T, et al. Incidence of neonatal diabetes in Austria-calculation based on the Austrian Diabetes Register. Pediatr Diabetes. 2010;11(1):18–23. doi: 10.1111/j.1399-5448.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- 2.Slingerland AS, Shields BM, Flanagan SE, et al. Referral rates for diagnostic testing support an incidence of permanent neonatal diabetes in three European countries of at least 1 in 260,000 live births. Diabetologia. 2009;52(8):1683–1685. doi: 10.1007/s00125-009-1416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattersley AT, Pearson ER. Minireview: pharmacogenetics and beyond: the interaction of therapeutic response, beta-cell physiology, and genetics in diabetes. Endocrinology. 2006;147(6):2657–2663. doi: 10.1210/en.2006-0152. [DOI] [PubMed] [Google Scholar]
- 4.Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci U S A. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Støy J, Edghill EL, Flanagan SE, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo C, Porzio O, Liu M, et al. Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J Clin Invest. 2008;118:2148–2156. doi: 10.1172/JCI33777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Njølstad PR, Sovik O, Cuesta-Munoz A, et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Eng J Med. 2001;344:1588–1592. doi: 10.1056/NEJM200105243442104. [DOI] [PubMed] [Google Scholar]
- 8.Njølstad PR, Sagen JV, Bjorkhaug L, et al. Permanent neonatal diabetes caused by glucokinase deficiency: inborn error of the glucose-insulin signaling pathway. Diabetes. 2003;52:2854–2860. doi: 10.2337/diabetes.52.11.2854. [DOI] [PubMed] [Google Scholar]
- 9.Edghill EL, Gloyn AL, Goriely A, et al. Origin of de novo KCNJ11 mutations and risk of neonatal diabetes for subsequent siblings. J Clin Endocrinol Metab. 2007;92:1773–1777. doi: 10.1210/jc.2006-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloyn AL, Cummings EA, Edghill EL, et al. Permanent neonatal diabetes due to paternal germline mosaicism for an activating mutation of the KCNJ11 gene encoding the Kir6.2 subunit of the beta-cell potassium adenosine triphosphate channel. J Clin Endocrinol Metab. 2004;89:3932–3935. doi: 10.1210/jc.2004-0568. [DOI] [PubMed] [Google Scholar]
- 11.Vaxillaire M, Populaire C, Busiah K, et al. Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes. 2004;53:2719–2722. doi: 10.2337/diabetes.53.10.2719. [DOI] [PubMed] [Google Scholar]
- 12.Gloyn AL, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27:220–231. doi: 10.1002/humu.20292. [DOI] [PubMed] [Google Scholar]
- 13.Vaxillaire M, Dechaume A, Busiah K, et al. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes. 2007;56:1737–1741. doi: 10.2337/db06-1540. [DOI] [PubMed] [Google Scholar]
- 14.Babenko AP, Polak M, Cave H, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 15.Shield JP, Flanagan SE, Mackay DJ, et al. Mosaic paternal uniparental isodisomy and an ABCC8 gene mutation in a patient with permanent neonatal diabetes and hemihypertrophy. Diabetes. 2008;57:255–258. doi: 10.2337/db07-0999. [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft FM, Proks P, Smith PA, et al. Stimulus-secretion coupling in pancreatic β-cells. J Cell Biochem. 1994;55(Suppl):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- 17.Proks P, Girard C, Haider S, et al. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;6:470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 19.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 20.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 21.Ashcroft FM. ATP-sensitive K channels and disease: from molecule to malady. Am J Physiol Endocrinol Metab. 2007;293:880–889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 22.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 24.Miki T, Liss B, Minami K, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 25.Li DP, Chen SR, Pan HL. Adenosine inhibits paraventricular pre-sympathetic neurons through ATP-dependent potassium channels. J Neurochem. 2010;113(2):530–542. doi: 10.1111/j.1471-4159.2010.06618.x. [DOI] [PubMed] [Google Scholar]
- 26.Bahi-Buisson N, Eisermann M, Nivot S, et al. Infantile spasms as an epileptic feature of DEND syndrome associated with an activating mutation in the potassium adenosine triphosphate (ATP) channel, Kir6.2. J Child Neurol. 2007;22(9):1147–1150. doi: 10.1177/0883073807306272. [DOI] [PubMed] [Google Scholar]
- 27.Amoroso S, Schmid-Antomarchi H, Fosset M, et al. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247:852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- 28.Shyng S, Nichols CG. Octameric stoichiometry of the KATP channel complex. J Gen Physiol. 1997;110:655–664. doi: 10.1085/jgp.110.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clement JP, Kunjilwar K, Gonzalez G, et al. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki N, Gonoi T, Clement JP, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 31.Sakura H, Ammala C, Smith PA, et al. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 32.Inagaki N, Tsuura Y, Namba N, et al. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- 33.Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39:51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Aguilar-Bryan L, Nichols CG, Wechsler SW, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 35.Chutkow WA, Simon MC, Le Beau MM, et al. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 36.Isomoto S, Kondo C, Yamada M, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 37.Sim JH, Yang DK, Kim YC, et al. ATP-sensitive K+ channels composed of Kir6.1 and SUR2B subunits in guinea pig gastric myocytes. Am J Physiol Gastrointest Liver Physiol. 2002;282:G137–G144. doi: 10.1152/ajpgi.00057x.2002. [DOI] [PubMed] [Google Scholar]
- 38.Antcliff JF, Haider S, Proks P, et al. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 2005;24:229–239. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proks P, Ashcroft FM. Modeling KATP channel gating and its regulation. Prog Biophys Mol Biol. 2009;99:7–19. doi: 10.1016/j.pbiomolbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Masia R, Koster JC, Tumini S, et al. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes. 2007;56:328–336. doi: 10.2337/db06-1275. [DOI] [PubMed] [Google Scholar]
- 41.Aittoniemi J, Fotinou C, Craig TJ, et al. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci. 2009;364(1514):257–267. doi: 10.1098/rstb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Nichols CG, Shyng SL, Nestorowicz A, et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 44.Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zingman LV, Alekseev AE, Bienengraeber M, et al. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 46.Mikhailov MV, Campbell JD, de Wet H, et al. 3-D structural and functional characterization of the purified KATP channel complex Kir6.2-SUR1. EMBO J. 2005;24:4166–4175. doi: 10.1038/sj.emboj.7600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zerangue N, Schwappach B, Jan YN, et al. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 48.Bienengraeber M, Alekseev AE, Abraham MR, et al. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- 49.Thomas PM, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1809–1812. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- 50.Thomas PM, Cote GJ, Wohllk N, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- 51.Gloyn AL, Pearson ER, Antcliff J, et al. Activating mutations in the gene encoding the ATP sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Eng J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 52.Colombo C, Delvecchio M, Zecchino C, et al. Transient neonatal diabetes mellitus is associated with a recurrent (R201H) KCNJ11 (KIR6.2) mutation. Diabetologia. 2005;48(11):2439–2441. doi: 10.1007/s00125-005-1958-1. [DOI] [PubMed] [Google Scholar]
- 53.Yorifuji T, Nagashima K, Kurokawa K, et al. The C42R mutation in the Kir6.2 (KCNJ11) gene as a cause of transient neonatal diabetes, childhood diabetes, or later-onset, apparently type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90(6):3174–3178. doi: 10.1210/jc.2005-0096. [DOI] [PubMed] [Google Scholar]
- 54.Riedel MJ, Steckley DC, Light PE. Current status of the E23K Kir6.2 polymorphism: implications for type-2 diabetes. Hum Genet. 2005;116(3):133–145. doi: 10.1007/s00439-004-1216-5. [DOI] [PubMed] [Google Scholar]
- 55.Tarasov AI, Nicolson TJ, Riveline JP, et al. A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive K+ channel activity and beta-cell glucose sensing is associated with type 2 diabetes in adults. Diabetes. 2008;57(6):1595–1604. doi: 10.2337/db07-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunne MJ, Cosgrove KE, Shepherd RM, et al. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev. 2004;84:239–275. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- 57.Dunne MJ, Kane C, Shepherd RM, et al. Familial persistent hyperinsulinemic hypoglycemia of infancy and mutations in the sulfonylurea receptor. N Engl J Med. 1997;336(10):703–706. doi: 10.1056/NEJM199703063361005. [DOI] [PubMed] [Google Scholar]
- 58.Shimomura K, Flanagan SE, Zadek B, et al. Adjacent mutations in the gating loop of Kir6.2 produce neonatal diabetes and hyperinsulinism. EMBO Mol Med. 2009;1:166–177. doi: 10.1002/emmm.200900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glaser B, Thornton P, Otonkoski T, et al. Genetics of neonatal hyperinsulinism. Arch Dis Child. 2000;82:F79–F86. doi: 10.1136/fn.82.2.F79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flanagan SE, Clauin S, Bellanne-Chantelot C, et al. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- 61.Taschenberger G, Mougey A, Shen S, et al. Identification of a familial hyperinsulinism causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J Biol Chem. 2002;277:17139–17146. doi: 10.1074/jbc.M200363200. [DOI] [PubMed] [Google Scholar]
- 62.Partridge CJ, Beech DJ, Sivaprasadarao A. Identification and pharmacological correction of a membrane trafficking defect associated with a mutation in the sulfonylurea receptor causing familial hyperinsulinism. J Biol Chem. 2001;276:35947–35952. doi: 10.1074/jbc.M104762200. [DOI] [PubMed] [Google Scholar]
- 63.Yan F, Lin CW, Weisiger E, et al. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J Biol Chem. 2004;279:11096–11105. doi: 10.1074/jbc.M312810200. [DOI] [PubMed] [Google Scholar]
- 64.Huopio H, Reimann F, Ashfield R, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest. 2000;106:897–906. doi: 10.1172/JCI9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuo M, Trapp S, Tanizawa Y, et al. Functional analysis of a mutant sulfonylurea receptor, SUR1–1420C, that is responsible for persistent hyperinsulinemic hypoglycemia of infancy. J Biol Chem. 2000;275:41184–41191. doi: 10.1074/jbc.M006503200. [DOI] [PubMed] [Google Scholar]
- 66.Shyng SL, Ferrigni T, Shepard JB, et al. Functional analyses of novel mutations in the sulfonylurea receptor 1 associated with persistent hyperinsulinemic hypoglycemia of infancy. Diabetes. 1998;47:1145–1151. doi: 10.2337/diabetes.47.7.1145. [DOI] [PubMed] [Google Scholar]
- 67.Pinney SE, MacMullen C, Becker S, et al. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118:2877–2886. doi: 10.1172/JCI35414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amato ED, Tammaro P, Craig TJ, et al. Variable phenotypic spectrum of diabetes mellitus in a family carrying a novel KCNJ11 gene mutation. Diabet Med. 2008;25:651–656. doi: 10.1111/j.1464-5491.2008.02443.x. [DOI] [PubMed] [Google Scholar]
- 69.Shimomura K, Girard CA, Proks P, et al. Mutations at the same residue (R50) of Kir6.2 (KCNJ11) that cause neonatal diabetes produce different functional effects. Diabetes. 2006;55:1705–1712. doi: 10.2337/db05-1640. [DOI] [PubMed] [Google Scholar]
- 70.Girard C, Shimomura K, Proks P, et al. Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes. Pflugers Arch. 2006;453:323–332. doi: 10.1007/s00424-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 71.Tarasov AI, Girard CA, Larkin B, et al. Functional analysis of two K ir6. 2 (KCNJ11) mutations, K170T and E322K, causing neonatal diabetes. Diabetes Obes Metab. 2007;9(Suppl 2):46–55. doi: 10.1111/j.1463-1326.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 72.Klupa T, Edghill EL, Nazim J, et al. The identification of a R201H mutation in KCNJ11, which encodes Kir6.2, and successful transfer to sustained-release sulphonylurea therapy in a subject with neonatal diabetes: evidence for heterogeneity of beta cell function among carriers of the R201H mutation. Diabetologia. 2005;48:1029–1031. doi: 10.1007/s00125-005-1731-5. [DOI] [PubMed] [Google Scholar]
- 73.Flanagan SE, Edghill EL, Gloyn AL, et al. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49:1190–1197. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]
- 74.Flanagan SE, Patch AM, Mackay DJ, et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slingerland AS, Hurkx W, Noordam K, et al. Sulphonylurea therapy improves cognition in a patient with the V59M KCNJ11 mutation. Diabet Med. 2008;25:277–281. doi: 10.1111/j.1464-5491.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 76.Doyle DA, Morais Cabral J, Pfuetzner RA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 77.Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 78.Proks P, Girard C, Baevre H, et al. Functional effects of mutations at F35 in the NH2-terminus of Kir6.2 (KCNJ11), causing neonatal diabetes, and response to sulfonylurea therapy. Diabetes. 2006;55(6):1731–1737. doi: 10.2337/db05-1420. [DOI] [PubMed] [Google Scholar]
- 79.Reimann F, Tucker SJ, Proks P, et al. Involvement of the N-terminus of Kir6.2 in coupling to the sulphonylurea receptor. J Physiol. 1999;518:325–336. doi: 10.1111/j.1469-7793.1999.0325p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Babenko AP, Bryan J. SUR-dependent modulation of KATP channels by an N-terminal Kir6.2 peptide: defining intersubunit gating interactions. J Biol Chem. 2002;277:43997–44004. doi: 10.1074/jbc.M208085200. [DOI] [PubMed] [Google Scholar]
- 81.Koster JC, Kurata HT, Enkvetchakul D, et al. A DEND mutation in Kir6.2 (KCNJ11) reveals a flexible N-terminal region critical for ATP-sensing of the KATP channel. Biophys J. 2008;95:4689–4697. doi: 10.1529/biophysj.108.138685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Proks P, Girard C, Ashcroft FM. Functional effects of KCNJ11 mutations causing neonatal diabetes: enhanced activation by Mg-ATP. Hum Mol Genet. 2005;14:2717–2726. doi: 10.1093/hmg/ddi305. [DOI] [PubMed] [Google Scholar]
- 83.Tarasov AI, Welters HJ, Senkel S, et al. A Kir6.2 mutation causing neonatal diabetes impairs electrical activity and insulin secretion from INS-1 beta-cells. Diabetes. 2006;55:3075–3082. doi: 10.2337/db06-0637. [DOI] [PubMed] [Google Scholar]
- 84.Männikkö R, Jefferies C, Flanagan SE, et al. Interaction between mutations in the slide helix of Kir6.2 associated with neonatal diabetes and neurological symptoms. Hum Mol Genet. 2010;19(6):963–972. doi: 10.1093/hmg/ddp554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gloyn AL, Reimann F, Girard C, et al. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925–934. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- 86.John SA, Weiss JN, Xie LH, et al. Molecular mechanism for ATP-dependent closure of the K+ channel Kir6.2. J Physiol. 2003;552:23–34. doi: 10.1113/jphysiol.2003.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Proks P, Arnold AL, Bruining J, et al. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- 88.Proks P, Shimomura K, Craig TJ, et al. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum Mol Genet. 2007;16:2011–2019. doi: 10.1093/hmg/ddm149. [DOI] [PubMed] [Google Scholar]
- 89.Fang K, Csanady L, Cha KW. The N-terminal transmembrane domain (TMD0) and a cytosolic linker (L0) of sulphonylurea receptor define the unique intrinsic gating of KATP channels. J Physiol. 2006;576:379–389. doi: 10.1113/jphysiol.2006.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masia R, de Leon DD, MacMullen C, et al. A mutation in the TMD0–L0 region of sulfonylurea receptor-1 (L225P) causes permanent neonatal diabetes mellitus (PNDM) Diabetes. 2007;56:1357–1362. doi: 10.2337/db06-1746. [DOI] [PubMed] [Google Scholar]
- 91.Patch AM, Flanagan SE, Boustred C, et al. Mutations in the ABCC8 gene encoding the SUR1 subunit of the KATP channel cause transient neonatal diabetes, permanent neonatal diabetes or permanent diabetes diagnosed outside the neonatal period. Diabetes Obes Metab. 2007;9(Suppl 2):28–39. doi: 10.1111/j.1463-1326.2007.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Babenko AP, Bryan J. SUR domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem. 2003;278:41577–41580. doi: 10.1074/jbc.C300363200. [DOI] [PubMed] [Google Scholar]
- 93.Tammaro P, Girard C, Molnes J, et al. Kir6.2 mutations causing neonatal diabetes provide new insights into Kir6.2-SUR1 interactions. EMBO J. 2005;24:2318–2330. doi: 10.1038/sj.emboj.7600715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Wet H, Rees MG, Shimomura K, et al. Increased ATPase activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:18988–18992. doi: 10.1073/pnas.0707428104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Wet H, Proks P, Lafond M, et al. A mutation (R826 W) in nucleotide-binding domain 1 of ABCC8 reduces ATPase activity and causes transient neonatal diabetes. EMBO Rep. 2008;9:648–654. doi: 10.1038/embor.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ellard S, Flanagan SE, Girard CA, et al. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;81:375–382. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Markworth E, Schwanstecher C, Schwanstecher M. ATP4–mediates closure of pancreatic beta-cell ATP-sensitive potassium channels by interaction with 1 of 4 identical sites. Diabetes. 2000;49:1413–1418. doi: 10.2337/diabetes.49.9.1413. [DOI] [PubMed] [Google Scholar]
- 98.Hani EH, Boutin P, Durand E, et al. Missense mutations in the pancreatic islet beta cell inwardly rectifying K+ channel gene (KIR6.2/BIR): a meta-analysis suggests a role in the polygenic basis of type II diabetes mellitus in Caucasians. Diabetologia. 1998;41:1511–1515. doi: 10.1007/s001250051098. [DOI] [PubMed] [Google Scholar]
- 99.Gloyn AL, Weedon MN, Owen KR, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 100.Barroso I, Luan J, Middelberg RP, et al. Candidate gene association study in type 2 diabetes indicates a role for genes involved in beta-cell function as well as insulin action. PLoS Biol. 2003;1:E20. doi: 10.1371/journal.pbio.0000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamming KS, Soliman D, Matemisz LC, et al. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K channel. Diabetes. 2009;58:2419–2424. doi: 10.2337/db09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sperling MA. Neonatal diabetes mellitus: from understudy to center stage. Curr Opin Pediatr. 2005;17(4):512–518. doi: 10.1097/01.mop.0000170517.20025.51. [DOI] [PubMed] [Google Scholar]
- 103.Polak M, Shield J. Neonatal and very-early-onset diabetes mellitus. Semin Neonatol. 2004;9:59–65. doi: 10.1016/S1084-2756(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 104.Greeley SA, Tucker SE, Worrell HI, et al. Update in neonatal diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(1):13–19. doi: 10.1097/MED.0b013e328334f158. [DOI] [PubMed] [Google Scholar]
- 105.Sagen JV, Raeder H, Hathout E, et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53:2713–2718. doi: 10.2337/diabetes.53.10.2713. [DOI] [PubMed] [Google Scholar]
- 106.Stanik J, Gasperikova D, Paskova M, et al. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab. 2007;92:1276–1282. doi: 10.1210/jc.2006-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 108.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Eng J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 109.Winkler M, Stephan D, Bieger S, et al. Testing the bipartite model of the sulfonylurea receptor binding site: binding of A-, B-, and A B-site ligands. J Pharmacol Exp Ther. 2007;322:701–708. doi: 10.1124/jpet.107.123224. [DOI] [PubMed] [Google Scholar]
- 110.Ashcroft FM. New uses for old drugs: neonatal diabetes and sulphonylureas. Cell Metab. 2010;11:179–181. doi: 10.1016/j.cmet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Stoy J, Greeley SA, Paz VP, et al. Diagnosis and treatment of neonatal diabetes: an United States experience. Pediatr Diabetes. 2008;9:450–459. doi: 10.1111/j.1399-5448.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Proks P, Reimann F, Green N, et al. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51(Suppl 3):S368–S376. doi: 10.2337/diabetes.51.2007.s368. [DOI] [PubMed] [Google Scholar]
- 113.Zung A, Glaser B, Nimri R, et al. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004;89:5504–5507. doi: 10.1210/jc.2004-1241. [DOI] [PubMed] [Google Scholar]
- 114.Manna TD, Battistim C, Radonsky V, et al. Glibenclamide unresponsiveness in a brazilian child with permanent neonatal diabetes mellitus and DEND syndrome due to a C166Y mutation in KCNJ11 (Kir6.2) gene. Arq Bras Endocrinol Metabol. 2008;52(8):1350–1355. doi: 10.1590/s0004-27302008000800024. [DOI] [PubMed] [Google Scholar]
- 115.Koster JC, Remedi MS, Dao C, et al. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes. 2005;54:2645–2654. doi: 10.2337/diabetes.54.9.2645. [DOI] [PubMed] [Google Scholar]
- 116.Pearson ER, Flechtner I, Njolstad PR, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Eng J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 117.Chen K, Wang R, Wen SY, et al. Relationship of P450 2C9 genetic polymorphisms in Chinese and the pharmacokinetics of tolbutamide. J Clin Pharm Ther. 2005;30(3):241–249. doi: 10.1111/j.1365-2710.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 118.Kirchheiner J, Roots I, Goldammer M, et al. Effect of genetic polymorphisms in cytochrome p450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet. 2005;44(12):1209–1225. doi: 10.2165/00003088-200544120-00002. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki K, Yanagawa T, Shibasaki T, et al. Effect of CYP2C9 genetic polymorphisms on the efficacy and pharmacokinetics of glimepiride in subjects with type 2 diabetes. Diabetes Res Clin Pract. 2006;72(2):148–154. doi: 10.1016/j.diabres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 120.Becker ML, Visser LE, Trienekens PH, et al. Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther. 2008;83(2):288–292. doi: 10.1038/sj.clpt.6100273. [DOI] [PubMed] [Google Scholar]
- 121.Slingerland AS, Nuboer R, Hadders-Algra M, et al. Improved motor development and good long-term glycemic control with sulfonylurea treatment in a patient with the syndrome of intermediate developmental delay, early-onset generalised epilepsy and neonatal diabetes associated with the V59M mutation in the KCNJ11 gene. Diabetologia. 2006;49:2559–2563. doi: 10.1007/s00125-006-0407-0. [DOI] [PubMed] [Google Scholar]
- 122.Mlynarski W, Tarasov A, Gach A, et al. Sulfonylurea improves CNS function in a case of intermediate DEND syndrome caused by a mutation in KCNJ11. Nat Clin Pract Neurol. 2007;3(11):640–645. doi: 10.1038/ncpneuro0640. [DOI] [PubMed] [Google Scholar]
- 123.Koster JC, Cadario F, Peruzzi C, et al. The G53D mutation in Kir6.2 (KCNJ11) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. J Clin Endocrinol Metab. 2008;93:1054–1061. doi: 10.1210/jc.2007-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Turkkahraman D, Bircan I, Tribble ND, et al. Permanent neonatal diabetes mellitus caused by a novel homozygous (T168A) glucokinase (GCK) mutation: initial response to oral sulphonylurea therapy. J Pediatr. 2008;153:122–126. doi: 10.1016/j.jpeds.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 125.Søvik O, Njølstad P, Følling I, et al. Hyperexcitability to sulphonylurea in MODY3. Diabetologia. 1998;41(5):607–608. doi: 10.1007/s001250050956. [DOI] [PubMed] [Google Scholar]
- 126.Pearson ER, Liddell WG, Shepherd M, et al. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1 alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med. 2000;17(7):543–545. doi: 10.1046/j.1464-5491.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- 127.Gribble FM, Reimann F. Differential selectivity of insulin secret-agogues mechanisms, clinical implications, and drug interactions. J Diabetes Complications. 2003;17(Suppl 2):11–15. doi: 10.1016/s1056-8727(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 128.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 129.Inagaki N, Gonoi T, Clement JP, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 130.Gribble FM, Tucker SJ, Seino S, et al. Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes. 1998;47(9):1412–1418. doi: 10.2337/diabetes.47.9.1412. [DOI] [PubMed] [Google Scholar]
- 131.Gribble FM, Ashcroft FM. Differential sensitivity of beta-cell and extrapancreatic K(ATP) channels to gliclazide. Diabetologia. 1999;42(7):845–848. doi: 10.1007/s001250051236. [DOI] [PubMed] [Google Scholar]
- 132.Song DK, Ashcroft FM. Glimepiride block of cloned beta-cell, cardiac and smooth muscle K(ATP) channels. Br J Pharmacol. 2001;133(1):193–199. doi: 10.1038/sj.bjp.0704062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miki T, Minami K, Zhang L, et al. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:E1178–E1184. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]
- 134.Chutkow WA, Samuel V, Hansen PA, et al. Disruption of SUR2-containing K(ATP) channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci U S A. 2001;98:11760–11764. doi: 10.1073/pnas.201390398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skupien J, Malecki MT, Mlynarski W, et al. Assessment of insulin sensitivity in adults with permanent neonatal diabetes mellitus due to mutations in the KCNJ11 gene encoding Kir6.2. Rev Diabet Stud. 2006;3(1):17–20. doi: 10.1900/RDS.2006.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malecki MT, Skupien J, Klupa T, et al. Transfer to sulphonylurea therapy in adult subjects with permanent neonatal diabetes due to KCNJ11-activating mutations: evidence for improvement in insulin sensitivity. Diabetes Care. 2007;30(1):147–149. doi: 10.2337/dc06-1628. [DOI] [PubMed] [Google Scholar]
- 137.Tessier D, Dawson K, Tétrault JP, et al. Glibenclamide vs gliclazide in type 2 diabetes of the elderly. Diabet Med. 1994;11(10):974–980. doi: 10.1111/j.1464-5491.1994.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 138.Hattersley A, Bruining J, Shield J, et al. ISPAD Clinical Practice Consensus Guidelines 2006–2007. The diagnosis and management of monogenic diabetes in children. Pediatr Diabetes. 2006;7(6):352–360. doi: 10.1111/j.1399-5448.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 139.Pun P, Clark R, Wan KW, et al. Neonatal diabetes mellitus: the impact of molecular diagnosis. NeoReviews. 2010;11(6):e306–e310. [Google Scholar]
- 140.DiStefano JK, Watanabe RM. Pharmacogenetics of anti-diabetes drugs. Pharmaceuticals. 2010;3(8):2610–2646. doi: 10.3390/ph3082610. [DOI] [PMC free article] [PubMed] [Google Scholar]